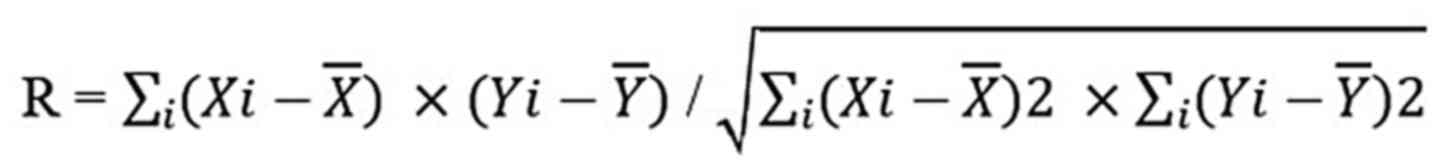Introduction
Lung cancer is the leading cause of cancer-related
mortality worldwide (1).
Cisplatin-vinorelbine is a standard adjuvant chemotherapy for
patients with resected stage II and III non-small cell lung cancer
(2). Adjuvant
cisplatin-vinorelbine treatment has been shown to improve the
overall survival rate by 8.9% and the disease-free survival rate by
9.2% at 5 years (3). The 5-year
survival rate is, however, unsatisfactory at 40.6% for patients
with p-stage IIA, 41.1% for IIB and 28.3% for IIIA (4). Chemotherapeutic failure occurs owing
to resistance to chemotherapeutic agents, which remains a challenge
in cancer treatment (5). The
development of resistance is associated with the overexpression of
energy-dependent drug efflux pumps, known as the ATP-binding
cassette (ABC) family of proteins, which eject anticancer drugs
from cells (6). Activated ABC
sub-family B member 1 (ABCB1) is commonly associated with drug
resistance (7,8).
The overexpression of ABCB1 and the activation of
phosphorylated Fyn (p-Fyn) play a crucial role in vinorelbine
resistance, and integrin β3 functions as an upstream regulator of
Src family kinases (SFKs), including Fyn, in vinorelbine-resistant
(VR) cells (9). Fyn, a
non-receptor tyrosine kinase, belongs to the Src family of kinases
and contributes to the development and progression of cancer by
regulating cell growth, death, morphogenic transformation and
motility in several types of cancer, including glioblastoma,
chronic myelogenous leukemia, prostate cancer and breast cancer
(10–13). However, the mechanisms through
which ABCB1 and p-Fyn contribute to vinorelbine resistance in lung
cancer remain unknown.
Reactive oxygen species (ROS) are products of oxygen
metabolism and play a critical role in cellular proliferation and
homeostasis. An imbalance between ROS generation and elimination
results in intracellular damage. Chemotherapeutic agents, including
vinorelbine, induce ROS generation to kill or inhibit the
antioxidant mechanism of cancer cells (14). The cancer cells that succeed in
controlling elevated ROS levels by upregulating cellular
antioxidant systems become more resistant to exogenous stimuli,
such as chemotherapy (15).
Previous studies have focused on the development of
NF-E2-related factor 2 (Nrf2) inhibitors to overcome cancer drug
resistance (16,17). Nrf2, a key transcription factor,
neutralizes cellular ROS and maintains cellular redox homeostasis
to protect cells against toxic xenobiotics (15). It also regulates the expression of
antioxidants, metabolism and detoxification, and the expression of
transporter genes by combining with the antioxidant response
element (ARE) to confer cytoprotection against various harmful
stimuli (18). Notably, Nrf2
promotes the survival of normal cells and creates an environment
that protects cancer cells against chemotherapy and radiotherapy
(19). Nrf2 is negatively
regulated by Kelch-like ECH-associated protein (Keap1). The loss of
Keap1 function leads to the constitutive activation of
Nrf2-mediated gene expression and induces resistance to
chemotherapeutic drugs (20).
However, only a limited number of studies have demonstrated the
role of Nrf2 in ABCB1-mediated vinorelbine resistance (8,21).
The present study evaluated the significance of
Nrf2, ABCB1 and p-Fyn in VR cells and clinical lung cancer tissue
samples. In addition, the possible association between protein
expression and the clinicopathological features of patients who
underwent adjuvant cisplatin-vinorelbine treatment was examined.
Furthermore, the functional consequences of the increased Nrf2
expression in vinorelbine resistance was determined.
Materials and methods
Cells and cell culture
NCI-H1299 cells (CRL-5803, 70026320, human lung
carcinoma) purchased from the American Type Culture Collection
(ATCC) were cultured in RPMI-1640 medium (MilliporeSigma)
supplemented with 10% (v/v) heat-inactivated fetal bovine serum
(Biosera), 100 units/ml penicillin and 100 mg/ml streptomycin
(FUJIFILM Wako Pure Chemical Corporation) at 37°C in a humidified
atmosphere containing 5% CO2. A549 cells (RCB-0098, 010,
human lung carcinoma, ATCC cat. no. CCL-185) purchased from RIKEN
Cell Bank were cultured in the same manner as H1299 cells, except
in DMEM (D5796, MilliporeSigma) instead of RPMI-1640 medium
(MilliporeSigma). The authenticity of the cell lines was confirmed
by ATCC or RCB. Mycoplasma negativity was confirmed using PCR for
parental cells and VR cells prior to use. All cells were preserved
with CELLBANKER 1 (Nippon Zenyaku Kogyo Co., Ltd.) in liquid
nitrogen.
Determination of ROS generation
The cells were seeded into 6-well plates at
4×104 cells per well and incubated for 24 h at 37°C in a
humidified atmosphere containing 5% CO2 with or without
500 nmol/l vinorelbine. ROS Assay Kit-Highly sensitive DCFH-DA
(Dojindo Laboratories, Inc.) was used to detect ROS according to
the manufacturer's protocol. Fluorescent images were obtained using
a BZ-X810 fluorescence microscope (Keyence Corporation). The
fluorescence intensity was determined using ImageJ 1.53 software
(National Institute of Health).
Reagents
Vinorelbine (FUJIFILM Wako Pure Chemical
Corporation) was dissolved in dimethyl sulfoxide (DMSO; Nacalai
Tesque, Inc.). VR cells were cultured with the indicated
concentrations from 5 to 500 nmol/l of vinorelbine for >4
months. Bardoxolone (Selleck Chemicals), a Keap1 inhibitor, was
dissolved in DMSO. Bardoxolone prevents the ubiquitination and
degradation of Nrf2 and, therefore, functions as an Nrf2 activator.
For the inhibition of Keap1, the cells were exposed to the
indicated concentrations from 0.1 to 2.0 µmol/l of bardoxolone for
24 h until they were harvested for lysate preparation using the
same procedure as described below in ‘Western blot analysis’.
Subcellular fractionation
The passage number of each cell line was <50. The
H1299 cells were seeded at 1×106 cells per 10-cm dish
and cultured at 37°C for 96 h until just before confluency.
Whole-cell lysates were then fractionated into cytoplasmic,
membrane and nuclear protein extracts using the Subcellular Protein
Fractionation kit for Cultured Cells (Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. To confirm that the
nuclear and cytoplasmic fractions predominantly consisted of
proteins derived from the nuclear and cytoplasmic compartments,
respectively, the extracts were probed with nucleus-specific
anti-lamin B1 antibody (cat. no. 12586, Cell Signaling Technology,
Inc.) and cytoplasm-specific anti-α-tubulin antibody (017-25031,
FUJIFILM Wako Pure Chemical Corporation).
Western blot analysis
To prepare total cell lysates, the cells were lysed
in radioimmunoprecipitation assay buffer (20 mM Tris, pH 7.4, 150
mM NaCl, 1% Nonidet p-40, 1% deoxycholic acid and 0.1% sodium
dodecyl sulfate) supplemented with protease inhibitors (1 mM
phenylmethylsulfonyl fluoride, 1 mM sodium vanadate, 1 ng/ml
aprotinin, 1 ng/ml leupeptin and 1 ng/ml pepstatin A). Protein
concentrations were determined using Bio-Rad Protein Assay Dye
Reagent Concentrate (#5000006JA; Bio-Rad Laboratories, Inc.). The
primary antibodies used were as follows: Anti-ABCB1 (1:1,000; cat.
no. 13978; Cell Signaling Technology), anti-Fyn (1:1,000; cat. no.
