Introduction
Cancer of the oral cavity is ranked among the top
five most frequent cancers occurring in India among both men and
women. Comprising 10.3% of all cancers, males (16.2%) appear to be
affected more by it than females (4.6%) in India (1). Oral tongue squamous cell carcinoma
(OTSCC) distresses ~16,000 people annually in USA (2) and is typically related to a long
history of smoking and/or heavy alcohol use (3). Even though the smoking rates continue
to drop, the incidence of squamous cell carcinoma (SCC) of the oral
cavity has remained constant (4)
with the increase of OSCC patients who have never smoked or whose
habit was non-significant. These individuals are often women in
their mid-forties or younger (3,5–8).
Oncogenic Human Papilloma Virus (HPV) has been implicated in the
recent rise and shown to improve disease-specific survival in
oropharyngeal cancers (9,10). Prognosis is particularly improved
in the case of tonsillar and base of tongue cancer (11).
Knowledge of the molecular and biological
characteristics of cancer cells is essential to develop therapeutic
strategies or identify drug targets for cancer treatment. Primary
cultures generated from different cell types, enable research into
microenvironment-driven characteristics displayed by the parent
tumors. However, due to the difficulty in long-term maintenance,
cell lines remain desirable source for conducting translational
research in laboratory settings (12). Additionally, the scarcity of
commercially available cancer-associated fibroblasts (CAFs) imposes
the need to establish and characterize novel CAF lines from patient
tumors. Microenvironment cross talks can be further studied in
detail if the epithelial and CAF cells can be obtained from the
same patients (13–15).
In vitro cell-based models of cancers have
proven to be an essential tool to enhance the current understanding
of tumor heterogeneity and stroma-tumor cross-talk (15–17).
For oral cancer, various articles report the establishment of
epithelial cell lines from buccal mucosa (18), gingivobuccal mucosa (19), oral cavity (20–22),
tongue (23,24), sinonasal (25), pyriform fossa (26), lower alveolus and retromolar
trigone (24). Zhao et al
(27) in 2011 further assembled
and characterized 85 cell lines from various head and neck tumor
sites. To elucidate the impact of different mutations on tumor
behaviour, 16 cell lines from Head and Neck Squamous Cell Carcinoma
(HNSCC) patients were previously established by Hayes et al
(28).
Earlier attempts were made to establish cell lines
from HNSCC patients who had undergone chemotherapy (CT) and
radiotherapy (RT) (29,30). More recently, one epithelial line
was established from a non-smoking patient, diagnosed with OTSCC
(31). Patil et al
(23) established the first-ever
cisplatin sensitive epithelial cell line established from an Indian
gutka chewer, diagnosed with SCC of the tongue. Pansare et
al (19) and Gawas et
al (18) reported establishing
four and three epithelial lines from tobacco chewers, from
gingivobuccal and buccal mucosa, respectively. Attempt to establish
four epithelial cell lines from patients diagnosed with
poorly-differentiated SCC of the tongue, moderately-differentiated
SCC of the lower alveolus and well-differentiated SCC of retromolar
trigone has been reported by Tatake et al in 1990 (24). Other epithelial cells were also
established from grade III SCC of the pyriform fossa (26). Though majority of the studies
established cells from treatment-naïve samples, Pansare et
al (19) established cell
lines with patients receiving CT or RT after radical surgery.
Studies of establishment of cell lines do not display any gender
bias.
Although abundant literature is available on the
establishment of cell lines from either chewers or smokers, cell
lines from non-habitual patients are comparatively few.
Additionally, a model system with epithelial and CAF from the same
patient, is not available.
The aim of the present study was to establish a
novel in vitro model system from tongue-tumor of two
non-habitual patients. Differential trypsinization assisted in the
isolation of two different cellular populations described.
Extensive characterization established the novelty of the cells and
their autologous nature provides a unique platform to study
tumor-stroma cross talk. To the best of our knowledge, such paired
cell systems isolated using explant culture (or any other cell
establishment methods) have not been reported in the literature so
far. The autologous pairs reported in the present study can be used
as a model system to identify theragnostic biomarkers as
appropriate.
Materials and methods
Tumor specimen and establishment
Tumor samples were collected after obtaining
informed consent from patients. The present study was approved
[approval no. NHH/MEC-CL-2015-405 (A)] by the ethics committee of
Narayana Health City (Bangalore, India). Tissues were collected
aseptically in RPMI-1640 (cat. no. AT222A; Himedia Laboratories,
LLC) with triple strength penicillin-streptomycin (cat. no.
15140122; Gibco; Thermo Fisher Scientific, Inc.) from two
65-year-old females with no risk habits, diagnosed with OTSCC.
Patient MhCT08 had undergone neoadjuvant therapy followed by
surgery and CT-RT and patient MhCT12 was a naive surgical sample.
Patient MhCT08 also developed lung metastasis (poorly
differentiated adenocarcinoma) one year post treatment. The
clinical details of the patients are mentioned in Table I. The tissue samples were
thoroughly washed 3 times at 5 min interval with 3X
penicillin-streptomycin followed by 10% povidone iodine solution
(Win Medicare Pvt. Ltd.) and finally with complete growth medium.
The tissue was cut into small sections and treated with 0.25%
trypsin (cat. no. 25200056; Gibco; Thermo Fisher Scientific, Inc.)
for 30 min at 37°C. The chopped and digested pieces were placed in
a serrated 60-mm petri dishes and supplemented with 10 ng/µl each
of human recombinant epidermal growth factor (hEGF; cat. no. E9644;
Sigma-Aldrich; Merck KGaA), N2 suppliment-1X (cat. no. 17502048;
Gibco; Thermo Fisher Scientific, Inc.), Epilife defined growth
supplement (EDGS; cat. no. S0125; Gibco; Thermo Fisher Scientific,
Inc.) along with 20% FBS (cat. no. RM10434; Himedia Laboratories,
LLC), in RPMI-1640 media with 1X penicillin-streptomycin. The media
was changed every 48 h to remove the dead cells. The epithelial
cells were enriched by differential trypsinization and further
sub-cultured. Briefly, the cells were trypsinized for two different
time points. After a min of trypsinization, floating cells
(fibroblasts) were removed and seeded in a separate flask. Since
fibroblasts can detach faster than epithelial cells, this
differential trypsinization technique yielded two separate cellular
populations. The separated cells were cultured in RPMI-1640 media
with 20% FBS and no additional growth supplements. The cells were
passaged for more than P50 and were characterized for cell-type
specificity at both early and late passages. Later passages of the
cells were maintained in RPMI-1640 medium, pH 7.2, supplemented
with 20% FBS and 1X penicillin-streptomycin solution. Both the
epithelial and fibroblast cells originated from the tongue tissue
of the patient. The established cell types were stained with
Pan-cytokeratin (PanCK; epithelial specific marker) and FSP-1
(fibroblast specific marker), to verify their identities after
differential trypsinization. Isolated epithelial and fibroblast
cells were denoted as MhCT08/12-E and MhCT08/12-F, respectively.
The names have been arrived at through an acronym: M, Mazumdar shaw
medical foundation; h, Human; C, Cancer of; T, Tongue; 08/12,
Patient code; E, Epithelial; and F, Fibroblast.
 | Table I.Clinical and pathological details of
the established cell lines. |
Table I.
Clinical and pathological details of
the established cell lines.
| Cell line | Patient's age
(years)/sex/habit | Tumor
diagnosis | Pathological
staging | Clinical
staging | Tumor
progression |
|---|
| MhCT08-E | 65/F/None | Tongue Squamous
cell | pT4N1Mx | cT4aN2c | Presentation:
Primary (Post NACT) |
| MhCT08-F |
| carcinoma |
|
| followed by CT-RT
after surgery. |
|
|
|
|
|
| Reported lung
metastasis one year post surgery (FNAC: Poorly |
|
|
|
|
|
| differentiated
adenocarcinoma) |
| MhCT12-E | 65/F/None | Tongue Squamous
cell | pT3N3bMx | cT4aN2c | Presentation:
Primary |
| MhCT12-F |
| carcinoma |
|
| Patient
Expired |
HeLa cell culture
HeLa cells were cultured in DMEM medium, pH 7.2,
(cat. no. 11995-065; Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 10% FBS and 1X penicillin-streptomycin solution
at 37°C.
Characterization
Growth curve
Cells were collected and seeded at a concentration
of 1×104 cells per well in a six-well plate. Cell counts
of three random wells were conducted every day for up to 5 days
using trypan blue (cat. no. T6146; Sigma-Aldrich; Merck KGaA). The
doubling time was calculated using the equation: Td=TX
log2/log(N/N0), where Td is the doubling time; T is the time
interval; N is the final cell number and N0 is the initial cell
number. Results are presented as the mean ± standard deviation (SD)
of three independent experiments.
