Introduction
As a primary malignant bone tumor, osteosarcoma (OS)
often occurs mainly in children and adolescents. Current typically
clinical therapy for OS patients is surgery in combination with
adjuvant chemotherapeutic agents. However, neither surgical nor
non-surgical treatment yields satisfactory effects in OS patients,
high mortality and recurrence rates are still obstacles to clinical
practice (1). Finding new
therapeutic targets is therefore urgently needed to improve the
life quality and survival for OS patients. Ferroptosis has emerged
as a novel form of programmed cell death in eukaryotic cells and it
is morphologically, genetically and biochemically distinct from
other types of cell death such as apoptosis, necroptosis and
autophagy (2). Ferroptosis is
characterized by iron-dependent lipid peroxidation accumulation,
which leads to accumulated intracellular production and subsequent
cell membrane rupture and death (3,4).
Although the phenomenon of ferroptosis has been present in multiple
cancers, the detailed mechanism, especially in OS, is largely
unknown. Elucidating the regulatory mechanism of ferroptosis is
therefore an effective strategy to find the potential targets for
OS therapeutics.
Long non-coding RNAs (lncRNAs) have been considered
as important regulators in various biological activities and
diseases and orchestrate a number of cell biological events ranging
from embryogenesis to cell fate determination (5). They have been demonstrated to actively
participate in various tumor processes, including carcinogenesis,
metastasis, epithelial to mesenchymal transition and drug
resistance (6,7). Furthermore, ferroptosis has been
reported to be involved in the cancer development, metastasis and
drug resistance these lncRNAs mediate. For instance, lncRNA-PMAN or
LINC00239 inhibit ferroptosis in gastric and colorectal cancer
(8,9). Moreover, lncRNA SNHG14 is markedly
upregulated in nutlin3a-resistant OS cells and contributes to drug
resistance through suppressing ferroptosis (10). HOXA transcript at the distal tip
(HOTTIP) is an identified onco-lncRNA and it is upregulated in OS
tissues (11). HOTTIP also
facilitates cell proliferation, invasion and migration as well as
chemoresistance in OS and is associated with poor prognosis in OS
patients (12). The present study
therefore considered whether ferroptosis is involved in HOTTIP
modulated OS progression.
In the present study, a CRISPR/Cas9 system was
applied to generate the HOTTIP knockout (KO) mice and the
transcriptome analysis from bone marrow revealed that several
signaling related ferroptosis were altered, suggesting that HOTTIP
may be involved in ferroptosis. It also demonstrated that HOTTIP
knockdown promoted, while its overexpression suppressed,
ferroptosis in OS cells in vitro and in vivo.
Furthermore, HOTTIP physically interacted with RNA binding protein
DiGeorge Critical Region 8 (DGCR8) and influenced its protein
stability. As a component of microprocessor complex, DGCR8
interacts with Drosha and facilitates miRNA maturation. The results
showed that HOTTIP knockdown potentiated miR-214-3p expression and
facilitated the suppression of its target gene glutathione
peroxidase 4 (GPX4) transcription, thereby leading to stimulating
ferroptosis. Taken together, the results demonstrated that HOTTIP
suppressed ferroptosis in OS cells via a novel
DGCR8/miR-214-3p/GPX4 regulatory axis, which provided insights to
develop HOTTIP as a promising therapeutic target for OS
patients.
Materials and methods
Reagents and antibodies
Erastin (cat. no. HY-15763), RSL3 (cat. no.
HY-100218A), ferrostatin-1 (cat. no. HY-100579), Z-VAD-FMK (cat.
no. HY-16658B) and necrosulfonamide (cat. no. HY-100573) were
purchased from MedChemExpress. Imidazole ketone erastin (cat. no.
T5523) was purchased from Topscience. Primary antibodies, X
cystine/glutamate antiporter (xCT; cat. no. T57046; 1:1,000; Abmart
Pharmaceutical Technology Co., Ltd.), GPX4 (cat. no. T56959;
1:1,000; Abmart Pharmaceutical Technology Co., Ltd.), DGCR8 (cat.
no. ET1609-32; 1:1,000; HuaBio), Ubiquitin (cat. no. T55965;
1:1,000; Abmart Pharmaceutical Technology Co., Ltd.) and β-actin
(cat. no. P30002; 1:1,000; Abmart Pharmaceutical Technology Co.,
Ltd.). Secondary anti-rabbit antibodies were from Cell Signaling
Technology, Inc. (cat. no. 7074S; 1:2,000).
RNA sequencing
RNA sequencing was provided by CloudSeq Biotech Inc.
A total of six male HOTTIP KO mice and male WT mice were provided
by Cyagen Biosciences. The mice were bred in a specified
pathogen-free environment at temperature and humidity controlled
(26°C and 50% humidity) environment with 12-h light/dark cycle, and
served a standard diet. The mice were 4–6 weeks old and weighed
20–22 g and consisted of three HOTTIP KO mice and three WT mice.
They were sacrificed and their bone marrow was isolated, total RNA
was extracted using Triquick Reagent (cat. no. R1100; Beijing
Solarbio Science & Technology Co., Ltd.). Total RNA was
isolated and reverse transcribed into cDNA to generated an indexed
Illumina library (Illumina, Inc.), including fragmentation (~300
bp), adapter ligation (unique dual indexes), and PCR amplification
(15 cycles). Library quality was validated via Agilent 2100
Bioanalyzer (Agilent Technologies, Inc.; peak size: 280-320 bp) and
quantified by Qubit Flex Fluorometer (Invitrogen; Thermo Fisher
Scientific, Inc.), followed by sequencing on an Illumina Novaseq
platform (Illumina, Inc.).
Cell culture
Human OS cell lines including MG63 and U2OS were
provided by Lingnan Medical Research Center, Guangzhou University
of Chinese Medicine (Guangzhou, China). Cell lines were
authenticated by STR profiling (ATCC) and tested negative for
mycoplasma. The two OS cell lines were cultured in Dulbecco's
modified Eagle's medium (DMEM; Shanghai VivaCell Biosciences, Ltd.)
with 10% fetal bovine serum (FBS; Shanghai ExCell Biology, Inc.)
and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher
Scientific, Inc.). These cells were incubated and maintained in a
humidified atmosphere at 37°C, 5% CO2.
Cell viability assays
The OS cells were seeded into 96-well plates and
cultured for 12 h. Then, cells were starved using 2% FBS for 12 h,
the Fer-1 group was preincubated with 2 µM Fer-1 (MedChemExpress).
Subsequently, cells were incubated with 10% FBS and induced by 10
µM Erastin (MedChemExpress) and 1 µM RSL3 (MedChemExpress) for 24
h. The cell viability was detected by Cell Counting Kit-8 (Beyotime
Institute of Biotechnology) examination and the absorbance was
measured at 450 nm using a Hybrid Multi-Mode Microplate Reader
(Tecan Group, Ltd.). All the experiments were performed in
triplicates.
Colony formation assays
The MG63 and U2OS cells were seeded into 6-well
plate and treated with 10 µM Erastin and 2 µM Fer-1 at 37°C in a 5%
CO2 incubator for two weeks. At room temperature, the
cells were fixed with 4% paraformaldehyde for half an hour and
stained with crystal violet staining solution for half an hour. The
images were captured using the ImmunoSpot analyzer (Cellular
Technology Ltd.) and the colony numbers were counted by ImmunoSpot
version 6.0 Academic system (Cellular Technology Ltd.).
Detection of ferroptosis
The OS cells were seeded and ferroptosis was induced
by Erastin and RSL3 at 37°C in a 5% CO2 incubator for 24
h. The samples were collected for the ferroptosis examination. The
level of intracellular iron was detected using an Iron Assay kit
(Applygen Technologies, Inc.) according to the manufacturer's
instructions. For intracellular ROS staining, the lipid ROS level
was examined by flow cytometry with a 5 µM DHE fluorescence probe
(Applygen Technologies, Inc.) in a visible spectrum of red range
(emission maximum 610 nm). The levels of malondialdehyde and
glutathione were evaluated by a lipid peroxidation (MDA) assay kit
and a reduced glutathione (GSH) assay kit (Njjcbio).
Reverse transcription-quantitative
(RT-q) PCR
Total RNA from 5×105 cells was extracted
using Animal Total RNA Isolation Kit (Chengdu Fuji Biotechnology
Co., Ltd.) and it was reversely transcribed using PrimeScript RT
Reagent Kit (Takara Bio, Inc.) following the manufacturer's
instructions. The RT-qPCR examinations were conducted using Power
Up TB Green Master Mix (Takara Bio, Inc.) on an ABI-QuantStudio 5
System (Thermo Fisher Scientific, Inc.). The cycling conditions
were as follows: Initial denaturation at 95°C for 5 min, followed
by 25–45 cycles of denaturation at 95°C for 15 sec, annealing at
60°C for 30 sec (depending on the target gene), and elongation at
72°C for 1 min, with a final extension at 72°C for 5 min. The
reactions were stopped during the exponential phase to ensure
accurate comparisons. The primer sequences are listed in Table I. GAPDH served as the endogenous
control and fold changes were calculated using the relative
quantification (2−ΔΔCq) method (13). All the experiments were performed in
triplicates.
 | Table I.The sequences of primer for reverse
transcription-quantitative PCR. |
Table I.
