Introduction
Efficient treatment of metastatic prostate cancer
(PC) remains a current clinical need (1). Initial androgen deprivation therapy
provides stabilization of disease for several years; however, the
development of resistance occurs in the large majority of patients
with time (2). Insensitivity to
existing treatments leads to disease progression, which
necessitates the identification of novel molecular targets and
targeted therapeutic approaches.
Prostate-specific membrane antigen (PSMA)-targeted
therapy using radioligands, such as [177Lu]Lu-PSMA-617,
has demonstrated promising results in clinical trials and improved
outcomes for patients with metastatic castration-resistant PC
(3). However, patients with low
PSMA expression are not eligible for this therapy (4); some patients do not respond to it and
some develop resistance despite initially good response (5).
Epithelial cell adhesion molecule (EpCAM) is a
potential therapeutic target in PC. EpCAM is a type I transmembrane
glycoprotein, which participates in cell adhesion and
proliferation, and was reported to be involved in oncogenic
signaling by engagement of elements of the Wnt pathway (6). Upregulated EpCAM expression is
detected in early stages of PC and is further induced in high-grade
tumors and metastatic lesions (7,8).
Intense EpCAM overexpression is found in 40–60% of prostate tumor
samples (9) and is associated with
metastasis and increased risk of PC recurrence (10,11).
Due to its involvement in mechanisms regulating resistance to
treatment, EpCAM has been suggested as a therapeutic target to
sensitize PC cells to chemotherapy and radiotherapy (12). The EpCAM protein surface level was
found to be up to 16-fold higher in PC cells compared to
non-cancerous prostate cells and up to 4-fold higher in malignant
compared to benign prostate tumors (7), which creates a potential therapeutic
window for targeted cytotoxic delivery.
A number of therapeutic agents targeting EpCAM have
been evaluated in preclinical and clinical trials for different
types of cancer (13). They
include monoclonal antibodies (mAbs), their fragments, as well as
their conjugates with cytotoxic drugs and immunotoxins (14,15).
The only EpCAM-targeting agent that has received approval in the
European Union is the rat-mouse bispecific anti-EpCAM/anti-CD3
antibody catumaxomab for the treatment of patients with malignant
ascites by intraperitoneal infusion (16). While being more effective than
conventional treatment, it has shown limited efficacy in solid
tumors, which could arise from several factors, such as
heterogeneity of EpCAM expression in patients, limited penetration
in the tumor due to the size of the targeting agent and limited
cytotoxic effect.
One way to increase the potency of targeted therapy
is conjugation of a cytotoxic payload, such as drugs, plant or
bacterial toxins, to a targeting molecule for selective eradication
of malignant cells. The limited therapeutic effect observed for
EpCAM-targeting mAbs promoted the development of targeted
immunotoxins (17). Several of
them have reached clinical trials, such as the mouse mAb MOC31 and
its scFv fragment (4D5MOCB) conjugated to Pseudomonas
aeruginosa exotoxin A (PE) (VB4-845, oportuzumab monatox)
(18–21) and a Fab fragment conjugated with
the plant-derived ribosome-inactivating protein de-bouganin
(VB6-845, citatuzumab bogatox) (22). Still, there is a number of
physiological barriers hampering the efficient tumor delivery of
cytotoxic payloads using bulky immunoglobulins, and the use of
smaller targeting vectors should be beneficial (17).
Designed ankyrin repeat proteins (DARPins) are a
type of engineered scaffold proteins with promising tumor-targeting
properties (23). Their use for
tumor targeting offers a number of advantages in comparison with
the traditional IgG scaffold. The small size (14–18 kDa) could
enable more efficient interstitial transport, faster extravasation
and deeper penetration of DARPins into the tumors compared to
therapeutics based on the IgG scaffold (150 kDa) (24). Their high affinity ensures a strong
binding to malignant cells and a good retention in tumors. Rapid
excretion of DARPins from blood provides high imaging contrast
already several hours after injection. DARPins have shown promising
results in preclinical studies for imaging of human epidermal
growth factor receptor 2 (HER2) (25–28)
and EpCAM expression (29–32) and could be used as companion
diagnostic agents during targeted therapy. The clinical trial
evaluating anti-HER2 DARPin G3 labeled with technetium-99m showed
its capacity of specific targeting HER2-positive breast cancers and
demonstrated the safety of this protein scaffold in humans, with no
toxicity or side effects observed (33).
Another advantage of DARPin scaffold over IgG
includes its robustness, high solubility and thermodynamic
stability. Conjugates of DARPins with protein-based toxins can be
genetically engineered and produced as single protein fusions in
large amounts with lower manufacturing costs (13,23,34).
In the present study, we investigated a fusion
protein consisting of an EpCAM-targeting DARPin Ec1 and a
re-engineered Pseudomonas exotoxin A variant (LoPE)
bacterial toxin for cytotoxic therapy of prostate cancer. The
N-terminal DARPin Ec1 (18 kDa) binds to EpCAM with picomolar
affinity [KD 68 pM (35)], while the C-terminal LoPE toxin (25
kDa) irreversibly inhibits eukaryotic elongation factor eEF2 that
leads to inhibition of protein synthesis in the cell (36). LoPE is the deimmunized C-terminal
catalytic subunit of bacterial Pseudomonas aeruginosa
Exotoxin A (PE toxin) with lower immunogenicity and general
toxicity in vivo in comparison with previous versions of PE
toxin (37).
The aim of the present study was to characterize the
functional parameters of Ec1-LoPE, such as binding specificity and
kinetics of binding to living cells, cellular processing,
internalization and cytotoxicity in PC cell lines and to evaluate
its potential for targeted therapy of PC in vivo.
Materials and methods
Cells
The human prostate cancer cell lines PC-3 and DU145
overexpressing EpCAM were purchased from the American Type Culture
Collection (ATCC; LGC Promochem). The cells were cultured in
Roswell Park Memorial Institute (RPMI)-1640 medium with L-glutamine
(L0500, Biowest) supplemented with 10% fetal bovine serum (FBS)
(F7524, Sigma-Aldrich; Merck KGaA) and penicillin-streptomycin
solution (L0022, Biowest) at 37°C and 5% CO2 atmosphere,
unless stated otherwise. Cells were detached using
trypsin-ethylenediaminetetraacetic acid (EDTA) solution (25200056,
Thermo Fisher Scientific, Inc.).