A0086, ABclonal Biotech Co., Ltd.), anti-phosphorylated Fyn (p-Fyn)
(1:1,000; cat. no. AP0510, ABclonal Biotech Co., Ltd.), anti-Nrf2
(1:1,000; cat. no. sc-722, Santa Cruz Biotechnology, Inc.),
anti-caspase-3 (1:1,000; cat. no. 9665, Cell Signaling Technology,
Inc.), anti-cleaved caspase-3 (1:500; cat. no. 9664, Cell Signaling
Technology, Inc.), anti-Bcl-2 (1:1,000; sc-7382, Santa Cruz
Biotechnology, Inc.), anti-MRP1/ABCC1 (1:1,000; cat. no. 72202,
Cell Signaling Technology, Inc.), anti-MRP2/ABCC2 (1:1,000; cat.
no. 12559, Cell Signaling Technology, Inc.), anti-MRP3/ABCC3
(1:1,000; cat. no. 14182, Cell Signaling Technology, Inc.) and
anti-β-actin (1:1,000; cat. no. A5441, MilliporeSigma). Gel
electrophoresis was performed using PowerPac™ HC
High-Current Power Supply (1645052; Bio-Rad), and 10% gel was used
for all antibodies except for cleaved caspase-3 and 15% gel was
used for cleaved caspase-3. The polyvinylidene difluoride membranes
(IPVH00010; Merck Millipore Ltd.) were incubated with 2% bovine
serum albumin (A9647-100G; MilliporeSigma) for 60 min at room
temperature (20–22°C) for blocking. Following incubation with the
primary antibodies overnight at 4°C, the polyvinylidene difluoride
membranes were incubated with horseradish peroxidase-conjugated
secondary antibodies (1:10,000; 711-035-152; donkey anti-rabbit
IgG, 715-035-150; donkey anti-mouse IgG, Jackson ImmunoResearch
Laboratories, Inc.) for 60 min at room temperature (20–22°C). The
protein bands were visualized using an Ez West Lumi Plus detection
kit (ATTO Corporation) and the LuminoGraph II imaging system (ATTO
Corporation). The protein expression was quantified using ImageJ
1.53 software (National Institute of Health).
Immunofluorescence assay
The cells were seeded at 2×104 cells/well
of a 2-chamber slide and were then fixed in 4% paraformaldehyde for
10 min at 37°C, permeabilized with 0.1% Triton X-100 for 15 min at
room temperature (20–22°C) and blocked in 2% bovine serum albumin
(Biosera) in phosphate-buffered saline for 60 min at room
temperature (20–22°C). Antigen recognition was performed by
incubation with primary antibodies against p-Fyn (1:400; cat. no.
AP0510, ABclonal Biotech Co., Ltd.) and integrin β3 (1:200; cat.
no. 336402, BioLegend, Inc.) overnight at 4°C, followed by
incubation with Alexa Fluor 568- (1:600; cat. no. ab175471, Abcam)
and 488- (1:600; A-21441, Invitrogen; Thermo Fisher Scientific,
Inc.) conjugated secondary antibodies. Nuclei were counterstained
with Invitrogen Prolong Gold Antifade Mountant with DAPI (P36931;
Invitrogen; Thermo Fisher Scientific, Inc.). Fluorescent images
were obtained using a TCS SP8 microscope (Leica Microsystems GmbH).
Co-localization analysis was performed using ImageJ 1.53 software
(National Institute of Health). Pearson's R value was determined as
follows:
where Xi or Yi is the individual intensity in the
pixel indexed with i, and X¯ or Y¯ is the mean intensity. R value
ranges between −1 and 1 continuously, and 0 < R < 1 and −1
< R < 0 suggest correlation and anti-correlation,
respectively.
Drug sensitivity assay
The cells were seeded in 96-well microplates at
5×103 cells/well and cultured at 37°C for 48 h. The
cells were then incubated with increasing concentrations of
vinorelbine from 0.1 nmol/l to 50 µmol/l for 96 h. The cells were
additionally incubated with 10 µl Cell Counting Kit-8 reagent
(Dojindo Laboratories, Inc.) for 2 h, and the absorbance at the 450
nm wavelength was measured using Microplate Manager 6 (Bio-Rad
Laboratories, Inc.). The half-maximal inhibitory concentration
(IC50) was calculated using Prism 7 software (GraphPad
Software, Inc.) with a three-parameter sigmoidal curve fit.
Cell proliferation assay
The cells were seeded in 96-well microplates at
5×103 cells/well and cultured at 37°C for 48 h. The
cells were additionally incubated with 10 µl Cell Counting Kit-8
reagent ((Dojindo Laboratories, Inc.) for 1 h, and the absorbance
at 450 nm wavelength was measured using Microplate Manager 6
(Bio-Rad Laboratories, Inc.). The ratio of cell proliferation was
determined by comparing the measured absorbance.
Small interfering (si)RNA-mediated
gene silencing
Ambion's Silencer™ Select Pre-Designed
siRNAs (ID nos. s9491, 9492 and 9493) targeting Nrf2 were purchased
from Thermo Fisher Scientific, Inc. The sequences of the siRNAs
used are presented in Table SI.
The cells were seeded at ~60% confluency in 6-cm dishes with
antibiotic-free medium containing 12.5 µl Lipofectamine
2000® (cat. no. 11668019; Thermo Fisher Scientific,
Inc.) and 1,000 µl Opti-MEM (cat. no. 31985062; Thermo Fisher
Scientific, Inc.), which gave a final siRNA concentration of 20
nmol/l, transfected for 48 h, and then harvested for lysate
preparation.
Patients and sample collection
In total, 104 surgical specimens were obtained from
patients who underwent lung cancer resection followed by adjuvant
cisplatin-vinorelbine treatment for pathological stage II to IIIA
(UICC, 7th edition) (22) between
December, 2006 and June, 2018 at the Department of Thoracic
Surgery, Kyoto University Hospital (Kyoto, Japan). The follow-up
period ranged from 3 to 159 months (median, 40 months). The
characteristics of the 104 patients are presented in Table SII. Disease-free survival and
overall survival were available for all patients. The study was
conducted in accordance with The Code of Ethics of the World
Medical Association (Declaration of Helsinki) for experiments
involving humans and was approved by the Ethics Committee of the
Graduate School of Medicine, Kyoto University (approval no.
G0028-7, R1706, R1486). Informed consent was obtained from all
patients.
Immunohistochemistry
Immunohistochemistry was performed to evaluate the
expression of Nrf2, ABCB1 and p-Fyn in formalin-fixed,
paraffin-embedded tissue sections from 3 to 4 µm in thickness of
the 104 surgical samples mentioned above. The slides were stained
using the VECTASTAIN Elite ABC HRP kit (Vector Laboratories, Inc.)
according to the manufacturer's protocol. Diaminobenzidine
(049-22831, FUJIFILM Wako Pure Chemical Corporation) was used as
the chromogen.
Statistical analysis
The log-rank test was used to compare two
Kaplan-Meier survival curves, and statistical analyses were
performed using Prism 7 (GraphPad) and JMP12 software (SAS
Institute Inc.). Fisher's exact test was used to compare the
patient clinicopathological characteristics. An unpaired t-test
with Welch's correction was used to compare the fluorescence
strength. A value of P<0.05 was considered to indicate a
statistically significant difference.
Results
Establishment of VR lung cancer
cells
VR cell lines were established by exposing H1299
cells to increasing concentrations of vinorelbine from 5 to 500
nmol/l. These cells were classified into three groups according to
the resistance level as follows: VR5, resistant cells cultured at 5
nmol/l; VR50, resistant cells cultured at 50 nmol/l; and VR500,
resistant cells cultured at 500 nmol/l (Fig. 1A). According to the datasheet of
Navelbine® Injection (Kyowa Kirin Co., Ltd.), 5 nmol/l
corresponds to the plasma concentration of vinorelbine at 24–48 h
following the intravenous injection of 20 mg/m2
vinorelbine in humans. The vinorelbine IC50 values for
the H1299 parental, VR5, VR50 and VR500 cells were 11.3, 43.9, 99.9
and 137.3 nmol/l, respectively (Fig.
1B). In Fig. S1, the ratio of
H1299 VR500 over H1299 parental cells in number were plotted at 2,
3, 4 and 5 days after seeding in 96-well microplates at
5×103 cells/well using cell proliferation assay. The
ratio of cells declined as the time passed to day 5, and this
result suggested that the H1299 VR500 cells were less proliferative
than the H1299 parental cells. To evaluate the apoptosis of the
H1299 cells treated with vinorelbine, lysates obtained from H1299
parental cells treated with or without 500 nmol/l vinorelbine and
the VR500 cells were immunoblotted with anti-caspase-3,
anti-cleaved caspase-3 and anti-Bcl-2 antibodies. Western blot
analysis revealed that the expression of cleaved caspase-3 was
augmented in the H1299 parental cells treated with 500 nmol/l
vinorelbine, and conversely, Bcl-2 expression was increased in the
VR500 cells (Fig. S2). The A549
VR500 cells were established in the same manner as the H1299 VR500
cells.
