Introduction
Epidermal growth factor receptor-tyrosine kinase
inhibitors (EGFR-TKIs) are considered as one of the most
significant targeted agents for the treatment of non-small cell
lung cancer (NSCLC) (1). However,
cardiac-related adverse events associated with EGFR-TKI therapy can
commonly occur (2) Gefitinib is an
oral EGFR-TKI, which is used to treat patients with T790M-positive
NSCLC, who have progressed on a standard EGFR-TKI (3). A study by Thein and Ball (4) and Soria et al (5) showed that gefitinib was associated
with an increased risk of cardiotoxicity, prolongation of the QT
interval and heart failure compared with controls. As a type of
EGFR-TKI, gefitinib serves a significant role in the treatment of
NSCLC. However, its potential cardiotoxicity and adverse effects
need to be further investigated.
Quercetin (3,3′,4′,5,7-pentahydroxyflavone), a
polyphenolic compound, is the most prevalent flavonoid in fruits,
vegetables and medicinal plants (6). It is widely recognized that quercetin
possesses antioxidant, anti-inflammatory, antimicrobial and
antiparasitic activities (7–9). The
anticancer effects of quercetin include its ability to promote cell
viability loss, apoptosis and autophagy via regulating the
phosphoinositide 3-kinase/protein kinase B/mammalian target of
rapamycin, Wnt/β-catenin and mitogen-activated protein kinase
(MAPK)/extracellular signal-regulated kinase 1/2 pathways (10–12).
Furthermore, it has been reported that quercetin exerts protective
effects against ischemic heart diseases, while it is involved in
myocardial remodeling and myocardial fibrosis (13,14).
The present study was based on the concern regarding
cardiac issues that could be caused by the treatment of patients
with NSCLC with gefitinib. Therefore, a potential therapeutic
approach for delaying gefitinib-induced cellular charring using
quercetin was proposed and its underlying mechanism of action was
thoroughly explored. Through in vitro and in vivo
experiments, the current study aimed to reveal the role of
quercetin in regulating the Src homology-2 domain-containing
protein tyrosine phosphatase (SHP2)/reactive oxygen species
(ROS)/AMP-activated protein kinase (AMPK)/X-box binding protein 1
(XBP-1)/Parkinsonism associated deglycase (DJ-1) signaling pathway
and its inhibitory effect on gefitinib-induced cell pyroptosis in
NSCLC, thus providing a significant reference and guidance for the
development of more effective and safer treatment strategies for
NSCLC, and further expanding the understanding of EGFR-TKI
therapy-related cardiac problems in clinical practice.
Materials and methods
Animal model
Animal experiments were approved by The Fourth
Hospital of Hebei Medical University Research Ethics Committee
(approval no. IACUC-4th Hos Hebmu-2024022; Shijiazhuang, China).
All animals were humanely cared according to the Fourth Hospital of
Hebei Medical University Guide for the Care and Use of Laboratory
Animals. A total of 24 mice, weighing 18.3±0.55 g, were housed in a
barrier facility with a 12/12-h light/dark cycle and ad
libitum access to food and water. A total of 5×106
AC16 cells in 100 µl of PBS were injected subcutaneously into the
right axilla of SPF-grade male nude mice (age, 4–5 weeks old). On
the 14th day after injection, mice were randomly divided into the
NSCLC, NSCLC + gefitinib and NSCLC + gefitinib + quercetin groups
(n=8 mice/group). After 6 weeks, the heart tissues were collected
following mice euthanasia by lethal doses of anesthetics. Animal
death was verified by the lack of response to toe pinch reflex.
Mice in the NSCLC + gefitinib group were injected with 40 mg/kg/day
gefitinib for 14 days (2), while
those in the NSCLC + gefitinib + quercetin group were
intraperitoneally injected with 50 mg/kg quercetin in combination
with gefitinib (15).
Ultrasonography
Doppler ultrasound was performed using the Vevo 2100
imaging system (Fujifilm VisualSonics, Inc.). Mice were first
placed in an anesthesia induction chamber filled with 2.5%
isoflurane in 1 l/min pure oxygen until being unresponsive to toe
pinching. Subsequently, mice were placed in the supine position on
a 37°C thermostatic heating pad supplied with anesthesia airflow
(1.5% isoflurane). The limbs of the mice were coated with
conductive gel and affixed to electrocardiographic electrodes
embedded in plates. Cardiac function was assessed via measuring
left ventricular ejection fraction (LVEF) and left ventricular fold
shortening (LVFS), which were calculated as the average of five
consecutive cardiac cycles.
Masson's trichrome staining
Mice were euthanized by intraperitoneal injection of
an overdose of sodium pentobarbital (100 mg/kg). Death was
confirmed 30 min after injection by observing cardiac arrest,
respiratory arrest, animal rigidity and dilated pupils. Notably,
none of the mice succumbed to humane endpoints during the
experimental process. Heart tissue samples were fixed in 4%
paraformaldehyde, embedded in paraffin and cut into 5-µm thick
sections. To assess fibrosis, Masson's trichrome staining was
performed using a modified Masson's trichrome staining kit [cat.
no. G1346-8 (50 ml); Beijing Solarbio Science & Technology Co.,
Ltd.]. Briefly, the heart tissue sections were incubated with
Brinell's solution at 56°C for 15 min and then rinsed with tap
water. Subsequently, the sections were incubated with Weigert's
iron hematoxylin solution followed by Biebrich scarlet-acid fuchsin
solution, phospho-molybdate-phospho-tungstic acid solution and
Aniline Blue solution. Finally, the slides were treated with 1%
acetic acid solution, dehydrated and mounted with mounting
solution.
Immunohistochemistry (IHC)
staining
The expression levels of SHP2 (1:50; cat. no.
ab300579), XBP-1 (20 µg/ml; cat. no. ab37152; both from Abcam),
phosphorylated (p)-stimulator of interferon genes (STING; 1:200;
cat. no. PA5-105674; Invitrogen; Thermo Fisher Scientific, Inc.)
and Nod-like receptor protein 3 (NLRP3; 1:500; cat. no. MA5-32255;
Thermo Fisher Scientific, Inc.) were detected in mouse heart
tissues using IHC. More specifically, EDTA microwave heat repair
was performed for 5–8 min followed by cooling at room temperature.
Subsequently, the tissue sections were incubated in 3%
H2O2 for 15 min and then with 10% goat serum
(MilliporeSigma) for 30 min at 37°C. Then, the tissue sections were
incubated with a primary antibody in a wet box at 4°C overnight,
followed by incubation with the corresponding goat anti-mouse
HRP-conjugated secondary antibody (1:500; cat. no. C31430100;
Thermo Fisher Scientific Inc.) for 30 min at room temperature. The
color was developed using the Ultra-Sensitive DAB kit (Beyotime
Institute of Biotechnology). Images of the stained tissue sections
were captured under a light microscope.
