Introduction
Gallbladder carcinoma (GBC) is a highly malignant
tumor of the biliary system with a median survival time of only 6
months (1–3). The primary pathological type of GBC
observed in patients is adenocarcinoma. The effects of current
chemotherapeutic regimens are not sufficient for GBC due to the
lack of effective drugs, making it particularly difficult to
control the mortality rate of GBC (3,4). Due to
the close relationship between inflammation and GBC, investigation
of inflammatory-related molecular mechanisms may highlight novel
specific targets for the treatment of GBC (4).
Golgi phosphoprotein 3 (GOLPH3) is a component of
the trans Golgi network (TGN) (5–7).
Physiologically, GOLPH3 stabilizes the Golgi structure and promotes
the sprouting of Golgi vesicles through binding with
phosphatidylinositol 4-phosphate [PtdIns(4)P] and myosin18A
(5,8,9). Excessive
activation of GOLPH3 induces the breakdown of the Golgi apparatus
(8–10). Recently, several studies have reported
that GOLPH3 serves an important role in the progression of several
types of cancer, such as hepatocellular carcinoma, gastric
adenocarcinoma and lung adenocarcinoma (11–14). In
addition, GOLPH3 is involved in the process of chemotherapeutic
resistance, further highlighting its close relationship with tumor
progression (8,15). It has been reported that GOLPH3
promotes tumor growth via several signaling pathways, including the
PI3K/AKT/mTOR pathway (14–17). However, the role and mechanism of
GOLPH3 in GBC remain unknown.
Nucleotide-binding domain leucine-rich repeat (NLR)
and pyrin domain containing receptor 3 (NLRP3) comprise the NLRP3
inflammasomes together with apoptosis associated speck like protein
and pro-caspase-1 (18,19). Physiological levels of NLRP3
activation protects the body's inflammatory immune system via
activation of Caspase-1, whereas uncontrolled activation will lead
to dysregulated inflammation, autoimmune diseases,
neurodegenerative diseases, and even malignant tumors (20–24).
Recently, a number of studies have reported that the overactivation
of NLRP3 is closely associated with the progression of several
malignant tumors (24–27). To date, there are numerous studies on
the downstream effects of NLRP3 (22–25). There
is evidence that the NLRP3/Caspase-1 pathway can promote the
occurrence and progression of adenocarcinoma, and IL-1β, an
important member of the NLRP3 inflammasome, has been found to be
closely related with the proliferation of GBC (25,28).
Nevertheless, the specific molecular mechanisms by which NLRP3 is
pathophysiologically activated remains unknown.
Thus, both GOLPH3 and NLRP3 are associated with
tumor progression. However, the relationship between GOLPH3 and
NLRP3 in tumorigenesis and progression remains unclear. Evidence
has shown that excessive activation of GOLPH3 can cause the
fragmentation of the Golgi apparatus, and the fragmentation of the
TGN is closely associated with the activation of NLRP3 (8,10,21). In addition, PtdIns(4)p is required
when NLRP3 is activated, of which, the free amount of PtdIns(4)p is
associated with GOLPH3 (5,21,29).
Moreover, there is evidence that mTOR can affect the activation of
NLRP3 inflammasomes by regulating reactive oxygen species, and the
activation of mTOR is also largely regulated by GOLPH3 (6,30).
Additionally, GOLPH3 and NLRP3 have been reported to both be
regulated by the same upstream protein PD2 (6,31).
Therefore, it is hypothesized that GOLPH3 may be an upstream factor
of NLRP3. In the present study, the expression levels of GOLPH3 and
NLRP3 in human GBC tissues were detected via immunohistochemistry,
and the clinical data and survival of these patients were analyzed.
Then, it was tested whether GOLPH3 can affect tumor proliferation
via regulation of the NLRP3 inflammasome in vitro. Through
these experiments, the aim was to find out whether GOLPH3 is an
upstream regulator of the NLRP3/Caspase-1 pathway in the
proliferation of GBC cells.
Materials and methods
Cell culture
The human GBC-SD cell line was purchased from
Guangzhou Cellcook Biotech Co., Ltd. Cells were grown in RPMI-1640
medium (Hyclone; Cytiva) supplemented with 10% FBS (Gibco; Thermo
Fisher Scientific, Inc.) in a humidified incubator with 5%
CO2 at 37°C.
Small interfering (si)RNA and plasmid
transfection
Knocking down of GOLPH3 and NLRP3 was performed
using specific siRNA Oligos (Guangzhou RiboBio Co., Ltd.). The
sequences of the siRNAs were as follows: si-negative control (NC)
sense, 5′-UUCUCCGAACGUGUCACGU-3′ and anti-sense,
5′-ACGUGACACGUUCGGAGAA-3′; si-NLRP3 (NLRP3-SiR) sense,
5′-GGCAGACCAUGUGGAUCUATT-3′ and anti-sense,
5′-UAGAUCCACAUGGUCUGCCTT-3′; si-GOLPH3-A (GOLPH3-SiA) sense,
5′-GGUGAGACAUGGAAUCCAU-3′ and anti-sense,
5′-AUGGAUUCCAUGUCUCACC-3′; and si-GOLPH3-B (GOLPH3-SiB) sense,
5′-GCAGCGCCUCAUCAAGAAA-3′ and anti-sense,
5′-UUUCUUGAUGAGGCGCUGC-3′.
Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc.) was used for transfection. A total of 1
day before transfection, 3.5×105 cells were plated in a
6-well plate, such that they were 60–70% confluent at the time of
transfection. Then, 30 nmol of the specific siRNA and 5 µl
Lipofectamine 2000 reagent was mixed with 250 µl Opti-MEM (Gibco;
Thermo Fisher Scientific, Inc.), and left to stand for 5 min. The
diluted siRNA and Lipofectamine 2000 reagent were combined and
incubated for another 20 min. Subsequently, the mixture was added
to the cells. All siRNA silencing experiments were performed three
times independently. siNC was used as the control group.
Additionally, treatment with Lipofectamine 2000 alone, under
equivalent culture conditions, was used as a blank control group
(Mock). All transfections lasted for 6 h in a humidified incubator
with 5% CO2 at 37°C. Then, 48 h after transfection,
subsequent experimentation was performed.
GOLPH3 was overexpressed using a GOLPH3
pcDNA-3×Flag-C plasmid (Guangzhou RiboBio Co., Ltd.). An empty
plasmid (3×Flag) was used as the negative control group. Treatment
with Lipofectamine 2000 alone, under equivalent culture conditions,
was used as a blank control group (Mock). The transfection protocol
of the overexpression plasmid was similar to that of the siRNA,
except that 2 µg plasmid was used for overexpression.
For co-transfections, cells were divided into the
following five groups: i) GOLPH3 + NLRP3-SiNC group, GOLPH3
pcDNA-3×Flag-C plasmid and si-NC; ii) GOLPH3 + NLRP3-SiR group,
GOLPH3 plasmid and si-NLRP3; iii) 3×Flag + NLRP3-SiR group, empty
plasmid (3×Flag) and si-NLRP3; iv) 3×Flag + NLRP3-SiNC group
(negative control), empty plasmid (3×Flag) and siNC; and v) mock
group (blank control), treatment with Lipofectamine 2000 alone
under equivalent culture conditions. The transfection protocol of
the co-transfection was similar to that of the siRNA and the
overexpression plasmid, except that 1 µg plasmid was used for
co-transfection.
EdU incorporation assay
Transfected cells were seeded into 96-well plates at
5×104 cells per well and incubated for 24 h. When the
confluency reached 60–70%, transfection was performed as described
above. After 48 h, the cells were cultured in EdU medium for 2 h
(Guangzhou RiboBio Co., Ltd.). Then, the cells were fixed with 4%
paraformaldehyde for 30 min at room temperature, and treated with
0.5% Triton X-100 for 10 min at room temperature. After washing
with PBS for 5 min with constant shaking, the cells were treated
with 100 µl 1X Apollo® reaction cocktail (Guangzhou
RiboBio Co., Ltd.) for 30 min. The DNA content of cells were
stained with 100 µl of Hoechst 33342 (5 µg/ml) for another 30 min
at room temperature. The results were observed under a fluorescence
microscope (magnification, ×200, Olympus DP80; Olympus
Corporation).
Cell viability assay
Cell viability was measured using a Cell Counting
Kit-8 (CCK-8) assay (Dojindo Molecular Technologies, Inc.) three
times independently. Cells (3.5×104) were plated in a
96-well plate and cultured for 24 h, at 50–60% confluence at the
time of transfection, and then transfected with siRNA or plasmid
for 48 h. A total of 10 µl CCK-8 solution was added to each well
and incubated for a further 2 h. The absorbance at 450 nm (OD450)
was measured using a microplate reader (Autobio Diagnostics Co.,
Ltd.).
