Heat shock proteins (HSPs), also known as molecular
chaperones, were discovered to be upregulated when cells were
exposed to conditions of stress, including heat shock, chemical
factors, and other pathological alterations (1). The HSP90 family, which are highly
conserved molecules, are involved in the regulation of the folding
of newly synthesized proteins, as well as in correcting incorrectly
folded proteins and impeding the aggregation of incorrectly folded
proteins (2). The HSP90 chaperone
machinery plays a crucial role in protecting overexpressed and
mutated proteins from misfolding and inducing their degradation
where appropriate (3).
Interactions between HSP90 and client proteins are essential
processes in tumor survival, proliferation and migration (4,5). In
total, >400 client proteins have been identified, with these
proteins being involved in a wide range of important biological
activities, including signaling cascades, DNA damage repair,
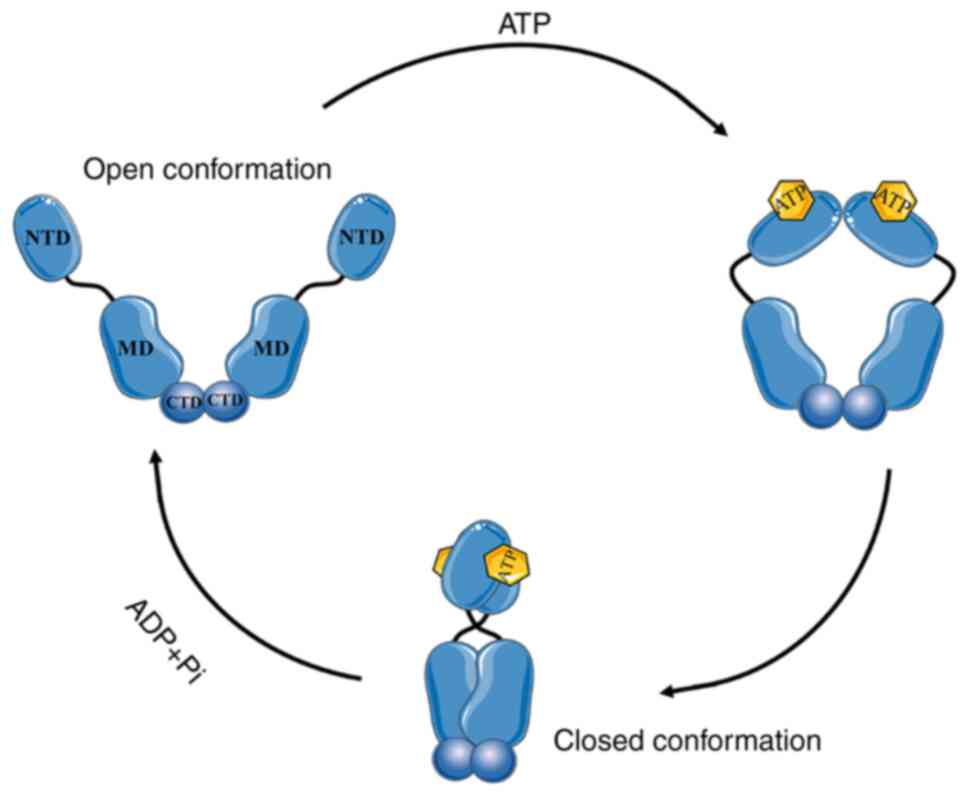
protein transportation and hormone receptor activation (6). HSP90 consists of three domains: The
N-terminal domain (NTD), the C-terminal domain (CTD) and the middle
domain (MD). The NTD has an ATP binding site and a client protein
binding site, the MD is vital for hydrolysis of ATP to ADP, and the
CTD contains one site for protein dimerization and another for
calmodulin binding; a charged linker domain connects the NTD to MD,
contributing to maintaining the function, interaction and
adaptability of the HSP90 chaperone (7–12).
HSP90 activates and facilitates the activities of its client
proteins through the ATPase cycle, which is completed by
dimerization (13). The HSP90
family includes four isoforms that are present in different
locations in cells. HSP90α and HSP90β are present in the cytoplasm
and nucleus, GRP94 is present in the endoplasmic reticulum, and
TRAP-1 is primarily located in the mitochondrion, but is also
present in the endoplasmic reticulum (1,14–16).
All four isoforms share a high degree of sequence homology in their
N-termini; thus, the ATP binding sites present in their N-termini
are interesting target locations, and considerable research has
been devoted to disrupting the molecular chaperone function through
targeting this domain (7,17). A total of 18 inhibitors of HSP90
have been identified and have entered clinical trials (18,19).
These inhibitors can be divided into five categories based on
chemical structure: i) Natural products and their derivatives; ii)
purine-based; iii) benzamide; iv) resorcinol-containing; and v)
miscellaneous. None of these inhibitors are currently used as
clinical treatments, due to their dose-limited toxicity and poor
bioavailability (20). CTD
inhibitors and the isoform-selective inhibitors that specifically
bind to HSP90α, HSP90β, GRP94, or TRAP-1 have also been developed,
attempting to improve their antitumor effects. In the present
review, the present armamentarium of HSP90 inhibitors as a
monotherapy in cancer management and the potential combination
therapies of HSP90 inhibitors are discussed, along with other
traditional clinical therapies, including chemotherapies, targeted
agents, immunotherapy, and radiotherapy. Furthermore, prospective
candidates for HSP90-targeting anti-neoplastic treatment are
proposed.
HSP90 is a crucial chaperone protein that functions
to maintain the correct folding of client proteins. It regulates
protein folding and degradation, several cell signaling pathways,
cell proliferation and survival, and cell apoptosis via interacting
with co-chaperones and client proteins (1). HSP90 consists of three distinct
domains: i) The NTD, which includes an ATP binding site and the
client protein binding site; ii) an MD that is responsible for the
hydrolysis of ATP to ADP; and iii) a CTD, which is comprised of a
protein dimerization site and a calmodulin-binding site; there is
also a linker domain connecting the NTD to the MD, contributing to
the maintenance of the functions, interactions, and adaptability of
the HSP90 chaperone (7–12). The NTD, which is also referred to
as the nucleotide-binding site, is necessary for the affinity
between client proteins and HSP90, and for the chaperone cycle, due
to the presence of the binding site for ATP which are crucial for
the HSP90 ATPase activity (11,21).
Thus, the NTD is considered a critical target in the development of
inhibitors. The CTD is primarily involved in the dimerization of
HSP90. There is also an ATP binding site that only opens when the
ATP-binding site in the NTD is unavailable, making the C-terminal
an allosteric regulator of the N-terminal ATPase activity (22,23).
There are special motifs, including MEEVD or KDEL on CTD, which
differ according to the different isoform types and the cellular
localization (24,25).
The HSP90 family includes four isoforms that are
present at different locations in a cell. HSP90α and HSP90β are
present in the cytoplasm and the nucleus, GRP94 is present in the
endoplasmic reticulum, and TRAP-1 is primarily located in the
mitochondrion, also being present in the endoplasmic reticulum
(1,14–16).
HSP90α and HSP90β are the most widely expressed isoforms and are
primarily located in the cytoplasm (32). They are involved in cell signaling,
energy metabolism and cell viability (1). Additionally, extracellular-secreted
HSP90α (eHSP90α) has been demonstrated to play crucial roles in the
invasion and migration of several types of cancer in vitro
and in vivo, suggesting that blocking eHSP90α is a rational
approach for cancer management (33–36).
GRP94 functions as a ‘quality supervisor’ of secreted proteins and
membrane proteins. GRP94 recognizes and binds to misfolded
proteins, attempting to correct them, preventing their degradation
in the cytoplasm (15,37). The inhibition of GRP94 results in
the accumulation of misfolded proteins, which translocate to the
cytoplasm and are marked for degradation. TRAP-1 is necessary for
mitochondrial homeostasis, and has been revealed to be involved in
regulating the mitochondrial redox state and curbing energy
metabolism (38–40). All four isoforms share a high
degree of sequence similarity in the N-terminus, thus the ATP
binding sites on the N-terminus are important targeting spots that
researchers have focused on to disrupt molecular chaperone function
(7,17).
HSP90 is a key mediator of >200 proteins, termed
‘HSP90 client proteins’. HSP90 facilitates protein-protein
interactions and is involved in cell signaling and the responses to
stress (27,41). Cancer cells use this mechanism to
protect the mutated and overexpressed oncoproteins from misfolding
and being degraded to ensure their survival and proliferation
(42–44). Several HSP90 client proteins
participate in signaling and other vital pathways that are of
utmost importance for malignancy (45). These client proteins include
receptor tyrosine kinases (HER2, EGFR, IGF-1R and MET), signaling
proteins (AKT and SRC), transcription factors (HIF-1 and TP53) and
cell cycle regulatory proteins (CDK4 and CDK6) (46–49).
There are several co-chaperones, including p50/Cdc37,
HSP90-organizing protein (HOP/Sti1, p23, Aha1 and HSP70), and a
variety of immunophilins that require HSP90 to function (46,50,51).
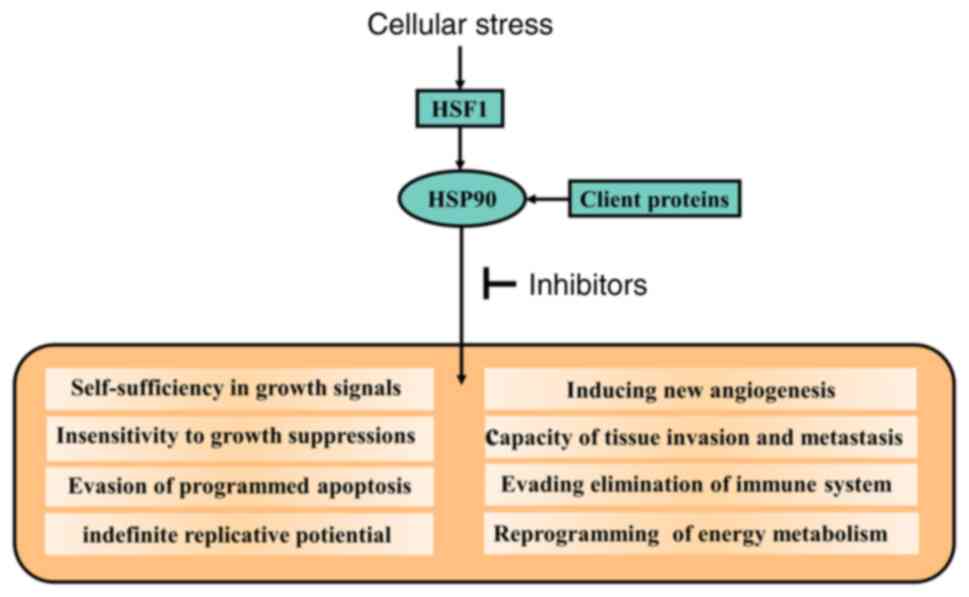
The HSP90 chaperone mechanisms and HSP90-client interactions are
involved in establishing the acquired capabilities of cancer cells
by regulating HSP90-dependent signal transduction (Fig. 2) (52). Thus, HSP90 inhibitors can
effectively suppress the tumor-promoting signaling pathways and
interrupt the functions of HSP90 in tumor cells.
