Introduction
It has previously been reported that the incidence
of esophageal cancer (EC) is ~604,100 per year, thus accounting for
3.1% of new cancer cases diagnosed and ranking 7th in cancer
incidence worldwide. In addition, it has been reported that there
are 544,000 cases of EC-associated mortality per year, accounting
for 5.5% of cancer-associated deaths and ranking 6th in cancer
deaths worldwide (1). EC is divided
into squamous cell carcinoma and adenocarcinoma according to
histological subtype (2). The
highest incidence of esophageal squamous cell carcinoma has been
reported to occur in Eastern Asia, especially in China (1). EC is difficult to diagnose at an early
stage due to the lack of specific biomarkers; therefore, patients
are nearly always diagnosed with advanced EC (3). The overall 5-year survival of patients
with EC is 15–20% worldwide (4);
therefore, a novel therapeutic strategy to improve the 5-year
survival rate of patients with EC is required.
During the COVID-19 pandemic, it was revealed that
traditional Chinese medicine (TCM), such as Jinhua Qinggan granules
and Lianhua Qingwen capsules, had a decisive role in the clinical
treatment of COVID-19 by targeting ACE (5). Furthermore, a large number of studies
have demonstrated that TCM serves an important role in the
treatment of cancer, including colorectal cancer (6), breast cancer (7), hepatocellular carcinoma (8), lung cancer (9) and EC (10–12).
Notably, the active ingredients of TCM are thought to inhibit
cancer progression by regulating certain genes or signaling
pathways.
4-Methoxydalbergione (4-MD) is a flavonoid with
methoxy groups, which is isolated and purified from Dalbergia
sissoo Roxb. 4-MD has been indicated to have anti-inflammatory
effects via inhibition of the NF-κB signaling pathway (13) and antitumor effects due to its high
cytotoxicity in tumor cells (14).
In addition, it has been demonstrated that 4-MD can inhibit
osteosarcoma (15), human
astroglioma (16) and bladder
cancer (17) by regulating multiple
signaling pathways, including JAK2/STAT3 and Akt/ERK, but not
NF-κB. However, the inhibitory effect of 4-MD on EC remains to be
determined.
It has been reported that the development of EC is a
multistep pathogenic process from inflammation to cancer (18,19).
The malignant degree of EC is positively associated with
inflammatory factors, such as tumor necrosis factor α (TNF-α)
(20), and the survival time of
patients with EC has been confirmed to be negatively correlated
with inflammation (21,22). Furthermore, EC is accelerated though
EC angiogenesis via activation of the NF-κB signaling pathway
(23). Therefore, inflammation may
accelerate the progression of EC. Whether 4-MD regulates the NF-κB
signaling pathway to inhibit EC remains unclear. Previous studies
have shown that NF-κB can promote the release of TNF-α (24,25),
the synthesis of prostaglandin E2 (PGE2) (26–28),
and the expression of cyclin-dependent kinase 1 (CDK1) (29,30),
cyclin D1 (31,32) and proliferating cell nuclear antigen
(PCNA) (33,34). However, whether 4-MD inhibits EC by
suppressing the release of PGE2 and TNF-α, and the expression of
proliferation-associated proteins through the NF-κB signaling
pathway requires further investigation. Therefore, the present
study aimed to explore the effects of 4-MD on cell proliferation
and invasion, and to determine the underlying mechanism by which
4-MD regulates the malignant characteristics of EC cells.
Materials and methods
Cell culture
ECA-109 esophageal squamous carcinoma cells were
purchased from the American Type Culture Collection and KYSE-150
esophageal squamous carcinoma cells were purchased from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
ECA-109 cells were cultured in Dulbecco's modified Eagle's medium
(cat. no. SH30021; Hyclone; Cytiva) and KYSE-150 cells were
cultured in RPMI 1640 (cat. no. 11875093; Gibco; Thermo Fisher
Scientific, Inc.). The media were supplemented with 10% fetal
bovine serum (cat. no. SH30396; Hyclone; Cytiva) and 1%
penicillin-streptomycin (cat. no. V900929; MilliporeSigma). Cells
were incubated in a humidified incubator containing 5%
CO2 at 37°C.
Drug preparation
4-MD (cat. no. CB31393122; Chemical Book) was
dissolved in DMSO to form a 10 mmol/l solution, which was stored at
−20°C. The storage solution was not diluted to the corresponding
concentration of the working solution until it was used.
Cell Counting Kit-8 (CCK-8) assay
The proliferation of cells was analyzed using the
CCK-8 assay. ECA-109 cells were seeded into 96-well plates at 1,000
cells/well, and were treated with different concentrations of 4-MD
(0, 3.125, 6.25, 12.5, 25, 50 and 100 µmol/l) for 24 h. The optical
density (OD) of the cells was evaluated using a spectrophotometer
at a wavelength of 450 nm. Using the OD of cells, the half maximal
inhibitory concentration (IC50) and IC50 95%
confidence interval (CI) were calculated using GraphPad Prism 8.3
(GraphPad Software, Inc.). In addition, ECA-109 and KYSE-150 cells
were seeded into 96-well plates at 1,000 cells/well, and were
pre-incubated with or without 1 µg/ml lipopolysaccharide (LPS; cat.
no. ST1470; Beyotime Institute of Biotechnology) for 30 min at 37°C
in an incubator with 5% CO2 to activate NF-κB (35), followed by treatment with or without
20 µmol/l 4-MD for 12, 24, 36 or 48 h at 37°C in an incubator with
5% CO2. Subsequently,10 µl CCK-8 (cat. no. C0038;
Beyotime Institute of Biotechnology) solution was added to each
well. After culture for 1 h in an incubator, the OD value of the
cells was evaluated.
Colony formation assay
ECA-109 and KYSE-150 cells were re-suspended in
culture medium. Subsequently, 1,000 cells/well were seeded into
24-well plates, and were treated with or without 20 µmol/l 4-MD and
1 µg/ml LPS for 10 days in a humidified incubator containing 5%
CO2 at 37°C. ECA-109 and KYSE-150 cells were then fixed
with 4% paraformaldehyde (cat. no. P0099; Beyotime Institute of
Biotechnology) for 30 min at room temperature (RT) and were stained
with crystal violet (cat. no. C0121; Beyotime Institute of
Biotechnology) for 5 min at RT. Finally, the cell colonies
containing ≥50 cells were observed, images were captured under a
light microscope and colonies were analyzed using ImageJ software
(version 1.8.0; National Institutes of Health).
Wound-healing assay
ECA-109 and KYSE-150 cells were used for
wound-healing assay. A total of 1ⅹ105 cells/well were
seeded into 12-well plates. Once cells reached 100% confluence, a
scratch was evenly drawn using a 10-µl pipette tip. Subsequently,
the cells were incubated in FBS-free medium in a 5% CO2
incubator at 37°C, and treated with or without 20 µmol/l 4-MD and 1
µg/ml LPS for a further 36 or 48 h. Finally, the images of wound
healing were captured under a light microscope. The wound area was
analyzed using ImageJ software. The rate of migration was
calculated using the following formula: Rate of migration
(%)=(scratch area at 0 h-scratch area at 48 h)/scratch area at 0 h
ⅹ100.
