Introduction
Apoptosis, a basic process of programmed cell death,
is necessary for homeostasis of all organisms. The occurrence and
development of cancer is not only associated with cell
proliferation, but also caused by dysregulated cell apoptosis
(1). To a certain extent, most
cancer treatment is based on a process to accelerate tumor cell
apoptosis. A thorough understanding of the apoptotic mechanisms is
critical for the development of potential therapeutic targets using
gene therapy techniques (2).
According to global cancer statistics of 2018, lung cancer was the
most common and leading cause of cancer in males (3). Therefore, the research of apoptotic
mechanisms has become a popular and urgent topic in lung cancer
research.
Tumor metastasis suppressor gene 1 (TMSG-1)
was discovered by Ma et al at Peking University in 1999
(4). Subsequently, Pan et
al cloned a new gene highly homologous to the longevity support
gene of yeast [longevity assurance homolog 1 (LAG1)] in the
human liver cDNA library (5). This
gene, termed LASS2, is highly homologous with TMSG-1
(5). The LASS2-encoded
protein contains two structural domains: Homeodomain structural
domain, and TRAM, LAG1 and CLN8 (TLC) structural domain (6). Reportedly, homeodomains play a vital
role in the regulation of the cell cycle and may inhibit tumor
invasion, metastasis, growth, and apoptosis by combining with C
subunit (ATP6L) of vacuolar H+-ATPase (V-ATPase)
(7). The TLC structural domain is
predominantly involved in lipid metabolism and synthesis of
ceramide, which can induce apoptosis in tumor cells, thereby
inhibiting tumor growth (8).
Previous studies have shown that the LASS2
gene is negatively correlated with the metastasis capacity of
prostate, breast, liver, and gastrointestinal cancers (9–12).
Recently, it was reported that LASS2 suppressed
proliferation and promoted apoptosis of HepG2 cells by regulating
the NF-κB signaling pathway (13).
However, the ability of LASS2 to induce apoptosis of lung
cancer cells, and the mechanism thereof remain unclear.
The typical mechanism of apoptosis is the caspase
activation pathway. The precise role that LASS2, mediated by
the caspase pathway, plays in the apoptosis of lung cancer cells is
unclear. In a previous study by the authors, it was determined that
the expression and gene positive rate of LASS2 in non-small
cell lung cancer tissues were significantly reduced compared with
those in para-carcinoma tissues (14). Additionally, it was revealed that
the mRNA and protein expression levels of LASS2 in lung cancer 95C
cell lines with low metastatic potential were significantly higher
than that in 95D cells with high metastatic potential (15). Therefore, the aim of the present
study was to determine the influence of LASS2 on apoptosis and
proliferation of 95D cells transfected with the LASS2
overexpression vector, and 95C cells transfected with
LASS2-RNAi to elucidate the underlying mechanism.
LASS2 was silenced and the protein expression levels of
cytochrome c, Bax, Bcl-2, caspase-3, and caspase-9 were
detected in 95D and 95C cells. The present study may reveal certain
theoretical mechanisms for lung cancer apoptosis, and provide some
potential targets for guiding lung cancer treatment.
Materials and methods
Cell lines and cell culture
The human lung cancer cell lines, 95D (cat. no.
XF0039; low expression of LASS2 and high metastatic
potential), and 95C (cat. no. XF0041; high expression of
LASS2 and low metastatic potential) were purchased from
Shanghai Fuxiang Biotechnology Co., Ltd (http://www.xiangbio.com/). Cells were cultured in
RPMI-1640 medium with 10% fresh fetal bovine serum (both Gibco;
Thermo Fisher Scientific, Inc.), and 1% penicillin and
streptomycin. Cells were maintained at 37°C with 5% CO2.
For the selection of the cell lines, the expression levels of
LASS2/TMSG1 mRNA and protein in 95C cells were significantly higher
than that of 95D cells (P<0.01). In addition, wound healing
assay also confirmed that the migration ability of 95D cells was
higher than that of 95C cells (t =23.56; P<0.05) (16).
Lentivirus transfection
experiment
The 2nd generation system was used in the lentivirus
transfection experiment. The interim cell line used in this study
was 293T cells (cat. no. SCSP-502; Cell Bank of Chinese Academy of
Sciences, Shanghai, China). The cells (1×105) were
seeded on a 6-well plate wherein 95D and 95C cells were transfected
with LASS2 overexpression lentiviral vectors (LV-LASS2
8649-2) and LASS2-RNAi (LV-LASS2-RNAi 30011-1; both from
Shanghai Genechem Co., Ltd., China), respectively, according to the
transfection instructions (Polybrene and Enhanced Infection
Solution; Shanghai Jikai Gene Medical Technology Co., Ltd). The
quantity of plasmids, Helper 1.0 (packaging) and Helper 2.0
(envelope) were 20, 15, and 10 µg, respectively. Transfection was
performed at room temperature for 12 h. Subsequently, the
transfection solution was replaced with complete medium, and the
stable strain selection experiment was carried out after culturing
for 48–72 h. The stable transfectants with LASS2
overexpression plasmid in 95D cells and LASS2 shRNA in 95C
cells were designated as LASS2 overexpression, and
silencing, respectively. The sequence of LASS2-RNAi (LASS2
shRNA) was 5′-AGTATTGGTACTACATGAT-3′, and the sequence of the
negative control (NC) was 5′-TTCTCCGAACGTGTCACGT-3′. The stable
transfectants with scrambled plasmid in 95D and 95C cells
(LV-CON054; Shanghai Genechem Co., Ltd., China) were used as
negative control, while the untransfected cells were designated as
normal controls. Preheated complete medium (500 µl) was added to
preheated transfection cells, followed by lentivirus (virus titer
was 1×108 TU/ml and multiplicity of infection was two
times), and polybrene infection solution (with concentration of
5–10 µg/ml), and enhanced infection solution were added to make the
final volume up to 1 ml. Fresh medium was then added after 8–12 h,
the culture medium was replaced by the complete medium with
puromycin after 48 h. The puromycin concentration for selection was
2 µg/ml, while for maintenance it was 0.5 µg/ml.
Flow cytometric analysis
Cells (1×105) were seeded in a 25
cm2 ventilated culture bottle and cultured to 70% cell
confluency. Following centrifugation at 187 × g at 4°C for 10 min,
the cell precipitation was washed with 1 ml precooled Dulbecco's
phosphate-buffered saline (DPBS; Biosharp Life Sciences). The cell
precipitation was suspended in 200-µl buffer solution with 10 µl
Annexin V-FITC and incubated at 4°C for 30 min in the dark. Then,
300-µl buffer solution, and 5 µl prodium iodide (PI) were added in
the mixed solution. by adding. The cells were then immediately
detected by BD FACSCalibur flow cytometer (Becton, Dickinson and
Company). NovoExpress software version 1.3.0 (Agilent Technologies,
Inc.) was used to analyze samples. The flow cytometric assay was
performed using three biological replicates with three technical
repetitions each.
Cell Counting Kit-8 (CCK-8) assay
The proliferation activity of cells in different
treatment groups was measured by CCK-8 assay (cat. no. C0038;
Beyotime Institute of Biotechnology). Briefly, the stable
transfected cells in logarithmic phase were digested with 1 ml
0.25% trypsin. Once the cells became round, the digestion process
was terminated using 1 ml complete medium. The cells were then
centrifuged at 187 g for 5 min, and resuspended by adding 1 ml
complete medium. Following dilution of the suspension to 500 cells
per 100 µl, 100 µl cell suspension was added into the 96-well
plate. After 48 h of preincubation, 10 µl CCK-8 solution was added
into each well. Finally, the cells were incubated for 4 h, and the
absorbance was measured at 450 nm using a microplate reader
(ELX800TM; Bio-Tek Instruments, Inc.). This experiment was repeated
independently three times.
