Introduction
Over the past decades, polysaccharides isolated from
naturally-occurring sources, such as fungi, plants, and algae, are
gradually recognized for their anti-tumor activity (1). In particular, these polysaccharides
have been previously shown to significantly prolong the survival of
patients with cancer whilst improving their quality of life when
used as cancer therapeutics (2,3).
Therefore, polysaccharides are potential candidates for cancer
therapy. Lentinan (LNT) is a polysaccharide that can be isolated
from the mushroom species Lentinus edodes. It has various
reported biologically active properties, including
immunomodulatory, anti-tumor, antiviral and antibacterial effects,
in addition to high efficacy and minimal side effects (4). As the first medicinal macrofungal
polysaccharide drug to enter the field of modern biotechnology
(5), its unique triple-helix
conformation and antitumor activity have particularly attracted
attention (6). Several studies have
shown that LNT can mediate anti-tumor effects directly and
indirectly (7–10). A previous study found that LNT can
exert inhibitory effects in a mammary-specific polyomavirus middle
T antigen overexpression mouse model of spontaneous breast cancer
(7). However, the anti-tumor
mechanism of LNT remains to be fully elucidated, where the relevant
signaling pathways involved remains poorly understood. In the
majority of cases, it is used as an adjuvant therapeutic agent in
clinical practice, potentially limiting global application.
According to data from a 2020 Global Cancer Report
published by the International Agency for Research on Cancer of the
World Health Organization, liver cancer has the sixth highest
incidence and third highest mortality rate of all cancers worldwide
(11). Despite the progress made
regarding its diagnosis and treatment methodologies, both morbidity
and mortality rates from liver cancer continue to rise (11). Identification of effective methods
for preventing and treating liver cancer remains in urgent demand.
From the perspective of hepatocarcinogenesis, changes in
transcription factor expression, dysregulated signaling pathways,
and alterations in the tumor microenvironment are all considered to
be factors that can promote this process (12,13).
If these factors can be restored to their pre-cancerous state, then
the malignant behavior of the tumor can be inhibited (14).
Recently, bioinformatics approaches have been used
to screen for genes that are abnormally expressed in primary liver
cancer (15,16). Among these aberrant genes, early
growth response 1 (EGR1) was found to be a potential
target for developing drugs against primary liver cancer (16). EGR1 belongs to the EGR
protein family member. The EGR1 gene is located in human
chromosome region 5q23-31, where the corresponding EGR1 protein is
an important transcription factor and belongs to the EGR protein
family member (17). It has been
reported that EGR1 is a transcription factor of the PTEN tumor
suppressor gene, which also transactivates p53, p73, p300/CBP, and
other pro-apoptotic and anti-oncogenes (18).
In the present study, the mechanisms by which LNT
can inhibit liver cancer physiology were investigated using both
in vitro and in vivo experimental methods. Various
cellular and molecular techniques in vitro were applied to
study the relationship between the impact of LNT on mouse
hepatocellular carcinoma (HCC) cell line Hepa1-6 and the abnormal
expression of EGR1 in HCC cells. Then the mouse model of
diethylnitrosamine (DEN)-induced primary liver cancer was used for
further verification in vivo. These results provide a
scientific basis for the future development and clinical
application of LNT as a treatment for primary liver cancer.
Materials and methods
Cell culture and treatment
The mouse HCC cell line Hepa1-6 was purchased from
The Cell Bank of Type Culture Collection of the Chinese Academy of
Sciences. Cells were cultured in RPMI-1640 medium (cat. no. MA0215;
Dalian Meilun Biology Technology Co., Ltd.) supplemented with 10%
FBS (cat. no. 164210-50; Procell Life Science & Technology Co.,
Ltd.) and 1% penicillin-streptomycin at 37°C in a 5%-CO2
incubator.
Cells were treated with a series of LNT dosages (0,
250, 500 and 1,000 µg/ml) for 24 h. LNT was prepared from
Lentinus edodes by the Engineering Technological Center of
Mushroom Industry and confirmed to be essentially consistent with
standards obtained from Jinling Pharmaceutical Co., Ltd. (Nanjing,
China).
Cell viability assay
Cellular growth and proliferation in different
groups were monitored via optical microscope and detected with Cell
Counting Kit-8 (CCK-8; cat. no. A311; Vazyme Biotech Co., Ltd.)
according to the manufacturer's protocol. Briefly, Hepa1-6 cells in
the logarithmic growth phase were inoculated into a 96-well
microplates at a density of 2×103 cells per well at 37°C
for 12 h. After the cells adhered to the plate wall, the
supernatant was discarded and the cells were treated with different
concentrations of LNT (0, 250, 500 and 1,000 µg/ml) in a humidified
atmosphere containing 5% CO2 for 12 and 24 h,
respectively. Then, 10 µl of CCK-8 reagent was added into each well
and incubated for 2 h at 37°C. The absorbance at 450 nm was
measured using a microplate reader (Infinite M200 PRO; Tecan Group,
Ltd.). Cell viability was calculated as follows: Cell viability
(%)=(As-Ab)/(Ac-Ab) ×100%, where Ab, As, and Ac were the values of
the blank medium, experimental group, and control group,
respectively.
Apoptosis staining
Hepa1-6 cells (6×104) were inoculated
into six-well plates, and cultured until adherence, before being
treated with different dosages of LNT for 24 h at 37°C. Next, in
situ fluorescence staining was performed using an Annexin
V-FITC Apoptosis Detection kit (cat. no. C1062L; Beyotime Institute
of Biotechnology). Fluorescence was observed under a fluorescence
microscope (BX51; Olympus Corporation). FITC and propidium iodide
(PI) staining were associated with green and red fluorescence,
respectively.
Flow cytometry
Hepa1-6 cells were inoculated and treated with LNT
according to the method that was performed for the apoptosis assay.
As a positive control, cells were also treated for 24 h at 37°C
with a mixture of 100 µmol/l FeSO4 and 500 µmol/l
H2O2. FITC and PI staining were performed
using an Annexin V-FITC Apoptosis Detection kit. Fluorescence was
measured by flow cytometry (Flowsight; Merck KGaA) and apoptosis
was analyzed for each group of cells using the FlowSight
software.
Western blotting (WB)
For samples in each group, total protein was
extracted from cells or tissues by WB and IP cell lysates (cat. no.
P0013; Beyotime Institute of Biotechnology) before the BCA Protein
Assay kit (cat. no. 23227; Thermo Fisher Scientific, Inc.) was used
for quantification. Protein samples (20 µg per well) were subjected
to SDS-PAGE on an 8% gel, transferred onto PVDF membranes (cat. no.
3010040001; Roche Diagnostics) after electrophoresis and blocked
with NcmBlot blocking buffer (cat. no. P30500; New Cell &
Molecular Biotech) for 20 min at room temperature. The membranes
were then incubated with primary antibodies (1:1,000) overnight at
4°C, before being incubated with HRP-conjugated anti-rabbit or
anti-mouse secondary antibodies for 1 h at room temperature.
Finally, an ECL luminescent solution (cat. no. MA0186-1; Dalian
Meilun Biology Technology Co., Ltd.) was added and the target
protein was detected by exposure using an Omega Lum C Gel Imaging
system (Gel Company, Inc.). Quantitative protein analysis was
performed using ImageJ software (version: 2.1.0/1.53c; National
Institutes of Health).
The following primary antibodies were used: Rabbit
EGR1 (cat. no. 55117-1-AP; ProteinTech Group, Inc.), rabbit PTEN
(cat. no. AF6351), rabbit phosphorylated (p)-AKT (cat. no. AF0016),
rabbit AKT (cat. no. AF6261), mouse Bcl-2 (cat. no. BF9103), rabbit
Bax (cat. no. AF0120), rabbit poly (ADP ribose) polymerase 1
(PARP1; cat. no. 13371-1-AP), rabbit heat shock protein 60 (Hsp60;
cat. no. AF0184), rabbit proliferating cell nuclear antigen (PCNA;
cat. no. AF0239) and mouse β-actin (cat. no. T0022; all from
Affinity Biosciences).
The following secondary antibodies were used: Goat
anti-Mouse IgG (H + L) Cross-Adsorbed Secondary Antibody, Alexa
Fluor™ 488 (cat. no. A-11001), and Goat anti-Rabbit IgG (H+L)
Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 (cat no.
A-11008; both from Thermo Fisher Scientific, Inc.).
