Introduction
Cervical cancer (CC) is the fourth most common type
of cancer among women, with an estimated 661,021 new cases and
348,189 cancer-related deaths worldwide in 2022; moreover, its
incidence in China has exhibited an increase in recent years as
well (1,2). Although numerous patients with
early-stage and locally advanced CC respond well to conventional
treatments, such as surgery, radiotherapy and chemotherapy, the
lack of effective treatment strategies remains an obstacle for
patients with advanced-stage or recurrent disease (3,4). Some
studies have yielded promising results regarding targeted therapies
for advanced-stage CC, such as inhibitors of vascular endothelial
growth factor and epidermal growth factor receptor (EGFR) (5–7). This
emphasizes the importance of exploring molecular mechanisms in CC
to seek for other therapeutic targets.
Eukaryotic translation initiation factor 3B (EIF3B),
a subunit of the EIF3 complex, is a scaffold protein that regulates
translation initiation and the cell cycle; it has been shown to
participate in the pathogenesis of several types of cancer,
including cholangiocarcinoma, pancreatic cancer, head and neck
squamous cell carcinoma (HNSCC), hepatocellular carcinoma (HCC)
(8–11). For instance, a previous study
revealed that the depletion of EIF3B suppressed cell proliferation
and migration, whereas it enhanced cell apoptosis in
cholangiocarcinoma (9). Another
study disclosed that EIF3B promoted the cell number, and invasion
and migration in HNSCC by regulating CCAAT/enhancer binding
protein-beta translation (11).
Methyltransferase-like (METTL) is a seven-chain
methyltransferase family with an S-adenosylmethionine binding
domain for nucleic acids, proteins and other small molecules
(12). METTLs regulate gene
transcription and translation via methylation modification and
further participate in malignant progression across a wider range
of cancer types (13). As a
translation initiation factor, EIF3B has been identified to
directly interact with METTL family members, and promotes cell
proliferation and migration, as well as invasion in HCC (14); this suggests the involvement of
EIF3B in METTL-mediated mRNA methylation and oncogenesis.
In a previous study, the authors reported that EIF3B
was not only overexpressed, but was also associated with an
elevated clinical stage, lymph node metastasis and unfavorable
survival profiles in patients with CC (15), indicating the underlying oncogenic
role of EIF3B in CC. However, whether EIF3B would interact with
METTL3 to show its carcinogenesis role have not yet been reported,
and their regulatory downstream pathways in CC is not clear. Hence,
the present study aimed to explore the detailed mechanism through
which EIF3B-METTL3 complex promotes CC progression by enhancing
EGFR/AKT signaling through m6A-mediated mRNA
translation.
Materials and methods
Cell lines and cell culture
Human cervical epithelial cells (HCerEpic; cat. no.
7060, ScienCell Research Laboratories, Inc.), as well as C-33 A
(cat. no. HTB-31), HeLa (cat. no. CCL-2), SiHa (cat. no. HTB-35)
and CaSki [cat. no. CRL-1550; all from American Type Culture
Collection (ATCC)] cells were cultured in cervical epithelial cell
medium, which contains basal medium and Cervical Epithelial Cell
Growth Supplement (cat. no. 7061; ScienCell Research Laboratories,
Inc.) or Eagle's minimum essential medium with Earle's salts,
L-glutamine, and non-essential amino acids, without sodium
bicarbonate (cat. no. M0643; MilliporeSigma). The medium was
supplemented with 10% fetal bovine serum (FBS; cat. no. ABS972;
Absin Bioscience Inc.). Cells at passages 3–10 were adopted for
subsequent experiments.
Transfection
Cells were plated to 6-well plates, and cultured to
60% confluence. Overexpression plasmid (5 µg) or small interference
RNA (siRNA, 75 pmol) of EIF3B (5 µg oeEIF3B or 75 pmol siEIF3B,
GenScript Biotech Corporation) and negative controls (5 µg oeNC and
75 pmol siNC, GenScript Biotech Corporation) were transfected into
HeLa or SiHa cells with the utilization of ExFect transfection
reagent (cat. no. T101-01; Vazyme Biotech Co., Ltd.) for 6 h at
37°C. Untransfected cells were set as the normal controls. In
total, three siRNA target sites of EIF3B were designed and
validated by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) to select the most efficient siRNA site
(Fig. S1A) 48 h after
transfection. The siRNA sequences were as follows: siEIF3B (sense:
CGGUGAUUGUAGUGGACAATT; antisense: UUGUCCACUACAAUCACCGTT) and siNC
(sense: UUCUCCGAACGUGUCACGUTT; antisense:
ACGUGACACGUUCGGAGAATT).