Flow cytometry
Cells at a concentration of 106 cells/100
µl were washed twice in PBS, permeabilized with 0.1% triton X100
(cat. no. 10655; Thermo Fisher Scientific, Inc.) for 30 min, and
incubated with primary antibody (1:50). The cell types were probed
with anti-PanCK (cat. no. 4545; Cell Signalling Technology, Inc.)
and anti-Fibroblast specific protein (FSP-1; cat. no. F4771;
Sigma-Aldrich; Merck KGaA) as primary antibodies for 1 h on ice.
Cells were then pelleted down by centrifugation at 500 × g for 5
min at 4°C and washed with PBS (cat. no. 10010-023; Gibco; Thermo
Fisher Scientific, Inc.) followed by incubation with the
corresponding AlexaFluor488-conjugated anti-mouse secondary
antibody (cat. no. A11029; Invitrogen; Thermo Fisher Scientific,
Inc.) for 30 min at room temperature in the dark. For the marker
expression analysis, cell sorting gates were established using an
unstained control population. The experiment was performed in
duplicates using the BD FACS Canto II system and analyzed using
BDFACS Diva software version 8.0.1 from BD Biosciences.
Immunofluorescence
Cells were seeded on coverslips at a density of
20,000 cells per 100 µl of complete medium and were allowed to
attach overnight. Coverslips were then treated with ice cold 100%
methanol (cat. no. AS059; HiMedia Laboratories, LLC) to fix the
cells for 15 min at −20°C followed by three PBS washes. Prior to
staining, the cells were blocked in 1X PBS containing 1% BSA (cat.
no. TC194; HiMedia Laboratories, LLC) and 0.3% triton-X 100 for 1 h
at room temperature. The cells were then incubated with anti-PanCK
and anti-FSP-1 as primary antibodies, overnight, at 4°C. After
three washes with 1X PBS, the cells were incubated in anti-mouse
Alexa488-conjugated secondary antibody for 1 h at room temperature
in dark. The processed coverslips were then mounted on slides with
DAPI histology mount (cat. no. F6057; HiMedia Laboratories, LLC)
and visualized under Zeiss Scope A1 fluorescent microscope.
DNA ploidy determination
Cells were harvested following trypsinization and
resuspended in PBS containing RNase (10 µg/ml; cat. no. 12091021;
Invitrogen; Thermo Fisher Scientific, Inc.) and propidium iodide
(40 µg/ml) (cat. no. P4170; Sigma-Aldrich; Merck KGaA) and
incubated at 37°C for 30 min for DNA staining. The DNA content was
compared with human mononuclear cells from peripheral blood, which
served as a control for diploid human genomic DNA content. The
cells were analyzed using a BD FACS Canto II system to determine
their fluorescence. The mean channel of cells that were in the G0
phase was divided by that of the lymphocytes to determine the DNA
index of each cell line. The DNA index was then used to predict the
ploidy of the cells.
Isolation of human lymphocytes
Lymphocytes were isolated from a 28-year old healthy
male from Karnataka in late August 2018 by layering whole blood
(diluted 1:3 with 1X PBS) above Ficoll-Paque Histopaque (cat. no.
LSM-1077; HiMedia Laboratories, LLC) at 1:1 ratio. Subsequently,
the Ficoll-Paque with whole blood was centrifuged at 400 × g for 25
min at 20°C. The resultant buffy coat containing lymphocytes was
taken out and washed twice with 1X PBS before further usage
(32,33).
Immunocytochemistry
The established cell lines were examined for the
presence of HPV infection status by staining with p16 (cat. no.
AM540-5M; BioGenex Laboratories) and E7 antibody (cat. no.
sc-58661; Santa Cruz Biotechnology, Inc.) via immunocytochemistry.
HeLa cells, a cervical cancer cell line, was used as a positive
control for the experiment. Cells (5×103) were cultured
on coverslips and fixed in 4% paraformaldehyde for 10 min (cat. no.
GRM3660; HiMedia Laboratories, LLC) followed by permeabilization
with 0.1% triton X-100 for 10 min, and probed with p16 or E7
overnight at 4°C. The coverslips were further processed with Dako
kit (cat. no. K5007; Dako; Agilent Technologies, Inc.). The
presence of target proteins was visualised using DAB as chromogen
and the cells were counterstained with haematoxylin (cat. no. S034;
HiMedia Laboratories, LLC) for 5 min at room temperature, mounted
with DPX mounting medium (cat. no. Q18404; Qualigen; Thermo Fisher
Scientific, Inc.) and examined under Nikon Eclipse E200 light
microscope. For only haematoxylin staining, the cells were stained
directly after permeabilization as aforementioned and the
coverslips were mounted with DPX mounting medium for examination
under Nikon Eclipse E200 light microscope.
PCR
Both the samples were screened for the presence of
HPV infection by PCR to amplify a 450-bp fragment from the
conserved region in all HPV variants with MY09/MY11 primers:
forward, 5′-CGTCCMARRGGAWACTGATC-3′ and reverse,
5′-GCMCAGGGWCATAAYAATGG-3′ as the reverse primer (34). PCR was performed using 200 ng of
DNA along with dNTPs (0.2 mM each), Taq polymerase (cat. no. D1806;
Sigma-Aldrich; Merck KGaA), 1X reaction buffer, and primers at a
final concentration of 0.1 µM each. Forward,
5′-AGCCATGTACGTTGCTATCCA-3′ and reverse, 5′-ACCGGAGTCCATCACGATG-3′
primers were used to amplify beta actin (amplicon 120 bp).
Amplifications were performed using the following thermocycling
conditions: 94°C for 5 min followed by denaturation at 95°C for 1
min, annealing for 1 min (50°C for MY09/MY11 and 60°C for beta
actin), and elongation at 72°C for 1 min. A final elongation step
was carried out at the end of the final cycle at 72°C for 10 min.
Genomic DNA extracted from the HeLa cell line was used as the
positive control and no template was added to the reaction mix as a
negative control. Amplified products were subjected to agarose gel
electrophoresis (1.2% gel) for amplicon visualization.
STR profiling
STR profiling of 10 loci was performed to establish
the genomic identity, cell line identity and exclude any
cross-contamination of MhCT08-E,F and MhCT12-E,F cells. STR
multiplex assay was outsourced to TheraCUES Innovations Pvt. Ltd.
and performed using GenePrint 10 (Promega Corporation).
SoftGenetics GeneMarker_HID version 3.0.0 was used for analyzing
the results. STR data were then examined in the reference STR
database of ATCC and CLASTR using the standard match threshold of
80%.
Estimation of proliferation
Conditioned media from CAFs was collected 48 h after
seeding. Epithelial cells were seeded at a concentration of
1×104 cells per well in a 96-well plate, overnight at
37°C in 5% CO2. They were allowed to grow further for 72
h in CAF-conditioned medium, provided neat or supplemented with 20
and 50% of fresh complete RPMI-1640 medium. Proliferative potential
of the cells was calculated every 24 h, using Alamar Blue Reagent
(cat. no. R7017; Sigma-Aldrich; Merck KGaA), prepared at a final
concentration of 0.2 mg/ml in sterile PBS. A total of 20 µl of
Alamar Blue was added per 100 µl of the growth medium in a 96-well
plate and incubated for 2 h at 37°C. Optical density at 570 and 600
nm was measured. Proliferation was calculated using the formula (O2
X A1)-(O1 X A2); where O1 is the molar extinction coefficient (E)
of oxidized Alamar blue at 570 nm, O2 is the E of the oxidized
Alamar blue at 600 nm, A1 is the absorbance of test wells at 570 nm
and A2 is the absorbance of test wells at 600 nm. From the table of
oxidation coefficients of Alamar Blue, values of O1 and O2 were
taken as 80856 and 117216, respectively. The fold proliferation was
calculated over the negative control i.e. media and the Alamar Blue
reagent without any cells. Results show the mean ± SD of three
independent experiments.
Wound healing assay
Conditioned media from CAFs was collected for 24, 48
and 72 h after seeding. Epithelial cells (0.3×106) were
seeded in each well of a six-well plate and cultured till 80%
confluency. The cell monolayer was gently scraped with a sterile
200-µl pipette tip and the wells were washed twice with 1X PBS to
remove cell debris. The cells were then treated with conditioned
medium collected at different time points, as indicated in the
figures. The width of the scratch was determined by images captured
under a light microscope at 0 and 24 h after creating the wound.
During the time of wound closure, the cells were maintained at 1%
FBS. Wound closure was then quantified using ImageJ software_1.53k
(National Institutes of Health) and the percentage of wound closure
was plotted. Percentage wound healing was calculated by the
formula: % wound confluence=(A-B)*100%)/A; where A is the width of
the initial scratch wound and B is the width of the scratch wound
at time 24 h. Results present the mean ± SD of three independent
experiments.
