Introduction
Liver cancer is one of the leading malignancies
worldwide. The etiology of liver cancer varies, which makes the
diagnosis and therapeutic strategy a significant challenge. For
example, hepatitis B virus infection is the prevalent cause of
liver cancer in China and South Africa, whereas >70% of liver
cancer cases in Japan and the US are associated with chronic
infection with hepatitis C virus (1).
Although numerous clinical trials and translational studies have
increased the understanding of the molecular mechanisms that drive
the initiation and progression of liver cancer, the prognosis of
advanced liver cancer remains poor, and novel therapeutics are
urgently required (2). For this
purpose, traditional Chinese medicine (TCM) has attracted
increasing interest as Chinese medicinal herbs can target multiple
signaling pathways that are involved in cancer cell survival and
proliferation (3,4). Additionally, TCM facilitates
conventional chemotherapy to promote liver cancer cell apoptosis
and reduce dose-related cytotoxicity of chemotherapeutics (5). Therefore, TCM deserves further
investigation as an optimal strategy for advanced liver cancer.
The Chinese medicinal herb Pulsatilla
chinensis (Bunge) Regel and its derivates exert various
bioactivities, including anti-inflammatory (6), anti-oxidative (7), anti-bacterial and anti-fungal effects
(8). Notably, Pulsatilla
chinensis (Bunge) Regel extracts have been demonstrated to
inhibit cancer cell proliferation in vitro and in
vivo, including in A549 lung cancer cells (9), SK-MEL-2 melanoma cells (10,11) and
MCF-7 breast cancer cells (12,13). These
results have prompted innovative research investigating the
isolation of biologically active components of this herb. Previous
studies have suggested that Pulsatilla saponin A is one of the
anticancer components, which kills cancer cells through the
induction of cell cycle arrest and apoptosis (14,15).
However, Pulsatilla saponin A exhibits severe hemolytic toxicity,
which could be a major obstacle for its clinical application as an
anticancer agent (16). Anemoside B4
(AB4) is another major component of Pulsatilla chinensis
(Bunge) Regel extracts. However, to the best of our knowledge, its
biological activity is largely unknown. AB4 is well tolerated and
exhibits minimal dose-related cytotoxicity. Therefore, deciphering
its pharmacological potential could be a promising strategy for the
treatment of patients with advanced liver cancer.
Notch signaling was initially noticed due to the
appearance of a notch in the wings of fruit flies, and was
subsequently found to serve a critical role in embryonic
development. Activation of Notch signaling by its ligand leads to
Notch intracellular domain (NICD) release and nucleus
translocation, where NICD cooperates with the DNA-binding protein
CSL to form a transcriptional active heterodimer, which activates
Notch-targeted gene expression (17).
Notably, aberrant activation of Notch has been detected in multiple
malignancies, and further in-depth laboratory investigations have
demonstrated that activation of Notch signaling not only directly
leads to tumorigenesis, but also cross-talks with numerous other
pathways implicated in tumor invasion, migration and metastasis
(18). For example, transgenic mice
carrying liver-specific constitutively activated Notch develop
liver cancer once they reach adult age, and upregulation of Notch1
expression is an unfavorable prognostic biomarker for patients with
liver cancer (18). Notch1 activation
contributes to liver cancer cell growth and proliferation, whereas
Notch1 downregulation inhibits invasion and migration by
inactivating the cyclooxygenase-2/Snail/E-cadherin signaling
pathway or via its interaction with PTEN and focal adhesion kinase
(19). These data indicate that Notch
is a potent therapeutic target for liver cancer. In agreement with
this notion, several ongoing phase I/II trials are evaluating the
safety and efficacy of Notch inhibitors in multiple solid tumors
(20–22). The γ-secretase inhibitors (GSIs) have
shown anticancer efficacy in multiple Notch-mutant solid tumors,
while the dose-related cytotoxicity of GSIs largely limits their
clinical application (23).
Therefore, increasing efforts have been made to develop novel Notch
inhibitors (24).
The present study reported the anticancer efficacy
of AB4 in liver cancer in vitro and in vivo. AB4
inhibited liver cancer cell proliferation and induced cell
apoptosis in a time- and dose-dependent manner. Further mechanical
experiments demonstrated that inhibition of Notch signaling might
be implicated in the anticancer efficacy of AB4. Overall, the
present study highlighted the anticancer activity of the medicinal
herb extract AB4, and targeting of Notch signaling by AB4 is a
potential therapeutic approach for advanced liver cancer.
Materials and methods
Reagents
AB4 was purchased from Absin Biotechnology Co., Ltd.
FBS, DMEM, trypsin and penicillin-streptomycin solution were
purchased from HyClone; Cytiva. Primary antibodies against cleaved
caspase-3 (#9661), cleaved poly(ADP-ribose) polymerase (PARP)
(#5625), cytochrome c (#4280), Notch1 (#4380), Jagged1
(#2620), NICD1 (#4380), hes family bHLH transcription factor 1
(Hes1) (#11988), Ki67 (#9027) and β-actin (#4967), and HRP-labeled
secondary anti-rabbit IgG (#7074) were obtained from Cell Signaling
Technology, Inc. The hes related family bHLH transcription factor
with YRPW motif 1 (Hey1) antibody (#ab11723) was from Abcam. The
Annexin V-FITC Apoptosis Detection kit was obtained from EMD
Millipore. DAPT, a γ-secretase inhibitor (GSI) that inhibits the
Notch pathway, MTT and DMSO were purchased from Sigma-Aldrich;
Merck KGaA. The 3,3′-diaminobenzidine reagent was purchased from
Dako; Agilent Technologies, Inc.
Cell culture
The hepatoblastoma HepG2 cell line and
hepatocellular carcinoma Huh-7 cell line were obtained from The
Cell Bank of Type Culture Collection of The Chinese Academy of
Sciences. Cell lines were authenticated using short-tandem repeat
profiling, tested for mycoplasma contamination and used at passage
numbers of <10. Cells were maintained in DMEM supplemented with
10% FBS and 100 U/ml penicillin-streptomycin solution. Cells were
cultured in a humidified incubator with 5% CO2 at
37°C.
MTT assay
Cells were seeded into 96-well plates at a density
of 5×103 cells per well. The cells were cultured
overnight to allow attachment, and then the cells were treated with
increasing concentrations of AB4 or DAPT (50 µmol/l) for different
time periods. Cells were subsequently incubated with 0.5 mg/ml MTT
for another 4 h. The formazan was dissolved in 100 µl DMSO and the
optical density at 570 nm was determined using a micro-array reader
(Bio-Rad Laboratories, Inc.). Each experiment was performed in
triplicate and the data are presented as the mean ± SD.
Colony formation assay
Cells were seeded into 6-well plates at a density of
1×103 cells per well. Following overnight incubation to
allow cell attachment, the cells were grown in medium containing
AB4 (5 and 10 µmol/l) or DAPT (5 µmol/l) for ~14 days. The medium
was refreshed every 3 days. At the end of the experiment, the cell
culture medium was discarded and the cells were washed with PBS
three times. After being fixed with 4% paraformaldehyde for 20 min,
the colonies were visualized using crystal violet staining under a
microscope.
Apoptosis assay
Apoptosis was determined using an Annexin V-FITC
Apoptosis Detection kit (EMD Millipore) according to the
manufacturer's protocols. Briefly, cells were seeded into 6-well
plates at a density of 2.5×105 cells per well, and then
treated with AB4 (50 µmol/l) and DAPT (50 µmol/l) for 24 h. Cells
were resuspended in 95 µl Annexin V-FITC binding buffer mixed with
5 µl Annexin V-FITC, followed by incubation with 10 µl PI. Flow
cytometry was performed using a FACS Calibur flow cytometer.