ELISA
The levels of TNF-α and PGE2 in the culture
supernatant of ECA-109 cells treated with or without LPS and 4-MD
were analyzed using TNF-α (cat. no. PT518; Beyotime Institute of
Biotechnology) and PGE2 (cat. no. HB833-Hu; Shanghai Hengyuan
Biotechnology Co., Ltd.) ELISA kits according to the manufacturers'
protocols. OD value was measure at 450 nm using a
spectrophotometer.
Antibodies
All antibodies were purchased from Beyotime
Institute of Biotechnology. The antibodies used for western
blotting (WB) were as follows: Cyclin D1 rabbit monoclonal antibody
(1:1,000; cat. no. AF1183), CDK1 rabbit polyclonal antibody (1:500;
cat. no. AF0111), PCNA rabbit monoclonal antibody (1:1,000; cat.
no. AF1363), phosphorylated (p)-IKBα (Ser32) rabbit monoclonal
antibody (1:1,000; cat. no. AF1870), IKBα rabbit monoclonal
antibody (1:1,000; cat. no. AF1282), P65 rabbit monoclonal antibody
(1:1,000; cat. no. AF1234), p-NF-κB P65 (Ser536) rabbit polyclonal
antibody (1:500; cat. no. AF5881), β-actin rabbit monoclonal
antibody (1:2,000; cat. no. AF5003) and HRP-labeled goat
anti-rabbit IgG (1:1,000; cat. no. A0208).
WB
ECA-109 cells were harvested and lysed in
pre-chilled RIPA buffer (cat. no. P0013B; Beyotime Institute of
Biotechnology) on ice for 30 min. The supernatants were collected
after centrifugation of lysates at 13,300 × g for 30 min at 4°C.
The concentrations of proteins were quantified using a NanoDrop
ND-2000 spectrophotometer (NanoDrop; Thermo Fisher Scientific,
Inc.). Subsequently, ~20 µg proteins were separated by SDS-PAGE on
10% gels. The proteins were then transferred onto PVDF membranes
(cat. no. IPVH00010; MilliporeSigma) and blocked with 5% skim milk
(cat. no. P0216; Beyotime Institute of Biotechnology) for 2 h at
room temperature (RT). The membranes were incubated with primary
antibodies overnight at 4°C, then incubated with the secondary
antibody for 2 h at RT. Finally, the bands were observed by
chemiluminescence using SuperSignal™ West Pico PLUS (cat. no.
34577; Thermo Fisher Scientific, Inc.), and images were captured
using a gel imaging system (Chemidoc MP; Bio-Rad Laboratories,
Inc.) and blots were analyzed using ImageJ software (version 1.8.0;
National Institutes of Health).
Statistical analysis
All data from three or five independent experiments
are presented as the mean ± SD, and were analyzed by SPSS 23.0 (IBM
Corp.) or GraphPad Prism 8.3 software (GraphPad Software, Inc.).
Unpaired Student's t-test was performed to analyze the differences
between two groups. One-way ANOVA followed by Sidak's multiple
comparisons test was used to analyze the differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
4-MD alleviates the proliferation and
migration of ECA-109 cells
To explore the inhibitory effect of 4-MD on EC
cells, ECA-109 cells were treated with different concentrations of
4-MD for 24 h. As shown in Fig. 1A,
the IC50 of 4-MD was 15.79 µmol/l and the 95% CI of
IC50 was 10.03-24.97 µmol/l. Subsequently, 20 µmol/l
4-MD was used for further studies. ECA-109 cells were treated with