Western blotting
The cells were lysed in RIPA lysis buffer (Beijing
Solarbio Science & Technology Co., Ltd.) for 20 min on ice and
collected using a cell scraper (Corning, Inc.). The total protein
concentration was determined using a BCA Protein assay kit (Beijing
Solarbio Science & Technology Co., Ltd.). Equivalent amounts of
protein (40 µg) were loaded per lane to a 10% gel, resolved via
SDS-PAGE, and subsequently transferred to a PVDF membrane. The
membrane was blocked using 3% BSA (Beijing Solarbio Science &
Technology Co., Ltd.) or 5% skimmed milk in TBS with 0.05% Tween-20
(TBST) for 1 h with constant shaking at room temperature. The
membranes were incubated with antibodies against GOLPH3 (1:2,000;
cat. no. ab98023; Abcam), NLRP3 (1:1,000; cat. no. ab263899;
Abcam), Caspase-1/P10 (1:1,000; cat. no. 22915-1-AP; ProteinTech
Group, Inc.), IL-1β (1:1,000; cat. no. ab9722; Abcam), IκB
(1:1,000; cat. no. ab76429; Abcam), phosphorylated (p)-IκB
(1:10,000; cat. no. ab133462; Abcam) and β-actin (1:5,000; cat. no.
66009-1-Ig; ProteinTech Group, Inc.) at 4°C overnight, and then
incubated with horseradish peroxidase (HRP)-conjugated goat
anti-mouse (cat. no. SA00001-1) and goat anti-rabbit (cat. no.
SA00001-2) secondary antibodies (all at 1:10,000; ProteinTech
Group, Inc.) for 1 h at room temperature after washing with TBST.
The bands were visualized using a chemiluminescence detection kit
(EMD Millipore) and densitometry was performed using ImageJ version
1.8.0.112 (National Institutes of Health). The relative amount of
protein was normalized by dividing the density value of the target
protein by the density value of the internal loading control.
Immunohistochemistry
Specimen acquisition
Pathological sections of gallbladders that had
previously been paraffin-embedded pathological sections from 25
patients with GBC that underwent radical resection of GBC (GBC
group) between January 2016 and January 2018 at the 904th Hospital
of Joint Logistic Support Force of PLA (Wuxi, China), as well as 10
patients with gallstones who underwent laparoscopic cholecystectomy
during the same period (control group) were obtained. Patients with
GBC were diagnosed with T1-3 gallbladder adenocarcinoma by
preoperative imaging, intraoperative exploration and postoperative
pathology, and there was no evidence of distant metastases in any
of the cases. The histopathology of the gallbladder in patients
with gallstones after surgery suggested cholecystitis. All
specimens were provided by the Department of Pathology, The 904th
Hospital of Joint Logistic Support Force of PLA (25 gallbladder
adenocarcinoma tissues, 10 gallbladder tissues with cholecystitis).
Written informed consent was obtained from all patients and the
study was approved by the Ethics Committee of the 904th Hospital of
Joint Logistic Support Force of PLA (approval no. 2019-10-006).
Tissue staining
Tissue staining was performed using an
immunohistochemical S-P kit (OriGene Technologies, Inc.), according
to the manufacturer's protocol. Briefly, 4% paraformaldehyde was
used for fixation at 4°C for 24 h. 5% BSA (Beijing Solarbio Science
& Technology Co., Ltd.) was used to block non-specific sites
for 30 min at 37°C. 3% hydrogen peroxide was used to block
endogenous peroxidase activity at room temperature for 10 min to
reduce non-specific background staining. The 4-µm sections were
incubated with GOLPH3 (1:100; cat. no. 19112-1-AP; ProteinTech
Group, Inc.), NLRP3 (1:50; cat. no. 19771-1-AP; ProteinTech Group,
Inc.) or Ki-67 antibodies (1:5,000; cat. no. 27309-1-AP;
ProteinTech Group, Inc.) with PBS as the blank control group) for 2
h at 37°C, followed by incubation with biotin-conjugated goat
anti-rabbit IgG antibody (1:200; cat. no. TA130016; OriGene
Technologies, Inc.) for 1 h at room temperature. Then, sections
were reacted with 3, 3′-diaminobenzidine chromogenic reagent. The
sections were counterstained with hematoxylin at room temperature
for 30 sec and immersed in graded series of alcohol solutions, then
dehydrated in 100% xylene and finally sealed with neutral glue. The
results were observed under an Olympus CX23 inverted microscope
(Olympus Corporation).
Evaluation
The results of immunohistochemistry were analyzed
using semi-quantitative integration (32). Randomly, five fields of view were
selected for each slice (magnification, ×200), and two pathologists
assessed the degree of staining. Staining intensity (SI) was scored
as follows: i) 0 points, undyed; ii) 1 point, lightly dyed or light
yellow; iii) 2 points, yellow; and iv) 3 points, darkly dyed, brown
or tan. The percentage of positively stained cells (PP) was scored
as follows: i) 0 points, no staining; ii) 1 point, ≤10% of cells
stained; iii) 2 points, 11–50% of cells stained; iv) 3 points,
51–80% of cells stained; and v) 4 points, >80% of cells stained.
The final score was the product of the SI and the PP score: 0–3
points, negative (−) and >3 points, positive (+).
Patients
The clinical data of the 35 patients (25 GBC cases,
10 gallstone cases) were collected. The 25 patients with GBC (GBC
group) all underwent R0 resection, including 10 male patients and
15 female patients, with a median age of 65.0 years (range, 36–85
years). The tumor staging was evaluated according to the 8th
Edition of the AJCC TNM staging system (33); amongst the patients with GBC, seven
cases were T1 stage, eight cases were T2 stage and 10 cases were T3
stage. In addition, 10 cases were accompanied by lymph node
metastasis, none of which had distant metastases. The median
survival of patients with GBC in the cohort was 19 months, and the
1, 3 and 5-year survival rates of the patients were 72, 24 and 12%,
respectively. Amongst the 10 patients with gallstones (Control),
four were men and six were women, with a median age of 65.5 years
(range, 50–74 years). There was no significant difference in the
sex or age between the GBC and control groups (P>0.05). None of
patients had received any targeted antitumor treatments before
surgery, such as chemotherapy, radiotherapy or targeted
therapy.
Surgery
All patients with GBC underwent radical resection of
GBC, and postoperative pathology confirmed R0 resection. Radical
resection included cholecystectomy, partial hepatectomy, or
regional lymph node dissection (hepatoduodenal ligament + posterior
pancreas + aortic root). The scope of surgical resection was based
on the tumor stage, and whether the extrahepatic bile duct was
removed was determined according to the degree of invasion of the
bile duct. All patients with cholelithiasis underwent laparoscopic
cholecystectomy.
Follow-up
After the operation, patients with GBC were
regularly followed up through clinics and telephone calls. In the
first year, the patients were followed up every 3 months, every 6
months in the second year, and every 12 months in the third year.
The follow-up was performed until 30 September 2020. The time
between surgery and death or the end of follow-up was considered
the overall survival.
Statistical analysis
All data were analyzed using SPSS version 23 (IBM
Corp.), and the experiments were repeated at least three times.
Quantitative data were analyzed using a unpaired Student's t-test
or a Mann-Whitney U test, and are expressed as the mean ± standard
deviation, or the median (interquartile range), respectively.
Qualitative data were analyzed using a χ2 test or a
Fisher's exact test. The Kaplan-Meier method was used for survival
analysis. The Log-rank test was used to analyze the Kaplan-Meier
curves. Pearson's linear correlation analysis was used to assess
correlations. P<0.05 was considered to indicate a statistically
significant difference.