Overexpression of EGR1 in cell
lines
To induce the overexpression of EGR1,
according to the mouse EGR1 mRNA sequence on NCBI
(NM-007913.5), the specific primers were designed by Primer Premier
5.0 software (Premier Biosoft International), and 15 bp eukaryotic
expression plasmid pCMV-Myc (cat no. 11910ES03; Shanghai Yeasen
Biotechnology Co., Ltd.) homologous sequence was added at both ends
(Table I). Total RNA was extracted
from Hepa1-6 cells by TRIzol Universal (cat. no. DP424; Tiangen
Biotech Co., Ltd.) and reverse transcribed into cDNA by
PrimeScript™ RT reagent kit (Perfect Real Time) (cat. no. RR037 A;
Takara Biotechnology Co., Ltd.). Using this as a template, PCR was
carried out with primers (cat. no. 10154; Shanghai Yeasen
Biotechnology Co., Ltd.). The reaction procedure is shown in
Table II. PCR products were
isolated by 1% agarose gel with YeaRed Nucleic Acid Gel Stain
(10,000X in DMSO) (cat. no. 10202ES30; Shanghai Yeasen
Biotechnology Co., Ltd.) and separated from the gel with a DNA
extraction kit (cat no. B518131; Sangon Biotech Co., Ltd.).
Subsequently, the pEASY®-Basic Seamless Cloning and
Assembly Kit (cat. no. CU201-02; Beijing Transgene Biotech Co.,
Ltd.) was applied to clone the amplified cDNA into the linear
pCMV-Myc plasmid fragment recovered by EcoRI restriction
enzymes (cat. no. R0101; New England Biolabs). The correctly
sequenced strains were amplified, and the plasmids were extracted
with TIANprep Mini Plasmid kit (cat. no. DP106; Tiangen Biotech
Co., Ltd.) for subsequent cell transfection.
 | Table I.Primer sequences used for reverse
transcription PCR. |
Table I.
Primer sequences used for reverse
transcription PCR.
| Gene name | Primer sequence
(5′→3′) |
|---|
| mEGR1 | F:
CATGGAGGCCCCGAATTATGAGCGGCCAAGG |
|
| R:
CTGGTCGACCGAATTGCAATTTCAATTGTCCTGG |
| pCMV-myc | F:
TCTAAAAAGCTGCGGAATTGT |
|
| R:
TCCAAACTCATCAATCAATGTATC |
 | Table II.Thermocycling conditions of PCR. |
Table II.
Thermocycling conditions of PCR.
| Temperature
(°C) | Duration | Number of
cycles |
|---|
| 98 | 3 min | 1 |
| 98 | 10 sec | 35 |
| 68 | 30 sec | 35 |
| 72 | 5 min | 1 |
| 4 | ∞ |
|
Cell transfection
Hepa1-6 cells (5×105) were seeded into a
six-well plate and cultured in 500 µl serum- and antibiotics-free
medium. When the cell density reached about 90%, the cells were
transfected with the plasmids. According to the protocol of Hieff
Trans™ Liposomal Transfection Reagent (cat no. 40802ES03; Shanghai
Yeasen Biotechnology Co., Ltd.), 4 µg of plasmid DNA and 10 µl of
Hieff TransTM liposome nucleic acid transfection reagent was mixed
with 250 µl of OPTI-MEMI medium and incubated at room temperature
(25°C) for 5 min, respectively. Subsequently, the diluted plasmid
DNA and liposome nucleic acid transfection reagent were mixed
evenly and incubated at room temperature (25°C) for 20 min to form
DNA- liposome complex. Then, 500 µl of DNA-liposome complex was
added into each plate well and cultured at 37°C in a
5%-CO2 incubator for 24 h. When necessary, after
transfection for 6 h, the cells were cultured with a complete
medium for improved transfection activity. Finally, mediums
containing gradient concentration of LNT (0, 250, 500, and 1,000
µg/ml) were applied to culture for another 24-h incubation. WB was
used to detect the expression level of EGR1 protein.
Separation of nuclear and cytoplasmic
fractions
After 24 h of treatment with gradient concentrations
of LNT at 37°C, nuclear and cytoplasmic proteins were extracted and
separated using a Nuclear and Cytoplasmic Protein Extraction kit
(cat. no. P0028; Beyotime Institute of Biotechnology). Finally, WB
was used to detect the expression of EGR1 protein in the two
intracellular compartments.
Immunofluorescence (IF) staining
Clean slides were placed into 24-well plates for
cell spreading, with six replicate wells set up for each group.
Cells at a density of 1×105 cells per well were allowed
to adhere before being treated with gradient concentrations of LNT
for 24 h at 37°C, after which they were fixed with 4%
paraformaldehyde solution at room temperature for 15 min. Cells
were then washed three times with PBS, penetrated with 0.5% Triton
X-100 for 20 min, and blocked with donkey serum (cat. no. MB4516;
Dalian Meilun Biology Technology Co., Ltd.) for 30 min at room
temperature (25°C). Subsequently, they were incubated with a
mixture of different primary antibodies, including mouse EGR1
(1:100; cat. no. H00001958-M03; Novus Biologicals, LLC) and rabbit
PTEN (1:100; cat. no. AF6351; Affinity Biosciences), overnight at
4°C. The cells were then washed with PBS and incubated with
fluorescently-labeled secondary antibody (1:200) for 1 h at room
temperature (25°C) in the dark. After staining nuclei with DAPI
(1:5,000; cat. no. C1002; Beyotime Institute of Biotechnology) for
5 min at room temperature, cells were sealed with anti-fluorescence
quenching sealing solution (cat. no. P0126; Beyotime Institute of
Biotechnology). Finally, images were collected using a laser
scanning confocal microscope (Leica TCS SP8; Leica Microsystems
GmbH).
Secondary antibodies used in IF staining were as
follows: Donkey anti-rabbit IgG (H + L) highly cross-adsorbed
secondary antibody labeled with Alexa Fluor Plus 555 (cat. no.
A32794) and donkey anti-mouse IgG (H + L) highly cross-adsorbed
secondary antibody labeled with Alexa Fluor 488 (cat. no. A-21202;
both from Invitrogen; Thermo Fisher Scientific, Inc.).
Mouse model of DEN-induced primary
liver cancer
All protocols involving animals in the present study
were reviewed and approved (approval no. 2020010) by the Animal
Ethics and Welfare Committee of Minnan Normal University
(Zhangzhou, China). Mice were obtained from Jiangsu Huachuang Xinuo
Pharmaceutical Technology (certificate no. 320928211100002573).
In total, 40 female mice (C57BL/6 background) with
gestational ages of 11–12 days were housed in a temperature (25°C)
and humidity (50–60%)-controlled room, where they were kept on a
12-h light/dark cycle and received autoclaved water and food ad
libitum under specific pathogen-free conditions. After the
pregnant mice gave birth, 1-week-old mice were acclimatized and fed
for 1 week, after which 2-week-old male offspring (weight, ~15 g)
were selected. A total of 40 mg/kg DEN (cat. no. HY-N7434;
MedChemExpress) was injected intraperitoneally. Following the
establishment of the model, mice were randomly divided into the
following four groups (8 mice/group): i) Model group; ii) LNT-low
dosage group (0.865 mg/kg); iii) LNT-middle dosage group (1.73
mg/kg); and iv) LNT-high dosage group (3.46 mg/kg). Meanwhile,
another 8 mice of the same batch without DEN induction were used as
the normal group. Each group of mice was treated via intragastric
administration for 8 weeks. The normal group and model group were
given an equivalent amount of saline. In the late stage of the
experiment, the mice in the control group began to present slow
movement and hair loss. At the end of the administration, the mice
were anesthetized by intraperitoneal injection of ketamine (100
mg/kg) and xylazine (10 mg/kg), and were sacrificed by cervical
dislocation. When cessation of breath and heartbeat was observed,
they were subjected to autopsy for observation.
Hematoxylin-eosin (H&E)
staining
Mouse liver tissues were fixed in a 10%
paraformaldehyde solution at 4°C, embedded in paraffin, and
sectioned into 5-µm sections. The sections were deparaffinized with
xylene and hydrated with gradient ethanol. H&E staining was
then performed according to standard techniques. The sections were
incubated with a hematoxylin solution (cat no. ZLI-9610; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.) for 2 min at room temperature (25°C) and washed
with water for 6 min. Then, the sections were soaked in 70% ethanol
solution containing 1% hydrochloric acid for 30 sec at room
temperature (25°C) and washed with water for 6 min again.
Subsequently, they were stained with eosin (cat no. ZLI-9613;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.) for 4 min at room temperature (25°C) and rinsed
with water for 6 min. Finally, the stained sections were examined
using an Olympus fluorescent inverted microscope (IX71; Olympus
Corporation).
