Introduction
Breast cancer (BC) is the most common type of cancer
in women (1). It has been estimated
that ~284,200 new BC cases were diagnosed in the USA in 2022
(2). BC classification is based on
molecular typing, including luminal A, luminal B, HER-2+
and triple-negative BC (TNBC). Patients with luminal A BC have the
best prognosis, followed by patients with luminal B BC (3). TNBC constituted 10–15% of all BC cases
in the United States between 2012 and 2016 (4); however, due to the deletion of
established molecular targets, patients with TNBC have a poor
prognosis. Therefore, it is necessary to identify novel molecular
targets for treatment. Recently, researchers have focused on the
pathogenic mechanisms underlying TNBC. For example,
cyclin-dependent kinase 1 (CDK1) has been reported to be highly
expressed in TNBC samples (5),
whereas CDK14 can act as a tumor suppressor gene in TNBC (6). Furthermore, salt-inducible kinase 2
inhibitors may decrease DNA double-strand break repair in TNBC
(7). In addition, a new
antibody-drug conjugate, sacituzumab govitecan, which targets
trophoblast cell-surface antigen 2 was approved by the United
States Food and Drug Administration (8).
The survival rate of patients with BC has increased
with the discovery of novel therapeutics; however, 20–30% of
patients with BC experience locoregional or distant disease
recurrence worldwide (9).
Radiotherapy is an integral treatment for patients with BC to
promote breast-conserving surgery. Increasing the radiosensitivity
of patients with BC is an effective approach to solving local
recurrence. Investigating the genes associated with radiotherapy
may provide additional insight into the effects of clinical
radiotherapy on BC. Numerous factors are involved in radiation
resistance, such as DNA repair, hypoxia and malignant behavior
(10). Activating transcription
factor 3 has been reported to increase radiation resistance via the
PI3K/Akt signaling pathway (11).
By contrast, microRNA (miRNA/miR)-142-3p decreases radiation
resistance in BC (12). Moreover,
miR-122 promotes cell survival in acquired radioresistant BC
(13). Despite these studies, a
limited number of gene markers are known to be associated with
radioresistant BC and the underlying mechanism is poorly
understood.
miRNAs are a group of noncoding RNAs with a small
number of nucleotides (usually 20–30). miRNA inhibits target gene
expression by mRNA degradation (14) and dysregulation of miRNAs has been
found in numerous types of cancer (15). Our previous study revealed that
miR-93-5p was upregulated in plasma exosomes from patients with BC
(16). A further study revealed
that miR-93-5p improved the sensitivity of BC cells to radiation
(17). Given the unknown underlying
mechanism, the present study aimed to identify the related
miR-93-5p pathway for patients with TNBC in clinical and in
vivo settings. The experimental design is summarized in
Fig. 1.
Materials and methods
Database analysis
The miR-93-5p target gene was identified using three
well-known bioinformatics prediction algorithms: TargetScan
(https://www.targetscan.org/vert_71/),
microRNA (http://cbio.mskcc.org/miRNA2003/miranda.html and miRDB
(http://www.mirdb.org/miRDB). The
starBase database (http://starbase.sysu.edu.cn) was used to identify the
potential target sequences of miR-93-5p and EphA4. The expression
of miR-93-5p was analyzed in 1,085 breast cancer and 104 normal
samples from healthy individuals. The expression of EphA4 and NF-κB
was analyzed in 1,104 cancer and 113 normal samples. The expression
data of genes in cancer were downloaded from The Cancer Genome
Atlas project via the Genomic Data Commons Data Portal (https://portal.gdc.cancer.gov/). The starBase
database was also employed to assess the correlations between
miR-93-5p expression and EphA4/NF-κB expression.
Cell culture and transduction
The MDA-MB-231 cell line (cat. no. MXC234) was
purchased from The Cell Bank of Type Culture Collection of The
Chinese Academy of Sciences. This cell line was cultured in RPMI
1640 (Corning, Inc.) medium containing 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100
µg/ml streptomycin. MCF-7 cells (HTB-22; American Type Culture
Collection) were cultured in Dulbecco's modified Eagle's medium
(Cytiva) supplemented with 10% FBS, 100 U/ml penicillin and 100
µg/ml streptomycin. The cells were cultured in an atmosphere
containing 5% CO2 at 37°C.
The 2nd generation system was used to package
lentiviruses. To induce transient expression of miR-93-5p'
(5′-CTGGGGGCTCCAAAGTGCTGTTCGTGCAGGTAGTGTGATTACCCAACCTACTGCTGAGCTAGCACTTCCCGAGCCCCCGG-3′;
Quanyang) the cloning vector (pCDH-CMV-MCS-EGFP-EF1-Puro; Quanyang)
was constructed using BamHI (cat. no. NEB R0136) and
EcoRI (cat. no. NEB R0101) restriction enzymes (both from
New England BioLabs, Inc.). The lentiviral plasmid (5 µg; 1 µg/µl)
(3.75 µg pH1 packaging plasmid and 1.25 µg pH2 envelope packaging
plasmid; Beijing Yingmao Shengye Biotechnology Co., Ltd.) was
transfected into 293T (cat. no. MXC006; Shanghai Meixuan
Biotechnology Co.) cells using Lipofectamine® 2000
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C for 4
h. The viral supernatant was collected, filtered and concentrated
by ultracentrifugation (80,000 × g, 4°C, 2 h) after a 48-h
incubation with new culture medium. MDA-MB-231 cells with stable
overexpression (OE) of miR-93-5p were established by lentiviral
infection. MDA-MB-231 cells were infected with lentivirus at a MOI
of 10 with polybrene at 37°C for 24 h. Puromycin (2 µg/ml) was used
to select a stable cell line. MDA-MB-231 cells were also infected
with the empty lentiviral vector as negative control.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
RNAiso PULS (Takara Bio, Inc.) was used to isolate
total RNA from MDA-MB-231 cells and TNBC tissues. One-step RT-qPCR
was performed using SYBR Premix Ex Taq (Takara Bio, Inc.) and a CFX
96 System (Bio-Rad Laboratories, Inc.). The RT-qPCR thermal cycling
conditions were as follows: 50°C for 10 min and 95°C for 5 min,
followed by 40 cycles at 95°C for 15 sec and 60°C for 30 sec. The
dissociation stage (95°C for 15 sec, 60°C for 1 min and 95°C for 15
sec) was performed after amplification. U6 was used as an internal
control for miRNA expression. The sequences of primers used in the
present study are listed in Table
I. The standard 2−ΔΔCq method was used to calculate
relative RNA abundance (18).
 | Table I.List of primers used for reverse
transcription-quantitative PCR. |
Table I.
List of primers used for reverse
transcription-quantitative PCR.
| Gene name | Sequence,
5′-3′ |
|---|
| miR-93-5p | F:
GCGCCAAAGTGCTGTTCGTGC |
|
| R:
TGCAGGGTCCGAGGTAT |
| U6 | F:
CTCGCTTCGGCAGCACA |
|
| R:
AACGCTTCACGAATTTGCGT |
| EphA4 | F:
CTGTTCAGGGAGAGCTTGGG |
|
| R:
CCTTGTCGTTGTCCGACTCA |
| NF-κB | F:
GCAGGAACTCAAGGGAGCTAA |
|
| R:
TCCACGAACTGGCTGTTGAG |
| GAPDH | F:
GACAGCCGCATCTTCTTGTG |
|
| R:
AATCCGTTCACACCGACCTT |
Luciferase assay
Wild-type (WT) or mutant (Mut) EphA4 was amplified
and cloned into a pmirGLO (Beyotime Institute of Biotechnology)
vector. Due to the poor survival of MDA-MB-231 cells
post-transduction, the luciferase assay was only performed in MCF-7
cells. MCF-7 cells (2×105) were seeded in 96-well plates
and were co-transfected with miR-93-5p mimic or miR-negative
control (NC) and EphA4 WT or Mut plasmids, using Lipofectamine 3000
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions. The miR mimic sequences were as
follows: Hsa-miR-93-5p mimic, sense 5′-CAAAGUGCUGUUCGUGCAGGUAG-3′,
anti-sense 5′-ACCUGCACGAACAGCACUUUGUU-3′; miR-NC (nonspecific
scrambled RNA), sense 5′-UUCUCCGAACGUGUCACGUTT-3′ and anti-sense
5′-ACGUGACACGUUCGGAGAATT-3′. Cells were harvested 48 h after
transfection. Finally, the luciferase activity was measured using a
Dual-Luciferase Reporter Assay System (Promega Corporation). Using
the Renilla luciferase as an internal control, the relative
luciferase activity is presented as a ratio of firefly luciferase
intensity to Renilla luciferase intensity.