The sequences of primer for reverse
transcription-quantitative PCR.
| Gene | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| HOTTIP |
CCTAAAGCCACGCTTCTTTG |
TGCAGGCTGGAGATCCTACT |
| DGCR8 |
GCCTCCTCATAGACCCGAACT |
CGGTAAAGCTCACGCTAATCTT |
| GAPDH |
GCACCACCAACTGCTTAGCA |
TCTTCTGGGTGGCAGTGATG |
Western blotting
Total protein was lysed using Radio
Immunoprecipitation Assay (RIPA) buffer supplemented with protease
and phosphatase inhibitor (Beyotime Institute of Biotechnology).
The supernatant fraction was collected by centrifugation and
quantified by BCA assay (Thermo Fisher Scientific, Inc.).
Subsequently, an equal volume of protein mixture (30 µg) were
separated by 10% SDS-PAGE and transferred to a PVDF membrane
(MilliporeSigma). The membranes were blocked with 5% fat-free milk
(Bio-Rad Laboratories, Inc.) for 1 h at room temperature and
incubated at 4°C overnight with primary antibodies. Next, the
membranes were incubated with secondary antibodies in dark at room
temperature for 1 h. The expression levels were visualized by
chemiluminescence (MilliporeSigma). Densitometric analysis was
carried out using ImageJ software (ver. 1.46; National Institutes
of Health).
Co-immunoprecipitation (Co-IP)
Co-IP was conducted with a magnetic IP kit (cat. no.
88804; Thermo Fisher Scientific, Inc.). Briefly, 1×107
cell lysates were gently rotated at 4°C overnight with anti-DGCR8
antibody, or normal rabbit IgG (Wuhan Servicebio Technology Co.,
Ltd.). Afterwards, pre-washed protein A/G magnetic beads were
incubated with rotating at 4°C for 16 h. The beads were collected
and washed three times. Western blotting was performed after
eluting bound proteins with elution buffer.
RNA immunoprecipitation (RIP)
RIP was performed as mentioned before (14) using RNA Binding Protein
Immunoprecipitation Kit (cat. no. JKR23003; Wuhan Gene Create
Biological Engineering Co., Ltd.). According to the manufacturer's
protocol, the A/G protein magnetic beads were pre-incubated with
anti-DGCR8 antibody or IgG (Wuhan Servicebio Technology Co., Ltd.)
at 4°C overnight. The 1×107 cells were washed with cold
PBS twice and lysed with 200 µl lysis buffer. Then, the lysates
were incubated with the pre-coated beads at 4°C for 16 h. The total
RNA was extracted with TRIzol® reagent (Invitrogen;
Thermo Fisher Scientific, Inc.) and detected by RT-qPCR
examination.
Stable cell line generation and miRNA
transfection
Stable HOTTIP overexpression or knockdown cell lines
were constructed using 2nd lentiviral system as previously reported
(15). Briefly, the 4 µg lentivirus
was generated in 293T cells (The Cell Bank of Type Culture
Collection of The Chinese Academy of Sciences) by co-transfecting
with two packaging vectors (3 µg psPAX2; 1 µg pMD2G). Lentiviral
particles were harvested 48 h post-transfection, followed by
infection of OS cells for 24 h. Cells (30% confluence) were seeded
in cell culture dishes and were infected with lentiviral plasmids
at multiplicity of infection (MOI) of 10. After 37°C and screening
of the cells for 7 days with medium containing 1 µg/ml puromycin
(Beyotime Institute of Biotechnology), these virus particles
infected OS cells and the stable cell lines were developed. The
sequence of short hairpin (sh)HOTTIP were Sense:
5′-nnnnnGGCACTTTATATGCTGTAAnnnnnnnnnTTACAGCATATAAAGTGCCnnnnnn-3′;
Anti-sense:
5′-nnnnnnnnnnGGCACTTTATATGCTGTAAnnnnnnnnnTTACAGCATATAAAGTGCCn-3′.
Has-miR-214-3p mimics were designed and synthesized by Beijing
Tsingke Biotech Co., Ltd. These oligoes were transfected in OS
cells using Lipofectamine® 3000 (Invitrogen; Thermo
Fisher Scientific, Inc.). The sequence of miR214-3p were Sense:
5′-ACAGCAGGCACAGACAGGCAGU-3′; Anti-sense:
5′-ACUGCCUGUCUGUGCCUGCUGU-3′.
Luciferase activity assays
The GPX4 3′UTR-untranslated region (3′UTR)
containing wild-type or mutated miR-214-3p binding sites were
respectively synthesized and inserted into pmirGLO luciferase
vector (Wuhan Gene Create Biological Engineering Co., Ltd.) and
then co-transfected with miR-214-3p mimics into OS cells using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at 37°C for 48 h. The cell lysates were collected
48 h post transfection and Firefly and Renilla luciferase
activities were detected by the Dual-Luciferase Reporter Assay kit
(Promega, USA). The luciferase activity was normalized to Renilla
luciferase activity.
Immunofluorescence
OS cells were seeded in confocal petri dishes and
cultured to 80% confluence. The dishes were then washed twice with
phosphate-buffered saline (PBS), fixed with 200 µl 4%
paraformaldehyde at room temperature for 15 min, and permeabilized
with 200 µl 0.1% Triton X for 15 min at room temperature. After
washing twice with PBS, the dishes were incubated with the
anti-DGCR8 antibody with 1:200 dilution at 4°C overnight. The next
day, the dishes were washed twice with PBS, then anti-rabbit
IgG-Cy3 Fluor 570 (Beyotime Institute of Biotechnology) was added
at 1:1,500 dilution and further incubated at room temperature for 1
h. The nucleus was then counterstained with DAPI (Shanghai Yeasen
Biotechnology Co., Ltd.) at room temperature for 5 min and the
images were captured using a Zeiss Axiophot 2 microscope (Zeiss
AG). The magnification of all images was ×40.
Osteosarcoma intra-tibia tumor-bearing
model
A total of 20 female Balb/c-nude mice (3–4 weeks
old, weight 10–13 g) were purchased from the Laboratory Animal
Center, Southern Medical University. The mice were bred in a
specified pathogen-free environment at temperature and humidity
controlled (26°C; 50% humidity) environment with 12-h light/dark
cycle and served a standard diet ad libitum. The animals
were randomly assigned into four groups (n=5), two groups (Group1
and Group2) were inoculated with 1.5×106/100 µl shHOTTIP
infected OS cells through trans-tibia injected into the medullary
cavity of the right tibia of mice; the other two groups (Group3 and
Group4) were injected with equivalent shNC infected OS cells at the
same site. Group1 and Group3 were intraperitoneally (i.p.) injected
with 20 mg/kg IKE (Shandong Topscience Biotech Co., Ltd.) and the
other two groups were treated with an equal volume of vehicle NaCl.
The IKE or NaCl was added once every other day and tumor formation
was monitored. The tumor volume was calculated using the formula
V=0.5 × L × W2 (L, length and W, width). The largest
tumor diameter allowed in this experiment was <20 mm. At the end
of the experiment, the mice were anesthetized with a dose of 100
mg/kg pentobarbital (intraperitoneal), followed by cervical
dislocation. Mortality was confirmed by the cessation of breathing
and heartbeat. And their tumor tissues were isolated. All animal
experimental procedures were approved by the Ethics and Animal
Research Committee of Southern Medical University (approval no.
SMUL2022219; Guangzhou, Guangdong).
Immunohistochemistry examination
Tumor tissues were fixed in 10% neutral formalin
fixative solution (BBI Solutions) embedded in paraffin at room
temperature overnight, and sectioned at 3-µm, which were
deparaffinized with xylene and then rehydrated with a succession of
decreasing alcohol concentrations (100, 95, 85, 70 and 50%
ethanol). Tissue sections were then placed in antigen retrieval
buffer (pH 9.0; Wuhan Servicebio Technology Co., Ltd.) in a
microwave oven on medium power for 10 min until boiling, then
cooled for 8 min and switched to medium-low power for 8 min. After
natural cooling, the slides were placed in PBS and washed three
times on a destaining shaker. Subsequently, 3% hydrogen peroxide
solution was added to the sample at room temperature for 25 min to
block endogenous peroxidase, followed by blocking with 3% BSA
(Sangon Biotech Co., Ltd.) at room temperature for 30 min. The
histological sections were then incubated at 4°C for 4 h with Ki67
antibody (cat. no. GB111499; Wuhan Servicebio Technology Co., Ltd.)
and GPX4 antibody (cat. no. T56959; Abmart Pharmaceutical
Technology Co., Ltd.) with 1:100 dilution, and with a horseradish
peroxidase-conjugated goat anti-rabbit IgG secondary antibody
(1:200; cat. no. SA00001-2; Proteintech Group, Inc.) at 37°C for 30
min. Sections were then counterstained with 0.1% hematoxylin
(Boster Biological Technology) at room temperature for 2 min and
were stained with the chromogen DAB (Boster Biological Technology).
Histological images were captured using a light microscope (cat.
no. AE2000; Motic Incorporation, Ltd.), the representative images
were taken with at ×40 magnification and the positive cells were
quantified with ImageJ software (ver. 1.46; National Institutes of
Health).
Bioinformatics analyses
Kyoto Encyclopedia of Genes and Genomes (KEGG)
pathway enrichment analysis was performed on differentially
expressed genes using the enrichKEGG function from the
clusterProfiler package in R software (ver. 3.20; http://www.genome.jp/kegg/kegg_ja.html).