Compensation experiment
METTL3 siRNA (siMETTL3, 75 pmol) or siNC (75 pmol)
were co-transfected with oeEIF3B (5 µg) or oeNC (5 µg) into HeLa
and SiHa cells. The ExFect transfection reagent (Vazyme Biotech
Co., Ltd.) was applied to complete transfection for 6 h at 37°C.
The most efficient siRNA of METTL3 was selected from three siRNA
target sites using RT-qPCR (Fig.
S1B) at 48 h after transfection. The sequences of siMETTL3 were
as follows: siMETTL3 (sense: CAGUGGAUCUGUUGUGAUATT; antisense:
UAUCACAACAGAUCCACUGTT).
RT-qPCR
RNA in cells was isolated using VeZol Reagent (cat.
no. R411-01; Vazyme Biotech Co., Ltd.). For the completion of
reverse transcription and qPCR, the RT SuperMix for qPCR (cat. no.
R222-01; Vazyme Biotech Co., Ltd.) and Universal SYBR qPCR Master
Mix (cat. no. Q711-02; Vazyme Biotech Co., Ltd.) were used
according to the manufacturer's instructions. The qPCR
thermocycling conditions were as follows: 95°C for 30 sec, 1 cycle;
95°C for 5 sec, 61°C for 30 sec, 40 cycles. The primers (5′-3′)
used were as follows: EIF3B forward, CGTATGTGCGTTGGTCTCCTAA and
reverse, CCTTGGTGGCTGAATCTCTGAAT; EGFR forward,
GGACAGCATAGACGACACCTTC and reverse, CCTGGCTTGGACACTGGAGA; METTL3
forward, TTGTAACCTATGCTGACCATTCCA and reverse,
ACCTTCTTGCTCTGTTGTTCCTT; GAPDH forward, GAGTCCACTGGCGTCTTCAC and
reverse, ATCTTGAGGCTGTTGTCATACTTCT. The results were analyzed with
the 2−ΔΔCq method (16).
Western blot analysis
Protein was extracted from the cells and lysed using
RIPA buffer (cat. no. P0013B; Beyotime Institute of Biotechnology)
and quantified using the BCA kit (cat. no. G2026; Wuhan Servicebio
Technology Co., Ltd.). Precast gel (4–20%) (cat. no. P0822;
Beyotime Institute of Biotechnology) and nitrocellulose membrane
(cat. no. 66485; Pall Life Sciences) were used to separate and blot
the 10 µg protein. The primary antibodies including EIF3B (1:5,000;
cat. no. 10319-1-AP), METTL3 (1:10,000; cat. no. 15073-1-AP),
N-cadherin (1:5,000; cat. no. 22018-1-AP), E-cadherin (1:5,000;
cat. no. 20874-1-AP), Vimentin (1:20,000; cat. no. 10366-1-AP),
EGFR (1:10,000; cat. no. 18986-1-AP), protein kinase B (AKT;
1:10,000; cat. no. 10176-2-AP), phosphorylated (p)-AKT (1:10,000;
cat. no. 28731-1-AP) and GAPDH (1:10,000; cat. no. 10494-1-AP; all
from Proteintech Group, Inc.) were incubated with the membrane
overnight at 4°C. The HRP-conjugated secondary antibody (1:20,000;
cat. no. GB23303; Wuhan Servicebio Technology Co., Ltd.) was
incubated with the membrane at 37°C for 1 h. The protein bands were
exposed using an ECL kit (G2020, Wuhan Servicebio Technology Co.,
Ltd.). Densitometric analysis was conducted by ImageJ v1.8
(National Institutes of Health).
Cell proliferation
The Cell Counting Kit-8 (CCK-8; cat. no. G4103;
Wuhan Servicebio Technology Co., Ltd.) was adopted to measure cell
proliferation at 0, 24, 48 and 72 h post-transfection. The
procedures were accomplished according to the manufacturer's
instructions. In brief, 3×103 cells were plated in
96-well plates a night before analysis. The mixture of 10 µl CCK-8
and 100 µl culture medium was cultured with cells for another 2 h
at 37°C. Afterward, the optical density value at 450 nm was
measured.
