Introduction
Gastric cancer (GC) is one of the most frequently
diagnosed types of cancer worldwide, with an estimated 1,089,103
newly diagnosed cases and 768,793 deaths worldwide in 2020
(1). Although there are numerous
different treatments for GC, the prognosis of patients with
advanced GC remains poor (2). Lymph
node invasion and distant metastasis are the main factors that
result in death and poor prognosis (3). Therefore, it is urgent to identify novel
diagnostic and prognostic biomarkers, which could contribute to
improved outcomes in patients with GC.
Serine/threonine kinase 17a (STK17A; also known as
death-associated protein kinase-related apoptosis-inducing protein
kinase 1; DRAK1) is a serine/threonine kinase. STK17A is a member
of the death-associated protein kinase (DAPK) family and acts as a
positive regulator of apoptosis (4,5). STK17A
was recently found to serve an important role in cell
proliferation, apoptosis, tumor metastasis and tumorigenesis
(6–11). However, the association between STK17A
expression and the development of GC remains unclear.
At present, research on the molecular function of
the DAPK family is mainly focused on the interaction between
phosphorylation of the myosin light chain (MLC) regulatory subunit
and the cytoskeleton, which leads to the regulation of actin stress
fibers, adhesion junctions and cell movement (12–14).
Recent genetic evidence indicates that DRAKs are involved in the
regulation of cell shape and adhesion and cytoskeletal dynamics
through phosphorylation of the Drosophila ortholog of MLC
(15–17). MLC has been identified as one of the
few targets for STK17A phosphorylation (4). A study on STK17A indicated that the
expression of STK17A induced apoptosis-related morphological
changes associated with membrane blistering, which was also induced
by MLC phosphorylation (4,18). In addition, STK17A affects cell
contractility by regulating weak metabolism, which further promotes
metastasis and spread. Weak metabolism is a type of cell death
triggered by cell detachment, which is necessary for survival in
the lymphatic and vascular systems (19). This indicates a new direction for
exploring the relationship between STK17A and tumor invasion,
progression and metastasis.
In recent years, research on the association between
STK17A and chemotherapy resistance has also yielded important
results. It has been demonstrated that STK17A exhibits low
expression in acquired drug-resistant cell phenotypes, which are
resistant to oxaliplatin and 5-fluorouracil (6). In MeWo cells, a malignant melanoma cell
line, STK17A was also revealed to be associated with
cross-resistance to DNA-damaging drugs (20). These results indicated that STK17A may
mediate the response of tumor cells to chemotherapy and may be
important for the development of cancer chemotherapeutics.
The aim of the present study was to investigate the
STK17A mRNA and protein expression in GC cells and human GC
tissues, and determine the effects of STK17A overexpression and
silencing on the proliferation and migration of GC cells.
Furthermore, its association with clinicopathological
characteristics and its significance for the prognosis of GC were
explored, in order to determine whether STK17A may be of value as a
prognostic factor and potential therapeutic target for GC.
Materials and methods
Clinical samples
A total of 39 GC and matched normal tissue samples
were obtained from patients with GC (aged from 41 to 76 years) who
underwent surgical excision at the First Affiliated Hospital of
Zhengzhou University (Zhengzhou, China) between October 2015 and
March 2016, and were used for RNA extraction. Of the 39 samples, 26
were male and 13 were female. The inclusion criteria were as
follows: Definite diagnosis of primary gastric cancer; successful
radical resection of gastric cancer and R0 resection. The exclusion
criteria were as fllows: Malignant tumors with other tissue
origins; death from causes other than gastric cancer. A total of
102 formalin-fixed paraffin-embedded GC samples from the period
between January 2008 and December 2010 were also collected from the
same hospital and STK17A immunohistochemistry was performed on all
samples. No patients had undergone chemotherapy or radiotherapy
prior to surgical resection. In addition, tumors were staged
according to the TNM staging criteria of the American Joint
Committee on Cancer (7th edition, 2010) (21). Written informed consent for the use of
tissue samples was obtained on their behalf from all from all
patients or their legal guardians. The use of GC tissues in the
present study was approved by the Ethics Committee of Zhengzhou
University.
Cell lines
Human GC cell lines used in this study included AGS
(RPMI-1640), HGC27 (RPMI-1640), KATOIII (IMDM), MKN45 (RPMI-1640),
NCI-N87 (RPMI-1640), and SNU1 (RPMI-1640), normal gastric cell
GES-1 (RPMI-1640), and 293T (DMEM) were obtained from the Cell Bank
of Type Culture Collection of Chinese Academy of Sciences. All
culture media were obtained from GIBCO BRL Life Technologies. All
cells were cultured in medium supplemented with 10% FBS and 1%
penicillin-streptomycin (all from Gibco; Thermo Fisher Scientific,
Inc.) at 37°C in humidified air with 5% CO2.
Reverse transcription-quantitative
(RT-q)PCR analysis
Fresh tissue samples were immediately frozen in
liquid nitrogen after surgical resection and then stored at −80°C
prior to RNA isolation. Total RNA was extracted from tissue samples
with TRIzol® reagent (Thermo Fisher Scientific, Inc.)
and reverse-transcribed to cDNA using the Reverse Transcription for
PCR Kit (Takara Bio, Inc.) according to the standard protocols
provided by the manufacturer. qPCR was performed with the ABI7900HT
Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher
Scientific, Inc.) using a SYBR Green PCR Kit (Applied Biosystems;
Thermo Fisher Scientific, Inc.). The primer sequences used for
STK17A and GAPDH are presented in Table
I. The following PCR conditions were used: Initial denaturation
at 95°C for 2 min, followed by 40 cycles of denaturation at 94°C
for 15 sec, and annealing/extension at 60°C for 1 min. The relative
expression of STK17A mRNA against GAPDH was calculated using the
2−ΔΔCq method (22).
 | Table I.Primer sequences of STK17A and
GAPDH. |
Table I.
