Introduction
Acute lymphoblastic leukemia (ALL) is a
hematological malignancy characterized by malignant transformation
and proliferation of lymphoid progenitor cells in bone marrow. The
prognosis of relapsed or refractory ALL (RR-ALL) remains poor, with
a reported median overall survival (OS) of 6 months and a 5-year
survival rate of 7% (1,2). Hematopoietic stem cell transplantation
(HSCT) is the only treatment option for achieving long-term
survival in RR-ALL. Although achieving complete remission (CR)
before the initiation of HSCT is preferable, conventional salvage
chemotherapy regimens are associated with CR rates of only 30–40%
(3).
Inotuzumab ozogamicin (IO), a novel therapeutic drug
for RR-ALL, is a humanized anti-cluster of differentiation (CD) 22
monoclonal antibody conjugated with calicheamicin. IO was approved
in August 2017 in the United States for the treatment of
CD22+ RR-ALL. The pivotal INO-VATE study compared
clinical efficacy of IO with that of conventional chemotherapies
used as the control; it revealed that CR and CR with incomplete
hematologic recovery were markedly higher in the IO group (80.7%)
compared with those in the control group (29.4%). On the other
hand, the duration of remission and median OS in the IO group were
only 4.6 months and 7.7 months, respectively, which is only 1.5
months longer than in the control group (4). The final report from the INO-VATE
study revealed 2-year OS rates of 22.8 and 10.8% in the IO group
and in the control group, respectively (5,6). Thus,
while IO is far more effective for treating RR-ALL than
conventional regimens, the duration of remission remains short. As
a result, effective combination strategies are needed to enhance
the anti-leukemic effects of IO.
IO comprises the cytotoxic antibiotic
N-acetyl-gamma-calicheamicin dimethylhydrazine, a calicheamicin
derivative, attached to a humanized monoclonal IgG4 antibody via
the 4-(4 acetylphenoxy) butanoic acid (acetyl butyrate) linker.
Once IO is administered to patients, the drug binds to the CD22
leukemic cell surface antigen. The complex is then internalized and
fuses with lysosomes inside the cell. Calicheamicin detaches from
the antibody site following the breakdown of the linker, inducing
DNA single-strand breaks (SSBs) and double-strand breaks (DSBs) in
the nucleus and subsequent apoptosis (7).
PARPs are a family of 17 proteins involved in the
repair of DNA strand breaks. The most well-studied members of the
PARP family are PARP-1 and PARP-2, which play crucial roles in the
repair of SSBs. PARP inhibitors are novel anticancer drugs
targeting the DNA damage response (8). Olaparib, a potent inhibitor of
PARP-1/2, was first approved for the treatment of breast cancer
susceptibility gene (BRCA)-mutant ovarian cancer in 2014.
Since then, several other PARP inhibitors have been developed and
approved, and their use has been expanded to the treatment of
various types of cancer (9,10). Talazoparib is the latest approved
PARP-1/2 inhibitor with the most potent PARP1 inhibitory activity
for the treatment of BRCA-mutant breast cancer. Loss of
BRCA function causes failure of the DSB repair pathway. In
BRCA-defective tumor cells, PARP inhibitors prevent the
repair of SSBs, which then convert to irreparable and toxic DSBs,
effectively exerting cytotoxicity through synthetic lethality
(11).
One strategy to increase the cytotoxicity of IO is
inhibition of DSB repair. Leukemic cells evoke a DSB repair
response following DNA damage triggered by IO. Inhibition of the
repair response is hypothesized to accumulate unrepaired DNA strand
breaks, thereby enhancing the cytotoxicity of IO. The present study
aimed to compare synergistic anti-leukemic effects between IO and
PARP inhibitors in B-ALL cells. For this purpose, the cytotoxicity
of IO was first evaluated in detail using Philadelphia
(Ph)− and Ph+ B-ALL cell lines in
vitro. Next, the alkaline comet assay was used to determine DNA
damage repair kinetics in cells treated with IO. Finally, attempts
were made to increase the cytotoxic effects of IO by adding either
olaparib or talazoparib, which are PARP inhibitors, under a
hypothesized interaction between these drugs.
Materials and methods
Cell cultures
The Reh Ph−B-ALL cell line (cat. no.
CRL-8286), the SUP-B15 Ph+ B-ALL cell line (cat. no.
CRL-1929) and the HL-60 acute promyelocytic leukemia cell line
(cat. no. CLL-240) were purchased from American Type Culture
Collection (ATCC). Reh and HL-60 cells were cultured in RPMI-1640
medium (ATCC) supplemented with 10% fetal bovine serum (FBS;
Sigma-Aldrich; Merck KGaA), at 37°C under 5% CO2 in a
humidified atmosphere. SUP-B15 cells were cultured in Dulbecco's
medium (FUJIFILM Wako Pure Chemical Corporation) with 20% FBS in
the same atmosphere. Reverse transcription PCR testing was used to
ensure the absence of mycoplasma contamination (Biotherapy
Institute of Japan, Inc.). Reh and SUP-B15 cells were confirmed to
be free of mycoplasma.
Chemicals and reagents
IO was kindly provided by Pfizer, Inc. and dissolved
in water for injection (FUSO Pharmaceutical Industries, Ltd.) to a
stock concentration of 250 µg/ml. Olaparib and talazoparib were
obtained from Selleck Chemicals and dissolved in dimethylsulfoxide
(Wako Yakuhin Co., Ltd.) to a stock concentration of 10 mM.
Determination of CD22 positivity
Flow cytometry was performed to determine CD22
expression on the cell surface of Reh and SUP-B15 cells using
antibodies against CD22. The analysis of CD22 expression was
performed by LSI Medience Corporation. CD22 expression was measured
using a flow cytometer (FACSCanto II; Becton, Dickinson and
Company) using undiluted IOTest CD22-Fluorescein isothiocyanate
(FITC; cat. no. IM0779U; Beckman Coulter, Inc.) and undiluted IgG2a
mouse-FITC (cat. no. A12689; Beckman Coulter, Inc.). Twice-diluted
IgG1 mouse-RD1 (cat. no. 6602884; Beckman Coulter, Inc.) was used
as a control. The acute promyelocytic leukemia cell line HL-60 was
used as a CD22− control.
Cell proliferation inhibition
assay
Cells (2.0×105/ml Reh cells and
5.0×105/ml SUP-B15 cells) were continuously exposed to
various concentrations of IO (1×10−3−1×102
ng/ml), olaparib (1×10−3−1×102 µM) and
talazoparib (1×10−1−1×104 nM) to evaluate
proliferation inhibition effects for 48 h. Cell viability was
assessed using a Cell Counting Kit-8 (CCK-8) assay (cat. no. CK04;
Dojindo Laboratories, Inc.) in accordance with the manufacturer's
instructions. A total of 10 µl of the CCK-8 reagent was added to
100 µl of cell suspension and incubated at 37°C for 3 h. Absorbance
was measured at 450 nm using a plate reader (SPECTRA MAX iD3;
Molecular Devices, LLC).