Bioinformatics analysis
For bioinformatics analysis the ‘geoquery’ (version
2.64.2; bioconductor.org/packages/release/bioc/html/geoquery.html),
‘limma’ (version 3.52.2;
bioconductor.org/packages/release/bioc/html/limma.html), ‘ggplot2’
(version 3.3.6; ggplot2.tidyverse.org/) and ‘ComplexHeatmap’
(version 2.13.1; jokergoo.github.io/ComplexHeatmap/) software in
‘R’ (version 4.2.1; www.R-project.org/) package were utilized. The
GSE18842 dataset was downloaded from the Gene Expression Omnibus
(GEO) database via the ‘GEOquery’ package. The missing values were
completed using the ‘impute’ package (16). The data were normalized using the
‘normalizeBetweenArrays’ function in ‘limma’ package and box plots
were constructed with the ‘ggplot2’ package. For the differential
analysis a threshold of |LogFC|>1 and P<0.05 was set and the
results were and visualized using the ‘ggplot2’ package. The rows
were normalized and clustered to Euclidean distance. The columns
were not clustered. The heatmaps of the differentially expressed
genes were constructed using the ‘ComplexHeatmap’ package. The
target genes of quercetin were retrieved from the DrugBank database
(https://go.drugbank.com/). A total of 29 target
genes were identified and were then analyzed for shared genes with
NSCLC. The results were visualized using the ‘ggplot2’ (version
3.3.6) and ‘VennDiagram’ (version 1.7.3;
cran.r-project.org/web/packages/VennDiagram/index.html) packages.
In addition, proteins with a confidence level of ≥0.9 were selected
in STRING database (https://string-db.org). The proteins in the input list
were analyzed for protein-protein interactions (PPI) and the hub
genes, which were enriched using ‘R’ (version 4.2.1) package, were
visualized using the Cytoscape software (https://cytoscape.org/). Additionally, the
‘clusterProfiler’ (version 4.4.4; http://bioconductor.org/packages/release/bioc/html/clusterProfiler.html),
‘GOplot’ (version 1.0.2; github.com/GuangchuangYu/GOplot),
‘ggplot2’ (version 3.3.6) in ‘R’ (version 4.2.1) package and the
‘ID conversion’ and ‘org.Hs.eg.db’ packages were used. The
‘Species’ option was set to ‘Human (Homo sapiens)’. P<0.05 was
considered to indicate differentially expressed genes. Gene
clusters and pathways with biometric differences between Hub genes
were screened out and the enrichment analysis results were
visualized using the ‘ggplot2’ package.
Cell culture and grouping
AC16 cardiomyocytes were purchased from the Cell
Bank of the Chinese Academy of Sciences. Cells were co-cultured in
Coning chambers. When needed, cells were treated with gefitinib
(0.1 µmol/l, cat. no. MB1112; Dalian Meilun Biology Technology Co.,
Ltd.) and incubated at 37°C in an incubator with 5% CO2.
For cell transfection, cardiomyocytes were cultured in serum-free
medium at 37°C and 5% CO2. When reached 80% confluency,
cells were transfected with the indicated short hairpin (sh) RNAs
or scrambled shRNA (Thermo Fisher Scientific, Inc.) for 48 h. The
plasmid (2.0 µg) was transfected with Lipofectamine 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Cells were incubated at 37°C (5%
CO2) for 12 h and the medium was replaced with growth
medium containing FBS. Incubation was continued for 48 h after
transfection. The shRNA sequence used for SHP2 was
5′-GAAGCACAGUACCGAUUUA-3′. All cell lines were cultured in DMEM and
were treated with 4.3 mM ATP, 100 nM gefitinib (17), 80 µM quercetin and 200 µM
H2O2 for 48 h.
Shp-2 knockdown efficiency
RNA was harvested from tumour cells collected from
mice using TRIzol reagent (Ambion; Thermo Fisher Scientific, Inc.)
and cDNA was obtained using HiScript III RT SuperMix (Vazyme
Biotech Co., Ltd.) catalyzed by using mRNA as template. The mRNA
primer sequences were used as shown in Table SI. chamQ SYBR qPCR Master Mix
(Vazyme Biotech Co., Ltd.) was used for fluorescence
quantification. GAPDH was used as an internal control, and the
relative gene expression was normalized using the 2−∆∆Cq
method (18). Transfection
efficiency, verified using PCR, is demonstrated in Fig. S1.
Western blot analysis
Heart tissues or cells were homogenized and lysed in
RIPA buffer (154 mM NaCl, 0.25% sodium deoxycholate, 1% NP-40, 0.8
mM EDTA and 65.2 mM Tris base) supplemented with a mixture of
protease inhibitors. Then, 20 µg of total proteins were separated
by 10% SDS-PAGE (Invitrogen; Thermo Fisher Scientific, Inc.) and
were then transferred onto a PVDF membrane. The membrane was
blocked with 0.1% TBS-Tween-20 (TBST) containing 5% skimmed milk
for 1 h. The primary antibodies used were as follows: Anti-NADPH
oxidase 4 (NOX4; 1:1,000; cat. no. ab112414), anti-SHP2 (1:1,000;
cat. no. ab300579), anti-p-AMPK (1:1,000; cat. no. 133448),
anti-XBP-1 (1:1,000; cat. no. ab31752), anti-DJ-1 (1:1,000; cat.
no. ab76008), anti-PTEN-induced putative kinase (PINK; 1:1,000;
cat. no. 216144), anti-beclin1 (1:1,000; cat. no. ab302669),
anti-p-STING (1:1,000; cat. no. ab2239074), p-interferon regulatory
factor 3 (IRF3; 1:1,000; cat. no. ab76493), NLRP3 (1:1,000; cat.
no. ab263899), anti-gasdermin D (GSDMD; 1:1,000; cat. no. 219800),
IL-β (1:1,000; cat. no. ab283818), peroxisome
proliferator-activated receptor-γ (PPAR-γ; 1:1,000; cat. no.
ab178860), PGC-1 (1:1,000; cat. no. ab310323) and anti-GAPDH
(1:1,000; cat. no. ab8245; all from Abcam). The membrane was
incubated with the corresponding HRP-conjugated secondary
antibodies (Goat Anti-Rabbit IgG H&L; 1:5,000; cat. no.
ab205718; Abcam) at room temperature for 1 h, followed by washing
with TBST for three times. The protein bands were visualized using
an ECL kit (Thermo Fisher Scientific, Inc.). Quantification was
performed using the Quantity One system (Bio-Rad Laboratories,
Inc.). GAPDH served as an internal control.
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazol-carbocyanine
iodide (JC-1) assay
Equal amounts of cardiomyocytes (5×105
cells/ml) were inoculated onto 8-well culture slides (BD Falcon™;
BD Biosciences) and 24 h later were processed as previously
described (19). Following washing
with PBS, cells were incubated in fresh medium supplemented with
JC-1 for 15 min. The cells were then washed with PBS to remove the
staining solution and were supplemented with fresh medium.
Subsequently, cells were immediately observed under a fluorescence
microscope.
Statistical analysis
All data are expressed as the mean ± SEM of at least
three independent experiments. The differences between two groups
were compared using Student's t-test (paired t-test). Comparisons
between multiple groups were performed by one-way analysis of
variance followed by Tukey/Bonferroni post hoc test. All
statistical analyses were performed using GraphPad Prism 5.0
software (GraphPad Software, Inc.; Dotmatics). P<0.05 was
considered to indicate a statistically significant difference.