HSP90 chaperone mechanisms in cancer cells differ
considerably from those in normal cells (53). Firstly, in cancer cells, the rapid
proliferation rate and reduced control in protein synthesis quality
result in increased and constant cellular stress. In response to
this situation, heat shock factor 1 (HSF1) is released and forms a
trimer. The trimer translocates to the nucleus and upregulates
HSP90 expression. Actually, increased levels of HSP90 improve the
chances of cell survival and in maintaining function during
tumorigenesis (3). Studies have
demonstrated that the expression of HSP90 is 2- to 10-fold higher
in cancer cells than in normal cells, and the overexpression of
HSP90 has been found in numerous tumor cells including breast,
lung, colorectal, ovarian, endometrial, esophageal, bone, urinary
and prostate cancer (46,54–56).
It has also been revealed that the high expression of HSP90 is
associated with a poor prognosis in clinical treatment. HSP90
expression is a determining factor in the survival outcomes in
hormone and protein kinase-dependent breast cancer (57). A previous study also revealed the
association between a high level of HSP90 expression and a less
favorable response to anti-neoplastic treatment (58). In another study, biostatisticians
analyzed HSP90 expression levels from >4,000 breast cancer
patients from 23 databases and overall survival data from >1,000
patients. It was concluded that upregulation of HSP90 and HSF1 was
observed in breast cancer, resulting in a more aggressive profile
of cancer and a poorer prognosis (59).
HSP90-client interactions and post-translational
modifications also differ between tumor and healthy cells (4,5).
Mutated oncoproteins expressed in cancer cells are involved in the
uncontrolled growth and proliferation of cancer cells. The majority
of these oncoproteins are also client proteins of HSP90 (60). Of note, >400 client proteins
have been discovered to be involved in cell signaling by binding to
HSP90 (6). Additionally,
post-translational modifications regulate HSP90-client interactions
(53). HSP90 inhibitors display a
high affinity to HSP90 in tumor cells. Inhibitors can interrupt the
function of HSP90 in cancer cells, while at the same dose, HSP90 is
not inhibited in normal cells (53). The study by Kamal et al
(61) revealed that HSP90 in
cancer cells exists as a multichaperone complex. Complexes can
promote the ATPase activity of HSP90 and promote the affinity of
HSP90 to its inhibitor 100-fold. Moulick et al (62) also stated that there are
biologically distinct HSP90 complexes present in tumor cells. Those
HSP90 functions differ from its physiological roles and ensure
tumor cell survival, by interacting with oncogenic proteins;
however, the majority of HSP90 present in tumor cells is similar to
that in normal cells. Notably, the higher the ATPase activity of
HSP90 in tumors, the better the affinity is to HSP90 inhibitors,
which is evidence supporting that transformation and malignancy do
not just center around overexpression of HSP90 (61).
Cell surface HSP90 expression is considerably higher
in tumor cells than in normal cells, highlighting HSP90 as a
prospective target in anti-neoplastic treatment (57,63).
To adapt to the reduced ATP-extracellular environment,
extracellular HSP90 can function independently of ATP (64). Due to the consistent environmental
stress in tumor cells, HSP90 is secreted consecutively, while HSP90
in healthy cells is secreted only under conditions of stress
(65). A study revealed that
blocking or neutralizing the secretion of HSP90 can inhibit the
invasion and migration of cancer (35,66).
A clinical study revealed that plasma concentrations of HSP90 are
positively associated with tumor malignancy in cancer patients
(33). The inhibition of
extracellular HSP90 can be an attractive approach for preventing
malignant tumor progression.
Inhibitors targeting the N-terminal domain.
Significant attention has been paid to discovering inhibitors
targeting the NTD of HSP90 in the last few decades (20). At present, there are 18 inhibitors
of HSP90 that have been designed and have entered clinical trials
(18,19). These inhibitors can be divided into
five categories based on their chemical structures: i) natural
products and their derivatives; ii) purine-based; iii) benzamide;
iv) resorcinol-containing; and v) miscellaneous compounds (Table I).
Geldanamycin (GDA) was discovered as an HSP90
inhibitor in the early 1990s; its binding to the NTD interrupts
ATPase activity and blocks the binding of the N-domain to client
proteins, resulting in the degradation of oncoproteins and thus, in
the abrogation of several cellular processes (67). It is worth mentioning that there is
a reactive quinone in GDA which can also induce cell death
independently of HSP90 inhibition (68). The toxicity and instability of GDA
in vivo indicate that it has limited use in clinical trials
(69). It has been demonstrated
that the C-17 position of GDA is crucial concerning its function
and toxicity. Based on this discovery, derivatives of GDA,
including tanespimycin (17-allylamino-17-demethoxygeldanamycin;
17-AAG), retaspimycin hydrochloride
(17-allylamino-demethoxygeldanamycin; 17-AAGH2; IPI-504),
17-amino-17-demethoxygeldanamycin (17-AG; IPI-493) and
17-dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG) were
designed to correct the deficiency in HSP90-targeted treatment
(67). 17-AAG has entered and has
been evaluated in several phase II trials, where it has been
demonstrated to exhibit a short-lived anti-HSP90 biological
activity, and a poor solubility in water, resulting in poor
bioavailability; these factors have limited the performance of
further trials and other structural modifications (70–72).
17-AAGH2 was synthesized to improve the metabolic profile by
eliminating the requirement for reduction (73). It is administered intravenously and
is prepared by reducing the quinone portion of 17-AAG to
hydroquinone using sodium hydrosulfite, forming hydrochloride salt
(74). 17-AAGH2 monotherapy has
exhibited good tolerance and antitumor activity in patients with
gastrointestinal stromal tumors and in non-small cell lung cancer
(NSCLC) (74,75). 17-AG (IPI-493) is a semi-synthetic
benzoquinone ansamycin derivative, developed into an oral
formulation that shows increased efficacy regarding HSP90
inhibition (76). 17-DMAG is a
semi-synthetic derivative of geldanamycin. Compared with 17-AAG,
17-DMAG exhibits improved water solubility, bioavailability,
reduced metabolism and antitumor efficacy (77). However, the majority of trials on
this agent were postponed or terminated, and none of those
derivatives entered phase III trials.
Purine-based inhibitors have a purine (or
purine-like) scaffold with amine and aryl substituents. PU-3 is the
first reported fully synthetic purine-based inhibitor of HSP90
(69). It displays antitumor
efficacy by interrupting the proliferation and differentiation of
breast cancer cells. Researchers designed derivatives by enhancing
the purine chemical scaffold; in doing so, they synthesized new
purine or purine-like compounds: BIIB021, BIIB028, MPC-3100, PU-H71
and Debio093 (78). BIIB021 alters
the metabolic activity of GIST and improves the response rate in
gastrointestinal stromal tumors. Additionally, it exhibits oral
bioavailability and is well-tolerated in patients with no
substantial hepatotoxicity observed. BIIB028 is an optimization of
BIIB021, as the antitumor efficacy of BIIB021 was achieved with
substantially high doses and the chemicals required for synthesis
are toxic. Researchers found that the N-7 position on the purine
scaffold is an optimal site for modification, selecting BIIB028 as
the optimal candidate for a series of alkynol analogs. A clinical
trial confirmed HSP90 inhibition and objective responses of BIIB028
in refractory metastatic or locally advanced solid tumors (79–82).
MCP-3100 and Debio0932 also entered three trials but failed to move
beyond phase II (69). PU-H71 has
been reported to downregulate the expression of the oncoproteins
involved in cell proliferation and cell apoptosis, whilst promoting
the degradation of oncoproteins without exhibiting hematological,
renal, or hepatic toxicity. These anticancer effects were also
observed in triple-negative breast cancer cells, B-cell lymphomas
and Ewing sarcoma. PU-H71 is now listed in six clinical trials
(NCT03166085; NCT03373877; NCT01581541), and three of these
(NCT03935555; NCT01393509; NCT01269593) are still active or
recruiting, indicating that PU-H71 is the most promising
purine-based inhibitor of HSP90 (83–85).
The only benzamide inhibitor, SNX-5422, was
discovered in the pyrazole and was separated and purified using an
ATP-affinity column. It has been investigated in multiple clinical
trials demonstrating that SNX-5422 exhibited antiproliferative
potency at low nanomolar concentrations and high oral
bioavailability (86,87).
A resorcinol-containing inhibitor was identified
using high-throughput screening (88). To optimize the solubility of this
compound, researchers added substituents to it and synthesized the
novel compound AUY922 (luminespib) (89), which demonstrated biological
efficacy against tumor proliferation, invasion, and metastasis
in vivo (90,91). STA-9090 (ganetespib) was
synthesized by modifying the resorcinol scaffold. STA-9090
displayed improved performance in downregulating the expression of
oncoproteins and the associated pathways than AUY922. STA-9090,
having high biological activity and more optimal binding efficacy
to HSP90, was discovered as part of a fragment-based drug design
approach, combining NMR and X-ray crystallography (92). Notably, STA-9090 has entered nearly
40 trials and has thus far progressed to phase III trials, and it
has been revealed to exhibit superior clinical potential regarding
HSP90 inhibition (93). KW-2478
was discovered by Nakashima et al (94). The anti-proliferative and cell
apoptosis-inducing effects were observed in KW-2478-treated tumor
cells. KW-2478 interfered with the interactions between HSP90 and
client proteins, such as fibroblast growth factor receptor3,
proto-oncogene c-Maf, and cyclin D1, and depleted the
transcriptional kinase Cdk9 and the translational inhibitor
phosphorylated 4E-BP1 (94–96).
KW-2478 was found to be well tolerated and displayed no
dose-limiting toxicity in clinical trials (97).
Other HSP90 inhibitors have been identified,
targeting the NTD. However, they are not classified into any of the
four categories discussed above or do not present with publicly
available structures. These compounds include FW-04-806, CH5164840
and XL888. FW-04-806 interferes with the interaction between HSP90
and Cdc37, disassociating the HSP90-Cdc37 complex compound and
promoting the degradation of the associated client proteins
(98). A decrease in cell
viability and proliferation and an increase in programmed cell
death were observed in HER2-positive breast cancer cells treated
with FW-04-806 (98,99). CH5164840 is a macrocyclic compound
bearing a 2-amino-6-arylpyrimidine moiety; it has been shown to
exhibit high oral bioavailability in mice (F=70.8%) and potential
antitumor efficacy in colorectal cancer (CRC) (100). XL888 was designed by modifying
5-position amine substituent on the
4-carboxamido-2-methylbenzamide, resulting in the reduction of
HSP90 client protein expression in vitro, and in presenting
with antitumor effects in vivo (101).