Targeting protein
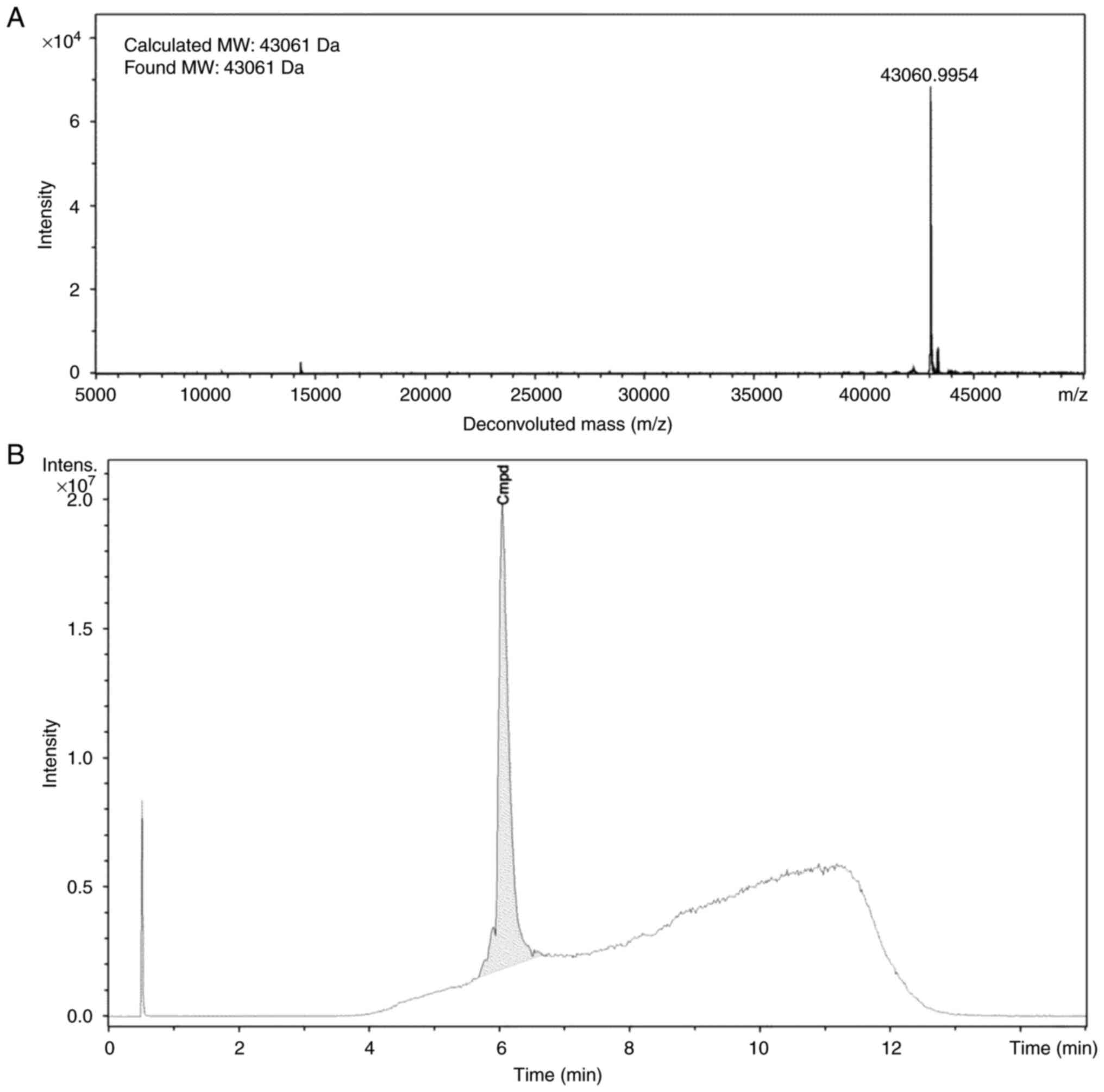
Ec1-LoPE (Fig. 1)
was produced and characterized as described previously (38). To confirm the identity, the
molecular weight of the protein was determined using electrospray
ionization mass spectrometry (Impact II instrument, Bruker Corp.).
The spectrometer worked on line with Dionex UltiMate 3000
ultra-high performance liquid chromatography (UHPLC) system (Thermo
Fisher Scientific, Inc.) equipped with a ProSwift RP-4H column
(1×50 mm, Thermo Fisher Scientific, Inc.). The chromatographic
system used two solvents: Solvent A (3% acetonitrile, 0.1% formic
acid in water) and solvent B (95% acetonitrile, 0.1% formic acid in
water), and the flow rate was 200 µl/min. The following gradient
profile was used: 4% solvent B for 2 min, 4–90% solvent B within 6
min, 90% solvent B for 2 min, 90–4% solvent B within 1 min followed
by 4% solvent B for 4 min. The found molecular weight (43,061 kDa)
was in excellent agreement with the calculated molecular weight
(43,061 kDa) (Fig. 2A). According
to HPLC analysis, the purity of Ec1-LoPE was >99% (Fig. 2B).
Radiolabeling
Ec1-LoPE having (His)6-tag at the
C-terminus was radiolabeled site-specifically with
[99mTc][Tc(CO)3(H2O)3]+
as described previously (38).
Briefly, technetium-99m pertechnetate
[99mTc]TcO4− was obtained by
eluting a commercial 99Mo/99mTc generator
(Mallinckrodt) with sterile 0.9% NaCl. To generate the
[99mTc][Tc(CO)3(H2O)3]+,
approximately 500 µl of
[99mTc]TcO4− eluate containing 3–4
GBq activity was added to a CRS kit vial (PSI) and incubated at
100°C for 30 min followed by cooling down at room temperature for
10 min. Solution of
[99mTc][Tc(CO)3(H2O)3]+
(100 MBq, 15 µl) was mixed with 30 µl of 0.1 M HCl and added to 30
µg of Ec1-LoPE [25 µl in phosphate-buffered saline (PBS)]. The
reaction mixture was incubated for 60 min at 40°C. A
pre-purification challenge was performed by adding a 1,000-fold
molar excess of histidine (110 µg in 11 µl of PBS) to the reaction
mixture and incubating at 40°C for 10 min to remove any
loosely-bound
[99mTc]Tc(CO)3(H2O)3]+.
The radiolabeled compound was separated from free
[99mTc]Tc(CO)3(H2O)3]+
by passage through a NAP-5 size-exclusion column (GE Healthcare)
pre-equilibrated and eluted with PBS. Radiochemical yield and
purity of [99mTc]Tc(CO)3-Ec1-LoPE were
measured using instant thin-layer chromatography (iTLC) silica gel
strips eluted in PBS. In this system, the radiolabeled compound
stays at the application point; all forms of free radionuclide move
with the solvent front. The activity distribution along the iTLC
strip was measured with a Storage Phosphor System (CR-35 BIO Plus,
Elysia-Raytest) and analyzed with AIDA Image Analysis software
(Elysia-Raytest).
In vitro stability test
The evaluation of radiolabel stability of
[99mTc]Tc(CO)3-Ec1-LoPE was performed by
incubating it with a 1,000-fold molar excess of histidine in PBS at
room temperature for 4 h. The control samples were incubated in
PBS. Samples were analyzed using radio-iTLC in PBS. The values were
normalized to the starting radiochemical purity taken as 100%.
In vitro specificity
The binding specificity of
[99mTc]Tc(CO)3-Ec1-LoPE to
EpCAM-overexpressing cells PC-3 and DU145 was performed by using
in vitro saturation assay (38). Briefly, the cells were seeded in a
6-well plate at a density of 5×105 cells per well one
day before the experiment and allowed for attachment overnight. A
set of three wells was used for each group. The next day, blocking
was performed by incubating one group of cells with 200 nM of
non-radiolabeled DARPin Ec1 in 0.5 ml of culture medium at room
temperature for 15 min to saturate EpCAM receptors. The same volume
of culture medium only was added to another group of cells. After
15 min, 0.5 ml of [99mTc]Tc(CO)3-Ec1-LoPE was
added to each well to a final concentration of 2 nM and incubated
at 37°C for 1 h. After incubation, medium was discarded, cells were
washed once with 1 ml of PBS. The cells were then incubated with
0.5 ml of trypsin solution at 37°C to allow for detachment and
resuspended with 0.5 ml of culture medium. The cell suspension was
collected, counted for cell number, followed by a wash with 1 ml
PBS, which was also collected. Three standard samples containing
0.5 ml of [99mTc]Tc(CO)3-Ec1-LoPE were
collected. The activity in standard samples and fractions
containing cells was measured using an automatic gamma spectrometer
(2480 Wizard, Perkin Elmer), percentage of cell-associated activity
per 1×106 cells was calculated.