Results
Quercetin improves cardiac function in
gefitinib-treated NSCLC mice
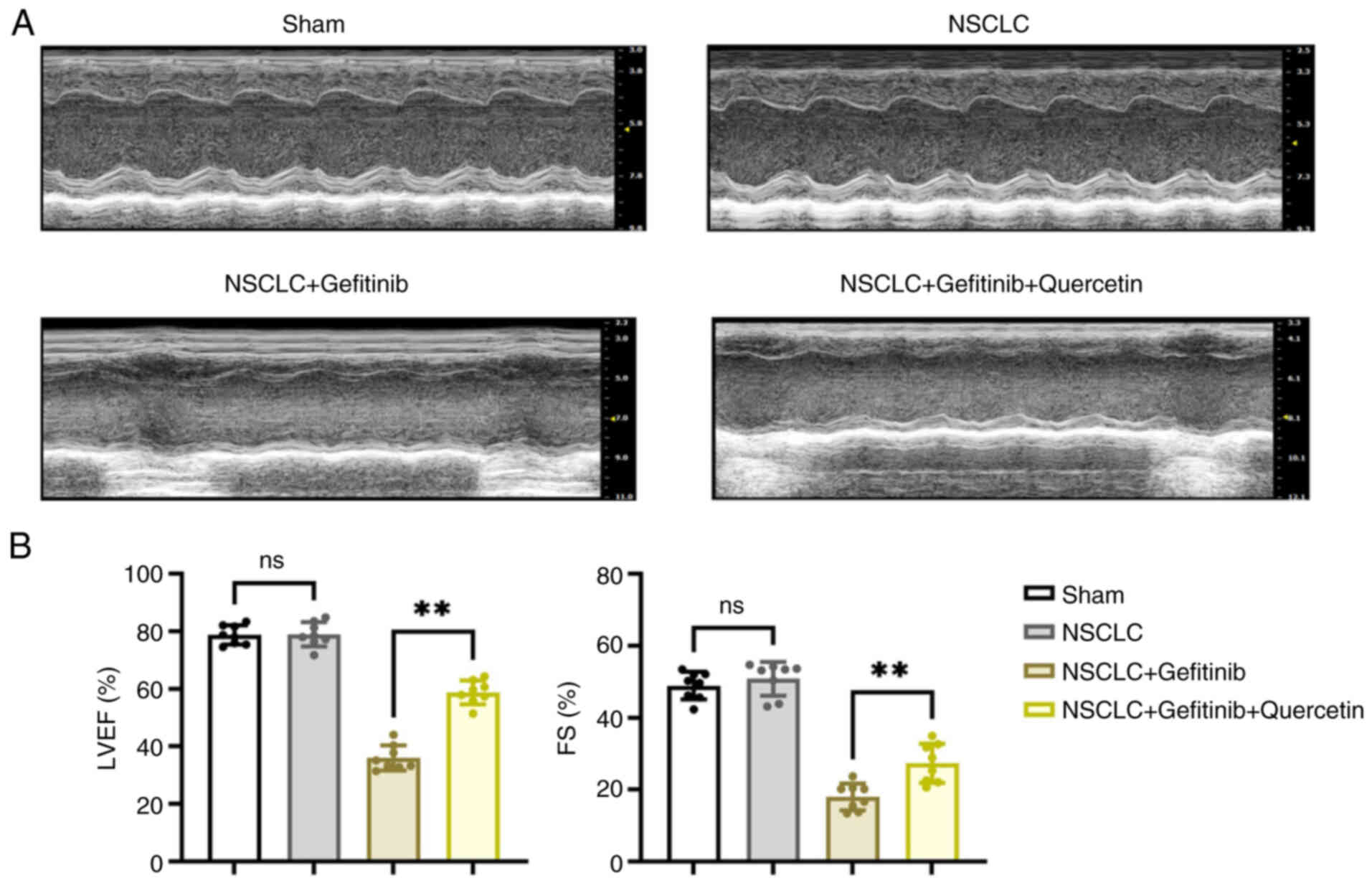
Firstly, the present study aimed to uncover the role
of quercetin in improving cardiac function in gefitinib-treated
NSCLC mice. The ultrasonic inspection results are shown in Fig. 1. Treatment of NSCLC mice with
gefitinib significantly attenuated cardiac function, as evidenced
by the reduced FS and LVEF (~50%). This finding indicated that
cardiac contraction and relaxation were significantly impaired.
However, mice co-treatment with quercetin significantly improved
cardiac function via increasing FS and LVEF by ~30%.
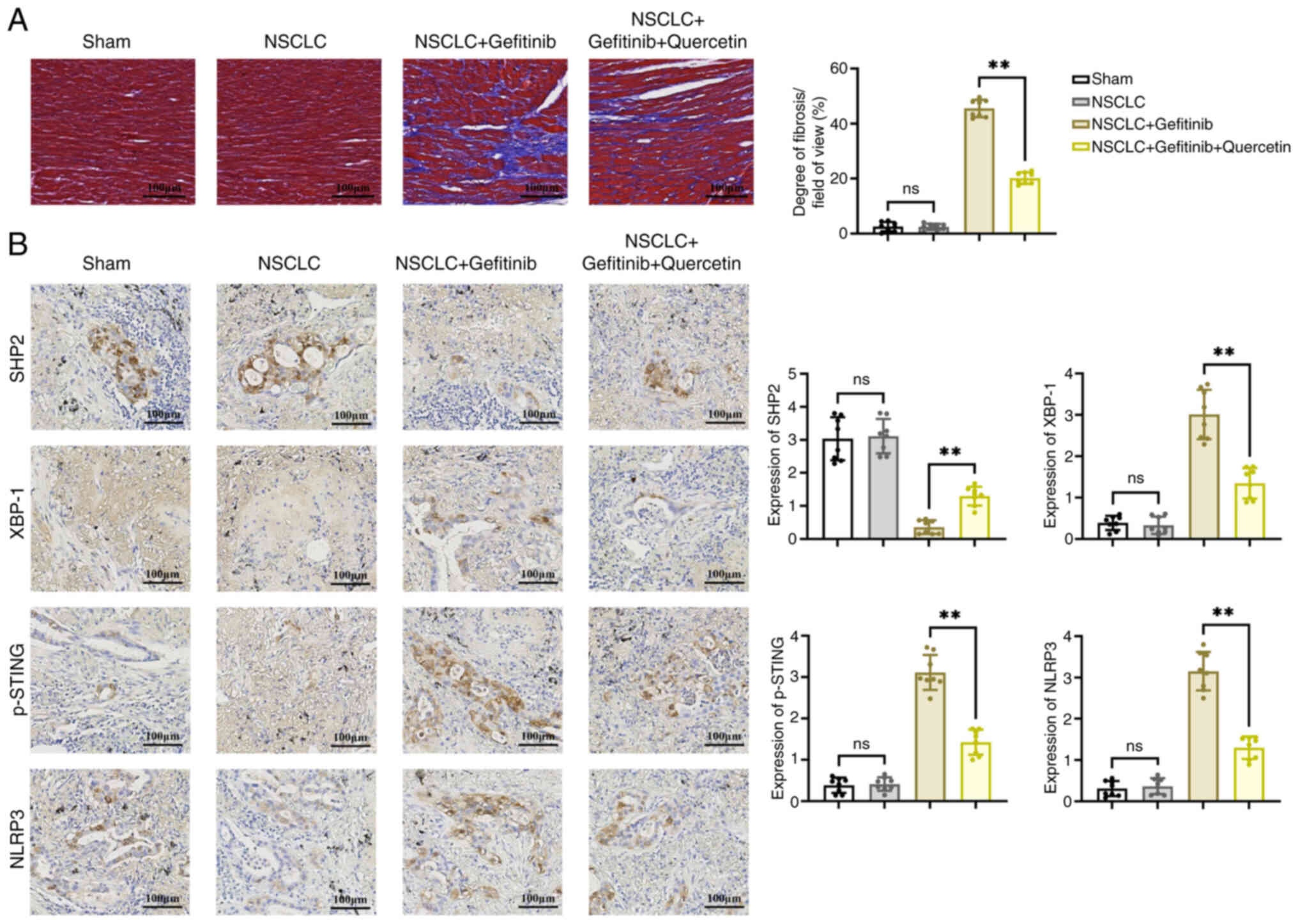
Quercetin ameliorates cardiac fibrosis
in gefitinib-treated mice with NSCLC via the SHP2/XBP-1/p-STING
signaling pathway
To detect changes in collagen content, fibrogenesis
was assessed by Masson's trichrome staining. The results revealed
that NSCLC had no significant effect on cardiomyocyte function.