Annexin-V binding assay
To detect the induction of apoptosis in Reh and
SUP-B15 cells, a total of 2.0×105/ml cells were treated
with various concentrations of IO, olaparib and talazoparib for 24
or 48 h. After drug administration, cells were stained with
annexin-V and propidium iodide using Annexin V FLUOS Staining Kit
(cat. no. 49734400; Sigma-Aldrich; Merck KGaA) and analyzed by flow
cytometry (FACSDiva Software version 6.1.3; Becton, Dickinson and
Company) according to the manufacturer's instructions. Untreated
cells were used as negative controls and cells treated with drug
concentrations sufficient to induce apoptosis were used as positive
controls.
Calculation of combination index
(CI)
The effects of combining IO and PARP inhibitors were
calculated using the CI method (12), with values determined using CompuSyn
(version 2.11; Informert Technologies, Inc.). CI values were
classified as follows: i) CI >1.1, antagonism; ii) CI, 0.9–1.1,
additive; iii) CI, 0.85–0.9, slight synergism; iv) CI, 0.7–0.85,
moderate synergism; v) CI, 0.3–0.7, synergism; vi) CI, 0.1–0.3,
strong synergism; and vii) CI <0.1, very strong synergism
(13).
Alkaline comet assay
Alkaline comet assay was conducted to quantify the
amount of DNA strand breaks using an OxiSelect Comet Assay Kit
(cat. no. STA-351; Cell Biolabs, Inc.) according to the
manufacturer's instructions. A total of 1×105 Reh
cells/ml were incubated with 2 ng/ml of IO in the presence or
absence of 1 µM olaparib or 100 nM talazoparib for 1 or 6 h at
37°C. A total of 1×105 SUP-B15 cells/ml were incubated
with 20 ng/ml IO in the presence or absence of 10 µM olaparib or 1
nM talazoparib under the same conditions used for Reh cells.
Resuspended cells were then mixed with melted 90% agarose gel and
transferred onto the base layer. These embedded cells were treated
with lysis buffer (included in OxiSelect Comet Assay Kit) and
alkaline solution. After electrophoresis, samples were washed and
fixed in ice-cold 70% ethanol at room temperature for 5 min. Slides
were dried, stained with Vista Green DNA dye (OxiSelect Comet Assay
Kit), and visualized using an epifluorescence microscope (BX50F;
Olympus corporation). The Olive tail moment was calculated using
Comet (version 4.0; Oxford Instruments plc). For each sample, 50
cells were selected and analyzed.
Statistical analysis
Data analysis and graph creation were conducted
using GraphPad Prism (version 10.0.3; Dotmatics). The results are
shown as the mean ± standard division, and two-way ANOVA with
Bonferroni corrections for multiple comparisons on the interaction
between IO and PARP inhibitors. All of the results were derived
from at least triplicate independent experiments. P<0.05 was
considered to indicate a statistically significant difference.
Results
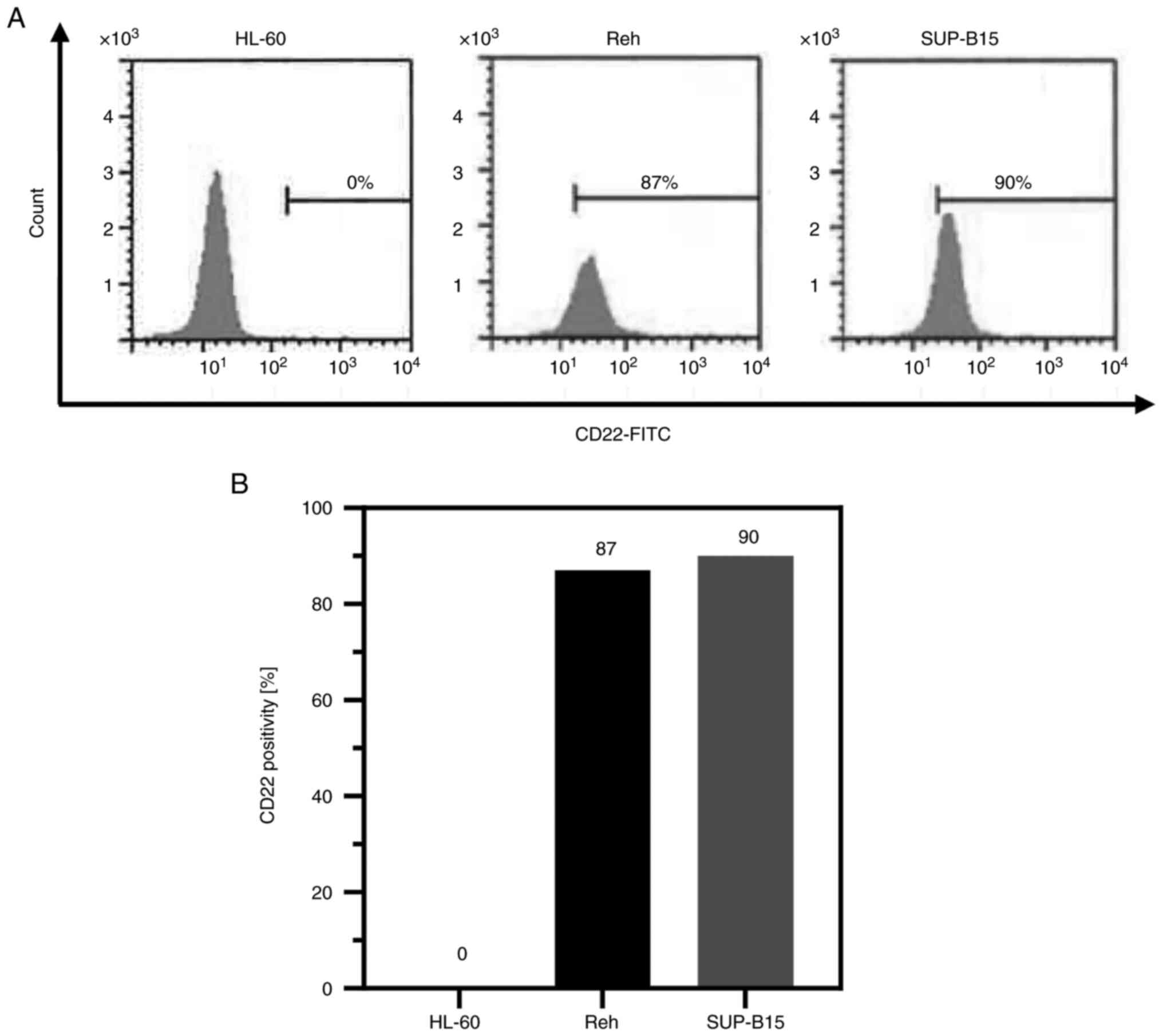
Cell surface CD22 expression
Cell surface CD22 expression, which is indispensable
to the internalization of IO in B-ALL cells, was determined by flow
cytometry (14). The expression
levels of CD22 in Reh and SUP-B15 cells were 87 and 90%,
respectively (Fig. 1). These two
cell lines expressed sufficient levels of CD22 for IO to show
cytotoxicity (7). CD22 was not
expressed in the negative control HL-60 cells.