Results
GOLPH3 and NLRP3 expression is
upregulated in human GBC tissues and its expression is associated
with Ki-67
First, the clinical relevance of GOLPH3 and NLRP3,
as well as their relationship in clinical GBC samples were
evaluated. The expression of GOLPH3, NLRP3 and the proliferative
marker Ki-67 (34) in the gallbladder
tissues of the 25 patients with GBC and 10 patients with gallstones
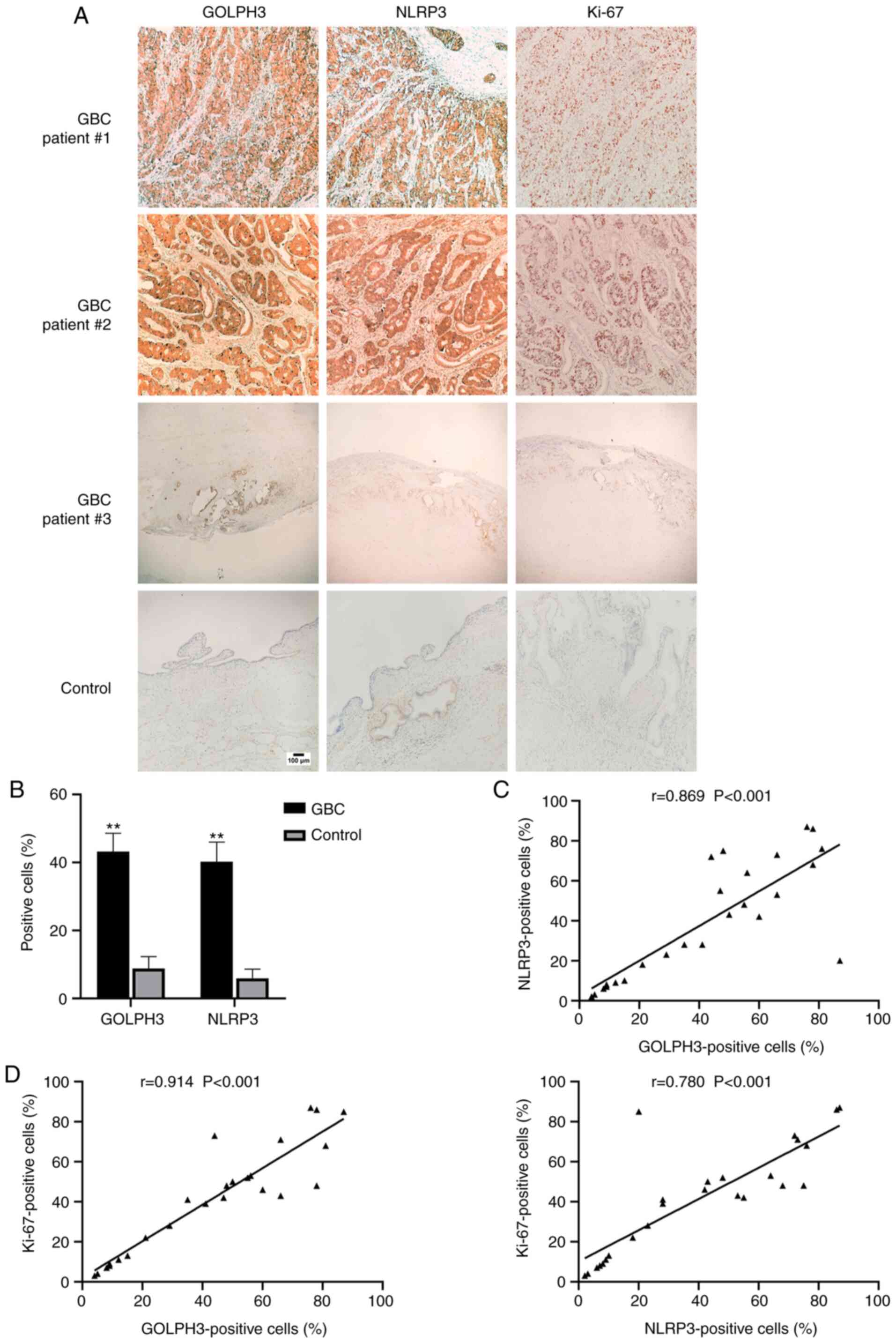
were assessed using immunohistochemical staining (Fig. 1). As shown in Fig. 1A and B, the percentage of GOLPH3- and
NLRP3-positive cells in the gallbladder tissue of GBC cases was 4–5
times higher than that of the non-cancer tissues (GOLPH3 GBC,
43.2±27.1% and Control, 8.8±11.1%; NLRP3 GBC, 40.2±29.0% and
Control, 5.9±8.7%), and the proportion of patients with GOLPH3- and
NLRP3-positive expression in the GBC cases was significantly higher
than that in the patients with gallstones (Table I). In addition, the percentage of
GOLPH3-positive cells was positively correlated with the percentage
of NLRP3-positive cells in GBC tissues (Fig. 1C). Additionally, the percentage of
GOLPH3- and NLRP3-positive cells were both positively correlated
with the percentage of Ki-67-positive cells in the GBC tissues
(Fig. 1D). The results of the
immunohistochemistry experiments also showed a similar trend
(Fig. 1A).
 | Table I.Differences in the expression of
GOLPH3 and NLRP3 in different gallbladder tissues. |
Table I.
Differences in the expression of
GOLPH3 and NLRP3 in different gallbladder tissues.
|
|
| GOLPH3 | NLRP3 |
|---|
|
|
|
|
|
|---|
| Group | n | Negative, n
(%) | Positive, n
(%) | Negative, n
(%) | Positive, n
(%) |
|---|
| GBC | 25 | 9 (36.0) | 16 (64.0) | 10 (40.0) | 15 (60.0) |
| Gallstones | 10 | 8 (80.0) | 2 (20.0) | 9 (90.0) | 1 (10.0) |
| P-value |
| 0.027 |
| 0.010 |
|
Expression of GOLPH3 and NLRP3 is
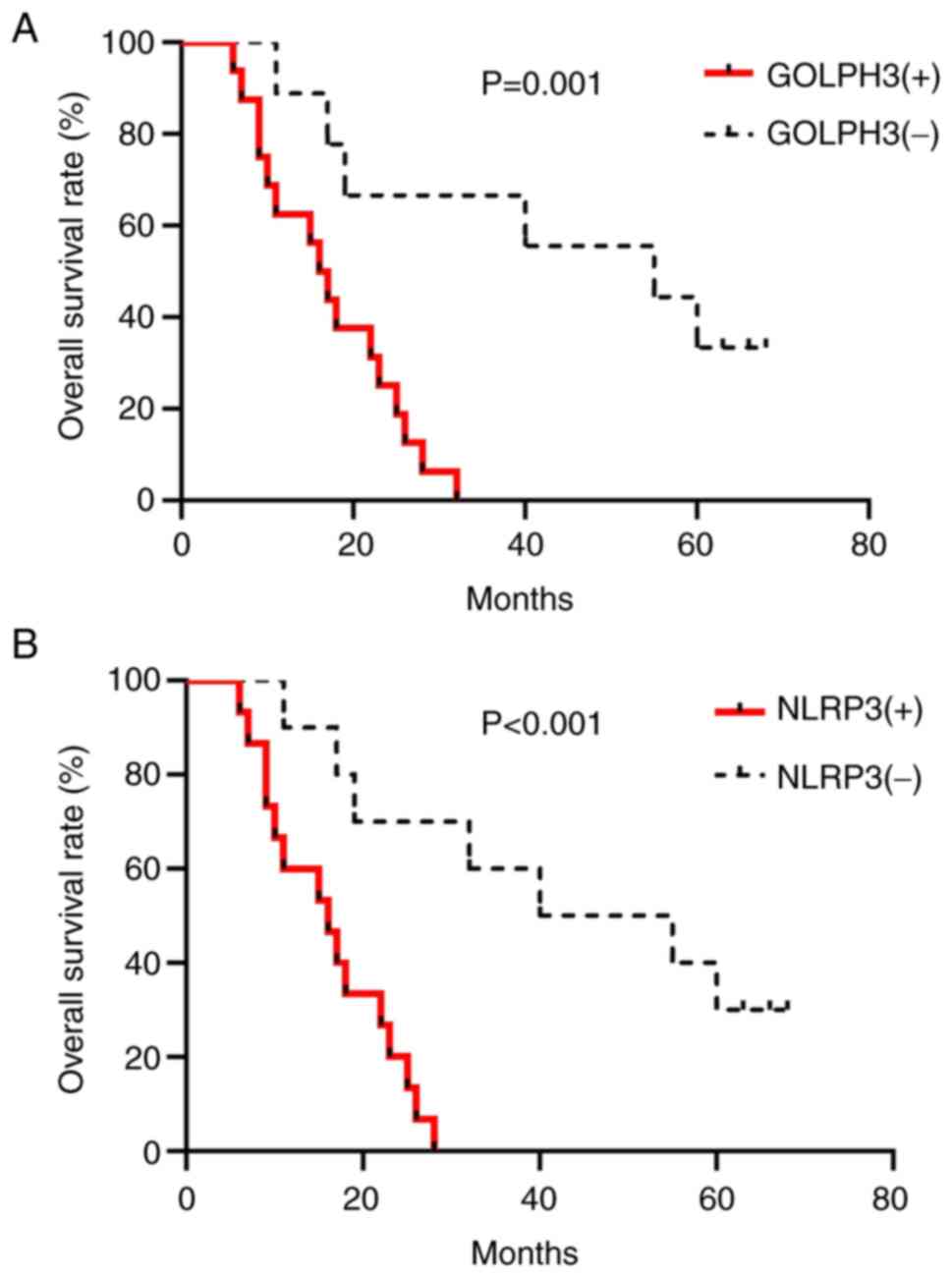
associated with a poor prognosis in patients with GBC
To explore the clinical significance of GOLPH3 and
NLRP3, the clinicopathological data of the 25 patients with GBC was
analyzed. The results showed that there was no significant
difference in the age or sex of the GOLPH3- or NLRP3-positive and
negative patients (Table II).