Cell apoptosis
Cell apoptosis was assessed at 48 h using the
terminal deoxynucleotidyl transferase (TdT) dUTP nick-end labeling
(TUNEL) assay kit (cat. no. C1089; Beyotime Institute of
Biotechnology). According to the protocols, the cells were fixed in
4% paraformaldehyde at room temperature for 30 min and incubated
with TUNEL working solution for 1 h in the dark at 37°C. The nuclei
were stained with DAPI (Beyotime Institute of Biotechnology) for 5
min at 37°C in the dark. The cells were sealed with mounting medium
(Beyotime Institute of Biotechnology) after being rinsed with PBS.
The images of 3 fields were captured using an inverted fluorescence
microscope (Motic Incorporation, Ltd.).
Transwell assay
A Matrigel matrix basement membrane-precoated
Transwell insert (cat. no. 3422; Corning, Inc.) was applied to
complete the evaluation at 48 h. The resuspended single cells in
FBS-free medium were cultivated in inserts for 24 h at 37°C. Images
of the invasive cells were captured after being stained with 0.1%
crystal violet (cat. no. GC307002-25g; Wuhan Servicebio Technology
Co., Ltd.) at 37°C for 5 min under an inverted microscope (Motic
Incorporation, Ltd.).
Co-immunoprecipitation (co-IP)
The HeLa and SiHa cells were lysed using RIPA lysis
buffer. Protein A+G Magnetic Beads (20 µl; cat. no. P2108; Beyotime
Institute of Biotechnology) were incubated with METTL3 antibody
(1:100) or IgG antibody (1:200; cat. no. 30000-0-AP; Proteintech
Group, Inc.) for 1 h at room temperature. Subsequently, the lysate
was incubated with protein A+G agarose beads overnight at 4°C.
Finally, the eluate was analyzed using western blot analysis.
The Cancer Genome Atlas (TCGA) data
analysis
The analyses of CC data sourced from TCGA database
were performed using The University of Alabama at Birmingham Cancer
data analysis Portal (UALCAN) (https://ualcan.path.uab.edu/index.html), including the
expression of METTL3 expression and its correlation with the
survival in patients with cervical squamous cell carcinoma (CESC)
(17).
Statistical analysis
Data are presented as the mean ± standard deviation
(SD). Statistical analyses were performed using GraphPad Prism 9.0
(Dotmatics). Differences between groups were analyzed using the
Wilcoxon's rank sum test or one-way ANOVA followed by Dunnett's or
Tukey's multiple comparisons tests as appropriate. The correlation
of METTL3 with survival was conducted by the log-rank test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Aberrant expression of EIF3B in CC
cell lines
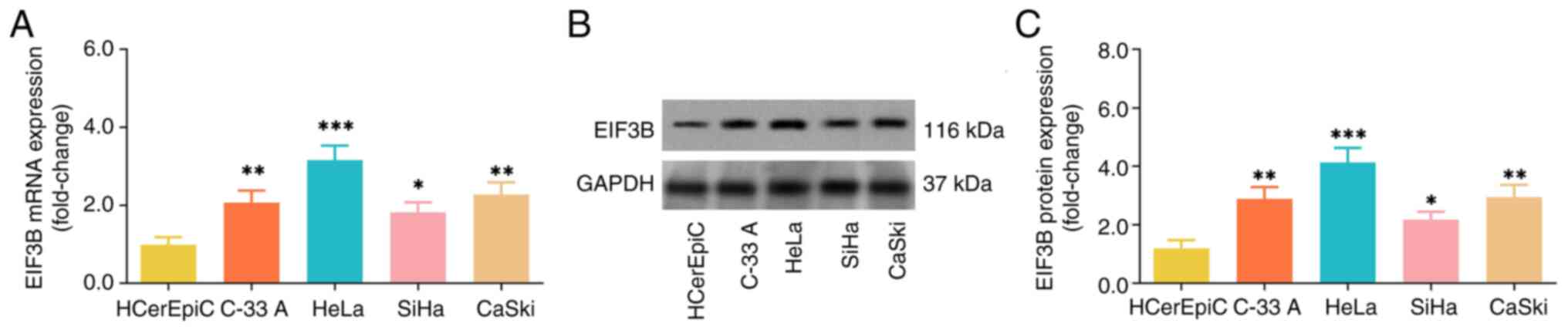
The EIF3B mRNA and protein expression levels were
elevated in several CC cell lines, including C-33A, HeLa, SiHa and
CaSki, compared with the HCerEpiC cells (Fig. 1). Since the EIF3B mRNA and protein
expression levels were highest in the HeLa cells and lowest in the
SiHa cells among all tested CC cells, the two cell lines were
selected for use in subsequent experiments.