ROS formation in lung cancer cells
induced by vinorelbine
The H1299 parental cells and VR500 cells were
uniformly seeded into 6-well plates at 4×104 cells/well.
The parental cells were incubated for 24 h with or without 500
nmol/l vinorelbine, and the VR500 cells were incubated for 24 h
with 500 nmol/l vinorelbine as above. Intracellular ROS formation
was detected in both parental cells and VR500 cells treated with
500 nmol/l vinorelbine (Fig. 1C).
To quantify the strength of ROS formation, the area of fluorescence
per cell and the mean intensity of fluorescence in the parental
cells and VR500 cells were calculated. The area of fluorescence per
cell in the parental and VR500 cells was 449.5±480.8 and
1,474±1,731 µm2 (P<0.0001). The mean fluorescence
intensity in the parental cells and VR500 cells was 51.47±10.84 and
71.83±4.674 µm2 (P<0.0001; Fig. 1D).
ABCB1 overexpression, and p-Fyn and
Nrf2 nuclear accumulation in VR cells
The total expression of ABCB1, Nrf2 and p-Fyn was
augmented in the crude lysate of the VR cells (Fig. 2A), indicating that Nrf2 contributes
to vinorelbine resistance, in addition to ABCB1 and p-Fyn. In the
MRP family, MRP3/ABCC3 expression was augmented in a manner similar
to ABCB1 (Fig. S3). In the A549
VR500 cells, ABCB1 expression was augmented, whereas Nrf2 and p-Fyn
expression was not upregulated (Fig.
S4). Given that the diverse activities of SFKs, including Fyn,
are dependent on their subcellular localization (23,24),
lysates obtained from the subcellular extracts of H1299 parental,
VR5, VR50 and VR500 cells were immunoblotted with anti-ABCB1,
anti-Fyn, anti-p-Fyn and anti-Nrf2 antibodies to investigate the
subcellular localization and expression of ABCB1, p-Fyn and Nrf2 in
the VR cell lines. ABCB1 expression was observed in the membrane
extracts and compared with that in the nuclear and cytoplasmic
extracts (Fig. 2B). The VR cells
exhibited the nuclear and cytoplasmic localization of p-Fyn.
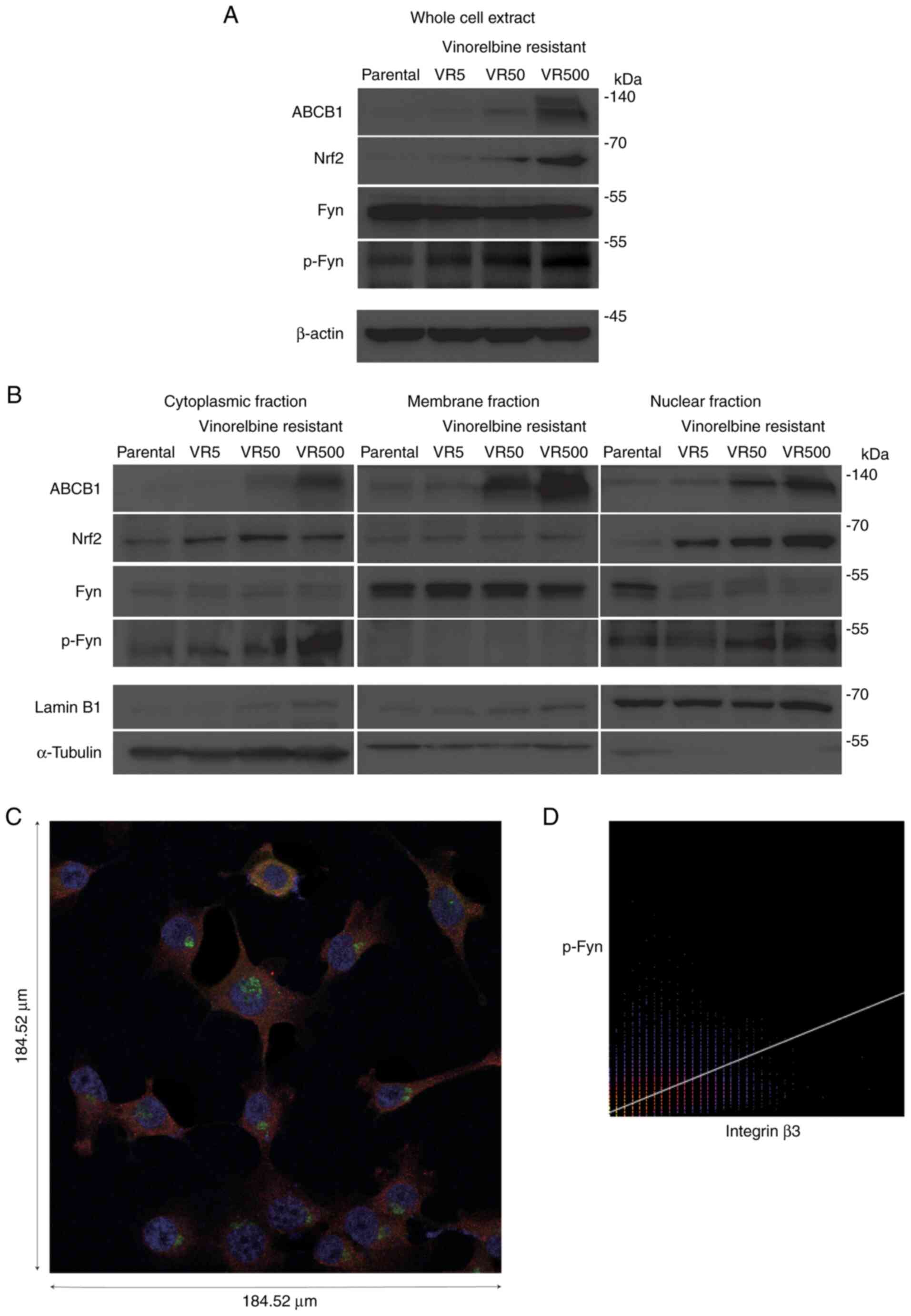 | Figure 2.Subcellular localization of ABCB1,
Nrf2 and p-Fyn in VR cells. (A and B) Parental and VR cell lines
were harvested, and cytoplasmic, membrane, nuclear, and whole-cell
extracts were prepared. All lysates were immunoblotted with
anti-ABCB1, anti-Nrf2, and anti-Fyn, anti-p-Fyn antibodies. Lamin
B1, a-tubulin and β-actin were probed as loading controls for
nuclear, cytoplasmic and whole-cell extracts, respectively. (C)
Immunofluorescence image illustrating p-Fyn localization in the
nucleus, as well as on the membrane adjacent to integrin β3. Alexa
568 (red) staining indicates integrin β3, Alexa 488 (green)
indicates p-Fyn, and DAPI (blue) indicates the nucleus. Scale bars
were 184.52 µm. (D) Co-localization analysis for integrin β3 and
p-Fyn using ImageJ software based on the immunofluorescence image.
Pearson's R value was 0.65, and this indicated a positive linear
relationship between integrin β3 and p-Fyn. VR,
vinorelbine-resistant; VNR, vinorelbine; ABCB1, ABC subfamily B
member 1; Nrf2, NF-E2-related factor 2; p-Fyn, phosphorylated
Fyn. |
Immunofluorescence was used to confirm the precise
subcellular localization of p-Fyn downstream of integrin β3. The
VR5 cells were incubated with primary antibodies against p-Fyn and
integrin β3. The fluorescence images revealed that p-Fyn was
located in the nucleus, as well as the cytoplasm adjacent to
integrin β3 in the membrane (Fig.
2C). Co-localization analysis demonstrated a positive linear
association between integrin β3 and p-Fyn (Fig. 2D). These findings suggest that
p-Fyn functions in both the cytoplasm downstream of integrin β3 and
the nucleus.