Western blot analysis
After the indicated treatment, cells were harvested
and lysed in lysis buffer. Equal amounts of extracted protein
samples (10-30 µg) were separated by 10% SDS-PAGE and transferred
onto PVDF membranes. The membranes were blocked with 5% BSA in TBS
with 1% Tween-20 for 1 h, and then incubated with the corresponding
primary antibodies (dilution, 1:1,000) overnight at 4°C. Protein
bands were detected using HRP-conjugated secondary antibodies
(dilution, 1:5,000) and visualized using enhanced chemiluminescence
(Millipore).
Xenograft tumor model
A total number of 20 specific pathogen-free male
nude mice (4 weeks old; 12±2 g) were purchased from Beijing Vital
River Laboratory Animal Technology Co., Ltd. The mice were housed
in temperature (25°C)- and humidity (50%)-controlled feeding rooms
under a 12 h light/dark cycle and provided with unrestricted
amounts of rodent chow and drinkable water. All experiments were
approved by the Ethic Committee of The Hospital 971 of The Navy of
Chinese People's Liberation Army (Qingdao, China) and were
performed in accordance with Animal Ethics Guidelines
(#401LL-2017010). HepG2 cells (~2×106) were resuspended
in 0.2 ml DMEM and subcutaneously injected into the right flank of
the nude mice. When the xenograft tumor volume reached 150
mm3, the tumor-bearing mice were randomly divided into
four groups (n=5 in each group), which received vehicle as a
control, low dose AB4 (60 mg/kg), high dose AB4 (120 mg/kg) or DAPT
(100 mg/kg) for 19 days. Xenograft tumor growth was monitored and
recorded every 2 days. At the end of the experiment, the mice were
humanely sacrificed by intraperitoneal injection of pentobarbital
(100 mg/kg), and tumors were carefully isolated and processed for
histological examination.
Immunohistochemistry (IHC)
Paraformaldehyde-fixed and paraffin-embedded tissue
blocks were cut into 4-µm sections. The sections were dewaxed and
dehydrated, followed by antigen retrieval and endogenous peroxidase
blocking. IHC was performed with anti-Notch1, anti-Hes1 and
anti-Ki67 antibodies (dilution, 1:100) at 4°C overnight.
Subsequently, the slides were washed with PBS and incubated with
HRP-conjugated secondary antibodies (dilution, 1:200) for 1 h at
room temperature. Indicated proteins were visualized using a
DakoEnVision Detection Kit (Dako; Agilent Technologies, Inc.).
Finally, all sections were rinsed with running water,
counterstained with hematoxylin and dehydrated in graded
ethanol.
Statistical analysis
All analyses were performed using SPSS v20.0
software (IBM Corp.) and the results are presented as the mean ±
standard deviation. Statistically significant differences among
groups were determined using one-way ANOVA and Dunnet's least
significant difference post hoc tests. P<0.05 was considered to
indicate a statistically significant difference.
Results
Anticancer effect of AB4 in liver
cancer in vitro
To determine the potential anticancer effects of AB4
in liver cancer, two liver cancer cell lines, namely the HepG2
(hepatoblastoma) and Huh-7 (hepatocellular carcinoma) cell lines,
were treated with increasing concentrations of AB4. The toxic
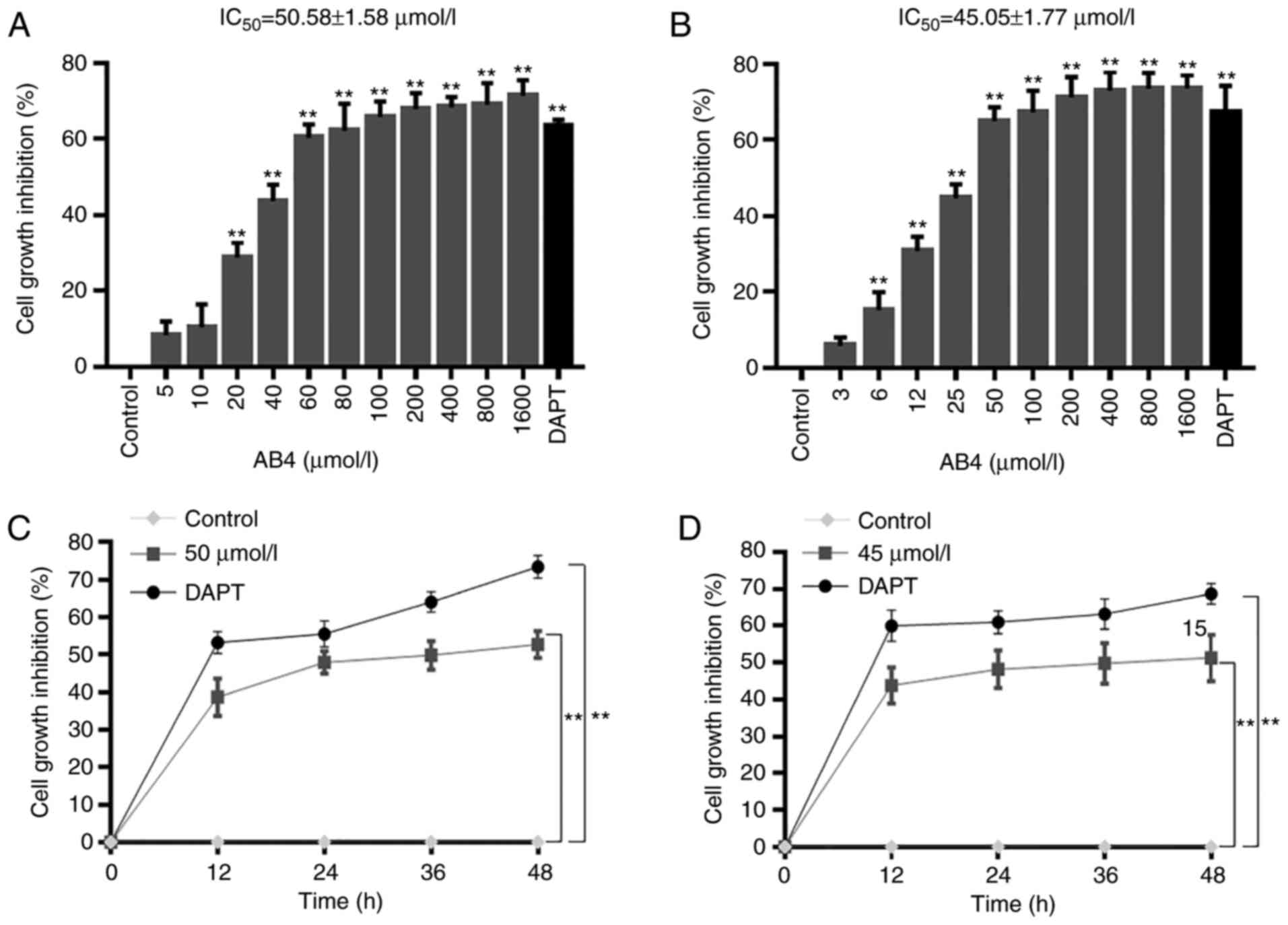
effect of AB4 was evaluated using an MTT assay. As shown in
Fig. 1A, 24 h of AB4 treatment
dose-dependently inhibited HepG2 cell proliferation. AB4
concentrations ranging between 20 and 1,600 µmol/l readily elicited
a growth inhibitory effect, and the estimated half maximal
inhibitory concentration (IC50) at 24 h was ~50 µmol/l.