Western blotting
The total protein of the cell was extracted using
RIPA Lysate and PMSF (RIPA: PMSF, 100:1; Beyotime Institute of
Biotechnology), and the protein concentration was determined using
the BCA method (Pierce; Thermo Fisher Scientific, Inc.). Cells were
washed twice with pre-chilled phosphate-buffered saline (PBS) and
lysed in lysis buffer. After being incubated on ice for 30 min, the
cells were centrifuged at 10,000 × g for 10 min at 4°C. The protein
concentration was measured using a BCA protein assay kit according
to the manufacturer's instructions. The sample was then mixed with
5X SDS sample buffer solution in equal volumes, and placed in a
boiling water bath for 5 min. Thereafter, 10 µg of protein from
each sample was separated in 10% SDS-PAGE, and transferred to
nitrocellulose membranes for sealing. The nitrocellulose membranes
were then blocked with 5% skim milk which included 2.5 g skim milk
and 50 ml TBST (BioFroxx; neoFroxx GmbH) at room temperature for 1
h. The primary antibody was then added, in which the working
concentration was 1:1,000 for β-actin (cat. no. AA128-1; Beyotime
Institute of Biotechnology), 1:300 for LASS/TMSG-1 (cat. no.
bs-5077R), 1:200 for Bcl-2 (cat. no. bs-4563R), 1:200 for Bax (cat.
no. bs-0127R), 1:200 for cytochrome c (cat. no. bs-0013R),
1:200 for caspase-9 (cat. no. bs-0050R), and 1:200 for caspase-3
(cat. no. bs-0081R). The antibodies of LASS, Bcl-2, Bax, cytochrome
c, caspase-9 and caspase-3 were purchased from Beijing
Biosynthesis Biotechnology Co., Ltd.; BIOSS). The membranes were
incubated for 12 h at 4°C, and then they underwent PBST membrane
cleaning. The membranes were then incubated for 1 h at room
temperature with a secondary antibody (working concentration was
1:15,000), and another PBST membrane cleaning was performed. The
secondary antibody used was Dylight 800 AffiniPure goat anti-rabbit
IgG (cat. no. A23920; Abbkine Scientific Co., Ltd.). Finally, the
NC membranes were scanned on the Odyssey CLX infrared laser imaging
system (Image Studio 3.1; LI-COR Biosciences) in the dark. Results
were observed, and the gray value was recorded. Three biological
replicates were prepared for each experiment.
Statistical analysis
SPSS 21.0 (IBM Corporation) was used for statistical
analyses and the experimental results were expressed as the mean ±
standard deviation (M ± SD). One-way ANOVA followed by Tukey's post
hoc test was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
LASS2 overexpression and silencing
confirmation
The highly metastatic 95D cells were transfected
with LASS2 gene overexpression vector, and 95C cells with
low metastaticity were transfected with LASS2 gene silencing
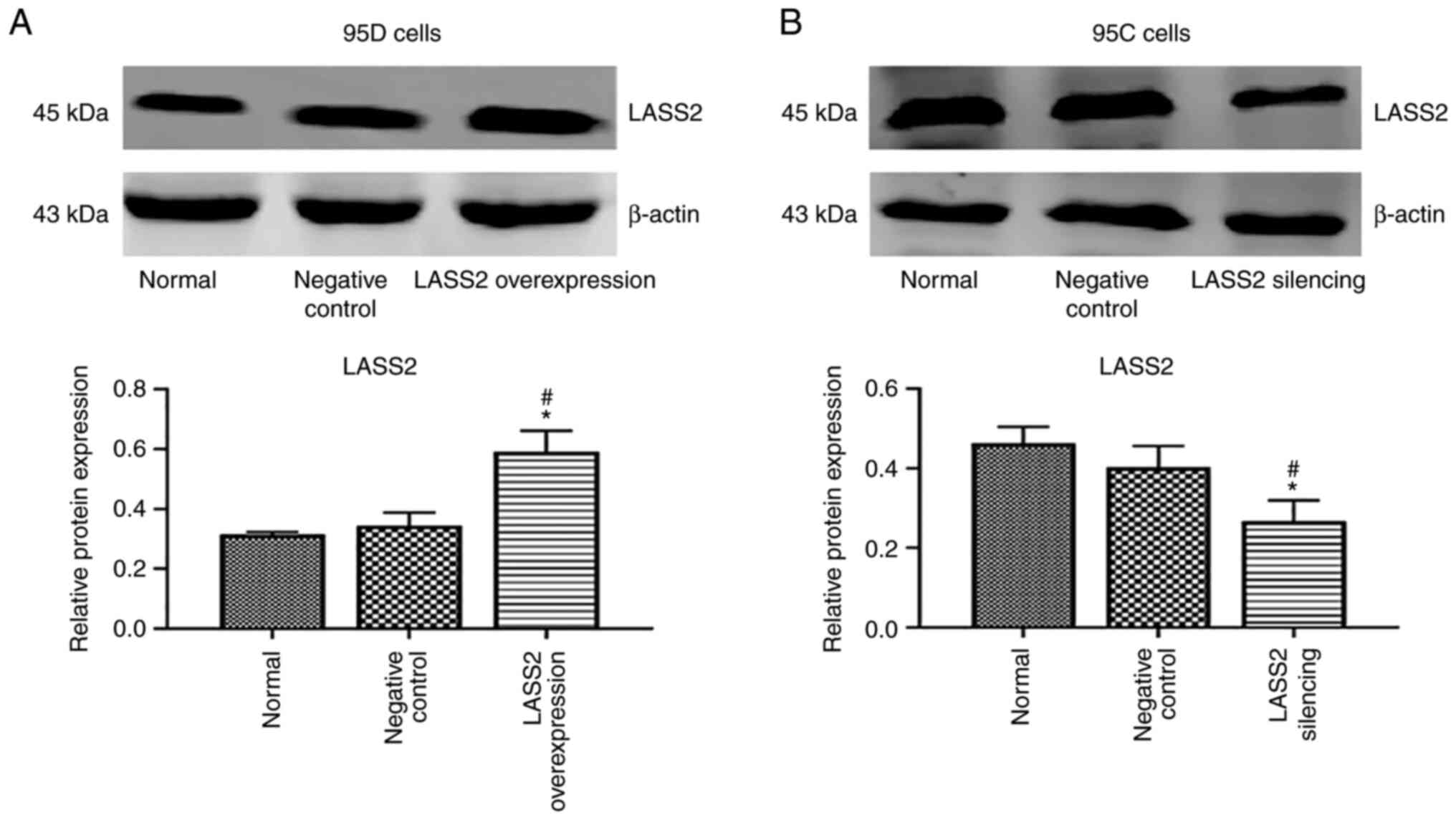
vector. The results revealed that the level of LASS2 protein in the
LASS2-overexpressed group was significantly increased
compared with the normal and negative control groups (P<0.05;
Fig. 1A). Additionally, the level
of LASS2 protein in the LASS2-silenced group was
significantly reduced compared with the normal and negative control
groups (P<0.05; Fig. 1B).