The result of 25 pathways were visualized using the Pathview
package (ver. 3.20, R Core Team, 2024) (16). Gene Ontology (GO) analysis was
performed on genes that were increased or decreased by >2-fold
in the HOTTIP KO sample compared with the WT sample. The analysis
was conducted using the gseGO function in the clusterProfiler
package (ver. 4.14.3) in R software (ver. 4.4.1, R Core Team,
2024), based on the Gene Set Enrichment Analysis approach
(https://www.gsea-msigdb.org/gsea/index.jsp). GO terms
which had adjusted P-value <0.01 were considered markedly
regulated GO. Up- or downregulated GO terms were featured by R
software. Heatmaps and volcano plots were generated using TBtools
(v 1.055; http://github.com/CJ-Chen/Tbtools/releases). Venn
diagrams showing the intersection between target genes identified
in microRNA databases (Targets can. http://www.targetscan.org/) and Ferroptosis Marker
(FerrDb v2 database. http://www.zhounan.org/). The online bioinformatics
programs, miRbase (https://www.mirbase.org/), Targets can and RNAhybrid
(https://bibiserv) were applied to predict the binding
site of miR-214-3p with GPX4.
Statistical analysis
Data were shown as mean ± SD from at least three
independent experiments. Data were analyzed by two-tailed unpaired
Student's t-test between two groups and by one-way ANOVA followed
by the Tukey post hoc test for multiple comparisons, Bonferroni
correction was applied where appropriate. Statistical analysis was
carried out using GraphPad Prism software version 8 (Dotmatics).
P<0.05 was considered to indicate a statistically significant
difference.
Results
HOTTIP may be a potential regulator of
ferroptosis
HOTTIP has been reported to serve as an oncogene in
OS and the emergence of ferroptosis provides a novel opportunity to
clarify the underlying mechanism. To clarify the relationship
between HOTTIP and ferroptosis, the transcriptome alternation
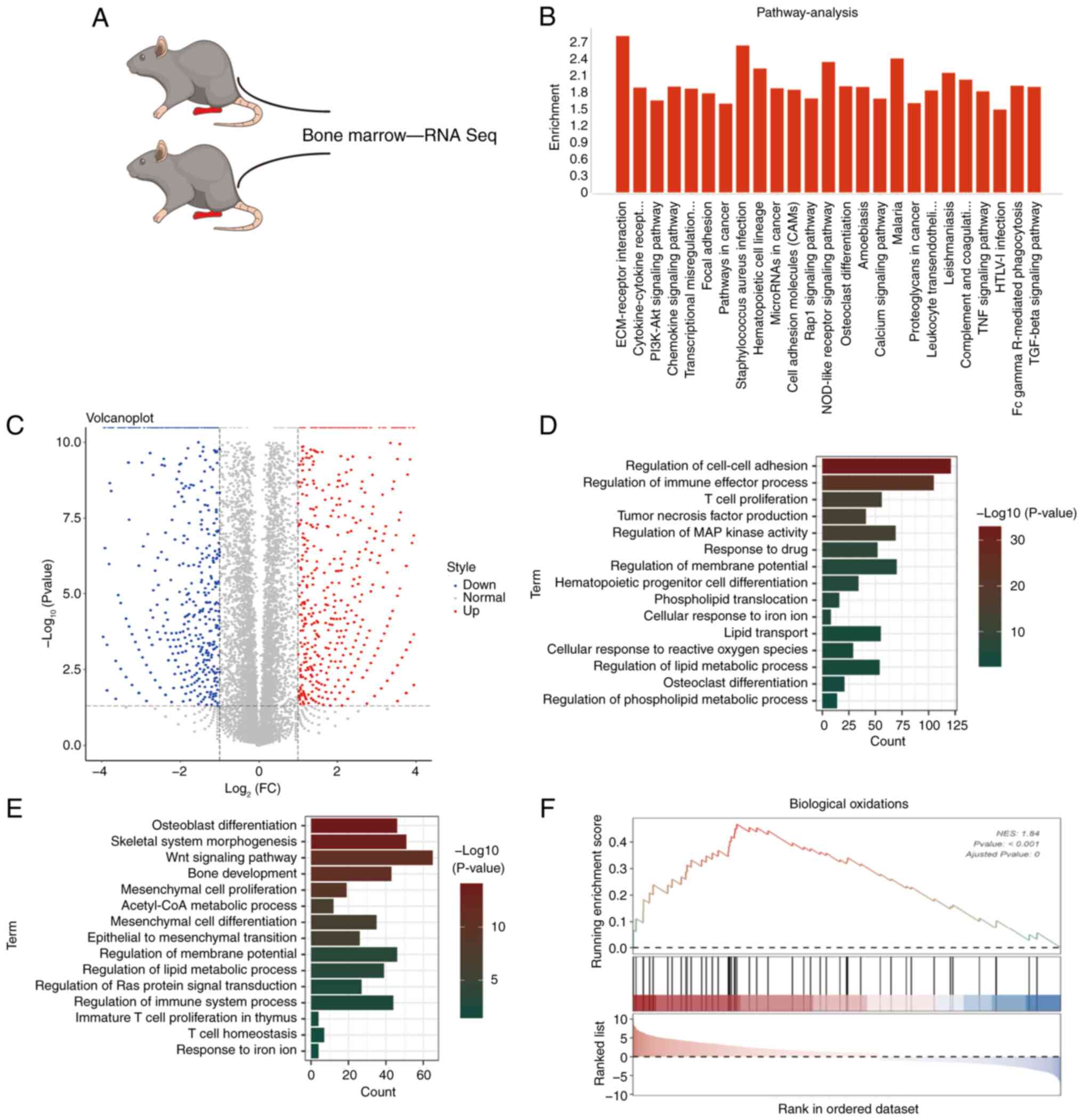
between bone marrow derived from KO and wild-type (WT) mice was
analyzed (Fig. 1A). The
RNA-sequencing data indicated that the differently expressed gene
mainly focused on 25 signaling pathways using the KEGG database
(Fig. 1B). According to their
relative expression level, the changed genes were divided into
upregulation and downregulation groups (Fig. 1C). Gene ontology (GO) analysis
showed a number of biological processes (BPs) were abnormally
influenced such as lipid transport, cellular response to iron ion,
cellular response to reactive oxygen species and regulation of
lipid metabolic process (Fig. 1D and
E). Furthermore, the related ferroptosis genes were predicted
from Gene Set Enrichment Analysis (GSEA) website and it was found
that Biological Oxidations gene set was clearly upregulated
(Fig. 1F). Considering that OS is a
type of primary malignant bone tumor and most OS come from bone
marrow (17), it was therefore
hypothesized that HOTTIP may be involved in ferroptosis of OS.
HOTTIP actively participates in the
Erastin-induced ferroptosis of OS cells
To determine the involvement of HOTTIP in
ferroptosis, the present study successfully established a
ferroptotic cell death model with OS cells. As shown in Fig. 2A and B, the cell viability was
markedly suppressed after respectively treated with the canonical
inducers of ferroptosis Erastin or RSL3. The potent ferroptosis
inhibitor ferrostatin-1 (Fer-1) partially reversed these
suppressive effects, whereas the apoptosis inhibitor Z-VAD-FMK and
necroptosis inhibitor necrostatin-1 (Necro) appeared to possess no
rescue abilities in Erastin or RSL3 treated OS cells (Fig. 2A and B). The colony formation assay
also confirmed that Erastin clearly inhibited the numbers and sizes
of colonies and Fer-1 markedly attenuated this suppressive effect
(Fig. 2C). As a new programmed cell
death, reactive oxygen species (ROS) and iron accumulation are two
important characters of ferroptosis (18). The level of intracellular iron was
determined using the Iron assay kit and the accumulation of total
Fe was promoted by Erastin while Fer-1 markedly rescued this
promotion (Fig. 2D and E). The flow
cytometry assays were used to detect the intracellular ROS and the
results displayed that ROS signal was increased by Erastin and this
increase was rescued by Fer-1 in OS cells (Fig. 2F and G). GSH depletion and MDA
accumulation are essential for ferroptosis. As expected, the
intracellular level of MDA was increased whereas GSH level was
suppressed by Erastin in MG63 and U2OS cells (Fig. 2H-K). These results indicated that
Erastin successfully triggered the ferroptosis in OS cells,
indicating that the ferroptotic cell death model was successfully
constructed. Using this cell model, it was found that HOTTIP was
markedly suppressed in Erastin-treated OS cells (Fig. 2L), suggesting that HOTTIP may
participate in ferroptosis of OS cells.