As a positive control, treatment with 50 µmol/l DAPT, a GSI, led to
>60% cell growth inhibition. In agreement with this, AB4 also
dose-dependently inhibited Huh-7 cell proliferation. The estimated
IC50 of AB4 at 24 h was ~45 µmol/l in Huh-7 cells
(Fig. 1B).
The present study evaluated the time-dependent
cytotoxicity of AB4 in both cell lines. HepG2 and Huh-7 cells were
treated with AB4 at a final concentration of 50 and 45 µmol/l,
respectively. Treatment with 50 µmol/l AB4 resulted in HepG2 cell
growth inhibition to a comparable magnitude of that in cells
treated with 50 µmol/l DAPT. This effect peaked at 24 h and was
maintained at a similar level at 36 and 48 h (Fig. 1C). Furthermore, all these
aforementioned findings could also be observed in Huh-7 cells, in
which treatment with 45 µmol/l AB4 time-dependently suppressed
Huh-7 cell proliferation (Fig. 1D).
These results revealed the anticancer effects of AB4 in liver
cancer cells. Therefore, AB4 at a final concentration of 50 or 45
µmol/l was used in further experiments.
AB4 inhibits liver cancer cell colony
formation
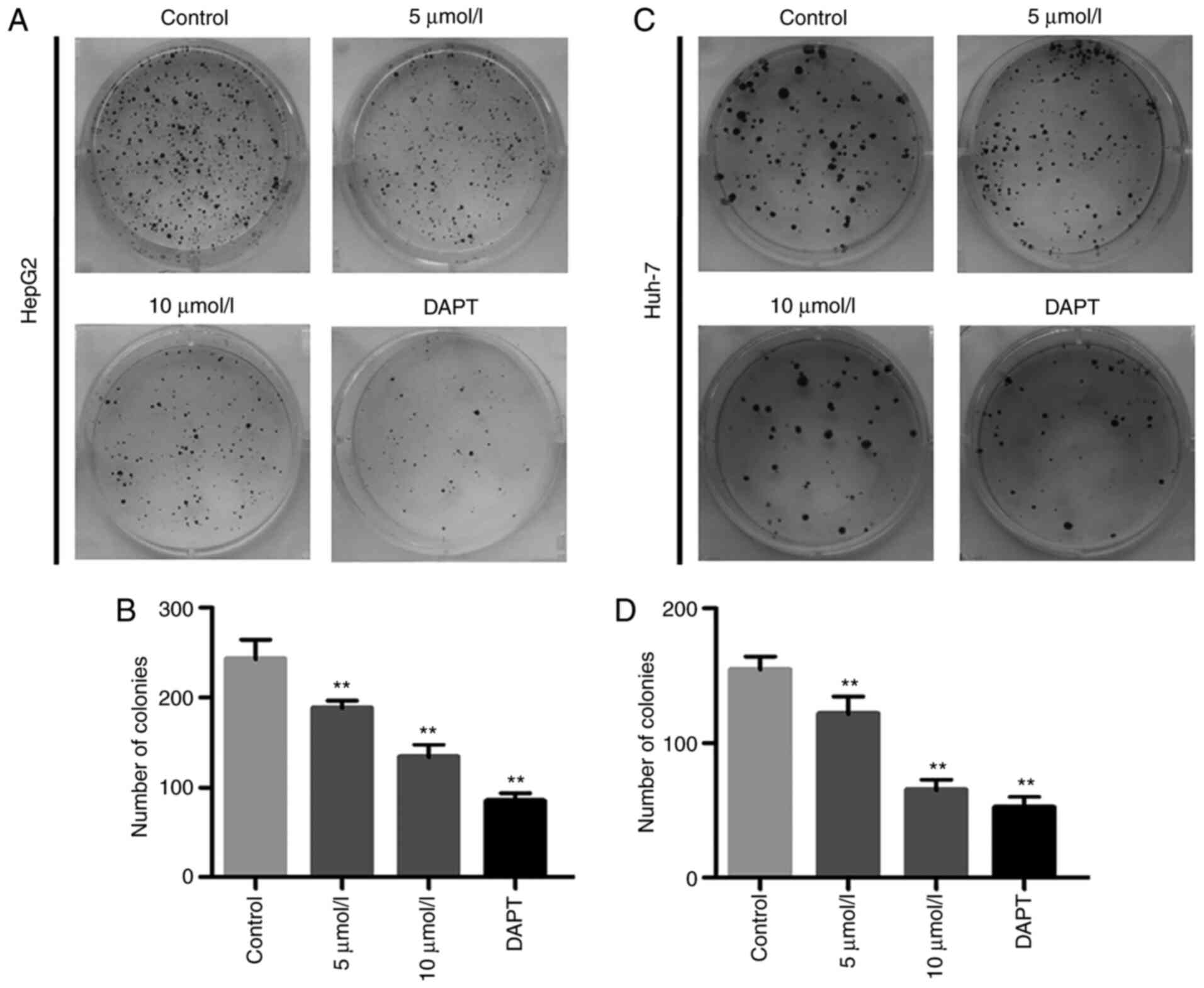
Having established that AB4 elicited cytotoxicity in
liver cancer cell lines after 24 h of treatment, the present study
next investigated whether AB4 also efficiently killed HepG2 and
Huh-7 cells in experiments with relatively longer durations. Cells
were seeded into 6-cm culture plates and cell colonies were readily
visualized after 2 weeks. Chronic exposure to low concentrations of
AB4 (5 and 10 µmol/l) markedly inhibited colony formation in both
cell lines. As shown in Fig. 2,
chronic exposure to AB4 also dose-dependently decreased the numbers
of HepG2 and Huh-7 cell colonies. Notably, the absolute colony
number in the 10 µmol/l AB4 group was approximately half of that in
the vehicle control group. Additionally, DAPT at a final
concentration of 5 µmol/l efficiently inhibited HepG2 and Huh-7
cell colony formation. These findings demonstrated the cytotoxicity
of AB4 in liver cancer cell lines and prompted the investigation of
the underlying mechanism responsible for its anticancer
efficacy.