Effects of LASS2 overexpression on
apoptosis of 95D cells and silencing on apoptosis of 95C cells
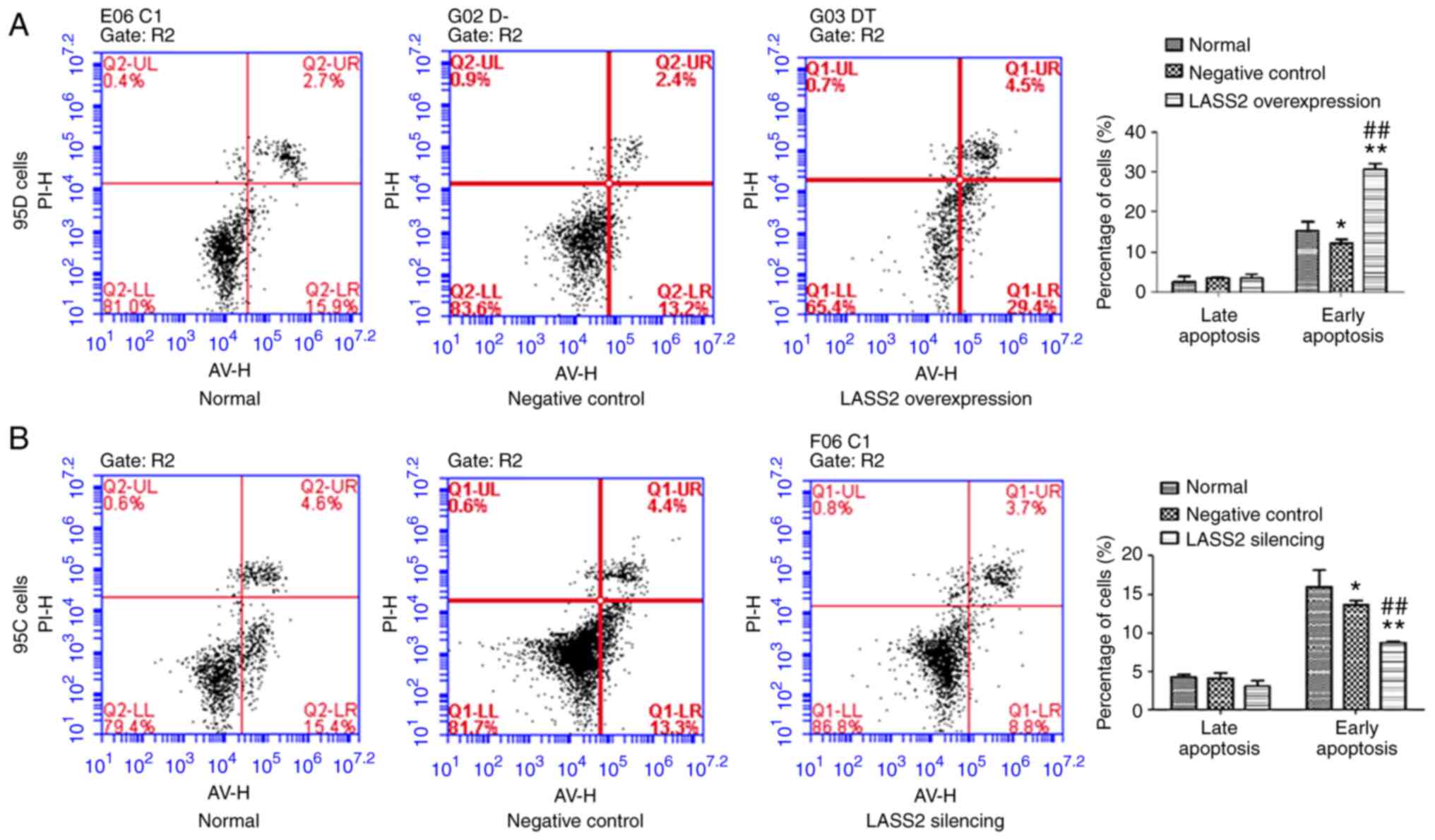
As revealed in Fig.
2A, the percentage of early apoptotic cells in the
LASS2-overexpressed group was significantly increased
compared with the normal and negative control groups (P<0.01),
while there was no significant difference in late apoptosis among
the three groups. Conversely, compared with the normal and negative
control groups, the percentage of early apoptotic cells in the
LASS2-silenced group was significantly reduced (P<0.01).
Similarly, no significant difference was detected in late apoptosis
among these groups (Fig. 2B).
Effects of LASS2 overexpression and
silencing on proliferation of 95D and 95C cells
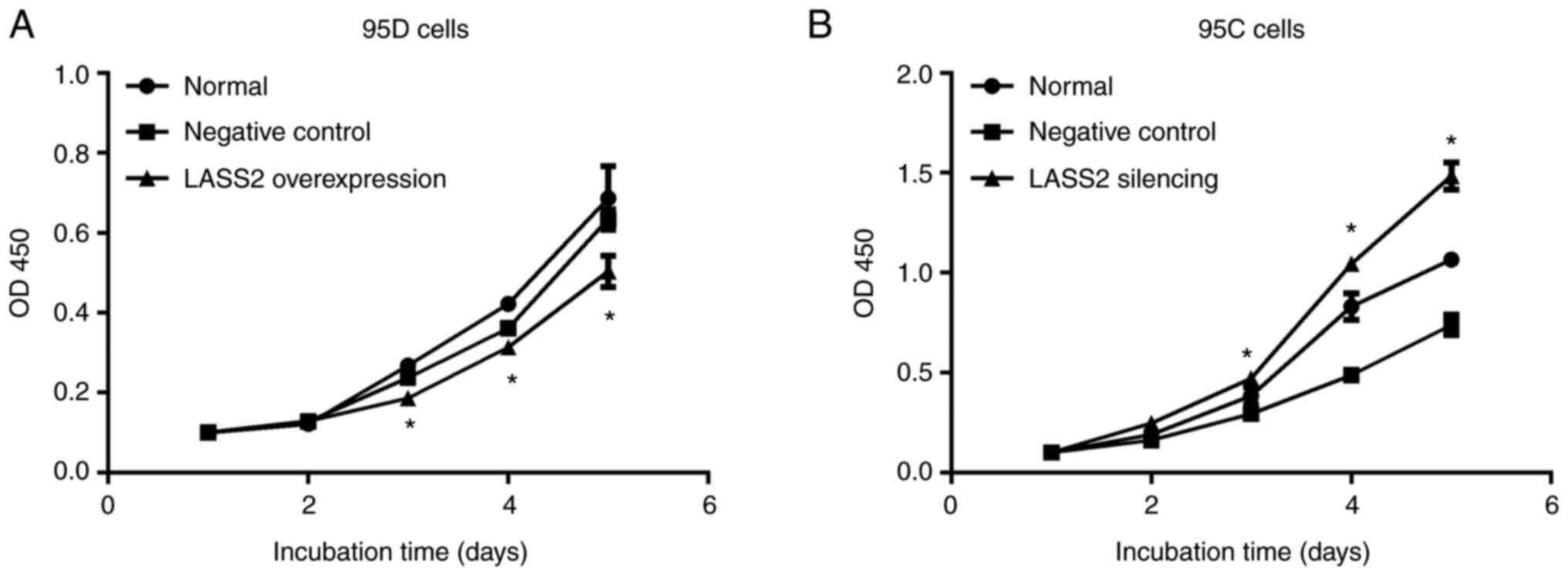
After inoculation from day 1 to 5, the proliferation
ability of 95D cells in the LASS2-overexpressed group was
significantly decreased after day 3, compared with the normal and
negative control groups (P<0.05; Fig. 3A). Additionally, the proliferation
ability of 95C cells in LASS2-silenced group was
significantly increased after day 3, compared with the control and
negative control groups (P<0.05; Fig. 3B).