However, treatment with gefitinib exacerbated ventricular fibrosis
compared with the NSCLC group, as evidenced by the large and
intense accumulation of collagen. Furthermore, co-treatment with
quercetin inhibited the gefitinib-induced ventricular fibrosis
(Fig. 2A). In addition, IHC
staining of mouse heart tissues revealed that the protein
expression levels of SHP2 were reduced, while those of XBP-1,
p-STING and NLRP3 were increased in the NSCLC + gefitinib group
compared with the NSCLC group. However, mice treatment with
quercetin abrogated the effects of gefitinib on the expression
levels of the aforementioned proteins (Fig. 2B).
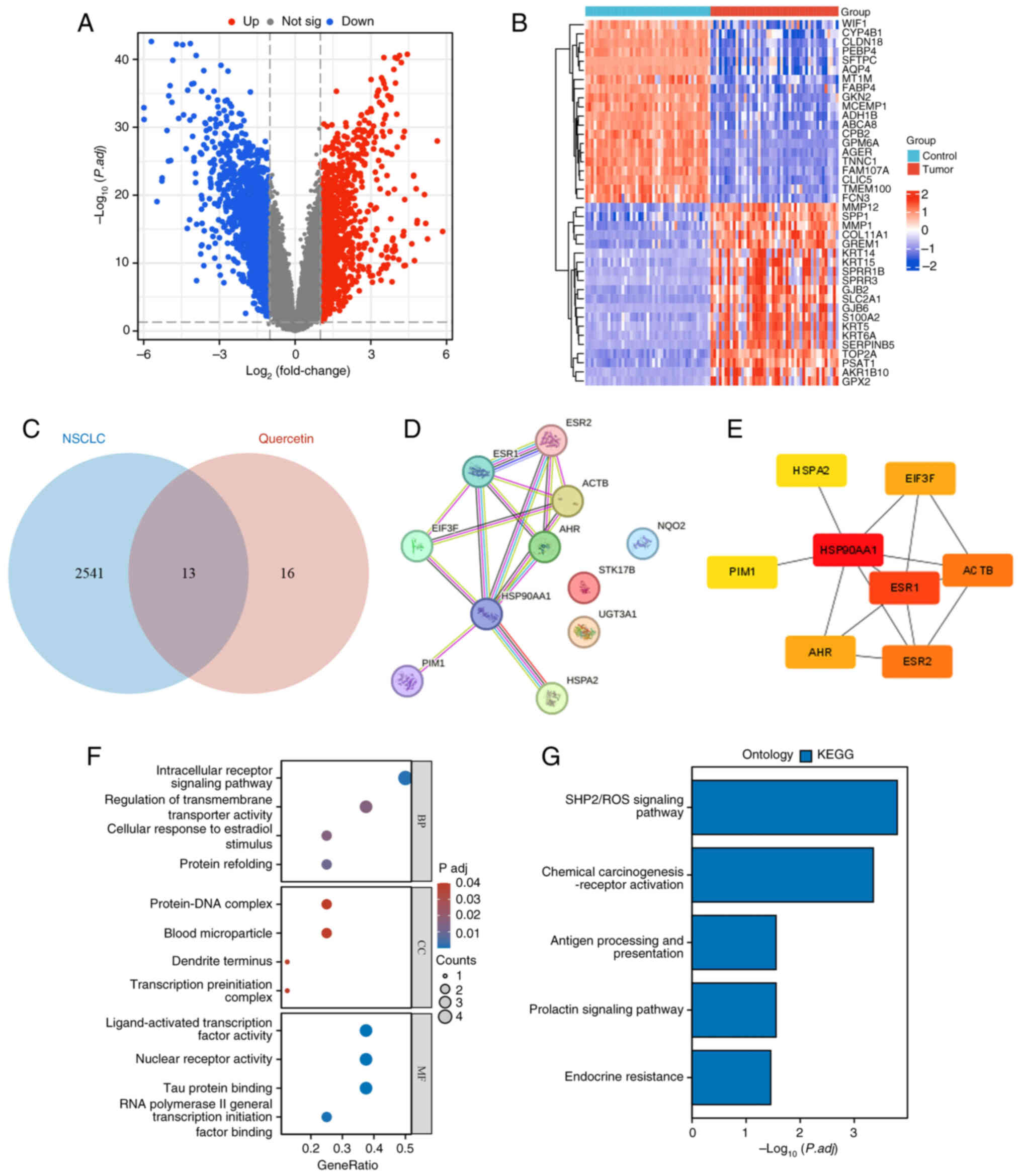
Quercetin is associated with ‘SHP2/ROS
signaling’, ‘chemical carcinogenesis-receptor’ and ‘antigen
processing and presentation’
The 91 sets of samples obtained in the GSE18842
dataset were divided into two groups, namely the control and cancer
groups. A total of 2,545 differentially expressed molecules were
identified, including 1,119 upregulated and 1,426 downregulated
ones. The differentially expressed genes are visualized in a
volcano plot (Fig. 3A). The top 20
upregulated and downregulated genes were also visualized in the
form of a heat map (Fig. 3B). The
13 common genes between quercetin and NSCLC were visualized by a
Wayne diagram (Fig. 3C).
Additionally, the STRING online database was used to construct a
PPI network of the 11 target proteins (Fig. 3D). The interaction network between
the drug and the eight hub genes was constructed using ‘Cytoscape’
(version 3.9.1) (Fig. 3E). Gene
Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG)
enrichment analyses were performed to create a biological process
network of the differentially expressed genes. These analyses are
used to classify the results of functional annotation into the
following three categories: Biological process, cellular component
and molecular function. GO (Fig.
3F) and KEGG (Fig. 3G) analyses
revealed that quercetin was mainly enriched in the terms ‘SHP2/ROS
signaling pathway’, ‘chemical carcinogenesis-receptor’ and ‘antigen
processing and presentation’.
Quercetin regulates myocardial cell
mitochondrial autophagy via the ROS/SHP2 and p-AMPK/XBP-1S/DJ-1
pathways
To further explore whether quercetin could regulate
mitochondrial autophagy the ROS/SHP2 axis, cardiomyocytes were
transfected with shSHP2 or stimulated with
H2O2. The western blot results from the in
vitro experiments revealed that the expression levels of the
ROS-related proteins, NOX4, XBP-1 and DJ-1, and those of the
mitochondrial autophagy-related proteins, PINK, parkin and beclin1,
were increased, while those of p-AMPK were reduced in
cardiomyocytes in the NSCLC + gefitinib group. Treatment with
quercetin abrogated the aforementioned effects. However, SHP2
silencing or cell treatment with H2O2
suppressed the protein expression levels of PINK, parkin, beclin1
and p-AMPK, and promoted the expression levels of NOX4, XBP-1 and
DJ-1 proteins (Fig. 4A and B).