AB4 induces liver cancer cell
apoptosis
Extensive studies have indicated that liver cancer
cells undergo apoptosis in response to various chemotherapeutic
agents. Therefore, the present study investigated whether the
anticancer effects of AB4 in liver cancer were dependent on the
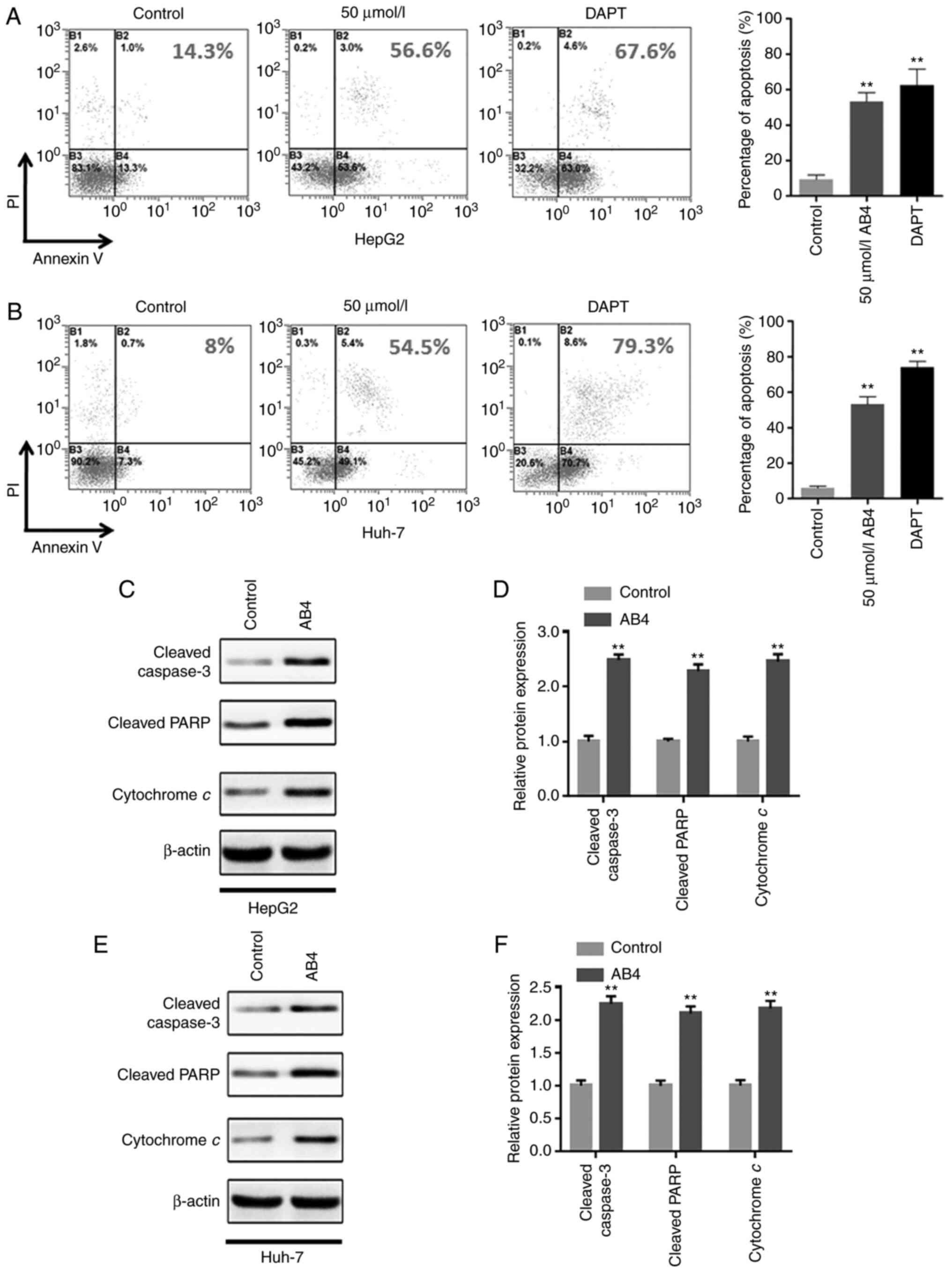
induction of cancer cell apoptosis. Following the indicated
treatment, the cell apoptosis status was determined by flow
cytometry. Fig. 3A shows that 24 h of
50 µmol/l AB4 treatment led to >50% of HepG2 cells undergoing
apoptosis. Importantly, most of the apoptotic cells were at the
early apoptotic stage. This was consistent with the notion that
cytotoxic drugs primarily induce cancer cell apoptosis, rather than
inducing necrosis. In agreement with this, treatment with AB4 at
the same concentration and for the same treatment duration induced
nearly 50% of Huh-7 cells to undergo early-stage apoptosis
(Fig. 3B). Furthermore, treatment
with the GSI DAPT also led to robust early-stage apoptosis in both
cell lines. These compelling results indicated that apoptosis
induction is a crucial mechanism of liver cancer cell death
following AB4 treatment. In addition, the similar findings obtained
for AB4 and DAPT indicated that both drugs may act on the same
signaling pathway to induce liver cancer cell apoptosis.
To validate these findings, western blot analysis
was performed to evaluate the expression levels of
apoptosis-related proteins following the indicated treatment. It
was revealed that AB4 treatment resulted in cytochrome c
release, caspase-3 activation and PARP cleavage (Fig. 3C and D). Notably, the changes of the
aforementioned apoptosis-related proteins were consistent in both
cell lines (Fig. 3E and F). Overall,
these findings indicated that AB4 may be a potential anticancer
candidate drug for liver cancer, and acts primarily through the
induction of cancer cell apoptosis.
Anticancer effect of AB4 in liver
cancer in vivo
Since AB4 exhibited anticancer activity in liver
cancer cell lines, the present study next determined whether this
anticancer effect could also be observed in vivo. Using a
xenograft model, HepG2 cells were injected into the right flank of
4-week-old male null mice. In this case, only HepG2 cells were used
because this cell line rapidly grows and readily forms subcutaneous
tumors in null mice. Treatment was initiated when the volume of the
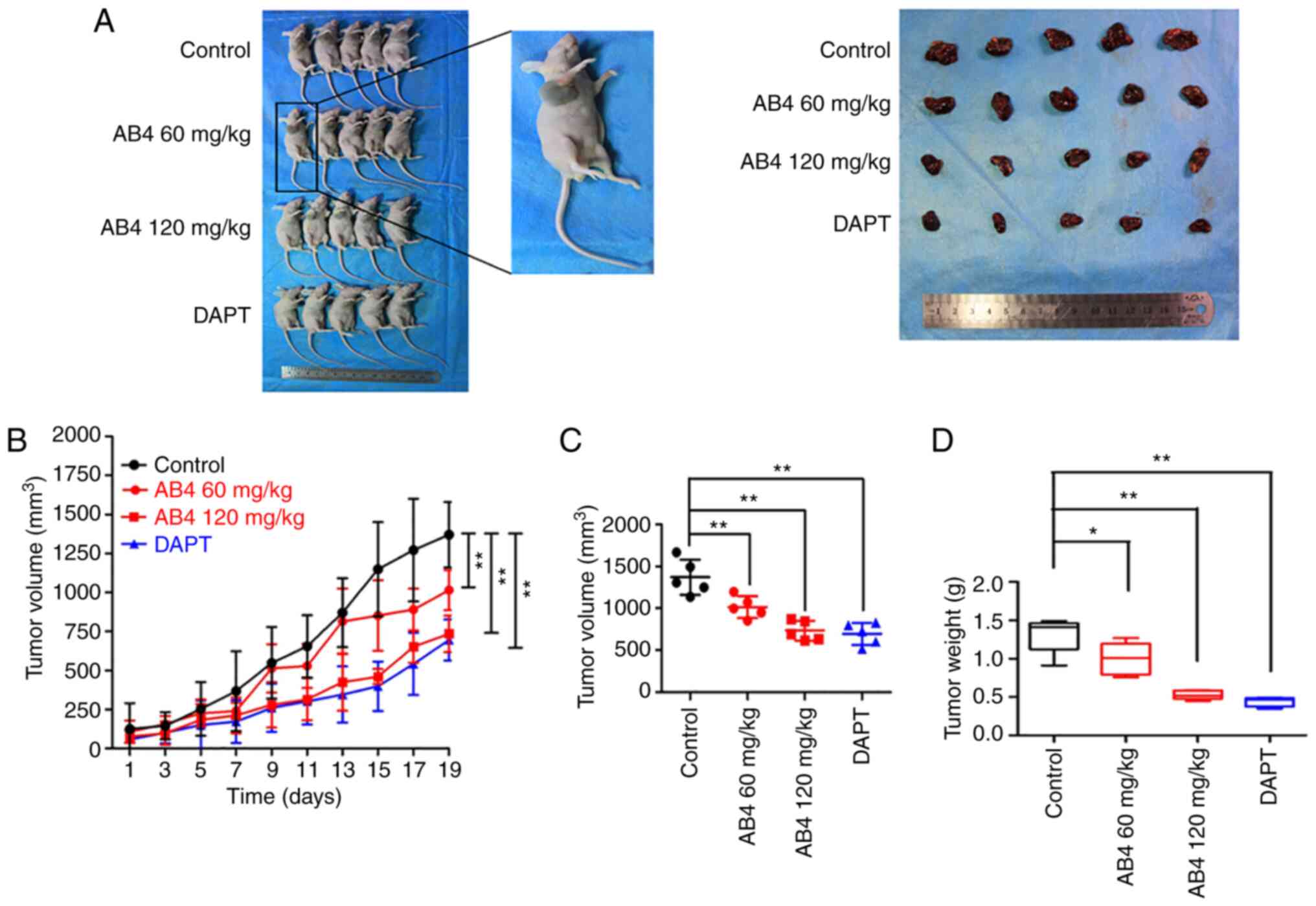
subcutaneous tumors reached 100 mm3. Fig. 4A shows the gross observation of
subcutaneous tumors after 19 days of the indicated treatment. The
dynamic change of tumor size was measured every 2 days. In Fig. 4B, it is shown that the xenograft
tumors in the vehicle group markedly grew in null mice. At the end
of the experiment, the average tumor volume exceeded 1,200
mm3 (maximum tumor size, 1,600 mm3; Fig. 4C). By contrast, the tumor volume
gradually decreased in the AB4 group, and this effect was
positively associated with the dose of AB4. To be specific, the
subcutaneous tumor began to shrink on day 15 in the AB4 low dose
group (60 mg/kg), whereas tumor growth began to shrink on day 9 in
mice fed with a high dose of AB4 (120 mg/kg; Fig. 4C). After 19 days of the indicated
treatment, the subcutaneous tumor size in the low and high dose
groups was ~83 and 50% of that in the vehicle control group,
respectively (Fig. 4B).