Tumor xenograft model
MDA-MB-231 cells (5×107) with or without
miR-93-5p OE were injected subcutaneously into 4-week-old BALB/c
female mice. All BALB/c nude mice (weight, 18±2 g) were purchased
from Silaike Laboratory Animal Co., Ltd. A total of 24 mice were
randomly divided into the following four groups (n=6 rats/group:
negative control; miR-93-5p OE; radiation therapy (RT) and
miR-93-5p OE + RT groups. All mice were housed in a specific
pathogen-free sterile environment with a constant temperature of
25°C, under a 12-h light/dark cycle, a relative humidity of 50–70%,
with free access to adequate food and water supply. Mice were
acclimated for 1 week before tumor injection and tumors of ~1 mm in
size were felt ~1 week after inoculation. The body weights of the
mice were measured every 3 days. The NC (negative control) group
mice were injected by MDA-MB-231 cells transfected with an empty
vector with a lentivirus. In the RT and miR-93-5p OE + RT groups, 4
Gy RT was administered three times in 1 week on days 1, 4 and 7 and
began when the tumor diameter was 5–7 mm (16 days after injection).
The mice were sacrificed by cervical dislocation on the next day
after the third irradiation. The humane endpoint was determined as
a tumor volume of 1 cm3 and no animals reached the
humane endpoint in the present study. All mice were humanely
sacrificed 4 weeks after tumor injection. Death was verified by the
cessation of breathing and heartbeat. After the mice were
sacrificed, the maximum (L) and minimum (W) lengths and weights of
tumors were determined. The tumor volume was estimated to be (L × W
× W)/2. Hematoxylin and eosin (H&E) staining was performed to
evaluate tissue morphology. Animal ethics approval was obtained
from the Medical College of Yangzhou University of Animal Ethics
Committee (approval no. YXYLL-2021-64).
Pathological section analysis
The collected mouse tumor tissues were fixed in 4%
paraformaldehyde for 24 h at room temperature, dehydrated, and
immersed in paraffin. Subsequently, 4 µm sections were cut on a
glass slide using a Leica RM2145 microtome (Leica Microsystems,
Ltd.) and allowed to dry at 40°C for 2 h before staining. Xylene
was used to dewax the slides and ethanol was used to rehydrate the
slides. H&E staining was performed for 5 min in Mayer's
hematoxylin and for 2 min in eosin at room temperature. Images of
the slides were captured using a Cewei LW300LFT LED light
microscope with a maximum magnification of ×200. H&E staining
was used to observe pathological damage under a microscope.
Western blotting
Proteins were extracted from MDA-MB-231 cells using
RIPA lysis buffer (Beyotime Institute of Biotechnology) and protein
concentration was determined using a BCA Kit (Beyotime Institute of
Biotechnology). Proteins (20 µg) were separated by SDS-PAGE on 12%
gels and were then transferred to 0.45 µm PVDF membranes
(MilliporeSigma). The membranes were blocked with 5% skim milk
powder for 2 h at room temperature and incubated overnight at 4°C
with the following primary antibodies: EphA4 1:1,000; cat. no.
A8346; ABclonal Biotech Co., Ltd.), NF-κB (1:1,000; cat. no. 8242T;
Cell Signaling Technology, Inc.), GAPDH (1:5,000; cat. no. 5174;
Cell Signaling Technology, Inc.). The membranes were then incubated
with a HRP-conjugated goat anti-rabbit IgG secondary antibody
(1:5,000; cat. no. 111-035-003; Jackson ImmunoResearch
Laboratories, Inc.) at room temperature for 2 h, and the protein
bands were detected using an ECL detection system (Bio-Rad
Laboratories, Inc.). GAPDH was used as a control. The relative
expression levels were calculated as follows: (band intensity of
target protein/band intensity of control protein); this was
measured using Image-Pro Plus 6.0 software (Media Cybernetics,
Inc.).
Patients and clinical parameters
This prospective study investigated 43 TNBC
formalin-fixed and paraffin-embedded (FFPE) samples and normal
adjacent tissue (≥0.5 cm away from TNBC tissues) from patients who
underwent surgery between January 2018 and March 2021 at the
Jiangsu Taizhou People's Hospital (Taizhou, China). FFPE samples
were fixed in 4% paraformaldehyde for 24 h at room temperature,
dehydrated and embedded in paraffin and cut into 10 µm sections.
None of the patients had received RT or chemotherapy before
surgery. The mean age was 53.02 years (age range, 29–74 years), and
the patients neither drank nor smoked. At the time of diagnosis,
the current TNM status of each patient was classified according to
the American Joint Committee on Cancer 8th edition (19). The Human Ethics Review Committee of
Jiangsu Taizhou People's Hospital approved the present study
(approval no. KY 2021-043-01). The requirement for informed consent
was waived by the Human Ethics Review Committee of Jiangsu Taizhou
People's Hospital.
RNA extraction
FFPE sections were sliced to a thickness of 10 µm
for subsequent RT-qPCR experiments. Finally, total RNA was
extracted from FFPE using the mRNA prep Pure FFPE Kit (cat. no.
DP502; Tiangen Biotech Co., Ltd.) according to the instructions
provided by the manufacturer.
Statistical analysis
The present study investigated the relationships
between miR-93/EphA4/NF-κB expression and clinicopathological
characteristics using Spearman's correlation analysis and
χ2 test. The mean ± SD of three separate experiments
were used to calculate all data. Differences between two groups
were assessed using an unpaired Student's t-test, and between
paired variables were assessed using a paired Student's t-test.
Multiple group comparisons were assessed using one-way ANOVA
followed by Bonferroni test to determine significance. P<0.05
was considered to indicate a statistically significant difference.
SPSS version 18.0 (SPSS, Inc.) was used for all statistical
analyses.