Invasion assay
ECM gel (cat. no. E1270; Sigma-Aldrich; Merck KGaA)
was prepared at a final concentration of 1 mg/ml in DMEM serum free
media. A total of 100 µl of ECM was coated on the Transwell inserts
(pore size 8.0 µm) and incubated for 2 h at 37°C to allow gel
formation. Cells (1×104) were then seeded in serum free
medium on the top chamber of the Transwell insert (cat. no. TCP083;
HiMedia Laboratories, LLC) with conditioned medium collected at
different time points (as indicated in figures) as the
chemoattractant in the lower chamber with 10% FBS. The cells were
allowed to invade for 48 h. For imaging, the cells on the inside of
the Transwell were removed gently using cotton swabs, followed by
staining with 2% crystal violet (cat. no. S012; HiMedia
Laboratories, LLC) for 10 min at room temperature. The Transwell
inserts were washed with 1X PBS twice to remove any unbound crystal
violet and then air-dried before imaging. For quantification of
invasion, bound crystal violet was eluted by incubating the
Transwell inserts in 10% acetic acid (cat. no. Q21057; Qualigen;
Thermo Fisher Scientific, Inc.) in water with shaking for 10 min at
room temperature. The eluent was then transferred to a 96-well
clear microplate, and absorbance was read at 590 nm. Results show
the mean ± SD of three independent experiments.
Sphere formation assay
3D sphere formation was carried out as detailed by
Arya et al, 2016 (35).
Briefly, 0.5×106 cells were encapsulated in 7.5% 3D
GelMA hydrogels and cultured for 14 days, with a partial medium
change (normal or CAF conditioned medium, as indicated in the
figures) on alternative days. After 14 days, the spheroids were
harvested using enzymatic degradation of the hydrogel and images
were captured. The spheroid size was calculated using the formula
4/3πr3, where ‘r’ represents the geometric mean of the
two diameters of the spheroids. Results present the mean ± SD of
three independent experiments.
Statistical analysis
All the quantitative data is expressed as the mean ±
standard error of the mean. Statistical significance was calculated
using paired Student's t-test unless stated otherwise. Two-way
ANOVA followed by Bonferroni test was performed for proliferation
assay and one-way ANOVA followed by Dunnett's test was performed
for invasion and migration assays. P<0.05 was considered to
indicate a statistically significant difference. All the
statistical calculations were performed using GraphPad Prism
software (version 5.00; GraphPad Software, Inc.).
Results
Tissue samples from both patients, MhCT12 and
MhCT08, were sub-cultured for more than 40 passages. Both yielded
epithelial as well as CAF cells and were further characterized in
various ways.
Characterization of established cell
lines
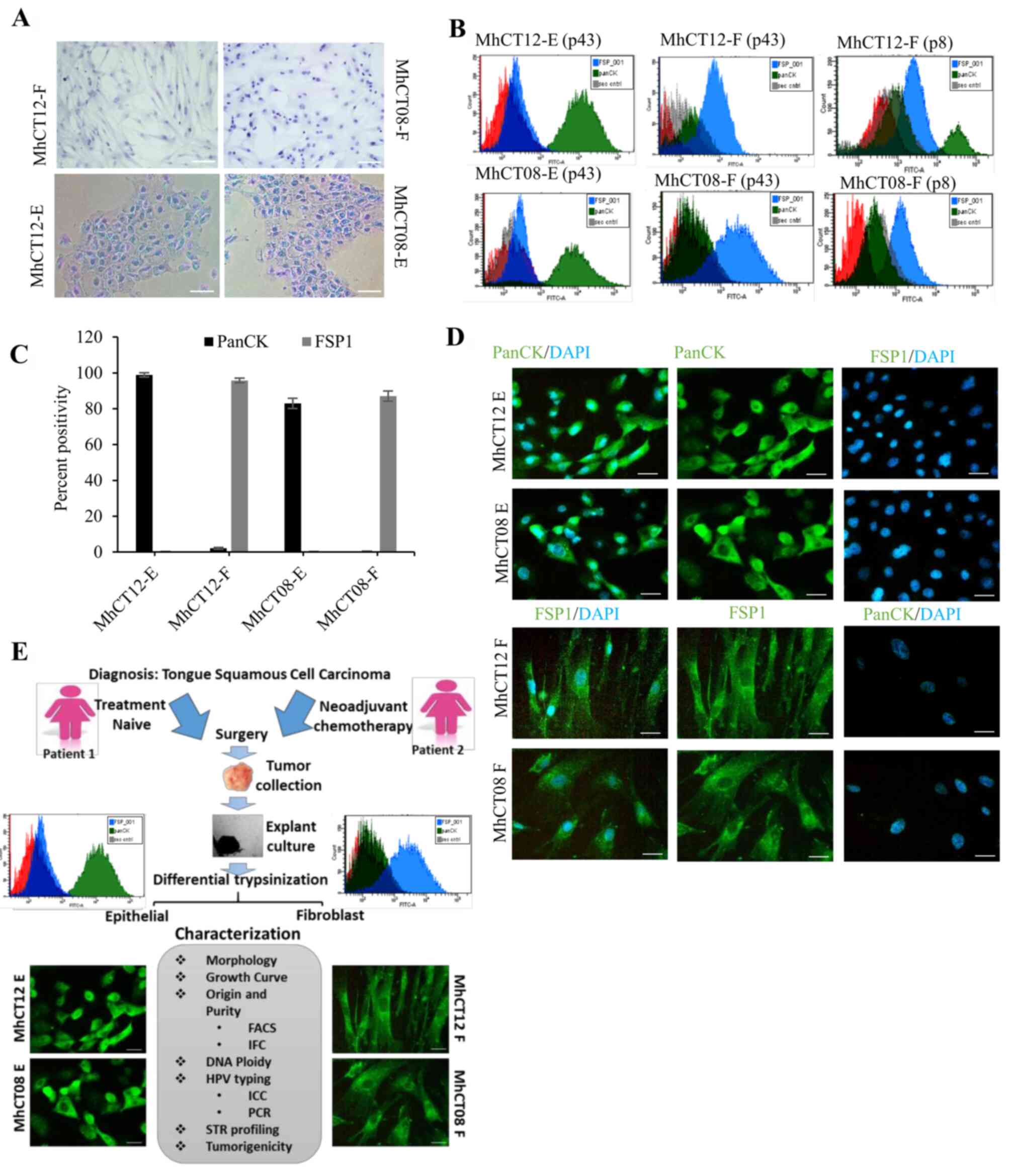
Morphology
To determine the morphological features of
established cell lines, the cells were observed under a light
microscope. Both the epithelial cells had typical polygonal
morphology while both the fibroblast cell types were observed to
have typical spindle-shaped morphology (36,37)
as revealed in Fig. 1A. The
haematoxylin-stained images were captured after passage 35 for each
cell type.
Purity
Explant culture from the tissue yielded two types of
cellular populations. To confirm their origin and to rule out any
cross contamination, checking the purity of the established cell
lines became indispensable to perform further experiments. The
epithelial and CAF nature of the cell lines was confirmed by the
presence of lineage-specific markers, using flow cytometry
(Fig. 1B). The removal of
fibroblasts from the epithelial population was confirmed by
negative staining with fibroblast specific FSP-1 antibody (38,39).
Similarly, a pure population of fibroblast was confirmed by
negative staining with epithelial specific PanCK antibody (40). FACS was performed at both early
(p8) and late passage (p43) of cells (Fig. 1B) and percentage of total
positively-stained cells was represented graphically (Fig. 1C). As revealed in Fig. 1B and C, MhCT12-E and MhCT08-E cells
showed 98 and 81% PanCK positivity, respectively, with less than
0.2% FSP positivity, confirming that epithelial populations were
not contaminated with fibroblasts. Similarly, MhCT12-F and MhCT08-F
exhibited 94.9 and 85% FSP-1-positivity, respectively. Furthermore,
fibroblast cultures at later passages revealed exclusively FSP-1
enriched populations as compared with the early passages. The FSP-1
positive population increased from 83.4% at early passage to 94.9%
at late passage of MhCT12-F cells. Similarly, MhCT08-F cultures
also displayed increase from 53.2% at early passage to 85% at later
passage. Immuno-cytochemical analysis also revealed that the
established fibroblast cultures stained positively with FSP-1, a
fibroblast specific marker, and did not exhibit any staining with
PanCK, an epithelial specific marker. Similarly, the established
epithelial cells were stained positively with PanCK, but did not
show any positive stain with FSP-1 (Fig. 1D). Collectively, these results
verified the purity of established cell lines in the present
study.