Protein expression of Bcl-2 and Bax
following LASS2 overexpression in 95D cells and silencing in 95C
cells
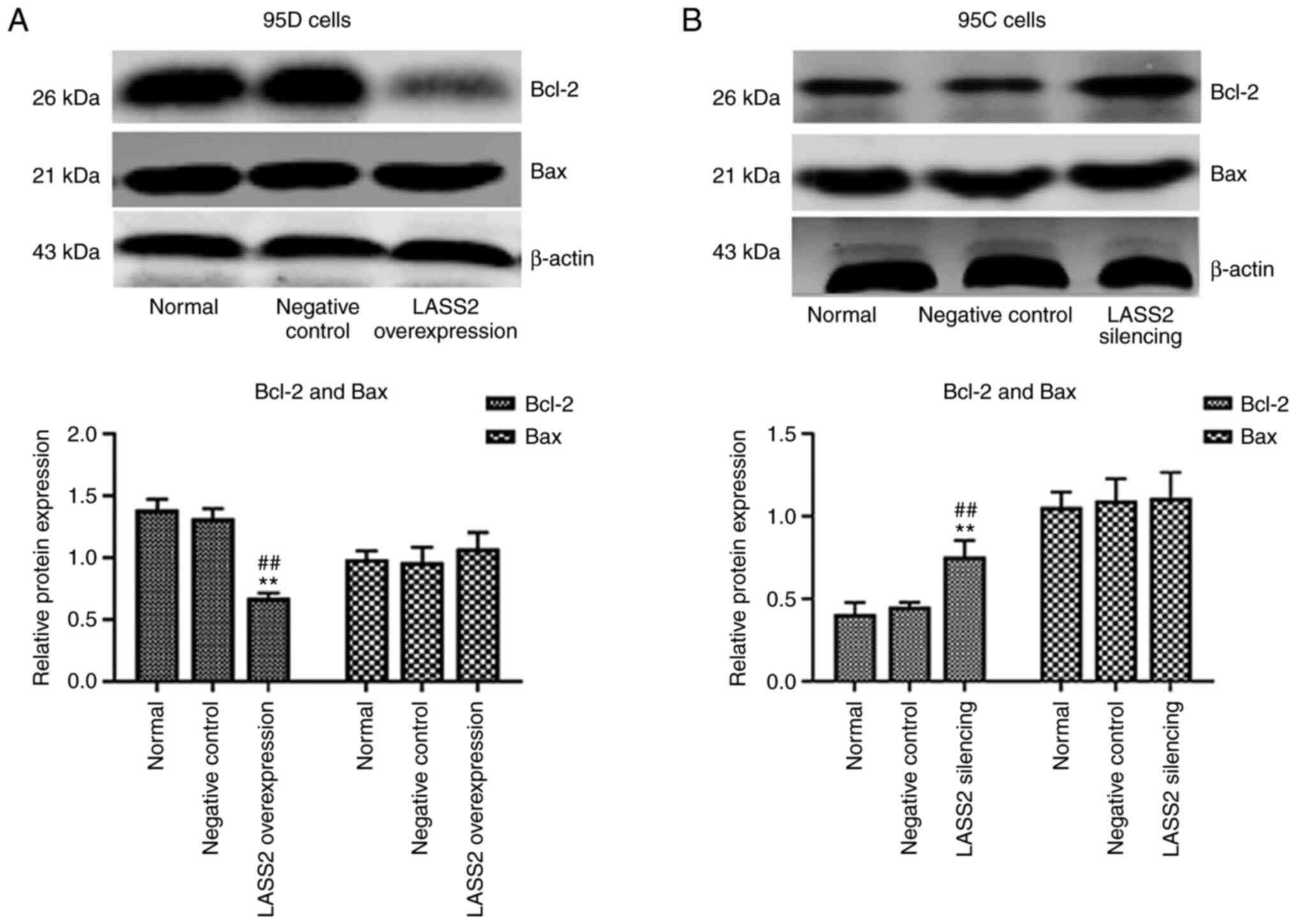
In comparison with the normal and negative control
groups, the expression level of Bcl-2 in 95D cells was
significantly decreased in the LASS2-overexpressed group
(P<0.01), while that of Bax underwent no change (Fig. 4A). In addition, the ratio of
Bax/Bcl-2 was increased. Conversely, the expression level of Bcl-2
in the LASS2 gene-silenced group was significantly increased
(P<0.05; Fig. 4B), and no
significant change in the level of Bax was detected. The ratio of
Bax/Bcl-2 was decreased in 95C cells following silencing of
LASS2.
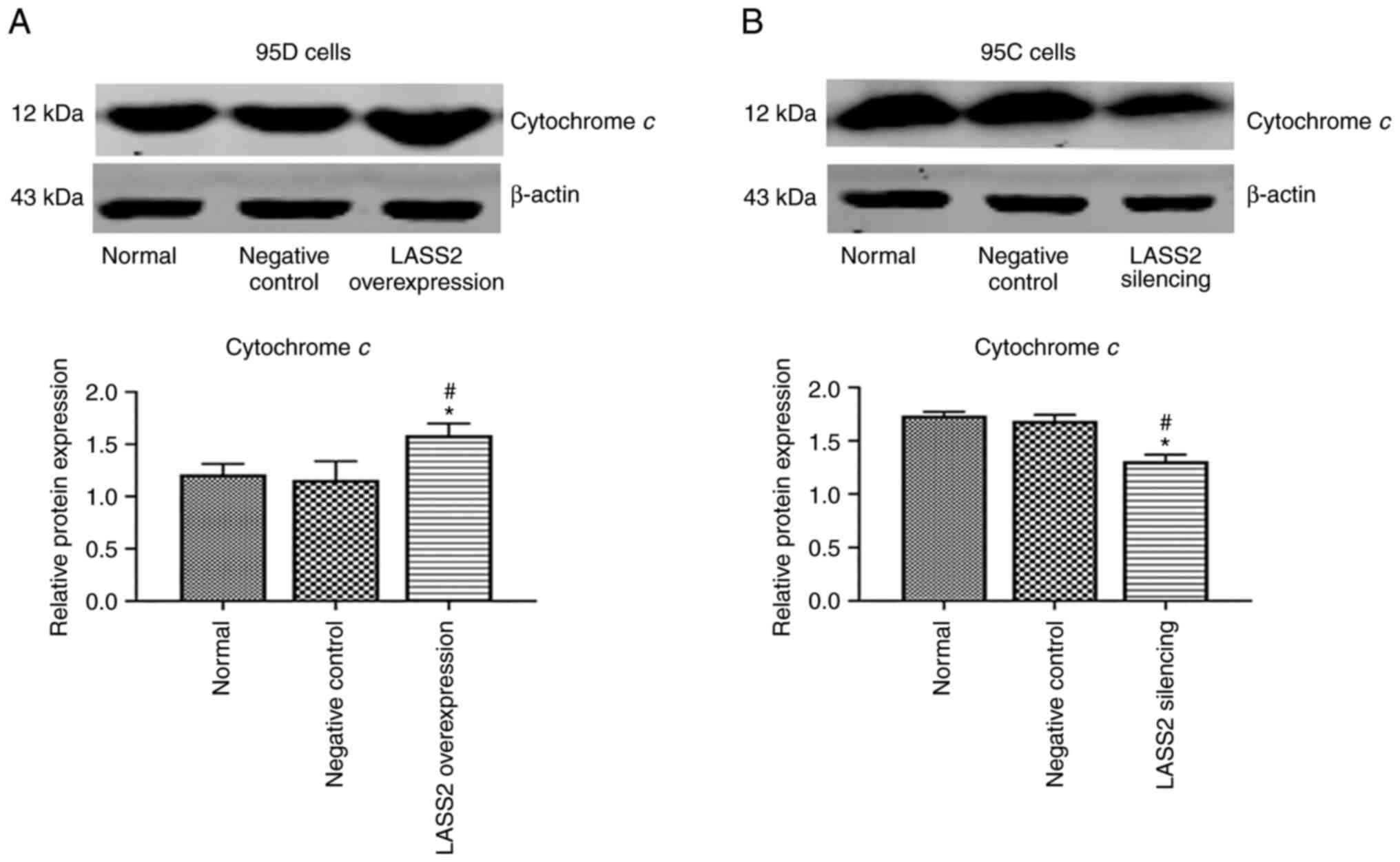
Protein expression of cytochrome c
after LASS2 overexpression in 95D cells and silencing in 95C
cells
As revealed in Fig.
5A, following LASS2 overexpression, the protein level of
cytochrome c in 95D cells was significantly increased as
compared with the control and negative control groups (P<0.05).
Additionally, the protein level of cytochrome c in 95C cells
with silenced LASS2 was significantly reduced than the
control and negative controls (P<0.05; Fig. 5B).