Immunohistochemical (IHC) assay
Tissue expression levels of EGR1 and PTEN proteins
were assessed by IHC. Liver tissue sections were deparaffinized
with xylene and hydrated with decreasing concentrations of ethanol,
after which they were treated for antigen retrieval with 0.01 mM
citric acid buffer in a microwave. Endogenous peroxidase activity
was inhibited by endogenous hydrogen peroxide (3%
H2O2) treatment. Sections were then blocked
with goat serum (cat no. MB4508-1; Dalian Meilun Biology Technology
Co., Ltd.) for 1 h at room temperature (25°C) and incubated
overnight at 4°C with rabbit EGR1 (1:200), rabbit PTEN (1:200) or
rabbit Ki-67 (1:200; cat. no. ab15580; Abcam). The sections were
then treated by IHC kits (cat. no. PV-9001/PV-9002; Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.) following the manufacturer's protocol. Sections
were stained with DAB and counterstained with hematoxylin. Finally,
sections were dehydrated in increasing concentrations of ethanol
and xylene before being observed and imaged using an Olympus IX71
inverted microscope.
Statistical analysis
All statistical analyses were performed using
GraphPad Prism 8 (Dotmatics). All experiments were repeated
independently in triplicate. Data are expressed as the mean ±
standard error of the mean (SEM) and analyzed by the independent
sample t-test (between two groups) or one-way ANOVA variance
analysis with Tukey's multiple comparisons (among-group
comparisons), respectively. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of LNT on apoptosis in mouse
HCC cells
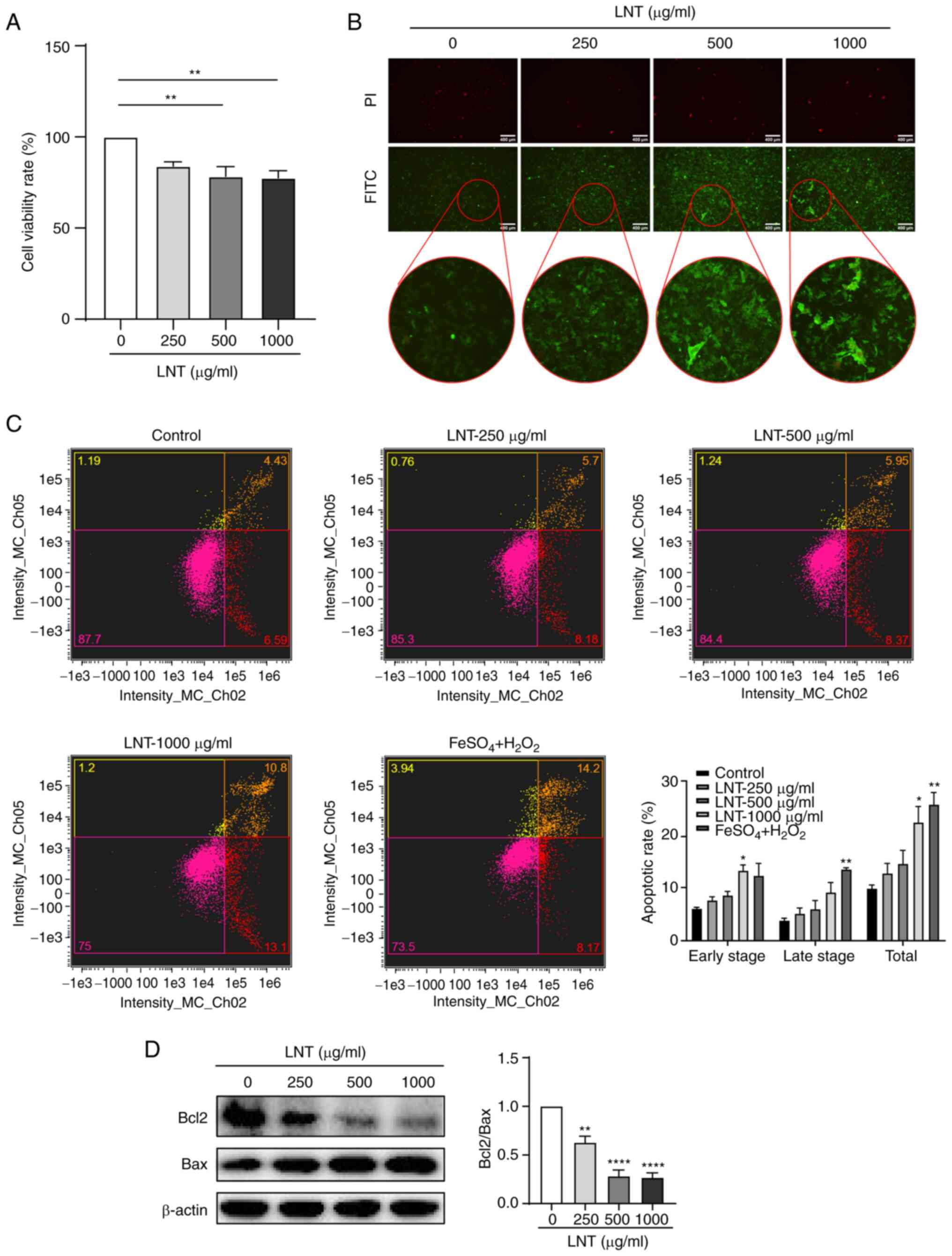
CCK-8 method was used to explore the effect of LNT
on the proliferation of Hepa1-6 cells. As revealed in Fig. 1A, the effect of LNT on Hepa1-6 cell
viability rate increased with the increasing concentration of LNT.
The lower concentration of LNT had no apparent toxicity to cells.
However, medium and high concentrations of LNT (500~1,000 µg/ml)
had a specific inhibitory effect on Hepa1-6 cell viability rate.
The Annexin V-FITC staining method was used to detect the apoptosis
of Hepa1-6 cells in situ. Early-stage apoptotic cells were
stained with green fluorescence (FITC) only, while late-stage
apoptotic cells or necrotic cells were double-stained with green
and red fluorescence (PI). However, healthy cells were not
fluorescently stained. As demonstrated in Fig. 1B, after the Hepa1-6 cells were
treated with increasing concentrations of LNT, the green
fluorescence became correspondingly more intense. When the LNT
concentration reached 1,000 µg/ml, the degree of early-stage
apoptosis appeared the most severe. In addition, flow cytometric
analysis demonstrated that the total apoptotic rate of Hepa1-6
cells increased gradually with the increase of LNT concentration,
and the LNT treatment at 1,000 µg/ml was more significant.
(Fig. 1C). Bcl-2 and Bax, two
markers in the Bcl-2 gene family, are closely associated with
apoptosis (19). Bcl-2 is
considered a representative anti-apoptotic marker (20), whilst Bax is a pro-apoptotic marker
in the Bcl-2 family (21,22). After treatment with LNT for 24 h,
total protein was extracted from each group for detection using WB.
With increasing LNT concentrations, expression of the
anti-apoptotic protein Bcl-2 was decreased, whilst expression of
the pro-apoptotic protein Bax was increased, resulting in a
significant reduction in the Bcl-2/Bax ratio (Fig. 1D). This suggested that LNT could
induce apoptosis in Hepa1-6 cells.
LNT induces apoptosis of Hepa1-6 cells
through the EGR1/PTEN/AKT axis
A previous study has shown that inhibition of AKT
phosphorylation in Hepa1-6 cells and mouse HCC models can cause
changes in Bax expression, leading to apoptosis (23). Furthermore, increased expression of
PTEN can inhibit AKT phosphorylation, thereby affecting downstream
signaling pathways through apoptosis or cell cycle arrest, exerting
a tumor suppressor function (24–28).
EGR1 is considered a transcription factor of PTEN (29,30).
Therefore, the present study next assessed whether LNT treatment
can cause changes in the expression of EGR1, PTEN, and Akt
phosphorylation in Hepa1-6 cells. From the WB data, it was found
that the protein expression levels of EGR1 and PTEN were both
significantly increased in Hepa1-6 cells with increasing LNT
concentrations. By contrast, the level of Akt phosphorylation
demonstrated the opposite trend, particularly p-Akt/Akt ratio
decreased significantly (Fig.
2A).