HPV detection
HPV infection status has been known to affect
patient prognosis. It has been reported that HPV-positive cancers
have a more favourable patient prognosis when compared with
HPV-negative cancers (41,42). To determine HPV infection status of
the established cell lines, PCR and immunocytochemical analysis
were performed. Immunocytochemical analysis revealed that both the
epithelial and fibroblast cells were positively stained for p16 and
E7 antibodies (Fig. S1A). P16
antibody against cyclin dependent kinase inhibitor 2A, stained the
nuclear region thus confirming the HPV positivity of the
established lines (Fig. S1A).
Fragments of 450 bp were also amplified using MY09/MY11 primers
against L1 region of the viral genome (43), thus confirming the HPV positive
status of the established cell lines (Fig. S1B) for either HPV 16 or 18.
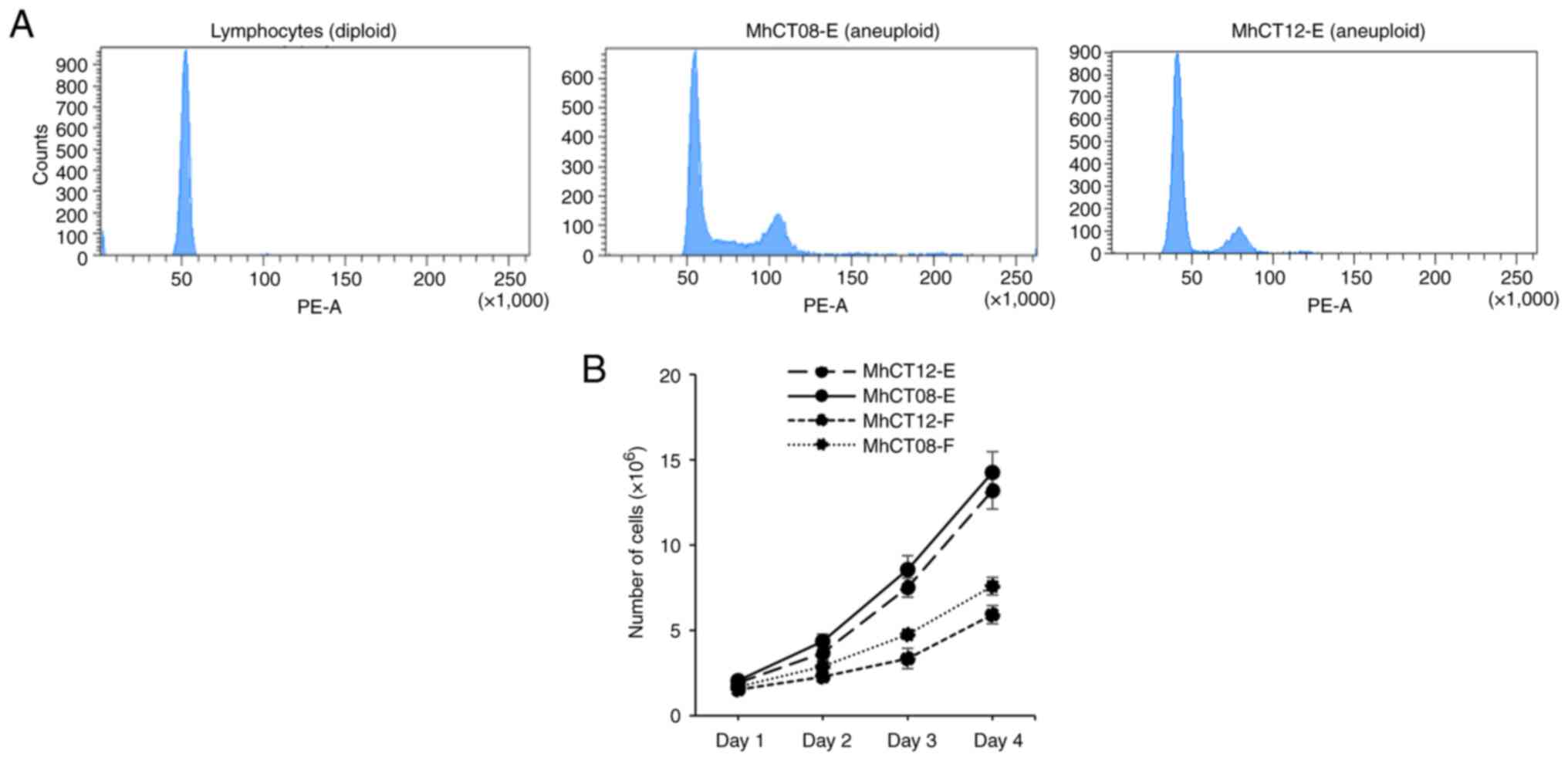
Ploidy determination
One of the major hallmarks of cancer is flawed DNA
amplification cycle which leads to tumor progression (44). The DNA content of the cell lines
was determined by performing ploidy analysis (45). Since normal lymphocytes rarely
exhibit any altered DNA content (46) and their average DNA value has been
designated as diploid (47),
normal lymphocytes from a healthy donor were used as a diploid
control for the experiment. The DNA indices of MhCT08 and MhCT12
cells, and diploid lymphocytes were 1.1, 0.8 and 1 respectively
(Fig. 2A). The results, therefore,
indicated that both the patient samples have abnormal DNA content
which may be responsible for the immortalization of these
cells.
Growth characteristics
The cell lines exhibited different doubling times
(Fig. 2B). Doubling time of 23.61
and 25.54 h was observed for MhCT08-E and MhCT12-E cell lines,
respectively. Both the fibroblast cell lines had higher doubling
time than epithelial cells as revealed with MhCT12-F doubling at
every 39.73 h and MhCT08-F doubling every 32.23 h.
STR profiling
The STR profile of the established cell lines was
performed to indicate that they are distinct from that of any other
cell lines deposited in ATCC and Expasy Cellosaurus STR (CLASTR)
database (Table II). STR
profiling indicated that both MhCT08-F and MhCT12-F and epithelial
cells are novel. According to the American Type Culture Collection,
an STR profile match of ≥80% between cell lines indicates their
‘relatedness’ to each other (48).
The percentage match (>80%) between epithelial and fibroblast
cells from the same patient distinctly proved the autologous nature
of the cells (Table III). No
cross-contamination, particularly with Cal27 and HSC3 cell lines
(simultaneously handled by author ND) was observed as shown in
Table III. Two pairs of
autologous cultures were thus established using explant culture
method. Differential trypsinization yielded two separate cellular
populations-Epithelial and Fibroblast which were extensively
characterized in various ways as outlined in Fig. 1E.
 | Table II.STR profiles of the established cell
lines. |
Table II.
STR profiles of the established cell
lines.
|
| Sample name | MhCT08-E | MhCT12-F | MhCT08-F | MhCT12-E |
|---|
|
|
|
|
|
|
|
|---|
| SI no | Marker | Allele #1 | Allele #2 | Allele #1 | Allele #2 | Allele #1 | Allele #2 | Allele #1 | Allele #2 |
|---|
| 1 | TH01 | 6 | 8 | 3 | 8 | 6 | 8 | 6 | 8 |
| 2 | D21S11 | 28 | 30 | 31 | 32.2 | 28 | 30 | 31 | 32.2 |
| 3 | D5S818 | 11 | 11 | 11 | 12 | 11 | 11 | 12 | 12 |
| 4 | D13S317 | 11 | 11 | 8 | 11 | 11 | 12 | 8 | 11 |
| 5 | D7S820 | 7 | 8 | 11 | 11 | 7 | 8 | 11 | 11 |
| 6 | D16S539 | 10 | 11 | 9 | 13 | 10 | 11 | 9 | 13 |
| 7 | CSF1PO | 11 | 12 | 10 | 11 | 11 | 12 | 10 | 10 |
| 8 | AMEL | X | X | X | X | X | X | X | X |
| 9 | vWA | 15 | 16 | 14 | 17 | 15 | 16 | 14 | 17 |
| 10 | TPOX | 12 | 12 | 8 | 11 | 8 | 12 | 8 | 11 |
 | Table III.Proof of novelty of established cell
lines by STR (*<50% match cut-off from ATCC and CLASTR public
databases). The numbers indicate the ‘relatedness’ of the cell
lines to each other. According to ATCC, a STR profile match of ≥80%
between cell lines indicate their ‘relatedness’ to each other. Cell
lines with between a 55 to 80% match require further analysis for
authentication of relatedness as per ATCC guidelines (48). ATCC, American Type Culture
Collection; CLASTR, Cellosarus STR Similarity Search Tool. |
Table III.