The anticancer effects of the GSI DAPT were also
evaluated in vivo. Interestingly, this drug elicited
profound tumor-suppressive effects in HepG2 cells in null mice
(Fig. 4A-C). Notably, the 100 mg/kg
DAPT regimen induced more pronounced anticancer effects. At the end
of the experiment, the tumor weight in the DAPT group was ~33% of
that in the vehicle control group (vs. 40% in the 120 mg/kg AB4
group; Fig. 4D). Both the in
vitro and in vivo experiments highlighted that AB4 and
DAPT exhibited similar anticancer efficacy in hepatocellular
carcinoma (HCC), suggesting the possibility that both drugs may
manipulate the same signaling pathway.
AB4 promotes liver cancer cell
apoptosis in vivo
In order to investigate whether AB4 promotes
apoptosis in vivo, the present study evaluated the
histological alterations of xenograft tumors after the indicated
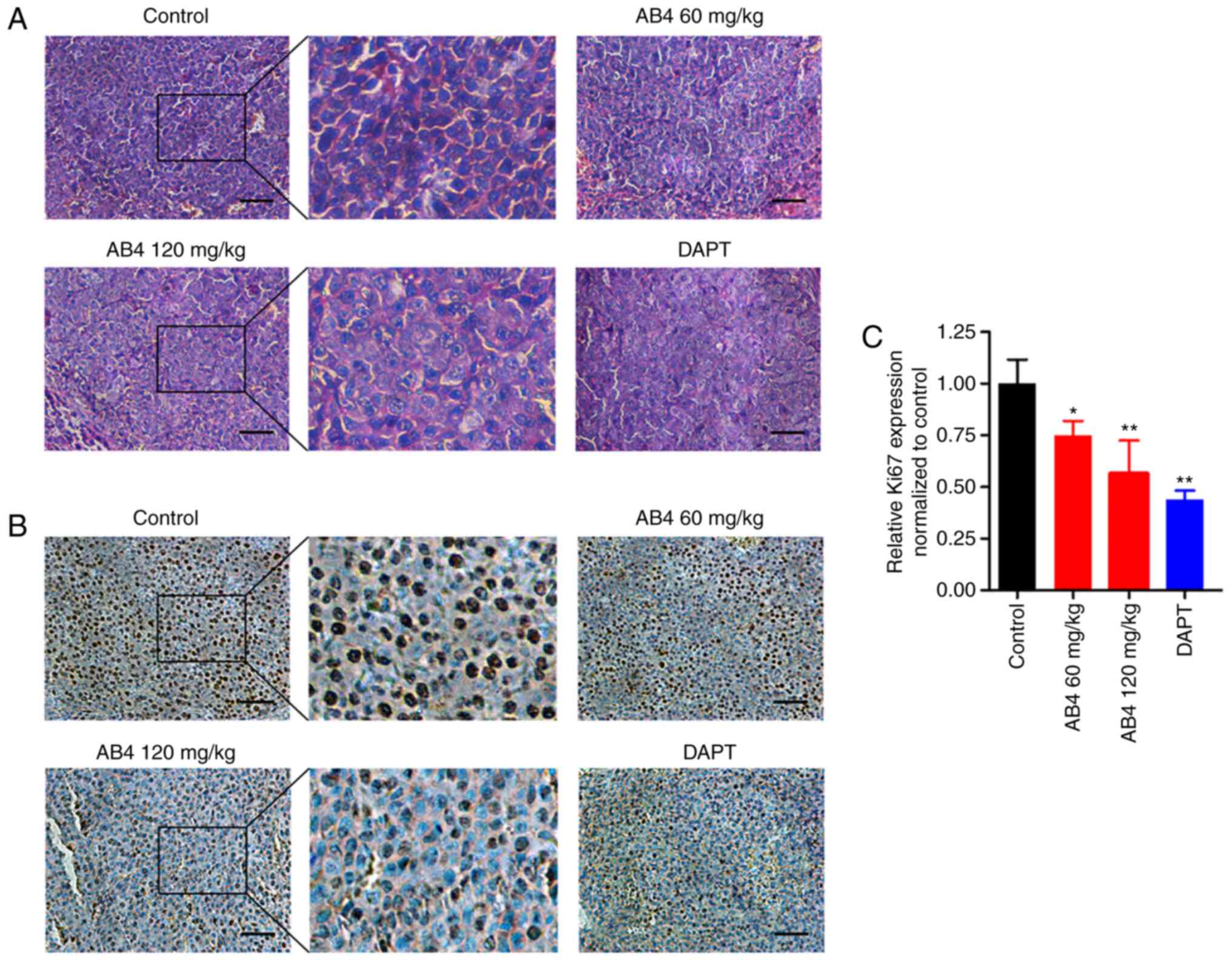
treatment. Fig. 5A shows
representative H&E staining images of xenograft tumors from
formalin-fixed paraffin-embedded tissue blocks. It was evident that
AB4 treatment led to nucleus fragmentation, a morphological
hallmark of cells undergoing apoptosis. In agreement with this, AB4
dose-dependently inhibited HCC cell proliferation, as examined by
Ki67 staining, in xenograft tumors (Fig.
5B). Specifically, qualitative analysis of the Ki67 score
indicated that AB4 at a dose of 60 mg/kg reduced the IHC score to
~75% of that in the vehicle group, while this effect was more
pronounced in the 120 mg/kg AB4 group. Again, DAPT produced similar
anticancer effects in xenograft tumors (Fig. 5C). These data strongly suggested that
AB4 is a potential anticancer drug in liver cancer in vitro
and in vivo, and mainly acts through the induction of
apoptosis, and AB4 and DAPT may act on the same signaling
pathway.