Effect of EIF3B on CC cell
proliferation, apoptosis, epithelial-mesenchymal transition (EMT)
process and invasion
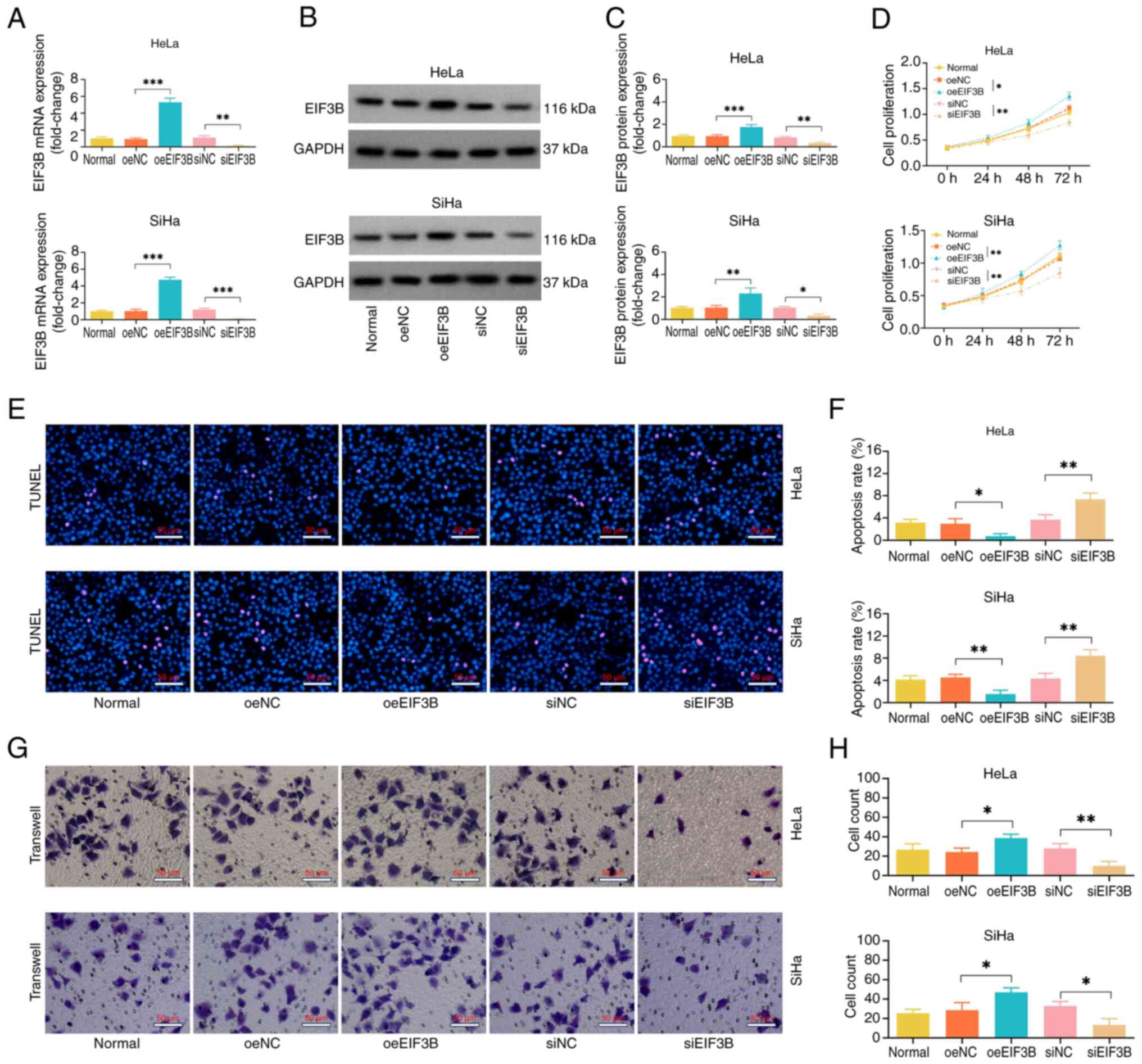
oeEIF3B increased, while siEIF3B decreased EIF3B
mRNA and protein expression levels in the HeLa and SiHa cell lines,
indicating the successful transfection of oeEIF3B and siEIF3B
(Fig. 2A-C).
CCK-8, TUNEL and Transwell assays were used to
measure cell proliferation, apoptosis and invasion, respectively.