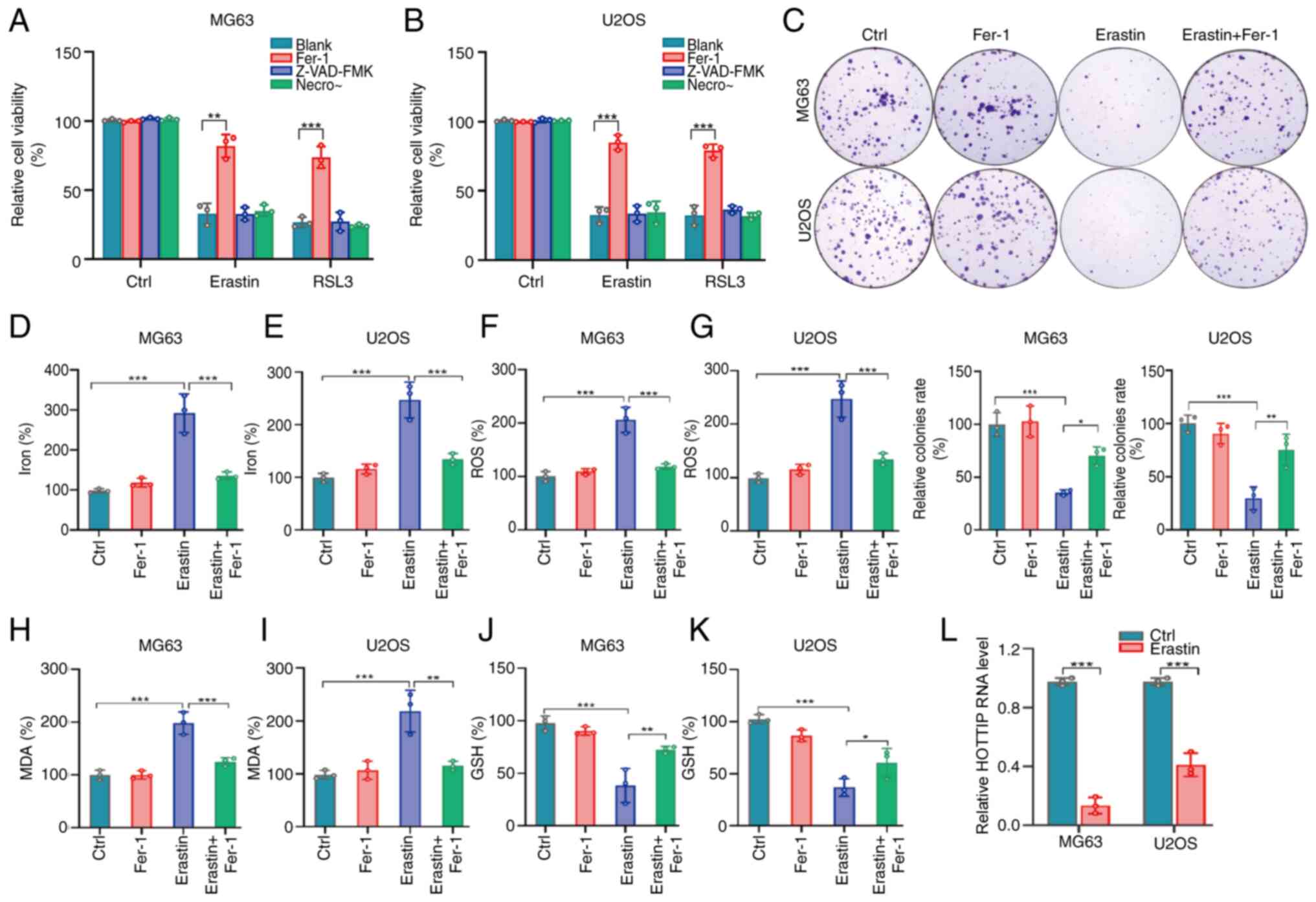 | Figure 2.HOTTIP actively participates in the
Erastin-induced ferroptosis of OS cells. (A) MG63 and (B) U2OS
cells were treated with Erastin (10 µM) or RSL3 (1 µM) in the
absence or presence of 2 µM Fer-1, 10 µM Z-VAD-FMK, or 1 µM Necro
for 24 h, the relative cell viability was measured. (C) MG63 and
U2OS cells were treated with 10 µM Erastin for 14 days and the
colony formation was examined. MG63 and U2OS cells were treated
with Erastin for 24 h, the levels of (D and E) iron, (F and G)
lipid ROS, (H and I) MDA and (J and K) GSH were determined by
commercial kits. (L) MG63 and U2OS cells were treated with Erastin
for 24 h and HOTTIP expression was monitored by quantitative PCR.
*P<0.05; **P<0.01; ***P<0.001. HOTTIP, HOXA transcript at
the distal tip; OS, osteosarcoma; Fer-1, ferrostatin-1; Necro,
necrosulfonamide; ROS, reactive oxygen species; MDA,
malondialdehyde; GSH, glutathione. |
HOTTIP partly suppresses ferroptosis
in OS cells
To identify the real function of HOTTIP in
ferroptosis, HOTTIP-overexpressing stable OS cells were established
(Fig. S1A). According to cell
viability assays, ectopic expression of HOTTIP clearly attenuated
the suppressive cell viability triggered by Erastin or RSL3
(Fig. 3A). The accumulation of
intracellular iron (Fig. 3B),
intracellular ROS signal (Fig. 3C)
and the expression level of MDA (Fig.
3D) were all suppressed while the GSH expression was promoted
(Fig. 3E) in this
HOTTIP-overexpressing cells. The canonical markers of ferroptosis
including xCT and GPX4 were markedly promoted by HOTTIP
overexpression in OS cells (Fig.
3F-H). On the other hand, the HOTTIP silencing stable OS cells
were developed (Fig. S1B) and it
was showed that silencing of HOTTIP markedly enhanced the
suppressive effects of the Erastin or RSL3 on cell viability
(Fig. 3I). Fer-1 successfully
reversed this suppressed cell viability in OS cells. Further
investigation showed that the accumulation of intracellular iron,
intracellular ROS signal and the expression level of MDA were
clearly promoted by HOTTIP knockdown (Fig. 3J-L). Conversely, GSH expression was
markedly suppressed in HOTTIP silenced OS cells (Fig. 3M). The expression of xCT and GPX4
was suppressed in HOTTIP silenced OS cells with Erastin or RSL3
treatment (Fig. 3N-P). All these
results revealed that HOTTIP inhibited the ferroptosis in OS
cells.
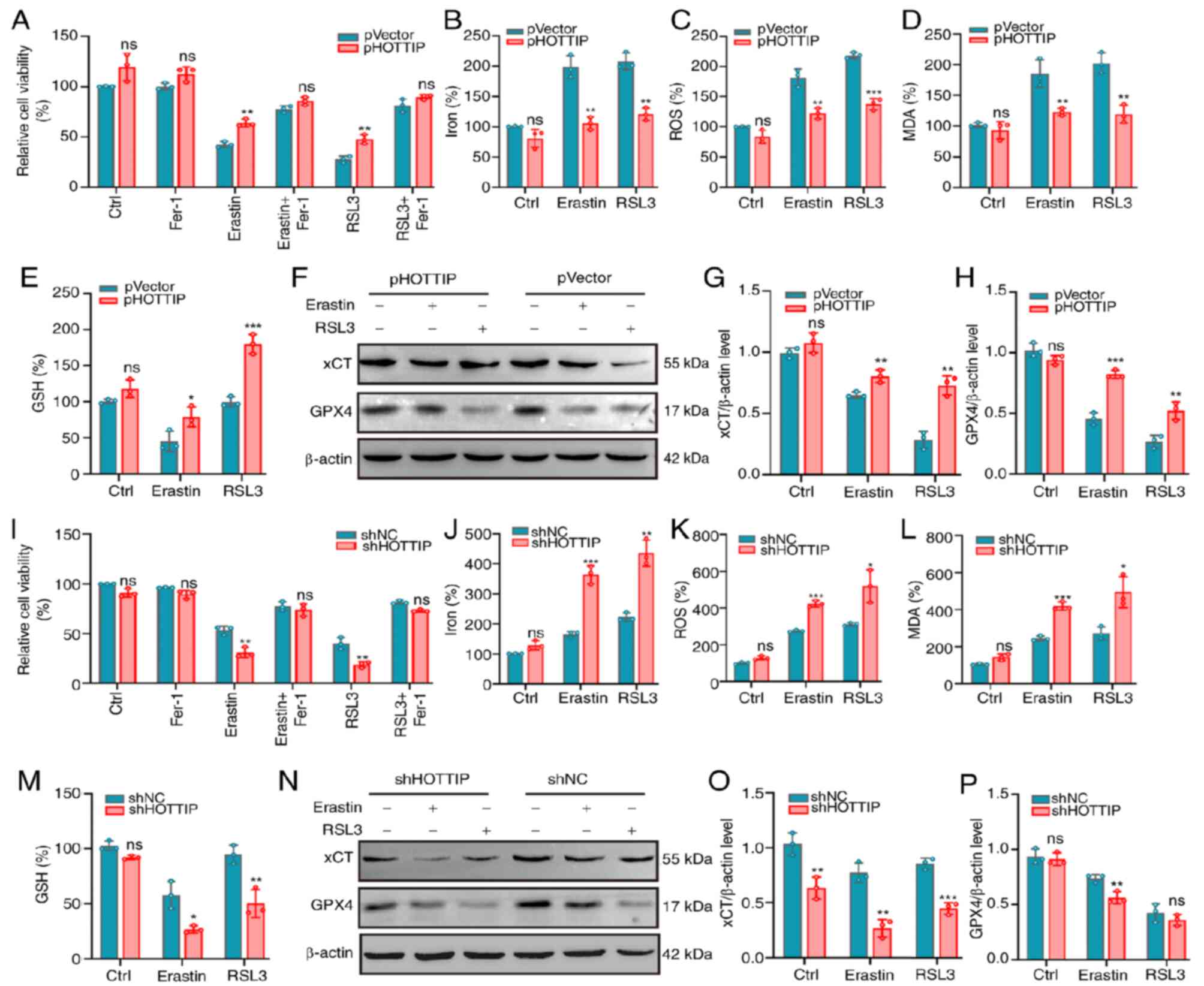 | Figure 3.HOTTIP may suppress ferroptosis in OS
cells. (A) PHOTTIP or pVector infected MG63 cells were treated with
10 µM Erastin or 1 µM RSL3 in the absence or presence of 2 µM Fer-1