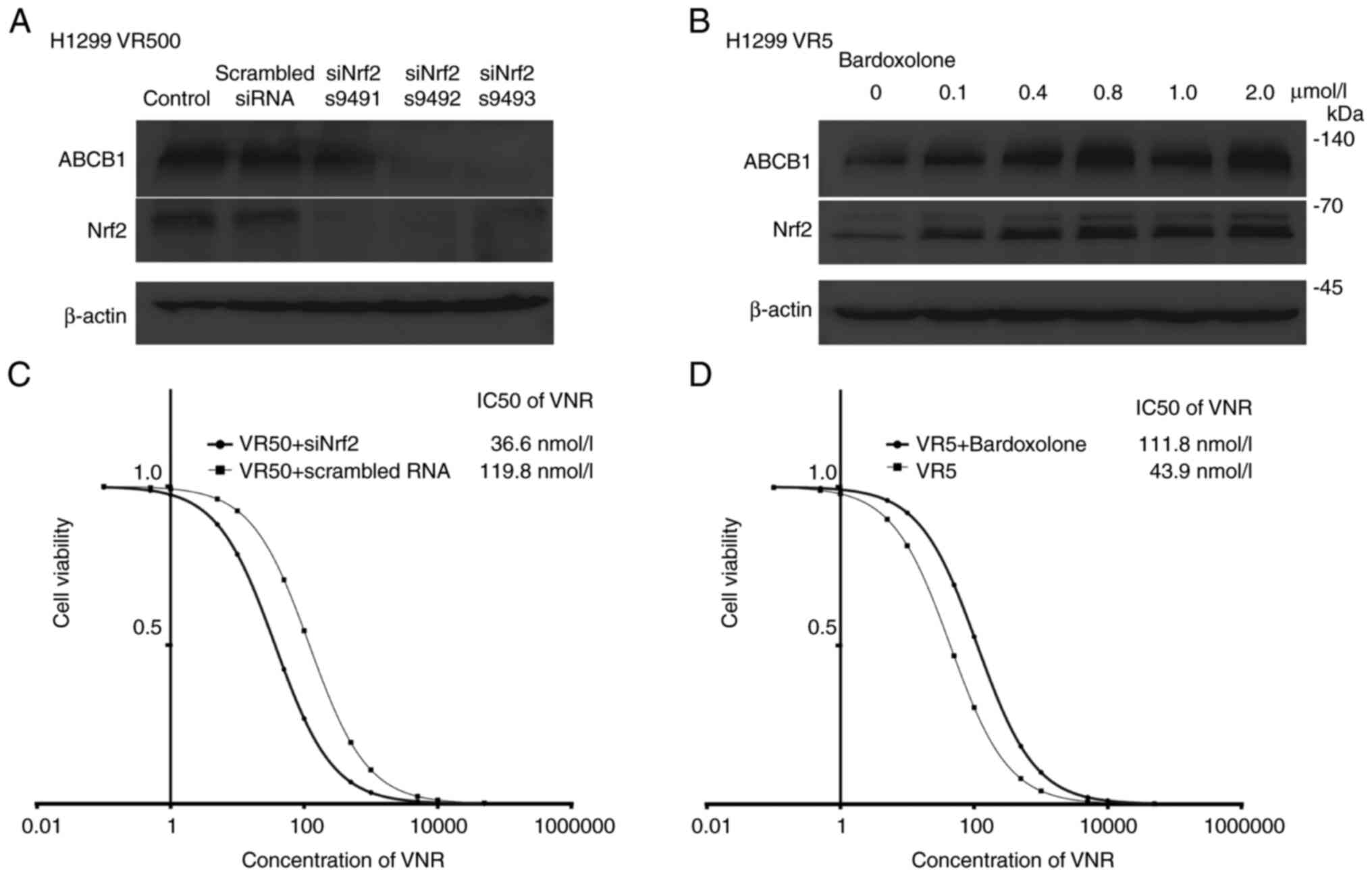
Dependence of ABCB1 expression on Nrf2
in VR cells
To elucidate whether the decreased activity of Nrf2
downregulates ABCB1 expression in VR cells, knockdown experiments
were performed using siRNA. The VR500 cells in which Nrf2 was
overexpressed, as described above in the paragraph entitled
‘ABCB1 overexpression, and p-Fyn and Nrf2 nuclear accumulation
in VR cells’ were transfected with siRNAs targeting Nrf2 and
incubated for 48 h. Scrambled RNA was used as a negative control.
In the VR500 cells, siRNAs with different sequences targeting Nrf2
similarly decreased Nrf2 expression and downregulated ABCB1
expression (Fig. 3A).
To examine whether activated Nrf2 upregulates ABCB1
expression, Keap1 was inhibited using bardoxolone. The VR5 cells in
which the Nrf2 level was lower than that in the VR500 cells were
treated with the indicated concentrations of bardoxolone for 24 h.
Bardoxolone treatment increased the expression of both Nrf2 and
ABCB1 in a concentration-dependent manner up to 0.8 µmol/l
(Figs. 3B and S5).
Effect of Nrf2 knockdown and Keap1
inhibition on the sensitivity and resistance to vinorelbine
As Nrf2 regulates the expression and functions of
ABCB1 (21), the present study
analyzed the drug resistance profile of VR cells to examine whether
Nrf2 contributes to vinorelbine resistance. Nrf2 knockdown lowered
the vinorelbine IC50 value in VR50 cells from 119.8 to
36.6 nmol/l, and restored vinorelbine sensitivity (P<0.001;
Fig. 3C). By contrast, bardoxolone
exposure for 96 h at 0.8 µmol/l increased the vinorelbine
IC50 value in the VR5 cells from 43.9 to 111.8 nmol/l
and contributed to vinorelbine resistance (P<0.001; Fig. 3D).
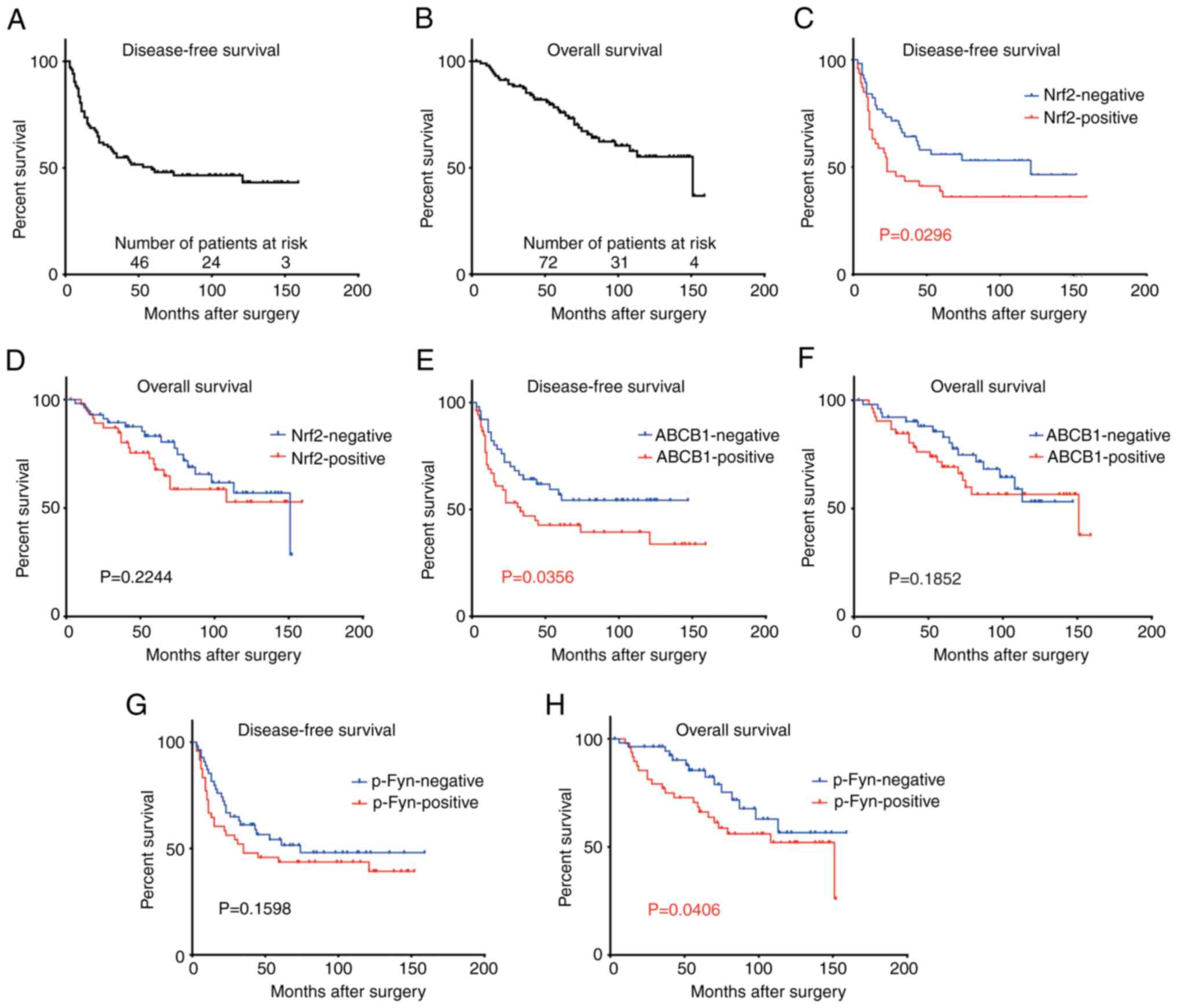
Immunohistochemical analysis of
clinical lung cancer tissue samples
To elucidate whether the expression of Nrf2, ABCB1
and p-Fyn is associated with the prognosis of patients with
completely resected lung cancer, 104 tissue samples obtained from
patients who underwent adjuvant cisplatin-vinorelbine treatment
were stained immunohistochemically. The survival curves and
clinicopathological data of the patients are presented in Fig. 4 and Table SII, respectively. The clinical
samples were stained for Nrf2, ABCB1 and p-Fyn; representative
staining patterns are illustrated in Fig. 5A-F. Nrf2, ABCB1 and p-Fyn were
predominantly expressed in the nucleus, cytoplasm and nucleus,
respectively. This finding is compatible with the subcellular
localization of each protein shown in Fig. 2B. Of the 104 patients, 47 (45%)
were positive for Nrf2, 53 (50%) for ABCB1, and 48 (46%) for p-Fyn
(Tables I and SII). Nrf2 and ABCB1 were significantly
associated with disease-free survival (P=0.029 and 0.035,
respectively), and p-Fyn was associated with overall survival
(P=0.040) (Fig. 4). The expression
of Nrf2 was significantly associated with that of p-Fyn (P=0.005),
but not with that of ABCB1 (P=0.244). The frequency of positive
Nrf2 expression was significantly associated with squamous cell
carcinoma (P=0.022), but not with adenocarcinoma (P=0.097) or other
cancers (P=0.726) (Table I).