Notch signaling as a therapeutic
target of AB4
The present experiments strongly indicated the
anticancer effects of AB4 in liver cancer in vitro and in
vivo. Additionally, the present study highlighted that
inhibition of Notch signaling by the GSI DAPT exerted anticancer
effects in a similar manner to AB4. These findings strongly
suggested the possibility that AB4 and DAPT may act on the same
signaling pathway and prompted the investigation of whether AB4 and
liver cancer converged at the Notch signaling pathway.
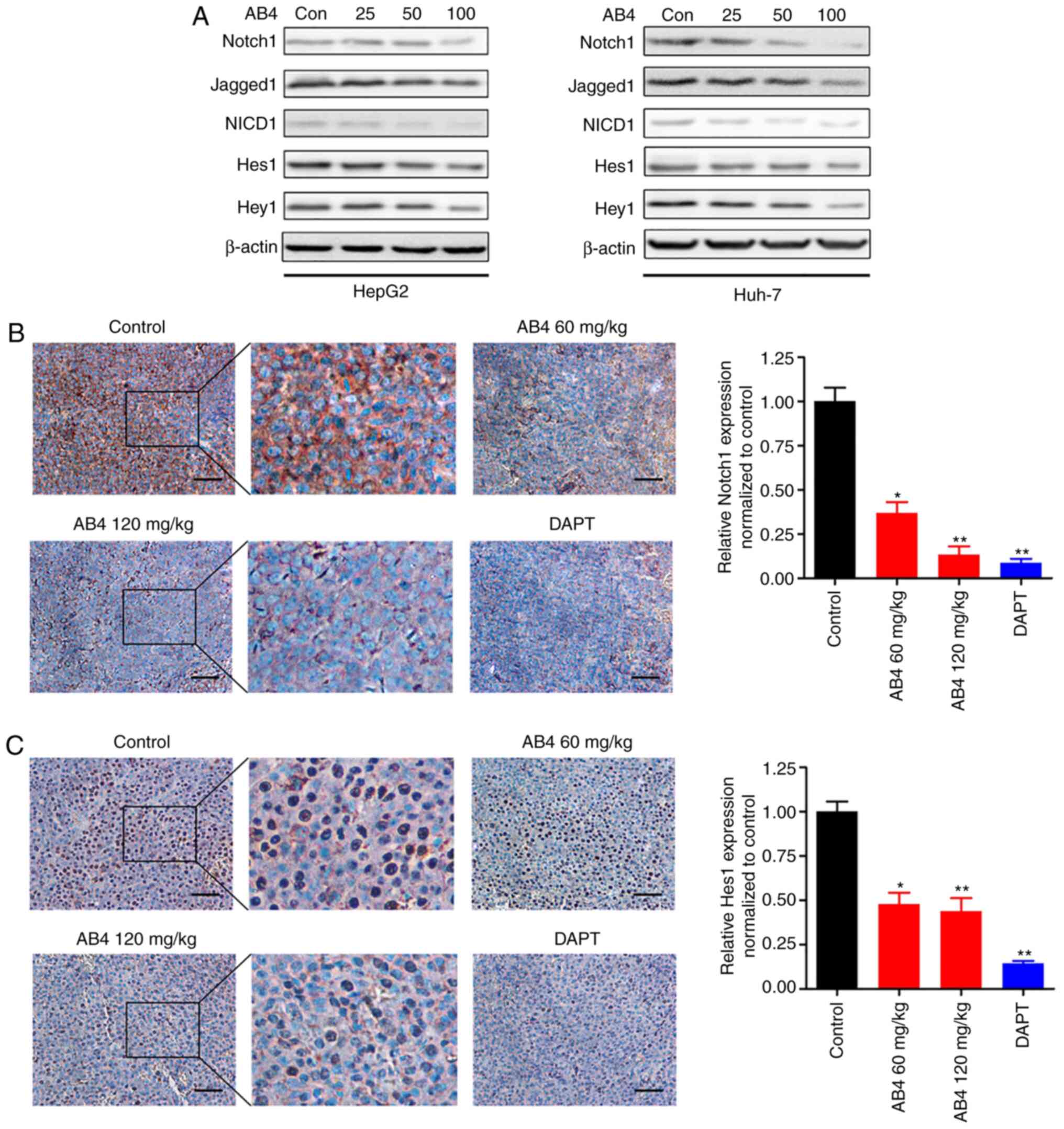
To address these questions, the present study
evaluated the effect of AB4 treatment on the expression levels of
key components of the Notch signaling pathway, including Notch1,
Jagged1, NICD1, Hes1 and Hey1. As illustrated in Fig. 6A, AB4 dose-dependently suppressed
Notch signaling in HepG2 and Huh-7 cells, and this effect was more
evident for higher doses of AB4 (50 and 100 µmol/l). Treatment with
AB4 at final concentrations of 50 and 100 µmol/l primarily
inhibited Notch1 expression and, to a lesser extent, inhibited
Jagged1 and NICD1 expression, suggesting that Notch1 could be the
major molecular target of AB4. Therefore, the expression levels of
downstream targeted proteins, such as Hes1 and Hey1, were
suppressed. Consistent with these findings, the Notch1 protein was
primarily expressed in the cytoplasm in xenograft tumors, whereas
its expression was readily suppressed following treatment with AB4
(Fig. 6B). Furthermore, AB4 treatment
inhibited Hes1 expression in vivo. Fig. 6C shows representative IHC images of
Hes1 expression after the indicated treatment. In contrast to the
Notch1 protein, the Hes1 protein was primarily expressed in the
nucleus in xenograft tumors. However, AB4 treatment markedly
reduced its expression. As a positive control, DAPT readily
suppressed the protein expression of Notch1 and Hes1 in xenograft
tumors. Overall, Notch signaling may be a pharmacological target of
AB4. Inhibition of Notch signaling by AB4 treatment suppressed
liver cancer cell proliferation and induced cancer cell
apoptosis.
Discussion
The present study revealed the anticancer efficacy
of a natural medicinal herb extract, AB4, an extract from the
medical herb Pulsatilla chinensis (Bunge) Regel, in liver
cancer in vitro and in vivo. It was revealed that AB4
readily inhibited HepG2 and Huh-7 cell proliferation and induced
cancer cell apoptosis. Notably, the present study provided
preliminary evidence showing that direct inhibition of Notch
signaling by GSI DAPT killed both cell lines in a similar manner
compared with AB4. Furthermore, it was reported that AB4 treatment
blocked the Notch signaling pathway in vitro and in
vivo. It was concluded that AB4 is a novel therapeutic agent
for liver cancer, and it was speculated that targeting of Notch
signaling underlies its anticancer activity.
Natural occurring phytochemicals have attracted
increasing interest, since they are well tolerated and have broad
spectrum biological activities (25).
Indeed, several commercially available anticancer agents, such as
taxol (26) and vincristine (27), are derived from medicinal herbs.
Pulsatilla chinensis (Bunge) Regel is another paradigm of
natural occurring medicinal herbs, and the bioactivities of its
components have been widely investigated. Pulsatilla saponin A is
the most well documented anticancer bioactive component of
Pulsatilla chinensis (Bunge) Regel (14). However, this agent elicits severe
cytotoxicity in untransformed cells as well. Therefore, exploring
other well tolerated anticancer components is urgently necessary
(16). In contrast to Pulsatilla
saponin A, AB4, is well tolerable and has minimal cytotoxicity in
untransformed cells. It has been widely used as a ‘blood-cooling’
and anti-infectious regimen in TCM (28). However, most studies have focused on
the isolation, purification, pharmacokinetics and distribution
pattern of AB4, and its pharmacological effect on cancer cells is
largely unknown. Therefore, given the compelling clinical need for
liver cancer treatment, this preliminary study was conducted to
examine the anticancer efficacy of AB4.