However, the survival of GOLPH3-positive patients (16/25) or
NLRP3-positive patients (15/25) was worse than the patients with
negative expression (GOLPH3, median 16 vs. 55 months, P=0.001;
NLRP3, median 16 vs. 40 months, P<0.001; Fig. 2A and B). Next, the relationship
between the positive expression of GOLPH3 or NLRP3 and the
clinicopathological characteristics of patients with GBC was
assessed. The results suggested that GOLPH3- or NLRP3-positive
patients had more advanced tumors (the TNM stage), deeper
infiltration, poorly differentiated tumors and higher carbohydrate
antigen 19-9 (CA199) or C-reactive protein (CRP) levels (Table II).
 | Table II.Clinicopathological characteristics
of the 25 patients with GBC. |
Table II.
Clinicopathological characteristics
of the 25 patients with GBC.
| A, GOLPH3
expression |
|
|
|
|
|---|
|
|---|
| Variable | Negative | Positive | χ2 | P-value |
|---|
| Sex, n (%) |
|
| 0.000 | 1.000 |
|
Male | 4 (44.4) | 6
(37.5) |
|
|
|
Female | 5 (55.6) | 10 (62.5) |
|
|
| With gallstones, n
(%) |
|
| 0.405 | 0.602 |
|
Yes | 7 (77.8) | 14 (87.5) |
|
|
| No | 2 (22.2) | 2
(12.5) |
|
|
| With hypertension,
n (%) |
|
| 0.762 | 0.434 |
|
Yes | 5 (55.6) | 6
(37.5) |
|
|
| No | 4 (44.4) | 10 (62.5) |
|
|
| Bile duct invasion,
n (%) |
|
| 0.011 | 0.915 |
|
Yes | 3 (33.3) | 5
(31.3) |
|
|
| No | 6 (66.7) | 11 (68.8) |
|
|
| Infiltration depth,
n (%) |
|
| 4.890 | 0.040b |
| T1+T2 | 8 (88.9) | 7
(43.8) |
|
|
| T3 | 1 (11.1) | 9
(56.3) |
|
|
| Lymph node
metastasis, n (%) |
|
| 1.852 | 0.229 |
|
Yes | 2 (22.2) | 8
(50.0) |
|
|
| No | 7 (77.8) | 8
(50.0) |
|
|
| Differentiation, n
(%) |
|
| 7.677 | 0.012b |
| Poor | 4 (44.4) | 15 (93.8) |
|
|
| High/medium | 5 (55.6) | 1
(6.3) |
|
|
| TNM stage, n
(%) |
|
| 6.173 | 0.033b |
| I+II | 8 (88.9) | 6
(37.5) |
|
|
| III | 1 (11.1) | 10 (62.5) |
|
|
|
Chemotherapya, n (%) |
|
| 0.043 | 0.835 |
|
Yes | 3 (33.3) | 6
(37.5) |
|
|
| No | 6 (66.7) | 10 (62.5) |
|
|
| Age, years, mean ±
SD | 63.89±7.47 | 64.63±12.94 | −0.156c | 0.878 |
| White blood cells
(×109/l), mean ± SD | 6.40±1.58 | 6.37±1.40 | 0.061c | 0.951 |
| Total bilirubin,
µmol/l, median (interquartile range) | 14.20 (10.20,
19.05) | 21.65 (12.00,
139.68) | 94.000d | 0.229 |
| CRP, g/l, median
(interquartile range) | 1.80 (0.95,
3.20) | 5.25 (2.90,
16.70) |
121.000d | 0.004b |
| CEA, µg/l, median
(interquartile range) | 1.97 (1.68,
4.01) | 2.12 (1.56,
5.82) | 72.000d | 1.000 |
| CA199, U/ml, median
(interquartile range) | 10.99 (9.71,
26.32) | 71.74 (20.98,
423.59) |
108.000d | 0.043b |
|
| B, NLRP3
expression |
|
| Variable | Negative | Positive | χ2 | P-value |