Primer sequences of STK17A and
GAPDH.
| Gene | Sequences |
|---|
| STK17A | q-F:
5′-TCTGAGTCGGCTGTTGATTTC-3′ |
|
| q-R:
5′-GGGGTGCTTTAGACATTCTTCA-3′ |
| GAPDH | q-F:
5′-GCTGAACGGGAAGCTCACTG-3′ |
|
| q-R:
5′-GTGCTCAGTGTAGCCCAGGA-3′ |
Tissue microarray (TMA) and
immunohistochemical staining
Using 102 pairs of GC tissue samples and
corresponding normal tissues, TMAs were constructed as previously
described (23). The archived
paraffin-embedded tissue blocks (21)
were cut into 4-µm sections on adhesion slides and then sections
were dried in a thermostat oven for 2 h at 65°C. Immunocytochemical
staining was performed using the streptavidin-biotin complex method
to investigate the expression of STK17A. In brief, tissue sections
were deparaffinized in xylene and rehydrated in ethanol, followed
by antigen retrieval in 0.1 mol/l citrate buffer solution (pH 6.0)
in a microwave oven for ~15 min. After washing in PBS, the sections
were blocked in 10% goat serum for 10 min at room temperature to
eliminate nonspecific binding. The sections were then incubated
overnight with primary anti-STK17A polyclonal antibody (1:150
dilution; ab97530, Abcam) at 4°C in humidity. In the next step, the
sections were sequentially incubated with a biotin-labeled goat
anti-rabbit IgG (cat. no. SP-9000; Beijing Zhongshan Golden Bridge
Biotechnology, Co., Ltd.) at a concentration of 1:100, at room
temperature for 30 min. Finally, diaminobenzidine (Beijing
Zhongshan Golden Bridge Biotechnology, Co., Ltd.) was used for
color development for 1 min at room temperature, followed by
hematoxylin (Beijing Zhongshan Golden Bridge Biotechnology, Co.,
Ltd.). counterstaining for 2 min at room temperature.
Two independent pathologists evaluated the
immunohistochemical results in a blinded manner. STK17A-positive
staining intensity was scored as follows: 0, negative; 1, weak; 2,
moderate; and 3, strong. In addition, the percentage of
STK17A-positive cells was scored as follows: 0, <5; 1, 5–25; 2,
26–50; 3, 51–75; and 4, >75%. The final staining scores were
calculated by multiplying the scores for the percentage of
STK17A-positive cells and staining intensity. To divide patients
into high- and normal-expression groups, the cutoff value was
determined by receiver operating characteristic curve analysis. The
sections were studied using a light microscope (Olympus BX 53;
Olympus Corporation).
Western blot analysis
Total proteins were extracted from GC tissues and
tumor cells using ice-cold RIPA lysis buffer (Thermo Fisher
Scientific, Inc.). The protein concentration was determined using a
bicinchoninic acid protein assay kit. Proteins (20 µg) were heated
in loading buffer at 100°C for 10 min and separated by 10%
SDS-PAGE, followed by transfer onto PVDF membranes. After blocking
with 5% skimmed milk at room temperature for 1 h, the membranes
were probed at 4°C overnight with primary antibodies against
N-cadherin (1:1,000 dilution; product no. 13116), E-cadherin
(1:1,000 dilution; product no. 3195), vimentin (1:1,000 dilution;
product no. 5741) and GAPDH (1:1,000 dilution; product no. 5174;
all from Cell Signaling Technology, Inc.) and STK17A (1:1,000
dilution; Abcam), After washing in TBST three times, the blots were
incubated with the HRP-conjugated secondary antibodies (anti-rabbit
IgG; 1:8,000 dilution, product no. 7074; Cell Signaling Technology,
Inc.) at room temperature for 1 h. Finally, an enhanced
chemiluminescence detection system (Imager 600; Amersham; Cytiva)
was used to quantify the protein levels with enhanced
chemiluminescent reagent (ECL; Epizyme, Inc.).
Cell Counting Kit-8 (CCK-8) assay
The transfected tumor cells were plated in a 96-well
plate at a density of 1,000 cells/well. CCK-8 reagent (10 µl;
Dojindo Molecular Technologies, Inc.) was added to 100 µl RPMI-1640
culture medium at 1–4 and 5 days and the sample was incubated in a
cell culture incubator for 2 h at 37°C. Absorbance was detected at
450 nm using a microplate reader to generate a cell proliferation
curve. At least three determinations were performed in
triplicate.
Colony formation assay
Tumor cells and control cells were plated in
triplicate in 6-well plates at a density of 1,000 cells/well. After
7 days of culture, the cells were fixed with 4% paraformaldehyde
for 30 min at room temperature, and then stained with 0.1% crystal
violet solution for 30 min at room temperature. Finally, images
were captured and the number of cell colonies was counted, and
clones with ≥50 cells were scored as actual colonies. At least
three determinations were performed in triplicate.
Wound healing assay
Cells (AGS, HGC27, MKN45 and SNU1) were uniformly
seeded in 6-well plates and cultured to 90% confluence. The cell
monolayer was washed with PBS and a 100-µl pipette tip was used to
scrape the bottom of the plate and create linear scratches. Then,
the cell culture medium was replaced with RPMI-1640 serum-free
medium. After scratching, images of the wound area were captured
under a light microscope (Olympus CKX 53; Olympus Corporation) at 0
and 16 h. At least three determinations were performed in
triplicate.
Transwell assay
Transwell experiments were performed using 24-well
(8.0 µM) Transwell inserts (BD Biosciences). The cells were
resuspended in serum-free RPMI-1640 medium, and 1×105
cells were seeded into the upper chamber of a 24-well plate.
RPMI-1640 medium supplemented with 10% FBS was added to the lower
chamber. After culturing the cells in an incubator containing 5%
CO2 at 37°C for 16 h, the cells remaining on the surface
of the membrane were removed with a cotton swab, and the migrated
cells were fixed with 4% methanol solution for 30 min at room
temperature, stained with 0.1% crystal violet solution for 30 min
at room temperature and images were captured under a light
microscope.
Transfection
PEZ-Lv201-STK17A, psi-LVRU6GP-shRNA targeting STK17A
and the corresponding control plasmids were purchased from
GeneCopoeia, Inc.. Lentivirus containing STK17A and shRNA was
packaged using the 3rd generation ViraPower™ Lentiviral Packaging
Mix (Invitrogen; Thermo Fisher Scientific, Inc.) in 293T cells. The
day before transfection, 293T cells were plated in 10-cm dishes
cultured in DMEM with 10% FBS. When cell density reached ~60-80%,
the cells were transfected with the above plasmids using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.). The mass of lentiviral plasmid used was 20 µg
and the ratio of the lentiviral plasmid, packaging vector and
envelope vector was 4:3:2. After, the transfected cells were
incubated at 37°C in a 5% CO2 incubator for ~8-12 h, the
culture medium was replaced with ~5-6 ml fresh DMEM with 10% FBS.