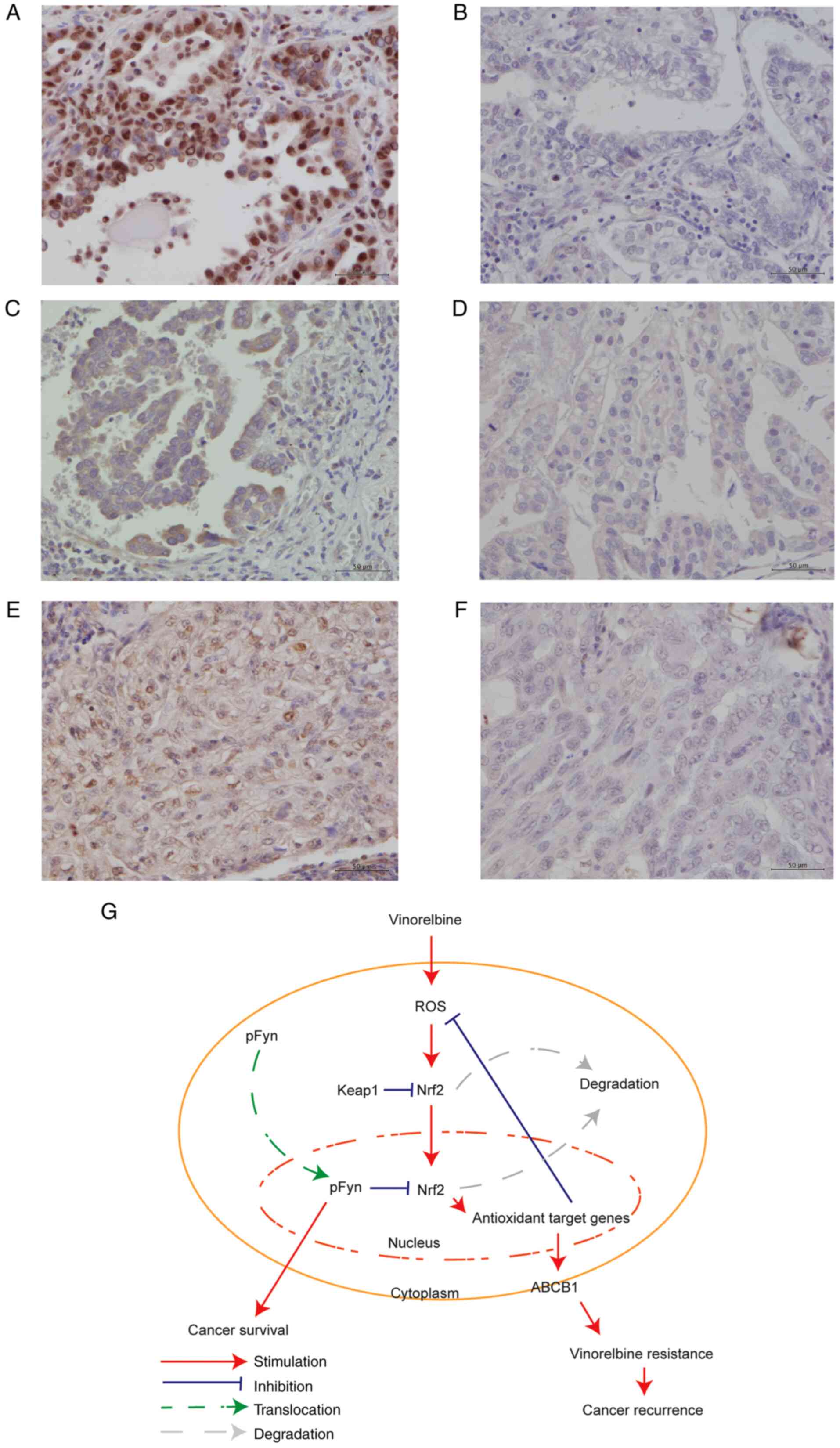 | Figure 5.Examples of immunohistochemistry
staining and proposed model of the signaling pathway. (A-F)
Representative images of Nrf2, ABCB1 and p-Fyn immunohistochemical
staining showing (A) Nrf2-positive, (B) Nrf2-negative, (C)
ABCB1-positive, (D) ABCB1-negative, (E) p-Fyn-positive, and (F)
p-Fyn-negative cells. Original magnification, ×400; scale bars, 50
µm. (G) Proposed model of the signaling pathway for vinorelbine
resistance. ABCB1, ABC subfamily B member 1; Nrf2, NF-E2-related
factor 2; p-Fyn, phosphorylated Fyn. |
 | Table I.Association of Nrf2 expression with
the expression of ABCB1 and p-Fyn, and histological types of lung
cancer in the 104 patients. |
Table I.
Association of Nrf2 expression with
the expression of ABCB1 and p-Fyn, and histological types of lung
cancer in the 104 patients.
|
|
| Nrf2
expression |
|
|
|---|
|
|
|
|
|
|
|---|
|
| Total n=104
(100%) | Positive n=47
(45.2%) | Negative n=57
(54.8%) | Odds ratio (95%
CI) | P-value |
|---|
| ABCB1
expression |
|
|
| 1.609
(0.738-3.505) | 0.244 |
|
Positive | 53 (51.0) | 27 (26.0) | 26 (25.0) |
|
|
|
Negative | 51 (49.0) | 20 (19.2) | 31 (29.8) |
|
|
| p-Fyn
expression |
|
|
| 3.222
(1.439-7.212) | 0.005 |
|
Positive | 48 (46.2) | 29 (27.8) | 19 (18.2) |
|
|
|
Negative | 56 (53.8) | 18 (17.3) | 38 (36.5) |
|
|
| Histology |
|
|
|
|
|
|
Squamous cell carcinoma |
|
|
| 3.022
(1.195-7.643) | 0.022 |
|
Yes | 26 (25.0) | 17 (16.4) | 9 (8.6) |
|
|
|
No | 78 (75.1) | 30 (28.9) | 48 (46.1) |
|
|
|
Adenocarcinoma |
|
|
| 0.482
(0.211-1.101) | 0.097 |
|
Yes | 69 (66.4) | 27 (26.0) | 42 (40.4) |
|
|
|
No | 35 (33.6) | 20 (19.2) | 15 (14.4) |
|
|
| Other
types |
|
|
| 0.709
(0.160-3.136) | 0.726 |
|
Yes | 8 (7.7) | 3 (2.9) | 5 (4.8) |
|
|
|
No | 96 (92.3) | 44 (42.3) | 52 (50) |
|
|
Discussion
Vinorelbine induces intracellular
ROS
In the present study, Nrf2 expression was augmented
in the nuclei, as well as the whole cell of VR cells, and
intracellular ROS formation was detected in both parental and VR
cells exposed to vinorelbine. Notably, VR cells, which had been
cultured with vinorelbine for months and acquired resistance to
vinorelbine, exhibited higher levels of ROS than the parental cells
treated with vinorelbine for 24 h. The formation of excessive ROS
is essential for the induction of apoptosis by commonly used
chemotherapeutic agents such as cisplatin, bleomycin, paclitaxel,
adriamycin, etoposide and vinca alkaloids (15). Vinorelbine, a semi-synthetic vinca
alkaloid, arrests cell growth at the prometaphase by inhibiting
microtubule polymerization and induces the accumulation of
mitochondrial ROS followed by prolonged JNK activation, DNA damage,
a decrease in Mcl-1 expression, mitochondrial dysfunction and
caspase-mediated apoptosis (14).