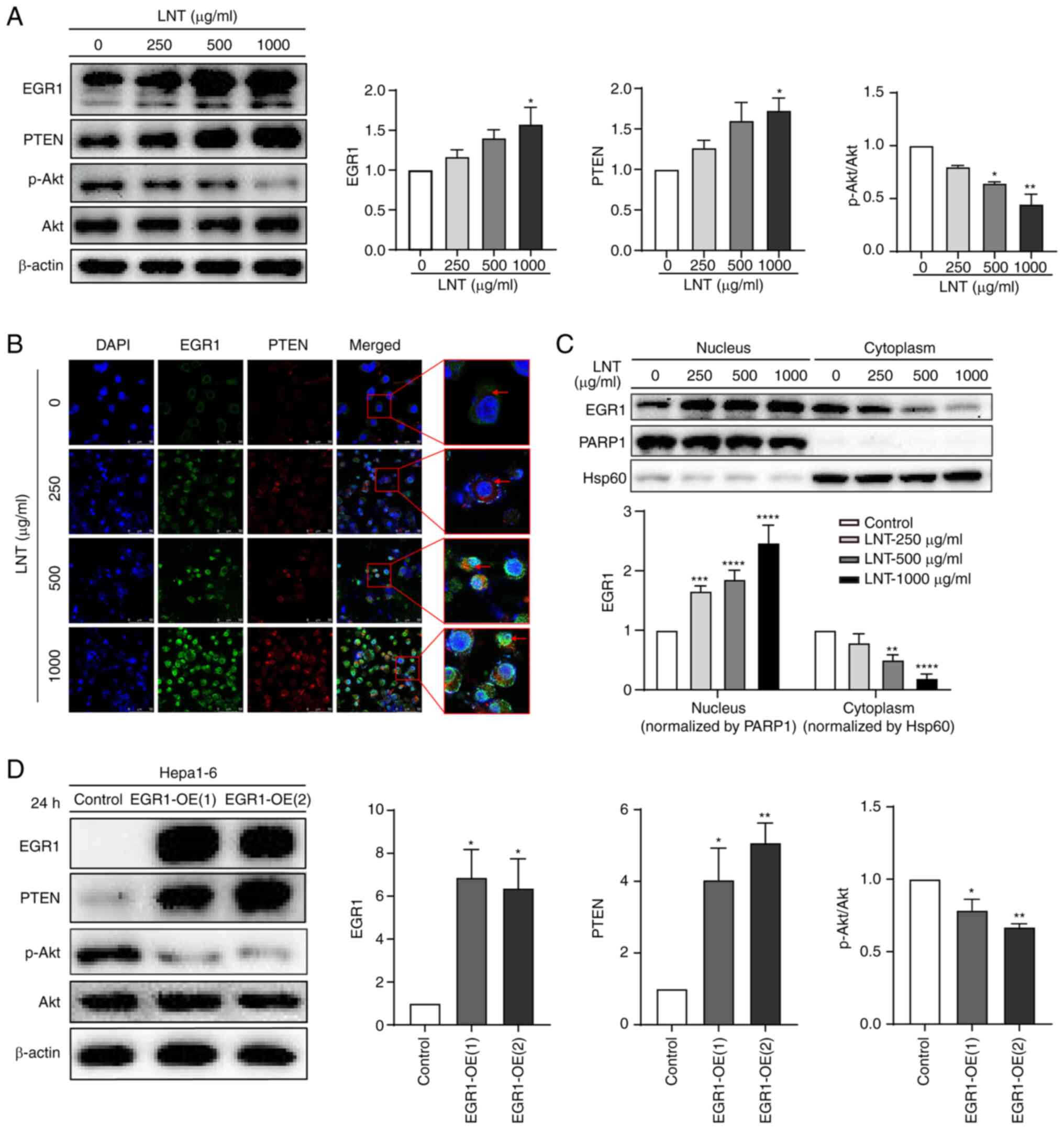 | Figure 2.Effects of LNT on the EGR1/PTEN/AKT
axis in Hepa1-6 cells. (A) Protein expression levels of EGR1, PTEN,
p-Akt and phosphorylation of Akt after treatment with a gradient of
LNT concentrations as detected by WB. All data for protein
expression were normalized using β-actin as a loading reference.
(B) Immunofluorescence co-staining was used to assess the
localization and expression of EGR1 and PTEN in Hepa1-6 cells
treated with a gradient of LNT concentrations. Images were observed
at ×630 magnification. Scale bars, 50 µm (white). (C) WB was used
to detect the expression of EGR1 in the nuclear and cytoplasmic
fractions following treatment with a gradient of LNT
concentrations. (D) WB was used to detect the expression of EGR1,
PTEN, p-Akt, and phosphorylation of Akt after EGR1
overexpression. *P<0.05, **P<0.01, ***P<0.001 and
****P<0.0001. LNT, Lentinan; EGR1, early growth response 1; p-,
phosphorylated; PARP1, poly-(ADP ribose) polymerase 1; Hsp60, heat
shock protein 60; OE, overexpression; WB, western blotting. |
Based on the upregulation of EGR1 expression after
LNT treatment in Hepa1-6 cells, cell localization of EGR1 was,
therefore, further explored under the same treatment conditions. By
co-staining for EGR1 and PTEN with IF, the expression levels and
cellular localization patterns of EGR1 and PTEN in Hepa1-6 cells
treated with a gradient of LNT concentrations were assessed. These
results showed that, in the control group, the weak fluorescence
intensity of EGR1 (green) and PTEN (red) was indicative of a low
expression level for EGR1 and PTEN. In the LNT-treated group, the
fluorescence intensity of EGR1 (green) and PTEN (red) gradually
increased, that is, the expression levels of EGR1 and PTEN
gradually increased with the increase of LNT concentration, and the
green fluorescence emitted by EGR1 was significantly enhanced in
the nucleus after administration (Fig.
2B). A subsequent nuclear-cytoplasmic separation experiment
revealed that with the increase of LNT concentration, EGR1
expression gradually increased in the nucleus and decreased in the
cytoplasm, and the difference was significant (Fig. 2C). These results suggested that LNT
could regulate both the expression and nuclear translocation of
EGR1 in Hepa1-6 cells. After EGR1 enters the nucleus, it may
function as a transcription factor to promote PTEN expression.
To further explore whether EGR1 is an upstream
signaling molecule of PTEN and p-Akt, an EGR1 overexpression
system was constructed. EGR1, PTEN, p-Akt and Akt expression were
all detected by WB. EGR1 overexpression was found to lead to
significantly increased PTEN expression and p-Akt/Akt ratio
decreased (Fig. 2D). These results
suggested that EGR1 was the upstream signal of PTEN and Akt.
Taken together, it was identified that LNT treatment
could restore EGR1 expression in mouse HCC cells and allow EGR1 to
serve its normal function as a transcription factor, in turn
promoting PTEN expression. This then inhibits the AKT signaling
pathway, which alters the expression of downstream proteins Bcl-2
and Bax to ultimately trigger the apoptosis of mouse HCC cells.
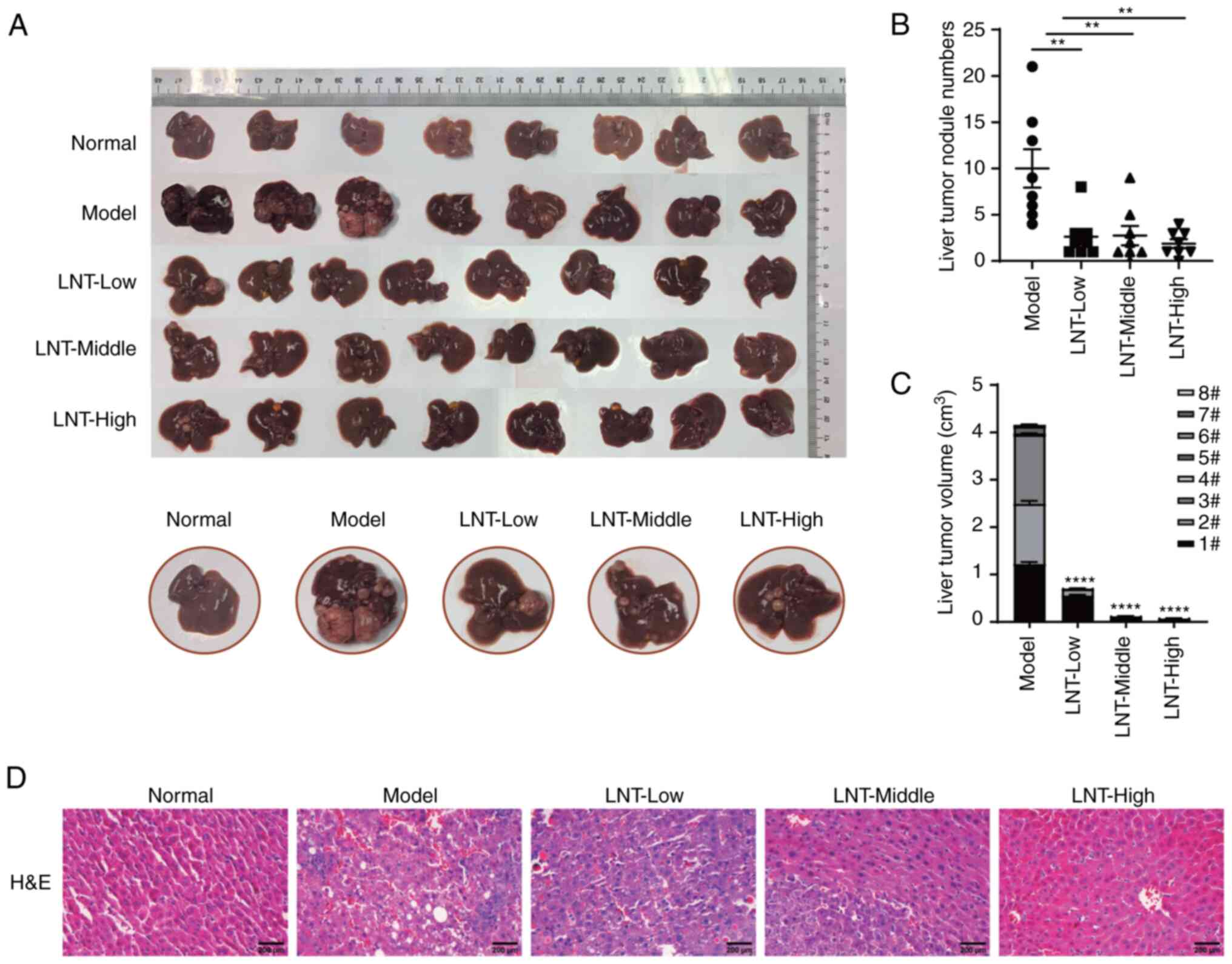
DEN-induced primary liver cancer model
in C57BL/6 mice
A primary liver cancer model was established using
C57BL/6 mice induced by DEN, after which normal, model and three
LNT administration groups were defined. Hyperplastic nodules could
be observed on the liver surfaces of mice in the model group
compared with those in the normal group. After treatment with LNT,
the number of hyperplastic nodules on the liver was significantly
reduced in the LNT-treated group compared with that in the model
group (Fig. 3A and B). In addition,
the tumor volume was significantly decreased (Fig. 3C). H&E staining was used to
further observe the pathological situation of tumor tissue. The
results revealed that in the model group, the tissue structure of
the liver tumor changed significantly and fatty degeneration
appeared in certain areas. The morphology and structure of the
LNT-treated group were improved compared with the model group. The
tumor area was relatively small, and the liver tissue structure in
the high-dose group was close to the normal group (Fig. 3D).