The supernatants containing viruses were harvested at 24 and 48 h
and concentrated by ultracentrifugation for 90 min at 50,000 × g,
4°C, and then stored at −80°C until use.
Cells were infected with lentiviruses according to
the protocol of the manufacturer. Briefly, cells were plated in
24-well plates at 1×105 cells/well, lentiviruses were
added into culture medium separately (the volume of lentiviruses
was calculated as an MOI of 20), and medium was refreshed after 12
h. Puromycin was used to screen the stable cells after 72 h of
lentiviral infection at 1.0 µg/ml as a final concentration.
Statistical analysis
SPSS 21.0 software (IBM Corp.) was used to perform
statistical analysis. Paired two-tailed t-test was performed to
compare STK17A expression between cancer tissues and their paired
adjacent tissues. In addition, the statistical significance of
associations between STK17A protein expression and
clinicopathological characteristics was analyzed using the
χ2 test. Survival rates were determined using the
Kaplan-Meier method, and survival curves were compared using
log-rank testing, while the prognostic value of STK17A was analyzed
with the Cox-proportional hazard model. Data from three separate
experiments are presented as the mean values ± standard deviation
(SD). The Student's t-test was used for comparisons between single
experimental groups and the control groups. Multigroup comparisons
of the means were carried out by one-way analysis of variance
(ANOVA) test, followed by Dunnett's post hoc test. P<0.05 was
considered to indicate statistically significant differences.
Results
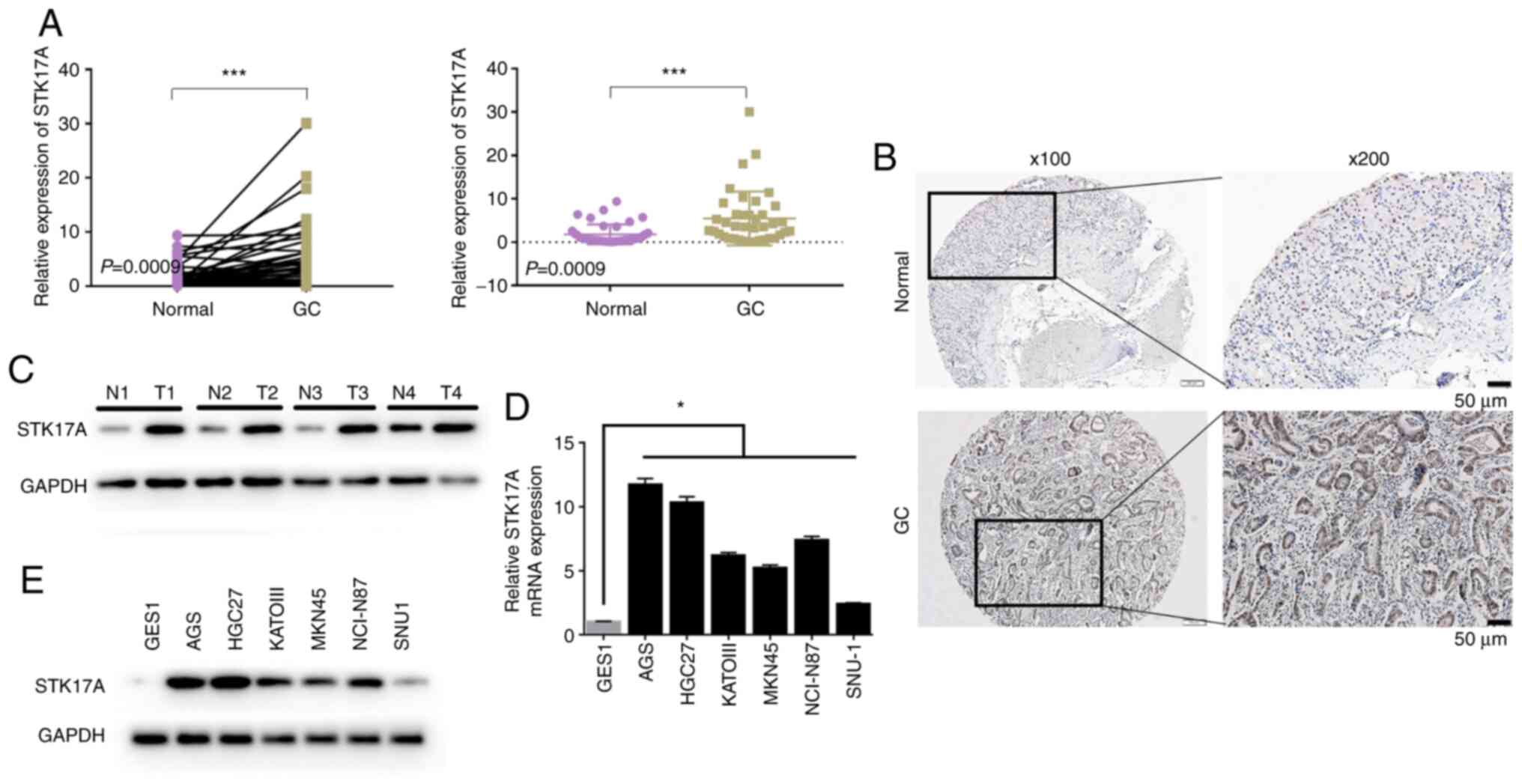
STK17A is upregulated in GC
Of the 39 GC tissue specimens, 31 (79.5%) exhibited
higher STK17A mRNA expression compared with their paired adjacent
specimens (P=0.0009; Fig. 1A). There
was also a significant difference in STK17A protein levels between
GC and adjacent normal tissues (P<0.001; Fig. 1B and C). Furthermore, STK17A
expression was markedly upregulated in all six GC cell lines
detected compared to GES1 by PCR (Fig.
1D) and western blotting (Fig.
1E).