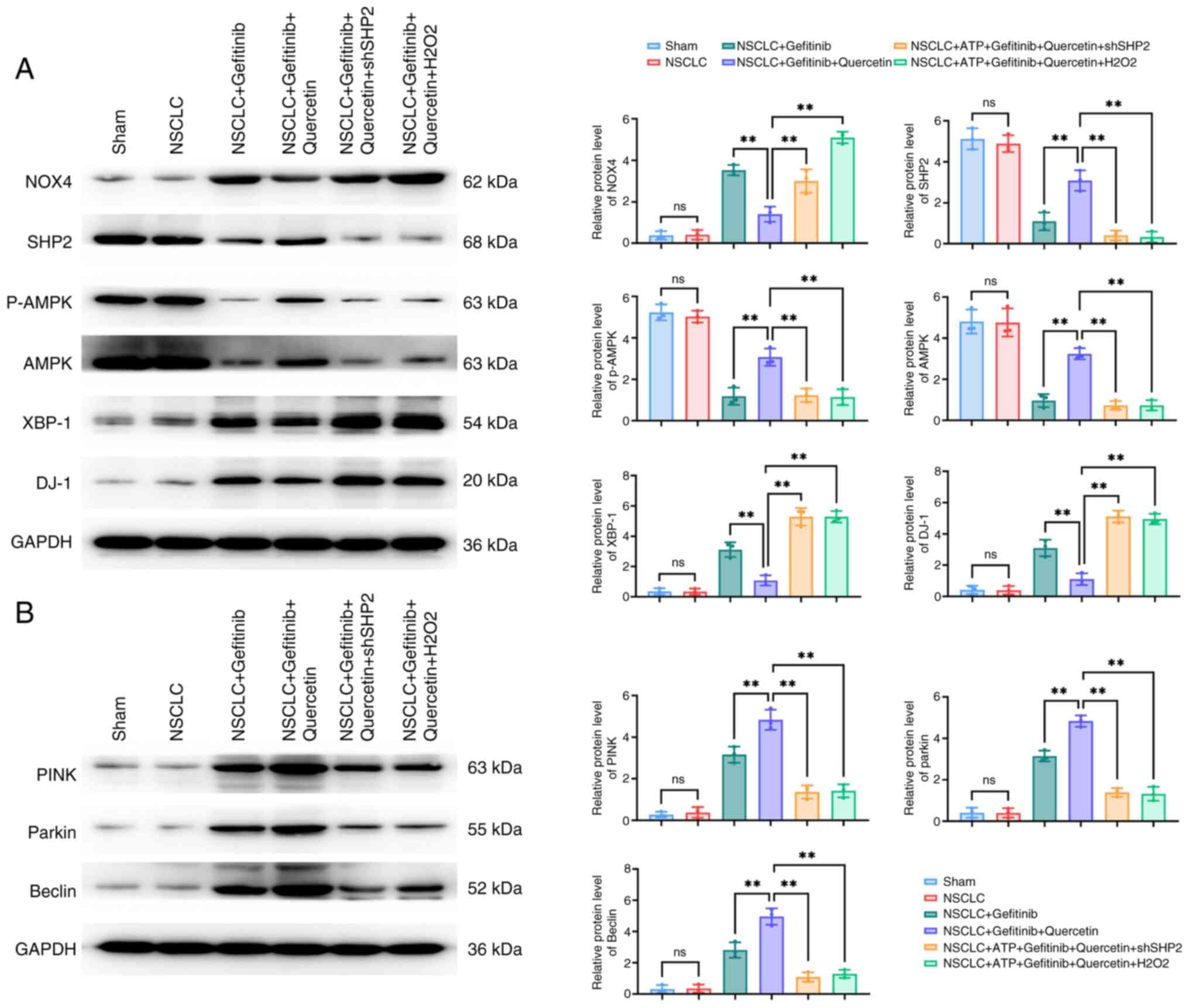 | Figure 4.Quercetin regulates
p-AMPK/XBP-1/DJ-1-mediated mitochondrial autophagy in
cardiomyocytes via ROS/SHP2. (A and B) The expression levels of (A)
the p-AMPK/XBP-1S/DJ-1 axis-related and (B) mitochondrial
autophagy-related, PTEN-induced putative kinase/parkin/beclin1,
proteins in ROS/SHP2-regulated cardiomyocytes treated with
quercetin were detected by western blot analysis. **P<0.01.
p-AMPK, phosphorylated AMP-activated protein kinase; XBP-1, X-box
binding protein 1; ROS, reactive oxygen species; SHP2, Src
homology-2 domain-containing protein tyrosine phosphatase; NOX4,
NADPH oxidase 4; sh-, short hairpin; NSCLC, non-small cell lung
cancer; ns, not significant (P>0.05). |
Quercetin regulates myocardial cell
mitochondrial function and the expression of apoptosis-related
proteins via ROS/SHP2
Mitochondrial membrane potential (MMP) was assessed
using JC-1 staining. The results demonstrated that MMP was
significantly reduced in the NSCLC + gefitinib group, as evidenced
by the enhanced blue/red fluorescence ratio. Additionally, western
blot analysis demonstrated that the expression levels of PPAR-γ and
PGC-1 were reduced, while those of the cellular scorch
death-related proteins, p-STING/p-IRF3/NLRP3/GSDMD/IL-1, were
significantly increased in the NSCLC + gefitinib group. However,
MMP was enhanced after quercetin treatment, accompanied by
PPAR-γ/PGC-1 upregulation and p-STING/p-IRF3/NLRP3/GSDMD/IL-1β
downregulation. Furthermore, cell transfection with shSHP2 or
treatment with H2O2 significantly reduced
MMP, downregulated PPAR-γ and PGC-1, and upregulated p-STING,
p-IRF3, NLRP3, GSDMD and IL-1β (Fig.
5).