There are three domains in HSP90, the C-terminus,
N-terminus, and the middle domain. As discussed above, there is
more research focus on the NTD domain of HSP90. Several compounds
have been demonstrated as potential candidates for HSP90 inhibitors
that target NTD. While HSF1 has been demonstrated to be a key
factor of resistance to N-terminal inhibitors, it binds to HSP90
and forms homotrimers after N-terminal inhibition, thus inducing
the pro-survival heat shock response (102). To overcome this ‘resistance
machinery’, inhibitors targeting the CTD have been investigated.
NTD inhibitors block HSP90 activity and several have entered
clinical trials; however, there is also an ATP binding region in
CTD (103–105). This indicates that both the C-
and N-terminal regions function as co-chaperones and client
proteins and subsequently regulate the associated biological
activities; however, the mechanisms involved differ notably
(106). N-terminus inhibitors
competitively bind to ATP binding sites to abrogate ATPase
activity, whereas C-terminus inhibitors disrupt the activities of
co-chaperones containing TPR motifs, resulting in aberrant
chaperone function. C-terminus inhibitors are emerging candidates
for the development of novel cancer chemotherapeutics (27,107,108). Novobiocin is an inhibitor of the
DNA gyrase ATP-binding site, and is able to interrupt HSP90 protein
folding machinery, leading to the hydrolysis of client proteins and
the induction of the apoptosis of cancer cells. Novobiocin can
selectively restrain the open-conformation HSP90 and block the
progression of the open and close conformation cycle through a
cascade of cumulative dynamic changes (105,107,109,110). KU-32 and KU-569 are derivatives
of novobiocin. They bind to the C-terminus and lead to a structural
shift in the chaperone, which can simultaneously enhance ATP
binding and promote ATPase activity (109,111,112). LB76 is the first C-terminus
inhibitor designed de novo, by interrupting co-chaperone
binding. LB76 is derived from an HSP90 co-chaperone and selectively
pulls down HSP90 from cell lysates. Further investigation confirmed
that the identity of the binding region was a MEEVD motif in the
C-terminus. LB76 restrains the protein-folding function of HSP90,
thus blocking protein-protein interactions between HSP90 and
co-chaperones (113,114). A de novo delivery system
for LB76 produced by polymer nanoparticles and functioning by
delivering LB76 into cells and releasing them in a pH-responsive
manner, displayed improved inhibitory activity (115). The non-toxic profile and
significant HSP90 inhibitory activity make LB70 a promising
cancer-targeting candidate therapeutic. KU711 and KU757 target the
C-domain of HSP90 in tumor-initiating cells that have stem
cell-like properties. They exhibit anti-malignant potential in
breast cancer stem cells and head and neck squamous cell carcinoma
cancer stem cells by inhibiting invasion, EMT, and self-renewal
(116,117). KU711 has been demonstrated to
selectively inhibit HSP90 function in thyroid cancer stem cells and
induce a potent antitumor response to cell growth, invasion, and
migration (118). KU135, compared
with the N-terminal inhibitor 17-AAG, displayed more potent
anti-proliferative effects in human leukemic cells and most
melanoma cells. 17-AAG functions by inducing cell cycle arrest
whereas KU135 induces cell cycle arrest and cell apoptosis. The
dual effects on tumor cells are in line with the expected effects
of antitumor agents (119,120).
KU363 was synthesized at the University of Kansas-Lawrence, and
KU135 exhibited antiproliferative effects in different cancer
models of bronchioalveolar carcinoma and epithelial lung carcinoma
(121). It was demonstrated to
induce cell apoptosis, and limit cell viability and proliferation
in head and neck squamous cell carcinoma cells in vitro and
in vivo (122). NCT-50 is
a C-terminal-targeting hybrid compound of novobiocin and deguelin.
It demonstrated a significant effect on inhibiting the viability,
colony-forming ability, and angiogenic ability of NSCLC cells.
NCT-50 overcomes the neurotoxicity observed with deguelin and
displays more significant pro-apoptotic efficacy on NSCLC cells
than either deguelin or novobiocin (123).
As mentioned above, the mammalian HSP90 family is
comprised of four isoforms, HSP90α, HSP90β, GRP94 and TRAP1. Among
these, HSP90α and HSP90β are the most abundantly expressed isoforms
and are primarily located in the cytoplasm (32). The extracellular form of HSP90α is
key for cancer cell invasion and migration (124). GRP94 is present in the
endoplasmic reticulum, and functions in ‘quality control’ of a
small subset of proteins. It recognizes and binds to misfolded
proteins, correcting, and refolding said proteins. GRP94 inhibitors
block this process, resulting in the translocation of these
misfolded proteins to the cytoplasm and hence their subsequent
degradation (37). In addition,
there are Ca2+ binding sites on GRP94 which play vital
roles in tumor processes (125).
TRAP1, located in the mitochondria, plays a crucial role in
mitochondrial homeostasis. It is involved in the regulation of the
organelle's redox state and in the disruption to the energy
metabolism of cancer cells (1,38).
Developing isoform-selective inhibitors allows for the alleviation
of the challenges faced with the use of ‘pan-inhibitors’,
permitting more targeted and personalized therapies.
HSP90α and HSP90β. For cancer cells, HSP90α- and
HSP90β-selective inhibitors are optimal choices considering that
they are the most abundantly expressed isoforms and they function
by interacting with oncogenic client proteins (1,32).
SNX-0723 is a promising candidate, due to its central nervous
system permeability and selectivity to HSP90α and HSP90β both. The
selectivity of SNX-0723 to cytosolic HSP90 isoforms is ~100-fold
vs. GRP94 and ~300-fold vs. TRAP1. Additionally, its affinity to
both HSP90α and HSP90β is similar (126,127). Previously, researchers identified
a benzolactam-hydroindolone derivative of SNX-0723 that contains a
cyclopentyl substituent, compound 31, that exhibited similar
pharmacokinetics as SNX-0723, although with a reduced cellular
toxicity and ~1,000-fold affinity for HSP90α and HSP90β vs. GRP94
and TRAP1 (128). TAS-116 was the
first reported cytosolic-isoform selective inhibitor to enter
clinical trials. In human xenograft mouse models, TAS-116 was found
to induce HSP90 client protein degradation and reduce tumor burden
(129). In another study, a total
of 61 patients with advanced solid tumors participated in a
clinical trial to identify the safety, maximum tolerated dose, and
overall response rates of TAS-116 in monotherapy intervention
(130). TAS-116 exhibited
antitumor activity with acceptable adverse reactions was
acceptable, suggesting further development of this HSP90
inhibitor.
TAS-116 had an acceptable safety profile with
notable antitumor activity, supporting the further development of
this HSP90 inhibitor. GRP94-selective inhibitors were the first
isoform-selective inhibitors being of utmost scientific interest.
Using an assay to determine the differences in the N-terminal
ATP-binding site between GRP94 and HSP90, it was eventually
revealed that GRP94 could be selectively targeted (131). The GRP94-selective inhibitor,
BnIm, was developed by replacing the cis-amide with a bioisosteric
imidazole ring that mimicked the amide heteroatoms. No degradation
of HSP90α/HSP90β client proteins was observed in BnIm-treated
cancer cells, indicating its selectivity towards GRP94 vs. the
cytosolic isoforms. Additionally, no cytotoxic effects were
observed (132). In addition,
researchers established the structure-activity relationships of the
BnIm scaffold, and further enhancements were made by replacing the
imidazole ring with other heterocycles and by modifying the benzyl
appendage. This second generation of compounds demonstrated a
two-fold higher affinity than BnIm and a 32-fold higher selectivity
for GRP94 than HSP90α (133).
PU-WS13 is a purine derivative that disrupts the cell surface
oncoprotein HER2. This compound substantially decreases HER2 levels
in overexpressing HER2 breast cancer cells (134). Compound 54 has a phenyl ring at
the meta-position, an isopropyl appendage at the fourth carbon of
the benzene ring, and a cyclohexanol with an amine linker at the
ortho-position of the benzamide scaffold, exhibiting >1,000-fold
affinity to GRP94 than to HSP90α (135).
Pan-inhibitors of HSP90 have demonstrated negative
efficacy with regard to its TRAP1 inhibitory effect due to its poor
mitochondrial permeability, where TRAP1 is located. Shepherdin is
the first peptidomimetic with the ability to permeate into the
mitochondria and target TRAP1 (136). There is a highly positively
charged moiety at the N-terminus of shepherdin which can interrupt
mitochondrial integrity, making it swell and subsequently release
cytochrome c (137). For
mitochondrial penetration, investigators used the mitochondrial
permeating TPP moiety to replace the corresponding ammonium group
on PU-H71 to develop the novel new compound SMTIN-P01. SMTIN-P01
induced membrane depolarization and cytochrome c release, resulting
in cytotoxicity to cancer cells (138). DN401 is the most selective TRAP1
inhibitor developed to date. Its selectivity for TRAP1 is
>9-fold compared with the other isoforms. It was developed by
modifying the pyridine ring to a pyrazolopyrimidine scaffold with a
pyridinyl appendage (139).
To date, although a few HSP90 inhibitors have been
produced that demonstrate selective affinity to HSP90 and disrupt
its biological activities, no HSP90 inhibitor has been applied in
clinical cancer therapies, which indicates that the full prospects
of HSP90 inhibitors have not been administered as a monotherapy in
cancer treatment. The inhibitors that have entered clinical trials
have had to be postponed or terminated due to their moderate
effects. More importantly, resistance to HSP90 inhibitors has been
demonstrated as the major reason for their limited effects as a
monotherapy (140). HSF1 is a
crucial factor for the underlying resistance to NTD inhibitors. It
forms homotrimers after binding to the inhibited HSP90 and induces
the pro-survival heat shock response (102). Additionally, the overexpression
of the multidrug resistance efflux pump P-glycoprotein 1 overcomes
the anticancer effects of benzoquinone-based HSP90 inhibitors
(141). The overexpression of UDP
glucuronosyltransferase 1A results in resistance to
resorcinol-based HSP90 inhibitors (142). Based on these findings,
combination therapy strategies have been investigated.
A previous study revealed that CRC cells treated
with the resorcinol-containing inhibitor, AUY-922, exhibited a
higher sensitivity to 5-FU-based chemotherapy both in vitro
and in vivo, which supports the combined use of AUY-922 with
5-FU as a feasible therapeutic strategy (141). A phase I clinical study testing
the efficacy of the combination therapy AUY-922 and capecitabine
demonstrated that in 19 patients with advanced CRC, 63% of these
patients demonstrated a partial response or stable disease when
treated with the combination therapy (143). The administration of AUY922 in
combination with doxorubicin resulted in increased levels of
caspase-3 expression, a biomarker of mitochondrial apoptosis, as
well as decreased levels of VEGF mRNA, an effect that was not
observed in monotherapy treatments (144,145). The combination of irinotecan and
17-AAG was also assessed in a phase I study in patients with solid
tumors. Of the 27 patients, a decrease in tumor volume was observed
in 6 patients, 5 of 10 patients with p53-mutant tumor had stable
disease and 2 of 6 patients with p53-wild-type tumor presented with
stable disease (146).