Results
miR-93-5p directly targets EphA4
3′-UTR
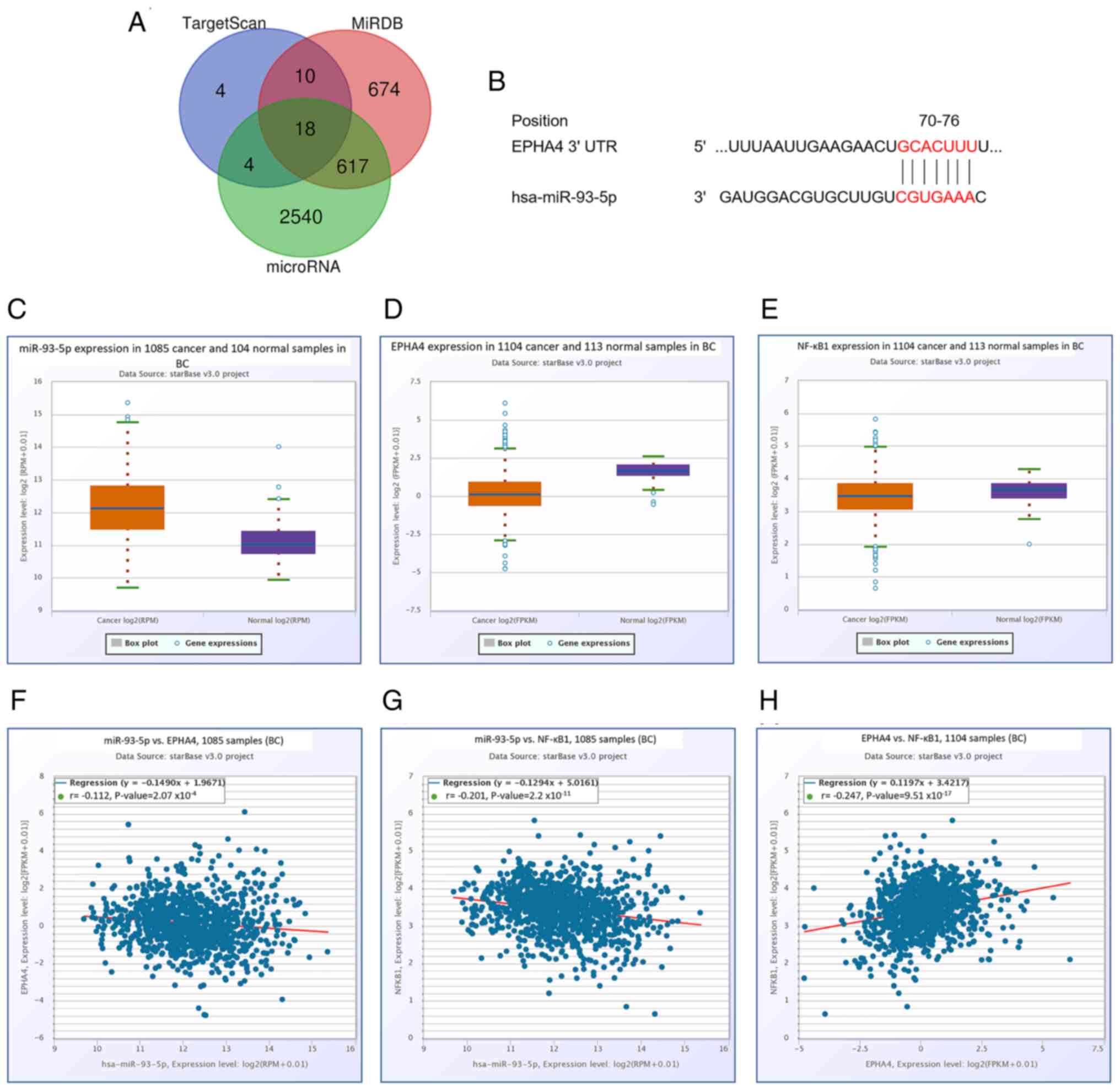
The miR-93-5p target gene was identified using three
well-known bioinformatics prediction algorithms: TargetScan,
microRNA and miRDB. Based on the results, 18 genes were predicted
to be miR-93-5p candidates (Fig.
2A). EphA4 was one of the genes that was considered a potential
target for miR-93-5p (Fig. 2B). The
online bioinformatics tool starBase demonstrated that miR-93-5p
expression was significantly higher in BC samples compared with
those in normal controls (P<0.001; Fig. 2C), whereas EphA4 expression was
significantly lower (P<0.001; Fig.
2D). The expression levels of NF-κB were also low in BC samples
(P<0.01; Fig. 2E). In addition,
a significant negative correlation was identified between miR-93-5p
and EphA4 expression (P<0.001; Fig.
2F), and the expression of miR-93-5p was negatively correlated
with NF-κB (P<0.001; Fig. 2G),
whereas a positive correlation was identified between EphA4 and
NF-κB (P<0.001; Fig. 2H);
however, all correlations were weak (r-values were
<0.3/-0.3).
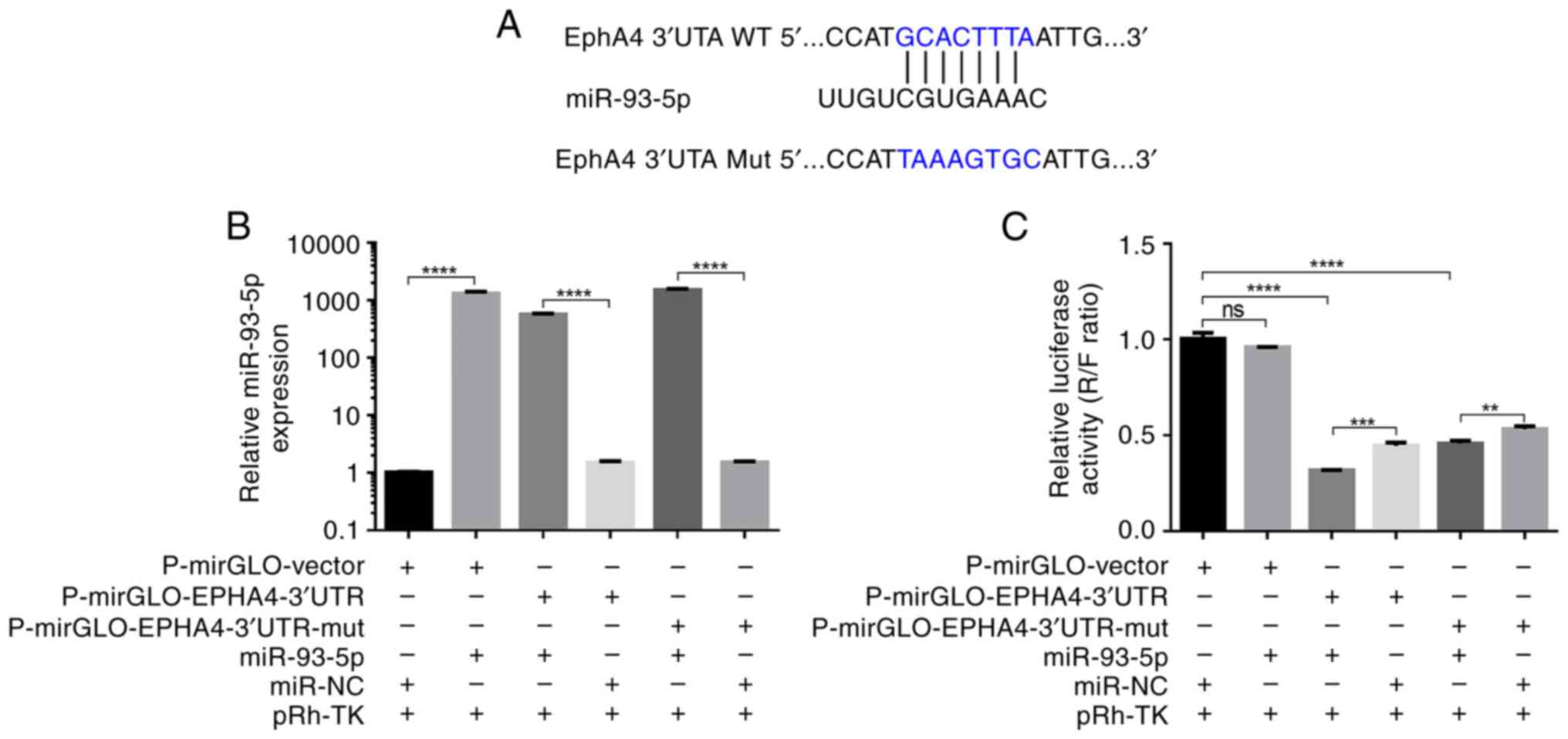
Luciferase reporter vectors containing WT and Mut
EphA4 3′-UTRs were generated to further validate the interaction
between miR-93-5p and EphA4 (Fig.