Cellular processing
Cellular processing and internalization of
[99mTc]Tc(CO)3-Ec1-LoPE by PC-3 and DU145
cells were evaluated during continuous incubation using acid wash
method (39). Briefly, cells were
seeded one day before the experiment in 3-cm Petri dishes at a
density of 7×105 cells per dish. At the day of the
experiment, the radiolabeled compound (2 nM, 1 ml) was added to
each dish and the dishes were incubated at 37°C in a humidified
incubator. Three standard samples containing 1 ml of
[99mTc]Tc(CO)3-Ec1-LoPE were collected and
used as total added activity. At 1, 2, 4 and 6 h a set of three
dishes was removed, medium was discarded, cells were washed once
with 1 ml of ice-cold PBS and discarded. To collect the
membrane-bound activity, the cells were incubated with 1 ml of 0.2
M glycine buffer containing 4 M urea (pH 2.0) for 5 min on ice,
washed with 1 ml of the same buffer and with 1 ml of PBS. These
steps were performed on ice to prevent internalization. To collect
the internalized activity, the cells were incubated with 1 ml of 1
M NaOH at 37°C for 30 min to lyse the cells. The cell lysate was
collected, followed by washing with 1 ml of PBS. The activity in
the standards, membrane-bound and internalized fractions was
measured using a gamma spectrometer and percentage cell-associated
activity was calculated.
Affinity
The binding affinity of
[99mTc]Tc(CO)3-Ec1-LoPE to DU145 cells was
measured using LigandTracer Yellow instrument (Ridgeview
Diagnostics) as described previously (38). The experiment was performed in
duplicate at room temperature to prevent internalization. In brief,
2×106 cells were seeded to a local area of an 89-mm
Petri dish one day before the experiment. The binding kinetics was
measured by adding increasing concentrations of the radiolabeled
compound (3 and 9 nM) every 1.5 h. Thereafter, the medium
containing the radiolabeled compound was replaced with cell culture
medium to measure the dissociation rate. The binding curve was
analyzed using TraceDrawer Software (Ridgeview Instruments, version
1.9) and the equilibrium dissociation constant
(KD) was calculated. The interaction
heterogeneity was estimated using Interaction Map analysis
(Ridgeview Diagnostics).
Cytotoxicity
The in vitro cytotoxicity assay was performed
using PC3 and DU-145 cells. Cells were seeded in a 96-well plate at
a density of 5,000 cells per well in 100 µl culture medium and
allowed to attach overnight. On the experimental day, the medium
was removed, serial dilutions of Ec1-LoPE in culture medium were
added for each concentration (n=4-5) and the plate was incubated in
a humidified incubator. After 72 h, the medium was replaced, and
cell viability was evaluated with the Cell Counting Kit-8 (CCK-8;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, 10 µl of CCK-8 kit was added to each well
containing 100 µl culture medium, followed by incubation at 37°C
for 1 to 2 h. The absorbance at 450 nm was measured using a
microplate reader. The value from the wells containing medium only
was taken as the background; the value from the wells containing
cells with medium (no Ec1-LoPE) was taken as 100% viability
control. The data were analyzed by GraphPad Prism (version 9.0.2
for Windows, GraphPad Software, Inc.) using a log(inhibitor) vs.
response-variable slope (four parameters) model to obtain a
half-maximal inhibitory concentration (IC50) value. To
test the specificity of Ec1-LoPE cytotoxic action, one group of
cells was incubated with a 100-fold molar excess of DARPin Ec1 for
10 min before the addition of Ec1-LoPE (10 nM) to saturate binding
sites on EpCAM and prevent binding of Ec1-LoPE. The cell viability
was evaluated as described above.
Statistics
Data were analyzed using GraphPad Prism (version
9.0.2 for Windows, GraphPad Software, Inc.). Unpaired 2-tailed
t-test was performed to determine significant differences
(P<0.05).
Results
Radiolabeling and stability
Ec1-LoPE was successfully labeled with
[99mTc]Tc(CO)3(H2O)3]+
with a radiochemical yield of 51±13% (n=7) and radiochemical purity
over 99% (n=7) after purification using size-exclusion column
(Table I). Radiolabeled
[99mTc]Tc(CO)3-Ec1-LoPE demonstrated high
stability during incubation with a 1,000-fold molar excess of
histidine for up to 4 h at room temperature (Table I).
 | Table I.Radiolabeling of EpCAM-targeting
Ec1-LoPE with [99mTc]Tc(CO)3 and in
vitro stability under a 1,000-fold molar excess of histidine
compared to PBS (control). |
Table I.
Radiolabeling of EpCAM-targeting
Ec1-LoPE with [99mTc]Tc(CO)3 and in
vitro stability under a 1,000-fold molar excess of histidine
compared to PBS (control).
|
|
|
| Protein-associated
activity (%) (n=2) |
|---|
|
|
|
|
|
|---|
|
|
|
| 1 h | 4 h |
|---|
|
|
|
|
|
|
|---|
|
| Radiochemical yield
(%) | Radiochemical
purity (%) | x1,000
Histidine | PBS (control) | x1,000
Histidine | PBS (control) |
|---|
|
[99mTc]Tc(CO)3- | 51±13 | 99±0 | 100±0 | 100±0 | 100±0 | 100±0 |
| Ec1-LoPE | (n=7) | (n=7) |
|
|
|
|
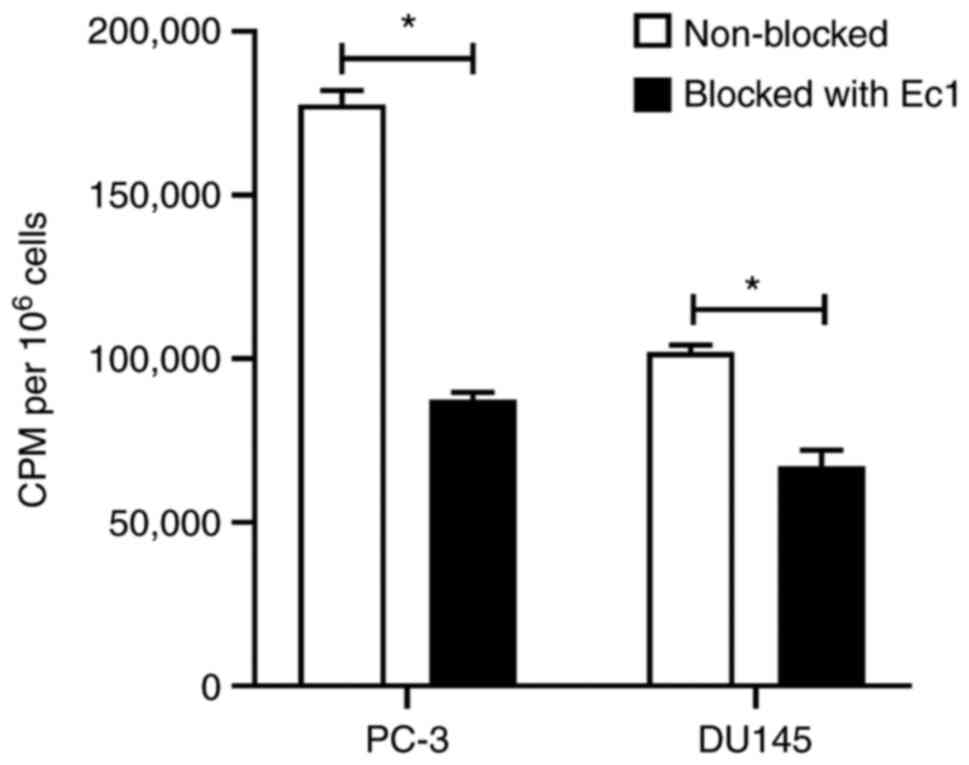
In vitro binding specificity
The specificity of
[99mTc]Tc(CO)3-Ec1-LoPE binding to
EpCAM-expressing PC-3 and DU145 cells was studied by saturating the
EpCAM receptors with a large excess of unlabeled DARPin Ec1. A
significant (P<0.001) reduction in cell-associated activity was
observed in the groups where EpCAM was pre-saturated, indicating
specific binding of [99mTc]Tc(CO)3-Ec1-LoPE
(Fig. 3). Cell-associated activity
was higher for PC-3 cells compared to DU145 cells.