4-MD, and the proliferation of cells was analyzed by CCK-8 and
colony formation assays. Compared with those in the untreated
control group, the OD values of cells treated with 4-MD were
significantly lower at 36 and 48 h (Fig. 1B). In addition, the colonies of
cells treated with 4-MD were fewer and smaller than those in the
control group (Fig. 1C). A
wound-healing assay was performed to evaluate the migration of
ECA-109 cells treated with or without 4-MD for 48 h. The migration
rate of control cells was ~80%, whereas the migration rate of cells
treated with 4-MD was ~60%, thus suggesting that the migration of
ECA-109 cells was suppressed by 4-MD (Fig. 1D). The expression levels of
proliferation-associated proteins were determined by WB. The
results revealed that the protein expression levels of cyclin D1,
CDK1 and PCNA were significantly downregulated in cells treated
with 4-MD compared with those in the control group (Fig. 1E). These results indicated that 4-MD
inhibited proliferation and migration, and downregulated the
expression levels of cyclin D1, CDK1 and PCNA in ECA-109 cells.
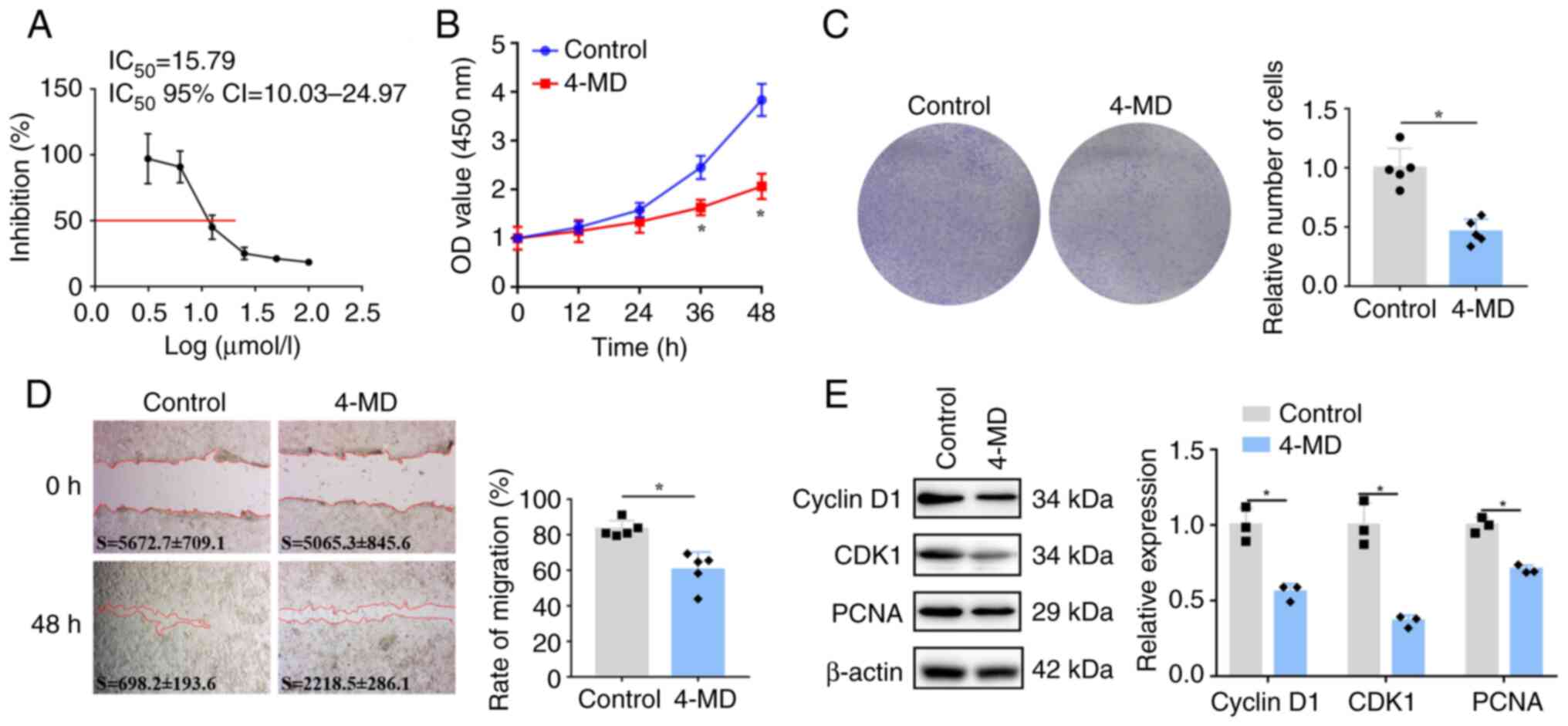 | Figure 1.4-MD inhibits the proliferation and
migration of ECA-109 cells. (A) ECA-109 cells were treated with
different concentrations of 4-MD for 24 h. The IC50 of
4-MD was 15.79 µmol/l, as determined by the CCK-8 assay. Cells were
then treated with 20 µmol/l 4-MD. (B) Proliferation and (C) colony
formation (×40 magnification) were analyzed by CCK-8 and colony
formation assays, respectively. (D) Migration was measured by a
wound-healing assay (×40 magnification); 4-MD inhibited the
migration of ECA-109 cells. S represents the scratch area in the
images; there was no significant difference in the scratch area at
0 h between the groups. (E) Expression levels of cyclin D1, CDK1
and PCNA were evaluated by WB in ECA-109 cells. Data were obtained
from three (for WB) or five (for CCK-8, colony formation and
wound-healing assays) independent experiments, and are presented as
the mean ± SD. *P<0.05 vs. control (unpaired Student's t-test).
4-MD, 4-methoxydalbergione; CCK-8, Cell Counting Kit-8; CDK1,
cyclin-dependent kinase 1; IC50, half maximal inhibitory
concentration; OD, optical density; PCNA, proliferating cell
nuclear antigen; WB, western blotting. |
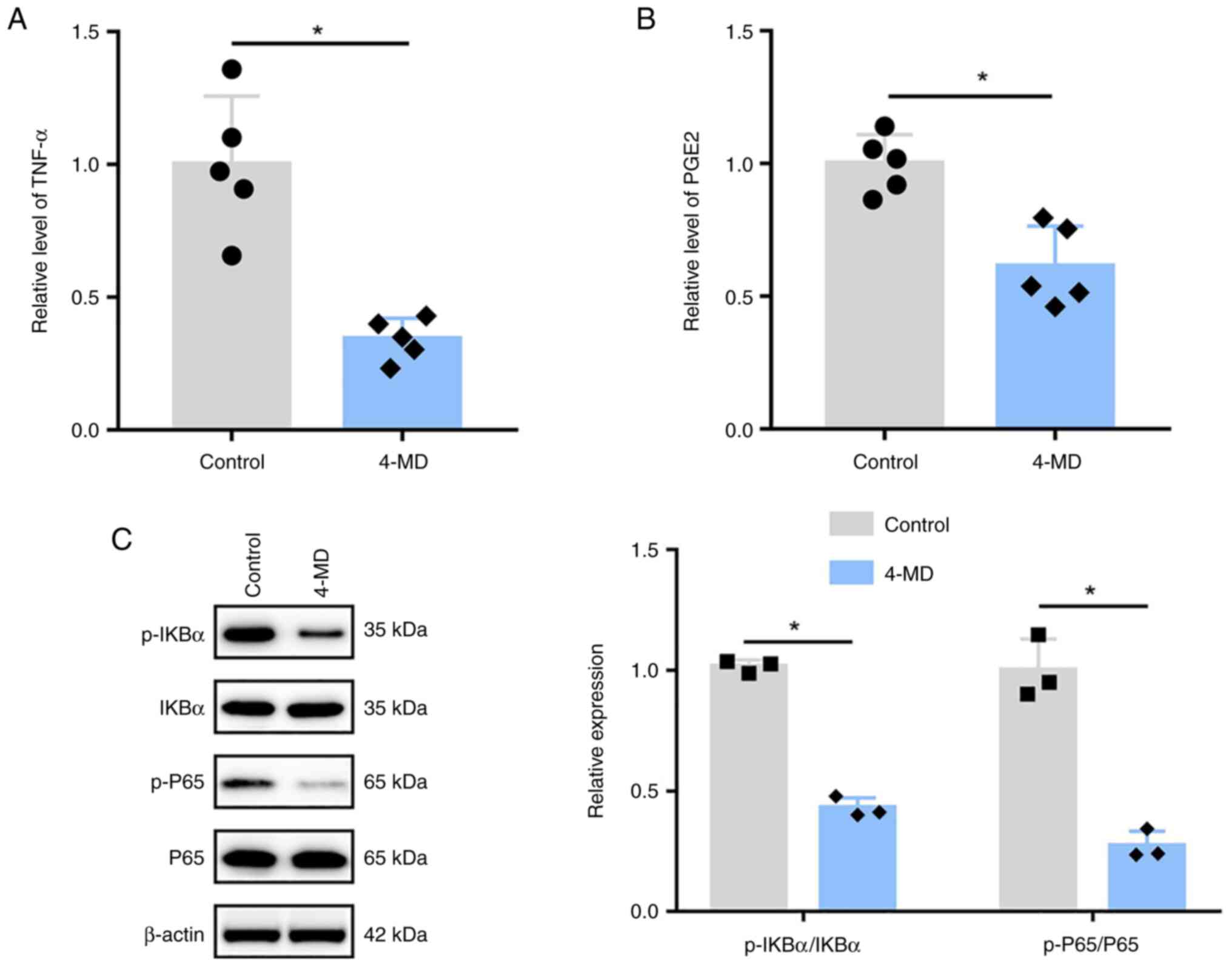
4-MD reduces the production of
inflammatory cytokines in ECA-109 cells
To investigate the inhibitory effects of 4-MD on
inflammation, the levels of inflammatory cytokines, including TNF-α
and PGE2, were measured in ECA-109 cells by ELISA. As shown in
Fig. 2A and B, the production of
TNF-α and PGE2 was significantly decreased in ECA-109 cells treated
with 4-MD. In addition, WB was performed to measure the expression
levels of IKBα and P65. Compared with those in the control groups,
the expression levels of p-IKBα and p-P65 were decreased in cells
treated with 4-MD, thus suggesting that 4-MD could inactivate NF-κB
in ECA-109 cells (Fig. 2C).