3A). A dual-luciferase reporter assay confirmed that miR-93-5p
can directly bind to the EphA4 3′-UTR. Transfection of MCF-7 cells
with miR-93-5p mimic or miR-NC revealed that cells transfected with
the miR-93-5p mimic had significantly increased miR-93-5p
expression (miR-93-5p vs. miR-NC; miR-93-5p + EphA4 vs. miR-NC +
EphA4; miR-93-5p + EphA4-Mut vs. miR-NC + EphA4-Mut; P<0.0001;
Fig. 3B). When miR-93-5p and the
p-mirGLO-EphA4-3′UTR were co-transfected into MCF-7 cells, the
luciferase signal was significantly lower than that in cells
transfected with miR-NC (P<0.001; Fig. 3C). In addition, miR-93-5p suppressed
luciferase activity when co-transfected with a luciferase reporter
containing Mut EphA4 3′-UTR (Fig.
3C). These findings indicated that the predicted binding site
and the designed Mut EphA4 were not suitable. However, it still can
be seen that the strength of the binding site in Mut EphA4 3′-UTR
was weaker than that in WT EphA4 3′-UTR. These findings indicated
that miR-93-5p directly targets the EphA4 3′-UTR.
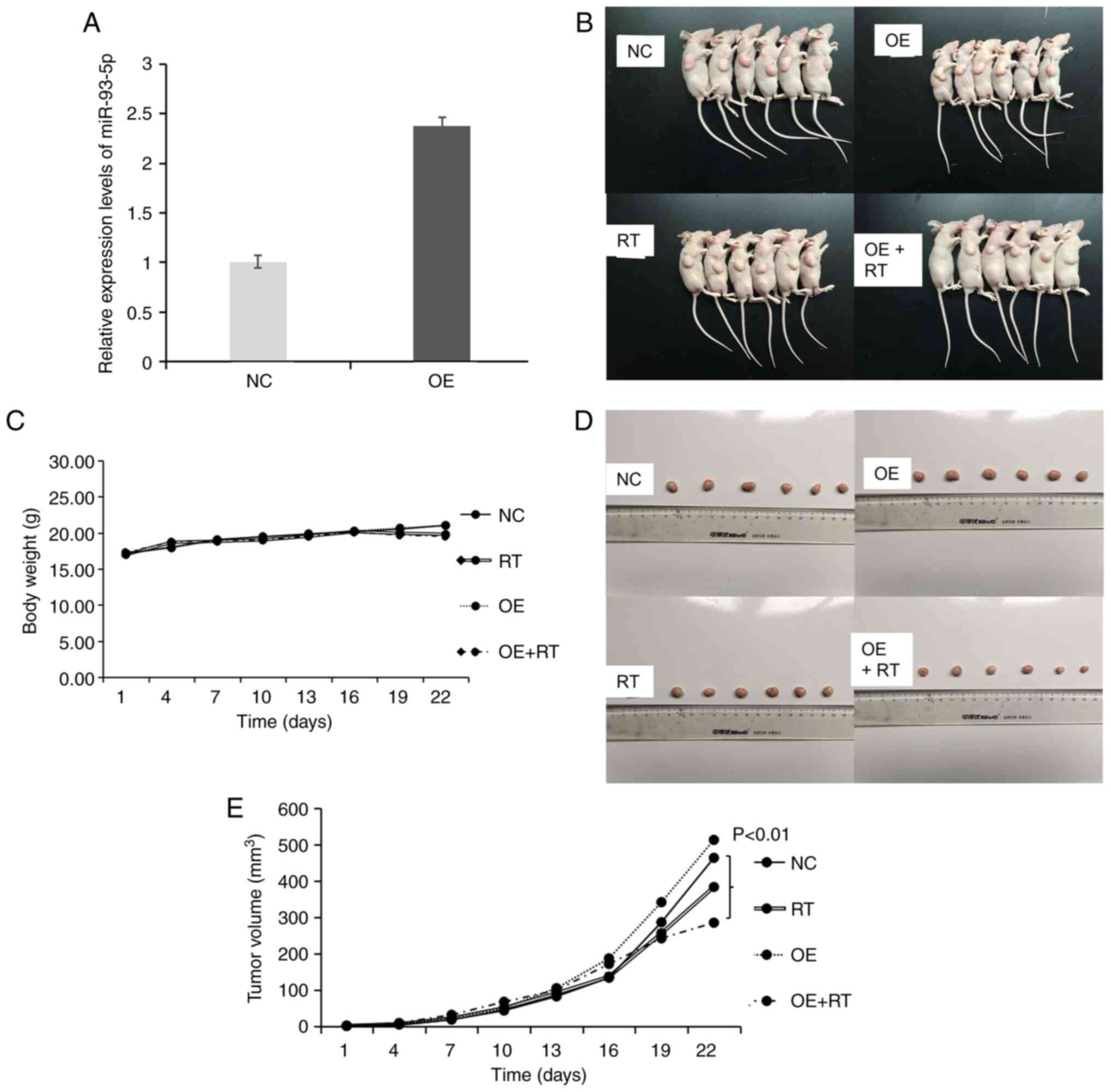
miR-93-5p OE improves the
radiosensitivity of MDA-MB-231 cells to RT in vivo
Our previous study reported that miR-93-5p could
improve the radiosensitivity of MDA-MB-231 cells (17); therefore, animal experiments were
performed to validate the role of miR-93-5p in vivo. First,
miR-93-5p OE vectors were constructed. RT-qPCR confirmed that
miR-93-5p was overexpressed in the MDA-MB-231 OE group (Fig. 4A). Subsequently, experiments with
tumor xenografts were conducted to examine whether miR-93-5p OE
rendered TNBC tumors more susceptible to RT in vivo
(Fig. 4B). Notably, the body weight
of the tumor-bearing nude mice in each group was not significantly
different (Fig. 4C). Xenograft
tumor weights were measured after the nude mice were sacrificed
(Fig. 4D). Comparing tumor sizes
revealed that the OE of miR-93-5p in the MDA-MB-231 cells used to
generate xenografts combined with concurrent RT significantly
reduced tumor formation compared with that in the NC group
(P<0.01; Fig. 4E).