for 24 h and the relative cell viability was measured. PHOTTIP or
pVector infected cells were treated with Erastin or RSL3 for 24 h,
the levels of (B) iron, (C) lipid ROS, (D) MDA and (E) GSH were
determined by commercial kits. (F-H) The ferroptosis relevant
proteins expressions of xCT and GPX4 were examined by western
blotting. β-actin served as a loading control. (I) The shHOTTIP or
shNC infected U2OS cells were treated with 10 µM Erastin or 1 µM
RSL3 in the absence or presence of 2 µM Fer-1 for 24 h, the
relative cell viability was measured. (J-M) The infected cells were
treated with Erastin or RSL3 for 24 h, the levels of Iron (J),
lipid ROS (K), MDA (L) and GSH (M) were determined by commercial
kits; (N-P) The ferroptosis relevant proteins expressions of xCT
and GPX4 were examined by western blotting. β-actin served as a
loading control. *P<0.05; **P<0.01; ***P<0.001; ns: not
significant. HOTTIP, HOXA transcript at the distal tip; OS,
osteosarcoma; Fer-1, ferrostatin-1; ROS, reactive oxygen species;
MDA, malondialdehyde; GSH, glutathione; xCT, X cystine/glutamate
antiporter; GPX4, glutathione peroxidase 4; sh, short hairpin; NC,
negative control. |
Knockdown of HOTTIP clearly promotes
ferroptosis in vivo
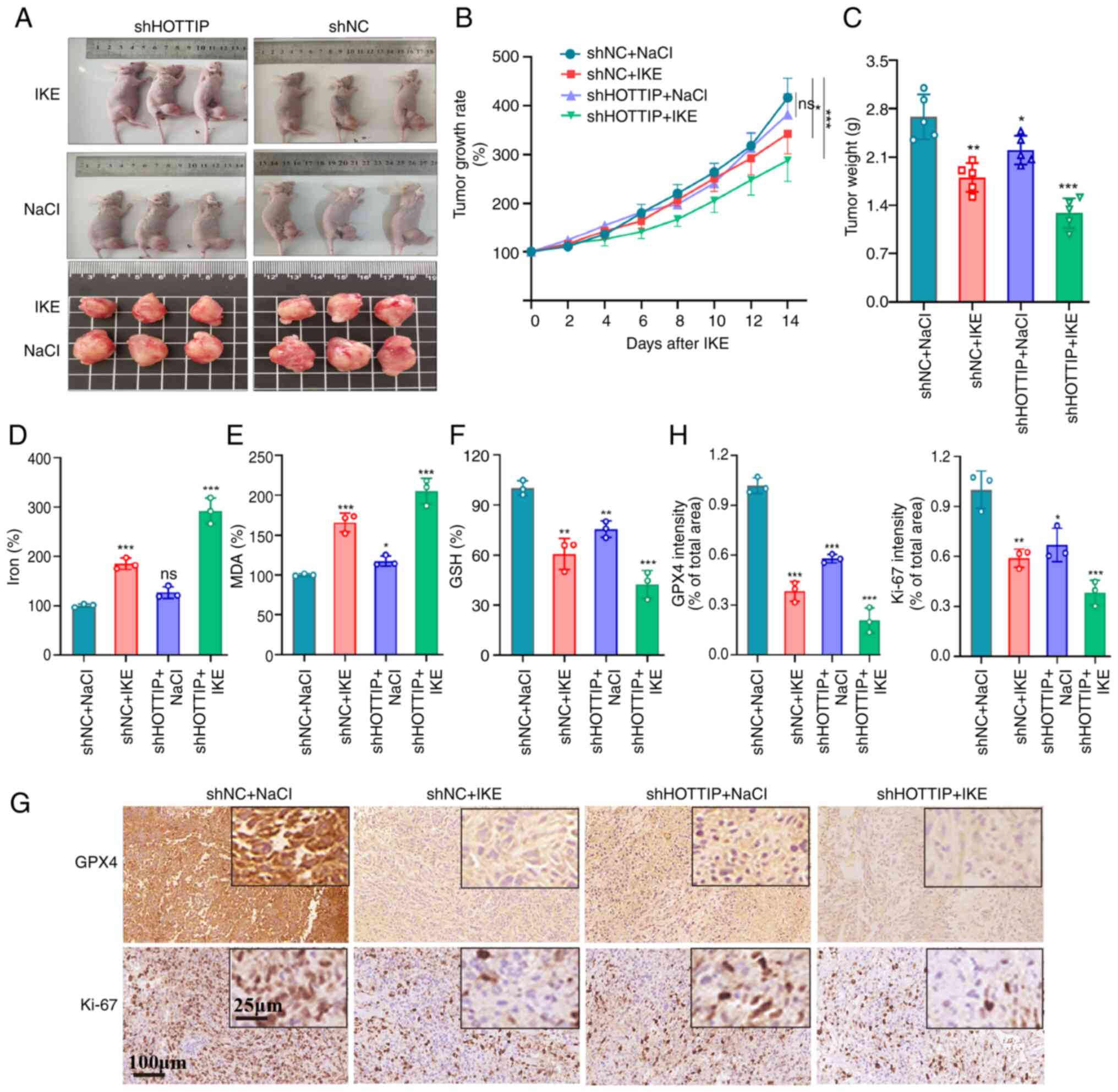
To further confirm the in vivo function of
HOTTIP in ferroptosis, an orthotopic intra-tibia tumor-bearing
model was established using shHOTTIP infected OS cells. A canonical
in vivo ferroptosis inducer, imidazole ketone erastin (IKE),
was given intraperitoneally (i.p.) with 20 mg/kg once every other
day. As expected, the IKE groups carried smaller burden when
compared with control (NaCl) group and the shHOTTIP group displayed
smaller tumor when compared with shNC group (Fig. 4A). Similar results were observed in
tumor growth rate (Fig. 4B) and
weight (Fig. 4C). The levels of Fe,
MDA and GSH were examined in animal tissues and the similar results
to the in vitro investigation were recorded (Fig. 4D-F). Further immunohistochemical
staining indicated that the expressions of Ki-67 and GPX4 were
markedly suppressed by IKE treatment and shHOTTOP group exhibited
more sensitive to IKE treatment (Fig.
4G and H). These data suggested that HOTTIP knockdown
facilitates the ferroptosis-based anti-tumor therapy in OS.
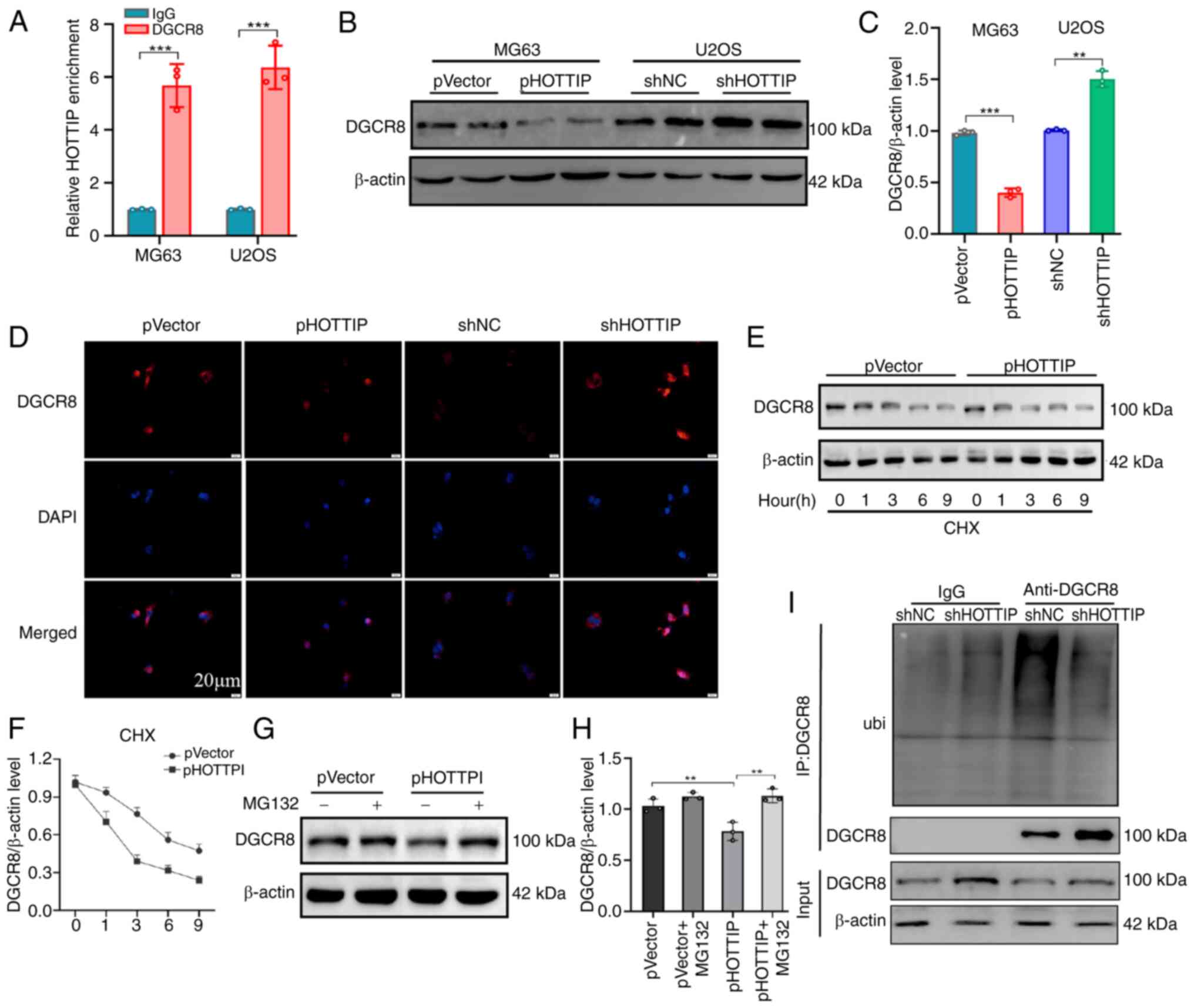
HOTTIP recruits DGCR8 and influences
its protein stability
LncRNAs are recognized to exert their function
through physically interacting with RNA binding proteins. DGCR8, a
known RNA-binding protein, was predicted to directly bind HOTTIP
and the RIP assay validated that DGCR8 successfully pulled down
HOTTIP RNA transcript (Fig. 5A).