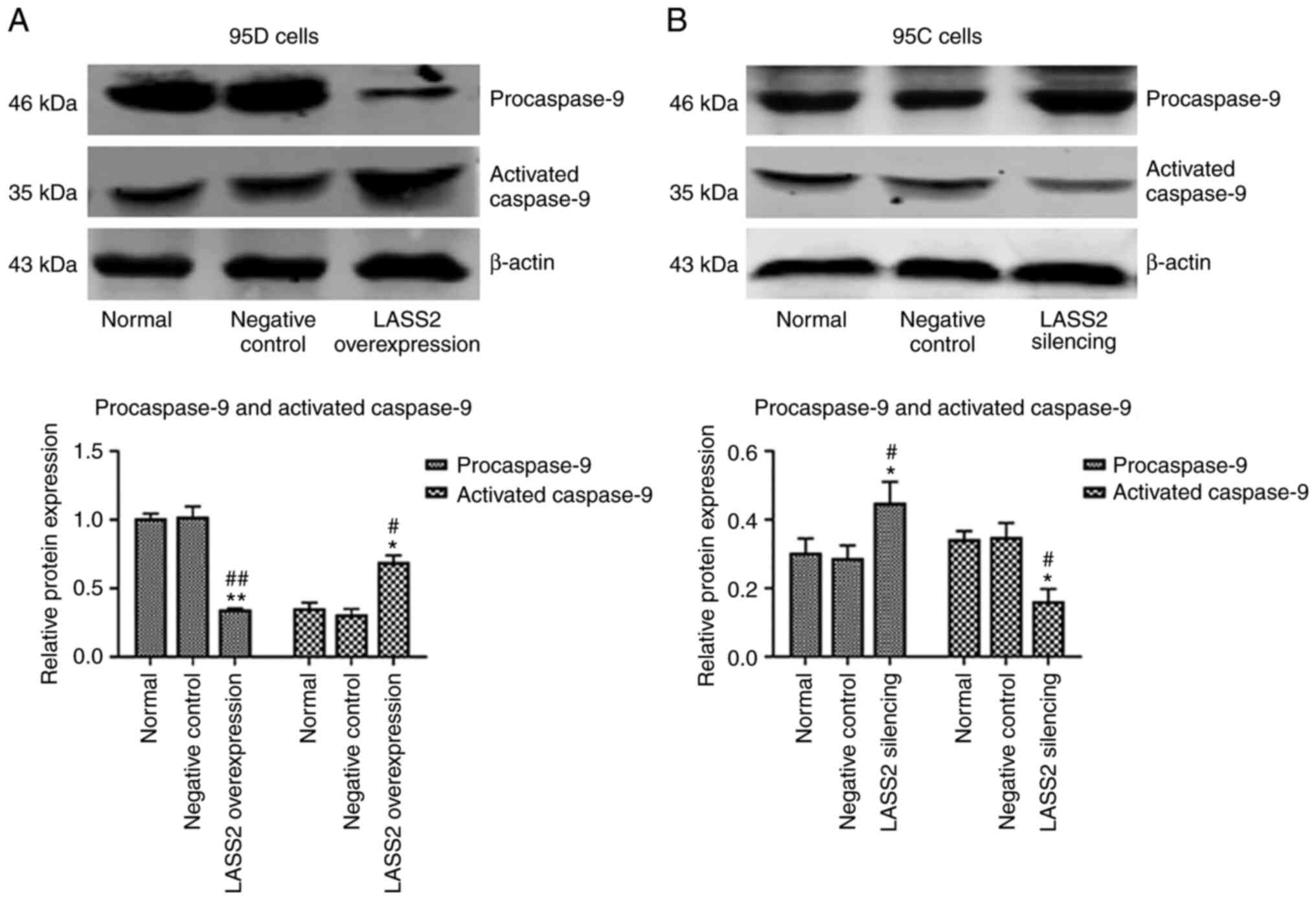
Expression of procaspase-9 and
activated caspase-9 after LASS2 overexpression and silencing
The effects of LASS2 overexpression and
silencing on procaspase-9 and activated caspase-9 expression levels
in 95D and 95C cells were detected, respectively. The results
revealed that the level of procaspase-9 protein in the LASS2
overexpression group was significantly lower than that in the
normal and negative control groups (P<0.01), while the activated
caspase-9 expression in 95D cells was significantly increased
(P<0.05; Fig. 6A). As revealed
in Fig. 6B, the expression of
procaspase-9 in 95C cells with silenced LASS2 was
significantly enhanced (P<0.05), while the activated caspase-9
was significantly reduced compared with the normal and negative
control groups (P<0.05).
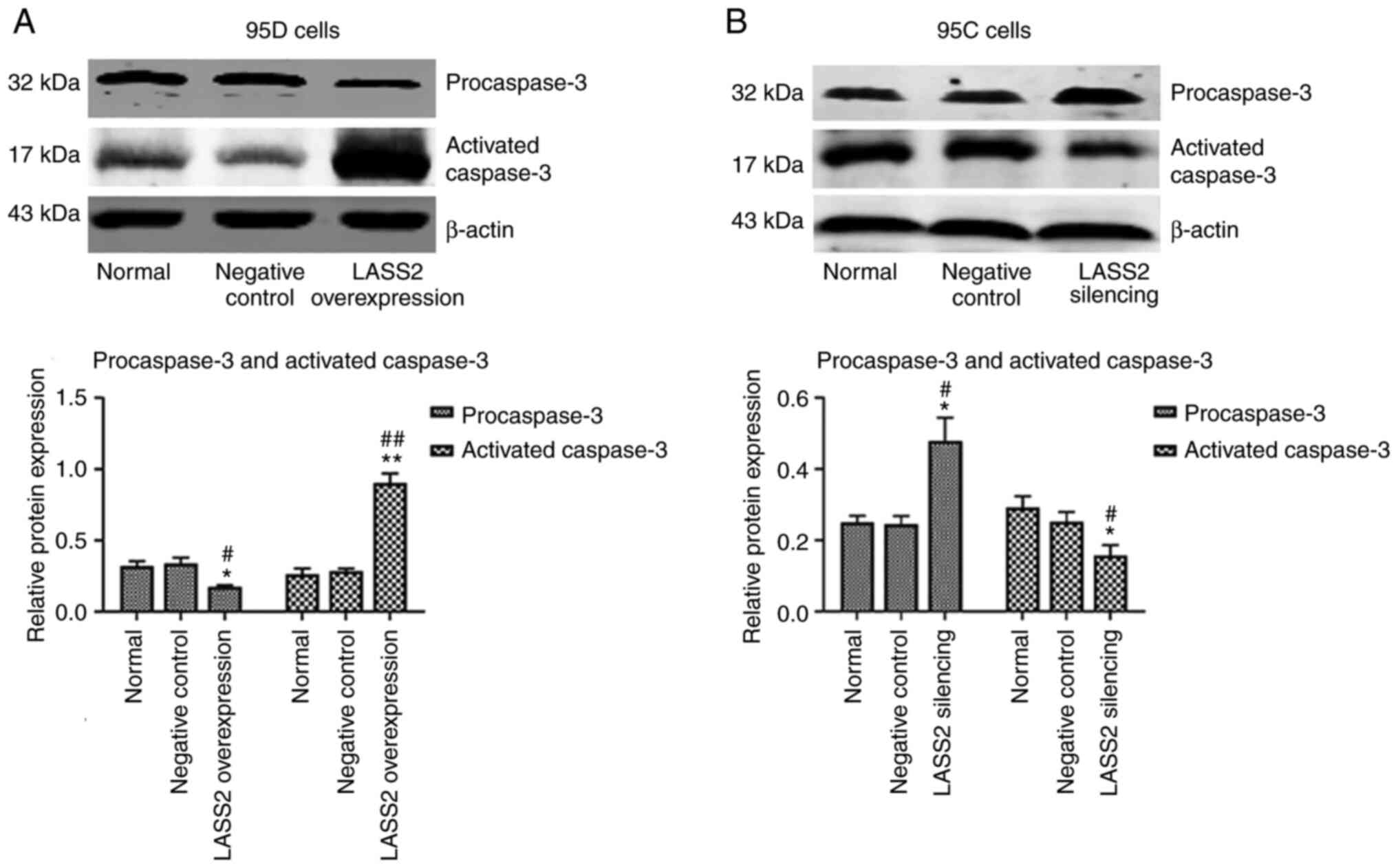
Expression of procaspase-3 and
activated caspase-3 after LASS2 overexpression and silencing
The effects of LASS2 overexpression and
silencing on procaspase-3 and activated caspase-3 expression levels
in 95D or 95C cells were detected, respectively. As revealed in
Fig. 7A, the procaspase-3 protein
level in 95D cells with LASS2 overexpression was
significantly decreased compared with the normal (P<0.05) and
negative control groups (P<0.05). Furthermore, activated
caspase-3 protein was significantly increased (P<0.01), which
indicated that the number of active fragments converted from the
procaspase-3 protein increased to participate in the process of
tumor cell apoptosis. Notably, the expression levels of
procaspase-3 and activated caspase-3 in 95C cells with silenced
LASS2 gene were the opposite of those observed in the 95D
cells (Fig. 7B).