Under conditions of oxidative stress, increased levels of
intracellular ROS promote the dissociation of Nrf2 and Keap1. Free
Nrf2 translocates to the nucleus, where it binds to ARE and
transactivates downstream cytoprotective genes to induce cell
defense processes and enhance cell resistance (15,25).
Oxidants, xenobiotics and electrophiles, including chemotherapeutic
agents hamper the Keap1-mediated proteasomal degradation of Nrf2
and induce the transcription of target genes (26). The results of the present study
suggested that persistent exposure to vinorelbine induced
intracellular ROS in VR cells, which may subsequently stimulate
Nrf2.
Activated Nrf2 upregulates ABCB1 in VR
cells
Whole-cell extracts obtained from VR cells exhibited
an increased expression level of ABCB1 and Nrf2. The level of
MRP3/ABCC3, which belongs to ABC sub-family C, was also augmented
in VR cells, indicating that MRPs, as well as ABCB1 were associated
with vinorelbine resistance. In a previous study, the authors
compared the gene and protein expression of H1299 parental cells
with that of VR cells using DNA microarray, and this
microarray-based comparison did not reveal any specific change in
MRP expression, but revealed a 10-fold or more change in ABCB1
expression (9). Therefore, the
present study focused on a mechanism involving ABCB1 in vinorelbine
resistance. Nrf2, a key transcription factor, protects cells from
oxidative damage and harmful xenobiotics (27). Under unstimulated conditions, Nrf2
is retained in the cytoplasm by the anchor protein, Keap1, and is
constantly ubiquitinated and degraded in the proteasome (20). Upon exposure to oxidative or
xenobiotic stress, the Keap1-mediated proteasomal degradation of
Nrf2 is inhibited, and Nrf2 translocates to the nucleus, where it
forms a heterodimer with the small Maf protein and binds to ARE
(28). The Nrf2 effector genes
bearing ARE include those that encode the majority of antioxidant
and phase II detoxifying enzymes (29). In addition to these enzyme-coding
genes, Nrf2 transactivates a wide variety of other genes, including
several ATP-dependent drug efflux pumps (30,31).
This suggests that activated Nrf2 in cancer cells provides
advantages in terms of survival and drug resistance (32). Nrf2 expression enhances the
resistance of myelogenous leukemia and colorectal, breast,
pancreatic, and gallbladder cancers to chemotherapeutic agents,
such as imatinib, oxaliplatin, tamoxifen, gemcitabine and
5-fluorouracil, respectively (33–37).
The results of the present study indicated that ABCB1 and Nrf2 are
both involved in vinorelbine resistance, and that activated Nrf2
upregulates ABCB1 expression. These findings suggest that ABCB1 is
dependent on Nrf2 and its downstream activity in vinorelbine
resistance.
Decrease in Nrf2 expression enhances
sensitivity to vinorelbine
The results of the present study revealed that the
suppression of Nrf2 potentiated the cytotoxicity of vinorelbine,
and the upregulation of Nrf2 conferred resistance to vinorelbine.
These findings suggested that Nrf2 plays a crucial role in
regulating the susceptibility to vinorelbine, and that the
upregulation of Nrf2 in VR cells confers vinorelbine resistance.
Thus, lowering the expression of Nrf2 may present a novel
therapeutic approach for VR lung cancer.
p-Fyn accumulates in VR cell
nuclei
p-Fyn accumulated in the nuclei of VR cells and was
located in the cytoplasm adjacent to integrin β3 in the membrane.
In the focal adhesion pathway, Fyn functions downstream of several
important cell surface receptors, including integrin β3, and
upstream of several cellular signals important for cancer
progression. Although the integrin cytoplasmic domains are short
and do not have any known catalytic activity, the engagement of
integrins by extracellular matrix ligands triggers outside-in
signals that collaborate with growth factor-initiated signals to
determine cell fate and function (38,39).
Deregulated integrin signaling empowers cancer cells with the
ability to proliferate without restraint, invade through tissue
boundaries, and survive in foreign microenvironments (40). SFKs bind to multiple integrin β
cytoplasmic domains to transmit integrin-dependent signals pivotal
for cell movement and proliferation; in particular, Fyn selectively
binds to the integrin β3 domain (38). Similar to other Src family members,
Fyn regulates cell shape and migration (12) and is located primarily in the
cytoplasm, although it is also observed in other cellular
compartments, including the nucleus (41). Fyn activity is regulated in
different subcellular compartments, and different equilibrium
states between Fyn and the corresponding kinase are maintained in
the cytoplasm and nucleus (23).
Resting-state Fyn is localized near the perinuclear region in
endosomes, whereas activated Fyn is trafficked to the plasma
membrane via the actin cytoskeleton (42,43).
The nuclear expression of p-Fyn is highly associated with the poor
prognosis of patients with resected lung adenocarcinoma (44). Here, immunofluorescence images
revealed the nuclear accumulation of p-Fyn in VR cells. These
results are consistent with those of previous studies by the
authors (9,44). The nuclear or cytoplasmic
localization of Fyn in breast cancer cells has been shown to be
associated with early recurrence in patients treated with endocrine
therapy (13,41). However, the role of nuclear p-Fyn
in vinorelbine resistance remains unclear.
Nuclear accumulation of p-Fyn follows
Nrf2 activation
In the present study, subcellular fractionation and
immunofluorescence demonstrated that p-Fyn accumulated in the
nucleus, as well as the cytoplasm. The regulation of Nrf2,
particularly its abundance in the nucleus, is important for
controlling the expression of cell-protective genes in response to
oxidative stress. The continuous accumulation of Nrf2 in the
nucleus can cause disease conditions (45). For example, in Keap1-deficient
mice, the persistent accumulation of Nrf2 in the nucleus causes
hyperkeratosis in the esophagus and forestomach, leading to
neonatal death (45). As a
persistent increase in the expression of cell-protective genes
threatens cell survival (46),
aggregated Nrf2 should be subsequently exported out of the nucleus
and degraded.
The abundance of Nrf2 inside the nucleus is tightly
controlled by positive and negative regulators that affect nuclear
import, ARE binding, nuclear export, and degradation of Nrf2 under
normal and stressful conditions (47). The nuclear export of Nrf2 is
activated after Fyn accumulates in the nucleus (48). Fyn is responsive to oxidative
stress (47). It is exported out
of the nucleus soon after exposure to oxidative stress, which
allows Nrf2 to bind to ARE and activate NAD(P)H:quinone
oxidoreductase 1. The nuclear export of Fyn is an integral part of
the ARE/Nrf2-mediated activation of cytoprotective genes (47). Chemical stress induces activated
GSK-3β, which phosphorylates Fyn and accumulates p-Fyn in the
nucleus, resulting in the ubiquitination and degradation of nuclear
Nrf2 (48). The results of the
present study suggested that the nuclear accumulation of p-Fyn
following Nrf2 activation in VR cells triggers the nuclear export
and degradation of Nrf2.
Expression of Nrf2 and ABCB1 predicts
a poor disease-free survival
The immunohistochemical findings demonstrated that
Nrf2 and ABCB1 were significantly associated with disease-free
survival. Previous studies have demonstrated an association between
ABCB1 expression and chemotherapy resistance in colorectal cancer
(49), breast cancer (50) and chronic myelogenous leukemia
(51). Nrf2 expression
significantly promotes tumor size, histological grade, distant
metastasis and lymph node metastasis, and reduces sensitivity to
chemotherapy or radiotherapy (8,17,52).
A positive Nrf2 expression in the nucleus is associated with a poor
prognosis of patients with esophageal squamous cell carcinoma
following chemoradiotherapy (53).
The nuclear Nrf2 expression in malignant lung cancer cells is
related to resistance to chemotherapy in squamous cell carcinoma
(54). Consistent with these
reports, the results of the present study revealed that Nrf2
expression was significantly associated with squamous cell
carcinoma, implying that squamous cell carcinoma is a predictor of
vinorelbine resistance. It was also found that Nrf2 and ABCB1 were
closely associated with a poor susceptibility to vinorelbine and
subsequent tumor relapse, and p-Fyn was not associated with
disease-free survival, but with overall survival. These findings
reveal that vinorelbine resistance is not a direct cause of the
nuclear accumulation of p-Fyn, but rather the overall prognosis,
which emerges from Nrf2 activation in the nucleus.