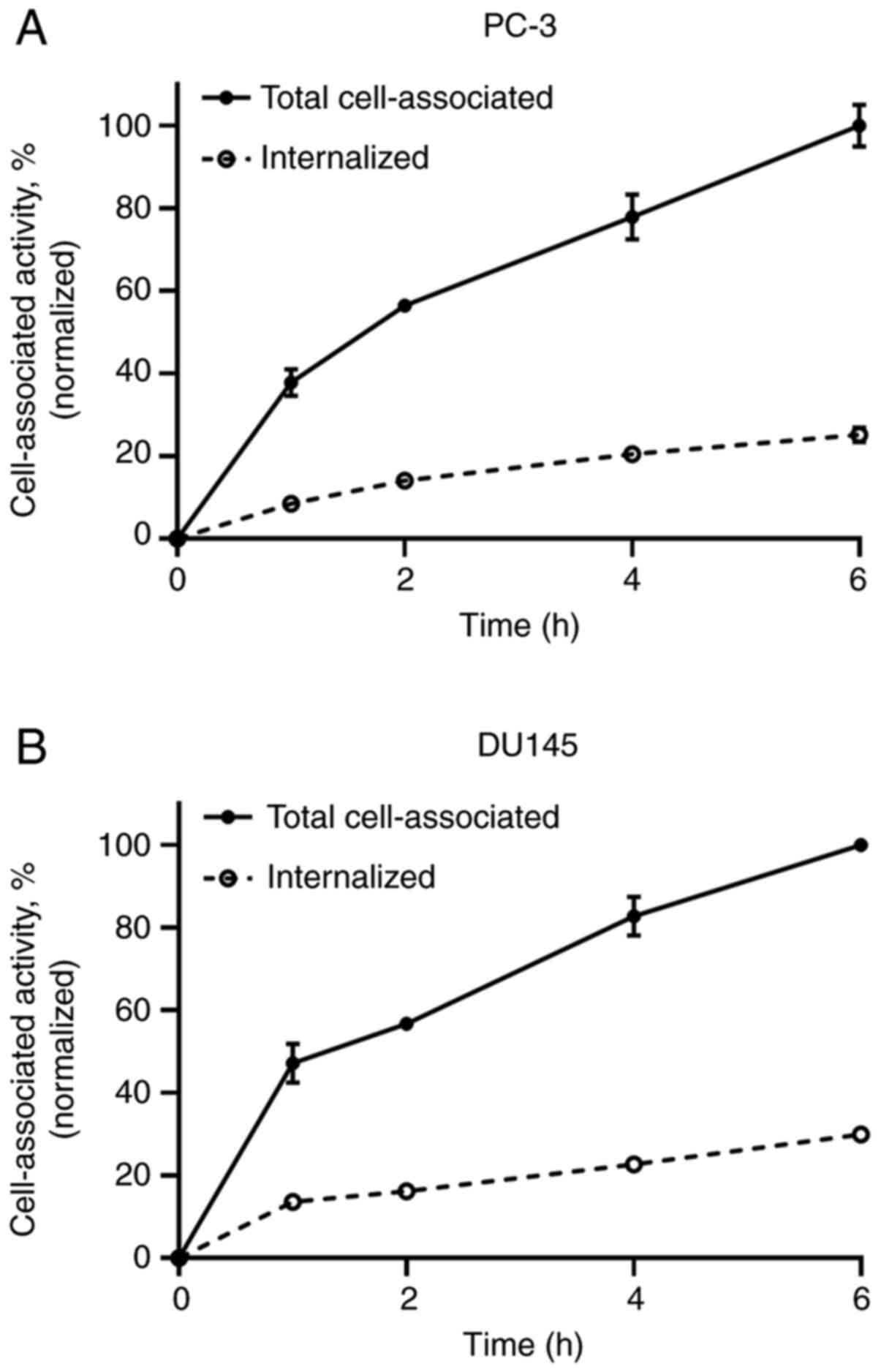
Cellular processing
The binding of [99mTc]Tc-labeled Ec1-LoPE
to both PC-3 and DU145 cells was rapid, and cell-associated
activity was increased with incubation time. The internalized
fraction reached 25±2% in the PC-3 cells and 30±0% in the DU145
cells after 6 h of incubation (Fig.
4).
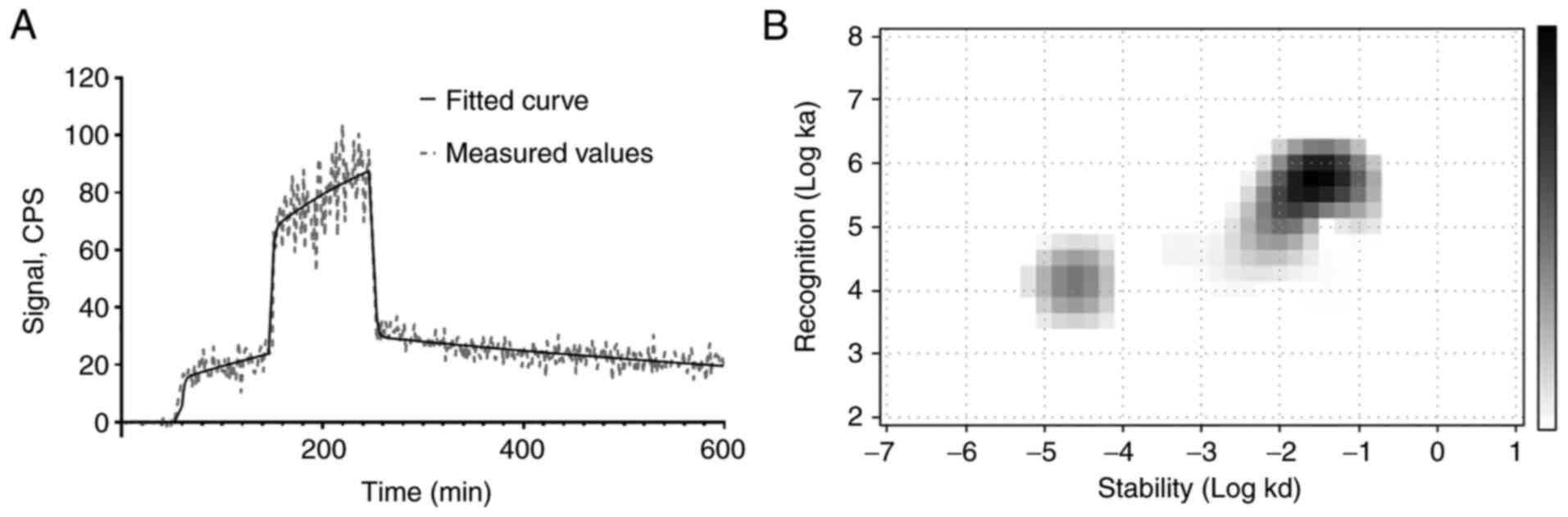
Binding kinetics
The binding of
[99mTc]Tc(CO)3-Ec1-LoPE to living DU145 cells
was characterized by rapid association, while the dissociation
included a rapid and a slow phase (Fig. 5). The Interaction Map analysis
indicated the presence of two interactions, one with affinity in
the low nanomolar range (KD1=2.1±0.3 nM) and
another one approximately 20 times weaker
(KD2=45±21 nM) (Table II).
 | Table II.Equilibrium dissociation constants
(KD) for the interaction between
[99mTc]Tc-labeled Ec1-LoPE and EpCAM-expressing DU145
cells. |
Table II.