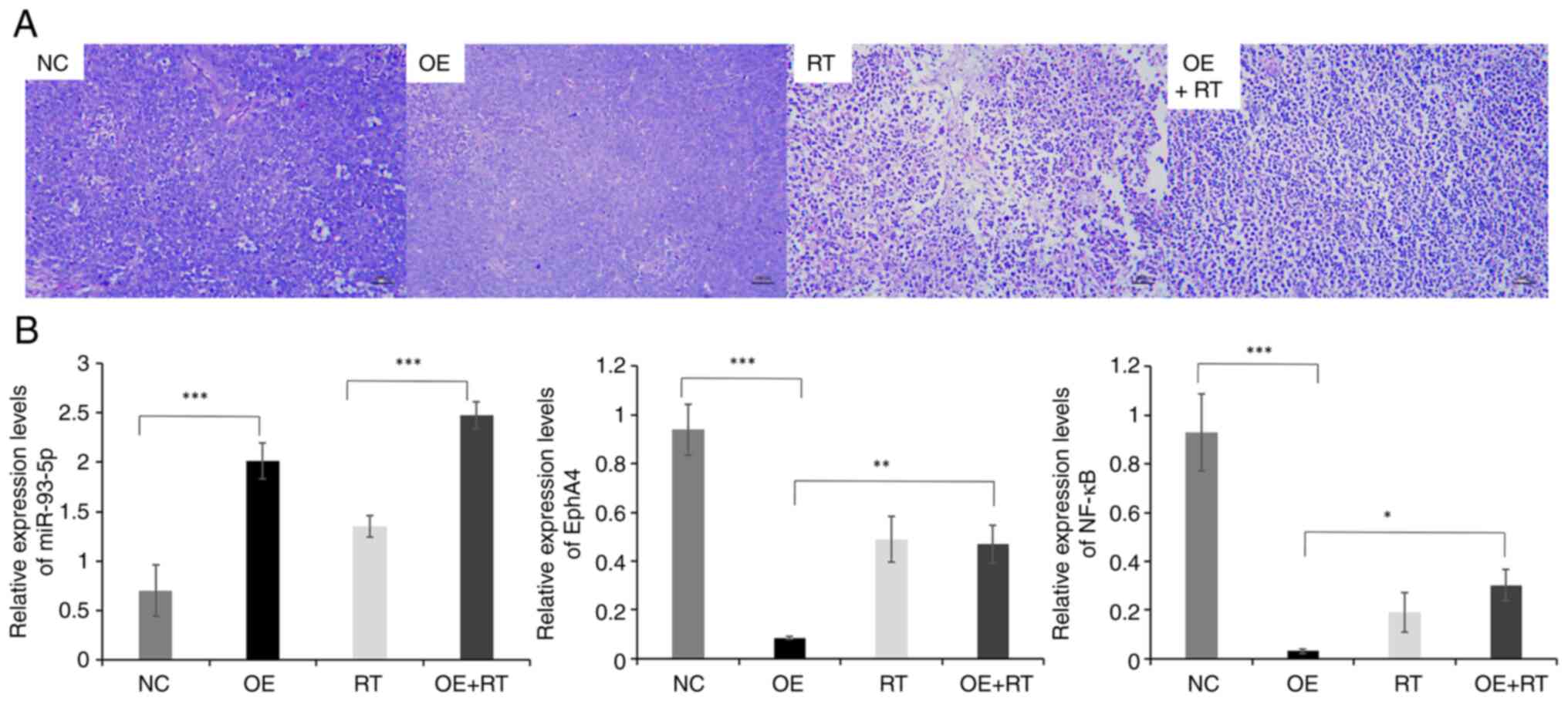
The H&E-stained images are shown in Fig. 5A. H&E staining revealed that the
tumor cells in the NC and OE groups grew well, with large and round
cell nuclei. Dividing cells and abundant microvessels were also
observed in the NC and OE groups. Distinct shrinkage of the
cytoplasm, wrinkled cell nuclei, some lysed nuclei and a large
necrotic area were visible in the RT group. Furthermore, necrosis
in the OE + RT group was much more severe than in RT group.
To further understand the mechanisms underlying the
effects of miR-93-5p, downstream regulatory genes were
progressively explored. Based on the bioinformatics analysis, it
was hypothesized that miR-93-5p targeting EphA4/NF-κB could improve
the effects of RT on TNBC cells. The expression levels of
miR-93-5p, EphA4 and NF-κB in each group (three tumors/group) are
displayed in Fig. 5B. EphA4 and
NF-κB expression levels were decreased in the miR-93-5p OE group
compared with those in the NC group (P<0.001). Moreover, the
expression levels of EphA4 and NF-κB were increased in the
miR-93-5p OE + RT group compared with those in the OE group
(P<0.001). However, the expression levels of EphA4 and NF-κB
were not significantly different between the RT and OE + RT groups.
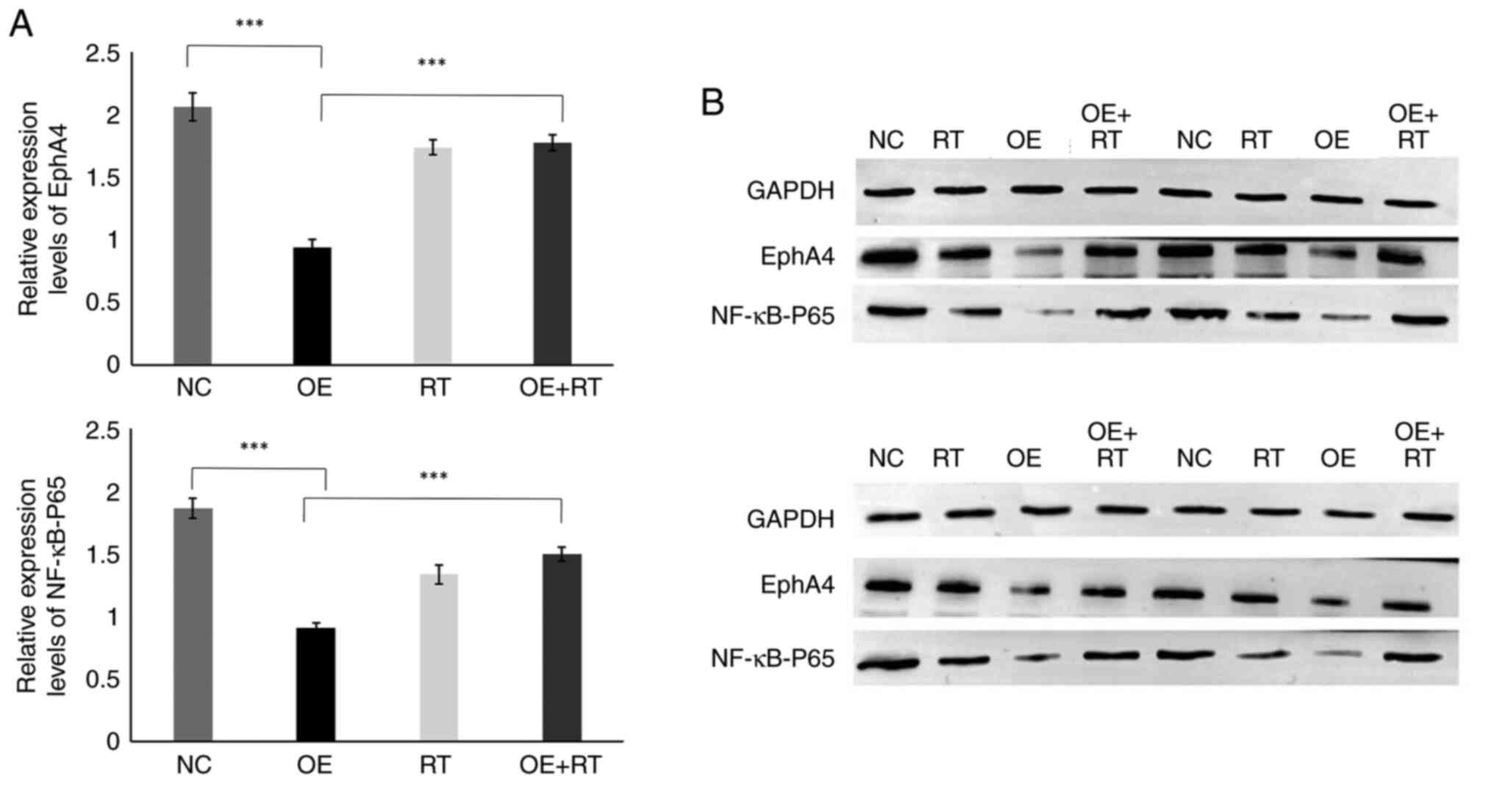
In addition, western blotting was performed and the blots were
semi-quantified (Fig. 6A and B).