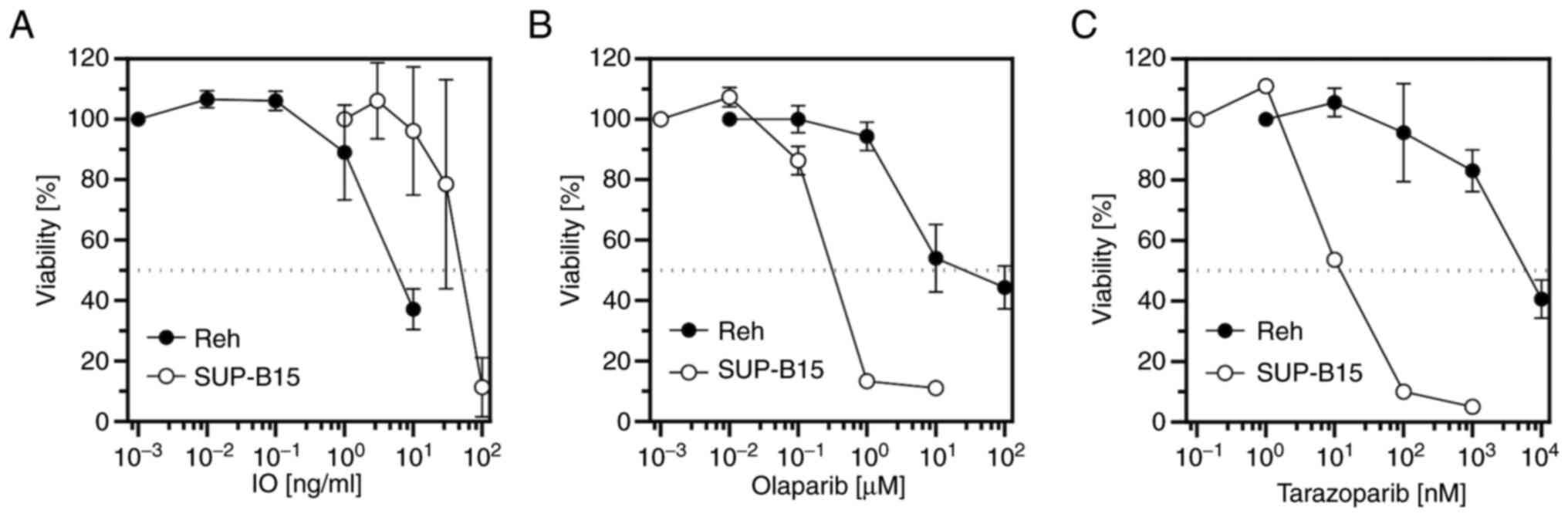
Cell proliferation inhibition and
induction of apoptosis by IO or PARP inhibitors
The anti-proliferative activity of IO in Reh and
SUP-B15 cells was examined. Cells were incubated with increasing
concentrations of IO for 48 h, then cell viability was measured by
water soluble tetrazolium assay using CCK-8. IO inhibited the
proliferation of these cells in a dose-dependent manner (Fig. 2A). The half-maximal inhibitory
concentration (IC50) values for IO are shown in Table I. Similarly, cell proliferation
inhibition by the PARP inhibitors olaparib and talazoparib in Reh
and SUP-B15 cells was examined. Olaparib and talazoparib inhibited
cell proliferation in a dose-dependent manner in both cell lines
(Fig. 2B and C). The
IC50 values of olaparib and talazoparib are shown in
Table II, revealing that
talazoparib was more potent than olaparib. An Annexin-V binding
assay was conducted to determine IO-induced apoptosis. When Reh and
SUP-B15 cells were treated with various concentrations of IO for 24
or 48 h, IO increased early and late cell apoptosis in a
dose-dependent manner. Moreover, exposure to IO induced more
apoptosis at 48 h than at 24 h for every IO concentration tested
(Fig. 3). In Fig. 3C, untreated SUP-B15 cells underwent
>20% apoptosis. SUP-B15 cells are prone to elimination by
apoptosis very easily and spontaneously. Previous studies using
SUP-B15 cell line also revealed some extent of cell death of
untreated SUP-B15 cells (15,16).
The live cell percentage of untreated SUP-B15 was ~83–88% in these
studies. These present results indicated that IO was cytotoxic to
both Ph− and Ph+ B-ALL cells, and talazoparib
was more cytotoxic to both Ph− and Ph+ B-ALL
cells than olaparib.
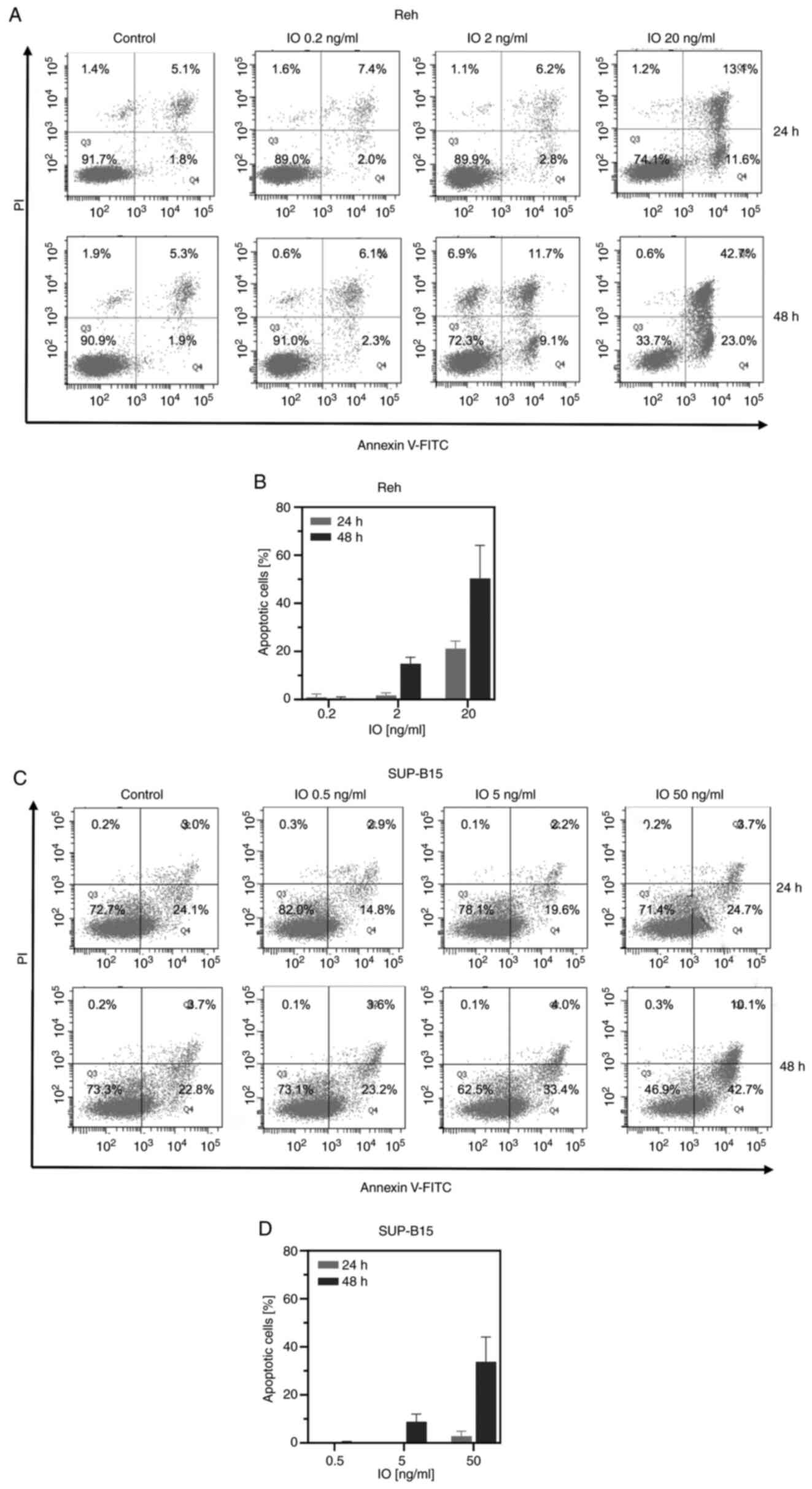 | Figure 3.Induction of apoptosis by IO. Cells
were incubated with (A and B) 0.2, 2, or 20 ng/ml IO for Reh cells;
(C and D) 0.5, 5, or 50 ng/ml IO for SUP-B15 cells for 24 or 48 h,
followed by (A and C) Annexin-V and propidium iodide co-staining
and flow cytometry. The left lower quadrant shows live cells, and
the left upper quadrant shows necrotic cells. The right lower and
upper quadrants, as annexin-V+ quadrants, show early and late
apoptotic cells, respectively. The percentage of cells in every
quadrant is indicated. (B and D) Histograms represent the
percentage of apoptotic cells (drug-treated apoptotic cells minus
control apoptotic cells). Error bars represent standard deviation
from triplicate experiments. IO, inotuzumab ozogamicin. |
 | Table I.IC50 values for IO, Ola
and Tala. |
Table I.