Additionally, the multifunctional nanoceria platform loaded with
both doxorubicin and STA-9090 as a combination therapy was
previously reported in NSCLC. The results reported >80% of NSCLC
cell death levels within 48 h in vitro. STA-9090 synergizes
with and enhances the therapeutic efficacy of doxorubicin,
minimizing the potential cardiotoxicity of doxorubicin via reactive
oxygen species (ROS) production. Furthermore, apoptosis, necrosis
and migration assays supported the negative effects of STA-9090 on
the proliferation and migration of cancer cells (147).
CH5164840 combined with the EGFR inhibitor,
erlotinib, has demonstrated improved antitumor effects in
EGFR-overexpressing xenograft models. Additionally, ERK signaling
was suppressed by the combined application of erlotinib and
CH5164840 in vivo (148).
17-AAG has been demonstrated as a positive factor for prognosis in
breast cancer cells treated with the VEGF inhibitor, bevacizumab,
in a preclinical study (149).
The combination of AUY922 and erlotinib was tested in phase I
trials that consisted of 18 patients with EGFR-mutant lung cancer.
Partial responses were observed; however, the toxic effects were a
major limiting factor (150). A
combination of STA-9090 and Ziv-Aflibercept, an antiangiogenic
agent, was evaluated in patients with advanced adenocarcinoma
(three colon adenocarcinomas, one small bowel adenocarcinoma, and
one rectal adenocarcinoma). Of 5 patients, 3 achieved stable
disease, although dose-related toxicity was observed (151). 17-DMAG in combination with the
anti-EGFR agent lapatinib overcame the acquired lapatinib
resistance in an ER-positive HER2-overexpressing breast cancer cell
line. Suppression of cell proliferation and HSP90 expression was
observed in monotherapy and combination therapy, with the latter
demonstrating a comparably increased synergistic effect (152). 17-AAG in combination with
trastuzumab in HER2-positive breast cancer did not demonstrate
suitable antitumor efficacy, whereas the combination therapy
demonstrated antitumor efficacy in ALK-mutated lung cancers
(50,153). 3-methyladenine (3-MA) is an
autophagy inhibitor that selectively inhibits the P13K signaling
pathway. The effect of combining 17-AAG with 3-MA evaluated in
preclinical trials revealed that the combination therapy resulted
in a notable increase in apoptosis and a lower level of autophagy
vs. monotherapy (154). The HSP90
molecular chaperone mechanism regulates the Raf kinase signaling
pathway; thus, the inhibition of HSP90 affects the Raf kinase
signaling pathway. Based on this mechanism, a combination of 17-AAG
and Raf kinase inhibitor sorafenib was evaluated in a phase I
trial. Antitumor efficacy was observed in 9 out of 12 renal cancer
patients and 4 out of 6 melanoma patients (155). A phase Ib trial of TAS-116
combined with nivolumab investigated the tumor response and
corresponding adverse response at the same dose in CRC and other
solid tumors patients. Positive tumor responses were observed and
the optimum concentration for safety profiles and antitumor
activity of TAS-116 was 160 mg (156). FW-04-806 is reported to promote
the antitumor efficacy of lapatinib in inhibiting cell
proliferation and inducing cell apoptosis, and in particular, in
reducing HER3 levels which were increased by lapatinib to inhibit
HER2. The results highlighted the potential of this combination
therapy for HER2-positive breast cancer (99). Bortezomib, a proteasome inhibitor,
has demonstrated improved anticancer effects when combined with
IPI-504, KW-2478 and PU-H71. The HSP90 inhibitors can overcome
intrinsic and acquired resistance to the proteasome inhibitor in
mantle cell lymphoma, multiple myeloma and Ewing sarcoma. In
addition, the synergistic effect of HSP90 inhibitors and bortezomib
is likely to reduce the toxic effects of HSP90 inhibitors, due to a
reduction in the required doses (85,95,157,158).
In melanoma cells, HSP90 inhibitors have been
demonstrated to be suitable candidates for increasing the
sensitivity of tumor cells to T-cells out of a list of 850
bioactive drugs. In addition, the inhibition of HSP90 enhances the
antitumor effects of anti-CTLA4 and anti-PD-1 therapy in
vivo (159). Furthermore,
several HSP90 clients such as mutated EGFR, rearranged ALK, HIF-1α
and JAK2 have been revealed to play essential roles in regulating
immune checkpoint blockade by promoting PD1 and PD-L1 expression
(160). These results suggested
that HSP90 inhibitors can be a complementary strategy to immune
checkpoint blockade for cancer therapy. In a preclinical study,
combining STA-9090 and the anti-PD-L1 antibody STI-A1015
demonstrated better efficacy in colon carcinoma and melanoma in
vivo than monotherapies (160). The combination of 17-DMAG and
agonists of EphA2 has also been found to improve the recruitment of
therapeutic T-cells by reconditioning the tumor microenvironment,
leading to an increase in antigen presentation and tumor cell
recognition (161).
In addition to the three classifications of
combination therapy discussed above, efforts have been made in de
novo therapeutic strategies. Radiotherapy is one of the most
frequently used cancer treatments. HSP90 client proteins, such as
BRCA1, BRCA2, CHK1, DNA-PKcs, ATM, FANCA and the MRE11/RAD50/NBN
complex are involved in DNA damage response pathways and have
become a major cause of resistance to radiotherapy. Based on the
preclinical study that demonstrated the favorable effect of AT13387
in combination with radiotherapy in vitro (162), an in vivo study was
performed on mice and positive results were obtained: AT13387
increased sensitivity to radiotherapy, enhanced apoptosis,
attenuation of migratory capacity, and a reduced DNA damage
response were observed (163). A
functional antioxidant nanomedicine composed of nanoceria
encapsulated with a two-drug cocktail of lactonic sophorolipids, a
constituent of natural sophorolipid known to inhibit histone
deacetylase activity, and the HSP90 inhibitor, STA9090, were
evaluated in NSCLC. The combination resulted in a marked reduction
of cell viability and suppression of cell migration; nanoceria
without any encapsulated drugs did not display any additional toxic
burden (164). A novel
multifunctional nano-platform for targeted delivery of heat, ROS,
and 17AAG/17DMAG simultaneously was proposed for prostate cancer
treatment (165). The common
adverse effects of geldanamycin derivatives are hepatoxicity, renal
failure, and gastrointestinal toxicities. Moreover, poor water
solubility is a major limiting factor for its clinical use. In that
study, nano-platforms were formulated to allow targeted delivery of
HSP90 inhibitors, thus improving therapeutic efficacy whilst
minimizing their off-site toxic effects (165).
An abundance of HSP90 inhibitors have been
discovered over the past decades, with certain inhibitors
demonstrating excellent antitumor efficacy both in vitro and
in vivo and entered clinical trials. However, limited
clinical effects and insurmountable toxicity have forced these
trials to be postponed or terminated. Phage display technology is
the most commonly used and robust in vitro method to select
specific peptides or antibodies against almost any antigen
(166). Phage technology screens
out the peptides required from a complex mixture pool of billions
of displayed peptides on phages in a combinatorial library via the
high affinity of peptides to phages with a specific target
(167). Peptides that are applied
as a targeting tool in cancer treatment may demonstrate advantages
in high affinity, favorable absorbability, endogenous degradability
and ease of synthesis, with fewer adverse reactions, improved
safety profiles, and ease of modifications with a variety of linker
chemistries (168,169). Peptide scFv47 was screened from
commercially available Tomlinson I and J phage display libraries
and was characterized as an HSP90α-selective binder. Experiments
in vitro revealed the ability of scFv47 to bind specifically
to HSP90α and inhibit ATPase in human breast cancer cells (170). In a recent study, a potentially
druggable peptide with strongly selective binding to the N-terminal
of HSP90 was screened using a T7-phage display system from an
undisclosed-cryptand. This peptide was demonstrated to exhibit
strong antibody-like affinity (KD, 62 nM) to the N-terminal of
HSP90 driven by enthalpy and demonstrated HSP90-inhibitory
biological activity by binding to the ATPase site in the NTD.
Notably, it is the first reported strong NTD-specific homing
peptide against HSP90 screened using a T7-phage display system from
the library of an undisclosed-cryptand36 with two lariat arms
(171). It is hypothesized that
the HSP90-homing peptide obtained for target recognition is not the
final achievement, these homing peptides may form the basis of
novel antibody-based HSP90 targeted strategies for anticancer
treatment.
Over the past few decades, several HSP90 inhibitors
have been developed and entered clinical trials. Thus far, all the
HSP90 inhibitors that have entered clinical trials target the NTD.
However, toxicity and poor bioavailability prevent NTD-targeting
inhibitors from being used clinically (20). Resistance to NTD inhibitors has
been demonstrated to be another major contributor to the poor
effects of NTD inhibitors (102).
Studies have revealed the presence of ATP binding sites on CTD;
this allows co-chaperones and unfolded client proteins to bind to
either the CTD or the NTD (103,105). A deeper understanding of the four
different isoforms provides a novel direction for the development
of HSP90 inhibitors, allowing progression from the development of
‘pan-inhibitors’ and instead developing more specific treatments.
Isoform-specific inhibitors can achieve antitumor effects with
reduced toxicity compared with pan-inhibition. Targeting a specific
isoform may be of additional value in each disease state, as
compared to pan-inhibition. Furthermore, the results of clinical
and preclinical investigations suggested that HSP90 inhibitors can
enhance the efficacy of other anti-neoplastic treatments, including
chemotherapies, targeted agents, immunotherapy, and radiotherapy.
Herein, the potential therapeutic strategies involving HSP90
targeting for the management of cancer were discussed. Certain
peptide inhibitors that target HSP90 have been screened using phage
display technology and revealed to exhibit high affinity to HSP90
in vitro and in vivo. Although additional studies are
required before HSP90-targeting peptide drugs can be developed,
novel antibody-based HSP90-targeting strategies based on these
targeting peptides are prospective approaches for future cancer
treatments.
Not applicable.
Funding: Not applicable.
Not applicable.
ZNL wrote the manuscript and YL reviewed the final
versions. All authors have read and approved the final manuscript.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Condelli V, Crispo F, Pietrafesa M,
Lettini G, Matassa DS, Esposito F, Landriscina M and Maddalena F:
HSP90 molecular chaperones, metabolic rewiring, and epigenetics:
Impact on tumor progression and perspective for anticancer therapy.
Cells. 8:5322019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hoter A, El-Sabban ME and Naim HY: The
HSP90 family: Structure, regulation, function, and implications in
health and disease. Int J Mol Sci. 19:25602018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Whitesell L and Lindquist SL: HSP90 and
the chaperoning of cancer. Nat Rev Cancer. 5:761–772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang H and Burrows F: Targeting multiple
signal transduction pathways through inhibition of Hsp90. J Mol Med
(Berl). 82:488–499. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chiosis G: Targeting chaperones in
transformed systems-a focus on HSP90 and cancer. Expert Opin Ther
Targets. 10:37–50. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Workman P: Combinatorial attack on
multistep oncogenesis by inhibiting the Hsp90 molecular chaperone.