The relative expression levels of EphA4 and NF-κB were
significantly lower in the miR-93-5p OE group compared with those
in the NC group (P<0.001). Moreover, the relative expression
levels of EphA4 and NF-κB were higher in the miR-93-5p OE + RT
group compared with those in the OE group (P<0.001). Similar to
the RT-qPCR results, the relative expression levels of EphA4 and
NF-κB were not significantly different between the RT and OE + RT
groups. These findings indicated that RT may prevent the
miR-93-5p/EphA4/NF-κB pathway.
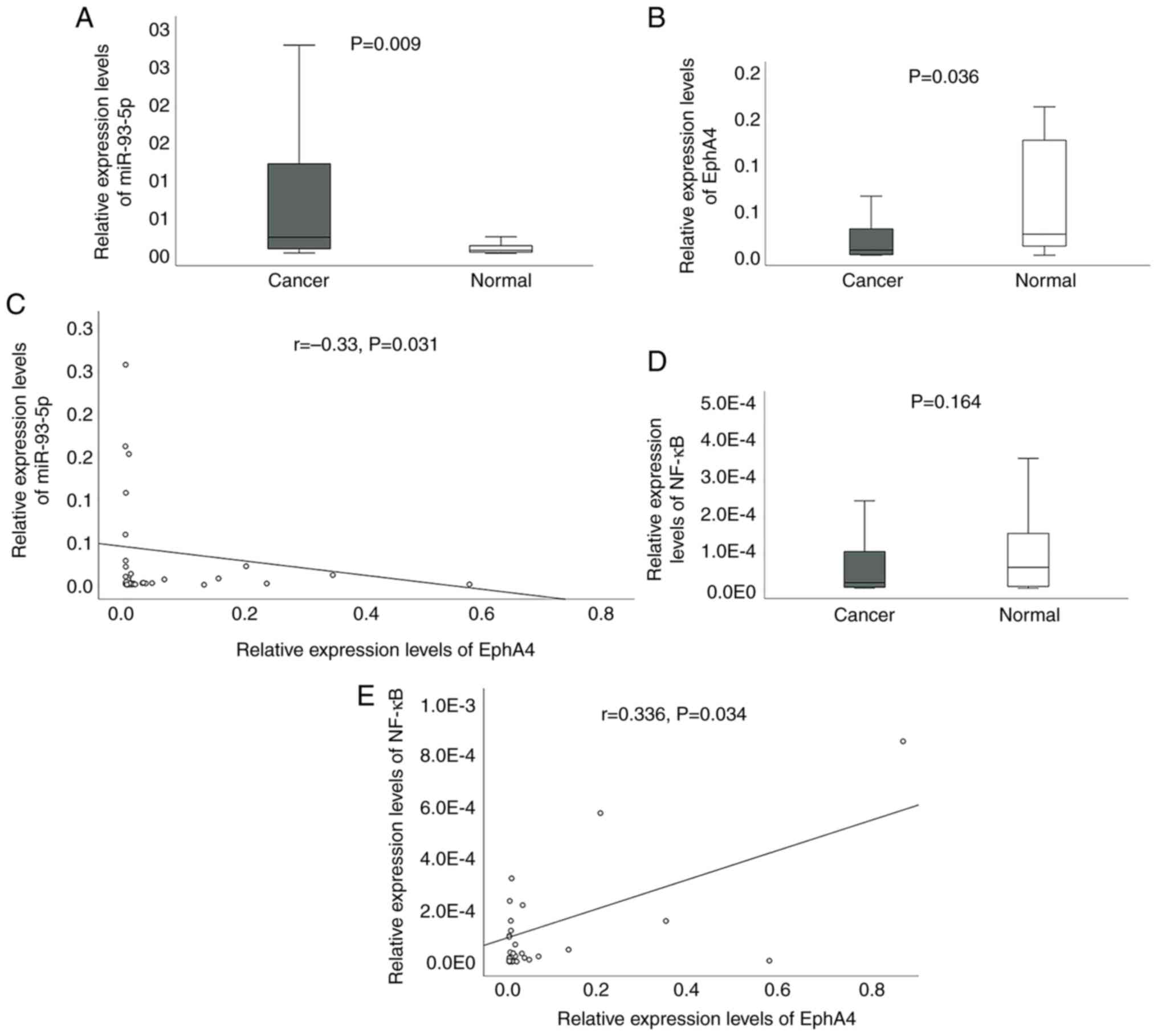
miRNA-93-5p is downregulated in
patients with TNBC
RT-qPCR was used to evaluate the expression levels
of miR-93-5p, EphA4 and NF-κB in 43 pairs of TNBC and adjacent
tissues. The clinicopathological associations between miR-93-5p,
EphA4 and NF-κB expression in TNBC are presented in Table II. Notably, the expression levels
of miR-93-5p were lower in patients with TNBC with positive lymph
nodes (P=0.049). NF-κB expression was higher in patients with a
larger tumor size (P=0.049) and later TNM stage (P=0.0498).
miR-93-5p expression was upregulated (P=0.009), whereas EphA4 was
downregulated in TNBC (P=0.036) (Fig.
7A and B). Correlations between expression levels were
determined using the Spearman's test. The analysis revealed an
inverse relationship (r=−0.33, P=0.031) between miR-93-5p and EphA4
(Fig. 7C). Although NF-κB
expression did not differ between normal and cancerous tissues
(P=0.164; Fig. 7D), a positive
correlation was identified between NF-κB and EphA4 expression
(r=0.336, P=0.034; Fig. 7E). The
correlation analysis also found no significant differences between
miR-93-5p and NF-κB (data not shown).
 | Table II.Clinicopathologic associations of
miR-93-5p, EphA4 and NF-κB expression in triple-negative breast
cancer (n=43). |
Table II.
Clinicopathologic associations of
miR-93-5p, EphA4 and NF-κB expression in triple-negative breast
cancer (n=43).
| Clinical
characteristic | Total number | miR-93-5p, n | EphA4, n | NF-κB, n |
|---|
| Age, years |
|
|
|
|
|
≤50 | 16 | 16 | 16 | 15 |
|
>50 | 27 | 27 | 27 | 25 |
|
P-value |
| 0.31 | 0.37 | 0.908 |
| Tumor size |
|
|
|
|
| ≤2 cm
(T1) | 17 | 17 | 17 | 15 |
| >2
cm (T2-T3) | 26 | 26 | 26 | 25 |
|
P-value |
| 0.442 | 0.549 | 0.0498 |
| Lymph node
metastasis |
|
|
|
|
| 0
(N0) | 29 | 29 | 29 | 26 |
| ≥1
(N1-N3) | 14 | 14 | 14 | 14 |
|
P-value |
| 0.049 | 0.901 | 0.206 |
| Stage |
|
|
|
|
| I | 15 | 15 | 15 | 13 |
|
II–III | 28 | 28 | 28 | 27 |
|
P-value |
| 0.375 | 0.396 | 0.0498 |
| Ki67 |
|
|
|
|
|
<50% | 13 | 13 | 13 | 12 |
|
≥50% | 30 | 30 | 30 | 28 |
|
P-value |
| 0.444 | 0.349 | 0.425 |
Discussion
TNBC is a fatal subtype of BC, with a high
proclivity for distant metastases and few therapeutic choices
(20). At present, the overall
survival of patients with TNBC remains poor. Recently, targeted
cancer therapy has attracted the attention of a number of
researchers. Strictinin is a targeted ROR1 inhibitor that may
decrease the proliferation of TNBC (21). Clofazimine reduces TNBC growth by
targeting the Wnt signaling pathway (22). However, available biomarkers cannot
deliver the desired effect in terms of diagnosis and prognosis in
patients with TNBC. Thus, identifying novel molecular markers and
therapeutic targets is critical for reducing the recurrence and
mortality of TNBC.
miRNAs have been regarded as potential oncogenes and
tumor suppressors in numerous types of cancer. In a previous study,
miR-93-5p was shown to be dysregulated in exosomes from patients
with BC (16). A single miRNA can
regulate various target genes. Sun et al (23) determined that miR-93-5p could
promote the progression of cervical cancer by targeting the
THBS2/MMPs signaling pathway. Wu et al (24) reported that miR-93-5p could inhibit
glioma cell proliferation and metastasis by targeting MMP2. Wang
et al (25) demonstrated
that miR-93-5p increased the apoptosis and adriamycin resistance of
BC cells by inhibiting the expression of Bcl-2 and P-GP protein. In
the present study, bioinformatics analysis was used to identify the
target gene of miR-93-5p. A dual-luciferase reporter experiment
confirmed that EphA4 was a candidate target gene of miR-93-5p.