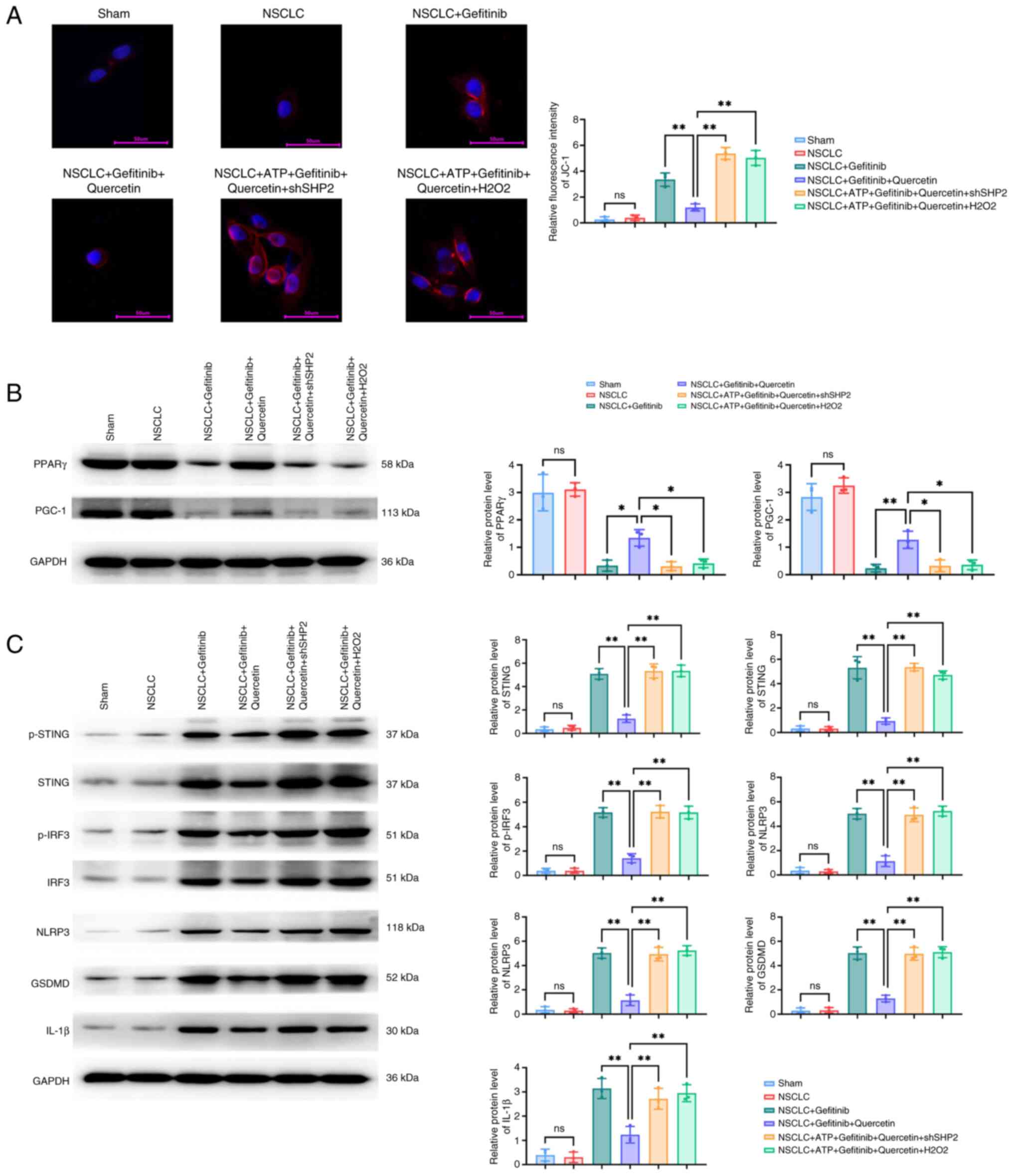 | Figure 5.Protein expression levels of the
p-STING/p-IRF3/NLRP3/GSDMD/IL-1β pathway-related proteins in
quercetin-treated and ROS/SHP2-regulated cardiomyocytes. (A)
Immunofluorescence staining of JC-1 in cardiomyocytes treated with
quercetin. The protein expression levels of the (B) mitochondrial
autophagy-related proteins, PPAR-γ and PPAR-γ coactivator-1, and
(C) those of the p-STING/p-IRF3/NLRP3/GSDMD/IL-1β pathway were
detected in quercetin-treated ROS/SHP2-regulated cardiomyocytes by
western blot analysis. *P<0.05 and **P<0.01. p-STING,
phosphorylated stimulator of interferon genes; IRF3, interferon
regulatory factor 3; NLRP3, Nod-like receptor protein 3; GSDMD,
gasdermin D; ROS, reactive oxygen species; SHP2, Src homology-2
domain-containing protein tyrosine phosphatase; PPAR-γ, peroxisome
proliferator-activated receptor γ; PGC-1, PPAR-γ coactivator;
NSCLC, non-small cell lung cancer; ns, not significant
(P>0.05). |
Discussion
Lung cancer is the most common type of cancer
worldwide. By the time it is diagnosed, it has usually spread. As a
result, surgery is not commonly applicable and, therefore,
medication, usually chemotherapy, is needed. NSCLC is the most
frequent type of lung cancer, which is more commonly treated with
TKIs (20). More specifically, TKIs
are considered as the standard treatment approach for EGFR-mutated
NSCLC with brain metastases. A recent study revealed that EGFR-TKIs
combined with chemotherapy could improve progression-free survival
in patients with EGFR-mutated advanced NSCLC (21).
It has been reported that the mechanism of action of
gefitinib in lung cancer is extremely complex and diverse. Firstly,
it can activate the inositol-requiring enzyme 1α/XBP-1 signaling
pathway via releasing intracellular Ca2+ and entering
the endoplasmic reticulum through the ATP/P2X7 purinergic receptor
pathway (22,23). This process not only inhibits the
mitochondrial autophagy process, but also further activates the
DJ-1/transcription factor EB signaling pathway (24,25),
which is in turn involved in maintaining the normal metabolic
function and homeostasis in cells. In addition, gefitinib was also
experimentally found to act via activating the ROS signaling
pathway. ROS is possibly involved in this process through the
SHP2-induced inhibition of the MAPK signaling pathway (26), thus further blocking mitochondrial
autophagy (27,28). Other studies also indicated that
mitochondrial autophagy could maintain cell survival and function
via inhibiting the mtDNA/cGAS signaling pathway-induced activation
of the STING/IRF3/NLRP3/GSDMD/IL-1β signaling pathway, thus
attenuating cell pyroptosis (29,30).
Numerous in vitro and in vivo studies
have suggested that quercetin exerts a variety of functions, such
as anti-inflammatory, antioxidant, antihypertensive, hypoglycemic,
neurovascular protective, anticancer, anti-aging and
immune-enhancing properties (31).
Quercetin, as a natural compound, has also attracted marked
attention in the field of anticancer therapy. Emerging evidence has
suggested that quercetin exerts a particular inhibitory effect on
several types of cancer, including lung cancer. Therefore, a
previous study revealed that quercetin inhibited tumor cell
proliferation, invasion and metastasis, while inducing cell
apoptosis (32). In addition,
quercetin could also display anticancer effects via regulating the
tumor microenvironment and affecting tumor angiogenesis (33,34).
Quercetin has also attracted increasing attention in NSCLC.
Therefore, previous experimental studies showed that quercetin
treatment enhanced the efficacy of chemotherapy or targeted
therapy, reduced tumor resistance to chemotherapeutic drugs and
prolong patient survival. In addition, it has been reported that
quercetin can also play an anti-lung cancer role via regulating the
activation of lung cancer-related signaling pathways, cell
proliferation, apoptosis and metastasis.
In the present study, the effects of quercetin on
gefitinib-induced heart problems in patients with NSCLC and its
mechanism of action were investigated. Therefore, the experimental
results identified that gefitinib could promote cardiac fibrosis
and cellular focal death, while the application of quercetin could
effectively suppress the occurrence of these adverse effects. More
specifically, quercetin could attenuate gefitinib-induced cell
pyroptosis via modulating mitochondrial autophagy mediated by the
SHP2/ROS/AMPK/XBP-1/DJ-1 signaling pathway.
The bioinformatics analysis results verified the
association between quercetin and SHP2/ROS signaling, thus further
supporting the effect of quercetin on regulating heart disorders.
The results of the in vitro experiments revealed that
quercetin treatment abrogated the effects of gefitinib on ROS
production and the expression of related proteins, accompanied by
the enhanced expression of mitochondrial autophagy-related proteins
and p-AMPK, thus maintaining the stability of cardiac function. In
addition, the results showed that quercetin could protect
mitochondrial integrity and attenuate the decrease in MPP, as
evidenced by the assessment of mitochondrial membrane damage via
JC-1 staining. This finding provided additional evidence for the
effect of quercetin on preventing cellular focal death.