Proof of novelty of established cell
lines by STR (*<50% match cut-off from ATCC and CLASTR public
databases). The numbers indicate the ‘relatedness’ of the cell
lines to each other. According to ATCC, a STR profile match of ≥80%
between cell lines indicate their ‘relatedness’ to each other. Cell
lines with between a 55 to 80% match require further analysis for
authentication of relatedness as per ATCC guidelines (48). ATCC, American Type Culture
Collection; CLASTR, Cellosarus STR Similarity Search Tool.
| Samples | MhCT08-E | MhCT08-F | MhCT12-E | MhCT12-F | Cal 27, HSC3 |
|---|
| MhCT08-E | - | 90 | 25 | 35 | -* |
| MhCT08-F | 90 | - | 30 | 40 | -* |
| MhCT12-E | 25 | 30 | - | 90 | -* |
| MhCT12-F | 35 | 40 | 90 | - | -* |
Tumorigenic properties of established
epithelial cell lines
Furthermore, to assess the tumorigenic potential of
the epithelial cell lines, various assays were performed
post-treatment with CAF-conditioned medium.
Proliferation
Epithelial cells revealed a significant increase
(P<0.05) in proliferation when treated with CAF-conditioned
medium. Since no significant difference was observed between neat
and conditioned mediums supplemented with fresh growth mediums,
only neat-conditioned medium was selected for subsequent
experiments. A slight decrease in proliferation was observed at 72
h, which may be attributed to the over-confluency of the cells
leading to floating cells. Maximum proliferation under the effect
of neat CAF-conditioned medium was observed at 48 h for both the
cell lines (Fig. 3A and B).
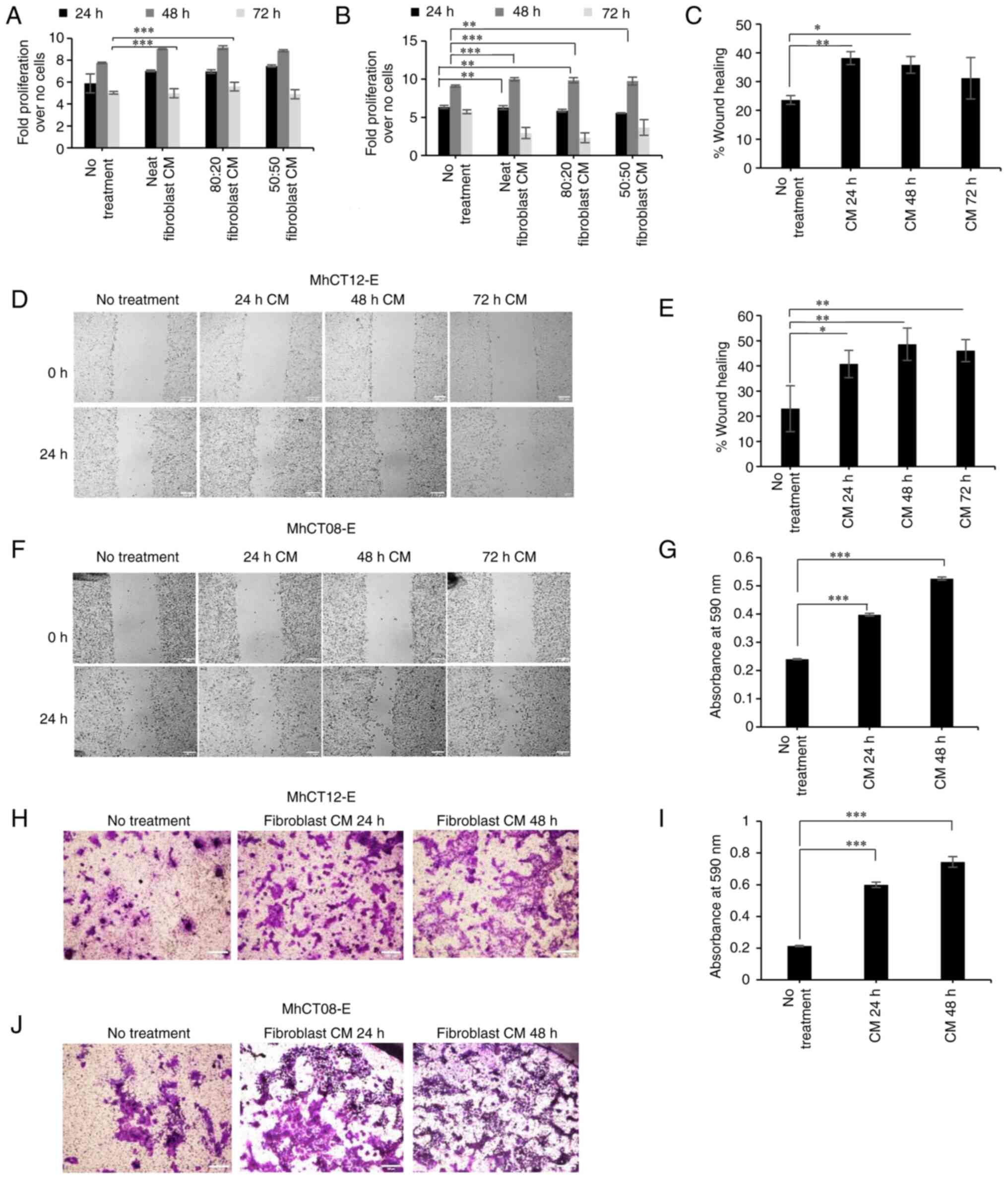 | Figure 3.Tumorigenic properties of established
cell lines. (A and B) Proliferation of (A) MhCT12-E and MhCT08-E
(B) under the effect of CAF-conditioned medium at different time
points. (C-F) Percentage of wound closure represented (C and E)
graphically and (D and F) pictorially for MhCT12-E and MhCT08-E
cell lines, respectively, under the effect of CAF-conditioned
medium collected at indicated time points. Scale bar=100 µm. (G-J)
Invasive potential as evaluated by crystal violet staining
represented (G and I) graphically and (H and J) pictorially for
MhCT12-E and MhCT08-E cell lines, respectively, under the effect of
CAF-conditioned medium collected at indicated time points. Scale
bar=100 µm. *P<0.05, **P<0.005 and ***P<0.001. CAF,
cancer-associated fibroblasts. CM, conditioned medium. Neat,
Complete conditioned medium; 80:20, 80% conditioned medium + 20%
complete fresh medium; 50:50, 50% conditioned medium + 50% complete
fresh medium. |
Wound healing assay
Treatment with neat-conditioned media from the
autologous fibroblast pair significantly increased the migration
potential of MhCT12-E cells from 23 to 38% (Fig. 3C) and 22 to 48% for MhCT08-E cells
(Fig. 3E) (P<0.05) (Fig. 3D and F). Treatment with
CAF-conditioned medium significantly increased the wound healing
potential of their respective epithelial counterparts demonstrating
the CAF-like nature of the established fibroblasts.
Invasion assay
Treatment with conditioned media from the autologous
fibroblast pair significantly increased the invasive potential of
both MhCT08-E and MhCT12-E cell types (P<0.05; Fig. 3G-J).