However, the luciferase reporter also showed miR-93-5p suppressed
Mut EphA4 3′-UTR. The strength of the binding site in Mut EphA4
3′-UTR was weaker than that in WT EphA4 3′-UTR. These findings
indicated that a limitation of the present study may be that the
predicted binding site of Mut EphA4 may not be suitable. Additional
unknown binding sites may exist between mut-EphA4 and miR-93-5p. In
future, experiments should be designed with shorter gene fragments
for mut-EphA4 to avoid interference from other sequences.
Eph is the largest branch of the receptor tyrosine
kinases family (26). EphA4 is the
only Eph family member that can bind to ephrin-A and ephrin-B
ligands (27). EphA4 is mainly
involved in nervous system disorders and cancer development
(28). A previous study on the
nervous system revealed that treatment targeting EphA4 could
improve ischemic stroke (29).
miR-93 has also been reported to promote neurite growth of spinal
cord neurons by targeting EphA4 (30). In addition, treatment targeting
EphA4 can eliminate the chemoresistance of cervical cancer cells
(31), and EphA4 has been shown to
be associated with the failure of RT for rectal cancer (32). Moreover, the aggressive phenotype of
colorectal cancer cells that have endured RT is controlled by
EphA4-mediated signaling (33).
EphA4 deficiency has been linked to high grade, advanced TNM stage,
lymph node metastases and a poor prognosis in BC (34). Moreover, a previous study reported
that miR-335 can inhibit EphA4 in BC to suppress its progression
(35).
The radiosensitivity of cancer cells is a major
factor in determining the effectiveness of cancer RT.
Radiosensitivity is a multi-gene, intricate process with an unclear
mechanism. Zheng et al (36)
reported that Linc-RA1 was upregulated in radioresistant glioma
cells and promoted glioma radioresistance in vitro and in
vivo. In our previous study, it was revealed that miR-93-5p
increased the radiosensitivity of TNBC cells (17). To further investigate the underlying
mechanisms, the present study transfected MDA-MB-231 cells with a
miR-93-5p mimic and used this cell line to generate a tumor
xenograft model. NF-κB is a transcription factor that was found in
the nuclear extract of B lymphocytes in 1986 (37). NF-κB is closely connected to various
activities, including tumor initiation, development and metastasis,
and the NF-κB pathway is critical for tumor cell development and
radiation resistance (38). In the
present study, the difference in NF-κB expression in patients with
TNBC with a large tumor size or in the later stages of TNBC were
only of borderline significance. The borderline significant
findings may be related to the small sample size. Lu et al
(39) reported that EphA4 activates
NF-κB and induces BC stem cells to secrete various cytokines to
maintain stem cell status. The present study also detected changes
in EphA4 and NF-κB. In cancer tissue, miR-93-5p expression was
increased, whereas EphA4 expression was decreased. Additionally, a
negative correlation between EphA4 expression and miR-93-5p
expression was identified in clinical samples. In animal
experiments, a decrease in EphA4 and NF-κB expression was detected
in the miR-93-5p OE group. These findings indicated that miR-93-5p
may regulate EphA4 and NF-κB. However, miR-93-5p OE in the
MDA-MB-231 cells used to generate the xenografts and the treatment
of mice with RT significantly decreased tumor development. This
result confirmed our previous results, which revealed that
miR-93-5p increased the radiosensitivity of BC in vitro
(17). Moreover, EphA4 and NF-κB
expression levels in the miR-93-5p OE + RT group were not
significantly different compared with those in the RT group, but
they were increased compared with in the miR-93-5p OE group. These
results indicated that RT may prevent tumor progression by
inhibiting the miR-93-5p/EphA4/NF-κB pathway. The findings of the
present study, alongside those of previous studies, indicated that
RT may affect gene expression, with miR-93-5p interacting with RT
and altering its effect. RT was shown to block the binding sites
between miR-93-5p and EphA4, which increased the expression levels
of miR-93-5p and had subsequent effects on EphA4 and NF-kB.
However, the potential mechanisms have not yet been fully
elucidated and require further investigation.
In conclusion, the present study revealed that
miR-93-5p targeted EphA4 in TNBC through the NF-κB pathway.
However, RT prevented tumor progression by inhibiting this pathway.
Therefore, future clinical studies should aim to elucidate the role
of miR-93-5p.
Acknowledgements
Not applicable.
Funding
This work was supported by the Jiangsu Provincial
Double-Innovation Doctor Program (grant nos. 202030205 and
202030206).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All of the authors contributed to the conception and
design of the study. QN and SS performed the experimental
operation, and data were collected by YG. The experimental design
and data analysis were performed by CP. The first draft of the
manuscript was written by QN, and all the authors commented on the
previous versions. QN and CP confirm the authenticity of all the
raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The Human Ethics Review Committee of Jiangsu Taizhou
People's Hospital approved the present study (approval no. KY
2021-043-01). The requirement for informed consent was waived by
the Human Ethics Review Committee of Jiangsu Taizhou People's
Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liang Y, Song X, Li Y, Chen B, Zhao W,
Wang L, Zhang H, Liu Y, Han D, Zhang N, et al: LncRNA BCRT1
promotes breast cancer progression by targeting miR-1303/PTBP3
axis. Mol Cancer. 19:852020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao JJ and Swain SM: Luminal A breast
cancer and molecular assays: A review. Oncologist. 23:556–565.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
DeSantis CE, Ma J, Gaudet MM, Newman LA,
Miller KD, Goding Sauer A, Jemal A and Siegel RL: Breast cancer
statistics, 2019. CA Cancer J Clin. 69:438–451. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pan C, Cong A and Ni Q: Microarray data
reveal potential genes that regulate triple-negative breast cancer.
J Int Med Res. 50:30006052211301882022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zhang M, Zhang L, Geng A, Li X, Zhou Y, Xu
L, Zeng YA, Li J and Cai C: CDK14 inhibition reduces mammary stem
cell activity and suppresses triple negative breast cancer
progression. Cell Rep. 40:1113312022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu Z, Mao W, Yang H, Santiago-O'Farrill
JM, Rask PJ, Mondal J, Chen H, Ivan C, Liu X, Liu CG, et al: SIK2
inhibition enhances PARP inhibitor activity synergistically in
ovarian and triple-negative breast cancers. J Clin Invest.
132:e1464712022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Coates JT, Sun S, Leshchiner I, Thimmiah
N, Martin EE, McLoughlin D, Danysh BP, Slowik K, Jacobs RA,
Rhrissorrakrai K, et al: Parallel genomic alterations of antigen
and payload targets mediate polyclonal acquired clinical resistance
to sacituzumab govitecan in triple-negative breast cancer. Cancer
Discov. 11:2436–2445. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goodman CR, Seagle BL, Friedl TWP, Rack B,
Lato K, Fink V, Cristofanilli M, Donnelly ED, Janni W, Shahabi S
and Strauss JB: Association of circulating tumor cell status with
benefit of radiotherapy and survival in early-stage breast cancer.