Subsequent study demonstrated that overexpression of HOTTIP
suppressed while its knockdown promoted DGCR8 expression at protein
level (Fig. 5B and C). The
immunofluorescence examination also confirmed that the expression
of DGCR8 was suppressed by HOTTIP overexpression whereas it was
promoted by HOTTIP knockdown (Fig.
5D). However, the mRNA expression level of DGCR8 was
inconsistent with HOTTIP overexpression or knockdown (Fig. S2). It was therefore hypothesized
that HOTTIP mediated DGCR8 expression via a post-transcriptional
manner. In order to validate this hypothesis, cycloheximide (CHX)
chase assays were conducted to monitor the stability of DGCR8
protein. A shorter half-life was observed in the
HOTTIP-overexpressing OS cells (Fig. 5E
and F). Given that the ubiquitin-proteasome pathway is the most
significant signaling to mediate the protein stability (19), an inhibitor of proteasome MG-132 was
added in the HOTTIP-overexpressing OS cells to examine the protein
stability of DGCR8. As shown in Fig. 5G
and H, MG-132 treatment markedly reversed the suppressive
effect of DGCR8 driven by HOTTIP overexpression. Next DGCR8
antibody was utilized to pull down endogenous DGCR8 proteins and
their modification examined by an ubiquitin antibody. Based on the
results of Co-IP assays, the decreased DGCR8 ubiquitination was
detected in HOTTIP silencing cells (Fig. 5I). This result proposed that
destabilization of DGCR8 mediated by HOTTIP may depend on the
proteasomal degradation. Collectively, the data demonstrated that
HOTTIP physically interacted with DGCR8 and modulated its stability
by promoting its ubiquitin-mediated degradation.
HOTTIP influences miR-214-3p
biogenesis and suppresses ferroptosis by blocking miR-214-3p
expression
Notably, DGCR8 is the RNA-binding partner of the
nuclease Drosha. The DGCR8/Drosha complex named by the
microprocessor (recognized and cleaved pri-miRNAs) is essential for
the processing of primary (pri-)miRNAs in the nucleus (20,21).
To identify which miRNA biogenesis was influenced in the present
study, miRNA sequencing was conducted using HOTTIP silencing OS
cells. As shown in Fig. 6A, among
the 10 promising candidates, hsa-miR-214-3p was the most
upregulated. Subsequent relevant assays were conducted in the
miR-214-3p transfected MG63 cell lines (Fig. S3A), cell viability examination
showed that miR-214-3p mimics markedly inhibited cell viability
while Fer-1 alleviated this suppressive ability (Fig. 6B). Further investigation of Fe
accumulation (Fig. 6C), ROS signal
(Fig. 6D) and MDA expression level
(Fig. 6E) showed that they were
promoted by this miR-214-3p mimics and Fer-1 treatment successfully
reversed this promotional effects. In term of GSH expression, the
converse results were observed in miR-214-3p transfected cells and
partially rescued by Fer-1 treatment (Fig. 6F).
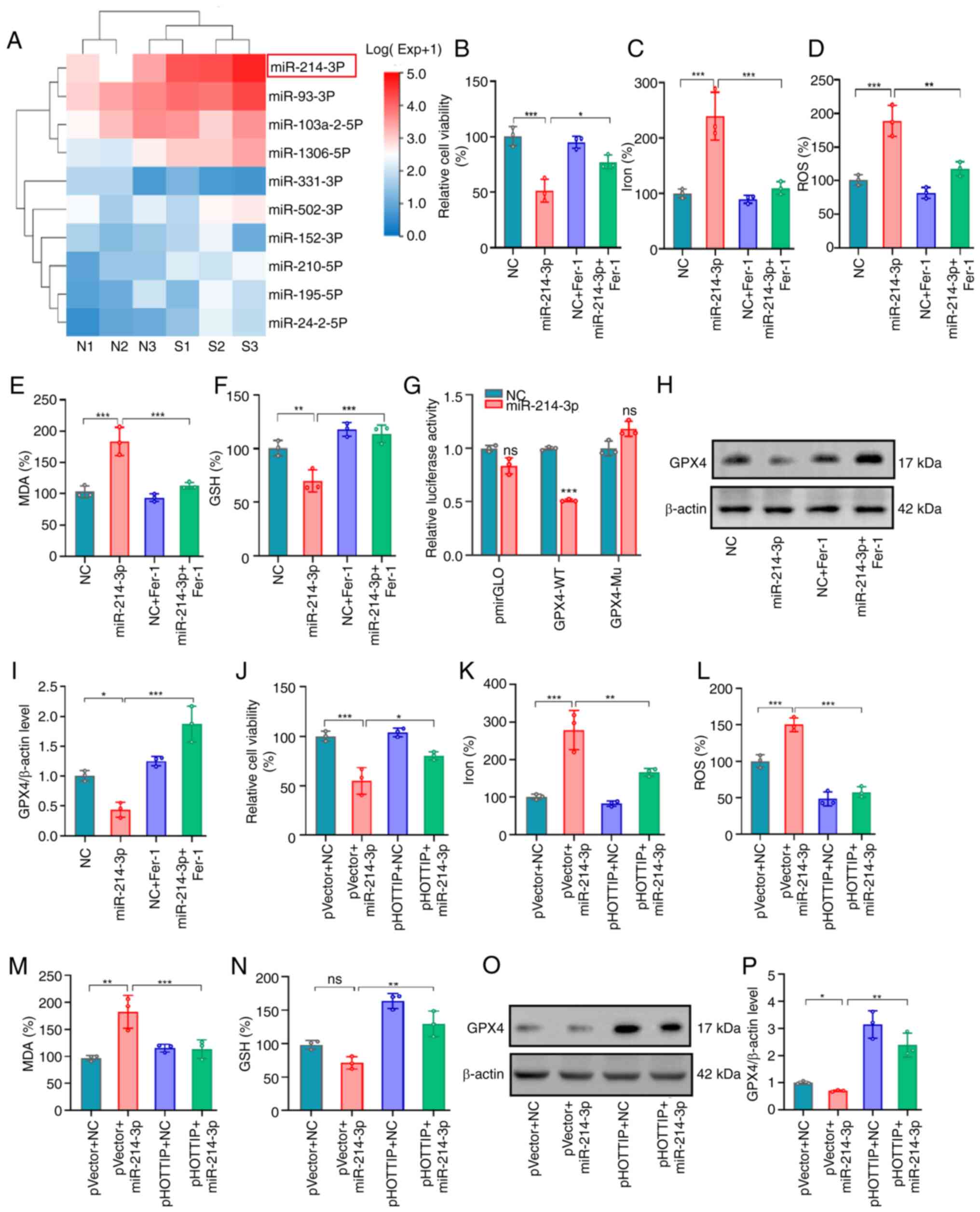 | Figure 6.HOTTIP influences miR-214-3p
biogenesis to suppress ferroptosis. (A) The miRNA sequencing was
conducted using HOTTIP silencing OS cells. (B) Relative cell
viability, (C) total iron, (D) lipid ROS, (E) MDA and (F) GSH
expression levels were examined in the miR-214-3p transfected OS
cells. (G) The interaction between miR-214-3p and GPX4 was
determined using luciferase reporter assay. (H and I) GPX4 protein
expression was examined in the miR-214-3p transfected OS cells.
miR-214-3p mimics were transfected into the HOTTIP overexpressing
cells and the (J) relative cell viability, (K) total iron, (L)
lipid ROS, (M) MDA and (N) GSH expression levels were measured with
or without Fer-1 treatment. (O and P) GPX4 protein expression
levels were also examined in the aforementioned described cells.
*P<0.05; **P<0.01; ***P<0.001; ns: not significant.
HOTTIP, HOXA transcript at the distal tip; miRNA, micro RNA; OS,
osteosarcoma; ROS, reactive oxygen species; MDA, malondialdehyde;
GSH, glutathione; Fer-1, ferrostatin-1; GPX4, glutathione
peroxidase 4. |
It is known that miRNAs trigger their function via
suppressing their targets expression. To find the targets of
miR-214-3p, an online program was searched to predict the potential
candidates and GPX4 was identified as the most promising one
(Fig. S4). Using the luciferase
activity assays, it was found that miR-214-3p mimics markedly
suppressed the luciferase activity of the GPX4-3′UTR-WT reporter
but the mutagenesis of these binding sites successfully abolished
this suppressive effect (Fig. 6G).
Further investigation showed that miR-214-3p mimics suppressed GPX4
expression while the suppressive effects were partially reversed by
Fer-1 (Fig. 6H and I).
To discover whether miR-214-3p participated in the
HOTTIP mediated ferroptosis, ectopic miR-214-3p mimics were applied
in the HOTTIP overexpressing cells to examine the rescue effects on
ferroptosis (Fig. S3B). The cell
viability examination revealed that ectopic expression of
miR-214-3p successfully inhibited the viability in OS cells and
HOTTIP overexpression reversed this suppressive effect (Fig. 6J). The similar rescued effects of
miR-214-3p mimics on Fe accumulation (Fig. 6K), ROS signal (Fig. 6L), MDA (Fig. 6M) and GSH expression (Fig. 6N) and GPX4 expression (Fig. 6O and P) were also detected in the
HOTTIP overexpressing OS cells.