Discussion
In the present study, the effect of LASS2
overexpression and silencing on proliferation and apoptosis of
human lung cancer 95D and 95C cells were investigated. The changes
of apoptotic-related molecular markers (cytochrome c, Bax,
Bcl-2, caspase-3 and caspase-9) were detected using western
blotting. It was confirmed that the overexpression of LASS2
in 95D cells promoted the early apoptosis and suppressed the
proliferation of tumor cells. The overexpression of LASS2
could decrease the expression level of Bcl-2 protein, increase that
of Bax/Bcl-2, promote the release of cytochrome c, and
activate downstream caspase-9 and caspase-3. Following LASS2
silencing in 95C cells, the opposite effects were observed.
LASS2 gene is a new tumor suppressor gene,
which plays an important role in inhibiting tumor metastasis. A
previous study reported that transfection of LASS2 using
Lipofectamine inhibited the invasion and metastasis of the highly
metastatic liver cancer cell line, HCCLM3 (17). Overexpression of LASS2 was
also determined to inhibit the proliferation, growth, and invasion
of the highly metastatic prostate cell line, PE-3ME8 (18). In another previous study on liver
cancer, downregulating the expression of LASS2 in liver
cancer cells, MHCC97-L, increased the extracellular hydrogen
concentration, demonstrating that inhibiting the binding of LASS2
to V-ATPase promotes the hydrogen transport of the transmembrane
proton pump and increases tumor cell invasion (19). It has been previously shown that
apoptosis plays an crucial role in the regulation of metastasis
(20). A previous in vivo
study revealed that an increase in the level of apoptosis reduced
the occurrence of metastasis in breast carcinoma cell lines
(21). Tumor cells undergo a
variety of apoptotic processes during metastasis, and their
corresponding metastases decrease (22). Therefore, alteration of induced
apoptotic genes can inhibit the metastatic ability and efficiency
of tumor cells (23).
Recently, studies have focused on LASS2
inhibition of growth, and metastasis through inhibition of V-ATPase
activity in various types of cancer such as prostate (24), and bladder cancer (25). Similarly, previous studies by the
authors revealed that the LASS2 gene inhibited V-ATPase
activity by binding the C subunit, ATP6L of V-ATPase. This resulted
in an increase of intracellular H+ concentration, and a
decrease of extracellular H+ concentration, thereby
reducing the activity of extracellular matrix metalloproteinase
which affects the invasion and migration of lung cancer cells
(26,27). It has been reported that
LASS2 overexpression significantly inhibits proliferation,
and promotes apoptosis of papillary thyroid cancer (28). Moreover, LASS2 was revealed
to inhibit proliferation and induce apoptosis in HepG2 cells by
affecting mitochondrial dynamics, the cell cycle, and the NF-κB
pathway (14). However, the
ability of LASS2 to affect the proliferation and early
apoptosis of lung cancer cells has not previously been reported.
V-ATPase is a transmembrane protein that can transport
H+ against an inverse concentration gradient to maintain
the acid homeostasis in the intracellular environment (29). It remains unclear whether
intracellular pH affects the microenvironment of tumor cells, and
causes tumor cell growth and apoptosis. V-ATPase inhibitor
treatment inhibited proliferation and increased cell death in
melanoma cells by reducing the pH gradient across the plasma
membrane, increasing the external pH and decreasing the internal pH
(30). Furthermore, it has been
demonstrated that the increase of H+ concentration in
cells affects the tumor microenvironment, which can induce the
cascade reaction of mitochondria, and stimulate apoptosis of tumor
cells (31). Thus, it was inferred
that the increase of intracellular H+ concentration and
decrease of extracellular H+ concentration mediated by
LASS2 overexpression may be responsible for proliferation
and early apoptosis of 95D cells. More experiments need to be
performed to confirm the aforementioned hypothesis.
The most important apoptotic pathway mediated by
H+ is considered to be dependent on the mitochondrial
apoptotic pathway (32), which
cannot only transmit the energy needed for life, but also regulate
the central location of cell apoptosis (33). In mitochondrion-dependent
apoptosis, the release of cytochrome c from mitochondria to
the cytoplasm, and activated caspases (such as caspase-9 and
caspase-10) are the initiators at the top of the caspase signaling
cascade (34). Additionally, Bax
and Bcl-2 as two main members of the Bcl-2 family, are the upstream
regulators of cytochrome c that affect caspase expression
(35). Bcl-2 is a membrane binding
protein that binds to Bax to inhibit opening of the mitochondrial
membrane permeability transition pore, and thereby prevent the
release of cytochrome c, while Bax elicits the opposite
effect (36). The ratio of
apoptotic protein and inhibitor of apoptotic protein (Bax/Bcl-2) in
the Bcl-2 family determines cell survival and death. In the present
study, it was determined that overexpression of LASS2
decreased Bcl-2 expression, increased the Bax/Bcl-2 ratio, promoted
the release of cytochrome c, and activated downstream
caspase-9 and caspase-3 in 95D cells.