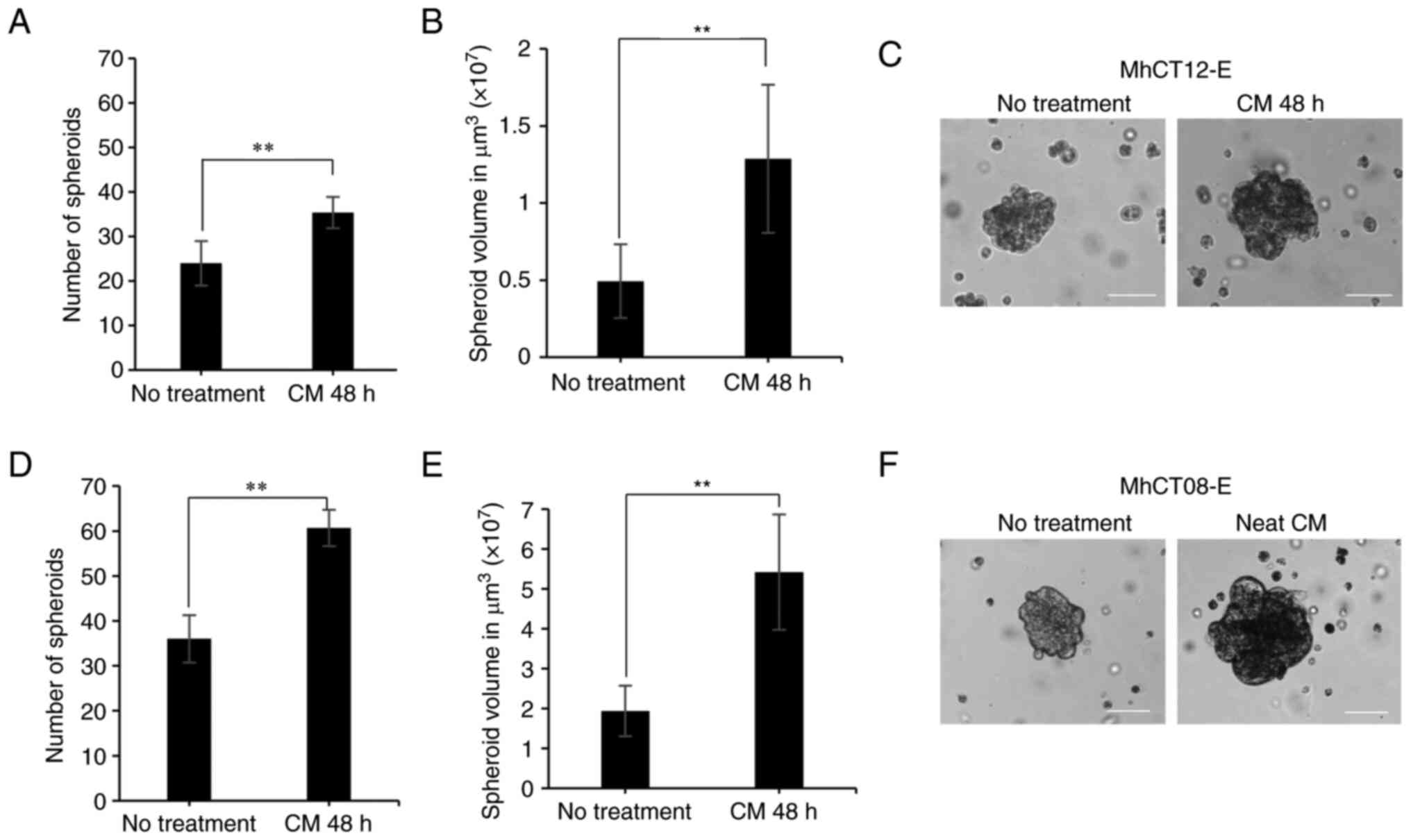
Sphere formation assay
Treatment with conditioned media from the autologous
fibroblast pair significantly increased the sphere formation
potential of both MhCT08-E (n=60, P<0.01) and MhCT12-E (n=35,
P<0.05) cell types, as compared with no treatment conditions of
MhCT08-E (n=36) and MhCT12-E (n=24) as revealed in Fig. 4. Treatment with MhCT08-F CAF
conditioned medium however influenced the epithelial cells to form
bigger spheres (5.4×107 µm3, P<0.01) as
compared with the treatment with MhCT12-F CAF-conditioned medium
(1.2×107 µm3, P<0.01).
Discussion
Patient-derived primary cultures are an invaluable
resource towards understanding the process of carcinogenesis. Given
the heterogeneity of the tumors, cell cultures that represent
multiple cell types of the tumor further enhance the significance
of the in vitro models. In the present study, the
establishment and characterization of two novel autologous
epithelial and CAF cultures from two 65-year old Indian females
without any risk habits, diagnosed with squamous cell carcinoma of
the tongue, were described.
Traditional risk factors for the development of oral
cancer include chewing, smoking, and alcohol consumption. The
established cultures reported from previous studies were from
different stages of tumor and included patients with/without
habits. In the present study, both patients were without any
reported risk habits. Overall in India, OSCC cases without any
known risk habits are comparatively very less (49–51).
Therefore, comparing these cell lines with the other established
cultures, from patients with risk habits, may provide insights into
the etiology of the disease progression in both cases. Furthermore,
considering the rise in cancer in women without any known risk
habits (51,52), increases the significance of this
model towards understanding the carcinogenic process of this cohort
of patients.
Most of the oral carcinoma cell lines established
are either from the western population, or from the patients with
habits. Such established platforms may provide insights into cancer
progression in patients with habits, but lack in the complexity of
disease progression in non-habitual patients diagnosed with oral
cancer. There are very few studies of establishment of oral cancer
cell lines from Indian subcontinent, even though oral cancer is
among the top five cancer-related deaths in India. The aim of the
present study was therefore to establish such a platform from
Indian subcontinent in patients without habits. Establishment of
this novel and unique platform will be helpful to understand the
underlying molecular mechanisms in non-habitual oral cancer
patients.
Multiple methods have been employed in the
development of primary cultures. Numerous studies have described
establishing cell lines by explant culture method (18,19,22,23,25).
Certain studies have also reported directly digesting the tumor
tissue and seeding the cell suspension (20,24).
Mulherkar et al (26)
described the establishment of NT-8e, an oral squamous cell
carcinoma cell line using a mouse xenograft model. The current
study used the explant culture method. A recent study demonstrated
the use of different mediums to enhance the differential growth of
epithelial and fibroblast populations (53). The cultures reported in the present
study were established in RPMI-1640 complete medium to eliminate
any phenotypic or genotypic changes arising due to patient-derived
xenograft generations. The cultures did not require any feeder
cells to grow and stabilized spontaneously, without the
intervention of viral vectors. Further, cell-based separation and
establishing homogeneous cultures are an essential requirement in
primary culture generation. CD90/CD44-based separation has been
successful in separating mesenchymal (CD90 positive) and epithelial
(CD90 negative) populations from tumor tissue (21). Kaur and Ralhan (22) described an innovative method of
treating the cultures with anti-fibroblast antibodies along with
complement rabbit serum treatment to remove fibroblast populations,
however this led to the destruction of the valuable CAFs. The
present study exploited the differential trypsinization method to
establish the novel autologous pairs described.
Cancer-associated stem cells (CSCs), characterized
by self-renewing oncogenic cells, form a small subpopulation of
tumor stroma (54). While the
explant culture method utilized in the study yielded distinct
epithelial and fibroblast populations, isolation of CSCs was not
undertaken. To overcome this limitation, further research should be
attempted, where, the explant may be supplemented with additional
growth factors. The isolated cells can then be sorted and enriched
based on various stem cell markers (CD44 and CD24) via flow
cytometry (55), followed by their
culture in defined growth medium supporting the pluripotency of
stem cells. However, since no such growth factors were added,
current explant culture method is limited in isolation and growth
of cancer-associated stem cells.
To the best of our knowledge, no previous studies
have attempted to establish fibroblast and epithelial cultures from
the same patient. The current study, at present, remains exclusive
in establishing both the epithelial and fibroblast pairs from the
same tumor sample. Most of the studies reported previously have
used either different growth mediums, growth supplements, or feeder
layers to establish epithelial cultures from tumors. However, the
cultures established in the present study were not only novel in
themselves, but also, did not require any additional supplements
for their growth maintenance. This unique platform will provide
answers to various research questions pertinent to tumor-stroma
cross talk.
Characterization of the cells to establish purity
and functional properties have been the mainstay of assigning an
identity to the primary cultures. PanCK and FSP-1 staining have
been reported to identify epithelial and fibroblast populations
exclusively (56–58). In the present study, epithelial and
fibroblast cells were stained with PanCK and FSP-1, respectively,
with high specificity. However, MhCT08 exhibited lesser percentage
positivity for FSP1 as compared with MhCT12 owing to the fact that
it originated from a more aggressive tumor, and probably has
fibroblast markers other than FSP-1. DNA ploidy determination can
correlate with the cancer grade, aggressiveness and metastatic
potential of a tumor and can provide insightful information about
cancer diagnosis and prognosis (59). The DNA index calculation implied
the aneuploid nature of the established cell lines, which may prove
chromosomal instability, thus promoting a heterogeneous tumor
evolution (44). However, since
the ploidy level from karyotype of the original tumor has not been
determined, the study is limited in drawing any hypothesis on the
role of chromosomal multi-ploidy in spontaneous establishment of
cell line from primary culture. Nevertheless, our analysis
indicated abnormal DNA content in both cell lines, which may be
responsible for their immortalization.
In vitro assays such as proliferation,
invasion-migration and sphere formation provide knowledge about the
cancer stem cell subpopulation within a heterogeneous cell
population (60,61). In a co-culture model, these studies
further provide information of the tumor-stromal cross talk. Both
MhCT12-E and MhCT08-E cell lines intrinsically had sphere formation
ability, which was significantly increased upon treatment with
corresponding CAF-conditioned medium. Similarly, the intrinsic
migration, invasion and proliferative potential of the epithelial
lines increased upon addition of CAF-conditioned medium. This may
be due to various signalling factors released by the CAFs, which
promote tumorigenesis and establishes the autologous pair of cells
as an effective model to study tumor-stroma crosstalk (62,63).
The in vivo tumor formation potential of the cell lines
cannot be commented on, as in vivo tumorigenecity assays
have not been performed yet. Unavailability of this data is a
limitation to the present study in revealing the in vivo
tumorigenicity of the established cultures.