Cancer Lett. 206:149–157. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Csermely P, Schnaider T, Soti C, Prohaszka
Z and Nardai G: The 90-kDa molecular chaperone family: Structure,
function, and clinical applications. A comprehensive review.
Pharmacol Ther. 79:129–168. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Prodromou C, Roe SM, O'Brien R, Ladbury
JE, Piper PW and Pearl LH: Identification and structural
characterization of the ATP/ADP-binding site in the Hsp90 molecular
chaperone. Cell. 90:65–75. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meyer P, Prodromou C, Hu B, Vaughan C, Roe
SM, Panaretou B, Piper PW and Pearl LH: Structural and functional
analysis of the middle segment of hsp90: Implications for ATP
hydrolysis and client protein and cochaperone interactions. Mol
Cell. 11:647–658. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Minami Y, Kimura Y, Kawasaki H, Suzuki K
and Yahara I: The carboxy-terminal region of mammalian HSP90 is
required for its dimerization and function in vivo. Mol Cell Biol.
14:1459–1464. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panaretou B, Prodromou C, Roe SM, O'Brien
R, Ladbury JE, Piper PW and Pearl LH: ATP binding and hydrolysis
are essential to the function of the Hsp90 molecular chaperone in
vivo. EMBO J. 17:4829–4836. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vorherr T, Knopfel L, Hofmann F, Mollner
S, Pfeuffer T and Carafoli E: The calmodulin binding domain of
nitric oxide synthase and adenylyl cyclase. Biochemistry.
32:6081–6088. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jackson SE: Hsp90: Structure and function.
Top Curr Chem. 328:155–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li J, Soroka J and Buchner J: The Hsp90
chaperone machinery: Conformational dynamics and regulation by
co-chaperones. Biochim Biophys Acta. 1823:624–635. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Marzec M, Eletto D and Argon Y: GRP94: An
HSP90-like protein specialized for protein folding and quality
control in the endoplasmic reticulum. Biochim Biophys Acta.
1823:774–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amoroso MR, Matassa DS, Sisinni L, Lettini
G, Landriscina M and Esposito F: TRAP1 revisited: Novel
localizations and functions of a ‘next-generation’ biomarker
(review). Int J Oncol. 45:969–977. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Soga S, Akinaga S and Shiotsu Y: Hsp90
inhibitors as anti-cancer agents, from basic discoveries to
clinical development. Curr Pharm Des. 19:366–376. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sanchez J, Carter TR, Cohen MS and Blagg
BSJ: Old and new approaches to target the Hsp90 chaperone. Curr
Cancer Drug Targets. 20:253–270. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Koren J III and Blagg BSJ: The right tool
for the job: An overview of Hsp90 inhibitors. Adv Exp Med Biol.
1243:135–146. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jhaveri K, Taldone T, Modi S and Chiosis
G: Advances in the clinical development of heat shock protein 90
(Hsp90) inhibitors in cancers. Biochim Biophys Acta. 1823:742–755.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Dutta R and Inouye M: GHKL, an emergent
ATPase/kinase superfamily. Trends Biochem Sci. 25:24–28. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Meng X, Devin J, Sullivan WP, Toft D,
Baulieu EE and Catelli MG: Mutational analysis of Hsp90 alpha
dimerization and subcellular localization: Dimer disruption does
not impede ‘in vivo’ interaction with estrogen receptor. J Cell
Sci. 109:1677–1687. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Soti C, Vermes A, Haystead TA and Csermely
P: Comparative analysis of the ATP-binding sites of Hsp90 by
nucleotide affinity cleavage: A distinct nucleotide specificity of
the C-terminal ATP-binding site. Eur J Biochem. 270:2421–2428.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sreedhar AS, Kalmar E, Csermely P and Shen
YF: Hsp90 isoforms: Functions, expression and clinical importance.
FEBS Lett. 562:11–15. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tsutsumi S, Mollapour M, Prodromou C, Lee
CT, Panaretou B, Yoshida S, Mayer MP and Neckers LM: Charged linker
sequence modulates eukaryotic heat shock protein 90 (Hsp90)
chaperone activity. Proc Natl Acad Sci USA. 109:2937–2942. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Rowlands M, McAndrew C, Prodromou C, Pearl
L, Kalusa A, Jones K, Workman P and Aherne W: Detection of the
ATPase activity of the molecular chaperones Hsp90 and Hsp72 using
the TranscreenerTM ADP assay kit. J Biomol Screen. 15:279–286.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wandinger SK, Richter K and Buchner J: The
Hsp90 chaperone machinery. J Biol Chem. 283:18473–18477. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ali MM, Roe SM, Vaughan CK, Meyer P,
Panaretou B, Piper PW, Prodromou C and Pearl LH: Crystal structure
of an Hsp90-nucleotide-p23/Sba1 closed chaperone complex. Nature.
440:1013–1017. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Richter K, Soroka J, Skalniak L, Leskovar
A, Hessling M, Reinstein J and Buchner J: Conserved conformational
changes in the ATPase cycle of human Hsp90. J Biol Chem.
283:17757–17765. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Terasawa K, Minami M and Minami Y:
Constantly updated knowledge of Hsp90. J Biochem. 137:443–447.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Meyer P, Prodromou C, Liao C, Hu B, Roe
SM, Vaughan CK, Vlasic I, Panaretou B, Piper PW and Pearl LH:
Structural basis for recruitment of the ATPase activator Aha1 to
the Hsp90 chaperone machinery. EMBO J. 23:1402–1410. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Langer T, Rosmus S and Fasold H:
Intracellular localization of the 90 kDA heat shock protein
(HSP90alpha) determined by expression of a EGFP-HSP90alpha-fusion
protein in unstressed and heat stressed 3T3 cells. Cell Biol Int.
27:47–52. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang X, Song X, Zhuo W, Fu Y, Shi H, Liang
Y, Tong M, Chang G and Luo Y: The regulatory mechanism of
Hsp90alpha secretion and its function in tumor malignancy. Proc
Natl Acad Sci USA. 106:21288–21293. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sidera K, Samiotaki M, Yfanti E, Panayotou
G and Patsavoudi E: Involvement of cell surface HSP90 in cell
migration reveals a novel role in the developing nervous system. J
Biol Chem. 279:45379–45388. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Tsutsumi S and Neckers L: Extracellular
heat shock protein 90: A role for a molecular chaperone in cell
motility and cancer metastasis. Cancer Sci. 98:1536–1539. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stellas D, Karameris A and Patsavoudi E:
Monoclonal antibody 4C5 immunostains human melanomas and inhibits
melanoma cell invasion and metastasis. Clin Cancer Res.
13:1831–1838. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Eletto D, Dersh D and Argon Y: GRP94 in ER
quality control and stress responses. Semin Cell Dev Biol.
21:479–485. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hua G, Zhang Q and Fan Z: Heat shock
protein 75 (TRAP1) antagonizes reactive oxygen species generation
and protects cells from granzyme M-mediated apoptosis. J Biol Chem.
282:20553–20560. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Sciacovelli M, Guzzo G, Morello V, Frezza
C, Zheng L, Nannini N, Calabrese F, Laudiero G, Esposito F,
Landriscina M, et al: The mitochondrial chaperone TRAP1 promotes
neoplastic growth by inhibiting succinate dehydrogenase. Cell
Metab. 17:988–999. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Masgras I, Sanchez-Martin C, Colombo G and
Rasola A: The chaperone TRAP1 as a modulator of the mitochondrial
adaptations in cancer cells. Front Oncol. 7:582017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhao R, Davey M, Hsu YC, Kaplanek P, Tong
A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, et al:
Navigating the chaperone network: An integrative map of physical
and genetic interactions mediated by the hsp90 chaperone. Cell.
120:715–727. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pratt WB, Morishima Y and Osawa Y: The
Hsp90 chaperone machinery regulates signaling by modulating ligand
binding clefts. J Biol Chem. 283:22885–22889. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zuehlke A and Johnson JL: Hsp90 and
co-chaperones twist the functions of diverse client proteins.
Biopolymers. 93:211–217. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Diaz-Villanueva JF, Diaz-Molina R and
Garcia-Gonzalez V: Protein folding and mechanisms of proteostasis.
Int J Mol Sci. 16:17193–17230. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pearl LH, Prodromou C and Workman P: The
Hsp90 molecular chaperone: An open and shut case for treatment.
Biochem J. 410:439–453. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Trepel J, Mollapour M, Giaccone G and
Neckers L: Targeting the dynamic HSP90 complex in cancer. Nat Rev
Cancer. 10:537–549. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Neckers L and Workman P: Hsp90 molecular
chaperone inhibitors: Are we there yet? Clin Cancer Res. 18:64–76.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Garg G, Khandelwal A and Blagg BS:
Anticancer inhibitors of Hsp90 function: Beyond the usual suspects.
Adv Cancer Res. 129:51–88. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Neckers L, Mimnaugh E and Schulte TW:
Hsp90 as an anti-cancer target. Drug Resist Updat. 2:165–172. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Modi S, Stopeck A, Linden H, Solit D,
Chandarlapaty S, Rosen N, D'Andrea G, Dickler M, Moynahan ME,
Sugarman S, et al: HSP90 inhibition is effective in breast cancer:
A phase II trial of tanespimycin (17-AAG) plus trastuzumab in
patients with HER2-positive metastatic breast cancer progressing on
trastuzumab. Clin Cancer Res. 17:5132–5139. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Ishikawa Y, Kozakai T, Morita H, Saida K,
Oka S and Masuo Y: Rapid detection of mycoplasma contamination in
cell cultures using SYBR Green-based real-time polymerase chain
reaction. In Vitro Cell Dev Biol Anim. 42:63–69. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Miyata Y, Nakamoto H and Neckers L: The
therapeutic target Hsp90 and cancer hallmarks. Curr Pharm Des.
19:347–365. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Barrott JJ and Haystead TA: Hsp90, an
unlikely ally in the war on cancer. FEBS J. 280:1381–1396. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Mori M, Hitora T, Nakamura O, Yamagami Y,
Horie R, Nishimura H and Yamamoto T: Hsp90 inhibitor induces
autophagy and apoptosis in osteosarcoma cells. Int J Oncol.
46:47–54. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ciocca DR and Calderwood SK: Heat shock
proteins in cancer: Diagnostic, prognostic, predictive, and
treatment implications. Cell Stress Chaperones. 10:86–103. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Jafari A, Rezaei-Tavirani M,
Farhadihosseinabadi B, Taranejoo S and Zali H: HSP90 and
Co-chaperones: Impact on tumor progression and prospects for
molecular-targeted cancer therapy. Cancer Invest. 38:310–328. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Birbo B, Madu EE, Madu CO, Jain A and Lu
Y: Role of HSP90 in cancer. Int J Mol Sci. 22:103172021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Pick E, Kluger Y, Giltnane JM, Moeder C,
Camp RL, Rimm DL and Kluger HM: High HSP90 expression is associated
with decreased survival in breast cancer. Cancer Res. 67:2932–2937.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Cheng Q, Chang JT, Geradts J, Neckers LM,
Haystead T, Spector NL and Lyerly HK: Amplification and high-level
expression of heat shock protein 90 marks aggressive phenotypes of
human epidermal growth factor receptor 2 negative breast cancer.