It was disclosed that oeEIF3B promoted the cell proliferative
capacity and invasive cell count but decreased the cell apoptotic
rate in the HeLa and SiHa cell lines (Fig. 2D-H). On the contrary, siEIF3B
exerted an opposite effect on these cellular functions.
In the HeLa cell line, the N-cadherin and vimentin
were elevated in oeEIF3B group compared with the oeNC group, while
E-cadherin was decreased in the oeEIF3B group compared with the
oeNC. The opposite trend was observed in the siEIF3B group compared
with the siNC group. Similarly, the SiHa cell line also showed the
similar results (Fig. S2A and B).
These findings indicated that the oeEIF3B might aggravate the EMT
and invasion processes.
Interaction of EIF3B with METTL3 and
EGFR/AKT signaling in CC
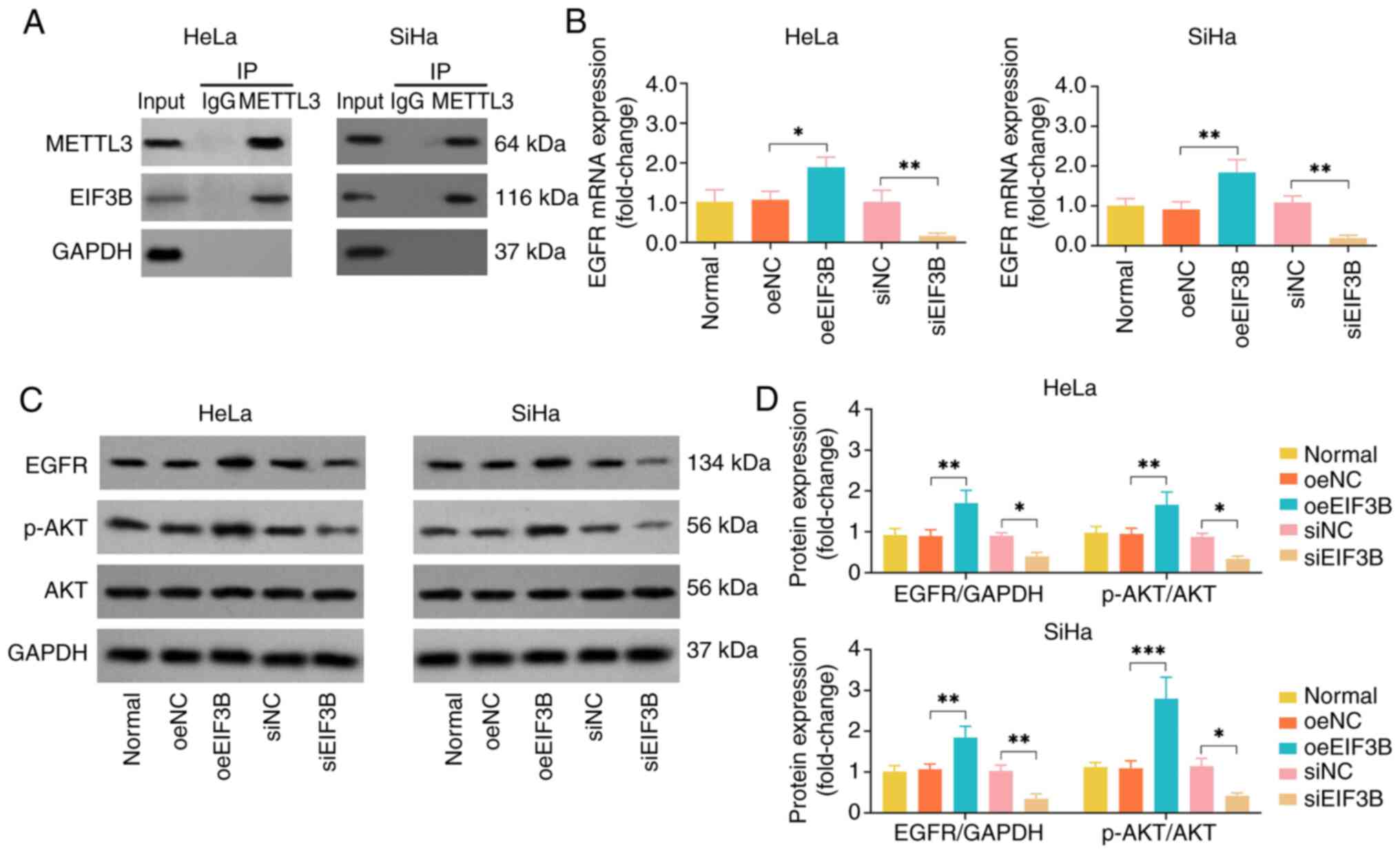
Considering that EIF3B has been previously reported
to specifically bind to the METTL to promote tumor progression
(14), the present study further
selected METTL3 as a protein partner of EIF3B in CC. The co-IP
assay revealed that EIF3B directly bound to METTL3 (Fig. 3A). The empty bands for the IgG
indicated that there was no non-specific adsorption occurred in the
experimental system. Subsequently, the potential downstream
EGFR/AKT signaling pathway was explored. oeEIF3B elevated EGFR and
p-AKT expression levels in the HeLa and SiHa cell lines; by
contrast, siEIF3B reduced EGFR and p-AKT expression levels
(Fig. 3B-D). These findings
suggested that EIF3B specifically binds to METTL3, and activates
the EGFR/AKT signaling pathway in CC.