Proposed model for signaling pathway
for vinorelbine resistance
The results of the present study support the
following model depicting the role of Nrf2 and ABCB1 in vinorelbine
resistance (Fig. 5G). In the
absence of stress, Nrf2 is bound to Keap1 and degraded through a
proteasome-dependent pathway, whereas the presence of ROS induced
by vinorelbine hinders the Keap1-mediated proteasomal degradation
of Nrf2. Nrf2 released from Keap1 is translocated into the nucleus
and activates the transcription of a broad spectrum of defensive
genes, including ABCB1. The increase in the expression of
chemoprotective genes neutralizes chemical stress and confers
vinorelbine resistance to the cells, which consequently promotes
tumor recurrence. As a persistent increase in defensive gene
expression threatens cell survival, Nrf2 is exported out of the
nucleus and degraded only after p-Fyn accumulates in the nucleus.
The nuclear accumulation of p-Fyn itself is a predictor of a poor
overall survival of patients with lung cancer.
The present study has the following limitations: The
sample size was small, as only datasets of patients with resected
stage II or IIIA lung cancer and adjuvant cisplatin-vinorelbine
treatment at a single institute were analyzed. In addition, the
present study was retrospective in nature. Further studies are thus
required to develop improved strategies for VR non-small cell lung
cancer.
In conclusion, the Nrf2-pFyn-ABCB1 axis plays a
pivotal role in vinorelbine resistance in non-small cell lung
cancer. Nrf2 upregulates ABCB1 and induces vinorelbine resistance,
and Nrf2 activation causes the nuclear accumulation of p-Fyn, which
triggers Nrf2 export out of the nucleus and subsequent degradation.
The present study revealed the possible pathway underlying
vinorelbine resistance in non-small cell lung cancer. It is hoped
that these findings will help researchers to develop strategies
with which to avoid this resistance or develop novel drug
targets.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank the Medical Research
Support Center, Graduate School of Medicine, Kyoto University for
TCS SP8 and the Center for Anatomical Pathological and Forensic
Medical Researches, Graduate School of Medicine, Kyoto University
for the formalin-fixed, paraffin-embedded tissue sections.
Funding
The present study supported by a Grant-in-Aid for Scientific
Research from the Ministry of Education, Culture, Sports, Science,
and Technology of Japan (no. 20H03770).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (ShT, TM, TT, HM, NC, MN, HI, RM, HK,
SaT, YYa, YYu, DN, AO, MH and HD) participated in the study design
and data interpretation, revised the manuscript, approved the
manuscript for publication, and agree to be accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. ShT, TM, TT, HM and NC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
The study was conducted in accordance with The Code
of Ethics of the World Medical Association (Declaration of
Helsinki) for experiments involving humans and was approved by the
Ethics Committee of the Graduate School of Medicine, Kyoto
University (approval no. G0028-7, R1706, R1486). Informed consent
was obtained from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ABC
|
ATP-binding cassette
|
|
ABCB1
|
ABC subfamily B member 1
|
|
ARE
|
antioxidant response element
|
|
DMSO
|
dimethyl sulfoxide
|
|
Keap1
|
kelch-Like ECH associated protein
1
|
|
Nrf2
|
NF-E2-related factor 2
|
|
p-Fyn
|
phosphorylated Fyn
|
|
ROS
|
reactive oxygen species
|
|
VR
|
vinorelbine-resistant
|
References
|
1
|
Bade BC and Dela Cruz CS: Lung cancer
2020: Epidemiology, etiology, and prevention. Clin Chest Med.
41:1–24. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Arriagada R, Bergman B, Dunant A, Le
Chevalier T, Pignon JP and Vansteenkiste J; International Adjuvant
Lung Cancer Trial Collaborative Group, : Cisplatin-based adjuvant
chemotherapy in patients with completely resected non-small-cell
lung cancer. N Engl J Med. 350:351–360. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Douillard JY, Tribodet H, Aubert D,
Shepherd FA, Rosell R, Ding K, Veillard AS, Seymour L, Le Chevalier
T, Spiro S, et al: Adjuvant cisplatin and vinorelbine for
completely resected non-small cell lung cancer: Subgroup analysis
of the lung adjuvant cisplatin evaluation. J Thorac Oncol.
5:220–228. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asamura H, Goya T, Koshiishi Y, Sohara Y,
Eguchi K, Mori M, Nakanishi Y, Tsuchiya R, Shimokata K, Inoue H, et
al: A Japanese lung cancer registry study: Prognosis of 13,010
resected lung cancers. J Thorac Oncol. 3:46–52. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nadkar A, Pungaliya C, Drake K, Zajac E,
Singhal SS and Awasthi S: Therapeutic resistance in lung cancer.
Expert Opin Drug Metab Toxicol. 2:753–777. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM: Mechanisms of cancer drug
resistance. Annu Rev Med. 53:615–627. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gottesman MM and Ling V: The molecular
basis of multidrug resistance in cancer: The early years of
P-glycoprotein research. FEBS Lett. 580:998–1009. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sadeghi MR, Jeddi F, Soozangar N, Somi MH,
Shirmohamadi M, Khaze V and Samadi N: Nrf2/P-glycoprotein axis is
associated with clinicopathological characteristics in colorectal
cancer. Biomed Pharmacother. 104:458–464. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakanishi T, Menju T, Nishikawa S,
Takahashi K, Miyata R, Shikuma K, Sowa T, Imamura N, Hamaji M,
Motoyama H, et al: The synergistic role of ATP-dependent drug
efflux pump and focal adhesion signaling pathways in vinorelbine
resistance in lung cancer. Cancer Med. 7:408–419. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang S, Qi Q, Chan CB, Zhou W, Chen J,
Luo HR, Appin C, Brat DJ and Ye K: Fyn-phosphorylated PIKE-A binds
and inhibits AMPK signaling, blocking its tumor suppressive
activity. Cell Death Differ. 23:52–63. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Singh MM, Howard A, Irwin ME, Gao Y, Lu X,
Multani A and Chandra J: Expression and activity of Fyn mediate
proliferation and blastic features of chronic myelogenous leukemia.
PLOS One. 7:e516112012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Posadas EM, Al-Ahmadie H, Robinson VL,
Jagadeeswaran R, Otto K, Kasza KE, Tretiakov M, Siddiqui J, Pienta
KJ, Stadler WM, et al: FYN is overexpressed in human prostate
cancer. BJU Int. 103:171–177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Elias D, Vever H, Lænkholm AV, Gjerstorff
MF, Yde CW, Lykkesfeldt AE and Ditzel HJ: Gene expression profiling
identifies FYN as an important molecule in tamoxifen resistance and
a predictor of early recurrence in patients treated with endocrine
therapy. Oncogene. 34:1919–1927. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chiu WH, Luo SJ, Chen CL, Cheng JH, Hsieh
CY, Wang CY, Huang WC, Su WC and Lin CF: Vinca alkaloids cause
aberrant ROS-mediated JNK activation, Mcl-1 downregulation, DNA
damage, mitochondrial dysfunction, and apoptosis in lung
adenocarcinoma cells. Biochem Pharmacol. 83:1159–1171. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xue D, Zhou X and Qiu J: Emerging role of
NRF2 in ROS-mediated tumor chemoresistance. Biomed Pharmacother.
131:1106762020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ren D, Villeneuve NF, Jiang T, Wu T, Lau
A, Toppin HA and Zhang DD: Brusatol enhances the efficacy of
chemotherapy by inhibiting the Nrf2-mediated defense mechanism.
Proc Natl Acad Sci USA. 108:1433–1438. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yoshino H, Murakami K, Nawamaki M and
Kashiwakura I: Effects of Nrf2 knockdown on the properties of
irradiated cell conditioned medium from A549 human lung cancer
cells. Biomed Rep. 8:461–465. 2018.PubMed/NCBI
|
|
18
|
Shin BY, Jin SH, Cho IJ and Ki SH:
Nrf2-ARE pathway regulates induction of Sestrin-2 expression. Free
Radic Biol Med. 53:834–841. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lau A, Villeneuve NF, Sun Z, Wong PK and
Zhang DD: Dual roles of Nrf2 in cancer. Pharmacol Res. 58:262–270.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Singh A, Misra V, Thimmulappa RK, Lee H,
Ames S, Hoque MO, Herman JG, Baylin SB, Sidransky D, Gabrielson E,
et al: Dysfunctional KEAP1-NRF2 interaction in non-small-cell lung
cancer. PLoS Med. 3:e4202006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Feng SL, Luo HB, Cai L, Zhang J, Wang D,
Chen YJ, Zhan HX, Jiang ZH and Xie Y: Ginsenoside Rg5 overcomes
chemotherapeutic multidrug resistance mediated by ABCB1
transporter: In vitro and in vivo study. J Ginseng Res. 44:247–257.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldstraw P: New TNM classification:
Achievements and hurdles. Transl Lung Cancer Res. 2:264–272.