Equilibrium dissociation constants
(KD) for the interaction between
[99mTc]Tc-labeled Ec1-LoPE and EpCAM-expressing DU145
cells.
|
|
KD1 |
KD2 |
|---|
|
[99mTc]Tc(CO)3- | 2.1±0.3 nM | 45±21 nM |
| Ec1-LoPE |
|
|
Cytotoxicity
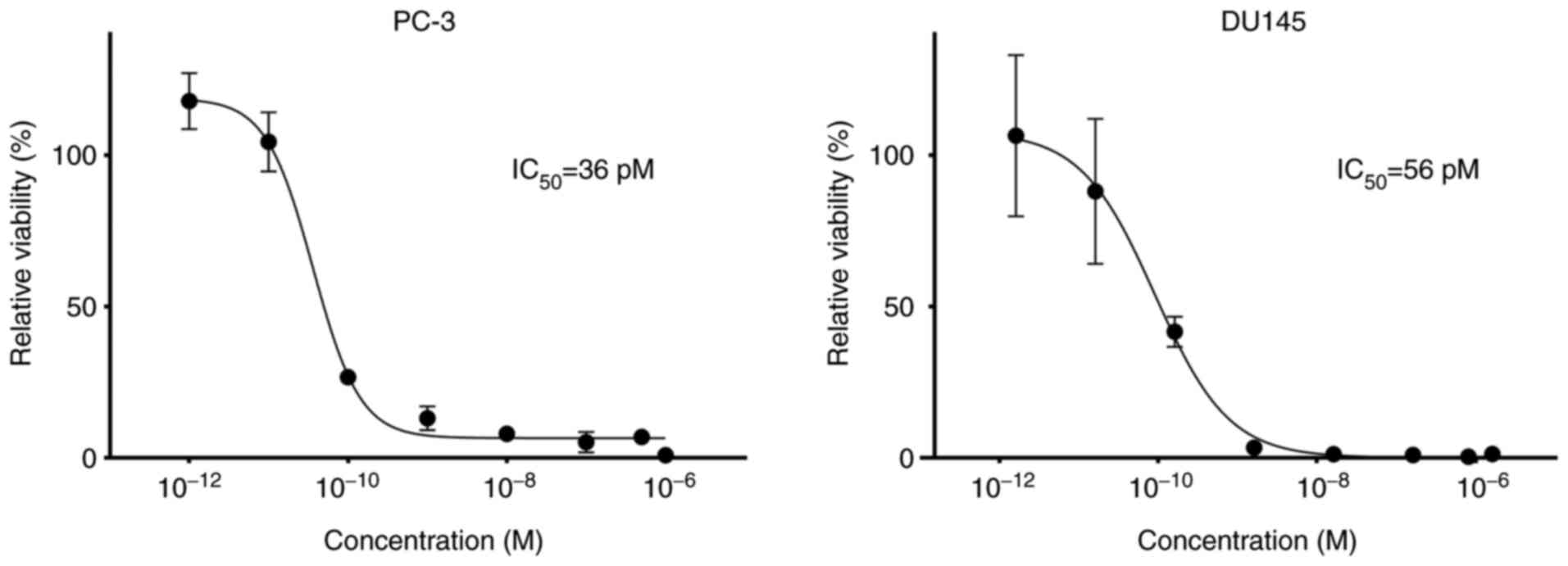
The results of cytotoxicity of Ec1-LoPE on
EpCAM-expressing PC-3 and DU145 cells are shown in Fig. 6. Ec1-LoPE demonstrated a
dose-dependent cytotoxic effect with subnanomolar IC50
values, 36 pM for PC-3 cells and 56 pM for DU145 cells.
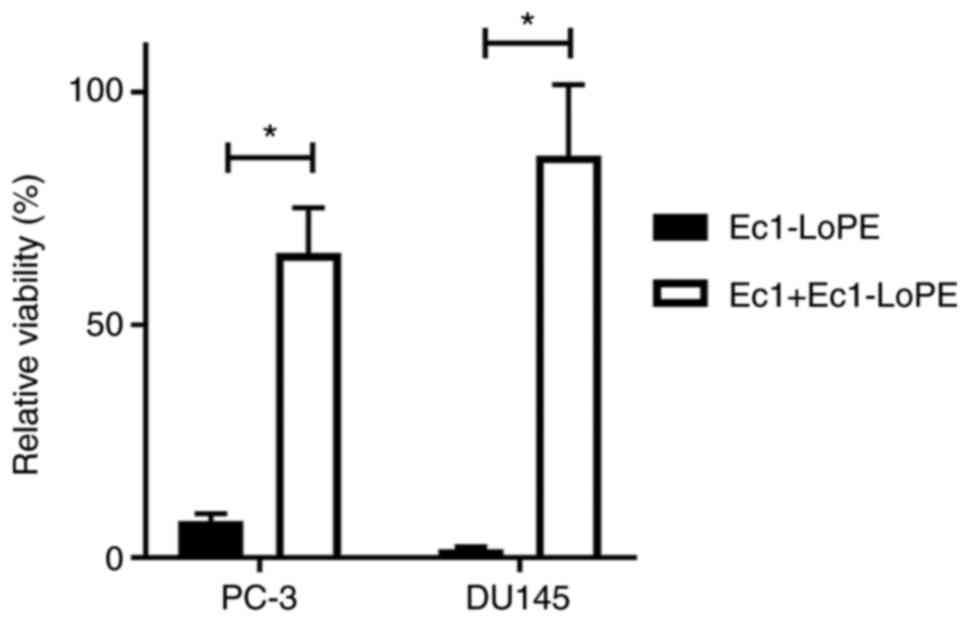
Pre-incubation of cells with a large excess of DARPin Ec1 before
the addition of Ec1-LoPE significantly (P<0.0001) reduced its
cytotoxic action (Fig. 7), which
suggested that the cytotoxic action of Ec1-LoPE was
EpCAM-dependent.
Discussion
Treatment of metastasized prostate cancer (PC) with
androgen deprivation therapy (ADT) provides an effective initial
response in the majority of patients; however, it is not curative
and tumors often develop resistance over the course of treatment.
In the next phase of cancer progression into metastatic
castration-resistant type, chemotherapy with docetaxel is added to
ADT to increase the therapeutic efficacy (40). While providing a cytotoxic effect,
conventional chemotherapeutic drugs do not discriminate between
normal and cancer cells resulting in numerous side effects due to
cumulative toxicity to normal organs.
Targeted therapeutic agents offer the advantage of
reducing cytotoxic side effects on normal cells by directing the
payload selectively to cancer cells. The most investigated targeted
cytotoxic agents are antibody-drug conjugates, with several of them
being approved for clinical use (41). In comparison with monoclonal
antibodies (mAbs), small engineered scaffold proteins, such as
designed ankyrin repeat proteins (DARPins), might be more effective
to overcome biological barriers and deliver a cytotoxic payload to
solid tumors, as well as to provide more even distribution in
tumors (24). Due to faster
clearance from blood and reduced exposure to normal tissues, a
larger therapeutic window may also be achieved. Fusion of a DARPin
to a bacterial toxin by genetic engineering enables recombinant
production of a therapeutic agent as a single protein, removing the
need for additional conjugation and purification steps (34). Genetic engineering provides an
opportunity for facile optimization of molecular design (e.g.,
addition of targeting modules or linkers) and biodistribution
characteristics.
Examples of scaffold proteins carrying bacterial
toxins as a payload include affibody molecules (42), ABD-derived affinity proteins
(ADAPTs) (43) and DARPins
(44) targeting human epidermal
growth factor receptor 2 (HER2). A number of agents have been
developed using DARPins targeting EpCAM (Ec1 or Ec4) carrying
Pseudomonas aeruginosa exotoxin A (ETA”, PE40), which have
shown potent antitumor activity in xenograft models of colon
cancer, small cell lung carcinoma (45) and breast cancer (46). To address the problem of
immunogenic response to the bacterial toxin, B-cell recognition
epitopes on the PE toxin were removed (36,47).
The new toxin variant termed LoPE was fused to HER2-targeting
DARPin 9_29 and showed lower non-specific toxicity and lower
immunogenicity in vivo than DARPin 9_29-PE40, while
preserving a potent cytotoxic action on ovarian cancer xenografts
in mice (37). We recently
demonstrated that Ec1-LoPE fusion targeting EpCAM caused a
significant inhibition of tumor growth, which was additionally
potentiated by a combination with HER2-targeting treatment in
breast cancer (48) and ovarian
cancer (38) models in mice.