Effect of the EIF3B-METTL3 complex on
CC cell proliferation, apoptosis and invasion
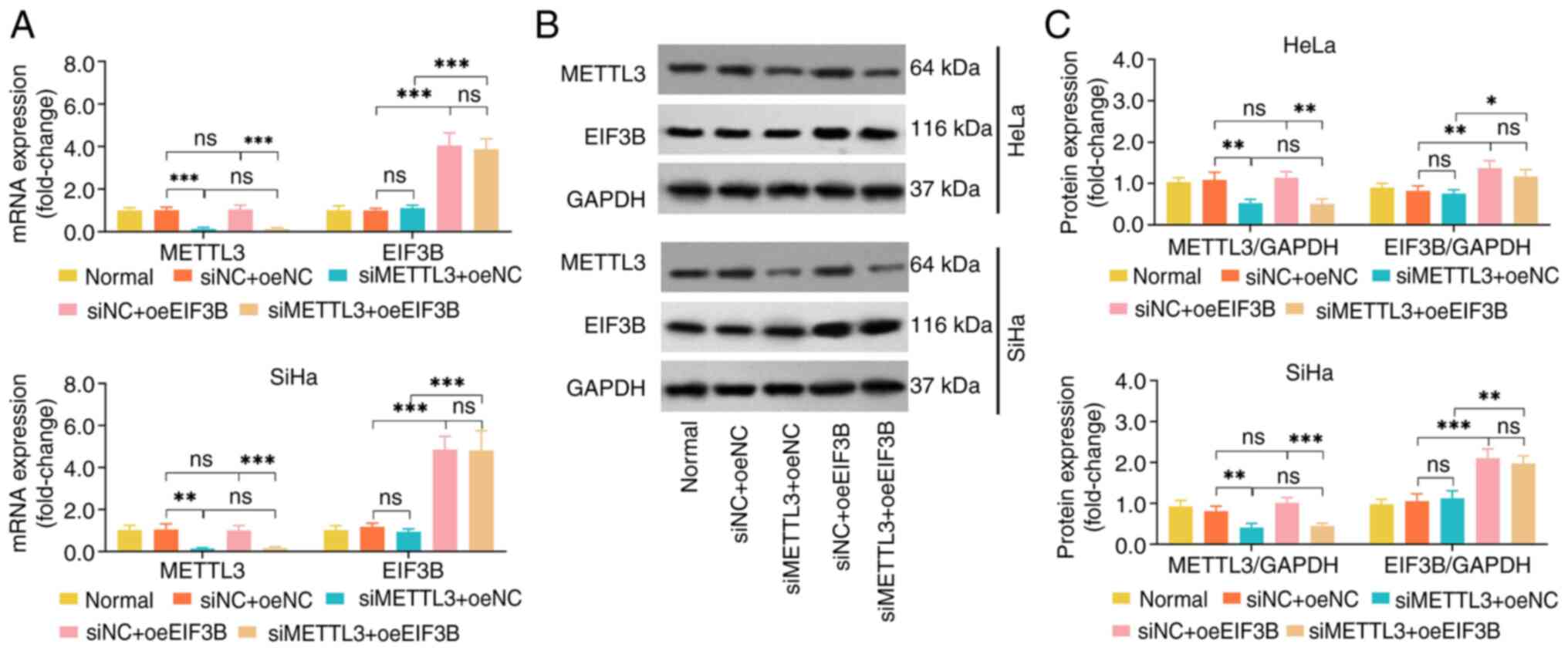
The METTL3 mRNA and protein expression levels were
suppressed by transfection with siMETTL3 in HeLa and SiHa cell
lines, which indicated that the transfection was successful
(Fig. 4A-C). In both the HeLa and
SiHa cell lines, oeEIF3B did not affect the expression of METTL3
mRNA and protein; moreover, siMETTL3 did not alter the mRNA and
protein expression of EIF3B. Together with the findings of the
co-IP assay, it was suggested that EIF3B and METTL3 formed a
complex to exert their functions in CC.