IC50 values for IO, Ola
and Tala.
|
| Drugs |
|---|
|
|
|
|---|
|
| IO | Ola | Tala |
|---|
|
IC50 |
|
|
|
|
Reh | 5.3 ng/ml | 24.0 µM | 4.9 µM |
|
SUP-B15 | 49.7 ng/ml | 0.3 µM | 0.01 µM |
 | Table II.IO sensitivity in combination with
Ola or Tala. |
Table II.
IO sensitivity in combination with
Ola or Tala.
|
| Drugs |
|---|
|
|
|
|---|
|
| IO + Ola | IO + Tala |
|---|
|
IC50 |
|
|
|
Reh | 0.8 ng/ml | 2.9 ng/ml |
|
SUP-B15 | 36.1 ng/ml | 39.6 ng/ml |
Cell proliferation-inhibiting effects
of combining IO with PARP inhibitors
Both Reh and SUP-B15 cells were incubated with IO
for 48 h with minimally toxic concentrations of either olaparib (1
µM in Reh cells; 10 nM in SUP-B15 cells) or talazoparib (100 nM in
Reh cells; 1 nM in SUP-B15 cells) to investigate the cell
proliferation inhibition effects of IO combined with olaparib or
talazoparib. Combined with olaparib or talazoparib, IC50
values of IO were apparently decreased (Table II). These cells became more
sensitive to IO administered in combination with PARP
inhibitors.
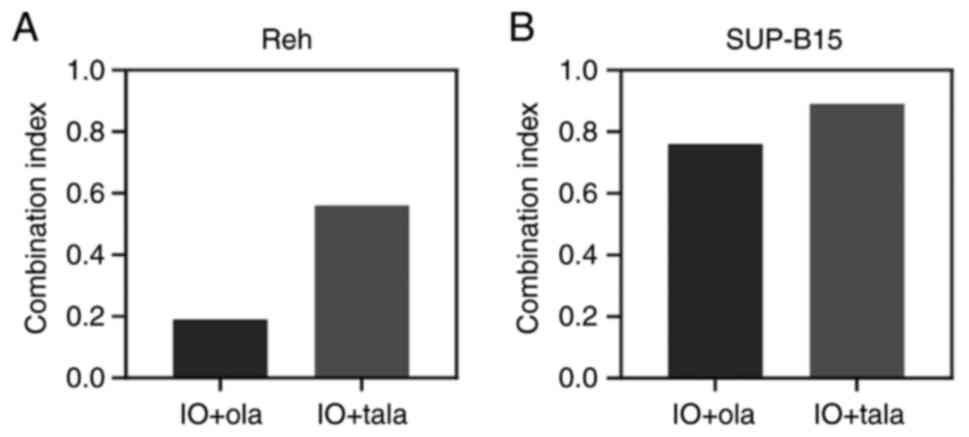
Determination of synergism between IO
and PARP inhibitors
To further investigate the combination effects
between IO and olaparib or talazoparib, the CI was calculated. Reh
and SUP-B15 cells were incubated with various concentrations of IO
in the presence of minimally toxic concentrations of olaparib or
talazoparib. When combined with olaparib or talazoparib, the CI
values were 0.19 and 0.56 for Reh cells, and 0.76 and 0.89 for
SUP-B15 cells, respectively (Fig.
4). These results revealed the synergism between IO and both
olaparib and talazoparib for Reh and SUP-B15 cells.