Upregulation of STK17A is associated
with advanced clinicopathological characteristics of GC
The associations between STK17A expression and the
clinicopathological characteristics of GC are presented in Table II. The analysis results demonstrated
that the overexpression of the STK17A protein was significantly
associated with Lauren classification (P=0.018), pTNM stage
(P<0.001), tumor invasion depth (P<0.001), lymph node
metastasis (P<0.001) and 5-year survival (P<0.001). No other
clinicopathological parameters, such as patient sex (P=0.815), age
(P=0.695), tumor location (P=0.449) or differentiation grade
(P=0.384). were found to be associated with STK17A expression
levels.
 | Table II.Relationship between STK17A protein
expression in gastric cancer tissue and its clinical pathological
parameters. |
Table II.
Relationship between STK17A protein
expression in gastric cancer tissue and its clinical pathological
parameters.
|
|
| STK17A
expression |
|
|---|
|
|
|
|
|
|---|
| Clinicopathological
parameters | All cases | High | Low | P-value |
|---|
| Sex |
|
|
|
|
|
Male | 79 | 41 | 38 | 0.815 |
|
Female | 23 | 11 | 12 |
|
| Age (years) |
|
|
|
|
|
<60 | 57 | 28 | 29 | 0.695 |
|
≥60 | 45 | 24 | 21 |
|
| Lauren
classification |
|
|
|
|
| Diffuse
type | 26 | 17 | 9 | 0.018 |
|
Intestinal and mixed type | 76 | 35 | 41 |
|
|
Differentiation |
|
|
|
|
| Well
and moderate | 52 | 30 | 22 | 0.384 |
|
Poor | 50 | 22 | 28 |
|
| Tumor invasion
depth |
|
|
|
|
|
T1/T2 | 25 | 2 | 23 | <0.001 |
|
T3/T4 | 77 | 50 | 27 |
|
| Location |
|
|
|
|
|
Upper | 17 | 10 | 7 | 0.449 |
|
Middle | 81 | 39 | 42 |
|
|
Lower | 4 | 3 | 1 |
|
| pTNM stage |
|
|
|
|
|
I/II | 49 | 9 | 40 | <0.001 |
|
III/IV | 53 | 43 | 10 |
|
| Lymph node
metastasis |
|
|
|
|
|
Yes | 52 | 41 | 11 | <0.001 |
| No | 50 | 11 | 39 |
|
| Survival
status |
|
|
|
|
|
Survival | 35 | 5 | 30 | <0.001 |
|
Death | 67 | 47 | 20 |
|
High expression of STK17A in GC
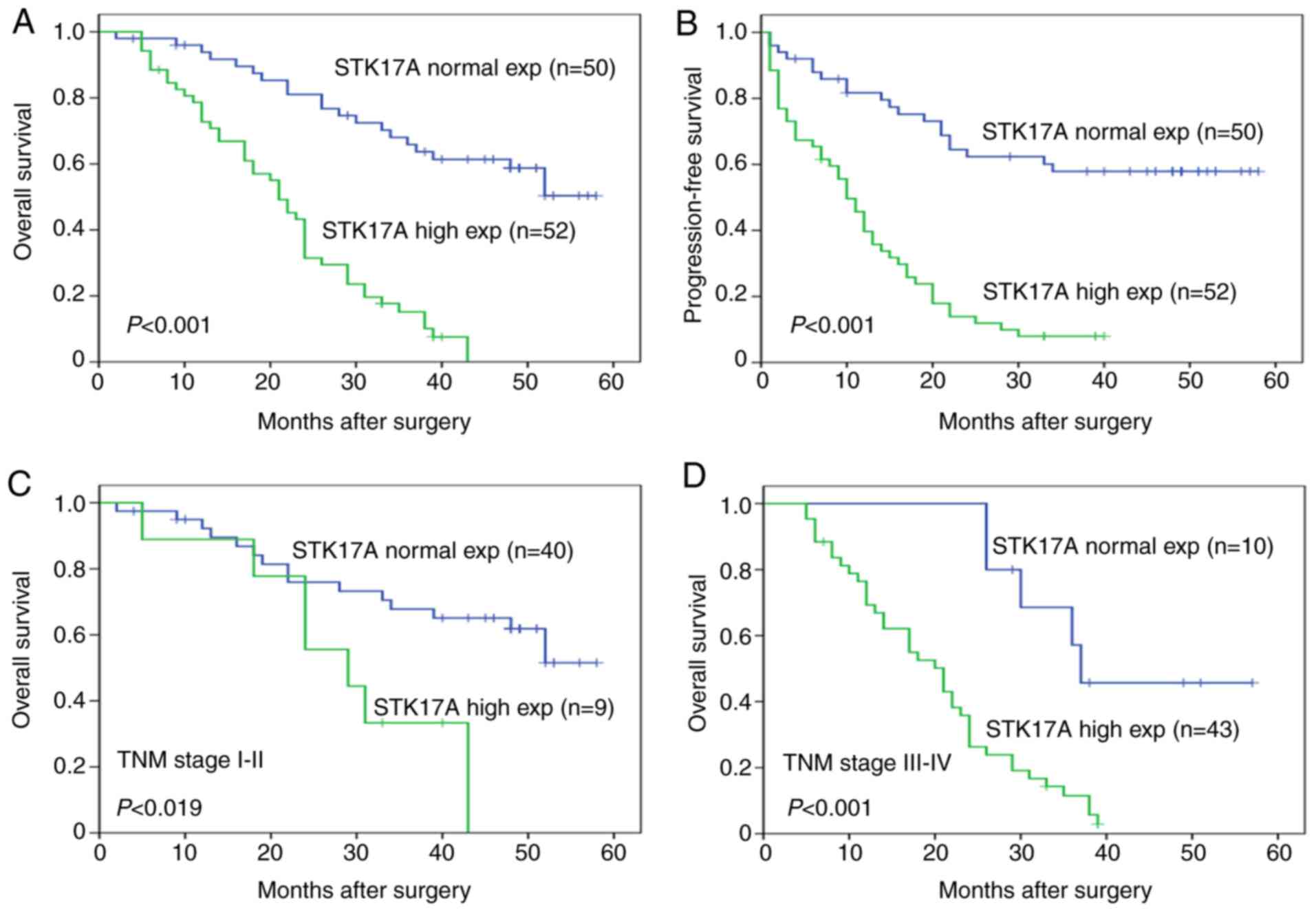
tissues is associated with poor patient survival
The study demonstrated that pTNM stage, lymph node
metastasis, depth of tumor invasion and STK17A protein expression
levels were prognostic factors for overall survival (OS) and
progression-free survival (PFS) in GC (Table III). Multivariate analysis further
indicated that STK17A protein expression and pTNM stage were
independent risk factors for OS and PFS (Table IV). The OS (Fig. 2A) and PFS (Fig. 2B) analyses revealed that patients with
high expression of STK17A exhibited shorter survival time compared
with patients exhibiting normal STK17A expression. Furthermore,
statistically significant differences in OS were found between
STK17A expression in patients with pTNM stage I–II (P=0.019) and
those with pTNM stage III–IV (P<0.001; Fig. 2C and D).
 | Table III.Univariate Cox regression analysis
for OS and PFS in gastric cancer. |
Table III.