Discussion
OS is a highly malignant tumor due to
chemoresistance and the tendency of recurrence following surgery,
and the prognosis of patients is not optimistic (22). Therefore, there is an urgent need
for novel and effective therapeutics, ferroptosis has also been
considered as a promising strategy for OS treatment (23,24).
As a new type of iron-dependent programmed cell death, ferroptosis
is characterized by iron-dependent lipid peroxidation (25). The results of the transcriptome
analysis indicated that the aforementioned biological processes
were influenced in bone marrow derived from HOTTIP KO mice,
suggesting that HOTTIP may be a potential mediator of ferroptosis.
It was therefore hypothesized that HOTTIP might be a potential
target for OS treatment possibly via suppressing ferroptosis
process.
Increasing evidence has revealed that lncRNAs could
serve as an independent regulator for ferroptosis to mediate
tumorigenesis in multiple cancers. For example, HOTTIP was
identified as an oncogene to facilitate cell proliferation,
invasion and migration in osteosarcoma by interaction with PTBP1 to
promote KHSRP level (26). It also
promoted epithelial-mesenchymal transition via a positive feedback
loop with c-Myc and contributed to chemoresistance by activating
the Wnt/β-catenin pathway in OS cells (27,28).
However, whether ferroptosis participates in the HOTTIP mediated
osteosarcoma remains unknown. The present study showed that HOTTIP
was markedly suppressed in OS cells during the Erastin-induced
ferroptosis process and HOTTIP overexpression suppressed, while its
knockdown promoted, ferroptosis in OS cells. To the best of the
authors' knowledge, this is the first report that HOTTIP functions
as a negative regulator of ferroptosis in OS.
It has been recognized that lncRNAs usually trigger
their functions by physically interacting with RNA binding proteins
(29,30). HOTTIP is reported to interact with
WDR5 to promote osteogenic differentiation (31). Furthermore, it also directly
recruits CTCF/cohesin complex to form R-loops to drive oncogene
transcription and leukemia development (32). To find the new direct targets, the
present study used bioinformatics analyses to predict the potential
binding protein and DGCR8 was the most promising one. The RIP
experiment validated the direct interaction between DGCR8 and
HOTTIP. Further investigations uncovered that HOTTIP modulated the
protein stability of DGCR8 by promoting its ubiquitin-mediated
degradation. Although the present study explored the role of HOTTIP
in mediating the ubiquitination of DGCR8 in OS cells, the more
detailed mechanism needs to be further investigated in the
future.
DGCR8 is a member of DGCR8/Drosha dimerization,
which recruits and cleavages pri-miRNA to generate pre-miRNA and
pre-miRNA is further processed and cut by Dicer to form mature
miRNA. DGCR8 has been reported to suppress migration and invasion
by facilitating miR-205/200b maturation in HCC cells (33). Similarly, the formation of
DDX5/Drosha/DGCR8 complex promotes miRNA-10b processing, which is
involved in mammary tumorigenesis and progression (34). Therefore, it was hypothesized that
HOTTIP influences pri-microRNA microprocessor by regulating DGCR8
expression in osteosarcoma. To examine which miRNA biogenesis was
influenced, miRNA sequencing was conducted using HOTTIP silencing
OS cells. In the present study, miR-214-3p was markedly upregulated
by HOTTIP silencing. However, the role of miR-214-3p in
tumorigenesis is confusing. miR-214-3p is reported to serve as an
oncogene via promoting OS cell proliferation, invasion and
migration (35–38). By contrast, it is also demonstrated
to suppress cell proliferation and tumor growth in OS by targeting
FNDC5 and KCNC4 (39,40). In the terms of ferroptosis,
miR-214-3p was found to aggravate ferroptosis by targeting GPX4 in
liver cancer, acute kidney injury (41) and cardiovascular diseases (42). The dual function may be related to
the diversity of target genes, differences in cellular environment
and signaling pathways, the complex relationship between tumor
proliferation and inhibition, differences in research background
and experimental conditions and potential feedback regulatory
mechanisms. For example, in hypoxic core areas of tumor, increased
ROS levels may make miR-214-3p more inclined to activate
ferroptosis. In the front of oxygen-rich invasion, it may
preferentially target pro-transfer genes. The present study showed
that miR-214-3p mimics promoted ferroptosis in OS cells by
targeting GPX4 while ferroptosis inhibitor Fer-1 reversely
attenuated this cell death, suggesting that miR-214-3p may act as a
ferroptosis stimulator to suppress OS tumorgenesis. Further rescue
experiments validated that the ectopic expression of HOTTIP
successfully reversed the ferroptosis induced by miR-214-3p. Based
on these results, it was concluded that HOTTIP suppressed
ferroptosis in OS, at least partly, through mediating
miR-214-3p/GPX4 regulatory axis. Notably, recent
clinicopathological studies highlight the prognostic relevance of
cancer-testis antigens, including NY-ESO-1 and MAGE-A4, in
osteosarcoma (43,44), suggesting that multi-omics
approaches integrating lncRNA and CTA networks may uncover novel
therapeutic vulnerabilities. Future studies should also evaluate
whether HOTTIP silencing potentiates the efficacy of conventional
chemotherapeutics, such as cisplatin, by further destabilizing
redox homeostasis in osteosarcoma cells.
The present study has some limitations. First, as a
critical module in miRNA biogenesis, DGCR8 also probably regulates
other miRNAs, such as miR-93-3p and miR-103a-2-5p, which might be
involve in HOTTIP mediated ferroptosis suppression and still need
further research. Second, there are difficulties in delivering
HOTTIP-targeting therapies, including the need for efficient
delivery systems and the potential for off-target effects. The
importance of further research to develop specific and effective
HOTTIP inhibitors should also be acknowledge. Third, only
ferroptosis studies were used here, the metastasis mechanism of OS,
which involved in local invasion and early metastasis in clinical
patients was not investigated.
In conclusion, the present study uncovered a novel
function of HOTTIP in inhibiting ferroptosis of OS cells. It
discovered that HOTTIP directly recruited DGCR8 and promoted its
ubiquitin-mediated degradation to disrupt the miRNA-214-3p/GPX4
regulatory axis. The findings gained from the present study
indicated that silencing of HOTTIP sensitized OS cells to
ferroptosis-mediated strategy, suggesting that the inhibiting
HOTTIP may be developed as an effective intervention for OS
patients in clinical practice.
Supplementary Material
Supporting Data
Acknowledgements
Human OS cell lines including MG63 and U2OS were
obtained from Lingnan Medical Research Center, Guangzhou University
of Chinese Medicine (Guangzhou, China). The HOTTIP overexpression
or knockdown lentivirus vector kindly provided by Dr Wei-ming Fu of
Pharmaceutical Sciences, Southern Medical University (Guangzhou,
China).
Funding
This work was supported by the National Natural Science
Foundation of China (grant no. 82272526) and the Sanming Project of
Medicine in Shenzhen (grant no. SZZYSM202311011).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author. The sequence data be found
in the NCBI SRA dataset with the accession number is PRJNA1250258
and the URL https://dataview.ncbi.nlm.nih.gov/object/PRJNA1250258.
Authors' contributions
JFZ conceived and supervised all the experiments.
JFZ and SCD designed the experiments. SCD, CJS and FXP conducted
the experiments. RJW, NL, YXM and STZ provided the technical
support. SCD and CJS analyzed the data. SCD and FXP confirm the
authenticity of all the raw data. JFZ and SCD prepared the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experimental procedures were approved by
the Ethics and Animal Research Committee of Southern Medical
University (Guangzhou, Guangdongl approval no. SMUL2022219).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CHX
|
cycloheximide
|
|
DGCR8
|
DiGeorge Critical Region 8
|
|
Fer-1
|
ferrostatin-1
|
|
GPX4
|
glutathione peroxidase 4
|
|
GSH
|
glutathione
|
|
HOTTIP
|
HOXA transcript at the distal tip
|
|
IKE
|
Imidazole ketone erastin
|
|
MDA
|
malondialdehyde
|
|
miRNA
|
micro RNA
|
|
Necro
|
necrosulfonamide
|
|
OS
|
osteosarcoma
|
|
ROS
|
reactive oxygen species
|
|
xCT
|
X cystine/glutamate antiporter
|
References
|
1
|
Meltzer PS and Helman LJ: New horizons in
the treatment of osteosarcoma. N Engl J Med. 385:2066–2076. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Dixon SJ, Lemberg KM, Lamprecht MR, Skouta
R, Zaitsev EM, Gleason CE, Patel DN, Bauer AJ, Cantley AM, Yang WS,
et al: Ferroptosis: An iron-dependent form of nonapoptotic cell
death. Cell. 149:1060–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jiang X, Stockwell BR and Conrad M:
Ferroptosis: Mechanisms, biology and role in disease. Nat Rev Mol
Cell Biol. 22:266–282. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen X, Kang R, Kroemer G and Tang D:
Broadening horizons: The role of ferroptosis in cancer. Nat Rev
Clin Oncol. 18:280–296. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liang WC, Ren JL, Wong CW, Chan SO, Waye
MMY, Fu WM and Zhang JF: LncRNA-NEF antagonized epithelial to
mesenchymal transition and cancer metastasis via cis-regulating
FOXA2 and inactivating Wnt/β-catenin signaling. Oncogene.