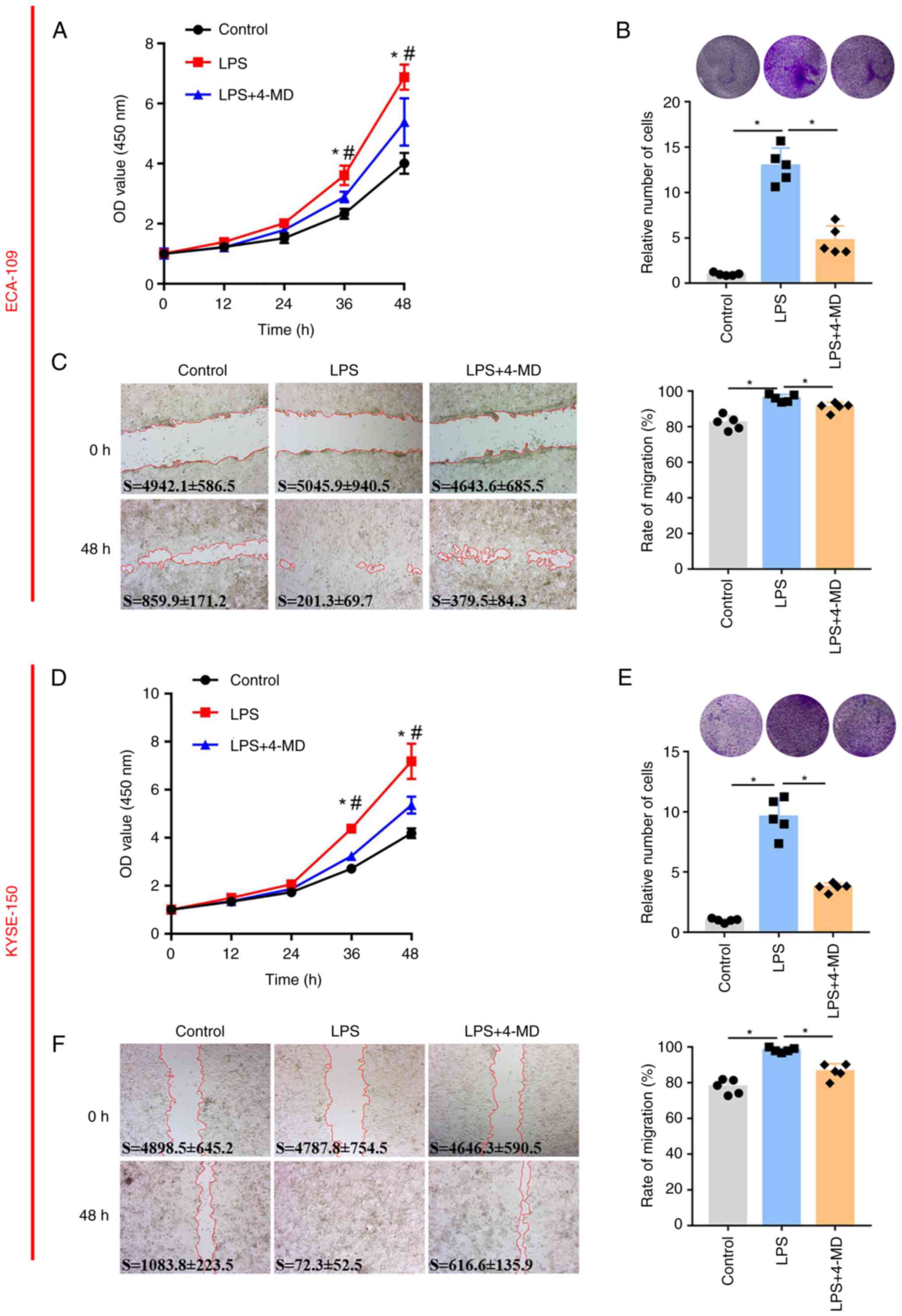
4-MD reverses the LPS-induced
proliferation and migration of EC cells
To further assess whether 4-MD inhibits the
proliferation and migration of EC cells by inactivating NF-κB,
ECA-109 cells were treated with LPS to activate NF-κB, and
subsequently treated with 4-MD. Results of the CCK-8 assay revealed
that the proliferation of cells pre-incubated with LPS was
significantly increased compared with that in the control group,
whereas cell proliferation was inhibited following treatment with
LPS and 4-MD, thus indicating that 4-MD partly inhibited the
LPS-induced increase in cell proliferation (Fig. 3A). Furthermore, the size and number
of cell colonies was increased in the LPS group compared with that
in the control group, whereas the colonies in the group treated
with LPS and 4-MD were fewer and smaller compared with in the group
treated with LPS alone, indicating that 4-MD suppressed the
acceleration of cell proliferation induced by LPS in ECA-109 cells
(Fig. 3B). Cell migration was
analyzed by wound healing. The migration rate of the control cells
was ~80% 48 h after scratching; however, the migration rate was
~100% in the group of cells treated with LPS, and was ~90% in the
group of cells treated with LPS and 4-MD, which suggested that 4-MD
could partly abolish the LPS-induced increase in cell migration
(Fig. 3C). To fully demonstrate the
inhibitory effect of 4-MD on another EC cell line, KYSE-105 cells
were treated with LPS and 4-MD, and proliferation and migration
were measured. Notably, the results of CCK-8, colony formation and
wound-healing assays in KYSE-105 cells were consistent with the
results in ECA-109 cells, which indicated that 4-MD could inhibit
the LPS-induced increase in the proliferation and migration of
KYSE-105 cells (Fig. 3D-F).
4-MD inhibits the production of TNF-α
and PGE2, and the expression levels of proliferation-related
proteins by inactivating NF-κB in ECA-109 cells
To elucidate the potential mechanism underlying the
suppressive effects of 4-MD on the proliferation and migration of
EC cells, ECA-109 cells were incubated with LPS and 4-MD. As shown
in Fig. 4A, the expression levels
of cyclin D1, CDK1 and PCNA were increased in cells treated with
LPS compared with those in the control group; meanwhile, the
expression levels of these proteins were downregulated in cells
treated with LPS and 4-MD compared with those in cells treated with
LPS alone.