|
| Sex, n (%) |
|
| 0.000 | 1.000 |
|
Male | 4 (40.0) | 6 (40.0) |
|
|
|
Female | 6 (60.0) | 9 (60.0) |
|
|
| With gallstones, n
(%) |
|
| 0.000 | 1.000 |
|
Yes | 8 (80.0) | 13 (86.7) |
|
|
| No | 2 (20.0) | 2
(13.3) |
|
|
| With hypertension,
n (%) |
|
| 0.244 | 0.697 |
|
Yes | 5 (50.0) | 6 (40.0) |
|
|
| No | 5 (50.0) | 9 (60.0) |
|
|
| Bile duct invasion,
n (%) |
|
| 0.031 | 0.861 |
|
Yes | 3 (30.0) | 5
(33.3) |
|
|
| No | 7 (70.0) | 10 (66.7) |
|
|
| Infiltration depth,
n (%) |
|
| 6.250 | 0.018b |
|
T1+T2 | 9 (90.0) | 6
(40.0) |
|
|
| T3 | 1 (10.0) | 9
(60.0) |
|
|
| Lymph node
metastasis, n (%) |
|
| 2.778 | 0.211 |
|
Yes | 2 (20.0) | 8
(53.3) |
|
|
| No | 8 (80.0) | 7
(46.7) |
|
|
| Differentiation, n
(%) |
|
| 6.177 | 0.023b |
|
Poor | 5 (50.0) | 14 (93.3) |
|
|
|
High/medium | 5 (50.0) | 1
(6.7) |
|
|
| TNM stage, n
(%) |
|
| 7.819 | 0.012b |
|
I+II | 9 (90.0) | 5
(33.3) |
|
|
|
III | 1 (10.0) | 10 (66.7) |
|
|
|
Chemotherapya, n (%) |
|
| 0.260 | 0.610 |
|
Yes | 3 (30.0) | 6
(40.0) |
|
|
| No | 7 (70.0) | 9
(60.0) |
|
|
| Age, years, mean ±
SD | 63.40±7.21 | 65.00±13.30 | −0.346c | 0.732 |
| White blood cells
(×109/L), mean ± SD | 6.47±1.51 | 6.32±1.44 | 0.255c | 0.801 |
| Total bilirubin,
µmol/l, median (interquartile range) | 14.80 (10.60,
22.10) | 21.20 (11.50,
177.30) | 93.500d | 0.311 |
| CRP, g/l, median
(interquartile range) | 1.90 (1.08,
3.63) | 5.90 (2.80,
18.80) |
121.000d | 0.010b |
| CEA, µg/l, median
(interquartile range) | 1.97 (1.48,
3.49) | 2.13 (1.57,
6.31) | 87.000d | 0.531 |
| CA199, U/ml, median
(interquartile range) | 10.55 (8.30,
20.79) | 109.76 (23.21,
479.20) |
123.000d | 0.007b |
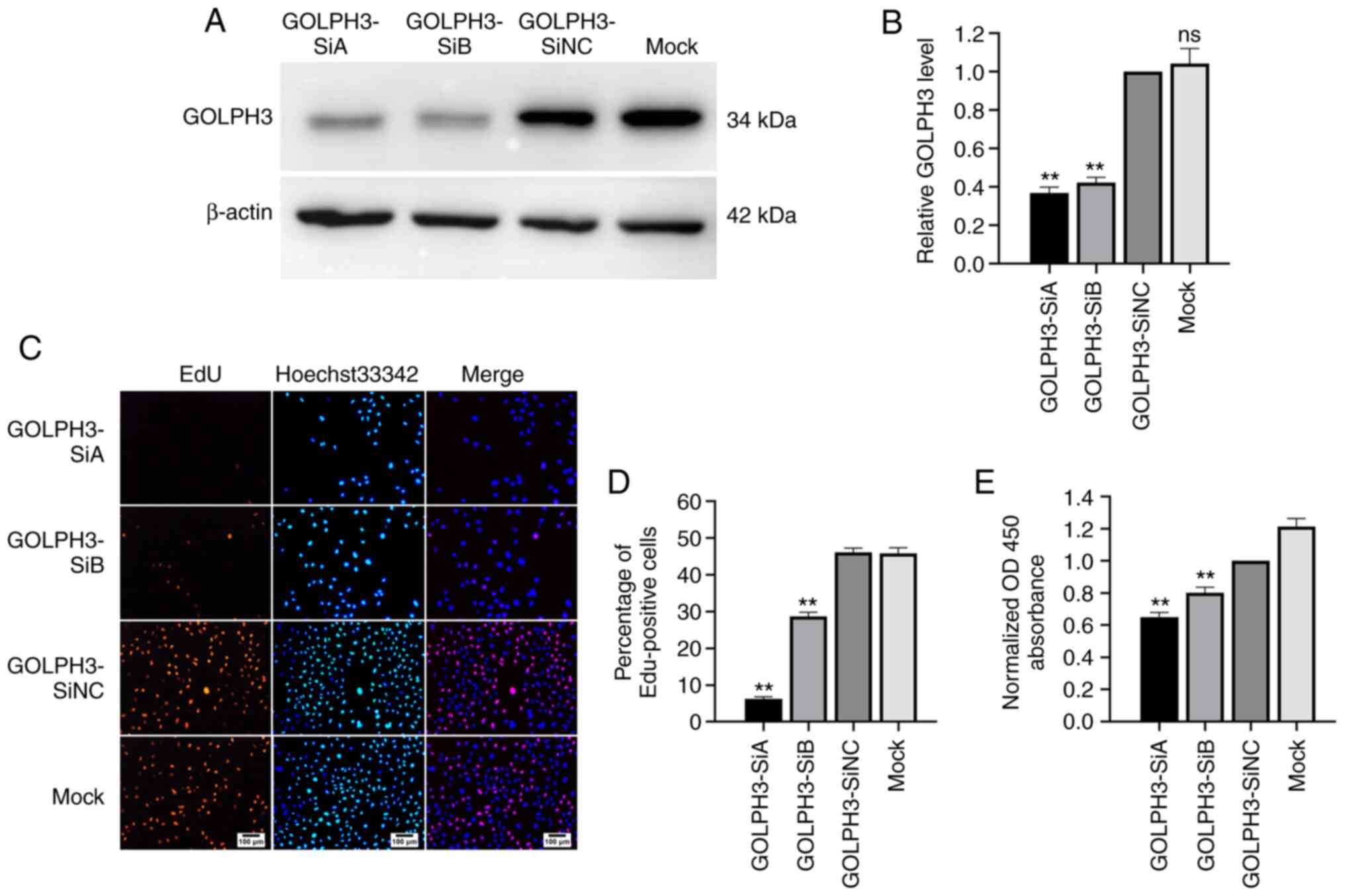
GOLPH3 promotes the proliferation of
human GBC cells
To explore the possible role of GOLPH3 in the
development of human GBC, GOLPH3-specific siRNAs were used to knock
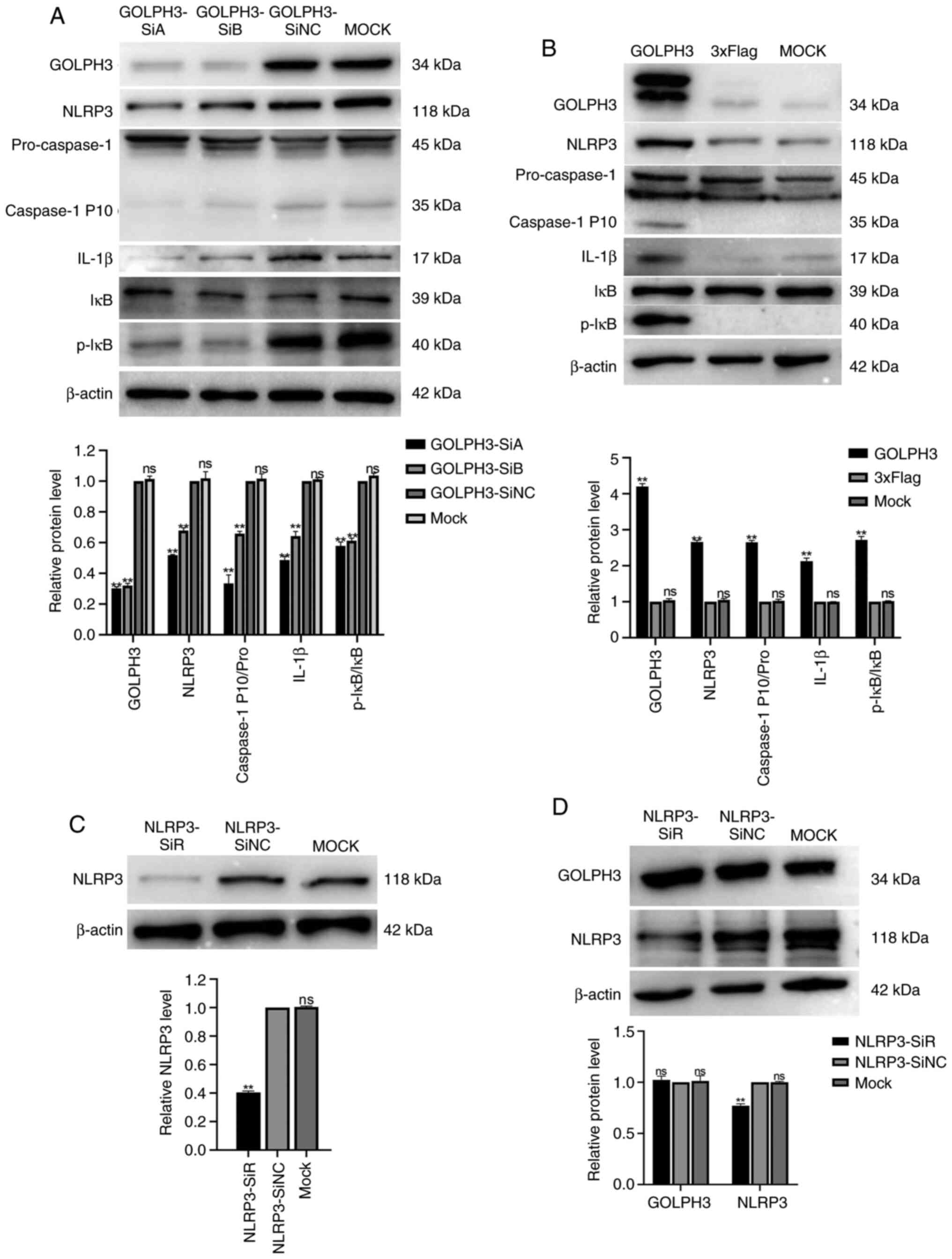
down the expression of GOLPH3 in GBC-SD cells. As shown in Fig. 3A and B, compared with the GOLDPH3-SiNC
group, the protein expression levels of GOLPH3 decreased by 55–65%
in the GBC-SD cells transfected with GOLDPH3-SiA and -SiB. Next,
cell proliferation after GOLPH3 knockdown was evaluated using EdU
and cell viability was evaluated using CCK-8 assays. The results of
the EdU assay showed that the EdU-positive cells of the GOLPH3-SiA
and -SiB groups were reduced by 40–80% compared with the
GOLDPH3-SiNC group (Fig. 3C and D).
Additionally, the results of the CCK-8 assay indicated that the
OD450 in the GOLPH3-SiA and -SiB groups decreased by 20–30%
compared with the GOLDPH3-SiNC group (Fig. 3E).