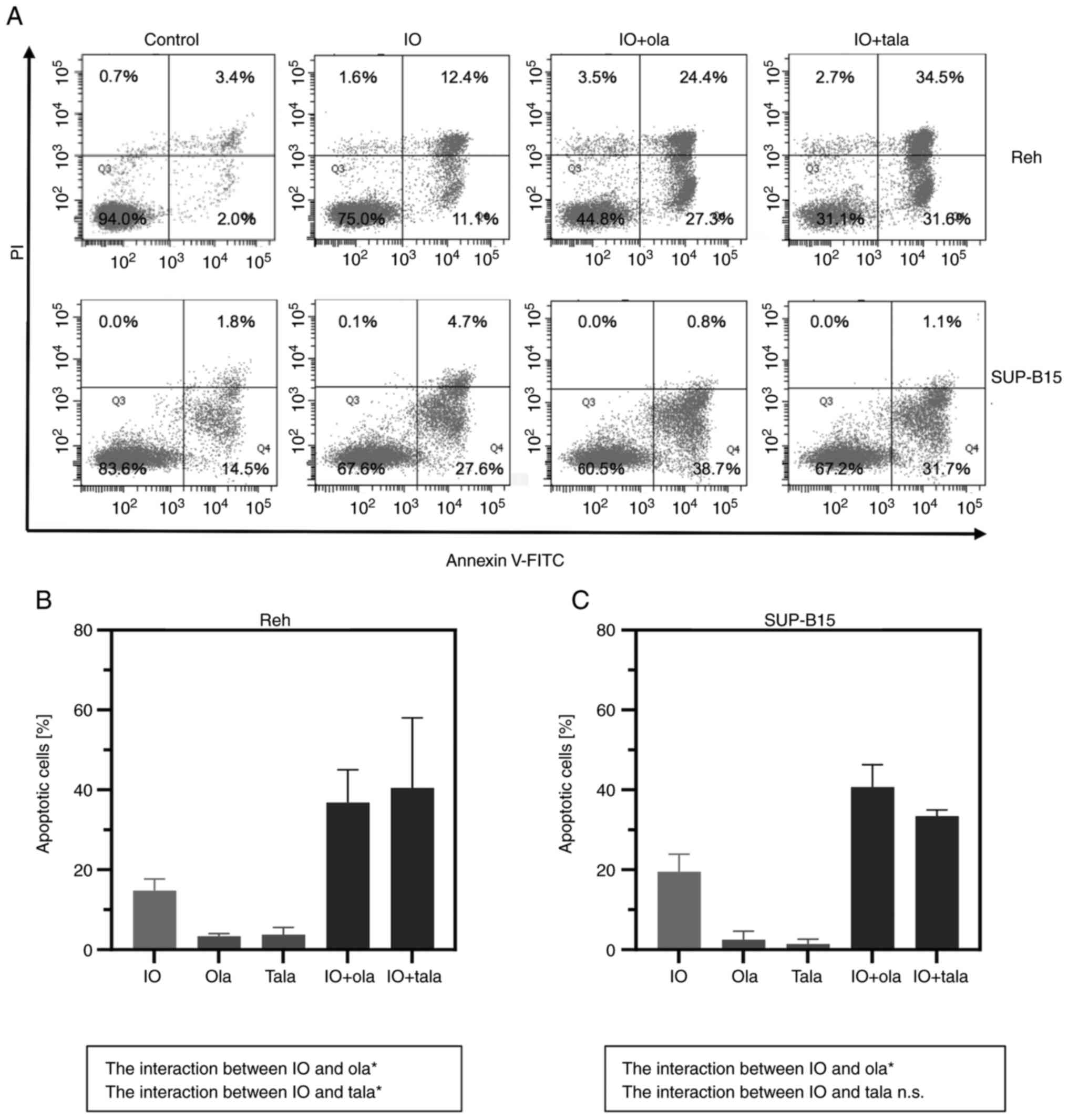
Induction of cell apoptosis by IO
combined with PARP inhibitors
Apoptosis was measured after cells were treated with
IO (0.2 ng/ml in Reh cells; 3.0 ng/ml in SUP-B15 cells) in the
presence or absence of minimally toxic concentrations of either
olaparib or talazoparib. Combining IO with olaparib or talazoparib
induced higher apoptosis than IO alone (Fig. 5). In both Reh and SUP-B15 cells, the
interaction between IO and olaparib was significant. In Reh cells,
the interaction between IO and talazoparib was also significant
(P<0.05; two-way ANOVA and Bonferroni multiple comparisons
test). The data indicated that combining IO with PARP inhibitors
augmented IO-induced apoptosis.
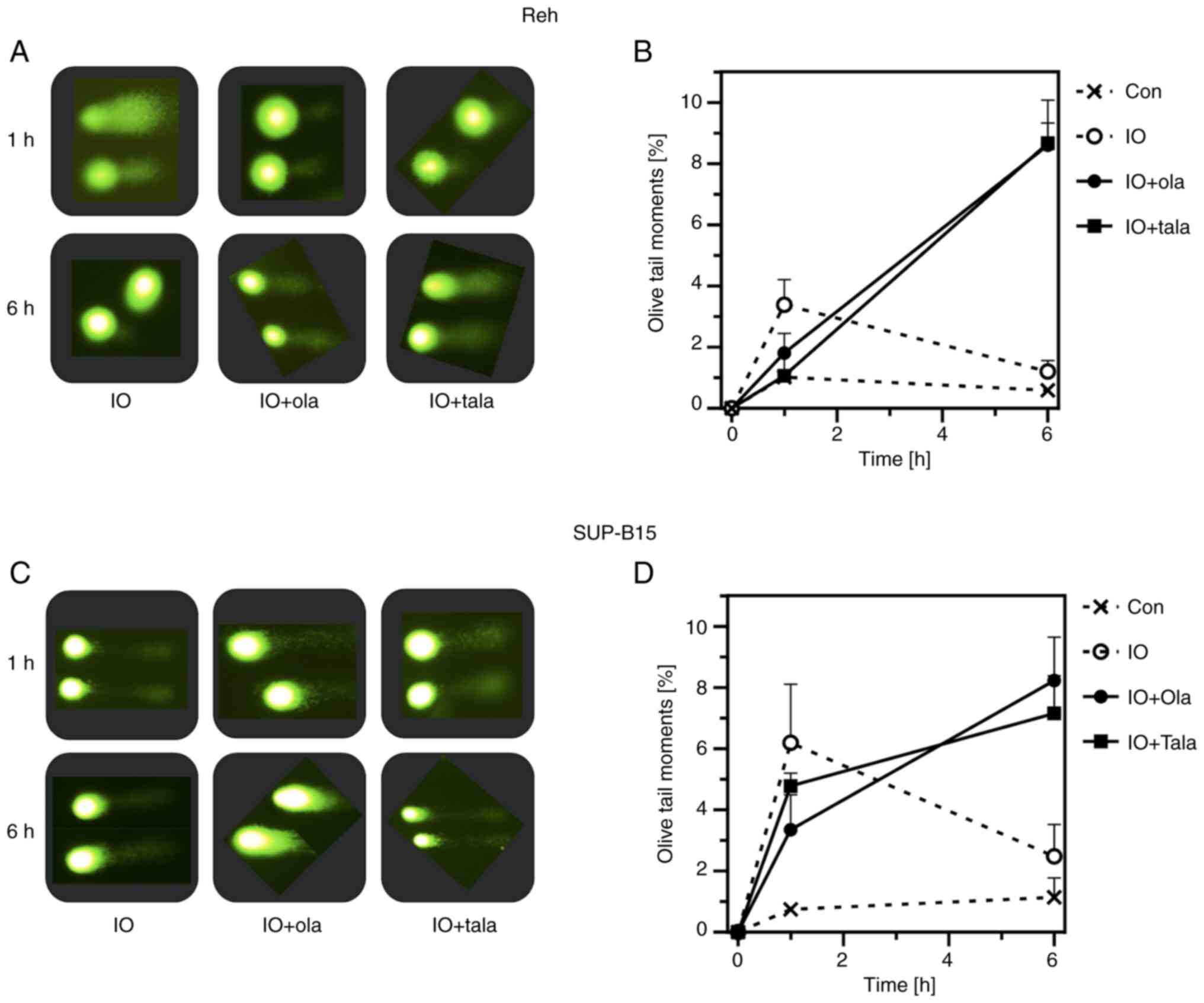
Inhibition of IO-induced DNA damage
repair by PARP inhibitors
The comet assay was conducted to verify DNA damage
and repair. The tail moment is an appropriate indicator of the
amount of DNA damage induced since this value takes under
consideration both the amount of DNA migration and the relative
amount of DNA in the tail (17).
Reh and SUP-B15 cells were treated with IO (2 ng/ml in Reh cells;
20 ng/ml in SUP-B15 cells) in the presence or absence of minimally
toxic concentrations of olaparib or talazoparib for 1 or 6 h.