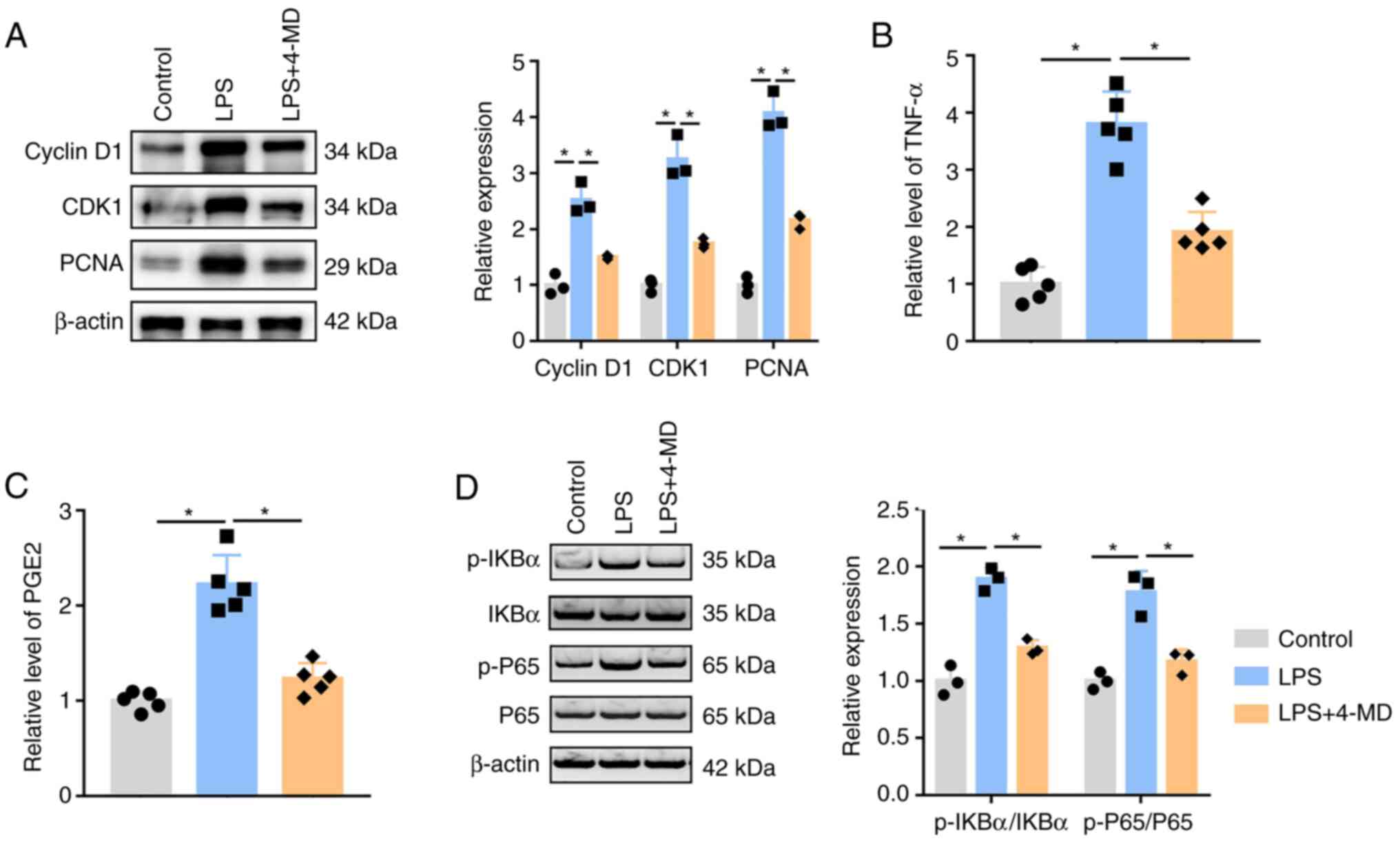 | Figure 4.4-MD downregulates the levels of
TNF-α, PGE2 and proliferation-related proteins by inhibiting NF-κB
in ECA-109 cells. (A) Expression levels of cyclin D1, CDK1 and PCNA
were measured by WB, which indicated that 4-MD inhibited the
LPS-induced upregulation of proliferation-related proteins in
ECA-109 cells. (B) TNF-α and (C) PGE2 levels were evaluated by
ELISA in the culture medium of ECA-109 cells treated with or
without LPS and 4-MD; 4-MD inhibited the LPS-induced increase in
the levels of TNF-α and PGE2. (D) Expression levels of
NF-κB-related proteins were analyzed by WB, which indicated that
4-MD abolished the LPS-induced activation of the NF-κB signaling
pathway in ECA-109 cells. Data were obtained from three (for WB) or
five (for ELISA) independent experiments, and are presented as the
mean ± SD. *P<0.05 (one-way ANOVA). 4-MD, 4-methoxydalbergione;
CDK11 cyclin-dependent kinase 1; LPS, lipopolysaccharide; p-,
phosphorylated; PCNA, proliferating cell nuclear antigen; PGE2,
prostaglandin E2; TNF-α, tumor necrosis factor α; WB, western
blotting. |
The production of TNF-α and PGE2 was analyzed by
ELISA. The levels of TNF-α (Fig.
4B) and PGE2 (Fig. 4C) were
increased in cells treated with LPS compared with those in the
control group. By contrast, compared with in cells treated with LPS
alone, the levels of TNF-α and PGE2 were decreased in cells treated
with LPS and 4-MD. Compared with in the control cells, the protein
expression levels of p-IKBα and p-P65 were increased in cells
treated with LPS, whereas p-IKBα and p-P65 expression levels were
significantly decreased in cells treated with LPS and 4-MD compared
with those in cells treated with LPS alone (Fig, 4D), which indicated that 4-MD could
inhibit LPS-induced activation of NF-κB.
Taken together, these results suggested that 4-MD
inhibited the proliferation and migration of EC cells by decreasing
TNF-α and PGE2 production, and downregulated cyclin D1, CDK1 and
PCNA expression through inactivating the NF-κB pathway.
Discussion
Approximately 25% of cancer is caused by
inflammation, including EC (36).
EC is an aggressive malignant tumor of the digestive system, which
is seriously life-threatening and has a complex pathogenesis.
Studies have shown that microbial infections serve a catalytic role
in EC development (37–39). Furthermore, it has been suggested
that inflammation triggered by infections is a notable cause of
cancer (40,41). Increasing evidence has shown that
inflammation is the main cause of EC induction (19). In the present study, the
inflammatory inducer LPS (42,43)
was used to promote inflammation in EC cells, which simulated the
inflammatory response of EC cells.
Esophagectomy is one of the main treatments for
advanced EC but is likely to impair quality of life (44). In a previous study, the single-cell
transcriptomic analysis of EC indicated that genes related to
macrophages and neutrophils were activated in early esophageal
tissue, which created a chronic inflammatory environment that
accelerated the progression of EC (18). During the follow-up of patients with
EC, it has been revealed that the levels of inflammatory cytokines
in recurrent EC are significantly higher than those in patients
without recurrence, and inflammation is positively associated with
the recurrence of EC, thus suggesting that inflammation could
accelerate EC recurrence (45).
Under inflammatory stimulation, the expression levels of
microRNA-302b have been reported to be decreased in EC cells,
resulting in increased expression levels of ERBB4, IRF2 and CXCR4,
which may promote tumor cell growth (46). These findings suggested that
inflammation could promote the development and recurrence of EC.
Therefore, inhibiting inflammation may be a therapeutic strategy
for the treatment of EC. Patients with EC undergoing continuous
oral administration of vitamin C exhibited reduced drug resistance
via the inactivation of NF-κB (47). Furthermore, curcumin has been shown
to enhance the sensitivity of EC to chemotherapy by reducing the
expression levels of cyclooxygenase-2 and lipoxygenase through
inhibiting the inflammatory response (48). Natural astaxanthin (49) and lycopene (50) may also significantly inhibit the
occurrence of EC by suppressing NF-κB activity. These results
indicated that NF-κB may be an effective molecular target for the
treatment of EC.