In addition, WB and IHC staining were further
applied to detect the difference of related protein expression.
Changes in the expression of the relevant proteins between the
normal and model groups are shown in Fig. 4A. Specifically, compared with the
normal group, the model group exhibited significantly reduced
expression levels of EGR1 and PTEN, whilst p-Akt/Akt ratio
increased, indicating that Akt was activated significantly. The
expression levels of proliferation-associated protein PCNA and the
anti-apoptotic protein Bcl-2 were significantly increased, whilst
those of the pro-apoptotic protein Bax were significantly
decreased. The ratio of Bcl-2/Bax, an indicator of cell apoptosis,
was significantly increased in the model group. These results
suggested that compared with those in the normal liver tissue,
there was no significant apoptosis in the liver cancer tissue of
the model group, where the expression levels of EGR1 and PTEN were
inhibited.
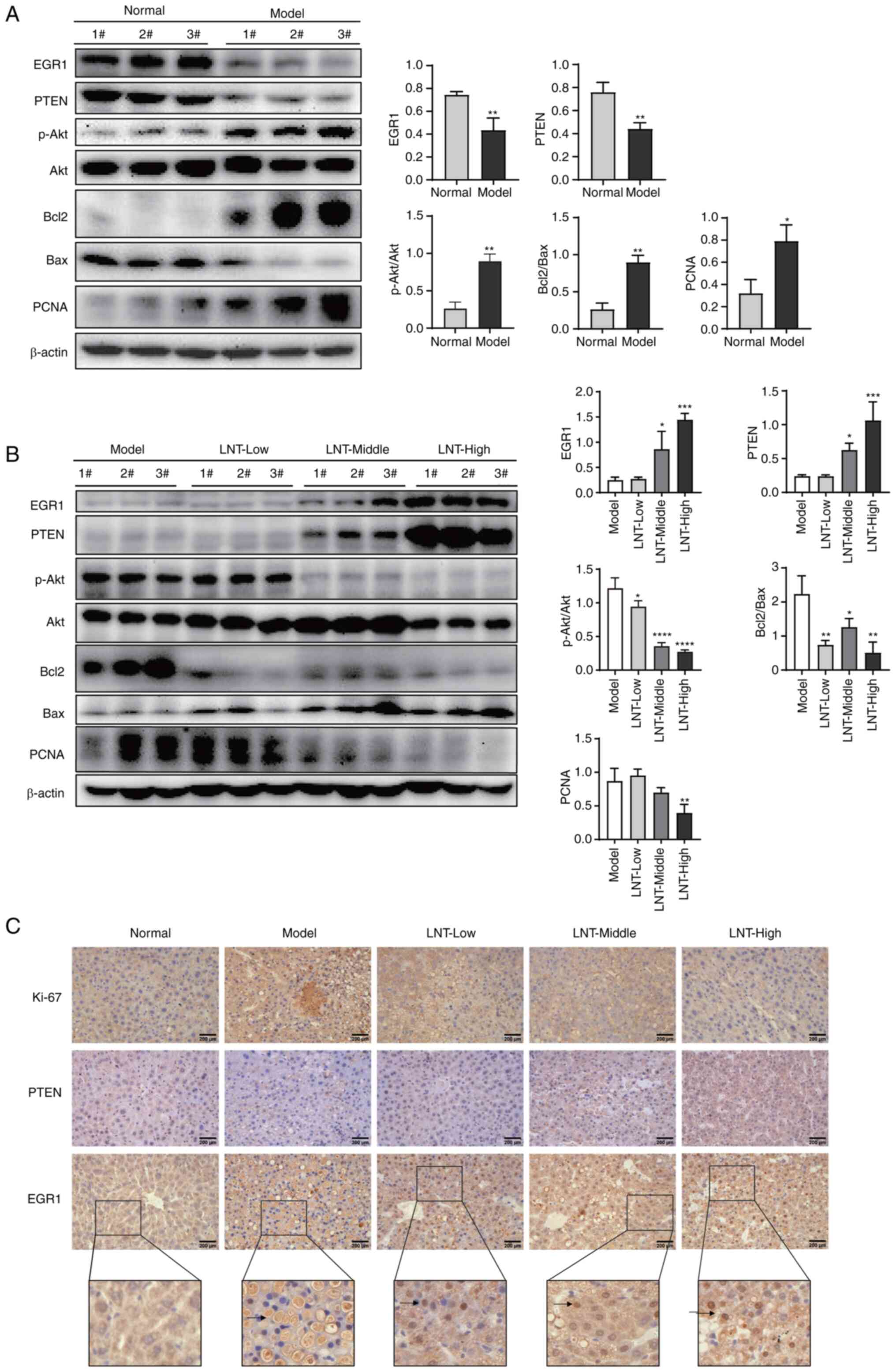 | Figure 4.Inhibitory effect of LNT on
DEN-induced primary liver cancer in mice. (A) The expression levels
of EGR1, PTEN and other proteins in the liver tissues of the normal
group and the model group, as analyzed by WB. (B) Expression of
EGR1, PTEN and other proteins in liver tissues of the model and
LNT-treated groups, as analyzed by WB. (C) Immunohistochemical
analysis of EGR1, PTEN and Ki-67 in the tissue sections.
Magnification, ×400. Scale bars, 200 µm (black). *P<0.05,
**P<0.01, ***P<0.001 and ****P<0.0001. LNT, Lentinan; DEN,
diethylnitrosamine; EGR1, early growth response 1; WB, western
blotting; p-, phosphorylated; PCNA, proliferating cell nuclear
antigen. |
Using WB, it was also identified that compared with
the model group the expression levels of EGR1 and PTEN were
significantly increased in the LNT-treated group (Fig. 4B). In addition, the p-Akt/Akt ratio
was significantly decreased, suggesting that AKT activity was
inhibited. The expression levels of Bcl-2 and PCNA expression were
significantly decreased, and Bax expression was increased, whilst
Bcl-2/Bax ratio decreased. IHC analysis revealed that the protein
Ki-67, which is associated with cancer cell proliferation, was
markedly reduced with increasing LNT dosages in the LNT
administration groups, compared with that in the model group. By
contrast, the expression levels of EGR1 and PTEN were markedly
increased, with EGR1 mainly distributing to the nucleus (Fig. 4C).
The results of these in vivo experiments are
consistent with those found in the in vitro experiments.