Taken together, the results of the present study
indicated that quercetin could be considered as a potential
therapeutic approach to effectively delay cardiac issues in
patients with NSCLC treated with gefitinib. Quercetin could inhibit
cell pyroptosis via modulating mitochondrial autophagy mediated by
the SHP2/ROS/AMPK/XBP-1/DJ-1 signaling pathway, thus providing a
significant reference and novel insights into the development of
more effective and safer therapeutic strategies for NSCLC. The
current study could also provide substantial guidance and insights
for exploring the mechanisms underlying the effect of EGFR-TKIs on
promoting the onset of cardiac issues in clinical practice
(Fig. 6).
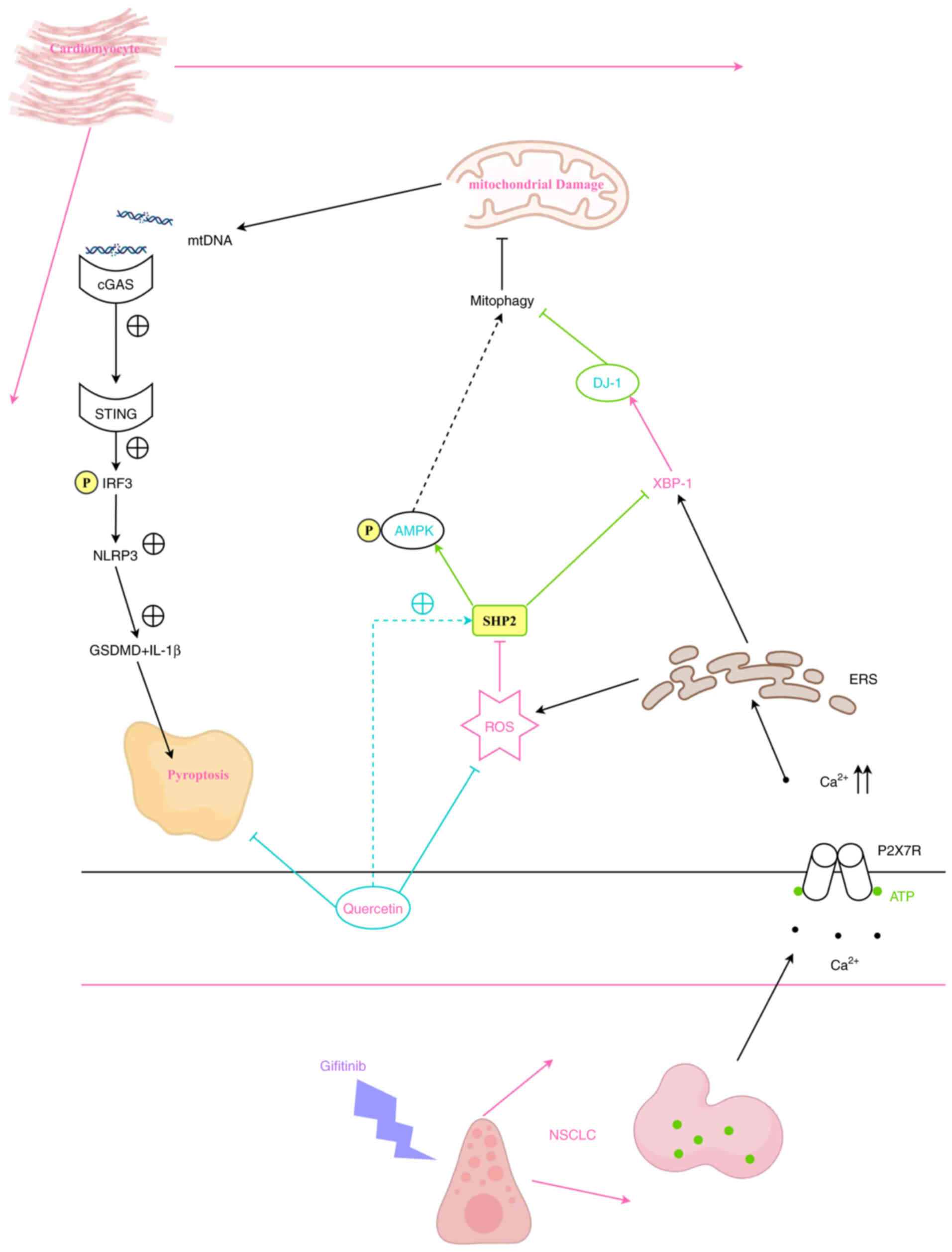 | Figure 6.Quercetin inhibits
gefitinib-activated cell death in NSCLC via regulating
SHP2/ROS/AMPK/XBP-1/DJ-1 signaling pathway-mediated mitochondrial
autophagy. NSCLC, non-small cell lung cancer; SHP2, Src homology-2
domain-containing protein tyrosine phosphatase; ROS, reactive
oxygen species; AMPK, AMP-activated protein kinase; GSDMD,
gasdermin D; STING, stimulator of interferon genes; cGAS, cyclic
GMP-AMP synthase; IRF3, interferon regulatory factor 3; mtDNA,
mitochondrial DNA; ERS, endoplasmic reticulum stress. |
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JZ conceived and designed the experiments. JZ, SQ,
YD, HD and NL performed the experiments. JZ, SQ and HD analysed the
data. JZ wrote the manuscript. JZ, SQ, YD, HD and NL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
The study protocol was approved (approval no.
IACUC-4th Hos Hebmu-2024022) by The Fourth Hospital of Hebei
Medical University Research Ethics Committee (Shijiazhuang,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pan Z, Wang K, Wang X, Jia Z, Yang Y, Duan
Y, Huang L, Wu ZX, Zhang JY and Ding X: Cholesterol promotes
EGFR-TKIs resistance in NSCLC by inducing EGFR/Src/Erk/SP1
signaling-mediated ERRα re-expression. Mol Cancer. 21:772022.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cheng C, Wang S, Dong J, Zhang S, Yu D and
Wang Z: Effects of targeted lung cancer drugs on cardiomyocytes
studied by atomic force microscopy. Anal Methods. 15:4077–4084.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mok TS, Wu YL, Ahn MJ, Garassino MC, Kim
HR, Ramalingam SS, Shepherd FA, He Y, Akamatsu H, Theelen WS, et
al: Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung
cancer. N Engl J Med. 376:629–640. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thein KZ, Swarup S, Ball S, Quirch M,
Vorakunthada Y, Htwe KK, D'Cunha N, Hardwicke F, Awasthi S and
Tijani L: 1388P Incidence of cardiac toxicities in patients with
advanced non-small cell lung cancer treated with osimertinib: A
combined analysis of two phase III randomized controlled trials.
Ann Oncol. 29:viii5002018. View Article : Google Scholar
|
|
5
|
Soria JC, Ohe Y, Vansteenkiste J,
Reungwetwattana T, Chewaskulyong B, Lee KH, Dechaphunkul A, Imamura
F, Nogami N, Kurata T, et al: Osimertinib in untreated EGFR-mutated
advanced non-small-cell lung cancer. N Engl J Med. 378:113–125.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang M, Chen X, Yu F, Zhang L, Zhang Y and
Chang W: The targeting of noncoding RNAs by quercetin in cancer
prevention and therapy. Oxid Med Cell Longev.