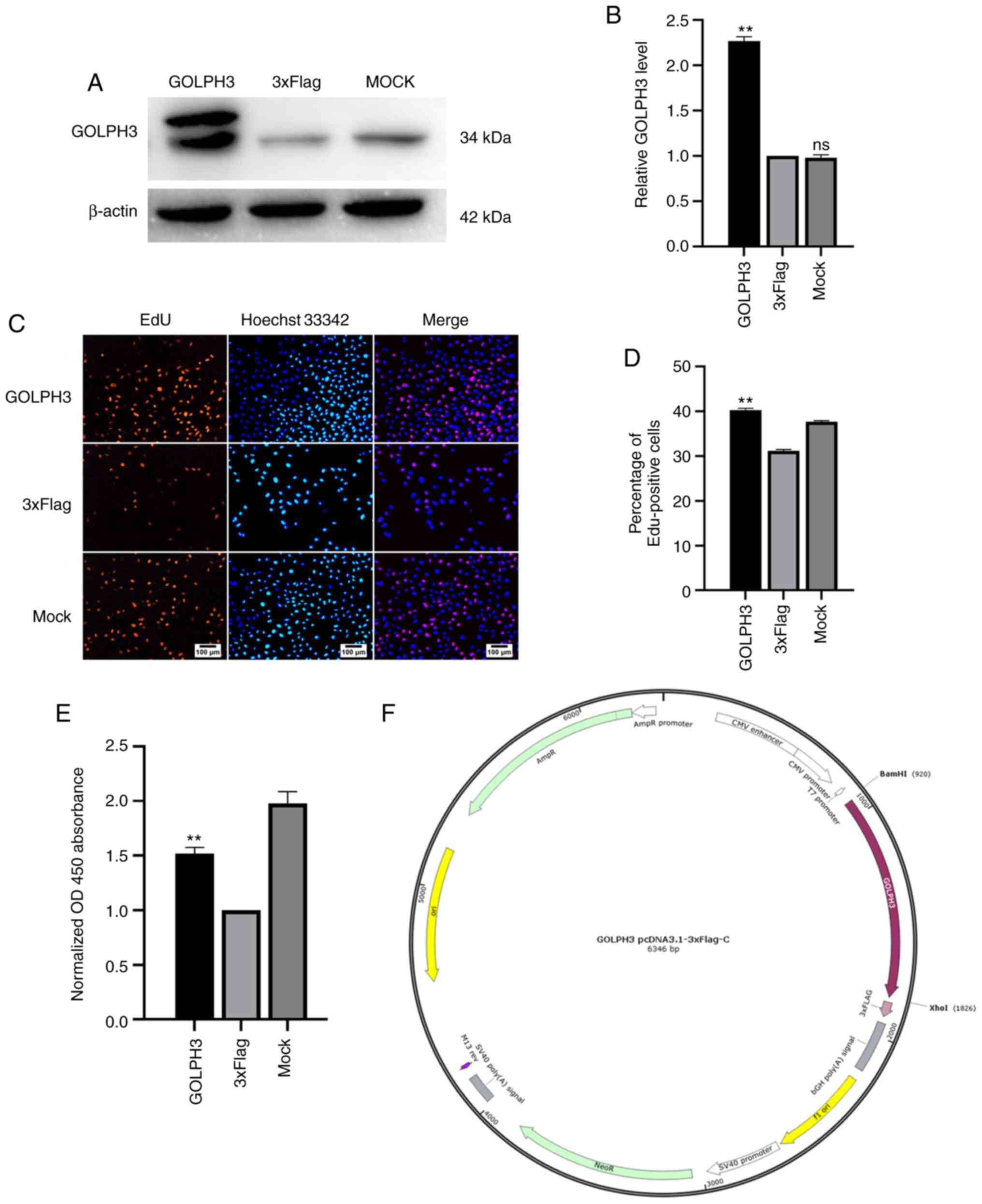
Next, GOLPH3 was overexpressed in GBC-SD cells, and
the change in the proliferation of GBC cells was assessed. The
results of western blotting showed that the GOLPH3-overexpression
plasmid (Fig. 4F) was successfully
transfected (Fig. 4A and B). The EdU
assay showed that when GOLPH3 was overexpressed, the percentage of
EdU positive cells increased by 30% compared with the 3×Flag group
(Fig. 4C and D). In addition, the
results of the CCK-8 assay suggested that the absorbance at 450 nm
of the GOLPH3-overexpressing cells increased by 50% compared with
the 3×Flag group (Fig. 4E). In
summary, GOLPH3 overexpression promoted the proliferation of GBC
cells.
GOLPH3 regulates the NLRP3/Caspase-1
pathway
It has been reported that GOLPH3 is related to
PtdIns(4)p and the mTOR pathway, and that PtdIns(4)p is an
important trigger for NLRP3 activation (21). There is also evidence showing that
overexpression of mTOR can upregulate the levels of NLRP3 (30). Therefore, whether GOLPH3 could
regulate NLRP3 was next assessed. Following knockdown or
overexpression of GOLPH3, the protein expression levels of NLRP3
were determined by western blotting. As shown in Fig. 5A and B, the expression levels of NLRP3
in the cells transfected with GOLPH3-SiA and -SiB were
significantly reduced, and significantly increased in the
GOLPH3-overexpressing cells, suggesting that GOLPH3 could indeed
regulate NLRP3. NLRP3 was knocked down in GBC-SD cells using siRNA,
and proof of transfection was determined via western blotting
(Fig. 5C). The expression levels of
GOLPH3 were not significantly altered following knockdown of NLRP3
in GBC-SD cells (Fig. 5D), suggesting
that NLRP3 may be located downstream of GOLPH3.
Whether GOLPH3 could activate Caspase-1 was next
determined. The results of western blotting showed that
overexpression of GOLPH3 increased Caspase-1 P10 levels, whereas
knockdown of GOLPH3 reduced Caspase-1 P10 levels (Fig. 5A and B). Additionally, IL-1β and
p-IκB, members of the NF-κB signaling pathway, showed similar
trends to NLRP3 in the experiments (Fig.
5A and B). These results suggested that GOLPH3 could activate
Caspase-1 through regulating NLRP3, and may have promoted the
proliferation of GBC via the IL-1β and NF-κB pathway.
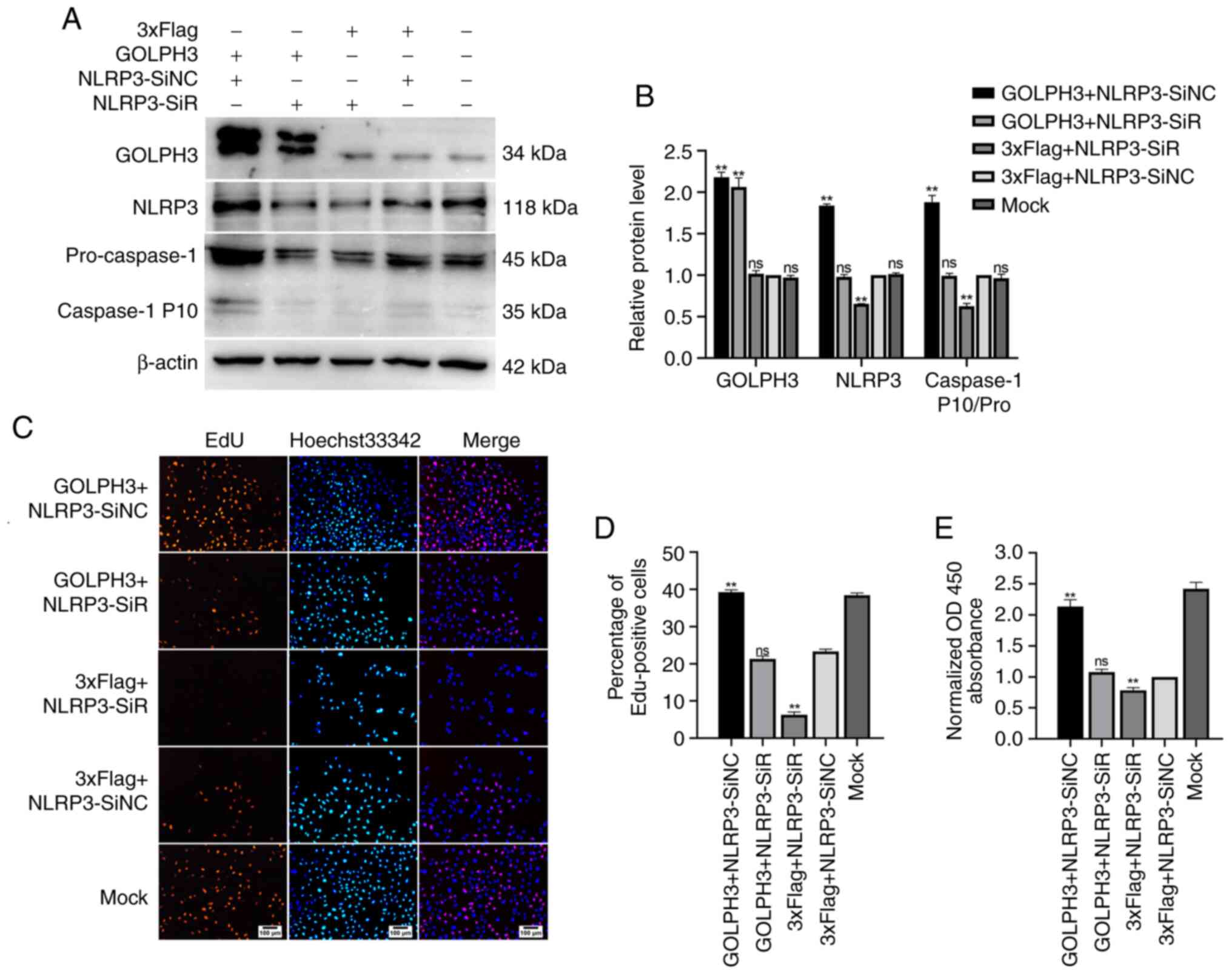
GOLPH3 promotes GBC cell proliferation
partially via regulation of NLRP3 in GBC-SD cells
The aforementioned results indicated that GOLPH3
could increase the levels of NLRP3, as well as Caspase-1 activity,
and GOLPH3 could also promote the proliferation of cells in GBC-SD.