Representative comet images are shown in Fig. 6A and C. A total of 1 h treatment
with IO induced the comet tail, indicating the induction of DNA
strand breaks. However, the image at 6 h did not reveal any tails,
suggesting that breaks were repaired after that time. Conversely,
in the presence of olaparib or talazoparib, comet tails remained at
6 h, indicating that breaks remained unrepaired by the inhibition
of PARP function. Line graphs revealed that the line for the Olive
tail moment of combination went upwards from 1 to 6 h after drug
administration; by contrast, that of IO alone went down (Fig. 6B and D). Olaparib and talazoparib
were thought to inhibit PARP-dependent SSB repair. These results
suggested that the enhancement of IO cytotoxicity by PARP
inhibitors was attributable to the inhibition of IO-induced DNA
damage repair.
Discussion
The present study demonstrated that the combination
of IO with olaparib or talazoparib demonstrated synergistic
anti-leukemic effects via the inhibition of DNA damage repair
mechanisms. As single agents, IO, olaparib and talazoparib all
inhibited cell proliferation in B-ALL cell lines. Combining IO with
either olaparib or talazoparib resulted in greater inhibition of
cell proliferation, showing synergistic effects. Furthermore, the
addition of minimally toxic concentrations of olaparib or
talazoparib combined with IO increased apoptosis. The comet assay
demonstrated that DNA strand breaks induced by IO alone had almost
disappeared by 6 h, suggesting successful completion of DNA repair.
However, in the presence of olaparib or talazoparib, DNA strand
breaks persisted even at 6 h, suggesting the inhibition of the DNA
repair function. These results indicated that the cytotoxicity of
IO was enhanced by olaparib or talazoparib via inhibition of DNA
damage repair.
SSBs are repaired by base excision repair (BER) and
nucleotide excision repair (18),
while DSBs are repaired by homologous recombination and
non-homologous end rejoining (19–21).
Calicheamicin, as the payload of IO, exhibits cytotoxicity by
inducing both SSBs and DSBs. The ratio of DSBs to SSBs among
cellular DNA is 1:3, close to the 1:2 ratio observed when
calicheamicin g1 cleaves purified plasmid DNA (22). PARP senses and binds to SSBs, then
forms long chains of poly(ADP-ribose) to recruit DNA repair
proteins involved in BER (23).
PARP inhibitors achieve cytotoxicity by inhibiting SSB repair. In
the presence of PARP inhibitors, calicheamicin-induced SSBs are not
repaired, and unrepaired SSBs convert to accumulating DSBs during
DNA replication, resulting in cell death. The strategy of combining
IO with PARP inhibitors thus achieves synergistic effects based on
a different concept from synthetic lethality. Previous studies have
reported that doxorubicin, which causes both SSBs and DSBs, similar
to calicheamicin, in combination with olaparib also demonstrated
synergistic effects in breast cancer and leukemia cells (24,25).
Gemtuzumab ozogamicin, an antibody-drug conjugate similar to IO,
reportedly demonstrated synergistic effects on CD33+
myeloid leukemia cells in combination with olaparib (26). Moreover, PARP1 was previously
revealed to be involved in DSB repair (27–30).
Combining IO with PARP inhibitors thus appears to represent a
reasonable strategy to enhance the cytotoxicity of IO.
Tirrò et al (31) investigated the cytotoxicity of IO in
CD22+ cells from the perspective of checkpoint kinase1
(Chk1) inhibition. When CD22+ cells were treated with
IO, the surviving cells were arrested in the G2/M phase during
which DNA repair was performed. The Chk1 inhibitor UCNO-1 abrogated
this IO-induced G2/M arrest. Such treatment increased cell death
rates among cells showing mutant p53. That study suggested the
cellular damage response as a therapeutic target for patients with
ALL receiving IO therapy. Takeshita et al (32) reported that cancer cells expressing
the multidrug resistance protein P-glycoprotein (P-gp) were
resistant to IO treatment in vitro. The cell lines used in
the present study did not exhibit P-gp upregulation, therefore the
results of the cytotoxicity experiments performed were not biased
in that regard. Nevertheless, the expression of P-gp should be
evaluated to further clarify the cytotoxicity of IO for RR-ALL in
the light of P-gp.
Jabbour et al (33) investigated the clinical efficacy of
IO combined with mini-hyper-CVD (cyclophosphamide, vincristine and
dexamethasone) chemotherapy with or without blinatumomab in newly
diagnosed elderly patients with Ph−ALL. A total of 135
patients were treated prospectively with standard hyper-CVAD
(cyclophosphamide, vincristine, doxorubicin and dexamethasone)
(n=77) or with the combination of IO plus mini-hyper-CVD with or
without blinatumomab (n=58). The identified 38 patients of each
cohort were compared with standard hyper-CVAD and mini hyper-CVD
plus IO using a propensity score matching. The 3-year event-free
survival rates were 34 and 64%, respectively (P=0.003). The 3-year
OS rates were 34 and 63%, respectively (P=0.004). Moreover, the
authors also investigated the clinical efficacy of IO combined with
mini-hyper-CVD chemotherapy. Mini-hyper CVD plus IO were performed
in combination with or without blinatumomab. Of 110 patients
(median age 37 years), 91 patients (83%) responded with 69 CR
(63%). Median OS was 17 months (34). Thus, more effective and less toxic
combination regimens are needed to improve the clinical efficacy of
ALL treatment.
A limitation of the present study includes the use
of cell lines only for experimentation. Although cancer cell lines
are widely used in basic research, cell lines do not completely
represent the molecular features of primary tumor cells (35). Cell lines likely represent a
subpopulation of the original tumor because of cell culture without
the original microenvironment, resulting in genetic or epigenetic
differences between cell lines and primary tumor cells (36). Therefore, experiments using primary
ALL cells for evaluation the efficacy of the combination therapy of
IO with PARP inhibitors are desired. The present study has another
limitation with regards to safety evaluation. The maximum drug
concentration of IO administration reached 200 ng/ml with the
half-life of 130 h in clinic (4,37).
Therefore, the IO concentrations used in the present study (2–100
ng/ml) were markedly lower, which can assure the safety. Moreover,
PARP inhibitors were used at minimally toxic concentrations in the
combination with IO [olaparib (1 µM in Reh cells and 10 nM in
SUP-B15 cells] or talazoparib (100 nM in Reh cells and 1 nM in
SUP-B15 cells)) here. These concentrations also were markedly lower
than the maximum drug concentration in clinic (olaparib 8.43 µg/ml,
talazoparib 13.78 ng/ml) (38,39).
Several clinical trials for combination therapy of chemotherapeutic
drugs with PARP inhibitors have been reported (40–42).
There was a major issue with a narrow therapeutic window. Both
chemotherapeutic drugs and PARP inhibitors are not selective for
tumor cells, therefore PARP inhibition of normal cells enhances the
toxicity of chemotherapeutic drugs, including myelosuppression. On
the other hand, IO targets CD22+ leukemic cells, not
other normal cells including neutrophils. The interaction between
IO and PARP inhibitors theoretically occurs in CD22+
leukemic cells, which will not increase adverse reactions in the
patient's body. To confirm the safety of this combination therapy,
experiments using patient-derived tumor xenografts as pre-clinical
models are also necessary (43).