High risk of HPV 16 and 18 infections have been
associated with oral cancer. More than 25% of all oral cancers are
associated with HPV16 infection, while a lower percentage of ~1-3%
is attributed to HPV18 infection (42). Factually, HPV-positive oral cancers
are associated with a favourable prognosis when compared with the
HPV-negative oral cancers (41).
Both MhCT12-E, F and MhCT08-E, F cultures were shown to be
HPV-positive by PCR and ICC with anti-HPV antibodies.
The autologous pair of CAFs and cancer epithelial
cells established in the present study serve as model systems to
mimic the tumor-stroma cross talk, using multiple co-culture modes
including conditioned medium-based assays, Transwell cultures and
3D models. This will aid in understanding the molecular mechanism
behind stroma-tumor interaction leading to progression, treatment
resistance, and other major problems in various types of cancer.
The described co-culture system mimics the actual crosstalk between
tumor cells and its static microenvironment, the CAFs. Moreover,
the CAFs themselves can be excellent model systems to understand
tumor progression. In summary, the cell lines developed in the
present study have persistent growth potential and stable cell
morphology. They hold great potential to be used as a novel drug
testing platform in co-culture environment in addition to providing
a useful tool to perform basic and translational research in the
area of tumor-stroma interaction in human tongue cancer arising
from no risk habits.
Supplementary Material
Supporting Data
Acknowledgements
The authors would like to thank Dr Sujan K Dhar of
MSMF for the help in analysing the STR profiling data for the cell
lines and Professor Anjali Karande of Indian Institute of Science,
Bangalore, for providing HeLa cells.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analysed during this study are
included in this published article.
Authors' contributions
MD and AS conceived and designed the study. CG
collected the patient samples and isolated fibroblast and
epithelial cells. ND performed the experiments. MAK and VP provided
the samples. MD and ND wrote the manuscript, analysed and
interpreted the data and confirm the authenticity of all the raw
data. AS reviewed the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved [approval no.
NHH/MEC-CL-2015-405 (A)] by the ethics committee of Narayana Health
City (Bangalore, India). Informed consent for the study was
obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
National Cancer Institute, . SEER Cancer
Stat Facts: Tongue Cancer. National Cancer Institute; Bethesda, MD:
2017, 2019, https://seer.cancer.gov/statfacts/html/tongue.htmlJune
7–2022
|
|
3
|
Gelband H, Jha P, Sankaranarayanan R and
Horton S: Disease control priorities, third edition (volume 3):
Cancer. The World Bank; 2015, View Article : Google Scholar
|
|
4
|
Jamal A, Homa DM, O'Connor E, Babb SD,
Caraballo RS, Singh T, Hu SS and King BA: Current cigarette smoking
among adults-United States, 2005-2014. MMWR Morb Mortal Wkly Rep.
64:1233–1240. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Patel SC, Carpenter WR, Tyree S, Couch ME,
Weissler M, Hackman T, Hayes DN, Shores C and Chera BS: Increasing
incidence of oral tongue squamous cell carcinoma in young white
women, age 18 to 44 years. J Clin Oncol. 29:1488–1494. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Durr ML, Van Zante A, Li D, Kezirian EJ
and Wang SJ: Oral tongue squamous cell carcinoma in never-smokers:
Analysis of clinicopathologic characteristics and survival.
Otolaryngol Head Neck Surg. 149:89–96. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Durr ML, Li D and Wang SJ: Oral cavity
squamous cell carcinoma in never smokers: Analysis of
clinicopathologic characteristics and survival. Am J Otolaryngol.
34:388–393. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heaton CM, Durr ML, Tetsu O, Van Zante A
and Wang SJ: TP53 and CDKN2a mutations in never-smoker oral tongue
squamous cell carcinoma. Laryngoscope. 124:E267–E273. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ernster JA, Sciotto CG, O'Brien MM, Finch
JL, Robinson LJ, Willson T and Mathews M: Rising incidence of
oropharyngeal cancer and the role of oncogenic human papilloma
virus. Laryngoscope. 117:2115–2128. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chaturvedi AK, Engels EA, Pfeiffer RM,
Hernandez BY, Xiao W, Kim E, Jiang B, Goodman MT, Sibug-Saber M,
Cozen W, et al: Human papillomavirus and rising oropharyngeal
cancer incidence in the United States. J Clin Oncol. 29:4294–4301.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ramqvist T, Grün N and Dalianis T: Human
papillomavirus and tonsillar and base of tongue cancer. Viruses.
7:1332–1343. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miserocchi G, Mercatali L, Liverani C, De
Vita A, Spadazzi C, Pieri F, Bongiovanni A, Recine F, Amadori D and
Ibrahim T: Management and potentialities of primary cancer cultures
in preclinical and translational studies. J Transl Med. 15:2292017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fu S, Dong L, Sun W, Xu Y, Gao L and Miao
Y: Stromal-epithelial crosstalk provides a suitable
microenvironment for the progression of ovarian cancer cells in
vitro. Cancer Invest. 31:616–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
National Cancer Institute, . Molecular
Crosstalk Promotes Tumor Growth. https://www.cancer.https://www.cancer.gov/news-events/cancer-currents-blog/2016/crosstalk-pancreaticJune
7–2022
|
|
15
|
Bremnes RM, Dønnem T, Al-Saad S, Al-Shibli
K, Andersen S, Sirera R, Camps C, Marinez I and Busund LT: The role
of tumor stroma in cancer progression and prognosis: Emphasis on
carcinoma-associated fibroblasts and non-small cell lung cancer. J
Thorac Oncol. 6:209–217. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni Y, Zhou X, Yang J, Shi H, Li H, Zhao X
and Ma X: The role of tumor-stroma interactions in drug resistance
within tumor microenvironment. Front Cell Dev Biol. 9:6376752021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bussard KM, Mutkus L, Stumpf K,
Gomez-Manzano C and Marini FC: Tumor-associated stromal cells as
key contributors to the tumor microenvironment. Breast Cancer Res.
18:842016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gawas NP, Navarange SS, Chovatiya GL,
Chaturvedi P and Waghmare SK: Establishment and characterization of
novel human oral squamous cell carcinoma cell lines from
advanced-stage tumors of buccal mucosa. Oncol Rep. 41:2289–2298.
2019.PubMed/NCBI
|
|
19
|
Pansare K, Gardi N, Kamat S, Dange P,
Previn R, Gera P, Kowtal P, Amin K and Sarin R: Establishment and
genomic characterization of gingivobuccal carcinoma cell lines with
smokeless tobacco associated genetic alterations and oncogenic
PIK3CA mutation. Sci Rep. 9:82722019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hamid S, Lim KP, Zain RB, Ismail SM, Lau
SH, Mustafa WM, Abraham MT, Nam NA, Teo SH and Cheong SC:
Establishment and characterization of Asian oral cancer cell lines
as in vitro models to study a disease prevalent in Asia. Int
J Mol Med. 19:453–460. 2007.PubMed/NCBI
|
|
21
|
Svobodova M, Raudenska M, Gumulec J,
Balvan J, Fojtu M, Kratochvilova M, Polanska H, Horakova Z,
Kostrica R, Babula P, et al: Establishment of oral squamous cell
carcinoma cell line and magnetic bead-based isolation and
characterization of its CD90/CD44 subpopulations. Oncotarget.
8:66254–66269. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaur J and Ralhan R: Establishment and
characterization of a cell line from smokeless tobacco associated
oral squamous cell carcinoma. Oral Oncol. 39:806–820. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Patil TT, Kowtal PK, Nikam A, Barkume MS,
Patil A, Kane SV, Juvekar AS, Mahimkar MB and Kayal LL:
Establishment of a tongue squamous cell carcinoma cell line from
indian gutka chewer. J Oral Oncol. 2014:2860132014.
|
|
24
|
Tatake RJ, Rajaram N, Damle RN, Balsara B,
Bhisey AN and Gangal SG: Establishment and characterization of four
new squamous cell carcinoma cell lines derived from oral tumors. J
Cancer Res Clin Oncol. 116:179–186. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
García-Inclán C, López-Hernández A,
Alonso-Guervós M, Allonca E, Potes S, Melón S, López F, Llorente JL
and Hermsen M: Establishment and genetic characterization of six
unique tumor cell lines as preclinical models for sinonasal
squamous cell carcinoma. Sci Rep. 4:49252014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Mulherkar R, Goud AP, Wagle AS, Naresh KN,
Mahimkar MB, Thomas SM, Pradhan SA and Deo MG: Establishment of a
human squamous cell carcinoma cell line of the upper aero-digestive
tract. Cancer Lett. 118:115–121. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao M, Sano D, Pickering CR, Jasser SA,
Henderson YC, Clayman GL, Sturgis EM, Ow TJ, Lotan R, Carey TE, et
al: Assembly and initial characterization of a panel of 85
genomically validated cell lines from diverse head and neck tumor
sites. Clin Cancer Res. 17:7248–7264. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hayes TF, Benaich N, Goldie SJ, Sipilä K,
Ames-Draycott A, Cai W, Yin G and Watt FM: Integrative genomic and
functional analysis of human oral squamous cell carcinoma cell
lines reveals synergistic effects of FAT1 and CASP8 inactivation.