Notably, the active ingredients of TCM have
attracted attention due to their inhibitory effects on cancer. A
previous study showed that sulforaphene activated the
GADD45B/MAP2K3/p38/p53 feedback loop, and downregulated the
expression levels of SCD and CDH3 to alleviate the progression of
EC (51). Echinatin has also been
shown to inhibit the proliferation and invasion of EC cells by
inducing AKT/mTOR signaling pathway-dependent autophagy and
apoptosis (52). These findings
indicated that the active ingredients of TCM may act on different
genes/signaling pathways to inhibit the progression of EC. 4-MD was
isolated and purified from D. sissoo Roxb. It has been shown
that 4-MD can suppress the proliferation and induce apoptosis of
human osteosarcoma cells by downregulating the JAK2/STAT3 pathway
in vitro and in vivo (15). 4-MD can also effectively arrest the
cell cycle of astroglioma cells in G2 phase, and
regulate multiple genes that enrich the cell cycle and the p53, TNF
and MAPK signaling pathways (16).
4-MD has been reported to attenuate the proliferation of bladder
cancer cells by inducing autophagy and suppressing the Akt/ERK
signaling pathway in vitro (17). The present study revealed that the
OD value and the number of cell colonies were reduced, the size of
colonies was smaller, and wound healing was significantly inhibited
in EC cells treated with 4-MD. Furthermore, WB showed that the
expression levels of cyclin D1, CDK1 and PCNA were decreased in EC
cells treated with 4-MD. These results indicated that 4-MD may be a
potential drug for the treatment of EC that functions by
downregulating cyclin D1, CDK1 and PCNA.
It has previously been reported that 4-MD can
suppress inflammatory responses in addition to being a potent
inhibitor of cancer. 4-MD has been demonstrated to exert
cytoprotection by inducing anti-inflammatory effects, specifically
via inhibiting the LPS-induced production of nitric oxide and PGE2
in microglia (53) and reducing
LPS-induced inflammation in RAW264.7 cells (54,55).
In the present study, the levels of TNF-α and PGE2 were decreased
in EC cells treated with 4-MD, as determined by ELISA.
Subsequently, the related proteins in the NF-κB signaling pathway
were analyzed by WB, and the results revealed that p-IKBα and p-P65
expression levels were significantly reduced, thus suggesting that
4-MD reduced inflammatory cytokines by inactivating NF-κB in EC
cells. To confirm that 4-MD inhibited the proliferation and
migration of EC cells by suppressing inflammation, EC cells were
treated with the NF-κB agonist LPS. Proliferation, migration, and
TNF-α and PGE2 levels were markedly increased, and the NF-κB
signaling pathway was activated in response to LPS. These results
were consistent with the finding that LPS can promote the
proliferation of EC cells (35,56).
Following activation of NF-κB, EC cells were treated with 4-MD.
Notably, 4-MD partially reversed the LPS-induced increases in
proliferation, migration and inflammatory cytokines (TNF-α and
PGE2), and activation of the NF-κB signaling pathway.
In conclusion, the results of the present study
demonstrated that 4-MD significantly inhibited proliferation and
migration by inactivating the NF-κB signaling pathway in EC cells,
thus providing a novel strategy for 4-MD-induced inhibition of
NF-κB in the treatment of EC. However, the present study has the
following limitations: First, esophageal inflammation is one of the
pathogeneses that accelerate the occurrence of EC. 4-MD can inhibit
the malignant characteristics of EC cells by reducing the release
of inflammatory factors; however, the present study does not show
that the antitumor effect of 4-MD is superior to other antitumor
drugs. Second, the present study revealed that 4-MD inhibited EC by
inactivating the NF-κB signaling pathway; however, whether the
inactivation of NF-κB is the dominant role of 4-MD in tumor
suppression requires further investigation. Third, 4-MD has only
been demonstrated to inhibit the proliferation of EC cells by
suppressing NF-κB in vitro; therefore, further studies are
needed to explore the inhibitory effect of 4-MD on EC in
vivo.
Acknowledgements
Not applicable.
Funding
This work is supported by the Scientific Research Fund of Hunan
Provincial Education Department (grant no. 21A0612), the Hunan
Provincial Natural Science Foundation of China (grant no.
2021JJ30482), the Guangxi Key Laboratory of Molecular Medicine in
Liver Injury and Repair (grant no. GXLIRMMKL-K202006) and the
Rehabilitation project of Hunan Disabled Persons' Federation (grant
no. 2022XK0223).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
QZ and FC designed the study and revised the
manuscript. ML wrote the manuscript, performed cell culture and
migration assays, and analyzed the data. YB, PY, LW, TZ, ZX and XL
performed the experiments, including the proliferation assay and
WB. KZ performed the WB experiment shown in Fig. 4A, analyzed these data, described the
relevant results and financially sponsored this study. All authors
read and approved the final manuscript. ML and FC confirm the
authenticity of all the raw data.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
4-MD
|
4-methoxydalbergione
|
|
EC
|
esophageal cancer
|
|
LPS
|
lipopolysaccharide
|
|
CCK-8
|
Cell Counting Kit-8
|
|
OD
|
optical density
|
|
TCM
|
traditional Chinese medicine
|
|
WB
|
western blotting
|
|
RT
|
room temperature
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rogers JE, Sewastjanow-Silva M, Waters RE
and Ajani JA: Esophageal cancer: Emerging therapeutics. Expert Opin
Ther Targets. 26:107–117. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fan J, Liu Z, Mao X, Tong X, Zhang T, Suo
C and Chen X: Global trends in the incidence and mortality of
esophageal cancer from 1990 to 2017. Cancer Med. 9:6875–6887. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Uhlenhopp DJ, Then EO, Sunkara T and
Gaduputi V: Epidemiology of esophageal cancer: Update in global
trends, etiology and risk factors. Clin J Gastroenterol.
13:1010–1021. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Huang K, Zhang P, Zhang Z, Youn JY, Wang
C, Zhang H and Cai H: Traditional Chinese Medicine (TCM) in the
treatment of COVID-19 and other viral infections: Efficacies and
mechanisms. Pharmacol Ther. 225:1078432021. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang S, Zhang Z, Li W, Kong F, Yi P,
Huang J, Mao D, Peng W and Zhang S: Network Pharmacology-Based
prediction and verification of the active ingredients and potential
targets of Zuojinwan for treating colorectal cancer. Drug Des Devel
Ther. 14:2725–2740. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Z, Zhang Q, Yu L, Zhu J, Cao Y and
Gao X: The signaling pathways and targets of traditional Chinese
medicine and natural medicine in triple-negative breast cancer. J
Ethnopharmacol. 264:1132492021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jingjing H, Hongna H, Xiaojiao W, Yan G,
Yuexue Z and Yueqiang H: Bie Jia Jian pill enhances the
amelioration of bone mesenchymal stem cells on hepatocellular
carcinoma progression. J Nat Med. 76:49–58. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kan Y, Song M, Cui X, Yang Q, Zang Y, Li
Q, Li Y, Cai W, Chen Y, Weng X, et al: Muyin extract inhibits
non-small-cell lung cancer growth by inducing autophagy and
apoptosis in vitro and in vivo. Phytomedicine. 96:1538342022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong L, Lu X, Chen X, Wu Y, Zhang Y, Shi H
and Li J: Qigesan inhibits esophageal cancer cell invasion and
migration by inhibiting Gas6/Axl-induced epithelial-mesenchymal
transition. Aging (Albany NY). 12:9714–9725. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wu Z, Zhao Y, Yu F, Shi H and Li J:
Qigefang inhibits migration, invasion, and metastasis of ESCC by
Inhibiting Gas6/Axl signaling pathway. Recent Pat Anticancer Drug
Discov. 16:285–294. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li H, Ma C, Chang S, Xi X, Shao S, Chen M,
Ren J, Sun M and Dong L: Traditional Chinese Medicine decoctions
improve longevity following diagnosis with stage IV esophageal
squamous cell carcinoma: A retrospective analysis. Int J Gen Med.