Thus, whether the effects of GOLPH3 on GBC cells were mediated via
an NLRP3/Caspase-1 pathway were determined. The results of the EdU
assay (Fig. 6C and D) showed that
knockdown of NLRP3 alone inhibited the proliferation of GBC cells,
and overexpression of GOLPH3 alone promoted it. However, in the
GOLPH3 + NLRP3-SiR co-transfection group, the number of
EdU-positive cells decreased, suggesting that the downregulation of
NLRP3 partially offset cell proliferation caused by GOLPH3
overexpression in GBC-SD cells. The CCK-8 assays also showed
similar results (Fig. 6E). In
addition, the activation of Caspase-1 (pro-caspase-1/Caspase-1 P10)
caused by GOLPH3 overexpression was partially abolished by
knockdown of NLRP3, suggesting that the activation of Caspase-1 by
GOLPH3 was also mediated by NLRP3 (Fig.
6A and B). Taken together, these results suggested that GOLPH3
promoted GBC cell proliferation, at least partially via a
NLRP3/Caspase-1 pathway.
Discussion
In the present study, it was shown that GOLPH3
contributed to GBC progression via upregulation of NLRP3
expression, which resulted in the activation of Caspase-1, IL-1β
and the NF-κB pathway. Additionally, GOLPH3, as well as NLRP3, were
both associated with a poor prognosis in patients with GBC, which
was supported by the following results. Firstly, knockdown of
GOLPH3 inhibited GBC cell proliferation, whereas GOLPH3
overexpression promoted cell proliferation. Secondly, GOLPH3
regulated the protein expression levels of NLRP3 and the activation
of Caspase-1, IL-1β, as well as the NF-κB pathway (p-IκB), whereas
NLRP3 did not regulate GOLPH3. Thirdly, cell proliferation induced
by overexpression of GOLPH3 was partially abolished by GOLPH3
knockdown. Additionally, GOLPH3 and NLRP3 were shown to be highly
expressed in human GBC tissues, and there was a significant
positive correlation between the expression of these two proteins,
and both were positively correlated with the expression of Ki-67 in
tissues. Finally, the survival of GOLPH3- or NLRP3-positive
patients with GBC was worse than that of cases with negative
expression, and GOLPH3 as well as NLRP3 positive expression were
associated with the tumor stage, degree of differentiation, depth
of invasion, and CA199 and CRP levels.
Several studies have reported that the activation of
the NLRP3/Caspase-1 pathway is associated with tumor progression
(24–27). Wang et al (25) found that when the NLRP3 inflammasome
is activated, Caspase-1 is spliced to further activate the Akt,
GSK-3p, ERK1/2 and CREB signaling pathways to promote tumor
proliferation. The role of NLRP3 in cancer has gained increasing
attention recently. However, the majority of previous studies have
focused on investigating the downstream effectors of NLRP3. There
has been no reports regarding the upstream effectors of NLRP3 in
tumor progression to date, to the best of our knowledge.
Conversely, although there is evidence showing that the TGN
(21), Ptdins(4)p (21), mTOR (30) and PD2 (31) are closely related to the activation of
NLRP3, these studies were all performed in macrophages. Whether
this relationship also exists in tumor cells, and which proteins
regulate these interactions remains unclear. In addition, several
studies have reported that GOLPH3 is involved in the progression of
various malignant tumors, such as lung cancer, pancreatic cancer,
gastric cancer and brain glioma, via different signaling pathways,
including the mTOR and FOXO1 pathway (6,11–14). However, the role of GOLPH3 in the
progression of GBC is yet to be elucidated.
In the present study, GOLPH3 and NLRP3 were shown to
be highly upregulated in clinical human GBC tissues, and there was
a positive correlation between these two molecules, and both were
also positively correlated with the expression of Ki-67 in the GBC
tissues. Moreover, GOLPH3 promoted the proliferation of GBC cells
and regulated the expression of NLRP3 as well as the activation of
Caspase-1. However, NLRP3 did not affect GOLPH3 levels, indicating
that GOLPH3 was located upstream of NLRP3. NLRP3 can activate
Caspase-1, and further activate the Akt, GSK-3p, ERK1/2 and CREB
pathways to promote adenocarcinoma proliferation (25). In the present study, it was shown that
not only NLRP3, but also GOLPH3 could promote the activation of
Caspase-1, and this activation could be offset by knocking down
NLRP3. Additionally, as IL-1β, an important member of NLRP3
inflammasome, has been reported to be associated with tumor
proliferation, and the NF-κB signaling pathway is upstream of the
inflammasome (21,25,28), the
levels of IL-1β and NF-κB pathway activation (based on p-IκB
levels) were determined. The results showed that GOLPH3 could also
regulate the protein expression levels of IL-1β and the activation
of IκB, which indicated that GOLPH3 may promote the proliferation
of GBC via IL-1β and the NF-κB pathway. Thus, GOLPH3 can also
regulate the expression of NLRP3, further activate Caspase-1, IL-1β
and the NF-κB pathway to promote the proliferation of gallbladder
adenocarcinoma cells.
As for the exact mechanism by which GOLPH3 regulates
NLRP3, this remains unknown. Based on the existing research, it is
hypothesized that on the one hand, the excessive activation of
GOLPH3 may cause the fragmentation of the TGN and break the binding
of GOLPH3 and PtdIns(4)p. The free PtdIns(4)p then bind to NLRP3
and activates the entire inflammasome, further affecting the
protein levels of NLRP3 (8,21). Additionally, GOLPH3 activates the mTOR
molecular pathway, which further increases the levels of NLRP3
(30). However, due to a lack of
direct and effective methods to detect the activation of NLRP3,
only the levels of NLRP3 expression were assessed in the present
study. It is unclear whether the effects of GOLPH3 on NLRP3 occur
by affecting the activity of NLRP3, or by directly affecting the
expression of NLRP3. It will be interesting to further reveal the
underlying mechanism by which GOLPH3 regulates NLRP3.
In addition, through the analysis of patient
clinical data, it was found that patients with positive expression
of GOLPH3 or NLRP3 had worse overall survival compared with the
negative patients, suggesting that this pathway also affects the
overall survival of patients in vivo. Additionally, GOLPH3-
and NLRP3-positive patients had less differentiated tumors, more
advanced tumors, deeper infiltration and high levels of CA199,
suggesting that the tumors in these patients were more aggressive
compared with the negative patients. Of note, it was found that the
expression of GOLPH3 and NLRP3 in vivo was associated with
the levels of CRP in the peripheral blood, which further revealed
the connection between this pathway and inflammation.
In conclusion, it was observed that GOLPH3 promoted
the proliferation of GBC cells by regulating NLRP3 and further
activating Caspase-1, IL-1β and the NF-κB pathway in vitro.
Additionally, the positive correlation between the expression
levels of these proteins was shown, and it was also shown that
their expression was positively correlated with the expression of
the proliferation marker Ki-67 in vivo. Patients with
positive expression of GOLPH3 or NLRP3 tended to have worse overall
survival, and the expression of both of these proteins was related
to the patients' tumor stage, degree of differentiation, depth of
invasion, and CA199 and CRP levels. This research provided evidence
regarding the existence of a GOLPH3-NLRP3-Caspase-1 signaling
pathway in human GBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KL contributed to the conception of the present
study. ZZ prepared the manuscript and contributed to performing the
experiments. KL and ZZ confirm the authenticity of all the raw
data. QZ analyzed the data and performed part of the experiments.