Therefore, it was considered that LNT could regulate EGR1
expression in mouse HCC cells, supporting its role as a
transcription factor that can promote the expression of PTEN. PTEN
then inhibits the PI3K/AKT signaling pathway and alters the
expression of related apoptotic proteins Bcl-2 and Bax, ultimately
inducing apoptosis and inhibiting the development of HCC.
Discussion
Over the past decade, the focus of cancer
therapeutics has shifted from anti-proliferative effects to the
development of agents that can induce cancer cell differentiation
and apoptosis (31).
Correspondingly, apoptosis-inducing drugs must selectively target
cancer cells whilst protecting normal cells from apoptosis.
Naturally-occurring compounds are characterized by low toxicity,
providing them the potential to greatly improve the patient quality
of life following treatment (32).
Therefore, it is of particular importance to identify natural
compounds that can selectively inhibit the initiation, development
and metastasis of cancer cells whilst eliminating cancer stem
cells, without toxic effects on normal cells (32). LNT was firstly discovered and
extracted by Chihara et al from the fruiting body of
Lentinula edodes (33,34).
It is a bioactive polysaccharide currently used as an adjuvant
therapy for cancer and other diseases (35–37).
Although it has been suggested that LNT is not directly toxic to
cancer cells (38,39), the present experimental results
demonstrated that LNT could induce apoptosis in mouse HCC cell line
Hepa1-6 cells in vitro. Previous studies have also reported
that LNT can directly induce cancer cell apoptosis in colon cancer
(40), cervical cancer (41) and lung adenocarcinoma (42).
In the present study, it was found that LNT could
restore the expression of EGR1 in mouse HCC cells. EGR1 belongs to
the Cys2His2-type family of zinc finger proteins and is encoded by
the human EGR1 gene (43).
It is a nuclear protein with a transcriptional function (17). At present, the role of EGR1 in liver
disease remains controversial. Various studies have suggested that
EGR1 can accelerate the development of liver injury (44–46).
However, Pritchard et al (47,48) in
two separate occasions reported that EGR1 can upregulate the
expression of hepatoprotective factors and attenuate carbon
tetrachloride-induced liver injury. The results of these two
studies are diametrically opposed. A recent study (49) found that whilst EGR1 expression is
upregulated during the initial stages of liver injury, silencing
EGR1 expression aggravated acetaminophen-induced liver injury
(AILI). By contrast, overexpression of EGR1 attenuated AILI. It has
been hypothesized that the expression of EGR1 in liver tissues can
vary across different stages of liver injury. During the early
stages of liver injury, the upregulation of EGR1 expression appears
to be a protective mechanism. However, in the stage of liver
cancer, the expression of EGR1 in HCC cells is suppressed and
exhibits low levels of expression compared with normal liver
tissue. This is consistent with the in vivo data in the
present study, where EGR1 expression in the model group was lower
than in the normal group. However, after LNT treatment, it not only
promoted the expression of EGR1 in mouse HCC cell but also found
that LNT could regulate EGR1 into the nucleus and play a
transcriptional role in vitro nuclear-separation
experiments, cell immunofluorescence experiments and in vivo
IHC experiments.
Subsequently, the present study also showed that
PTEN serves an important role in the LNT-induced regulation of EGR1
expression and induction of apoptosis. The PTEN gene is a
tumor suppressor with dual-specific phosphatase activity (50). Numerous studies have found that the
expression of PTEN protein is closely associated with primary liver
cancer (51–55). PTEN has been reported to be mutated,
inactivated and decreasingly expressed in numerous malignant tumors
(56), which need to be
supplemented by re-synthesis. In the present study, as the dosage
of LNT treatment increased, PTEN expression also increased. In
addition, PTEN expression was significantly increased in cells
overexpressing EGR1. Therefore, the changes in PTEN expression are
likely to be caused by the upregulation of EGR1 by LNT since PTEN
is considered as the direct downstream target of EGR1. Similar
results have been reported in papillary thyroid carcinoma and lung
cancer cells (29,30).
PTEN is essential for maintaining the balance of the
PI3K/AKT signaling pathway (24–28).
Several previous studies have reported that increased expression of
PTEN can inhibit AKT phosphorylation; inhibition of the AKT
signaling pathway can activate the expression of the pro-apoptotic
protein Bax whilst inhibiting the expression of the anti-apoptotic
protein Bcl-2, ultimately resulting in the apoptosis of cancer
cells (57–60). In the present study, as the LNT
concentration increased, the expression of PTEN also increased,
whilst the phosphorylation of Akt was significantly decreased.
Furthermore, Bcl-2 expression decreased and Bax expression
increased. Finally, apoptosis of mouse HCC cells occurred.
However, the present study has several limitations.
It is generally considered that Caspase-3 is the most crucial
terminal shearing enzyme in cell apoptosis. Unfortunately,
Caspase-3 and its cleavage products were not detected in the study
of LNT on mouse HCC apoptosis. Moreover, when the TUNEL staining
test was performed on the tissue sections of the primary liver
cancer mouse model in our previous experiment, an intense staining
background was identified, rendering it impossible to evaluate
whether apoptosis occurs accurately. The reason may be that liver
tissue is rich in endogenous peroxidase, which is easy to stain
non-specifically. Therefore, the TUNEL staining result was not
included in the results of the present study; this will be explored
in future study by the authors.
The results of the present study suggested that LNT
could inhibit the progression of mouse HCC by regulating the
expression and nuclear translocation of EGR1 in mouse HCC cells,
activating the expression of the tumor suppressor PTEN. This
inhibited activation of the AKT signaling pathway, promoting the
apoptosis of mouse HCC. Therefore, LNT can exert its
tumor-inhibitory function by increasing the expression of EGR1
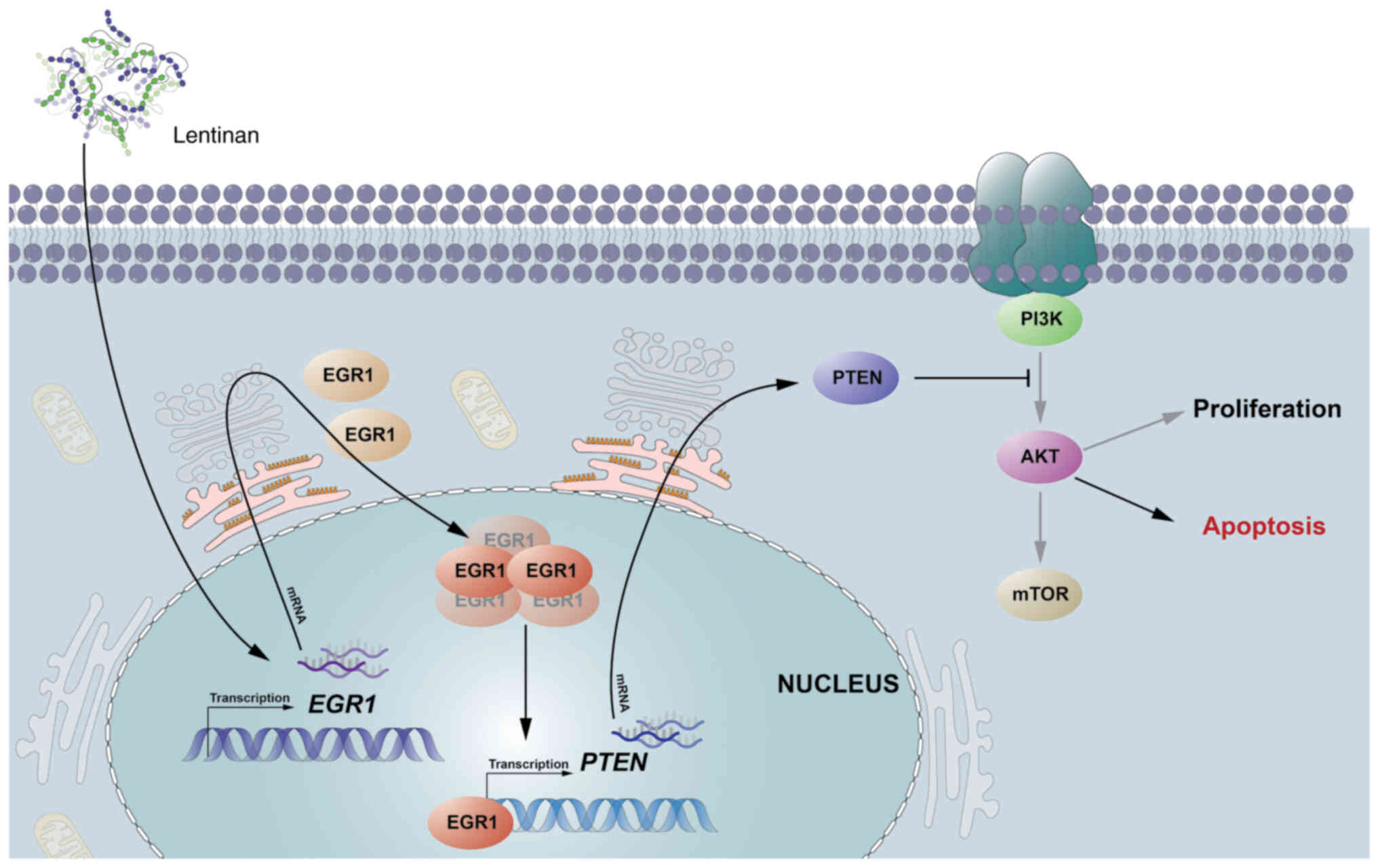
during hepatocarcinogenesis. A possible mechanism by which LNT
induces apoptosis and stops the progression of mouse HCC through
the EGR1/PTEN/AKT axis is shown in Fig.