Univariate Cox regression analysis
for OS and PFS in gastric cancer.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Clinical
parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age | 0.629–1.656 | 0.935 | 0.621–1.635 | 0.974 |
| Sex | 0.676–2.086 | 0.551 | 0.667–2.053 | 0.583 |
| Lauren
classification | 0.800–1.176 | 0.760 | 0.823–1.207 | 0.971 |
|
Differentiation | 0.595–1.061 | 0.120 | 0.603–1.708 | 0.146 |
| Tumor invasion
depth | 1.444–2.744 | <0.001 | 1.417–2.673 | <0.001 |
| pTNM stage | 1.569–3.181 | <0.001 | 1.503–3.014 | <0.001 |
| Lymph node
metastasis | 1.799–5.109 | <0.001 | 1.622–4.498 | <0.001 |
| STK17A
expression | 2.846–8.865 | <0.001 | 2.573–7.619 | <0.001 |
 | Table IV.Cox multivariate regression analysis
for OS and PFS in gastric cancer. |
Table IV.
Cox multivariate regression analysis
for OS and PFS in gastric cancer.
|
| OS | PFS |
|---|
|
|
|
|
|---|
| Parameters | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| STK17A
expression | 1.871–6.715 | <0.001 | 1.744–5.929 | <0.001 |
| pTNM stage | 1.042–2.299 | 0.031 | 1.022–2.218 | 0.039 |
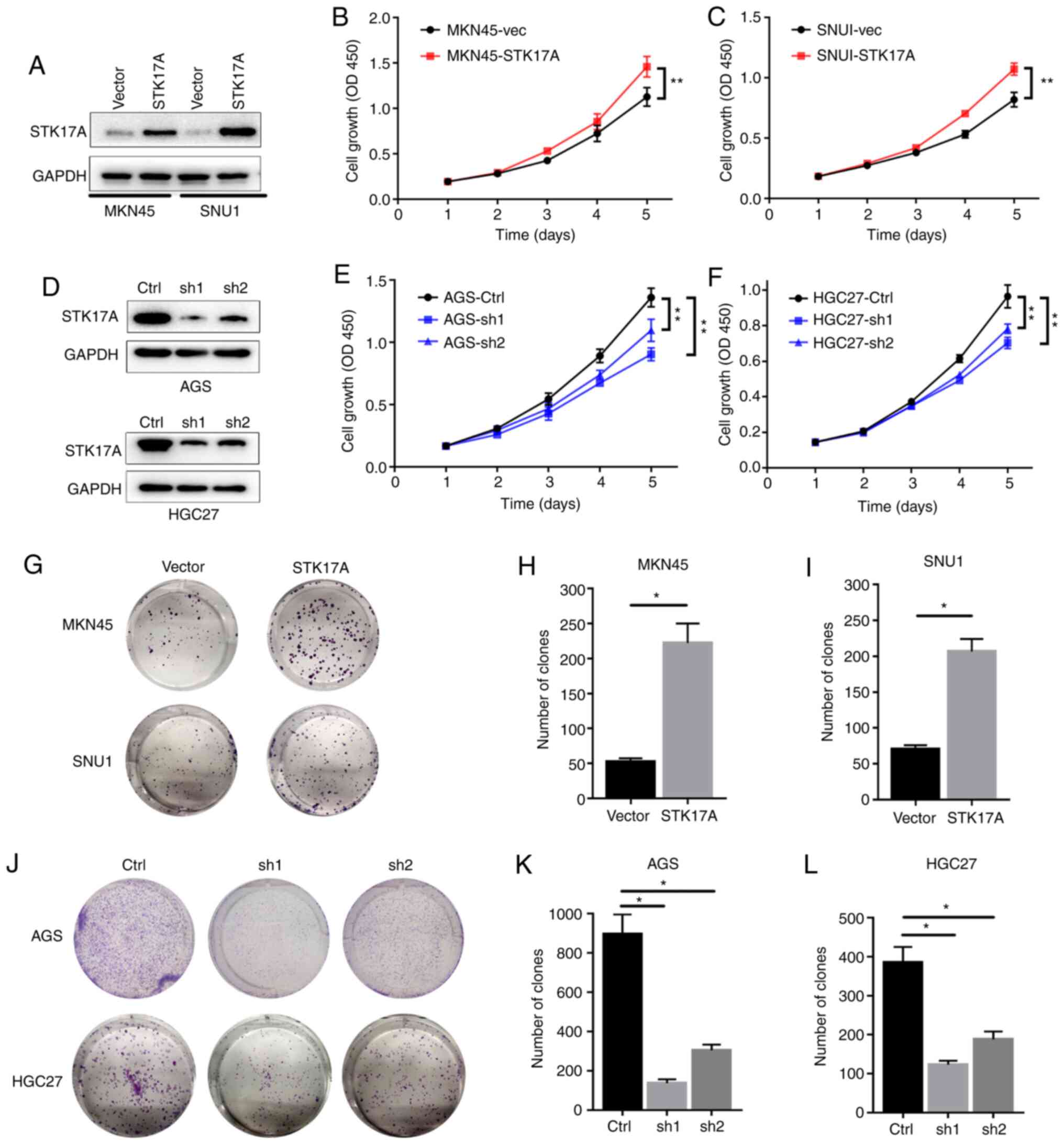
Upregulation of STK17A promotes the
proliferation of GC cells
As revealed in Fig. 3,
to investigate the functional effects of STK17A, STK17A was
overexpressed in MKN45 and SNU1 GC cells (Fig. 3A). CCK-8 assays revealed that
overexpression of STK17A significantly promoted the proliferation
of GC cells (Fig. 3B and C). Compared
to vector-transfected cells, overexpression of STK17A also
significantly increased the mean number of colonies in the colony
formation assay (Fig. 3G-I). In
parallel, STK17A-knockdown stable cell lines were established in
AGS and HGC27 cells (Fig. 3D). CCK-8
and colony formation assays both indicated that knockdown of STK17A
significantly reduced the proliferation of GC cells (Fig. 3E and F and J-L). These results
suggested that STK17A could promote the proliferation of GC
cells.