DC taught the laboratory techniques needed in the present study and
assisted in completing part of the experiments. LC contributed to
designing the experiments. LC and WX contributed to the manuscript
editing. YB contributed to data collection. WX performed the
follow-ups of patients in the present study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from all
patients and the study was approved by the Ethics Committee of the
904th Hospital of Joint Logistic Support Force of PLA (approval no.
2019-10-006; Wuxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hundal R and Shaffer EA: Gallbladder
cancer: Epidemiology and outcome. Clin Epidemiol. 6:99–109.
2014.PubMed/NCBI
|
|
2
|
Gamboa AC and Maithel SK: The landmark
series: Gallbladder cancer. Ann Surg Oncol. 27:2846–2858. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Song X, Hu Y, Li Y, Shao R, Liu F and Liu
Y: Overview of current targeted therapy in gallbladder cancer.
Signal Transduct Target Ther. 5:2302020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goetze TO: Gallbladder carcinoma:
Prognostic factors and therapeutic options. World J Gastroenterol.
21:12211–12217. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kuna RS and Field SJ: GOLPH3: A Golgi
phosphatidylinositol(4)phosphate effector that directs vesicle
trafficking and drives cancer. J Lipid Res. 60:269–275. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhou X, Xue P, Yang M, Shi H, Lu D, Wang
Z, Shi Q, Hu J, Xie S, Zhan W and Yu R: Protein kinase D2 promotes
the proliferation of glioma cells by regulating Golgi
phosphoprotein 3. Cancer Lett. 355:121–129. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sechi S, Frappaolo A, Karimpour-Ghahnavieh
A, Piergentili R and Giansanti MG: Oncogenic roles of GOLPH3 in the
physiopathology of cancer. Int J Mol Sci. 21:9332020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Farber-Katz SE, Dippold HC, Buschman MD,
Peterman MC, Xing M, Noakes CJ, Tat J, Ng MM, Rahajeng J, Cowan DM,
et al: DNA damage triggers Golgi dispersal via DNA-PK and GOLPH3.
Cell. 156:413–427. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Buschman MD, Xing M and Field SJ: The
GOLPH3 pathway regulates Golgi shape and function and is activated
by DNA damage. Front Neurosci. 9:3622015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bergeron JJM, Au CE, Thomas DY and Hermo
L: Proteomics identifies Golgi phosphoprotein 3 (GOLPH3) with a
link between Golgi structure, cancer, DNA damage and protection
from cell death. Mol Cell Proteomics. 16:2048–2054. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang S, Pan H, Wei W, Yang H, Liu J and
Yang R: GOLPH3: A novel biomarker that correlates with poor
survival and resistance to chemotherapy in breast cancer.
Oncotarget. 8:105155–105169. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Wang X, Feng B, Tang L, Li W, Zheng
X, Liu Y, Peng Y, Zheng G and He Q: Golgi phosphoprotein 3 (GOLPH3)
promotes hepatocellular carcinoma progression by activating mTOR
signaling pathway. BMC Cancer. 18:6612018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu J, Wei H, Lai L, Wang Y, Han X and
Zhang Z: Golgi phosphoprotein-3 promotes invasiveness of gastric
cancer cells through the mTOR signalling pathway. Clin Invest Med.
42:E38–E47. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao C, Zhang J, Ma L, Wu H, Zhang H, Su
J, Geng B, Yao Q and Zheng J: GOLPH3 promotes angiogenesis of lung
adenocarcinoma by regulating the Wnt/β-catenin signaling pathway.
Onco Targets Ther. 13:6265–6277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang Q, Zhuang J, Deng Y, Yang L, Cao W,
Chen W, Lin T, Lv X, Yu H, Xue Y and Guo H: miR34a/GOLPH3 axis
abrogates urothelial bladder cancer chemoresistance via reduced
cancer stemness. Theranostics. 7:4777–4790. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu T, An Q, Cao XL, Yang H, Cui J, Li ZJ
and Xiao G: GOLPH3 inhibition reverses oxaliplatin resistance of
colon cancer cells via suppression of PI3K/AKT/mTOR pathway. Life
Sci. 260:1182942020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zeng Z, Lin H, Zhao X, Liu G, Wang X, Xu
R, Chen K, Li J and Song L: Overexpression of GOLPH3 promotes
proliferation and tumorigenicity in breast cancer via suppression
of the FOXO1 transcription factor. Clin Cancer Res. 18:4059–4069.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lim Y and Kumar S: A single cut to
pyroptosis. Oncotarget. 6:36926–36927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Islam MT, Bardaweel SK, Mubarak MS, Koch
W, Gaweł-Beben K, Antosiewicz B and Sharifi-Rad J: Immunomodulatory
effects of diterpenes and their derivatives through NLRP3
inflammasome pathway: A review. Front Immunol. 11:5721362020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Latz E, Xiao TS and Stutz A: Activation
and regulation of the Inflammasomes. Nat Rev Immunol. 13:397–411.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen J and Chen ZJ: PtdIns4P on dispersed
trans-Golgi network mediates NLRP3 inflammasome activation. Nature.
564:71–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Feng YS, Tan ZX, Wu LY, Dong F and Zhang
F: The involvement of NLRP3 inflammasome in the treatment of
Alzheimer's disease. Ageing Res Rev. 64:1011922020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu F, Zhao Y, Pang Y, Ji M, Sun Y, Wang H,
Zou J, Wang Y, Li G, Sun T, et al: NLRP3 inflammasome upregulates
PD-L1 expression and contriwhilees to immune suppression in
lymphoma. Cancer Lett. 497:178–189. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tezcan G, Garanina EE, Zhuravleva MN,
Hamza S, Rizvanov AA and Khaiboullina SF: Rab GTPase mediating
regulation of NALP3 in colorectal cancer. Molecules. 25:48342020.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Y, Kong H, Zeng X, Liu W, Wang Z, Yan
X, Wang H and Xie W: Activation of NLRP3 inflammasome enhances the
proliferation and migration of A549 lung cancer cells. Oncol Rep.
35:2053–2064. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen Z, Liu Q, Zhu Z, Xiang F, Zhang M, Wu
R and Kang X: Ursolic acid protects against proliferation and
inflammatory response in LPS-Treated gastric tumour model and cells
by inhibiting NLRP3 inflammasome activation. Cancer Manag Res.
12:8413–8424. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen Z, He M, Chen J, Li C and Zhang Q:
Long non-coding RNA SNHG7 inhibits NLRP3-dependent pyroptosis by
targeting the miR-34a/SIRT1 axis in liver cancer. Oncol Lett.
20:893–901. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guo R, Qin Y, Shi P, Xie J, Chou M and
Chen Y: IL-1β promotes proliferation and migration of gallbladder
cancer cells via Twist activation. Oncol Lett. 12:4749–4755. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dippold HC, Ng MM, Farber-Katz SE, Lee SK,
Kerr ML, Peterman MC, Sim R, Wiharto PA, Galbraith KA, Madhavarapu
S, et al: GOLPH3 bridges phosphatidylinositol-4-phosphate and
actomyosin to stretch and shape the Golgi to promote budding. Cell.
139:337–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Zhang X, Pan Y, Shi G, Ren J, Fan H,
Dou H and Hou Y: mTOR regulates NLRP3 inflammasome activation via
reactive oxygen species in murine lupus. Acta Biochim Biophys Sin
(Shanghai). 50:888–896. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang Z, Meszaros G, He WT, Xu Y, de
Fatima Magliarelli H, Mailly L, Mihlan M, Liu Y, Puig Gámez M,
Goginashvili A, et al: Protein kinase D at the Golgi controls NLRP3
inflammasome activation. J Exp Med. 214:2671–2693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Friedrichs K, Gluba S, Eidtmann H and
Jonat W: Overexpression of p53 and prognosis in breast cancer.
Cancer. 72:3641–3647. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Lamartina L, Grani G, Arvat E, Nervo A,
Zatelli MC, Rossi R, Puxeddu E, Morelli S, Torlontano M, Massa M,
et al: 8th edition of the AJCC/TNM staging system of thyroid
cancer: What to expect (ITCO#2). Endocr Relat Cancer. 25:L7–L11.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hui AM, Shi YZ, Li X, Sun L, Guido T,
Takayama T and Makuuchi M: Proliferative marker Ki-67 in
gallbladder carcinomas: High expression level predicts early
recurrence after surgical resection. Cancer Lett. 176:191–198.
2002. View Article : Google Scholar : PubMed/NCBI
|