2022:43306812022.PubMed/NCBI
|
|
7
|
Güran M, Şanlıtürk G, Kerküklü NR,
Altundağ EM and Süha Yalçın A: Combined effects of quercetin and
curcumin on anti-inflammatory and antimicrobial parameters in
vitro. Eur J Pharmacol. 859:1724862019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ersoz M, Erdemir A, Derman S, Arasoglu T
and Mansuroglu B: Quercetin-loaded nanoparticles enhance
cytotoxicity and antioxidant activity on C6 glioma cells. Pharm Dev
Technol. 25:757–766. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sul OJ and Ra SW: Quercetin Prevents
LPS-induced oxidative stress and inflammation by modulating
NOX2/ROS/NF-kB in lung epithelial cells. Molecules. 26:69492021.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lan CY, Chen SY, Kuo CW, Lu CC and Yen GC:
Quercetin facilitates cell death and chemosensitivity through
RAGE/PI3K/AKT/mTOR axis in human pancreatic cancer cells. J Food
Drug Anal. 27:887–896. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hasan AAS, Kalinina EV, Tatarskiy VV,
Volodina YL, Petrova АS, Novichkova MD, Zhdanov DD and Shtil AA:
Suppression of the antioxidant system and PI3K/Akt/mTOR signaling
pathway in cisplatin-resistant cancer cells by quercetin. Bull Exp
Biol Med. 173:760–764. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li X, Zhou N, Wang J, Liu Z, Wang X, Zhang
Q, Liu Q, Gao L and Wang R: Quercetin suppresses breast cancer stem
cells (CD44(+)/CD24(−)) by inhibiting the PI3K/Akt/mTOR-signaling
pathway. Life Sci. 196:56–62. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Suo W, Zhang X, Lv J, Liu Z and
Liu R: Roles and mechanisms of quercetin on cardiac arrhythmia: A
review. Biomed Pharmacother. 153:1134472022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang L, Tan A, An X, Xia Y and Xie Y:
Quercetin dihydrate inhibition of cardiac fibrosis induced by
angiotensin II in vivo and in vitro. Biomed Pharmacother.
127:1102052020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Huang KY, Wang TH, Chen CC, Leu YL, Li HJ,
Jhong CL and Chen CY: Growth suppression in lung cancer cells
harboring EGFR-C797S mutation by quercetin. Biomolecules.
11:12712021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barretina J, Caponigro G, Stransky N,
Venkatesan K, Margolin AA, Kim S, Wilson CJ, Lehár J, Kryukov GV,
Sonkin D, et al: The cancer cell line encyclopedia enables
predictive modelling of anticancer drug sensitivity. Nature.
483:603–607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Heliste J, Jokilammi A, Vaparanta K,
Paatero I and Elenius K: Combined genetic and chemical screens
indicate protective potential for EGFR inhibition to cardiomyocytes
under hypoxia. Sci Rep. 11:166612021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sunasee R, Araoye E, Pyram D, Hemraz UD,
Boluk Y and Ckless K: Cellulose nanocrystal cationic derivative
induces NLRP3 inflammasome-dependent IL-1β secretion associated
with mitochondrial ROS production. Biochem Biophys Rep. 4:1–9.
2015.PubMed/NCBI
|
|
20
|
Greenhalgh J, Boland A, Bates V, Vecchio
F, Dundar Y, Chaplin M and Green JA: First-line treatment of
advanced epidermal growth factor receptor (EGFR) mutation positive
non-squamous non-small cell lung cancer. Cochrane Database Syst
Rev. 3:CD0103832021.PubMed/NCBI
|
|
21
|
Hou X, Li M, Wu G, Feng W, Su J, Jiang H,
Jiang G, Chen J, Zhang B, You Z, et al: Gefitinib plus chemotherapy
vs gefitinib alone in untreated EGFR-mutant non-small cell lung
cancer in patients with brain metastases: The GAP BRAIN open-label,
randomized, multicenter, phase 3 study. JAMA Netw Open.
6:e22550502023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wilkaniec A, Cieślik M, Murawska E, Babiec
L, Gąssowska-Dobrowolska M, Pałasz E, Jęśko H and Adamczyk A: P2X7
receptor is involved in mitochondrial dysfunction induced by
extracellular alpha synuclein in neuroblastoma SH-SY5Y cells. Int J
Mol Sci. 21:39592020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hong Y, Zhou X, Li Q, Chen J, Wei Y, Long
C, Shen L, Zheng X, Li D, Wang X, et al: X-box binding protein 1
caused an imbalance in pyroptosis and mitophagy in immature rats
with di-(2-ethylhexyl) phthalate-induced testis toxicity. Genes
Dis. 11:935–951. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Imberechts D, Kinnart I, Wauters F,
Terbeek J, Manders L, Wierda K, Eggermont K, Madeiro RF, Sue C,
Verfaillie C and Vandenberghe W: DJ-1 is an essential downstream
mediator in PINK1/parkin-dependent mitophagy. Brain. 145:4368–4384.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu M, Hang H, Huang M, Li J, Xu D, Jiao J,
Wang F, Wu H, Sun X, Gu J, et al: DJ-1 deficiency in hepatocytes
improves liver ischemia-reperfusion injury by enhancing mitophagy.
Cell Mol Gastroenterol Hepatol. 12:567–584. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhu G, Xie J, Kong W, Xie J, Li Y, Du L,
Zheng Q, Sun L, Guan M, Li H, et al: Phase separation of
disease-associated SHP2 mutants underlies MAPK hyperactivation.
Cell. 183:490–502.e18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li Y, Chen H, Xie X, Yang B, Wang X, Zhang
J, Qiao T, Guan J, Qiu Y, Huang YX, et al: PINK1-mediated mitophagy
promotes oxidative phosphorylation and redox homeostasis to induce
drug-tolerant persister cancer cells. Cancer Res. 83:398–413. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu H, Ho PW, Leung CT, Pang SY, Chang
EES, Choi ZY, Kung MH, Ramsden DB and Ho SL: Aberrant mitochondrial
morphology and function associated with impaired mitophagy and
DNM1L-MAPK/ERK signaling are found in aged mutant Parkinsonian
LRRK2R1441G mice. Autophagy. 17:3196–3220. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo T, Jia X, Feng WD, Wang JY, Xie F,
Kong LD, Wang XJ, Lian R, Liu X, Chu YJ, et al: Bergapten inhibits
NLRP3 inflammasome activation and pyroptosis via promoting
mitophagy. Acta Pharmacol Sin. 44:1867–1878. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liu Z, Wang M, Wang X, Bu Q, Wang Q, Su W,
Li L, Zhou H and Lu L: XBP1 deficiency promotes hepatocyte
pyroptosis by impairing mitophagy to activate mtDNA-cGAS-STING
signaling in macrophages during acute liver injury. Redox Biol.
52:1023052022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Di Petrillo A, Orrù G, Fais A and Fantini
MC: Quercetin and its derivates as antiviral potentials: A
comprehensive review. Phytother Res. 36:266–278. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Reyes-Farias M and Carrasco-Pozo C: The
anti-cancer effect of quercetin: Molecular implications in cancer
metabolism. Int J Mol Sci. 20:31772019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang B, Zhang W, Zhou X, Liu M, Hou X,
Cheng Z and Chen D: Development of dual-targeted nano-dandelion
based on an oligomeric hyaluronic acid polymer targeting
tumor-associated macrophages for combination therapy of non-small
cell lung cancer. Drug Deliv. 26:1265–1279. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang SM, Deng XT, Zhou J, Li QP, Ge XX and
Miao L: Pharmacological basis and new insights of quercetin action
in respect to its anti-cancer effects. Biomed Pharmacother.
121:1096042020. View Article : Google Scholar : PubMed/NCBI
|