Breast Cancer Res. 14:R622012. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Moran Luengo T, Mayer MP and Rudiger SGD:
The Hsp70-Hsp90 chaperone cascade in protein folding. Trends Cell
Biol. 29:164–177. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kamal A, Thao L, Sensintaffar J, Zhang L,
Boehm MF, Fritz LC and Burrows FJ: A high-affinity conformation of
Hsp90 confers tumour selectivity on Hsp90 inhibitors. Nature.
425:407–410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Moulick K, Ahn JH, Zong H, Rodina A,
Cerchietti L, Gomes DaGama EM, Caldas-Lopes E, Beebe K, Perna F,
Hatzi K, et al: Affinity-based proteomics reveal cancer-specific
networks coordinated by Hsp90. Nat Chem Biol. 7:818–826. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ferrarini M, Heltai S, Zocchi MR and
Rugarli C: Unusual expression and localization of heat-shock
proteins in human tumor cells. Int J Cancer. 51:613–619. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sims JD, McCready J and Jay DG:
Extracellular heat shock protein (Hsp)70 and Hsp90alpha assist in
matrix metalloproteinase-2 activation and breast cancer cell
migration and invasion. PLoS One. 6:e188482011. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Cheng CF, Fan J, Fedesco M, Guan S, Li Y,
Bandyopadhyay B, Bright AM, Yerushalmi D, Liang M, Chen M, et al:
Transforming growth factor alpha (TGFalpha)-stimulated secretion of
HSP90alpha: Using the receptor LRP-1/CD91 to promote human skin
cell migration against a TGFbeta-rich environment during wound
healing. Mol Cell Biol. 28:3344–3358. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Eustace BK, Sakurai T, Stewart JK,
Yimlamai D, Unger C, Zehetmeier C, Lain B, Torella C, Henning SW,
Beste G, et al: Functional proteomic screens reveal an essential
extracellular role for hsp90 alpha in cancer cell invasiveness. Nat
Cell Biol. 6:507–514. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Gorska M, Popowska U, Sielicka-Dudzin A,
Kuban-Jankowska A, Sawczuk W, Knap N, Cicero G and Wozniak F:
Geldanamycin and its derivatives as Hsp90 inhibitors. Front Biosci
(Landmark Ed). 17:2269–2277. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
68
|
Samuni Y, Ishii H, Hyodo F, Samuni U,
Krishna MC, Goldstein S and Mitchell JB: Reactive oxygen species
mediate hepatotoxicity induced by the Hsp90 inhibitor geldanamycin
and its analogs. Free Radic Biol Med. 48:1559–1563. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Biamonte MA, Van de Water R, Arndt JW,
Scannevin RH, Perret D and Lee WC: Heat shock protein 90:
Inhibitors in clinical trials. J Med Chem. 53:3–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Ronnen EA, Kondagunta GV, Ishill N,
Sweeney SM, Deluca JK, Schwartz L, Bacik J and Motzer RJ: A phase
II trial of 17-(Allylamino)-17-demethoxygeldanamycin in patients
with papillary and clear cell renal cell carcinoma. Invest New
Drugs. 24:543–546. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Solit DB, Osman I, Polsky D, Panageas KS,
Daud A, Goydos JS, Teitcher J, Wolchok JD, Germino FJ, Krown SE, et
al: Phase II trial of 17-allylamino-17-demethoxygeldanamycin in
patients with metastatic melanoma. Clin Cancer Res. 14:8302–8307.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Heath EI, Hillman DW, Vaishampayan U,
Sheng S, Sarkar F, Harper F, Gaskins M, Pitot HC, Tan W, Ivy SP, et
al: A phase II trial of 17-allylamino-17-demethoxygeldanamycin in
patients with hormone-refractory metastatic prostate cancer. Clin
Cancer Res. 14:7940–7946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Hanson BE and Vesole DH: Retaspimycin
hydrochloride (IPI-504): A novel heat shock protein inhibitor as an
anticancer agent. Expert Opin Investig Drugs. 18:1375–1383. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kim YS, Alarcon SV, Lee S, Lee MJ,
Giaccone G, Neckers L and Trepel JB: Update on Hsp90 inhibitors in
clinical trial. Curr Top Med Chem. 9:1479–1492. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Wagner AJ, Chugh R, Rosen LS, Morgan JA,
George S, Gordon M, Dunbar J, Normant E, Grayzel D and Demetri GD:
A phase I study of the HSP90 inhibitor retaspimycin hydrochloride
(IPI-504) in patients with gastrointestinal stromal tumors or
soft-tissue sarcomas. Clin Cancer Res. 19:6020–6029. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Floris G, Sciot R, Wozniak A, Van Looy T,
Wellens J, Faa G, Normant E, Debiec-Rychter M and Schoffski P: The
Novel HSP90 inhibitor, IPI-493, is highly effective in human
gastrostrointestinal stromal tumor xenografts carrying
heterogeneous KIT mutations. Clin Cancer Res. 17:5604–5614. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Mellatyar H, Talaei S,
Pilehvar-Soltanahmadi Y, Barzegar A, Akbarzadeh A, Shahabi A,
Barekati-Mowahed M and Zarghami N: Targeted cancer therapy through
17-DMAG as an Hsp90 inhibitor: Overview and current state of the
art. Biomed Pharmacother. 102:608–617. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wright L, Barril X, Dymock B, Sheridan L,
Surgenor A, Beswick M, Drysdale M, Collier A, Massey A, Davies N,
et al: Structure-activity relationships in purine-based inhibitor
binding to HSP90 isoforms. Chem Biol. 11:775–785. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lundgren K, Zhang H, Brekken J, Huser N,
Powell RE, Timple N, Busch DJ, Neely L, Sensintaffar JL, Yang YC,
et al: BIIB021, an orally available, fully synthetic small-molecule
inhibitor of the heat shock protein Hsp90. Mol Cancer Ther.
8:921–929. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Dickson MA, Okuno SH, Keohan ML, Maki RG,
D'Adamo DR, Akhurst TJ, Antonescu CR and Schwartz GK: Phase II
study of the HSP90-inhibitor BIIB021 in gastrointestinal stromal
tumors. Ann Oncol. 24:252–257. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Yan L, Zhang W, Zhang B, Xuan C and Wang
D: BIIB021: A novel inhibitor to heat shock protein 90-addicted
oncology. Tumour Biol. 39:10104283176983552017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Hong D, Said R, Falchook G, Naing A,
Moulder S, Tsimberidou AM, Galluppi G, Dakappagari N, Storgard C,
Kurzrock R and Rosen LS: Phase I study of BIIB028, a selective heat
shock protein 90 inhibitor, in patients with refractory metastatic
or locally advanced solid tumors. Clin Cancer Res. 19:4824–4831.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Caldas-Lopes E, Cerchietti L, Ahn JH,
Clement CC, Robles AI, Rodina A, Moulick K, Taldone T, Gozman A,
Guo Y, et al: Hsp90 inhibitor PU-H71, a multimodal inhibitor of
malignancy, induces complete responses in triple-negative breast
cancer models. Proc Natl Acad Sci USA. 106:8368–8373. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Cerchietti LC, Lopes EC, Yang SN, Hatzi K,
Bunting KL, Tsikitas LA, Mallik A, Robles AI, Walling J,
Varticovski L, et al: A purine scaffold Hsp90 inhibitor
destabilizes BCL-6 and has specific antitumor activity in
BCL-6-dependent B cell lymphomas. Nat Med. 15:1369–1376. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Ambati SR, Lopes EC, Kosugi K, Mony U,
Zehir A, Shah SK, Taldone T, Moreira AL, Meyers PA, Chiosis G, et
al: Pre-clinical efficacy of PU-H71, a novel HSP90 inhibitor, alone
and in combination with bortezomib in Ewing sarcoma. Mol Oncol.
8:323–336. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Fadden P, Huang KH, Veal JM, Steed PM,
Barabasz AF, Foley B, Hu M, Partridge JM, Rice J, Scott A, et al:
Application of chemoproteomics to drug discovery: Identification of
a clinical candidate targeting hsp90. Chem Biol. 17:686–694. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Huang KH, Veal JM, Fadden RP, Rice JW,
Eaves J, Strachan JP, Barabasz AF, Foley BE, Barta TE, Ma W, et al:
Discovery of novel 2-aminobenzamide inhibitors of heat shock
protein 90 as potent, selective and orally active antitumor agents.
J Med Chem. 52:4288–4305. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Cheung KM, Matthews TP, James K, Rowlands
MG, Boxall KJ, Sharp SY, Maloney A, Roe SM, Prodromou C, Pearl LH,
et al: The identification, synthesis, protein crystal structure and
in vitro biochemical evaluation of a new 3,4-diarylpyrazole class
of Hsp90 inhibitors. Bioorg Med Chem Lett. 15:3338–3343. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Brough PA, Aherne W, Barril X, Borgognoni
J, Boxall K, Cansfield JE, Cheung KM, Collins I, Davies NG,
Drysdale MJ, et al: 4,5-diarylisoxazole Hsp90 chaperone inhibitors:
Potential therapeutic agents for the treatment of cancer. J Med
Chem. 51:196–218. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Eccles SA, Massey A, Raynaud FI, Sharp SY,
Box G, Valenti M, Patterson L, de Haven Brandon A, Gowan S, Boxall
F, et al: NVP-AUY922: A novel heat shock protein 90 inhibitor
active against xenograft tumor growth angiogenesis, and metastasis.
Cancer Res. 68:2850–2860. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Jensen MR, Schoepfer J, Radimerski T,
Massey A, Guy CT, Brueggen J, Quadt C, Buckler A, Cozens R,
Drysdale MJ, et al: NVP-AUY922: A small molecule HSP90 inhibitor
with potent antitumor activity in preclinical breast cancer models.
Breast Cancer Res. 10:R332008. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Murray CW, Carr MG, Callaghan O, Chessari
G, Congreve M, Cowan S, Coyle JE, Downham R, Figueroa E,
Frederickson M, et al: Fragment-based drug discovery applied to
Hsp90. Discovery of two lead series with high ligand efficiency. J
Med Chem. 53:5942–5955. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Wang Y, Trepel JB, Neckers LM and Giaccone
G: STA-9090, a small-molecule Hsp90 inhibitor for the potential
treatment of cancer. Curr Opin Investig Drugs. 11:1466–1476.