JAMA Oncol. 4:e1801632018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Alamilla-Presuel JC, Burgos-Molina AM,
González-Vidal A, Sendra-Portero F and Ruiz-Gómez MJ: Factors and
molecular mechanisms of radiation resistance in cancer cells. Int J
Radiat Biol. 98:1301–1315. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhao W, Sun M, Li S, Chen Z and Geng D:
Transcription factor ATF3 mediates the radioresistance of breast
cancer. J Cell Mol Med. 22:4664–4675. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Troschel FM, Böhly N, Borrmann K, Braun T,
Schwickert A, Kiesel L, Eich HT, Götte M and Greve B: miR-142-3p
attenuates breast cancer stem cell characteristics and decreases
radioresistance in vitro. Tumour Biol. 40:10104283187918872018.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Perez-Añorve IX, Gonzalez-De la Rosa CH,
Soto-Reyes E, Beltran-Anaya FO, Del Moral-Hernandez O,
Salgado-Albarran M, Angeles-Zaragoza O, Gonzalez-Barrios JA,
Landero-Huerta DA, Chavez-Saldaña M, et al: New insights into
radioresistance in breast cancer identify a dual function of
miR-122 as a tumor suppressor and oncomiR. Mol Oncol. 13:1249–1267.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feketea G, Bocsan CI, Popescu C, Gaman M,
Stanciu LA and Zdrenghea MT: A review of macrophage MicroRNAs' role
in human asthma. Cells. 8:422019. View Article : Google Scholar
|
|
15
|
Xue T, Liang W, Li Y, Sun Y, Xiang Y,
Zhang Y, Dai Z, Duo Y, Wu L, Qi K, et al: Ultrasensitive detection
of miRNA with an antimonene-based surface plasmon resonance sensor.
Nat Commun. 10:282019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ni Q, Stevic I, Pan C, Müller V,
Oliveira-Ferrer L, Pantel K and Schwarzenbach H: Different
signatures of miR-16, miR-30b and miR-93 in exosomes from breast
cancer and DCIS patients. Sci Rep. 8:129742018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pan C, Sun G, Sha M, Wang P, Gu Y and Ni
Q: Investigation of miR-93-5p and its effect on the
radiosensitivity of breast cancer. Cell Cycle. 20:1173–1180. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Amin MB, Edge S, Greene F, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC cancer staging manual. 8th edition.
Springer International Publishing; New York, NY: 2017, View Article : Google Scholar
|
|
20
|
Tajbakhsh A, Rivandi M, Abedini S, Pasdar
A and Sahebkar A: Regulators and mechanisms of anoikis in
triple-negative breast cancer (TNBC): A review. Crit Rev Oncol
Hematol. 140:17–27. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fultang N, Illendula A, Chen B, Wu C,
Jonnalagadda S, Baird N, Klase Z and Peethambaran B: Strictinin, a
novel ROR1-inhibitor, represses triple negative breast cancer
survival and migration via modulation of PI3K/AKT/GSK3ß activity.
PLoS One. 14:e2177892019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmed K, Koval A, Xu J, Bodmer A and
Katanaev VL: Towards the first targeted therapy for triple-negative
breast cancer: Repositioning of clofazimine as a
chemotherapy-compatible selective Wnt pathway inhibitor. Cancer
Lett. 449:45–55. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun XY, Han XM, Zhao XL, Cheng XM and
Zhang Y: MiR-93-5p promotes cervical cancer progression by
targeting THBS2/MMPS signal pathway. Eur Rev Med Pharmacol Sci.
23:5113–5121. 2019.PubMed/NCBI
|
|
24
|
Wu H, Liu L and Zhu JM: MiR-93-5p
inhibited proliferation and metastasis of glioma cells by targeting
MMP2. Eur Rev Med Pharmacol Sci. 23:9517–9524. 2019.PubMed/NCBI
|
|
25
|
Wang Q, Su C, Li J and Wei C: Mechanism of
the enhancing effects of miR-93 on resistance of breast cancer
MCF-7 cells to adriamycin. Oncol Lett. 16:3779–3783.
2018.PubMed/NCBI
|
|
26
|
Ge YW, Liu ZQ, Sun ZY, Yu DG, Feng K, Zhu
ZA and Mao YQ: Titanium particle-mediated osteoclastogenesis may be
attenuated via bidirectional ephrin-B2/eph-B4 signaling in
vitro. Int J Mol Med. 42:2031–2041. 2018.PubMed/NCBI
|
|
27
|
Chen R, Yang X, Zhang B, Wang S, Bao S, Gu
Y and Li S: EphA4 negatively regulates myelination by inhibiting
schwann cell differentiation in the peripheral nervous system.
Front Neurosci. 13:11912019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Pan Y, Lu S, Lei L, Lamberto I and Wang Y,
Pasquale EB and Wang Y: Genetically encoded FRET biosensor for
visualizing EphA4 activity in different compartments of the plasma
membrane. ACS Sens. 4:294–300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Okyere B, Mills WR, Wang X, Chen M, Chen
J, Hazy A, Qian Y, Matson JB and Theus MH: EphA4/Tie2 crosstalk
regulates leptomeningeal collateral remodeling following ischemic
stroke. J Clin Invest. 130:1024–1035. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chen X, Yang H, Zhou X, Zhang L and Lu X:
MiR-93 targeting EphA4 promotes neurite outgrowth from spinal cord
neurons. J Mol Neurosci. 58:517–524. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kina S, Kinjo T, Liang F, Nakasone T,
Yamamoto H and Arasaki A: Targeting EphA4 abrogates intrinsic
resistance to chemotherapy in well-differentiated cervical cancer
cell line. Eur J Pharmacol. 840:70–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
de Marcondes PG and Morgado-Diaz JA: The
role of EphA4 signaling in radiation-induced EMT-like phenotype in
colorectal cancer cells. J Cell Biochem. 118:442–445. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de Marcondes PG, Bastos LG,
De-Freitas-Junior JC, Rocha MR and Morgado-Díaz JA: EphA4-mediated
signaling regulates the aggressive phenotype of irradiation
survivor colorectal cancer cells. Tumour Biol. 37:12411–12422.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Sun Y, Qian J, Lu M and Xu H: Lower and
reduced expression of EphA4 is associated with advanced TNM stage,
lymph node metastasis, and poor survival in breast carcinoma.
Pathol Int. 66:506–510. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dong Y, Liu Y, Jiang A, Li R, Yin M and
Wang Y: MicroRNA-335 suppresses the proliferation, migration, and
invasion of breast cancer cells by targeting EphA4. Mol Cell
Biochem. 439:95–104. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zheng J, Wang B, Zheng R, Zhang J, Huang
C, Zheng R, Huang Z, Qiu W, Liu M, Yang K, et al: Linc-RA1 inhibits
autophagy and promotes radioresistance by preventing H2Bub1/USP44
combination in glioma cells. Cell Death Dis. 11:7582020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sen R and Baltimore D: Multiple nuclear
factors interact with the immunoglobulin enhancer sequences. Cell.
1986.46:705–716. J Immunol. 177:7485–7496. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hou Y, Liang H, Rao E, Zheng W, Huang X,
Deng L, Zhang Y, Yu X, Xu M, Mauceri H, et al: Non-canonical NF-κB
antagonizes STING sensor-mediated DNA sensing in radiotherapy.
Immunity. 49:490–503.e4. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu H, Clauser KR, Tam WL, Fröse J, Ye X,
Eaton EN, Reinhardt F, Donnenberg VS, Bhargava R, Carr SA and
Weinberg RA: A breast cancer stem cell niche supported by
juxtacrine signalling from monocytes and macrophages. Nat Cell
Biol. 16:1105–1117. 2014. View Article : Google Scholar : PubMed/NCBI
|