In conclusion, the present study demonstrated
synergistic anti-leukemic effects from the combination of IO with
olaparib or talazoparib. There were no previous studies of clinical
trials on the combination of IO with PARP inhibitors. Therefore,
the present study is considered to be highly novel. The impact of
such combination is dependent on the inhibition of DNA damage
repair. In ALL cells, IO-induced DNA strand breaks were inhibited
by PARP inhibitors. Such combination may not increase damage to
normal cells, since IO specifically targets CD22+ cancer
cells. Targeting the inhibition of DNA damage repair may thus
represent a potent therapeutic strategy for ALL.
Acknowledgements
The authors wish to thank the Life Science Research
Laboratory in the Division of Bioresearch, University of Fukui for
the use of research instruments. Parts of the present study were
presented in the 65th American Society of Hematology Annual Meeting
and Exposition in December 9–12, 2023. Inotuzumab ozogamicin was
kindly provided by Pfizer, Inc.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
NI, MO, ST, NH and TY have participated sufficiently
in the conception and design of the study and were involved in the
acquisition, analysis and interpretation of the data, as well as
drafting the manuscript. NI acquired and analyzed the data,
produced the figures, and wrote the manuscript as principal
investigator. ST was involved in acquisition and interpretation of
data. MO and TY confirm the authenticity of all the raw data. NH
supervised the study design and critically revised the work for
important intellectual content. TY developed the study concept and
designed the work. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IO
|
inotuzumab ozogamicin
|
|
RR-ALL
|
relapsed or refractory acute
lymphoblastic leukemia
|
|
CD
|
cluster of differentiation
|
|
B-ALL
|
B-acute lymphoblastic leukemia
|
|
PARP
|
poly (ADP-ribose) polymerase
|
|
Ph
|
Philadelphia
|
|
IC50
|
half-maximal inhibitory
concentration
|
|
OS
|
overall survival
|
|
HSCT
|
hematopoietic stem cell
transplantation
|
|
CR
|
complete remission
|
|
SSBs
|
single strand breaks
|
|
DSBs
|
double strand breaks
|
|
BRCA
|
breast cancer susceptibility gene
|
|
ATCC
|
American Type culture collection
|
|
FBS
|
fatal bovine serum
|
|
FITC
|
fluorescein isothiocyanate
|
|
CCK-8
|
Cell Counting Kit-8
|
|
CI
|
combination index
|
|
BER
|
base excision repair
|
|
Chk1
|
checkpoint kinase 1
|
|
P-gp
|
P-glycoprotein
|
|
CVD
|
cyclophosphamide, vincristine and
dexamethasone
|
|
CVAD
|
cyclophosphamide, vincristine,
doxorubicin and dexamethasone
|
References
|
1
|
Annino L, Vegna ML, Camera A, Specchia G,
Visani G, Fioritoni G, Ferrara F, Peta A, Ciolli S, Deplano W, et
al: Treatment of adult acute lymphoblastic leukemia (ALL):
Long-Term follow-up of the GIMEMA ALL 0288 randomized study. Blood.
99:863–871. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeuchi J, Kyo T, Naito K, Sao H,
Takahashi M, Miyawaki S, Kuriyama K, Ohtake S, Yagasaki F, Murakami
H, et al: Induction therapy by frequent administration of
doxorubicin with four other drugs, followed by intensive
consolidation and maintenance therapy for adult acute lymphoblastic
leukemia: The JALSG-ALL93 study. Leukemia. 16:1259–1266. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gökbuget N, Stanze D, Beck J, Diedrich H,
Horst HA, Hüttmann A, Kobbe G, Kreuzer KA, Leimer L, Reichle A, et
al: Outcome of relapsed adult lymphoblastic leukemia depends on
response to salvage chemotherapy, prognostic factors, and
performance of stem cell transplantation. Blood. 120:2032–2041.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kantarjian HM, DeAngelo DJ, Stelljes M,
Martinelli G, Liedtke M, Stock W, Gökbuget N, O'Brien S, Wang K,
Wang T, et al: Inotuzumab ozogamicin versus standard therapy for
acute lymphoblastic leukemia. N Engl J Med. 375:740–753. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kantarjian HM, DeAngelo DJ, Stelljes M,
Liedtke M, Stock W, Gökbuget N, O'Brien SM, Jabbour E, Wang T,
Liang White J, et al: Inotuzumab ozogamicin versus standard of care
in relapsed or refractory acute lymphoblastic leukemia: Final
report and long-term survival follow-up from the randomized, phase
3 INO-VATE study. Cancer. 125:2474–2487. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantarjian HM, Su Y, Jabbour EJ,
Bhattacharyya H, Yan E, Cappelleri JC and Marks DI:
Patient-Reported outcomes from a phase 3 randomized controlled
trial of inotuzumab ozogamicin versus standard therapy for
relapsed/refractory acute lymphoblastic leukemia. Cancer.
124:2151–2160. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Vries JF, Zwaan CM, De Bie M, Voerman
JSA, den Boer ML, van Dongen JJM and van der Velden VHJ: The novel
calicheamicin-conjugated CD22 antibody inotuzumab ozogamicin
(CMC-544) effectively kills primary pediatric acute lymphoblastic
leukemia cells. Leukemia. 26:255–264. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rose M, Burgess JT, O'Byrne K, Richard DJ
and Bolderson E: PARP inhibitors: Clinical relevance, mechanisms of
action and tumor resistance. Front Cell Dev Biol. 8:5646012020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
DiSilvestro P, Colombo N, Harter P,
González-Martín A, Ray-Coquard I and Coleman RL: Maintenance
treatment of newly diagnosed advanced ovarian cancer: Time for a
paradigm shift? Cancers (Basel). 13:57562021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-Deficient tumours with inhibitors of
poly(ADP-Ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortesi L, Rugo HS and Jackisch C: An
overview of PARP inhibitors for the treatment of breast cancer.
Targ Oncol. 16:255–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chou TC: Drug combination studies and
their synergy quantification using the chou-talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhatla T, Wang J, Morrison DJ, Raetz EA,
Burke MJ, Brown P and Carroll WL: Epigenetic reprogramming reverses
the relapse-specific gene expression signature and restores
chemosensitivity in childhood B-Lymphoblastic leukemia. Blood.
119:5201–5210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Uy N, Nadeau M, Stahl M and Zeidan AM:
Inotuzumab ozogamicin in the treatment of relapsed/refractory acute
B cell lymphoblastic leukemia. J Blood Med. 9:67–74. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pardee TS, Stadelman K, Gee JJ, Caudell DL
and Gmeiner WH: The poison oligonucleotide F10 is highly effective
against acute lymphoblastic leukemia while sparing normal
hematopoietic cells. Oncotarget. 5:4170–4179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Punzo F, Argenziano M, Tortora C, Paola
AD, Mutarelli M, Pota E, Martino MD, Pinto DD, Marrapodi MM,
Roberti D, et al: Effect of CB2 stimulation on gene expression in
pediatric B-Acute lymphoblastic leukemia: New possible targets. Int
J Mol Sci. 23:86512022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kumaravel TS, Vilhar B, Faux SP and Jha
AN: Comet assay measurements: A perspective. Cell Biol Toxicol.
25:53–64. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Madhusudan S and Hickson ID: DNA Repair
inhibition: A selective tumour targeting strategy. Trends Mol Med.