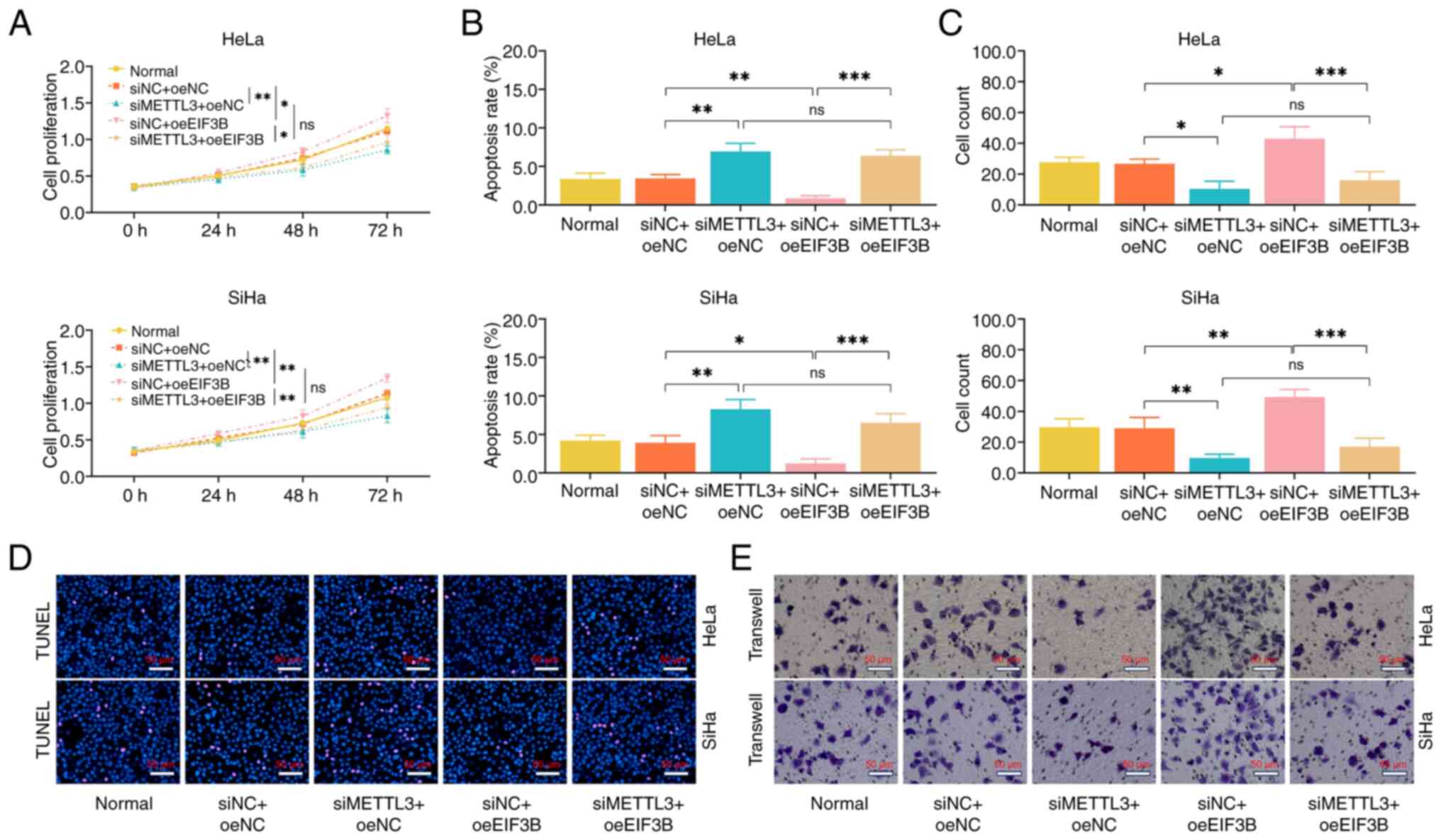
siMETTL3 suppressed cell proliferative capacity and
the invasive cell count but facilitated the apoptosis of the HeLa
and SiHa cell lines, regardless of the presence or absence of
oeEIF3B (Fig. 5A-E). Moreover,
following transfection with siMETTL3, oeEIF3B lost its regulatory
effect on cell proliferative capacity, apoptotic rate and invasive
ability of the HeLa and SiHa cell lines. These findings indicated
that EIF3B exerted its cancer-promoting effects through forming a
complex with METTL3 in CC.