2013.PubMed/NCBI
|
|
23
|
Huang Z, Ouyang M, Lu S, Wang Y and Peng
Q: Optogenetic control for investigating subcellular localization
of Fyn kinase activity in single live cells. J Mol Biol.
432:1901–1909. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Resh MD: Fyn, a Src family tyrosine
kinase. Int J Biochem Cell Biol. 30:1159–1162. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kitamura H and Motohashi H: NRF2 addiction
in cancer cells. Cancer Sci. 109:900–911. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Singh A, Boldin-Adamsky S, Thimmulappa RK,
Rath SK, Ashush H, Coulter J, Blackford A, Goodman SN, Bunz F,
Watson WH, et al: RNAi-mediated silencing of nuclear factor
erythroid-2-related factor 2 gene expression in non-small cell lung
cancer inhibits tumor growth and increases efficacy of
chemotherapy. Cancer Res. 68:7975–7984. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bryan HK, Olayanju A, Goldring CE and Park
BK: The Nrf2 cell defence pathway: Keap1-dependent and -independent
mechanisms of regulation. Biochem Pharmacol. 85:705–717. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Osburn WO and Kensler TW: Nrf2 signaling:
An adaptive response pathway for protection against environmental
toxic insults. Mutat Res. 659:31–39. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jaiswal AK: Nrf2 signaling in coordinated
activation of antioxidant gene expression. Free Radic Biol Med.
36:1199–1207. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hayashi A, Suzuki H, Itoh K, Yamamoto M
and Sugiyama Y: Transcription factor Nrf2 is required for the
constitutive and inducible expression of multidrug
resistance-associated protein 1 in mouse embryo fibroblasts.
Biochem Biophys Res Commun. 310:824–829. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Vollrath V, Wielandt AM, Iruretagoyena M
and Chianale J: Role of Nrf2 in the regulation of the MRP2 (ABCC2)
gene. Biochem J. 395:599–609. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Homma S, Ishii Y, Morishima Y, Yamadori T,
Matsuno Y, Haraguchi N, Kikuchi N, Satoh H, Sakamoto T, Hizawa N,
et al: Nrf2 enhances cell proliferation and resistance to
anticancer drugs in human lung cancer. Clin Cancer Res.
15:3423–3432. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tarumoto T, Nagai T, Ohmine K, Miyoshi T,
Nakamura M, Kondo T, Mitsugi K, Nakano S, Muroi K, Komatsu N and
Ozawa K: Ascorbic acid restores sensitivity to imatinib via
suppression of Nrf2-dependent gene expression in the
imatinib-resistant cell line. Exp Hematol. 32:375–381. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wang XJ, Li Y, Luo L, Wang H, Chi Z, Xin
A, Li X, Wu J and Tang X: Oxaliplatin activates the Keap1/Nrf2
antioxidant system conferring protection against the cytotoxicity
of anticancer drugs. Free Radic Biol Med. 70:68–77. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kim SK, Yang JW, Kim MR, Roh SH, Kim HG,
Lee KY, Jeong HG and Kang KW: Increased expression of
Nrf2/ARE-dependent anti-oxidant proteins in tamoxifen-resistant
breast cancer cells. Free Radic Biol Med. 45:537–546. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hu Q, Qin Y, Xiang J, Liu W, Xu W, Sun Q,
Ji S, Liu J, Zhang Z, Ni Q, et al: dCK negatively regulates the
NRF2/Are axis and ROS production in pancreatic cancer. Cell Prolif.
51:e124562018. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shibata T, Kokubu A, Gotoh M, Ojima H,
Ohta T, Yamamoto M and Hirohashi S: Genetic alteration of Keap1
confers constitutive Nrf2 activation and resistance to chemotherapy
in gallbladder cancer. Gastroenterology. 135:1358–1368.
1368.e1351–e1354. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Arias-Salgado EG, Lizano S, Sarkar S,
Brugge JS, Ginsberg MH and Shattil SJ: Src kinase activation by
direct interaction with the integrin beta cytoplasmic domain. Proc
Natl Acad Sci USA. 100:13298–13302. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Reddy KB, Smith DM and Plow EF: Analysis
of Fyn function in hemostasis and alphaIIbbeta3-integrin signaling.
J Cell Sci. 121:1641–1648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cooper J and Giancotti FG: Integrin
signaling in cancer: Mechanotransduction, stemness, epithelial
plasticity, and therapeutic resistance. Cancer Cell. 35:347–367.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Campbell EJ, McDuff E, Tatarov O, Tovey S,
Brunton V, Cooke TG and Edwards J: Phosphorylated c-Src in the
nucleus is associated with improved patient outcome in ER-positive
breast cancer. Br J Cancer. 99:1769–1774. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Kaplan KB, Swedlow JR, Varmus HE and
Morgan DO: Association of p60c-src with endosomal membranes in
mammalian fibroblasts. J Cell Biol. 118:321–333. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Sandilands E, Cans C, Fincham VJ, Brunton
VG, Mellor H, Prendergast GC, Norman JC, Superti-Furga G and Frame
MC: RhoB and actin polymerization coordinate Src activation with
endosome-mediated delivery to the membrane. Dev Cell. 7:855–869.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nishikawa S, Menju T, Takahashi K, Miyata
R, Sonobe M, Yoshizawa A and Date H: Prognostic significance of
phosphorylated Fyn in patients with lung adenocarcinoma after lung
resection. Ann Thorac Cardiovasc Surg. 25:246–252. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wakabayashi N, Itoh K, Wakabayashi J,
Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F,
Roop DR, et al: Keap1-null mutation leads to postnatal lethality
due to constitutive Nrf2 activation. Nat Genet. 35:238–245. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kang MI, Kobayashi A, Wakabayashi N, Kim
SG and Yamamoto M: Scaffolding of Keap1 to the actin cytoskeleton
controls the function of Nrf2 as key regulator of cytoprotective
phase 2 genes. Proc Natl Acad Sci USA. 101:2046–2051. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kaspar JW and Jaiswal AK: Tyrosine
phosphorylation controls nuclear export of Fyn, allowing Nrf2
activation of cytoprotective gene expression. FASEB J.
25:1076–1087. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Jain AK and Jaiswal AK: GSK-3beta acts
upstream of Fyn kinase in regulation of nuclear export and
degradation of NF-E2 related factor 2. J Biol Chem.
282:16502–16510. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Tokunaga Y, Hosogi H, Hoppou T, Nakagami
M, Tokuka A and Ohsumi K: Effects of MDR1/P-glycoprotein expression
on prognosis in advanced colorectal cancer after surgery. Oncol
Rep. 8:815–819. 2001.PubMed/NCBI
|
|
50
|
Li W and Song M: Expression of multidrug
resistance proteins in invasive ductal carcinoma of the breast.
Oncol Lett. 8:2103–2109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Park SH, Park CJ, Kim DY, Lee BR, Kim YJ,
Cho YU and Jang S: MRP1 and P-glycoprotein expression assays would
be useful in the additional detection of treatment non-responders
in CML patients without ABL1 mutation. Leuk Res. 39:1109–1116.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kawasaki Y, Ishigami S, Arigami T,
Uenosono Y, Yanagita S, Uchikado Y, Kita Y, Nishizono Y, Okumura H,
Nakajo A, et al: Clinicopathological significance of nuclear factor
(erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer.
BMC Cancer. 15:52015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zhang J, Jiao Q, Kong L and Yu J, Fang A,
Li M and Yu J: Nrf2 and Keap1 abnormalities in esophageal squamous
cell carcinoma and association with the effect of
chemoradiotherapy. Thorac Cancer. 9:726–735. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Solis LM, Behrens C, Dong W, Suraokar M,
Ozburn NC, Moran CA, Corvalan AH, Biswal S, Swisher SG, Bekele BN,
et al: Nrf2 and Keap1 abnormalities in non-small cell lung
carcinoma and association with clinicopathologic features. Clin
Cancer Res. 16:3743–3753. 2010. View Article : Google Scholar : PubMed/NCBI
|