11:503–511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Moynahan ME and Jasin M: Mitotic
homologous recombination maintains genomic stability and suppresses
tumorigenesis. Nat Rev Mol Cell Biol. 11:196–207. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ceccaldi R, Rondinelli B and D'Andrea AD:
Repair pathway choices and consequences at the double-strand break.
Trends Cell Biol. 26:52–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
O'Connor MJ: Targeting the DNA damage
response in cancer. Mol Cell. 60:547–560. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Elmroth K, Nygren J, Mårtensson S, Ismail
IH and Hammarsten O: Cleavage of cellular DNA by calicheamicin
gamma1. DNA Repair (Amst). 2:363–374. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Javle M and Curtin NJ: The role of PARP in
DNA repair and its therapeutic exploitation. Br J Cancer.
105:1114–1122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mariano G, Ricciardi MR, Trisciuoglio D,
Zampieri M, Ciccarone F, Guastafierro T, Calabrese R, Valentini E,
Tafuri A, Bufalo DD, et al: PARP inhibitor ABT-888 affects response
of MDA-MB-231 cells to doxorubicin treatment, targeting snail
expression. Oncotarget. 6:15008–15021. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wu J, Xiao S, Yuan M, Li Q, Xiao G, Wu W,
Ouyang Y, Huang L and Yao C: PARP inhibitor re-sensitizes
adriamycin resistant leukemia cells through DNA damage and
apoptosis. Mol Med Rep. 19:75–84. 2019.PubMed/NCBI
|
|
26
|
Yamauchi T, Uzui K, Nishi R, Shigemi H and
Ueda T: Gemtuzumab ozogamicin and olaparib exert synergistic
cytotoxicity in CD33-positive HL-60 myeloid leukemia cells.
Anticancer Res. 34:5487–5494. 2014.PubMed/NCBI
|
|
27
|
Ariumi Y, Masutani M, Copeland TD, Mimori
T, Sugimura T, Shimotohno K, Ueda K, Hatanaka M and Noda M:
Suppression of the poly(ADP-Ribose) polymerase activity by
DNA-dependent protein kinase in vitro. Oncogene. 18:4616–4625.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Galande S and Kohwi-Shigematsu T:
Poly(ADP-ribose) polymerase and Ku autoantigen form a complex and
synergistically bind to matrix attachment sequences. J Biol Chem.
274:20521–20528. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Haince JF, Kozlov S, Dawson VL, Dawson TM,
Hendzel MJ, Lavin MF and Poirier GG: Ataxia telangiectasia mutated
(ATM) signaling network is modulated by a novel
poly(ADP-ribose)-dependent pathway in the early response to
DNA-damaging agents. J Biol Chem. 282:16441–16453. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Haince JF, McDonald D, Rodrigue A, Déry U,
Masson JY, Hendzel MJ and Poirier GG: PARP1-dependent kinetics of
recruitment of MRE11 and NBS1 proteins to multiple DNA damage
sites. J Biol Chem. 283:1197–1208. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Tirrò E, Massimino M, Romano C, Pennisi
MS, Stella S, Vitale SR, Fidilio A, Manzella L, Parrinello NL,
Stagno F, et al: Chk1 inhibition restores inotuzumab ozogamicin
citotoxicity in CD22-positive cells expressing mutant P53. Front
Oncol. 9:572019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Takeshita A, Shinjo K, Yamakage N, Ono T,
Hirano I, Matsui H, Shigeno K, Nakamura S, Tobita T, Maekawa M, et
al: CMC-544 (inotuzumab ozogamicin) shows less effect on multidrug
resistant cells: Analyses in cell lines and cells from patients
with B-cell chronic lymphocytic leukaemia and lymphoma. Br J
Haematol. 146:34–43. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jabbour EJ, Sasaki K, Ravandi F, Short NJ,
Garcia-Manero G, Daver N, Kadia T, Konopleva M, Jain N, Cortes J,
et al: Inotuzumab ozogamicin in combination with low-intensity
chemotherapy (Mini-HCVD) with or without blinatumomab versus
standard intensive chemotherapy (HCVAD) as frontline therapy for
older patients with philadelphia chromosome-negative acute
lymphoblastic leukemia: A propensity score analysis. Cancer.
125:2579–2586. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kantarjian H, Haddad FG, Jain N, Sasaki K,
Short NJ, Loghavi S, Kanagal-Shamanna R, Jorgensen J, Khouri I,
Kebriaei P, et al: Results of salvage therapy with mini-hyper-CVD
and inotuzumab ozogamicin with or without blinatumomab in pre-B
acute lymphoblastic leukemia. J Hematol Oncol. 16:442023.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Javad N, Francisca V and James MM:
Bridging the gap between cancer cell line models and tumours using
gene expression data. Br J Cancer. 125:311–312. 2021. View Article : Google Scholar
|
|
36
|
Wilding JL and Bodmer WF: Cancer cell
lines for drug discovery and development. Cancer Res. 74:2377–2384.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ricart AD: Antibody-drug conjugates of
calicheamicin derivative: Gemtuzumab ozogamicin and inotuzumab
ozogamicin. Clin Cancer Res. 17:6417–6427. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Yonemori K, Tamura K, Kodaira M, Fujikawa
K, Sagawa T, Esaki T, Shirakawa T, Hirai F, Yokoi Y, Kawata T, et
al: Safety and tolerability of the olaparib tablet formulation in
Japanese patients with advanced solid tumours. Cancer Chemother
Pharmacol. 78:525–531. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Naito Y, Kuboki Y, Ikeda M, Harano K,
Matsubara N, Toyoizumi S, Mori Y, Hori N, Nagasawa T and Kogawa T:
Safety, pharmacokinetics, and preliminary efficacy of the PARP
inhibitor talazoparib in Japanese patients with advanced solid
tumors: Phase 1 study. Invest New Drugs. 39:1568–1576. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lee JM, Peer CJ, Yu M, Amable L, Gordon N,
Annunziata CM, Houston N, Goey AKL, Sissung TM, Parker B, et al:
Sequence-specific pharmacokinetic and pharmacodynamic phase I/Ib
study of olaparib tablets and carboplatin in Women's cancer. Clin
Cancer Res. 23:1397–1406. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bang YJ, Xu RH, Chin K, Lee KW, Park SH,
Rha SY, Shen L, Qin S, Xu N, Im SA, et al: Olaparib in combination
with paclitaxel in patients with advanced gastric cancer who have
progressed following first-line therapy (GOLD): A double-blind,
randomised, placebo-controlled, phase 3 trial. Lancet Oncol.
18:1637–1651. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Oza AM, Cibula D, Benzaquen AO, Poole C,
Mathijissen RHJ, Sonke GS, Colombo N, Spacek J, Vuylsteke P, Hirte
H, et al: Olaparib combined with chemotherapy for recurrent
platinum-sensitive ovarian cancer: A randomised phase 2 trial.
Lamcet Oncol. 16:87–97. 2015. View Article : Google Scholar
|
|
43
|
Lai Y, Wei X, Lin S, Qin L, Cheng L and Li
P: Current status and perspectives of patient-derived xenograft
models in cancer research. J Hematol Oncol. 10:1062017. View Article : Google Scholar : PubMed/NCBI
|