The present study highlighted the anticancer
efficacy of AB4 in the HepG2 and Huh-7 cell lines. The present
study particularly focused on liver cancer because liver cancer
cells are not sensitive to most cytotoxic agents. Consistently,
chemotherapy fails to elicit marked and durable antitumor activity
in clinical settings, and the 5-year survival of patients with
advanced liver cancer is rather limited. Despite numerous successes
having been achieved in the era of immunotherapy, the efficacy of
immunotherapy in patients with liver cancer is far from
satisfactory, and the response rate of anti-programmed cell death
protein 1 (PD-1)/programmed death-ligand 1 inhibitors, including
nivolumab and pembrolizumab, is <20% (29,30). Given
the pressing clinical needs, the present study reported that AB4
treatment readily suppressed liver cancer cell proliferation and
resulted in apoptosis in vitro and in vivo. AB4
exhibited durable (both short-term and long-term) cytotoxicity in
both cell lines and efficiently inhibited xenograft tumor
outgrowth. Indeed, these pharmacological effects are interesting,
suggesting AB4 as a novel candidate for liver cancer treatment.
Notably, biochemical experiments have demonstrated that AB4
protects human 293 cells from platinum-induced injuries by
increasing reactive oxygen species scavenger activity (31). In an adenine-induced kidney injury rat
model, AB4 administration markedly reduces blood urea nitrogen and
creatinine levels, suppresses the expression of pro-inflammatory
cytokines, and attenuates collagen deposition in the renal
interstitium (32). These findings
highlight a kidney-protective effect of AB4 and may indicate
important clinical significance. Since most cytotoxic agents are
excreted through the kidney, the kidney-protective properties of
AB4 could compensate chemotherapeutic agent-induced kidney injury.
Therefore, it is reasonable to hypothesize that the combination of
AB4 with other chemotherapeutic agents may reduce renal
toxicity.
Another notable finding of the present study was the
observation of the involvement of Notch signaling in the anticancer
activity of AB4. The present study focused on Notch signaling
because the GSI DAPT has similar anticancer efficacy compared with
AB4. This finding was in compliance with the notion that Notch
signaling is hyperactivated in liver cancer, thereby driving
carcinogenesis and tumor progression (33). However, targeting Notch via a
conventional pharmacological approach is technically challenging
and severe adverse effects could occur upon non-selective
inhibition of Notch (34). The
present study highlighted that AB4 treatment readily inhibited
Notch signaling in liver cancer in vitro and in vivo.
This finding not only elucidated the mechanism underlying the
anticancer efficacy of AB4, but also elicited important clinical
significance. Firstly, AB4 could be considered as a novel Notch
inhibitor and it was speculated that AB4 may exert broad spectrum
anticancer activity, particularly in cancers driven by Notch.
Secondly, a combinational strategy would result in more robust
anticancer efficacy. For example, our recent study suggested that
AB4 also inhibits the PI3K/Akt/mTOR signaling pathway (35). Therefore, it is reasonable to
hypothesize that AB4 could be combined with chemotherapeutic agents
to kill cancer cells through inhibition of multiple signaling
pathways. Furthermore, the combinational strategy is expected to be
well tolerated. Since AB4 possesses antioxidant and
anti-inflammatory properties, combination therapy could reduce
cytotoxic agent-induced hepatological and renal toxicity. In
addition, increasing lines of evidence have demonstrated that Notch
is a predictive biomarker for immunotherapy. Patients with
non-small cell lung cancer carrying Notch mutations tend to have a
higher response rate and longer progression-free survival (36). It was predicted that disrupting Notch
signaling by AB4 would facilitate with immunotherapy. Therefore, it
would be interesting to investigate the anticancer efficacy of AB4
and anti-PD-1 combination therapy.
In conclusion, the present study reported the
anticancer efficacy of AB4 in liver cancer. AB4 treatment readily
inhibited liver cancer cell proliferation and induced cell
apoptosis in vitro and in vivo. Furthermore, the
present study demonstrated that Notch is an important pharmacologic
target for AB4. Further experiments evaluating the combinational
strategy of AB4 would be interesting.
Acknowledgements
Not applicable.
Funding
This study was sponsored by grants from the National
Natural Science Foundation of China (#81703768 to QM, #81803400 to
ZX), Postdoctoral Science Foundation of China (#2018T111161 to QM),
and National Natural Science Foundation of Shandong Province
(ZR2018BH045 to ZX).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JZ and QM designed and supervised the research. ZX
and YL performed the majority of the experiments. GL, SX, XL and ZZ
analyzed the data. LS, BL, YZ and TM provided technical support in
conducting the experiments. All authors wrote the manuscript, read
the final manuscript and approved the submission.
Ethics approval and consent to
participate
All experiments were approved by the Ethic Committee
of The Hospital 971 of The Navy of Chinese People's Liberation Army
(Qingdao, China) and were performed in accordance with Animal
Ethics Guidelines (#401LL-2017010).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu X, Zhou J, Zhou N, Zhu J, Feng Y and
Miao X: SYNJ2BP inhibits tumor growth and metastasis by activating
DLL4 pathway in hepatocellular carcinoma. J Exp Clin Cancer Res.
35:1152016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han H, Wang L, Liu Y, Shi X, Zhang X, Li M
and Wang T: Combination of curcuma zedoary and kelp inhibits growth
and metastasis of liver cancer in vivo and in vitro via reducing
endogenous H2S levels. Food Funct. 10:224–234. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang X, Wang N, Cheung F, Lao L, Li C and
Feng Y: Chinese medicines for prevention and treatment of human
hepatocellular carcinoma: Current progress on pharmacological
actions and mechanisms. J Integr Med. 13:142–164. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wu L, Cao KX, Ni ZH, Li WD, Chen ZP, Cheng
HB and Liu X: Effects of Dahuang zhechong pill on
doxorubicin-resistant SMMC-7721 ×enografts in mice. J
Ethnopharmacol. 222:71–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li W, Yan XT, Sun YN, Ngan TT, Shim SH and
Kim YH: Anti-Inflammatory and PPAR transactivational effects of
oleanane-type triterpenoid saponins from the roots of Pulsatilla
koreana. Biomol Ther (Seoul). 22:334–340. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu L, Gu L, Tao X, Xu Y, Qi Y, Yin L, Han
X and Peng J: Effect of dioscin on promoting liver regeneration via
activating Notch1/Jagged1 signal pathway. Phytomedicine.
38:107–117. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu Y, He K and Wang X: Role of Chinese
herbal medicinal ingredients in secretion of cytokines by
PCV2-induced endothelial cells. J Immunotoxicol. 13:141–147. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen Z, Duan H, Tong X, Hsu P, Han L,
Morris-Natschke SL, Yang S, Liu W and Lee KH: Cytotoxicity,
hemolytic toxicity, and mechanism of action of Pulsatilla Saponin D
and its synthetic derivatives. J Nat Prod. 81:465–474. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bang SC, Lee JH, Song GY, Kim DH, Yoon MY
and Ahn BZ: Antitumor activity of Pulsatilla koreana
saponins and their structure-activity relationship. Chem Pharm Bull
(Tokyo). 53:1451–1454. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim Y, Bang SC, Lee JH and Ahn BZ:
Pulsatilla saponin D: The antitumor principle from Pulsatilla
koreana. Arch Pharm Res. 27:915–918. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wan JY, Zhang YZ, Yuan JB, Yang FQ, Chen
Y, Zhou LD and Zhang QH: Biotransformation and metabolic profile of
anemoside B4 with rat small and large intestine microflora by
ultra-performance liquid chromatography-quadrupole time-of-flight
tandem mass spectrometry. Biomed Chromatogr. 31:bmc.38732017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang Y, Bao J, Wang K, Jia X, Zhang C,
Huang B, Chen M, Wan JB, Su H, Wang Y and He C: Pulsatilla Saponin
D inhibits autophagic flux and synergistically enhances the
anticancer activity of chemotherapeutic agents against HeLa cells.