Repeated administration of Ec1-LoPE was well-tolerated with no
observable toxicities or weight loss in mice.
Contrary to the IgG scaffold, the use of small
engineered scaffold proteins for the development of targeted
anticancer therapeutics is very recent. Therefore, more studies are
necessary to improve the understanding of their therapeutic effect
in different cancer types. In this study, we used DARPin Ec1 as an
EpCAM-targeting module with a molecular weight of approximately
8-fold smaller than an mAb. The addition of LoPE toxin provided
Ec1-LoPE fusion protein (43 kDa), which is smaller than a Fab
fragment. Overexpression of EpCAM in a large fraction of prostate
cancers and in metastatic lesions makes it a possible target for
therapy of disseminated PC. This is further supported by a
correlation between EpCAM overexpression and increased risk of PC
recurrence (10,11).
To obtain quantitative information concerning the
functional characteristics of Ec1-LoPE in PC cell lines, we applied
site-specific radiolabeling approach using tricarbonyl
technetium-99m as a label. Tricarbonyl technetium-99m was attached
to the (His)6-tag at the C-terminus of Ec1-LoPE to form
a residualizing label, which stays inside the cells after
internalization and lysosomal degradation of the protein. This
labeling method should have a minimal impact on the binding
properties of Ec1-LoPE, since the label and the binding site are
spatially separated. Radiolabeling results were in agreement with
the previously reported data (38)
and the stability of the radiolabel was confirmed (Table I).
The binding of
[99mTc]Tc(CO)3-Ec1-LoPE to EpCAM-expressing
PC-3 and DU145 cells was specific as demonstrated by the saturation
experiment. The level of cell-associated activity per cell number
was higher for PC-3 cells than for DU145 cells. Massoner et
al reported that PC-3 and DU145 cells have a similar level of
EpCAM expression at both the mRNA and protein levels, while PC-3
cells were found to have a higher degree of EpCAM-positive cells
than DU145 by flow cytometry (7),
which might be an explanation for the differences in the level of
the cell-associated activity.
Previous studies have shown that the binding of Ec1
to EpCAM-expressing cells triggers receptor-mediated endocytosis
and enables intracellular delivery of cytotoxic agents (35,48).
Cellular processing experiment showed that approximately a quarter
of cell-bound activity (25±2%) of
[99mTc]Tc(CO)3-Ec1-LoPE was internalized by 6
h in PC-3 cells and 30±0% in DU145 cells, which suggests a
sufficiently high internalization rate. These results are in line
with a previous study in ovarian carcinoma cell lines, where
internalization of [99mTc]Tc(CO)3-Ec1-LoPE
was also rapid, with 31±0% in OVCAR3 cells and 38±3% in SKOV3 cells
by 6 h (38).
Analysis of
[99mTc]Tc(CO)3-Ec1-LoPE binding kinetics to
DU145 cells showed the presence of two interactions, a stronger
(KD1=2.1±0.3 nM) and a weaker one
(KD2=45±21 nM), both being in the nanomolar
range. A similar pattern of two interactions was observed for
[99mTc]Tc(CO)3-Ec1-LoPE binding to SKOV3 and
OVCAR3 cells, with the first equilibrium dissociation constant
(KD1) being in the subnanomolar range. Comparing
these results to DARPin Ec1 alone having KD of
approximately 0.3 nM to DU145 cells (average value for different
radiolabel types) (32), it can be
concluded that fusion of Ec1 with LoPE resulted in a slight
decrease in affinity, which might be expected due to the addition
of a bulky toxin moiety to the targeting Ec1 module.
Ec1-LoPE demonstrated a potent cytotoxic effect with
IC50 values in the low picomolar range in both PC-3 and
DU145 cells. High efficiency of cell growth inhibition might be due
to a combination of rapid internalization, efficient delivery and
high sensitivity to LoPE toxin action. In our recent study,
Ec1-LoPE efficiently inhibited tumor growth of SKOV3 ×enografts in
mice, while having an IC50 value of 0.53 µM in SKOV3
cells in the cytotoxicity assay (38). It must be noted that SKOV3 cells
were shown to be more resistant to the cytotoxic action of drug
(49) and toxin conjugates
(50) based on affibody molecules
and ADAPTs (43,51) in comparison to other cell lines.
Therefore, picomolar IC50 values of Ec1-LoPE in both
PC-3 and DU145 cells suggest its potential for a therapeutic effect
in PC models in vivo.
As the efficacy of targeted therapy depends on the
presence of a sufficient amount of molecular target in tumors, it
is important to select the patients with a high level of target
expression. For the EpCAM-targeting mAb adecatumumab the
probability of tumor progression was significantly lower in breast
cancer patients who had high EpCAM expression and were treated with
a high dose (14,52). High expression of EpCAM was also
found to be a precondition for response of PC to adecatumumab
(15). In addition to a
conventional biopsy-based approach, evaluation of target expression
could be performed by positron emission tomography (PET) or single
photon emission tomography (SPECT) imaging. Radionuclide molecular
imaging is a non-invasive method that provides whole-body
information about target expression in real time and can be
performed repeatedly to monitor changes in expression or receptor
occupancy. The use of a pair of targeting agents, one for
diagnostic imaging and another one for targeted therapy, forms a
theranostic approach to patient treatment, where imaging is used
for stratification of patients for targeted therapy. We recently
reported on the preclinical development of Ec1-based radionuclide
imaging probes, which efficiently visualize EpCAM expression in PC
xenografts (32). Such probes may
be used as companion diagnostics in the treatment of PC xenografts
with Ec1-LoPE enabling selection of patients that would most likely
benefit from such treatment.
In conclusion, DARPin Ec1 is an efficient targeting
domain for LoPE toxin. Ec1-LoPE showed EpCAM-specific binding to
EpCAM-expressing PC-3 and DU145 PC cells. Rapid internalization
mediated potent cytotoxic effect in both studied cell lines. Taken
together, these data support the further evaluation of Ec1-LoPE in
a therapeutic setting in PC models in vivo.
Acknowledgements
The authors would like to thank Mr Haozhong Ding and
Professor Torbjörn Gräslund for performing the LC-MS analysis of
Ec1-LoPE. The authors would like to thank Ms. Kamila Seytova for
proofreading the manuscript.
Funding
This research was funded by grants from the Swedish Prostate
Cancer Federation (Prostatacancerförbundet) and the Swedish Cancer
Society (Cancerfonden, 20 0181 P) to AV, by RFBR project
18-29-08030 in the part of Ec1-LoPE expression and
purification.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TX and YL performed the experiments and analyzed the
data. AS and EK performed the production and purification of
proteins. SMD participated in the molecular design, supervised the
production, purification and characterization of protein, and
coordinated the work. VT participated in the study design, labeling
chemistry development, data treatment and interpretation, and
coordinated the work. AV obtained funding, participated in the
study design, labeling chemistry development, data treatment and
interpretation, coordinated the work. TX and AV wrote the first
version of the manuscript. All authors read and approved the
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work and all data are appropriately investigated and
resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
ORCID nos.: TX 0000-0002-1826-4093; YL
0000-0001-5871-5779; AS 0000-0003-3425-0828; EK
0000-0002-4187-376X; SMD 0000-0002-3952-0631; VT
0000-0002-6122-1734; AV 0000-0002-4778-3909.