5.
In the present study, the effect of LNT on HCC cell
line Hepa1-6 and its possible mechanism were investigated in
vitro and in vivo. The results showed that LNT could
induce apoptosis of Hepa1-6 cells. In addition, LNT promotes the
expression of transcription factor EGR1 and regulates EGR1 into the
nucleus to promote the expression of tumor suppressor gene PTEN.
PTEN then inhibits activation of the AKT signaling pathway,
stimulating the occurrence of apoptosis in HCCs. Therefore, it is
suggested that LNT can induce apoptosis and cease the progression
of mouse HCC through the EGR1/PTEN/AKT signaling axis. These
results lay a foundation for exploring the mechanism of anti-HCC by
LNT and provide novel ideas for the treatment of primary liver
cancer.
Acknowledgements
The authors are grateful to the members of Pan
Laboratory from Minnan Normal University for discussion and
technical assistance.
Funding
The present study was supported by the National Science and
Technology Planning Project of China (grant no. 2021L3027), the New
Agricultural Science Education and Practice Project (Letter No.
[2020]/20 from Higher Education Department of Education Ministry),
the Natural Science Foundation of China (grant no. 81903665), the
Natural Science Foundation of Fujian Province (grant no.
2020J01822), the Fujian Province Foreign Cooperation Project (grant
no. 2022I0022) and the Cultivation Project of Minnan Normal
University (grant no. MSPY202101).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YTP and QCW conceptualized the present study. YTP,
QCW and YX developed methodology. JPY and YBL validated the data
and conducted investigation. JPY and XML performed formal analysis.
XML provided resources. ZCL and JFH curated the data. JPY prepared
the original draft. YTP and QCW wrote, reviewed and edited the
manuscript. AG and YX performed data visualization. QCW supervised
the study. YTP conducted project administration. YTP, QCW, ZCL and
YX acquired funding. YTP and QCW confirm the authenticity of all
the raw data. All authors have read and approved the final version
of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2020010) and supervised by the Animal Ethics and Welfare Committee
of Minnan Normal University (Zhangzhou, China). All animal
experiments were performed according to the relevant regulatory
standards and were performed in accordance with the Experimental
animal research Ethics and Welfare Committee of Minna Normal
University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
LNT
|
lentinan
|
|
HCC
|
hepatocellular carcinoma
|
|
EGR1
|
early growth response-1
|
|
DEN
|
diethylnitrosamine
|
|
IF
|
immunofluorescence
|
|
IHC
|
immunohistochemical
|
|
PCNA
|
proliferation cell nuclear antigen
|
References
|
1
|
Vannucci L, Krizan J, Sima P, Stakheev D,
Caja F, Rajsiglova L, Horak V and Saieh M: Immunostimulatory
properties and antitumor activities of glucans (Review). Int J
Oncol. 43:357–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Schepetkin IA and Quinn MT: Botanical
polysaccharides: Macrophage immunomodulation and therapeutic
potential. Int Immunopharmacol. 6:317–333. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wasser SP: Medicinal mushrooms as a source
of antitumor and immunomodulating polysaccharides. Appl Microbiol
Biotechnol. 60:258–274. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sheng K, Wang C, Chen B, Kang M and Wang
M, Liu K and Wang M: Recent advances in polysaccharides from
Lentinus edodes (berk.): Isolation, structures and bioactivities.
Food Chem. 358:1298832021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bisen PS, Baghel RK, Sanodiya BS, Thakur
GS and Prasad GB: Lentinus edodes: A macrofungus with
pharmacological activities. Curr Med Chem. 17:2419–2430. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Li S, Huang Y, Wang S, Xu X and Zhang L:
Determination of the triple helical chain conformation of β-glucan
by facile and reliable triple-detector size exclusion
chromatography. J Phys Chem B. 118:668–675. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang X, Li T, Liu S, Xu Y, Meng M, Li X,
Lin Z, Wu Q, Xue Y, Pan Y and Alitongbieke G: β-glucan from
Lentinus edodes inhibits breast cancer progression via the
nur77/hif-1alpha axis. Biosci Rep. 40:BSR202010062020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Q, Du Z, Zhang Y, Zheng Z, Li Q and
Wang K: Apoptosis induction activity of polysaccharide from
Lentinus edodes in H22-bearing mice through ROS-mediated
mitochondrial pathway and inhibition of tubulin polymerization.
Food Nutr Res. 64:43642020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sun M, Bu R, Zhang B, Cao Y, Liu C and
Zhao W: Lentinan inhibits tumor progression by immunomodulation in
a mouse model of bladder cancer. Integr Cancer Ther. 19:1–7. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Xu H, Qi Z, Zhao Q, Xue J, Zhu J, He Y,
Liu G and Qin S: Lentinan enhances the antitumor effects of
Delta-like 1 via neutrophils. BMC Cancer. 22:9182022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
Globocan estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin J, Shi J, Guo H, Yang X, Jiang Y, Long
J, Bai Y, Wang D, Yang X, Wan X, et al: Alterations in DNA damage
repair genes in primary liver cancer. Clin Cancer Res.
25:4701–4711. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gingold JA, Zhu D, Lee DF, Kaseb A and
Chen J: Genomic profiling and metabolic homeostasis in primary
liver cancers. Trends Mol Med. 24:395–411. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Song J, Zhou H, Gu D and Xu Y:
Hepatocellular carcinoma differentiation: Research progress in
mechanism and treatment. Front Oncol. 11:7903582021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Berger E, Vega N, Vidal H and Geloen A:
Gene network analysis leads to functional validation of pathways
linked to cancer cell growth and survival. Biotechnol J.
7:1395–1404. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu S, Yao X, Zhang D, Sheng J, Wen X,
Wang Q, Chen G, Li Z, Du Z and Zhang X: Analysis of transcription
factor-related regulatory networks based on bioinformatics analysis
and validation in hepatocellular carcinoma. Biomed Res Int.
2018:14313962018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang B, Guo H, Yu H, Chen Y, Xu H and Zhao
G: The role of the transcription factor Egr1 in cancer. Front
Oncol. 11:6425472021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu J, Zhang SS, Saito K, Williams S,
Arimura Y, Ma Y, Ke Y, Baron V, Mercola D, Feng GS, et al: Pten
regulation by AKT-EGR1-ARF-PTEN axis. EMBO J. 28:21–33. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Cui J and Placzek WJ: Post-transcriptional
regulation of anti-apoptotic Bcl2 family members. Int J Mol Sci.
19:3082018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lalier L, Cartron PF, Juin P, Nedelkina S,
Manon S, Bechinger B and Vallette FM: Bax activation and
mitochondrial insertion during apoptosis. Apoptosis. 12:887–896.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Huang Y, Ding J, Liu N, Peng S,
Wang J, Wang F and Zhang Y: Targeting akt by SC66 triggers GSK-3β
mediated apoptosis in colon cancer therapy. Cancer Cell Int.
19:1242019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yamaguchi H and Wang HG: The protein
kinase PKB/Akt regulates cell survival and apoptosis by inhibiting
Bax conformational change. Oncogene. 20:7779–7786. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen TA, Wang JL, Hung SW, Chu CL, Cheng
YC and Liang SM: Recombinant VP1, an Akt inhibitor, suppresses
progression of hepatocellular carcinoma by inducing apoptosis and
modulation of CCl2 production. PLoS One. 6:e233172011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hopkins BD, Hodakoski C, Barrows D, Mense
SM and Parsons RE: PTEN function: The long and the short of it.
Trends Biochem Sci. 39:183–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li C and Li X: Circpten suppresses
colorectal cancer progression through regulating PTEN/AKT pathway.