Cancer Lett. 383:106–114. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lin CJ, Grandis JR, Carey TE, Gollin SM,
Whiteside TL, Koch WM, Ferris RL and Lai SY: Head and neck squamous
cell carcinoma cell lines: Established models and rationale for
selection. Head Neck. 29:163–188. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Easty DM, Easty GC, Carter RL, Monaghan P
and Butler LJ: Ten human carcinoma cell lines derived from squamous
carcinomas of the head and neck. Br J Cancer. 43:772–785. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang SJ, Asthana S, van Zante A, Heaton
CM, Phuchareon J, Stein L, Higuchi S, Kishimoto T, Chiu CY, Olshen
AB, et al: Establishment and characterization of an oral tongue
squamous cell carcinoma cell line from a never-smoking patient.
Oral Oncol. 69:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dwivedi N, Mondal S, P K S, T S, Sachdeva
K, Bathula C, K V, K S N, Damodar S, Dhar SK and Das M: Relative
quantification of BCL2 mRNA for diagnostic usage needs stable
uncontrolled genes as reference. PLoS One. 15:e02363382020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chiu PL, Chang CH, Lin YL, Tsou PH and Li
BR: Rapid and safe isolation of human peripheral blood B and T
lymphocytes through spiral microfluidic channels. Sci Rep.
9:81452019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Asiaf A, Ahmad ST, Zargar MA, Mufti SM and
Mir SH: Prevalence of human papillomavirus infection in a Kashmiri
ethnic female population. Genet Test Mol Biomarkers. 16:904–909.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Arya AD, Hallur PM, Karkisaval AG,
Gudipati A, Rajendiran S, Dhavale V, Ramachandran B, Jayaprakash A,
Gundiah N and Chaubey A: Gelatin methacrylate hydrogels as
biomimetic three-dimensional matrixes for modeling breast cancer
invasion and chemoresponse in vitro. ACS Appl Mater Interfaces.
8:22005–22017. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Van der Scheuren B, Cassiman JJ and Van
den Berghe H: Morphological characteristics of epithelial and
fibroblastic cells growing out from biopsies of human skin. J
Invest Dermatol. 74:29–35. 1980. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cell Morphology|Thermo Fisher
Scientific-IN, . https://www.thermofisher.com/in/en/home/references/gibco-cell-culture-basics/cell-morphology.html
|
|
38
|
Park CK, Jung WH and Koo JS: Expression of
cancer-associated fibroblast-related proteins differs between
invasive lobular carcinoma and invasive ductal carcinoma. Breast
Cancer Res Treat. 159:55–69. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sahai E, Astsaturov I, Cukierman E,
DeNardo DG, Egeblad M, Evans RM, Fearon D, Greten FR, Hingorani SR,
Hunter T, et al: A framework for advancing our understanding of
cancer-associated fibroblasts. Nat Rev Cancer. 20:174–186. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Vaidya MM, Borges AM, Pradhan SA and
Bhisey AN: Cytokeratin expression in squamous cell carcinomas of
the tongue and alveolar mucosa. Eur J Cancer Part B Oral Oncol.
32B:333–336. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Nagel R, Martens-de Kemp SR, Buijze M,
Jacobs G, Braakhuis BJ and Brakenhoff RH: Treatment response of
HPV-positive and HPV-negative head and neck squamous cell carcinoma
cell lines. Oral Oncol. 49:560–566. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ghittoni R, Accardi R, Chiocca S and
Tommasino M: Role of human papillomaviruses in carcinogenesis.
Ecancermedicalscience. 9:5262015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Venceslau EM, Bezerra MM, Lopes ACM, Souza
ÉV, Onofre ASC, de Melo CM, de Lourdes Sierpe Jeraldo V and de
Miranda Onofre FB: HPV detection using primers MY09/MY11 and
GP5+/GP6+ in patients with cytologic and/or colposcopic changes. J
Bras Patol Med Lab. 50:280–285. 2014. View Article : Google Scholar
|
|
44
|
Ben-David U and Amon A: Context is
everything: Aneuploidy in cancer. Nat Rev Genet. 21:44–62. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Tabll A and Ismail H: The use of flow
cytometric DNA ploidy analysis of liver biopsies in liver cirrhosis
and hepatocellular carcinoma. Liver Biopsy. 88–108. 2011.
|
|
46
|
Wang S, Li N, Heald P, Fisk JM, Fadare O,
Howe JG, McNiff JM and Smith BR: Flow cytometric DNA ploidy
analysis of peripheral blood from patients with sezary syndrome:
Detection of aneuploid neoplastic T cells in the blood is
associated with large cell transformation in tissue. Am J Clin
Pathol. 122:774–782. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Petrakis NL: Microspectrophotometric
estimation of the desoxyribonucleic acid (DNA) content of
individual normal and leukemic human lymphocytes. Blood. 8:905–915.
1953. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Interrogating the Database|ATCC, .
https://www.atcc.org/search-str-database/interrogating-the-database
|
|
49
|
Smitha, Mohan C and Hemavathy S:
Clinicopathological features of oral squamous cell carcinoma: A
hospital-based retrospective study. J Dr NTR Univ Heal Sci.
6:29–34. 2017.
|
|
50
|
Ranganathan K, Rooban T and Rao U: Oral
squamous cell carcinoma in patients with and without predisposing
habits in glossal and extra-glossal site: An institutional
experience in South India. Indian J Cancer. 52:625–627. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Baskar K, Nerella M and Dharman S:
Assessment of patients having oral cancer without habits. Int J
Curr Res Rev. 12:69–72. 2020. View Article : Google Scholar
|
|
52
|
Saxena PS and Kumar PS: Non-habit related
oral squamous cell carcinoma: Possible etiologic factors and
probable prevention in indian scenario. Oral Surg Oral Med Oral
Pathol Oral Radiol. 128:e902019. View Article : Google Scholar
|
|
53
|
Oppel F, Shao S, Schürmann M, Goon P,
Albers AE and Sudhoff H: An effective primary head and neck
squamous cell carcinoma in vitro model. Cells. 8:5552019.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Yu Z, Pestell TG, Lisanti MP and Pestell
RG: Cancer Stem Cells. Int J Biochem Cell Biol. 44:2144–2151. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Jaggupilli A and Elkord E: Significance of
CD44 and CD24 as cancer stem cell markers: An enduring ambiguity.
Clin Dev Immunol. 2012:7080362012. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Lawson WE, Polosukhin VV, Zoia O,
Stathopoulos GT, Han W, Plieth D, Loyd JE, Neilson EG and Blackwell
TS: Characterization of fibroblast-specific protein 1 in pulmonary
fibrosis. Am J Respir Crit Care Med. 171:899–907. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Strutz F, Okada H, Lo CW, Danoff T, Carone
RL, Tomaszewski JE and Neilson EG: Identification and
characterization of a fibroblast marker: FSP1. J Cell Biol.
130:393–405. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Barak V, Goike H, Panaretakis KW and
Einarsson R: Clinical utility of cytokeratins as tumor markers.
Clin Biochem. 37:529–540. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ross JS: DNA ploidy and cell cycle
analysis in cancer diagnosis and prognosis. Oncology (Williston
Park). 10:867–882, 887-890. 1996.PubMed/NCBI
|
|
60
|
Wang H, Paczulla AM, Konantz M and
Lengerke C: In vitro tumorigenic assay: The tumor spheres assay.
Methods Mol Biol. 1692:77–87. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Pijuan J, Barceló C, Moreno DF, Maiques O,
Sisó P, Marti RM, Macià A and Panosa A: In vitro cell migration,
invasion, and adhesion assays: From cell imaging to data analysis.
Front Cell Dev Biol. 7:1072019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Steinbichler TB, Metzler V, Pritz C,
Riechelmann H and Dudas J: Tumor-associated fibroblast-conditioned
medium induces CDDP resistance in HNSCC cells. Oncotarget.
7:2508–2518. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Xu LN, Xu BN, Cai J, Yang JB and Lin N:
Tumor-associated fibroblast-conditioned medium promotes tumor cell
proliferation and angiogenesis. Genet Mol Res. 12:5863–5871. 2013.
View Article : Google Scholar : PubMed/NCBI
|