Glossary
Abbreviations
Abbreviations:
|
DARPin
|
designed ankyrin repeat protein
|
|
EpCAM
|
epithelial cell adhesion molecule
|
|
PBS
|
phosphate-buffered saline
|
|
KD
|
equilibrium dissociation constant
|
References
|
1
|
Battaglia A, De Meerleer G, Tosco L, Moris
L, Van den Broeck T, Devos G, Everaerts W and Joniau S: Novel
insights into the management of oligometastatic prostate cancer: A
comprehensive review. Eur Urol Oncol. 2:174–188. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jacob A, Raj R, Allison DB and Myint ZW:
Androgen receptor signaling in prostate cancer and therapeutic
strategies. Cancers (Basel). 13:54172021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sadaghiani MS, Sheikhbahaei S, Werner RA,
Pienta KJ, Pomper MG, Solnes LB, Gorin MA, Wang NY and Rowe SP: A
systematic review and meta-analysis of the effectiveness and
toxicities of lutetium-177-labeled prostate-specific membrane
antigen-targeted radioligand therapy in metastatic
castration-resistant prostate cancer. Eur Urol. 80:82–94. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thang SP, Violet J, Sandhu S, Iravani A,
Akhurst T, Kong G, Ravi Kumar A, Murphy DG, Williams SG, Hicks RJ
and Hofman MS: Poor outcomes for patients with metastatic
castration-resistant prostate cancer with low prostate-specific
membrane antigen (PSMA) expression deemed ineligible for
177Lu-labelled PSMA radioligand therapy. Eur Urol Oncol.
2:670–676. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Rosar F, Hau F, Bartholomä M, Maus S,
Stemler T, Linxweiler J, Ezziddin S and Khreish F: Molecular
imaging and biochemical response assessment after a single cycle of
[225Ac]Ac-PSMA-617/[177Lu]Lu-PSMA-617 tandem therapy in
mCRPC patients who have progressed on [177Lu]Lu-PSMA-617
monotherapy. Theranostics. 11:4050–4060. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Munz M, Baeuerle PA and Gires O: The
emerging role of EpCAM in cancer and stem cell signaling. Cancer
Res. 69:5627–5629. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Massoner P, Thomm T, Mack B, Untergasser
G, Martowicz A, Bobowski K, Klocker H, Gires O and Puhr M: EpCAM is
overexpressed in local and metastatic prostate cancer, suppressed
by chemotherapy and modulated by MET-associated miRNA-200c/205. Br
J Cancer. 111:955–964. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Poczatek RB, Myers RB, Manne U,
Oelschlager DK, Weiss HL, Bostwick DG and Grizzle WE: Ep-Cam levels
in prostatic adenocarcinoma and prostatic intraepithelial
neoplasia. J Urol. 162:1462–1466. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Went PT, Lugli A, Meier S, Bundi M,
Mirlacher M, Sauter G and Dirnhofer S: Frequent EpCam protein
expression in human carcinomas. Hum Pathol. 35:122–128. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benko G, Spajić B, Krušlin B and Tomas D:
Impact of the EpCAM expression on biochemical recurrence-free
survival in clinically localized prostate cancer. Urol Oncol.
31:468–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu Y, Wu Q, Gao J, Zhang Y and Wang Y: A
meta-analysis and the cancer genome atlas data of prostate cancer
risk and prognosis using epithelial cell adhesion molecule (EpCAM)
expression. BMC Urol. 19:672019. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ni J, Cozzi P, Hao J, Beretov J, Chang L,
Duan W, Shigdar S, Delprado W, Graham P, Bucci J, et al: Epithelial
cell adhesion molecule (EpCAM) is associated with prostate cancer
metastasis and chemo/radioresistance via the PI3K/Akt/mTOR
signaling pathway. Int J Biochem Cell Biol. 45:2736–2748. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Simon M, Stefan N, Plückthun A and
Zangemeister-Wittke U: Epithelial cell adhesion molecule-targeted
drug delivery for cancer therapy. Expert Opin Drug Deliv.
10:451–468. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmidt M, Scheulen ME, Dittrich C, Obrist
P, Marschner N, Dirix L, Schmidt M, Rüttinger D, Schuler M,
Reinhardt C and Awada A: An open-label, randomized phase II study
of adecatumumab, a fully human anti-EpCAM antibody, as monotherapy
in patients with metastatic breast cancer. Ann Oncol. 21:275–282.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marschner N, Rüttinger D, Zugmaier G,
Nemere G, Lehmann J, Obrist P, Baeuerle PA, Wolf A, Schmidt M,
Abrahamsson PA, et al: Phase II study of the human anti-epithelial
cell adhesion molecule antibody adecatumumab in prostate cancer
patients with increasing serum levels of prostate-specific antigen
after radical prostatectomy. Urol Int. 85:386–395. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Seimetz D, Lindhofer H and Bokemeyer C:
Development and approval of the trifunctional antibody catumaxomab
(anti-EpCAM × anti-CD3) as a targeted cancer immunotherapy. Cancer
Treat Rev. 36:458–467. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Macdonald J, Henri J, Roy K, Hays E, Bauer
M, Veedu RN, Pouliot N and Shigdar S: EpCAM Immunotherapy versus
specific targeted delivery of drugs. Cancers (Basel). 10:192018.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Di Paolo C, Willuda J, Kubetzko S, Lauffer
I, Tschudi D, Waibel R, Plückthun A, Stahel RA and
Zangemeister-Wittke U: A recombinant immunotoxin derived from a
humanized epithelial cell adhesion molecule-specific single-chain
antibody fragment has potent and selective antitumor activity. Clin
Cancer Res. 9:2837–2848. 2003.PubMed/NCBI
|
|
19
|
MacDonald GC, Rasamoelisolo M, Entwistle
J, Cuthbert W, Kowalski M, Spearman MA and Glover N: A phase I
clinical study of intratumorally administered VB4-845, an
anti-epithelial cell adhesion molecule recombinant fusion protein,
in patients with squamous cell carcinoma of the head and neck. Med
Oncol. 26:257–264. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Andersson Y, Engebraaten O, Juell S,
Aamdal S, Brunsvig P, Fodstad Ø and Dueland S: Phase I trial of
EpCAM-targeting immunotoxin MOC31PE, alone and in combination with
cyclosporin. Br J Cancer. 113:1548–1555. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Frøysnes IS, Andersson Y, Larsen SG,
Davidson B, Øien JT, Olsen KH, Giercksky KE, Julsrud L, Fodstad Ø,
Dueland S and Flatmark K: Novel treatment with intraperitoneal
MOC31PE immunotoxin in colorectal peritoneal metastasis: results
from the ImmunoPeCa phase 1 trial. Ann Surg Oncol. 24:1916–1922.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Cizeau J, Grenkow DM, Brown JG, Entwistle
J and MacDonald GC: Engineering and biological characterization of
VB6-845, an anti-EpCAM immunotoxin containing a T-cell
epitope-depleted variant of the plant toxin bouganin. J Immunother.
32:574–584. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Plückthun A: Designed ankyrin repeat
proteins (DARPins): Binding proteins for research, diagnostics, and
therapy. Annu Rev Pharmacol Toxicol. 55:489–511. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Thurber GM, Schmidt MM and Wittrup KD:
Antibody tumor penetration: Transport opposed by systemic and
antigen-mediated clearance. Adv Drug Deliv Rev. 60:1421–1434. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Goldstein R, Sosabowski J, Livanos M,
Leyton J, Vigor K, Bhavsar G, Nagy-Davidescu G, Rashid M, Miranda
E, Yeung J, et al: Development of the designed ankyrin repeat
protein (DARPin) G3 for HER2 molecular imaging. Eur J Nucl Med Mol
Imaging. 42:288–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Deyev S, Vorobyeva A, Schulga A, Proshkina
G, Güler R, Löfblom J, Mitran B, Garousi J, Altai M, Buijs J, et
al: Comparative evaluation of two DARPin variants: Effect of
affinity, size, and label on tumor targeting properties. Mol Pharm.