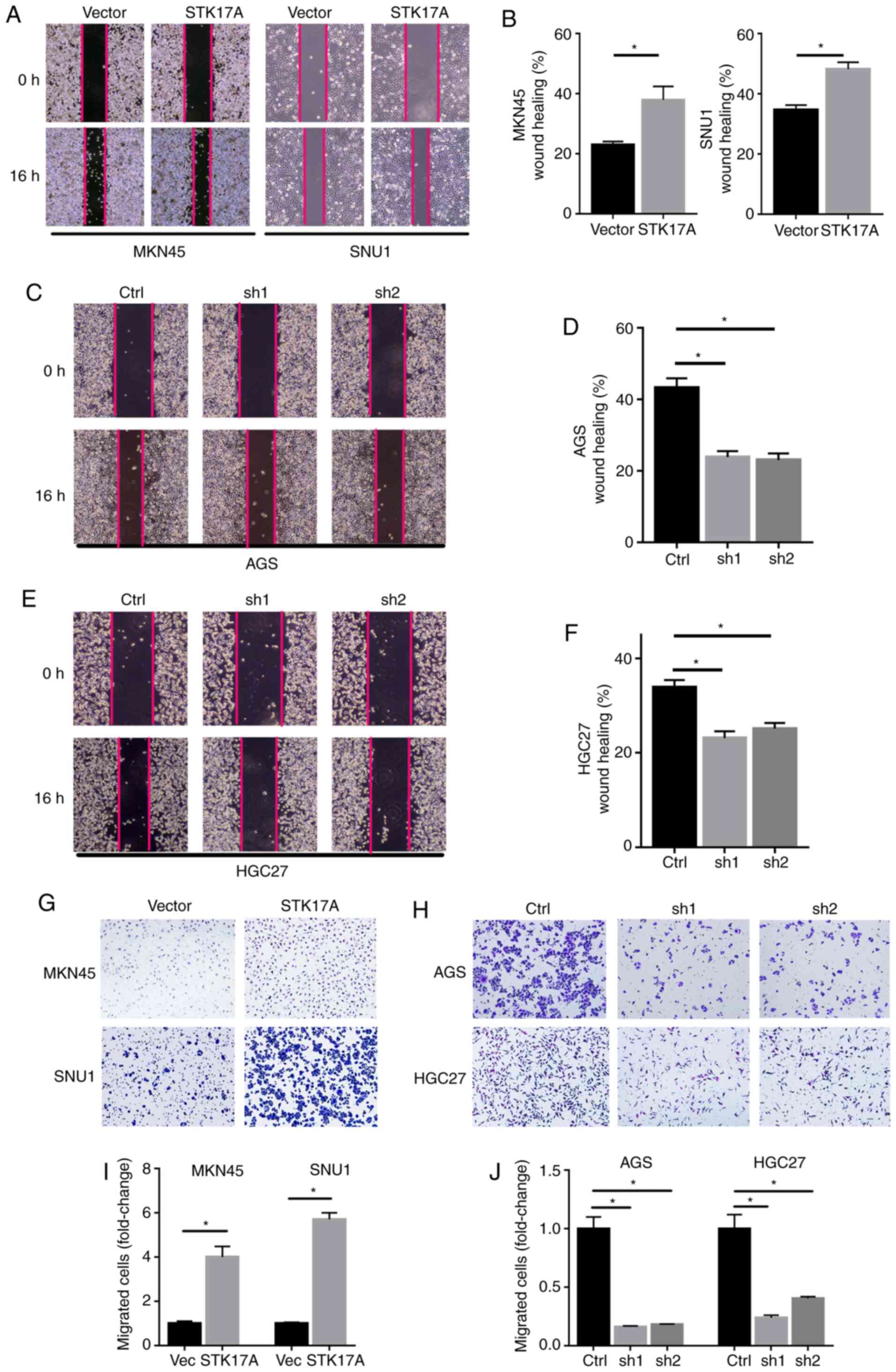
STK17A regulates the migration of GC
cells via epithelial-mesenchymal transition (EMT)
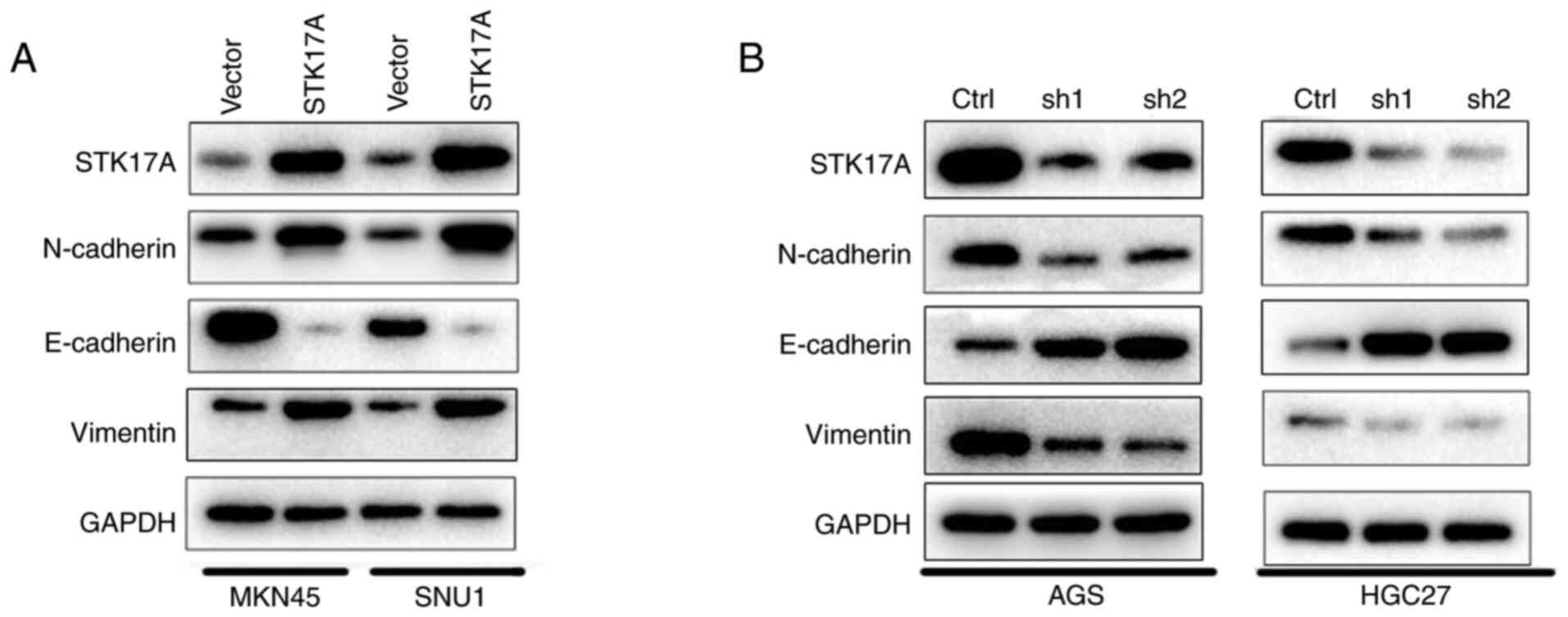
The effect of STK17A on the migration ability of GC
cells was further evaluated. In the wound healing assay, MKN45 and
SNU1 cells overexpressing STK17A exhibited more rapid wound closure
compared with vector-expressing cells (Fig. 4A and B). Conversely, AGS and HGC27
cells with STK17A knockdown exhibited slower wound closure
(Fig. 4C-F). Consistently, similar
results were obtained with the Transwell assay. STK17A
overexpression significantly enhanced the migration ability of
MKN45 and SNU1 cells (Fig. 4G), while
STK17A knockdown in AGS and HGC27 cells suppressed their migration
ability (Fig. 4H). Consistent with
the aforementioned results, the western blot analysis results
demonstrated that STK17A overexpression significantly increased the
expression of N-cadherin and vimentin, but inhibited the expression
of E-cadherin (Fig. 5A), while STK17A
downregulation produced the opposite results, as predicted
(Fig. 5B).
Discussion
With the continuous development of clinical
diagnosis and treatment technology, the clinical prognosis of
patients with GC has significantly improved. However, patients with
the similar clinicopathological characteristics exhibit different
clinical results when receiving the same therapy. Therefore, it is
imperative to identify new biomarkers, which may accurately predict
the prognosis of patients and develop individualized treatments for
patients with GC.
The present study demonstrated that STK17A was
upregulated at both the mRNA and protein levels in GC compared with
adjacent normal gastric tissues. It was observed that the STK17A
expression level was correlated with clinical and pathological
factors, specifically tumor invasion depth and lymph node
metastasis. On clinical outcome analysis, patients with STK17A
overexpression exhibited significantly shorter survival and poorer
prognosis. These data provided evidence that STK17A expression may
be associated with the malignant biological behavior of GC,
clinicopathological characteristics and prognosis of GC. Therefore,
STK17A may be considered as a potential prognostic biomarker. It
was demonstrated that GC patients with lymph node metastasis and
greater depth of tumor invasion exhibited higher expression levels
of STK17A, and these results suggested that STK17A may promote GC
growth and metastasis. Furthermore, it was demonstrated through
functional analysis that high STK17A expression enhanced the
proliferation and migration of GC cells. In addition, mechanistic
studies demonstrated that STK17A promoted GC migration via
mediating EMT.
EMT has been reported to be an important process in
tumor occurrence and metastasis. EMT is characterized by loss of
epithelial marker expression, increased mesenchymal marker