37:1445–1456. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang Y, Zeng X, Wang N, Zhao W, Zhang X,
Teng S, Zhang Y and Lu Z: Long noncoding RNA DANCR, working as a
competitive endogenous RNA, promotes ROCK1-mediated proliferation
and metastasis via decoying of miR-335-5p and miR-1972 in
osteosarcoma. Mol Cancer. 17:892018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhan A, Soleimani M and Mandal SS: Long
noncoding RNA and cancer: A new paradigm. Cancer Res. 77:3965–3981.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han Y, Gao X, Wu N, Jin Y, Zhou H, Wang W,
Liu H, Chu Y, Cao J, Jiang M, et al: Long noncoding RNA LINC00239
inhibits ferroptosis in colorectal cancer by binding to Keap1 to
stabilize Nrf2. Cell Death Dis. 13:7422022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin Z, Song J, Gao Y, Huang S, Dou R,
Zhong P, Huang G, Han L, Zheng J, Zhang X, et al: Hypoxia-induced
HIF-1α/lncRNA-PMAN inhibits ferroptosis by promoting the
cytoplasmic translocation of ELAVL1 in peritoneal dissemination
from gastric cancer. Redox Biol. 52:1023122022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li L, Zhang Y, Gao Y, Hu Y, Wang R, Wang
S, Li Y, He Y and Yuan C: LncSNHG14 promotes nutlin3a resistance by
inhibiting ferroptosis via the miR-206/SLC7A11 axis in osteosarcoma
cells. Cancer Gene Ther. 30:704–715. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu K, Ni JD, Li WZ, Pan BQ, Yang YT, Xia
Q and Huang J: The Sp1/FOXC1/HOTTIP/LATS2/YAP/β-catenin cascade
promotes malignant and metastatic progression of osteosarcoma. Mol
Oncol. 14:2678–2695. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li Z, Zhao L and Wang Q: Overexpression of
long non-coding RNA HOTTIP increases chemoresistance of
osteosarcoma cell by activating the Wnt/β-catenin pathway. Am J
Transl Res. 8:2385–2393. 2016.PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shi CJ, Lv MY, Deng LQ, Zeng WQ, Fu WM and
Zhang JF: Linc-ROR drive adriamycin resistance by targeting
AP-2α/Wnt/β-catenin axis in hepatocellular carcinoma. Cell Biol
Toxicol. 39:1735–1752. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shao J, Shi CJ, Li Y, Zhang FW, Pan FF, Fu
WM and Zhang JF: LincROR mediates the suppressive effects of
curcumin on hepatocellular carcinoma through inactivating
Wnt/β-catenin signaling. Front Pharmacol. 11:8472020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Draghici S, Khatri P, Tarca AL, Amin K,
Done A, Voichita C, Georgescu C and Romero R: A systems biology
approach for pathway level analysis. Genome Res. 17:1537–1545.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chang X, Ma Z, Zhu G, Lu Y and Yang J: New
perspective into mesenchymal stem cells: Molecular mechanisms
regulating osteosarcoma. J Bone Oncol. 29:1003722021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu J, Song X, Kuang F, Zhang Q, Xie Y,
Kang R, Kroemer G and Tang D: NUPR1 is a critical repressor of
ferroptosis. Nat Commun. 12:6472021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang Y, Yan D, Liu JB, Tang DL and Chen X:
Protein modification and degradation in ferroptosis. Redox Biol.
75:1032592024. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Weitz SH, Gong M, Barr I, Weiss S and Guo
F: Processing of microRNA primary transcripts requires heme in
mammalian cells. Proc Natl Acad Sci USA. 111:1861–1866. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Partin AC, Ngo TD, Herrell E, Jeong BC,
Hon G and Nam Y: Heme enables proper positioning of Drosha and
DGCR8 on primary microRNAs. Nat Commun. 8:17372017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hattinger CM, Patrizio MP, Fantoni L,
Casotti C, Riganti C and Serra M: Drug resistance in osteosarcoma:
Emerging biomarkers, therapeutic targets and treatment strategies.
Cancers (Basel). 13:28782021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lei T, Qian H, Lei P and Hu Y:
Ferroptosis-related gene signature associates with immunity and
predicts prognosis accurately in patients with osteosarcoma. Cancer
Sci. 112:4785–4798. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wang X, Xia G, Xiao S, Wu S, Zhang L,
Huang J, Zhang W and Cao X: A ferroptosis-related gene signature
associated with immune landscape and therapeutic response in
osteosarcoma. Front Oncol. 12:10249152022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Li J, Kang R, Klionsky DJ and Tang
D: Ferroptosis: Machinery and regulation. Autophagy. 17:2054–2081.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yao XY, Liu JF, Luo Y, Xu XZ and Bu J:
LncRNA HOTTIP facilitates cell proliferation, invasion, and
migration in osteosarcoma by interaction with PTBP1 to promote
KHSRP level. Cell Cycle. 20:283–297. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liao B, Chen R, Lin F, Mai A, Chen J, Li
H, Xu Z and Dong S: Long noncoding RNA HOTTIP promotes endothelial
cell proliferation and migration via activation of the
Wnt/β-catenin pathway. J Cell Biochem. 119:2797–2805. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang Y and Ji F: lncRNA HOTTIP facilitates
osteosarcoma cell migration, invasion and epithelial-mesenchymal
transition by forming a positive feedback loop with c-Myc. Oncol
Lett. 18:1649–1656. 2019.PubMed/NCBI
|
|
29
|
Herman AB, Tsitsipatis D and Gorospe M:
Integrated lncRNA function upon genomic and epigenomic regulation.
Mol Cell. 82:2252–2266. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bridges MC, Daulagala AC and Kourtidis A:
LNCcation: lncRNA localization and function. J Cell Biol.
220:e2020090452021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Liu R, Li Z, Song E, Hu P, Yang Q, Hu Y,
Liu H and Jin A: LncRNA HOTTIP enhances human osteogenic BMSCs
differentiation via interaction with WDR5 and activation of
Wnt/β-catenin signalling pathway. Biochem Biophys Res Commun.
524:1037–1043. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo H, Zhu G, Eshelman MA, Fung TK, Lai Q,
Wang F, Zeisig BB, Lesperance J, Ma X, Chen S, et al:
HOTTIP-dependent R-loop formation regulates CTCF boundary activity
and TAD integrity in leukemia. Mol Cell. 82:833–851.e11. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Liu X, Chen D, Chen H, Wang W, Liu Y, Wang
Y, Duan C, Ning Z, Guo X, Otkur W, et al: YB1 regulates
miR-205/200b-ZEB1 axis by inhibiting microRNA maturation in
hepatocellular carcinoma. Cancer Commun (Lond). 41:576–595. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Li Y, Xing Y, Wang X, Hu B, Zhao X, Zhang
H, Han F, Geng N, Wang F, Li Y, et al: PAK5 promotes RNA helicase
DDX5 sumoylation and miRNA-10b processing in a kinase-dependent
manner in breast cancer. Cell Rep. 37:1101272021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu S, Chang J, Ruan H, Zhi W, Wang X, Zhao
F, Ma X, Sun X, Liang Q, Xu H, et al: Cantharidin inhibits
osteosarcoma proliferation and metastasis by directly targeting
miR-214-3p/DKK3 axis to inactivate β-catenin nuclear translocation
and LEF1 translation. Int J Biol Sci. 17:2504–2522. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao K, Yin J and Dong J: Deregulated WWOX
is involved in a negative feedback loop with microRNA-214-3p in
osteosarcoma. Int J Mol Med. 38:1850–1856. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Cai H, Miao M and Wang Z: miR-214-3p
promotes the proliferation, migration and invasion of osteosarcoma
cells by targeting CADM1. Oncol Lett. 16:2620–2628. 2018.PubMed/NCBI
|
|
38
|
Zhao X, Wang Q, Lin F, Wang X, Wang Y,
Wang J and Wang C: RNA sequencing of osteosarcoma gene expression
profile revealed that miR-214-3p facilitates osteosarcoma cell
proliferation via Targeting ubiquinol-cytochrome c reductase core
protein 1 (UQCRC1). Med Sci Monit. 25:4982–4991. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Yao X, Wu L, Gu Z and Li J: LINC01535
promotes the development of osteosarcoma through modulating
miR-214-3p/KCNC4 axis. Cancer Manag Res. 12:5575–5585. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cheng G, Xu D, Chu K, Cao Z, Sun X and
Yang Y: The effects of MiR-214-3p and Irisin/FNDC5 on the
biological behavior of osteosarcoma cells. Cancer Biother
Radiopharm. 35:92–100. 2020.PubMed/NCBI
|
|
41
|
Zhou J, Xiao C, Zheng S, Wang Q, Zhu H,
Zhang Y and Wang R: MicroRNA-214-3p aggravates ferroptosis by
targeting GPX4 in cisplatin-induced acute kidney injury. Cell
Stress Chaperones. 27:325–336. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu F, Jiang LJ, Zhang YX, Xu ST, Liu SL,
Ye JT and Liu PQ: Inhibition of miR-214-3p attenuates ferroptosis
in myocardial infarction via regulating ME2. Biochem Biophys Res
Commun. 661:64–74. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hashimoto K, Nishimura S, Ito T, Oka N,
Kakinoki R and Akagi M: Clinicopathological assessment of
cancer/testis antigens NY-ESO-1 and MAGE-A4 in osteosarcoma. Eur J
Histochem. 66:33772022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chen A, Qiu Y, Yen YT, Wang C, Wang X, Li
C, Wei Z, Li L, Yu L, Liu F and Li R: Expression of cancer-testis
antigens MAGE-A1, MAGE-A4, NY-ESO-1 and PRAME in bone and soft
tissue sarcomas: The experience from a single center in China.
Cancer Med. 14:e707502025. View Article : Google Scholar : PubMed/NCBI
|