15:1665–1675. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Funakoshi-Tago M, Okamoto K, Izumi R, Tago
K, Yanagisawa K, Narukawa Y, Kiuchi F, Kasahara T and Tamura H:
Anti-inflammatory activity of flavonoids in Nepalese propolis is
attributed to inhibition of the IL-33 signaling pathway. Int
Immunopharmacol. 25:189–198. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirano T, Abe K, Gotoh M and Oka K: Citrus
flavone tangeretin inhibits leukaemic HL-60 cell growth partially
through induction of apoptosis with less cytotoxicity on normal
lymphocytes. Br J Cancer. 72:1380–1388. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Park KR, Yun HM, Quang TH, Oh H, Lee DS,
Auh QS and Kim EC: 4-Methoxydalbergione suppresses growth and
induces apoptosis in human osteosarcoma cells in vitro and in vivo
xenograft model through down-regulation of the JAK2/STAT3 pathway.
Oncotarget. 7:6960–6971. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li R, Xu CQ, Shen JX, Ren QY, Chen DL, Lin
MJ, Huang RN, Li CH, Zhong RT, Luo ZH, et al: 4-Methoxydalbergione
is a potent inhibitor of human astroglioma U87 cells in vitro and
in vivo. Acta Pharmacol Sin. 42:1507–1515. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du H, Tao T, Xu S, Xu C, Li S, Su Q, Yan
J, Liu B and Li R: 4-Methoxydalbergione inhibits bladder cancer
cell growth via inducing autophagy and inhibiting Akt/ERK signaling
pathway. Front Mol Biosci. 8:7896582022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yao J, Cui Q, Fan W, Ma Y, Chen Y, Liu T,
Zhang X, Xi Y, Wang C, Peng L, et al: Single-cell transcriptomic
analysis in a mouse model deciphers cell transition states in the
multistep development of esophageal cancer. Nat Commun.
11:37152020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ekheden I, Ludvigsson JF, Yin L, Elbe P
and Ye W: Esophageal abnormalities and the risk for
gastroesophageal cancers-a histopathology-register-based study in
Sweden. Eur J Epidemiol. 37:401–411. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Keeley BR, Islami F, Pourshams A, Poustchi
H, Pak JS, Brennan P, Khademi H, Genden EM, Abnet CC, Dawsey SM, et
al: Prediagnostic serum levels of inflammatory biomarkers are
correlated with future development of lung and esophageal cancer.
Cancer Sci. 105:1205–1211. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu C, Wang M, Zhou Q and Shi H:
Associations of changes in intestinal flora and inflammatory
factors with prognosis of patients with esophageal cancer. J
Healthc Eng. 2022:24263012022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sugawara K, Yagi K, Okumura Y, Aikou S,
Yamashita H and Seto Y: Survival prediction capabilities of
preoperative inflammatory and nutritional status in esophageal
squamous cell carcinoma patients. World J Surg. 46:639–647. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen Y, Wang D, Peng H, Chen X, Han X, Yu
J, Wang W, Liang L, Liu Z, Zheng Y, et al: Epigenetically
upregulated oncoprotein PLCE1 drives esophageal carcinoma
angiogenesis and proliferation via activating the PI-PLCℇ-NF-κB
signaling pathway and VEGF-C/Bcl-2 expression. Mol Cancer.
18:12019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sojoodi M, Erstad DJ, Barrett SC, Salloum
S, Zhu S, Qian T, Colon S, Gale EM, Jordan VC, Wang Y, et al:
Peroxidasin deficiency re-programs macrophages toward
pro-fibrolysis function and promotes collagen resolution in liver.
Cell Mol Gastroenterol Hepatol. 13:1483–1509. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Balzano T, Arenas YM, Dadsetan S, Forteza
J, Gil-Perotin S, Cubas-Nuñez L, Casanova B, Gracià F,
Varela-Andrés N, Montoliu C, et al: Sustained hyperammonemia
induces TNF-a IN Purkinje neurons by activating the TNFR1-NF-κB
pathway. J Neuroinflammation. 17:702020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yamada T, Osawa S, Ikuma M, Kajimura M,
Sugimoto M, Furuta T, Iwaizumi M and Sugimoto K: Guggulsterone, a
plant-derived inhibitor of NF-TB, suppresses CDX2 and COX-2
expression and reduces the viability of esophageal adenocarcinoma
cells. Digestion. 90:208–217. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhao J, Bi W, Xiao S, Lan X, Cheng X,
Zhang J, Lu D, Wei W, Wang Y, Li H, et al: Neuroinflammation
induced by lipopolysaccharide causes cognitive impairment in mice.
Sci Rep. 9:57902019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sousa FBM, Pacheco G, Oliveira AP, Nicolau
LAD, Lopes ALF, Ferreira-Fernandes H, Pinto GR and Medeiros JVR:
Mechanism of preservation of the intestinal mucosa architecture and
NF-κB/PGE2 reduction by hydrogen sulfide on cholera toxin-induced
diarrhea in mice. Life Sci. 284:1198692021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Voce DJ, Bernal GM, Cahill KE, Wu L,
Mansour N, Crawley CD, Campbell PS, Arina A, Weichselbaum RR and
Yamini B: CDK1 is up-regulated by temozolomide in an NF-κB
dependent manner in glioblastoma. Sci Rep. 11:56652021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Han D, Zhu S, Li X, Li Z, Huang H, Gao W,
Liu Y, Zhu H and Yu X: The NF-κB/miR-488/ERBB2 axis modulates
pancreatic cancer cell malignancy and tumor growth through cell
cycle signaling. Cancer Biol Ther. 23:294–309. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Gu P, Zhang M, Zhu J, He X and Yang D:
Suppression of CDCA3 inhibits prostate cancer progression via
NF-κB/cyclin D1 signaling inactivation and p21 accumulation. Oncol
Rep. 47:422022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang X, Liu X, Yang Y and Yang D: Cyclin
D1 mediated by the nuclear translocation of nuclear factor kappa B
exerts an oncogenic role in lung cancer. Bioengineered.