expression, and enhanced migratory and invasive cell behaviors
(24). Consistently, in the present
study, upregulation of STK17A inhibited the expression of the
epithelial marker E-cadherin, while it enhanced the expression of
the mesenchymal markers N-cadherin and vimentin. Furthermore,
STK17A was shown to promote tumor cell invasion and motility by
changing the cell-cell and cell-stroma interactions (25). It has been reported that EMT of GC
cells may affect the pathogenesis of GC through the Wnt/β-catenin,
PI3K/AKT, and HGF/c-Met signaling pathways, which provides an
important approach to the study of the relationship between cell
behavior and EMT (26–28).
It was previously demonstrated that, in some tumor
types, STK17A is considered to act as a tumor promoter: STK17A was
revealed to promote glioblastoma cell proliferation, and its
overexpression may be considered as a biomarker for advanced
cervical cancer (8,29). However, it was also demonstrated that
STK17A overexpression reduced cell proliferation in testicular and
ovarian cancer (7). These results
support the highly relevant specificity and dependence of STK17A
function on cancer type. These effects may be closely associated
with the p53-dependent signaling mechanism, as the study revealed
that STK17A is one of the direct and DNA damage-inducible p53
target genes. Moreover, STK17A has functional and shared response
elements upstream of the p53 pathway, which is involved in cell
signal transduction (7,30). These findings provide new insight into
the mechanism through which STK17A may promote GC cell
proliferation.
There remains a limitation in this research. It has
been demonstrated that STK17A has a close relationship with
chemoresistance in ovarian cancer cells (6). Unfortunately, the association between
STK17A and chemoresistance in GC has not been fully evaluated. This
issue may be addressed in a future study.
In summary, STK17A was revealed to be upregulated in
GC tissues in the present study, and high STK17A expression was
significantly associated with clinical metastasis and poor
prognosis in patients with GC. In addition, STK17A may promote cell
proliferation and migration via regulation of EMT, thereby
providing a potential therapeutic target for GC.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81872264).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZW, YQ and YJ conceived and designed the study. ZW,
CW, LL and JQ performed the experiments and analyzed the data. LS,
SL and LW collected the specimens and performed patient follow-up.
ZW and CW wrote the paper. YQ, BJ and YJ reviewed the manuscript
for important intellectual content and edited the manuscript. ZW
and CW performed the experiments required for revision. All authors
read and approved the manuscript and agree to be accountable for
all aspects of the research in ensuring that the accuracy or
integrity of any part of the work are appropriately investigated
and resolved.