16:995–1008. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Vorobyeva A, Sсhulga A, Konovalova E,
Güler R, Mitran B, Garousi J, Rinne S, Löfblom J, Orlova A, Deyev S
and Tolmachev V: Comparison of tumor-targeting properties of
directly and indirectly radioiodinated designed ankyrin repeat
protein (DARPin) G3 variants for molecular imaging of HER2. Int J
Oncol. 54:1209–1220. 2019.PubMed/NCBI
|
|
28
|
Vorobyeva A, Schulga A, Rinne SS, Günther
T, Orlova A, Deyev S and Tolmachev V: Indirect radioiodination of
DARPin G3 using N-succinimidyl-para-iodobenzoate improves the
contrast of HER2 molecular imaging. Int J Mol Sci. 20:30472019.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Deyev SM, Vorobyeva A, Schulga A,
Abouzayed A, Günther T, Garousi J, Konovalova E, Ding H, Gräslund
T, Orlova A and Tolmachev V: Effect of a radiolabel biochemical
nature on tumor-targeting properties of EpCAM-binding engineered
scaffold protein DARPin Ec1. Int J Biol Macromol. 145:216–225.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vorobyeva A, Konovalova E, Xu T, Schulga
A, Altai M, Garousi J, Rinne SS, Orlova A, Tolmachev V and Deyev S:
Feasibility of imaging EpCAM expression in ovarian cancer using
radiolabeled DARPin Ec1. Int J Mol Sci. 21:33102020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vorobyeva A, Bezverkhniaia E, Konovalova
E, Schulga A, Garousi J, Vorontsova O, Abouzayed A, Orlova A, Deyev
S and Tolmachev V: Radionuclide molecular imaging of EpCAM
expression in triple-negative breast cancer using the scaffold
protein DARPin Ec1. Molecules. 25:47192020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Deyev SM, Xu T, Liu Y, Schulga A,
Konovalova E, Garousi J, Rinne SS, Larkina M, Ding H, Gräslund T,
et al: Influence of the position and composition of radiometals and
radioiodine labels on imaging of Epcam expression in prostate
cancer model using the DARPin Ec1. Cancers (Basel). 13:35892021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bragina O, Chernov V, Schulga A,
Konovalova E, Garbukov E, Vorobyeva A, Orlova A, Tashireva L,
Sorensen J, Zelchan R, et al: Phase I trial of
99mTc-(HE)3-G3, a DARPin-based probe for
imaging of HER2 expression in breast cancer. J Nucl Med.
Aug 12–2021.(Epub ahead of print). View Article : Google Scholar
|
|
34
|
Shilova O, Shramova E, Proshkina G and
Deyev S: Natural and designed toxins for precise therapy: Modern
approaches in experimental oncology. Int J Mol Sci. 22:49752021.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stefan N, Martin-Killias P, Wyss-Stoeckle
S, Honegger A, Zangemeister-Wittke U and Plückthun A: DARPins
recognizing the tumor-associated antigen EpCAM selected by phage
and ribosome display and engineered for multivalency. J Mol Biol.
413:826–843. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liu W, Onda M, Lee B, Kreitman RJ, Hassan
R, Xiang L and Pastan I: Recombinant immunotoxin engineered for low
immunogenicity and antigenicity by identifying and silencing human
B-cell epitopes. Proc Natl Acad Sci USA. 109:11782–11787. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sokolova EA, Shilova ON, Kiseleva DV,
Schulga AA, Balalaeva IV and Deyev SM: HER2-specific targeted toxin
DARPin-LoPE: Immunogenicity and antitumor effect on intraperitoneal
ovarian cancer xenograft model. Int J Mol Sci. 20:23992019.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu T, Vorobyeva A, Schulga A, Konovalova
E, Vorontsova O, Ding H, Gräslund T, Tashireva LA, Orlova A,
Tolmachev V and Deyev SM: Imaging-guided therapy simultaneously
targeting HER2 and EpCAM with trastuzumab and EpCAM-directed toxin
provides additive effect in ovarian cancer model. Cancers (Basel).
13:39392021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wållberg H and Orlova A: Slow
internalization of anti-HER2 synthetic affibody monomer
111In-DOTA-ZHER2:342-pep2: Implications for development of labeled
tracers. Cancer Biother Radiopharm. 23:435–442. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cornford P, van den Bergh RCN, Briers E,
Van den Broeck T, Cumberbatch MG, De Santis M, Fanti S, Fossati N,
Gandaglia G, Gillessen S, et al: EAU-EANM-ESTRO-ESUR-SIOG
guidelines on prostate cancer. Part II-2020 update: Treatment of
relapsing and metastatic prostate cancer. Eur Urol. 79:263–282.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Drago JZ, Modi S and Chandarlapaty S:
Unlocking the potential of antibody-drug conjugates for cancer
therapy. Nat Rev Clin Oncol. 18:327–344. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Altai M, Liu H, Orlova A, Tolmachev V and
Gräslund T: Influence of molecular design on biodistribution and
targeting properties of an Affibody-fused HER2-recognising
anticancer toxin. Int J Oncol. 49:1185–1194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ding H, Altai M, Yin W, Lindbo S, Liu H,
Garousi J, Xu T, Orlova A, Tolmachev V, Hober S and Gräslund T:
HER2-specific Pseudomonas exotoxin A PE25 based fusions:
Influence of targeting domain on target binding, toxicity, and in
vivo biodistribution. Pharmaceutics. 12:3912020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Sokolova E, Proshkina G, Kutova O, Shilova
O, Ryabova A, Schulga A, Stremovskiy O, Zdobnova T, Balalaeva I and
Deyev S: Recombinant targeted toxin based on HER2-specific DARPin
possesses a strong selective cytotoxic effect in vitro and a potent
antitumor activity in vivo. J Control Release. 233:48–56. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Martin-Killias P, Stefan N, Rothschild S,
Plückthun A and Zangemeister-Wittke U: A novel fusion toxin derived
from an EpCAM-specific designed ankyrin repeat protein has potent
antitumor activity. Clin Cancer Res. 17:100–110. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Simon M, Stefan N, Borsig L, Plückthun A
and Zangemeister-Wittke U: Increasing the antitumor effect of an
EpCAM-targeting fusion toxin by facile click PEGylation. Mol Cancer
Ther. 13:375–385. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Proshkina GM, Kiseleva DV, Shilova O,
Ryabova AV, Shramova EI, Stremovskiy OA and Deyev SM: Bifunctional
toxin DARP-LoPE based on the HER2-specific innovative module of a
non-immunoglobulin scaffold as a promising agent for theranostics.
Mol Biol. 51:865–873. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shramova E, Proshkina G, Shipunova V,
Ryabova A, Kamyshinsky R, Konevega A, Schulga A, Konovalova E,
Telegin G and Deyev S: Dual targeting of cancer cells with
DARPin-based toxins for overcoming tumor escape. Cancers (Basel).
12:30142020. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Altai M, Liu H, Ding H, Mitran B, Edqvist
PH, Tolmachev V, Orlova A and Gräslund T: Affibody-derived drug
conjugates: Potent cytotoxic molecules for treatment of HER2
over-expressing tumors. J Control Release. 288:84–95. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Liu H, Seijsing J, Frejd FY, Tolmachev V
and Gräslund T: Target-specific cytotoxic effects on
HER2-expressing cells by the tripartite fusion toxin
ZHER2:2891-ABD-PE38X8, including a targeting affibody molecule and
a half-life extension domain. Int J Oncol. 47:601–609. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Garousi J, Ding H, von Witting E, Xu T,
Vorobyeva A, Oroujeni M, Orlova A, Hober S, Gräslund T and
Tolmachev V: Targeting HER2 expressing tumors with a potent drug
conjugate based on an albumin binding domain-derived affinity
protein. Pharmaceutics. 13:18472021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Schmidt M, Rüttinger D, Sebastian M,
Hanusch CA, Marschner N, Baeuerle PA, Wolf A, Göppel G, Oruzio D,
Schlimok G, et al: Phase IB study of the EpCAM antibody
adecatumumab combined with docetaxel in patients with
EpCAM-positive relapsed or refractory advanced-stage breast cancer.
Ann Oncol. 23:2306–2313. 2012. View Article : Google Scholar : PubMed/NCBI
|