13:6866–6879. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhao Y, Liu C, Gao Z, Shao D, Zhao X, Wei
Q and Ma B: G protein-coupled estrogen receptor 1 mediates
proliferation and adipogenic differentiation of goat
adipose-derived stem cells through ERK1/2-NF-κB signaling pathway.
Acta Biochim Biophys Sin (Shanghai). 54:494–503. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang W and Wang Y: Activation of
RIPK2-mediated NOD1 signaling promotes proliferation and invasion
of ovarian cancer cells via NF-κB pathway. Histochem Cell Biol.
157:173–182. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zu Y, Ping W, Deng T, Zhang N, Fu X and
Sun W: Lipopolysaccharide-induced toll-like receptor 4 signaling in
esophageal squamous cell carcinoma promotes tumor proliferation and
regulates inflammatory cytokines expression. Dis Esophagus. 30:1–8.
2017.PubMed/NCBI
|
|
36
|
Kawanishi S, Ohnishi S, Ma N, Hiraku Y and
Murata M: Crosstalk between DNA Damage and inflammation in the
multiple steps of carcinogenesis. Int J Sci. 18:18082017.
|
|
37
|
Holleczek B, Schottker B and Brenner H:
Helicobacter pylori infection, chronic atrophic gastritis and risk
of stomach and esophagus cancer: Results from the prospective
population-based ESTHER cohort study. Int J Cancer. 146:2773–2783.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhou Q, Wei Y, Zhai H, Li S, Xu R and Li
P: Comorbid early esophageal cancer and Gongylonema pulchrum
infection: A case report. BMC Gastroenterol. 21:3052021. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kawasaki M, Ikeda Y, Ikeda E, Takahashi M,
Tanaka D, Nakajima Y, Arakawa S, Izumi Y and Miyake S: Oral
infectious bacteria in dental plaque and saliva as risk factors in
patients with esophageal cancer. Cancer. 127:512–519. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fujiwara N, Kitamura N, Yoshida K,
Yamamoto T, Ozaki K and Kudo Y: Involvement of fusobacterium
species in oral cancer progression: A literature review including
other types of cancer. Int J Mol Sci. 21:62072020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Tian C, Chen K, Gong W, Yoshimura T, Huang
J and Wang JM: The G-Protein coupled formyl peptide receptors and
their role in the progression of digestive tract cancer. Technol
Cancer Res Treat. 19:15330338209732802020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu CH, Chen Z, Chen K, Liao FT, Chung CE,
Liu X, Lin YC, Keohavong P, Leikauf GD and Di YP:
Lipopolysaccharide-Mediated chronic inflammation promotes tobacco
carcinogen-induced lung cancer and determines the efficacy of
immunotherapy. Cancer Res. 81:144–157. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li M, Liu H, Shao H, Zhang P, Gao M, Huang
L, Shang P, Zhang Q, Wang W and Feng F: Glyburide attenuates B(a)p
and LPS-induced inflammation-related lung tumorigenesis in mice.
Environ Toxicol. 36:1713–1722. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Watanabe M, Otake R, Kozuki R, Toihata T,
Takahashi K, Okamura A and Mamura Y: Recent progress in
multidisciplinary treatment for patients with esophageal cancer.
Surg Today. 50:12–20. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zheng L, Jiang J, Liu Y, Zheng X and Wu C:
Correlations of recurrence after radical surgery for esophageal
cancer with glucose-lipid metabolism, insulin resistance,
inflammation, stress and serum p53 expression. J BUON.
24:1666–1672. 2019.PubMed/NCBI
|
|
46
|
Zhang M, Zhang L, Cui M, Ye W, Zhang P,
Zhou S and Wang J: miR-302b inhibits cancer-related inflammation by
targeting ERBB4, IRF2 and CXCR4 in esophageal cancer. Oncotarget.
8:49053–49063. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abdel-Latif MMM, Babar M, Kelleher D and
Reynolds JV: A pilot study of the impact of Vitamin C
supplementation with neoadjuvant chemoradiation on regulators of
inflammation and carcinogenesis in esophageal cancer patients. J
Cancer Res Ther. 15:185–191. 2019.PubMed/NCBI
|
|
48
|
Komal K, Chaudhary S, Yadav P, Parmanik R
and Singh M: The therapeutic and preventive efficacy of curcumin
and its derivatives in esophageal cancer. Asian Pac J Cancer Prev.
20:1329–1337. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Cui L, Xu F, Wang M, Li L, Qiao T, Cui H,
Li Z and Sun C: Dietary natural astaxanthin at an early stage
inhibits N-nitrosomethylbenzylamine-induced esophageal cancer
oxidative stress and inflammation via downregulation of NFκB and
COX2 in F344 rats. Onco Targets Ther. 12:5087–5096. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Cui L, Xu F, Wu K, Li L, Qiao T, Li Z,
Chen T and Sun C: Anticancer effects and possible mechanisms of
lycopene intervention on N-methylbenzylnitrosamine induced
esophageal cancer in F344 rats based on PPARү1. Eur J
Pharmacol. 881:1732302020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Han S, Wang Y, Ma J, Wang Z, Wang HD and
Yuan Q: Sulforaphene inhibits esophageal cancer progression via
suppressing SCD and CDH3 expression, and activating the
GADD45B-MAP2K3-p38-p53 feedback loop. Cell Death Dis. 11:7132020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hong P, Liu QW, Xie Y, Zhang QH, Liao L,
He QY, Li B and Xu WW: Echinatin suppresses esophageal cancer tumor
growth and invasion through inducing AKT/mTOR-dependent autophagy
and apoptosis. Cell Death Dis. 11:5242020. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Kim DC, Lee DS, Ko W, Kim KW, Kim HJ, Yoon
CS, Oh H and Kim YC: Heme Oxygenase-1-Inducing Activity of
4-Methoxydalbergione and 4′-Hydroxy-4-methoxydalbergione from
Dalbergia odorifera and their anti-inflammatory and cytoprotective
effects in murine hippocampal and BV2 microglial cell line and
primary rat microglial cells. Neurotox Res. 33:337–352. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sun K, Su C, Li W, Gong Z, Sha C and Liu
R: Quality markers based on phytochemical analysis and
anti-inflammatory screening: An integrated strategy for the quality
control of Dalbergia odorifera by UHPLC-Q-Orbitrap HRMS.
Phytomedicine. 84:1535112021. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Funakoshi-Tago M, Ohsawa K, Ishikawa T,
Nakamura F, Ueda F, Narukawa Y, Kiuchi F, Tamura H, Tago K and
Kasahara T: Inhibitory effects of flavonoids extracted from
Nepalese propolis on the LPS signaling pathway. Int
Immunopharmacol. 40:550–560. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gergen AK, Kohtz PD, Halpern AL, White AM,
Meng X, Fullerton DA and Weyant MJ: Statins Inhibit Toll-Like
Receptor 4-Mediated growth of human esophageal adenocarcinoma
cells. J Surg Res. 260:436–447. 2021. View Article : Google Scholar : PubMed/NCBI
|