2010.PubMed/NCBI
|
|
94
|
Nakashima T, Ishii T, Tagaya H, Seike T,
Nakagawa H, Kanda Y, Akinaga S, Soga S and Shiotsu Y: New molecular
and biological mechanism of antitumor activities of KW-2478, a
novel nonansamycin heat shock protein 90 inhibitor, in multiple
myeloma cells. Clin Cancer Res. 16:2792–2802. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Cavenagh J, Oakervee H, Baetiong-Caguioa
P, Davies F, Gharibo M, Rabin N, Kurman M, Novak B, Shiraishi N,
Nakashima D, et al: A phase I/II study of KW-2478, an Hsp90
inhibitor, in combination with bortezomib in patients with
relapsed/refractory multiple myeloma. Br J Cancer. 117:1295–1302.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Chang X, Zhao X, Wang J, Ding S, Xiao L,
Zhao E and Zheng X: Effect of Hsp90 inhibitor KW-2478 on HepG2
cells. Anticancer Agents Med Chem. 19:2231–2242. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Yong K, Cavet J, Johnson P, Morgan G,
Williams C, Nakashima D, Akinaga S, Oakervee H and Cavenagh J:
Phase I study of KW-2478, a novel Hsp90 inhibitor, in patients with
B-cell malignancies. Br J Cancer. 114:7–13. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Huang W, Ye M, Zhang LR, Wu QD, Zhang M,
Xu JH and Zheng W: FW-04-806 inhibits proliferation and induces
apoptosis in human breast cancer cells by binding to N-terminus of
Hsp90 and disrupting Hsp90-Cdc37 complex formation. Mol Cancer.
13:1502014. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Huang W, Wu QD, Zhang M, Kong YL, Cao PR,
Zheng W, Xu JH and Ye M: Novel Hsp90 inhibitor FW-04-806 displays
potent antitumor effects in HER2-positive breast cancer cells as a
single agent or in combination with lapatinib. Cancer Lett.
356:862–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Suda A, Koyano H, Hayase T, Hada K,
Kawasaki K, Komiyama S, Hasegawa K, Fukami TA, Sato S, Miura T, et
al: Design and synthesis of novel macrocyclic
2-amino-6-arylpyrimidine Hsp90 inhibitors. Bioorg Med Chem Lett.
22:1136–1141. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Bussenius J, Blazey CM, Aay N, Anand NK,
Arcalas A, Baik T, Bowles OJ, Buhr CA, Costanzo S, Curtis JK, et
al: Discovery of XL888: A novel tropane-derived small molecule
inhibitor of HSP90. Bioorg Med Chem Lett. 22:5396–5404. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Samarasinghe B, Wales CT, Taylor FR and
Jacobs AT: Heat shock factor 1 confers resistance to Hsp90
inhibitors through p62/SQSTM1 expression and promotion of
autophagic flux. Biochem Pharmacol. 87:445–455. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Soti C, Racz A and Csermely P: A
Nucleotide-dependent molecular switch controls ATP binding at the
C-terminal domain of Hsp90. N-terminal nucleotide binding unmasks a
C-terminal binding pocket. J Biol Chem. 277:7066–7075. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Schulte TW, Akinaga S, Soga S, Sullivan W,
Stensgard B, Toft D and Neckers LM: Antibiotic radicicol binds to
the N-terminal domain of Hsp90 and shares important biologic
activities with geldanamycin. Cell Stress Chaperones. 3:100–108.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Donnelly A and Blagg BS: Novobiocin and
additional inhibitors of the Hsp90 C-terminal nucleotide-binding
pocket. Curr Med Chem. 15:2702–2717. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Buchner J: Bacterial Hsp90-desperately
seeking clients. Mol Microbiol. 76:540–544. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Yun BG, Huang W, Leach N, Hartson SD and
Matts RL: Novobiocin induces a distinct conformation of Hsp90 and
alters Hsp90-cochaperone-client interactions. Biochemistry.
43:8217–8229. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhao J, Zhao H, Hall JA, Brown D, Brandes
E, Bazzill J, Grogan PT, Subramanian C, Vielhauer G, Cohen MS and
Blagg BS: Triazole containing novobiocin and biphenyl amides as
Hsp90 C-Terminal inhibitors. Medchemcomm. 5:1317–1323. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Astl L, Stetz G and Verkhivker GM:
Dissecting molecular principles of the Hsp90 chaperone regulation
by allosteric modulators using a hierarchical simulation approach
and network modeling of allosteric interactions: Conformational
selection dictates the diversity of protein responses and
ligand-specific functional mechanisms. J Chem Theory Comput.
16:6656–6677. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Marcu MG, Chadli A, Bouhouche I, Catelli M
and Neckers LM: The heat shock protein 90 antagonist novobiocin
interacts with a previously unrecognized ATP-binding domain in the
carboxyl terminus of the chaperone. J Biol Chem. 275:37181–37186.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Forsberg LK, Anyika M, You Z, Emery S,
McMullen M, Dobrowsky RT and Blagg BSJ: Development of
noviomimetics that modulate molecular chaperones and manifest
neuroprotective effects. Eur J Med Chem. 143:1428–1435. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Kumar Mv V, Ebna Noor R, Davis RE, Zhang
Z, Sipavicius E, Keramisanou D, Blagg BSJ and Gelis I: Molecular
insights into the interaction of Hsp90 with allosteric inhibitors
targeting the C-terminal domain. Medchemcomm. 9:1323–1331. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Rahimi MN and McAlpine SR: Protein-protein
inhibitor designed de novo to target the MEEVD region on the
C-terminus of Hsp90 and block co-chaperone activity. Chem Commun
(Camb). 55:846–849. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Huo Y, Buckton LK, Bennett JL, Smith EC,
Byrne FL, Hoehn KL, Rahimi MN and McAlpine SR: Delivering bioactive
cyclic peptides that target Hsp90 as prodrugs. J Enzyme Inhib Med
Chem. 34:728–739. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rahimi MN, Foster HG, Farazi SN, Chapman R
and McAlpine SR: Polymer mediated transport of the Hsp90 inhibitor
LB76, a polar cyclic peptide, produces an Hsp90 cellular phenotype.
Chem Commun (Camb). 55:4515–4518. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Subramanian C, Grogan PT, Wang T, Bazzill
J, Zuo A, White PT, Kalidindi A, Kuszynski D, Wang G, Blagg BSJ and
Cohen MS: Novel C-terminal heat shock protein 90 inhibitors target
breast cancer stem cells and block migration, self-renewal, and
epithelial-mesenchymal transition. Mol Oncol. 14:2058–2068. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Subramanian C, Kovatch KJ, Sim MW, Wang G,
Prince ME, Carey TE, Davis R, Blagg BSJ and Cohen MS: Novel
C-Terminal heat shock protein 90 inhibitors (KU711 and Ku757) are
effective in targeting head and neck squamous cell carcinoma cancer
stem cells. Neoplasia. 19:1003–1011. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
White PT, Subramanian C, Zhu Q, Zhang H,
Zhao H, Gallagher R, Timmermann BN, Blagg BS and Cohen MS: Novel
HSP90 inhibitors effectively target functions of thyroid cancer
stem cell preventing migration and invasion. Surgery. 159:142–151.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Samadi AK, Zhang X, Mukerji R, Donnelly
AC, Blagg BS and Cohen MS: A novel C-terminal HSP90 inhibitor KU135
induces apoptosis and cell cycle arrest in melanoma cells. Cancer
Lett. 312:158–167. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Shelton SN, Shawgo ME, Matthews SB, Lu Y,
Donnelly AC, Szabla K, Tanol M, Vielhauer GA, Rajewski RA, Matts
RL, et al: KU135, a novel novobiocin-derived C-terminal inhibitor
of the 90-kDa heat shock protein, exerts potent antiproliferative
effects in human leukemic cells. Mol Pharmacol. 76:1314–1322. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Nirmalanandhan VS, Duren A, Hendricks P,
Vielhauer G and Sittampalam GS: Activity of anticancer agents in a
three-dimensional cell culture model. Assay Drug Dev Technol.
8:581–590. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Cohen SM, Mukerji R, Samadi AK, Zhang X,
Zhao H, Blagg BS and Cohen MS: Novel C-terminal Hsp90 inhibitor for
head and neck squamous cell cancer (HNSCC) with in vivo efficacy
and improved toxicity profiles compared with standard agents. Ann
Surg Oncol. 19 (Suppl 3):S483–S490. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Hyun SY, Le HT, Nguyen CT, Yong YS, Boo
HJ, Lee HJ, Lee JS, Min HY, Ann J, Chen J, et al: Development of a
novel Hsp90 inhibitor NCT-50 as a potential anticancer agent for
the treatment of non-small cell lung cancer. Sci Rep. 8:139242018.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Li W, Sahu D and Tsen F: Secreted heat
shock protein-90 (Hsp90) in wound healing and cancer. Biochim
Biophys Acta. 1823:730–741. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Biswas C, Ostrovsky O, Makarewich CA,
Wanderling S, Gidalevitz T and Argon Y: The peptide-binding
activity of GRP94 is regulated by calcium. Biochem J. 405:233–241.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Ernst JT, Liu M, Zuccola H, Neubert T,
Beaumont K, Turnbull A, Kallel A, Vought B and Stamos D:
Correlation between chemotype-dependent binding conformations of
HSP90α/β and isoform selectivity-Implications for the
structure-based design of HSP90α/β selective inhibitors for
treating neurodegenerative diseases. Bioorg Med Chem Lett.
24:204–208. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Putcha P, Danzer KM, Kranich LR, Scott A,
Silinski M, Mabbett S, Hicks CD, Veal JM, Steed PM, Hyman BT and
McLean PJ: Brain-permeable small-molecule inhibitors of Hsp90
prevent alpha-synuclein oligomer formation and rescue
alpha-synuclein-induced toxicity. J Pharmacol Exp Ther.
332:849–857. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ernst JT, Neubert T, Liu M, Sperry S,
Zuccola H, Turnbull A, Fleck B, Kargo W, Woody L, Chiang P, et al:
Identification of novel HSP90α/β isoform selective inhibitors using
structure-based drug design. demonstration of potential utility in
treating CNS disorders such as Huntington's disease. J Med Chem.
57:3382–3400. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Ohkubo S, Kodama Y, Muraoka H,
Hitotsumachi H, Yoshimura C, Kitade M, Hashimoto A, Ito K, Gomori
A, Takahashi K, et al: TAS-116, a highly selective inhibitor of
heat shock protein 90α and β, demonstrates potent antitumor
activity and minimal ocular toxicity in preclinical models. Mol
Cancer Ther. 14:14–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Shimomura A, Yamamoto N, Kondo S, Fujiwara
Y, Suzuki S, Yanagitani N, Horiike A, Kitazono S, Ohyanagi F, Doi
T, et al: First-in-human phase I study of an oral HSP90 inhibitor,
TAS-116, in patients with advanced solid tumors. Mol Cancer Ther.