A previous study has suggested that cytosolic
acidosis leads to apoptosis of coronary endothelial cells by
activating caspase-12 and caspase-3 (33). Furthermore, an acidic extracellular
environment increases the Bax/Bcl-2 ratio to induce apoptosis and
inhibit proliferation of BM-EPCs in tumor microenvironments
(37). Additionally, it has been
reported that V-ATPase inhibitor activates the caspase-independent
apoptotic pathway via inhibiting Bcl-2 and Bcl-xL (38). Moreover, the V-ATPase inhibitor
induces tumor cell death through rapid intracellular acidification
and activation of several caspases (30). A low pH was demonstrated to change
the Bax/Bcl-2 ratio in placental cells (39). Conversely, the Bcl-2-like protein
13 knockdown in 3T3-L1 cells, revealed a higher extracellular
acidification rate than the control groups (40). The Bcl-2 family members are
important regulators of the mitochondrial pathway of apoptosis, and
can commit a cell to programmed death by permeabilizing the outer
mitochondrial membrane, followed by initiation of the caspase
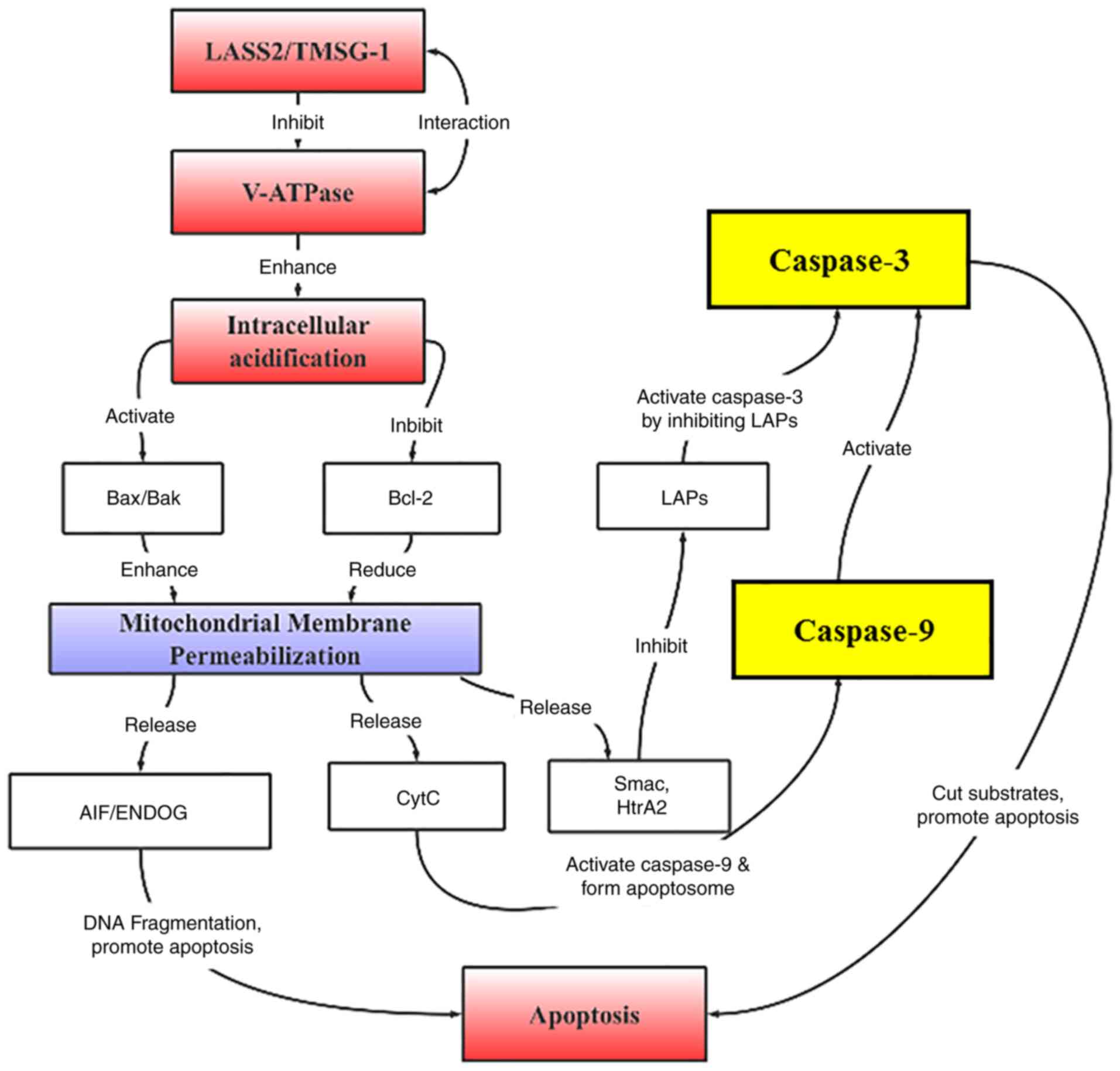
cascade (41). In light of
previous findings by the authors and the results of the present
study, it is hypothesized that LASS2 may promote the early
apoptosis of lung cancer via caspase-dependent mitochondrial
pathway (Fig. 8).
In the present study, only Annexin V (FITC) staining
was selected to detect apoptosis. Although Annexin V (FITC) can
label the entire cell population, and makes it easy to distinguish
live cells from dead cells (42),
double-stained Annexin V + PI is more suitable for suspension
cells, while the 95C and 95D cells lines were adherent cells. Thus,
the specimen collection may easily lead to cell membrane damage to
improve the false positive rate of apoptosis. In future research,
more methods of detecting apoptosis are required to mutually
confirm the experimental results. Certainly, more in vitro
and in vivo experiments are needed to confirm the results in
the future. Whether the expression of LASS2 affects the activity of
lung cancer cells by affecting other pathways is also a topic
worthy of discussion in the future. The main focus of the present
study, was the caspase-dependent apoptotic pathway. The effects of
LASS2/TMSG1 gene intervention on metastatic ability of 95D or 95C
cells will be examined in a future study. To observe the release of
cytochrome c, detection of the expression of cytochrome
c in the cytoplasm and mitochondria may be an effective
method to be studied in the future. In subsequent experiments, the
target site will also be investigated in depth.
In summary, LASS2 was revealed to enhance the
expression of Bax/Bcl-2 ratio, cytochrome c, caspase-9, and
caspase-3 to activate the caspase pathway to induce early apoptosis
of tumor cells, thereby inhibiting tumor cell proliferation.
Therefore, LASS2 may be a new therapeutic target in
anticancer gene therapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Natural Science
Foundation (grant no. 81660393), and the Inner Mongolia Natural
Science Foundation (grant no. 2019MS08115).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
YW and XX conceived and designed the research as
well as coordinated the study, helped draft the manuscript and
revised it for important intellectual content. SL and XX
participated in the acquisition of the data. LW and XX carried out
the analysis and interpretation of data. JW and HD participated in
the design of the study and performed the statistical analysis. XX
contributed to funding acquisition. YW and XX confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Gerard IE and Karen HV: Proliferation,
cell cycle and apoptosis in cancer. Nature. 411:342–348. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mcconkey BJ, Bold RJ and Termuhlen PM:
Apoptosis, cancer and cancer therapy. Surg Oncol. 6:133–142. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma C, Liu Y, Zheng J, Fang W, You J, Wang
J, Cui X and Wu B: Identification of tumor metastasisrelated gene
TMSG-1 by mRNA differential display. Sci China C Life Sci.
45:553–560. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan H, Qin WX, Huo KK, Wan DF, Yu Y, Xu
ZG, Hu QD, Gu KT, Zhou XM, Jiang HQ, et al: Cloning, mapping, and
characterization of a human homologue of the yeast longevity
assurance gene LAG1. Genomics. 77:58–64. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Xu XY, Pei F and You JF: TMSG-1 and its
roles in tumor biology. Chin J Cancer. 29:697–702. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu W, Wang L, Wang Y, Xu X, Zou P, Gong M,
Zheng J, You J, Wang H, Mei F and Pei F: A novel tumor metastasis
suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through
its homeodomain. J Cell Biochem. 114:570–583. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Teufel A, Maass T, Galle PR and Malik N:
The longevity assurance homologue of yeast lag1 (Lass) gene family
(review). Int J Mol Med. 23:135–140. 2009.PubMed/NCBI
|
|
9
|
Xu X, You J and Pei F: LASS2/TMSG1 gene
silencing promotes the invasiveness and metastatic of human
prostatic carcinoma cells through increase in vacuolar ATPase
activity. Zhonghua Bing Li Xue Za Zhi. 43:177–183. 2014.(In
Chinese). PubMed/NCBI
|
|
10
|
Fan S, Niu Y, Tan N, Wu Z, Wang Y, You H,
Ke R, Song J, Shen Q, Wang W, et al: LASS2 enhances
chemosensitivity of breast cancer by counteracting acidic tumor
microenvironment through inhibiting activity of V-ATPase proton
pump. Oncogene. 32:1682–1690. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen L, Lu X, Zeng T, Chen Y, Chen Q, Wu
W, Yan X, Cai H, Zhang Z, Shao Q and Qin W: Enhancement of
DEN-induced liver tumourigenesis in hepatocyte-specific
Lass2-knockout mice coincident with upregulation of the
TGF-β1-Smad4-PAI-1 axis. Oncol Rep. 31:885–893. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B: Expression and clinical
significance of TMSG1 in gastric cancer. China Practical Medicine.