Am J Chin Med. 43:1657–1670. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Q, Chen W, Jiao Y, Hou J, Wu Q, Liu Y
and Qi X: Pulsatilla saponin A, an active molecule from
Pulsatilla chinensis, induces cancer cell death and inhibits
tumor growth in mouse xenograft models. J Surg Res. 188:387–395.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xu L, Cheng G, Lu Y and Wang S: An active
molecule from Pulsatilla chinensis, Pulsatilla saponin A,
induces apoptosis and inhibits tumor growth of human colon cancer
cells without or with 5-FU. Oncol Lett. 13:3799–3802. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tong X, Han L, Duan H, Cui Y, Feng Y, Zhu
Y, Chen Z and Yang S: The derivatives of Pulsatilla saponin A, a
bioactive compound from Pulsatilla chinensis: Their
synthesis, cytotoxicity, haemolytic toxicity and mechanism of
action. Eur J Med Chem. 129:325–336. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Q, Bermingham NA, Finegold MJ and
Zoghbi HY: Requirement of Math1 for secretory cell lineage
commitment in the mouse intestine. Science. 294:2155–2158. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu JN, Jiang L, Jiang JH, Yang X, Li XY,
Zeng JX, Shi RY, Shi Y, Pan XR, Han ZP and Wei LX: Hepatocyte
nuclear factor-1beta enhances the stemness of hepatocellular
carcinoma cells through activation of the Notch pathway. Sci Rep.
7:47932017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Huang T, Zhou Y, Cheng AS, Yu J, To KF and
Kang W: NOTCH receptors in gastric and other gastrointestinal
cancers: Oncogenes or tumor suppressors? Mol Cancer. 15:802016.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Takebe N, Miele L, Harris PJ, Jeong W,
Bando H, Kahn M, Yang SX and Ivy SP: Targeting Notch, Hedgehog, and
Wnt pathways in cancer stem cells: Clinical update. Nat Rev Clin
Oncol. 12:445–464. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Papayannidis C, DeAngelo DJ, Stock W,
Huang B, Shaik MN, Cesari R, Zheng X, Reynolds JM, English PA,
Ozeck M, et al: A Phase 1 study of the novel gamma-secretase
inhibitor PF-03084014 in patients with T-cell acute lymphoblastic
leukemia and T-cell lymphoblastic lymphoma. Blood Cancer J.
5:e3502015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hughes DP, Kummar S and Lazar AJ: New,
tolerable γ-secretase inhibitor takes desmoid down a notch. Clin
Cancer Res. 21:7–9. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li L, Tang P, Li S, Qin X, Yang H, Wu C
and Liu Y: Notch signaling pathway networks in cancer metastasis: A
new target for cancer therapy. Med Oncol. 34:1802017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu CX, Xu A, Zhang CC, Olson P, Chen L,
Lee TK, Cheung TT, Lo CM and Wang XQ: Notch inhibitor PF-03084014
inhibits hepatocellular carcinoma growth and metastasis via
suppression of cancer stemness due to reduced activation of
Notch1-Stat3. Mol Cancer Ther. 16:1531–1543. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhu PL, Fu XQ, Li JK, Tse AK, Guo H, Yin
CL, Chou JY, Wang YP, Liu YX, Chen YJ, et al: Antrodia camphorata
mycelia exert anti-liver cancer effects and inhibit STAT3 signaling
in vitro and in vivo. Front Pharmacol. 9:14492018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang Y, Zhang C, Zhang S, Zhao Z, Wang J,
Song J, Wang Y, Liu J and Hou S: Kanglaite sensitizes colorectal
cancer cells to Taxol via NF-κΒ inhibition and connexin 43
upregulation. Sci Rep. 7:12802017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Peng S, Yang K, Xu Z, Chen S and Ji Y:
Vincristine and sirolimus in the treatment of kaposiform
haemangioendothelioma. J Paediatr Child Health. 55:1119–1124. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu QM, Shu Z, He WJ, Chen LY, Yang SL,
Yang G, Liu YL and Li XR: Antitumor activity of Pulsatilla
chinensis (Bunge) Regel saponins in human liver tumor 7402
cells in vitro and in vivo. Phytomedicine. 19:293–300. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu AX, Finn RS, Edeline J, Cattan S,
Ogasawara S, Palmer D, Verslype C, Zagonel V, Fartoux L, Vogel A,
et al: Pembrolizumab in patients with advanced hepatocellular
carcinoma previously treated with sorafenib (KEYNOTE-224): A
non-randomised, open-label phase 2 trial. Lancet Oncol. 19:940–952.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
El-Khoueiry AB, Sangro B, Yau T, Crocenzi
TS, Kudo M, Hsu C, Kim TY, Choo SP, Trojan J, Welling TH Rd, et al:
Nivolumab in patients with advanced hepatocellular carcinoma
(CheckMate 040): An open-label, non-comparative, phase 1/2 dose
escalation and expansion trial. Lancet. 389:2492–2502. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He L, Zhang Y, Kang N, Wang Y, Zhang Z,
Zha Z, Yang S, Xu Q and Liu Y: Anemoside B4 attenuates
nephrotoxicity of cisplatin without reducing anti-tumor activity of
cisplatin. Phytomedicine. 56:136–146. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gong Q, He LL, Wang ML, Ouyang H, Gao HW,
Feng YL, Yang SL, Du LJ, Li J and Luo YY: Anemoside B4 protects rat
kidney from adenine-induced injury by attenuating inflammation and
fibrosis and enhancing podocin and nephrin expression. Evid Based
Complement Alternat Med. 2019:80310392019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Villanueva A, Alsinet C, Yanger K, Hoshida
Y, Zong Y, Toffanin S, Rodriguez-Carunchio L, Solé M, Thung S,
Stanger BZ and Llovet JM: Notch signaling is activated in human
hepatocellular carcinoma and induces tumor formation in mice.
Gastroenterology. 143:1660–1669.e7. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rizzo P, Mele D, Caliceti C, Pannella M,
Fortini C, Clementz AG, Morelli MB, Aquila G, Ameri P and Ferrari
R: The role of notch in the cardiovascular system: Potential
adverse effects of investigational notch inhibitors. Front Oncol.
4:3842015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xue S, Zhou Y, Zhang J, Xiang Z, Liu Y,
Miao T, Liu G, Liu B, Liu X, Shen L, et al: Anemoside B4 exerts
anti-cancer effect by inducing apoptosis and autophagy through
inhibiton of PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Am
J Transl Res. 11:2580–2589. 2019.PubMed/NCBI
|
|
36
|
Zhang K, Hong X, Song Z, Xu Y, Li C, Wang
G, Zhang Y, Zhao X, Zhao Z, Zhao J, et al: Identification of
deleterious NOTCH mutation as novel predictor to efficacious
immunotherapy in NSCLC. Clin Cancer Res. 26:3649–3661. 2020.
View Article : Google Scholar : PubMed/NCBI
|