18:531–540. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Doi T, Kurokawa Y, Sawaki A, Komatsu Y,
Ozaka M, Takahashi T, Naito Y, Ohkubo S and Nishida T: Efficacy and
safety of TAS-116, an oral inhibitor of heat shock protein 90, in
patients with metastatic or unresectable gastrointestinal stromal
tumour refractory to imatinib, sunitinib and regorafenib: A phase
II, single-arm trial. Eur J Cancer. 121:29–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Rosser MF and Nicchitta CV: Ligand
interactions in the adenosine nucleotide-binding domain of the
Hsp90 chaperone, GRP94. I. Evidence for allosteric regulation of
ligand binding. J Biol Chem. 275:22798–22805. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Duerfeldt AS, Peterson LB, Maynard JC, Ng
CL, Eletto D, Ostrovsky O, Shinogle HE, Moore DS, Argon Y,
Nicchitta CV and Blagg BS: Development of a Grp94 inhibitor. J Am
Chem Soc. 134:9796–9804. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Patel PD, Yan P, Seidler PM, Patel HJ, Sun
W, Yang C, Que NS, Taldone T, Finotti P, Stephani RA, et al:
Paralog-selective Hsp90 inhibitors define tumor-specific regulation
of HER2. Nat Chem Biol. 9:677–684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Crowley VM, Khandelwal A, Mishra S,
Stothert AR, Huard DJ, Zhao J, Muth A, Duerfeldt AS, Kizziah JL,
Liebermann RL, et al: Development of glucose regulated protein
94-selective inhibitors based on the bnim and radamide scaffold. J
Med Chem. 59:3471–3488. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Jiang F, Guo AP, Xu JC, You QD and Xu XL:
Discovery of a Potent Grp94 selective inhibitor with
anti-inflammatory efficacy in a mouse model of ulcerative colitis.
J Med Chem. 61:9513–9533. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Plescia J, Salz W, Xia F, Pennati M,
Zaffaroni N, Daidone MG, Meli M, Dohi T, Fortugno P, Nefedova Y, et
al: Rational design of shepherdin, a novel anticancer agent. Cancer
Cell. 7:457–468. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Altieri DC, Stein GS, Lian JB and Languino
LR: TRAP-1, the mitochondrial Hsp90. Biochim Biophys Acta.
1823:767–773. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Lee C, Park HK, Jeong H, Lim J, Lee AJ,
Cheon KY, Kim CS, Thomas AP, Bae B, Kim ND, et al: Development of a
mitochondria-targeted Hsp90 inhibitor based on the crystal
structures of human TRAP1. J Am Chem Soc. 137:4358–4367. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Park HK, Jeong H, Ko E, Lee G, Lee JE, Lee
SK, Lee AJ, Im JY, Hu S, Kim SH, et al: Paralog specificity
determines subcellular distribution, action mechanism, and
anticancer activity of TRAP1 inhibitors. J Med Chem. 60:7569–7578.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Sveen A, Bruun J, Eide PW, Eilertsen IA,
Ramirez L, Murumagi A, Arjama M, Danielsen SA, Kryeziu K, Elez E,
et al: Colorectal cancer consensus molecular subtypes translated to
preclinical models uncover potentially targetable cancer cell
dependencies. Clin Cancer Res. 24:794–806. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Piper PW and Millson SH: Mechanisms of
resistance to Hsp90 inhibitor drugs: A complex mosaic emerges.
Pharmaceuticals (Basel). 4:1400–1422. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Bendell JC, Jones SF, Hart L, Pant S,
Moyhuddin A, Lane CM, Earwood C, Murphy P, Patton J, Penley WC, et
al: A Phase I Study of the Hsp90 inhibitor AUY922 plus capecitabine
for the treatment of patients with advanced solid tumors. Cancer
Invest. 33:477–482. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wang Y, Koay YC and McAlpine SR: How
selective are Hsp90 inhibitors for cancer cells over normal cells?
Chem Med Chem. 12:353–357. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Mohammadian M, Feizollahzadeh S, Mahmoudi
R, Toofani Milani A, Rezapour-Firouzi S and Karimi Douna B: Hsp90
Inhibitor; NVP-AUY922 in combination with doxorubicin induces
apoptosis and downregulates VEGF in MCF-7 breast cancer cell line.
Asian Pac J Cancer Prev. 21:1773–1778. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Tse AN, Klimstra DS, Gonen M, Shah M,
Sheikh T, Sikorski R, Carvajal R, Mui J, Tipian C, O'Reilly E, et
al: A phase 1 dose-escalation study of irinotecan in combination
with 17-allylamino-17-demethoxygeldanamycin in patients with solid
tumors. Clin Cancer Res. 14:6704–6711. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Sulthana S, Banerjee T, Kallu J, Vuppala
SR, Heckert B, Naz S, Shelby T, Yambem O and Santra S: Combination
therapy of NSCLC using Hsp90 inhibitor and doxorubicin carrying
functional nanoceria. Mol Pharm. 14:875–884. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Ono N, Yamazaki T, Tsukaguchi T, Fujii T,
Sakata K, Suda A, Tsukuda T, Mio T, Ishii N, Kondoh O and Aoki Y:
Enhanced antitumor activity of erlotinib in combination with the
Hsp90 inhibitor CH5164840 against non-small-cell lung cancer.
Cancer Sci. 104:1346–1352. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
149
|
Feng Q, Zhang C, Lum D, Druso JE, Blank B,
Wilson KF, Welm A, Antonyak MA and Cerione RA: A class of
extracellular vesicles from breast cancer cells activates VEGF
receptors and tumour angiogenesis. Nat Commun. 8:144502017.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Johnson ML, Yu HA, Hart EM, Weitner BB,
Rademaker AW, Patel JD, Kris MG and Riely GJ: Phase I/II study of
HSP90 inhibitor AUY922 and erlotinib for EGFR-Mutant lung cancer
with acquired resistance to epidermal growth factor receptor
tyrosine kinase inhibitors. J Clin Oncol. 33:1666–1673. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Meehan R, Kummar S, Do K, O'Sullivan Coyne
G, Juwara L, Zlott J, Rubinstein L, Doroshow JH and Chen AP: A
Phase I Study of ganetespib and Ziv-Aflibercept in patients with
advanced carcinomas and sarcomas. Oncologist. 23:1269–e1125. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Lee HJ, Shin S, Kang J, Han KC, Kim YH,
Bae JW and Park KH: HSP90 Inhibitor, 17-DMAG, alone and in
combination with lapatinib attenuates acquired lapatinib-resistance
in ER-positive, HER2-overexpressing breast cancer cell line.
Cancers (Basel). 12:26302020. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Jhaveri K and Modi S: HSP90 inhibitors for
cancer therapy and overcoming drug resistance. Adv Pharmacol.
65:471–517. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Chen F, Xie H, Bao H, Violetta L and Zheng
S: Combination of HSP90 and autophagy inhibitors promotes
hepatocellular carcinoma apoptosis following incomplete thermal
ablation. Mol Med Rep. 22:337–343. 2020.PubMed/NCBI
|
|
155
|
Vaishampayan UN, Burger AM, Sausville EA,
Heilbrun LK, Li J, Horiba MN, Egorin MJ, Ivy P, Pacey S and Lorusso
PM: Safety, efficacy, pharmacokinetics, and pharmacodynamics of the
combination of sorafenib and tanespimycin. Clin Cancer Res.
16:3795–3804. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Kawazoe A, Itahashi K, Yamamoto N, Kotani
D, Kuboki Y, Taniguchi H, Harano K, Naito Y, Suzuki M, Fukutani M,
et al: TAS-116 (Pimitespib), an Oral HSP90 inhibitor, in
combination with nivolumab in patients with colorectal cancer and
other solid tumors: An open-label, dose-finding, and expansion
Phase Ib trial (EPOC1704). Clin Cancer Res. 27:6709–6715. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Roué G, Pérez-Galán P, Mozos A,
López-Guerra M, Xargay-Torrent S, Rosich L, Saborit-Villarroya I,
Normant E, Campo E and Colomer D: The Hsp90 inhibitor IPI-504
overcomes bortezomib resistance in mantle cell lymphoma in vitro
and in vivo by down-regulation of the prosurvival ER chaperone
BiP/Grp78. Blood. 117:1270–1279. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Ishii T, Seike T, Nakashima T, Juliger S,
Maharaj L, Soga S, Akinaga S, Cavenagh J, Joel S and Shiotsu Y:
Anti-tumor activity against multiple myeloma by combination of
KW-2478, an Hsp90 inhibitor, with bortezomib. Blood Cancer J.
2:e682012. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Mbofung RM, McKenzie JA, Malu S, Zhang M,
Peng W, Liu C, Kuiatse I, Tieu T, Williams L, Devi S, et al: HSP90
inhibition enhances cancer immunotherapy by upregulating interferon
response genes. Nat Commun. 8:4512017. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Proia DA and Kaufmann GF: Targeting
heat-shock protein 90 (HSP90) as a complementary strategy to immune
checkpoint blockade for cancer therapy. Cancer Immunol Res.
3:583–589. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Rao A, Taylor JL, Chi-Sabins N, Kawabe M,
Gooding WE and Storkus WJ: Combination therapy with HSP90 inhibitor
17-DMAG reconditions the tumor microenvironment to improve
recruitment of therapeutic T cells. Cancer Res. 72:3196–3206. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Spiegelberg D, Dascalu A, Mortensen AC,
Abramenkovs A, Kuku G, Nestor M and Stenerlow B: The novel HSP90
inhibitor AT13387 potentiates radiation effects in squamous cell
carcinoma and adenocarcinoma cells. Oncotarget. 6:35652–35666.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Spiegelberg D, Abramenkovs A, Mortensen
ACL, Lundsten S, Nestor M and Stenerlow B: The HSP90 inhibitor
Onalespib exerts synergistic anti-cancer effects when combined with
radiotherapy: An in vitro and in vivo approach. Sci Rep.
10:59232020. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Naz S, Banerjee T, Totsingan F, Woody K,
Gross RA and Santra S: Therapeutic efficacy of lactonic
sophorolipids: Nanoceria-assisted combination therapy of NSCLC
using HDAC and Hsp90 inhibitors. Nanotheranostics. 5:391–404. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Lin TY, Guo W, Long Q, Ma A, Liu Q, Zhang
H, Huang Y, Chandrasekaran S, Pan C, Lam KS, et al: HSP90 inhibitor
encapsulated photo-theranostic nanoparticles for synergistic
combination cancer therapy. Theranostics. 6:1324–1335. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Bradbury AR and Marks JD: Antibodies from
phage antibody libraries. J Immunol Methods. 290:29–49. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Saw PE and Song EW: Phage display
screening of therapeutic peptide for cancer targeting and therapy.
Protein Cell. 10:787–807. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Wang J, Masehi-Lano JJ and Chung EJ:
Peptide and antibody ligands for renal targeting: Nanomedicine
strategies for kidney disease. Biomater Sci. 5:1450–1459. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Fosgerau K and Hoffmann T: Peptide
therapeutics: Current status and future directions. Drug Discov
Today. 20:122–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Petters E, Sokolowska-Wedzina A and
Otlewski J: Selection and characterization of single chain antibody
fragments specific for Hsp90 as a potential cancer targeting
molecule. Int J Mol Sci. 16:19920–19935. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
Mochizuki K, Matsukura L, Ito Y, Miyashita
N and Taki M: A medium-firm drug-candidate library of cryptand-like
structures on T7 phage: Design and selection of a strong binder for
Hsp90. Org Biomol Chem. 19:146–150. 2021. View Article : Google Scholar : PubMed/NCBI
|