8:1–2. 2013.
|
|
13
|
Yang Y, Yang X, Li L, Yang G, Ouyang X,
Xiang J, Zhang T and Min X: LASS2 inhibits proliferation and
induces apoptosis in HepG2 cells by affecting mitochondrial
dynamics, the cell cycle and the nuclear factor-κB pathways. Oncol
Rep. 41:3005–3014. 2019.PubMed/NCBI
|
|
14
|
Tingqi Z, Shirong L and Jingyuan W:
Expression of LASS2/TMSG1 in non small cell lung cancer and its
clinical significance. J Clin Exp Pathol. 36:323–325. 2020.
|
|
15
|
Haiqin X, Shirong L, Lixin W, Jingyuan W,
Hai L and Xiaoyan X: Expression and significance of LASS2/TMSG-1 in
human lung cancer cell lines with different metastatic
potentiality. Journal of Shanxi Medical University. 047:884–889.
2016.
|
|
16
|
Xu Haiqin LS, Weng Lixin, Wang Jingyuan,
Hai Ling and Xu Xiaoyan: Expression and significance of LASS2
/TMSG-1 in human lung cancer cell lines with different metastatic
potentiality. J Shanxi Med Univ. 47:884–889. 2016.
|
|
17
|
Chen SH, Bubb MR, Yarmola EG, Zuo J, Jiang
J, Lee BS, Lu M, Gluck SL, Hurst IR and Holliday LS: Vacuolar
H+-ATPase binding to microfilaments: Regulation in response to
phosphatidylinositol 3-kinase activity and detailed
characterization of the actin-binding site in subunit B. J Biol
Chem. 279:7988–7998. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Su J, You J, Zhen J, Cui X and Fang W:
Studies of tumor metastasis suppressor gene TMSG-1 inhibited
proliferation and invasion of prostate cancer cell line PC-3M-1E8
in vitro. Zhonghua Zhong Liu Za Zhi. 30:404–407. 2008.(In Chinese).
PubMed/NCBI
|
|
19
|
Tang N, You H, Jin J, Yu B and Qin W:
Small interfering RNA targeting LASS2 gene enhances invasion
capacity of hepatocellular carcinoma cells. Tumor. 29:399–403.
2009.
|
|
20
|
Mehlen P and Puisieux A: Metastasis: A
question of life or death. Nat Rev Cancer. 6:449–458. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wong NC, Mueller BM, Barbas CF, Ruminski
P, Quaranta V, Lin EC and Smith JW: Alphav integrins mediate
adhesion and migration of breast carcinoma cell lines. Clin Exp
Metastasis. 16:50–61. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Townson JL, Naumov GN and Chambers AF: The
role of apoptosis in tumor progression and metastasis. Curr Mol
Med. 3:631–642. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Márquez-Jurado S, Díaz-Colunga J, das
Neves RP, Martinez-Lorente A, Almazán F, Guantes R and Iborra FJ:
Mitochondrial levels determine variability in cell death by
modulating apoptotic gene expression. Nat Commun. 9:3892018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zou P, Yang Y, Xu X, Liu B, Mei F, You J,
Liu Q and Pei F: Silencing of vacuolar ATPase c subunit ATP6V0C
inhibits the invasion of prostate cancer cells through a
LASS2/TMSG1-independent manner. Oncol Rep. 39:298–306.
2018.PubMed/NCBI
|
|
25
|
Wang H, Zuo Y, Ding M, Ke C, Yan R, Zhan
H, Liu J, Wang W, Li N and Wang J: LASS2 inhibits growth and
invasion of bladder cancer by regulating ATPase activity. Oncol
Lett. 13:661–668. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu X YR, Li X, Yu H and Li S: Effect of
LASS2/TMSG-1 overexpression on the invasion and migration of 95D
human pulmonary carcinoma cell and its mechanism. Chinese Journal
of Clinical and Experimental Pathology. 32:901–907. 2016.
|
|
27
|
Zuo H LX, Li S, Yun F and Xu X: Effect of
LASS2/TMSG on the invation and migration of 95C human pulmonary
carcinoma cell. Chinese Journal of Cancer Prevention and Treatment.
22:1865–1873. 2015.
|
|
28
|
Zeng F, Huang L, Cheng X, Yang X, Li T,
Feng G, Tang Y and Yang Y: Overexpression of LASS2 inhibits
proliferation and causes G0/G1 cell cycle arrest in papillary
thyroid cancer. Cancer Cell Int. 18:1512018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beyenbach KW and Wieczorek H: The V-type
H+ ATPase: Molecular structure and function, physiological roles
and regulation. J Exp Biol. 209:577–589. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
De Milito A, Canese R, Marino ML, Borghi
M, Iero M, Villa A, Venturi G, Lozupone F, Iessi E, Logozzi M, et
al: pH-dependent antitumor activity of proton pump inhibitors
against human melanoma is mediated by inhibition of tumor acidity.
Int J Cancer. 127:207–219. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumar S, Kasseckert S, Kostin S, Abdallah
Y, Schafer C, Kaminski A, Reusch HP, Piper HM, Steinhoff G and
Ladilov Y: Ischemic acidosis causes apoptosis in coronary
endothelial cells through activation of caspase-12. Cardiovasc Res.
73:172–180. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yang T, Li MH, Liu J, Huang N, Li N, Liu
SN, Liu Y, Zhang T, Zou Q and Li H: Benzimidazole derivative,
BMT-1, induces apoptosis in multiple myeloma cells via a
mitochondrial-mediated pathway involving H+/K+-ATPase inhibition.
Oncol Rep. 31:2743–2750. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bock FJ and Tait SW: Mitochondria as
multifaceted regulators of cell death. Nat Rev Mol Cell Biol.
21:85–100. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen M and Wang J: Initiator caspases in
apoptosis signaling pathways. Apoptosis. 7:313–319. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Rosse T, Olivier R, Monney L, Rager M,
Conus S, Fellay I, Jansen B and Borner C: Bcl-2 prolongs cell
survival after Bax-induced release of cytochrome c. Nature.
391:496–499. 1998. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Oltvai ZN, Milliman CL and Korsmeyer SJ:
Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that
accelerates programmed cell death. Cell. 74:609–619. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang S, He P, Xu D, Li J, Peng X and Tang
Y: Acidic stress induces apoptosis and inhibits angiogenesis in
human bone marrow-derived endothelial progenitor cells. Oncol Lett.
14:5695–5702. 2017.PubMed/NCBI
|
|
38
|
Sasazawa Y, Futamura Y, Tashiro E and
Imoto M: Vacuolar H+-ATPase inhibitors overcome Bcl-xL-mediated
chemoresistance through restoration of a caspase-independent
apoptotic pathway. Cancer Sci. 100:1460–1467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Pérez-Pérez A, Toro A, Vilariño-Garcia T,
Guadix P, Maymó J, Dueñas JL, Varone C and Sánchez-Margalet V:
Leptin protects placental cells from apoptosis induced by acidic
stress. Cell Tissue Res. 375:733–742. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujiwara M, Tian L, Le PT, DeMambro VE,
Becker KA, Rosen CJ and Guntur AR: The mitophagy receptor
Bcl-2-like protein 13 stimulates adipogenesis by regulating
mitochondrial oxidative phosphorylation and apoptosis in mice. J
Biol Chem. 294:12683–12694. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Edlich F: BCL-2 proteins and apoptosis:
Recent insights and unknowns. Biochem Biophy Res Commun. 500:26–34.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wallberg F, Tenev T and Meier P: Analysis
of apoptosis and necroptosis by fluorescence-activated cell
sorting. Cold Spring Harbor Protoc 1. pdb.prot087387. 2016.
View Article : Google Scholar : PubMed/NCBI
|