Ethics approval and consent to
participate
Written informed consent for the use of tissue
samples was obtained from all patients or their legal guardians.
The use of GC tissues by the present study was approved by the
Ethics Committee of Zhengzhou University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel R, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. Feb 4–2021.(Online
ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cidon EU, Ellis SG, Inam Y, Adeleke S,
Zarif S and Geldart T: Molecular targeted agents for gastric
cancer: A step forward towards personalized therapy. Cancers
(Basel). 5:64–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steeg PS: Metastasis suppressors alter the
signal transduction of cancer cells. Nat Rev Cancer. 3:55–63. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sanjo H, Kawai T and Akira S: DRAKs, novel
serine/threonine kinases related to death-associated protein kinase
that trigger apoptosis. J Biol Chem. 273:29066–29071. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bialik S and Kimchi A: The
death-associated protein kinases: Structure, function, and beyond.
Annu Rev Biochem. 75:189–210. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gao J, Liu D, Li J, Song Q and Wang Q:
Effect of STK17A on the sensitivity of ovarian cancer cells to
paclitaxel and carboplatin. Oncol Lett. 12:1107–1112. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mao P, Hever MP, Niemaszyk LM, Haghkerdar
JM, Yanco EG, Desai D, Beyrouthy MJ, Kerley-Hamilton JS, Freemantle
SJ and Spinella MJ: Serine/threonine kinase 17A is a novel p53
target gene and modulator of cisplatin toxicity and reactive oxygen
species in testicular cancer cells. J Biol Chem. 286:19381–19391.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mao P, Hever-Jardine MP, Rahme GJ, Yang E,
Tam J, Kodali A, Biswal B, Fadul CE, Gaur A, Israel MA and Spinella
MJ: Serine/threonine kinase 17A is a novel candidate for
therapeutic targeting in glioblastoma. PLoS One. 8:e818032013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park Y, Kim W, Lee JM, Park J, Cho JK,
Pang K, Lee J, Kim D, Park SW, Yang KM and Kim SJ: Cytoplasmic
DRAK1 overexpressed in head and neck cancers inhibits TGF-β1 tumor
suppressor activity by binding to Smad3 to interrupt its complex
formation with Smad4. Oncogene. 34:5037–5045. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Short SP, Thompson JJ, Bilotta AJ, Chen X,
Revetta FL, Washington MK and Williams CS: Serine threonine kinase
17A maintains the epithelial state in colorectal cancer cells. Mol
Cancer Res. 17:882–894. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wei W and Liu C: Prognostic and predictive
roles of microRNA-411 and its target STK17A in evaluating
radiotherapy efficacy and their effects on cell migration and
invasion via the p53 signaling pathway in cervical cancer. Mol Med
Rep. 21:267–281. 2020.PubMed/NCBI
|
|
12
|
Temmerman K, Simon B and Wilmanns M:
Structural and functional diversity in the activity and regulation
of DAPK-related protein kinases. FEBS J. 280:5533–5350. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang WJ, Kuo JC, Yao CC and Chen RH:
DAP-kinase induces apoptosis by suppressing integrin activity and
disrupting matrix survival signals. J Cell Biol. 159:169–179. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lin Y, Hupp TR and Stevens C:
Death-associated protein kinase (DAPK) and signal transduction:
Additional roles beyond cell death. FEBS J. 277:48–57. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiger AA, Baum B, Jones S, Jones MR,
Coulson A, Echeverri C and Perrimon N: A functional genomic
analysis of cell morphology using RNA interference. J Biol.
2:272003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Neubueser D and Hipfner DR: Overlapping
roles of Drosophila Drak and Rok kinases in epithelial
tissue morphogenesis. Mol Biol Cell. 21:2869–2879. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Robertson F, Pinal N, Fichelson P and
Pichaud F: Atonal and EGFR signalling orchestrate rok- and
Drak-dependent adherens junction remodelling during ommatidia
morphogenesis. Development. 139:3432–3441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Coleman ML, Sahai EA, Yeo M, Bosch M,
Dewar A and Olson MF: Membrane blebbing during apoptosis results
from caspase-mediated activation of ROCK I. Nat Cell Biol.
3:339–345. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gilmore AP: Anoikis. Cell Death Differ. 12
(Suppl 2):S1473–S1477. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tang H, Liu YJ, Liu M and Li X:
Establishment and gene analysis of an oxaliplatin-resistant colon
cancer cell line THC8307/L-OHP. Anticancer Drugs. 18:633–639. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC cancer staging manual. 7th edition.
New York NY: Springer; 2010
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li Y, Nie CJ, Hu L, Qin Y, Liu HB, Zeng
TT, Chen L, Fu L, Deng W, Chen SP, et al: Characterization of a
novel mechanism of genomic instability involving the
SEI1/SET/NM23H1 pathway in esophageal cancers. Cancer Res.
70:5695–5705. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Inoue T, Umezawa A, Takenaka T, Suzuki H
and Okada H: The contribution of epithelial-mesenchymal transition
to renal fibrosis differs among kidney disease models. Kidney Int.
87:233–238. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen DH, Yu JW and Jiang BJ: Contactin 1:
A potential therapeutic target and biomarker in gastric cancer.
World J Gastroenterol. 21:9707–9716. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lamouille S, Xu J and Derynck R: Molecular
mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell
Biol. 15:178–196. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mesteri I, Schoppmann SF, Preusser M and
Birner P: Overexpression of CMET is associated with signal
transducer and activator of transcription 3 activation and
diminished prognosis in oesophageal adenocarcinoma but not in
squamous cell carcinoma. Eur J Cancer. 50:1354–1360. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Thomas A, Mahantshetty U, Kannan S,
Deodhar K, Shrivastava SK, Kumar-Sinha C and Mulherkar R:
Expression profiling of cervical cancers in Indian women at
different stages to identify gene signatures during progression of
the disease. Cancer Med. 2:836–648. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Cekirge HS, Peynircioglu B and Saatci I:
Endovascular treatment of an ‘anterior cerebral artery’ aneurysm in
a patient with ‘embryonic unfused middle cerebral artery’ anomaly:
A case report. Neuroradiology. 47:690–694. 2005. View Article : Google Scholar : PubMed/NCBI
|