Mol Ther Nucleic Acids. 26:1418–1432. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li R, Xing QW, Wu XL, Zhang L, Tang M,
Tang JY, Wang JZ, Han P, Wang SQ, Wang W, et al: Di-n-butyl
phthalate epigenetically induces reproductive toxicity via the
PTEN/AKT pathway. Cell Death Dis. 10:3072019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui Z, Gao H, Yan N, Dai Y, Wang H, Wang
M, Wang J, Zhang D, Sun P, Qi T, et al: Lncrna plncrna-1
accelerates the progression of prostate cancer by regulating
PTEN/Akt axis. Aging (Albany NY). 13:12113–12128. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang X, Liu C, Li H and Guo L: Effects of
mir-21 on proliferation and apoptosis of wt cells via PTEN/Akt
pathway. Exp Ther Med. 19:2155–2160. 2020.PubMed/NCBI
|
|
29
|
Guo H and Zhang L: Egr1/2 inhibits
papillary thyroid carcinoma cell growth by suppressing the
expression of PTEN and BAX. Biochem Genet. 59:1544–1557. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yamamoto C, Basaki Y, Kawahara A,
Nakashima K, Kage M, Izumi H, Kohno K, Uramoto H, Yasumoto K,
Kuwano M and Ono M: Loss of PTEN expression by blocking nuclear
translocation of EGR1 in gefitinib-resistant lung cancer cells
harboring epidermal growth factor receptor-activating mutations.
Cancer Res. 70:8715–8725. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Denisenko TV, Sorokina IV, Gogvadze V and
Zhivotovsky B: Mitotic catastrophe and cancer drug resistance: A
link that must to be broken. Drug Resist Updat. 24:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Dutta S, Mahalanobish S, Saha S, Ghosh S
and Sil PC: Natural products: An upcoming therapeutic approach to
cancer. Food Chem Toxicol. 128:240–255. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chihara G, Maeda Y, Hamuro J, Sasaki T and
Fukuoka F: Inhibition of mouse sarcoma 180 by polysaccharides from
lentinus edodes (berk.) sing. Nature. 222:687–688. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yu Z, Ming G, Kaiping W, Zhixiang C,
Liquan D, Jingyu L and Fang Z: Structure, chain conformation and
antitumor activity of a novel polysaccharide from lentinus edodes.
Fitoterapia. 81:1163–1170. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang Y, Zhang M, Jiang Y, Li X, He Y,
Zeng P, Guo Z, Chang Y, Luo H, Liu Y, et al: Lentinan as an
immunotherapeutic for treating lung cancer: A review of 12 years
clinical studies in China. J Cancer Res Clin Oncol. 144:2177–2186.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen YW, Hu DJ, Cheong KL, Li J, Xie J,
Zhao J and Li SP: Quality evaluation of lentinan injection produced
in china. J Pharm Biomed Anal. 78–79. 176–182. 2013.
|
|
37
|
Zhang M, Zhang Y, Zhang L and Tian Q:
Mushroom polysaccharide lentinan for treating different types of
cancers: A review of 12 years clinical studies in China. Prog Mol
Biol Transl Sci. 163:297–328. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Murata Y, Shimamura T, Tagami T, Takatsuki
F and Hamuro J: The skewing to Th1 induced by lentinan is directed
through the distinctive cytokine production by macrophages with
elevated intracellular glutathione content. Int Immunopharmacol.
2:673–689. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Maeda YY and Chihara G: Lentinan, a new
immuno-accelerator of cell-mediated responses. Nature. 229:6341971.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Zhang Y, Liu Y, Zhou Y, Zheng Z, Tang W,
Song M, Wang J and Wang K: Lentinan inhibited colon cancer growth
by inducing endoplasmic reticulum stress-mediated autophagic cell
death and apoptosis. Carbohydr Polym. 267:1181542021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ya G: A Lentinus edodes polysaccharide
induces mitochondrial-mediated apoptosis in human cervical
carcinoma HeLa cells. Int J Biol Macromol. 103:676–682. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Chen Q, Zheng Y, Chen X, Ge P, Wang P and
Wu B: Upregulation of miR-216a-5p by lentinan targeted inhibition
of JAK2/STAT3 signaling pathway to reduce lung adenocarcinoma cell
stemness, promote apoptosis, and slow down the lung adenocarcinoma
mechanisms. Front Oncol. 11:7780962021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang Y, Wu F, Zhang J, Sun R, Li F, Li Y,
Chang S, Wang L, Wang X, Liu L and Huang C: Egr1 interacts with
DNMT3L to inhibit the transcription of miR-195 and plays an
anti-apoptotic role in the development of gastric cancer. J Cell
Mol Med. 23:7372–7381. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Bi JG, Zheng JF, Li Q, Bao SY, Yu XF, Xu P
and Liao CX: Microrna-181a-5p suppresses cell proliferation by
targeting Egr1 and inhibiting Egr1/TGF-β/Smad pathway in
hepatocellular carcinoma. Int J Biochem Cell Biol. 106:107–116.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pritchard MT and Nagy LE: Ethanol-induced
liver injury: Potential roles for egr-1. Alcohol Clin Exp Res.
29:146S–150S. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Pritchard MT, Roychowdhury S, McMullen MR,
Guo L, Arteel GE and Nagy LE: Early growth response-1 contributes
to galactosamine/lipopolysaccharide-induced acute liver injury in
mice. Am J Physiol Gastrointest Liver Physiol. 293:G1124–G1133.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Pritchard MT, Cohen JI, Roychowdhury S,
Pratt BT and Nagy LE: Early growth response-1 attenuates liver
injury and promotes hepatoprotection after carbon tetrachloride
exposure in mice. J Hepatol. 53:655–662. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Pritchard MT, Malinak RN and Nagy LE:
Early growth response (EGR)-1 is required for timely cell-cycle
entry and progression in hepatocytes after acute carbon
tetrachloride exposure in mice. Am J Physiol Gastrointest Liver
Physiol. 300:G1124–G1131. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Lei X, Xu Q, Li C, Niu B, Ming Y, Li J,
Tang Y, Li X, Tang J, Wu J, et al: Egr1 confers protection against
acetaminophen-induced hepatotoxicity via transcriptional
upregulating of Acaa2. Int J Biol Sci. 18:3800–3817. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Worby CA and Dixon JE: PTEN. Annu Rev
Biochem. 83:641–669. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Fu X, Wen H, Jing L, Yang Y, Wang W, Liang
X, Nan K, Yao Y and Tian T: Microrna-155-5p promotes hepatocellular
carcinoma progression by suppressing PTEN through the PI3K/Akt
pathway. Cancer Sci. 108:620–631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Tan L, Xu Z, Mao Q, Zhou S, Zhu J, Zhang X
and Li H: Purified PTEN-long induces liver cancer cells to undergo
autophagy and apoptosis. Front Surg. 9:7676112022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wang B, Song K, Chen L, Su H, Gao L, Liu J
and Huang A: Targeted inhibition of ACK1 can inhibit the
proliferation of hepatocellular carcinoma cells through the
PTEN/AKT/mTOR pathway. Cell Biochem Funct. 38:642–650. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Xin X, Wu M, Meng Q, Wang C, Lu Y, Yang Y,
Li X, Zheng Q, Pu H, Gui X, et al: Long noncoding RNA HULC
accelerates liver cancer by inhibiting PTEN via autophagy
cooperation to miR15a. Mol Cancer. 17:942018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhang R, Zhong L, Sun K, Liu J, Wang Q,
Mao D, Fang G and Long F: A study on curcumol influencing
proliferation and apoptosis of hepatocellular carcinoma cells
through DJ-1/PTEN/PI3K/AKT pathway. Biomed Res Int.
2022:99127762022.PubMed/NCBI
|
|
56
|
Sulis ML and Parsons R: PTEN: From
pathology to biology. Trends Cell Biol. 13:478–483. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Pilling AB and Hwang C: Targeting
prosurvival BCL2 signaling through Akt blockade sensitizes
castration-resistant prostate cancer cells to enzalutamide.
Prostate. 79:1347–1359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Cui Y, Su Y, Deng L and Wang W:
Ginsenoside-rg5 inhibits retinoblastoma proliferation and induces
apoptosis through suppressing BCL2 expression. Chemotherapy.
63:293–300. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Zhang L, Ge C, Zhao F, Zhang Y, Wang X,
Yao M and Li J: Nrbp2 overexpression increases the chemosensitivity
of hepatocellular carcinoma cells via Akt signaling. Cancer Res.
76:7059–7071. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Belkhiri A, Dar AA, Zaika A, Kelley M and
El-Rifai W: T-darpp promotes cancer cell survival by up-regulation
of BCL2 through Akt-dependent mechanism. Cancer Res. 68:395–403.
2008. View Article : Google Scholar : PubMed/NCBI
|