Effect of the EIF3B-METTL3 complex on
the EGFR/AKT signaling pathway in CC
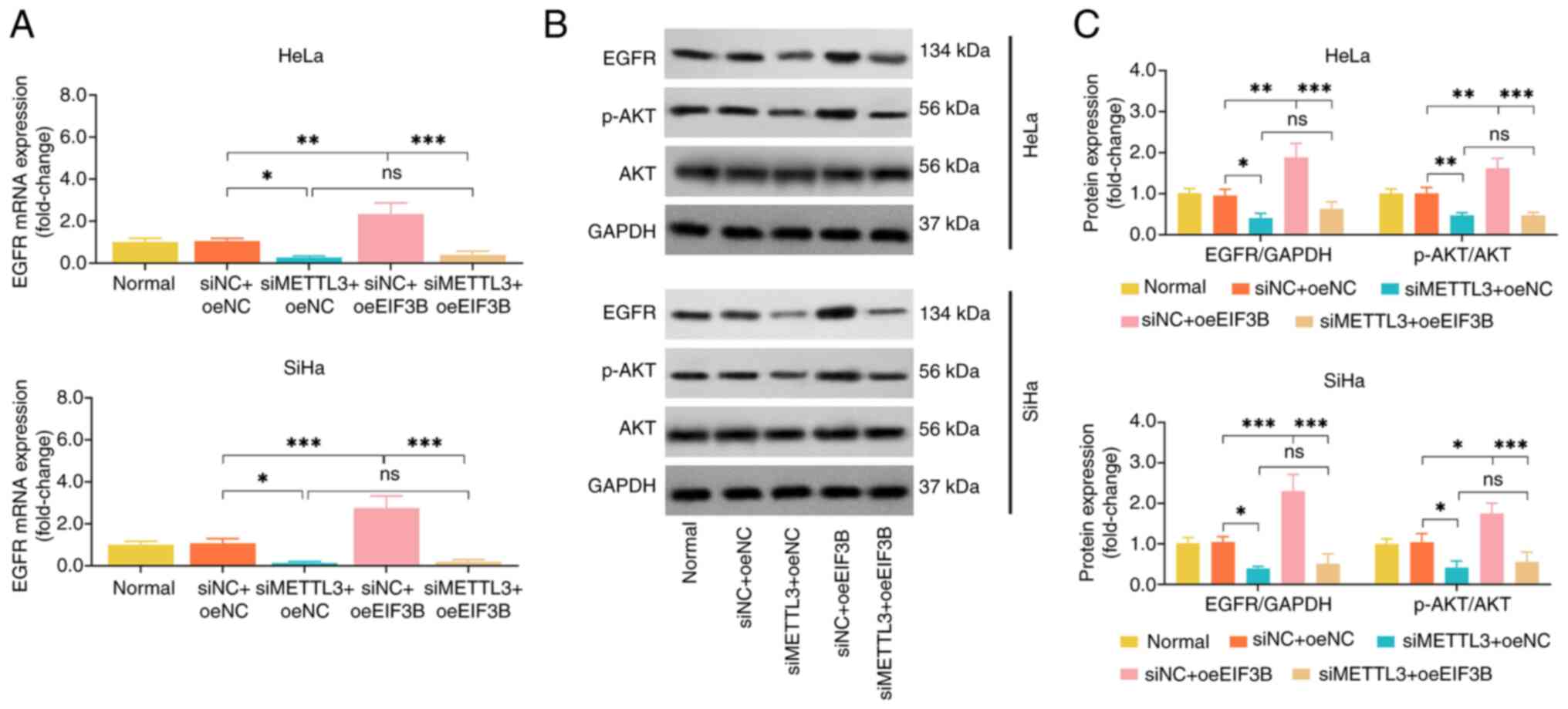
siMETTL3 reduced the EGFR and p-AKT expression
levels in HeLa and SiHa cell lines, and its regulatory effect was
not affected by oeEIF3B. Nevertheless, the promoting effect of
oeEIF3B on EFGR and p-AKT expression was attenuated by transfection
with siMETTL3 (Fig. 6A-C). These
findings suggested that EIF3B activated EGFR/AKT signaling via
forming a complex with METTL3 in CC.
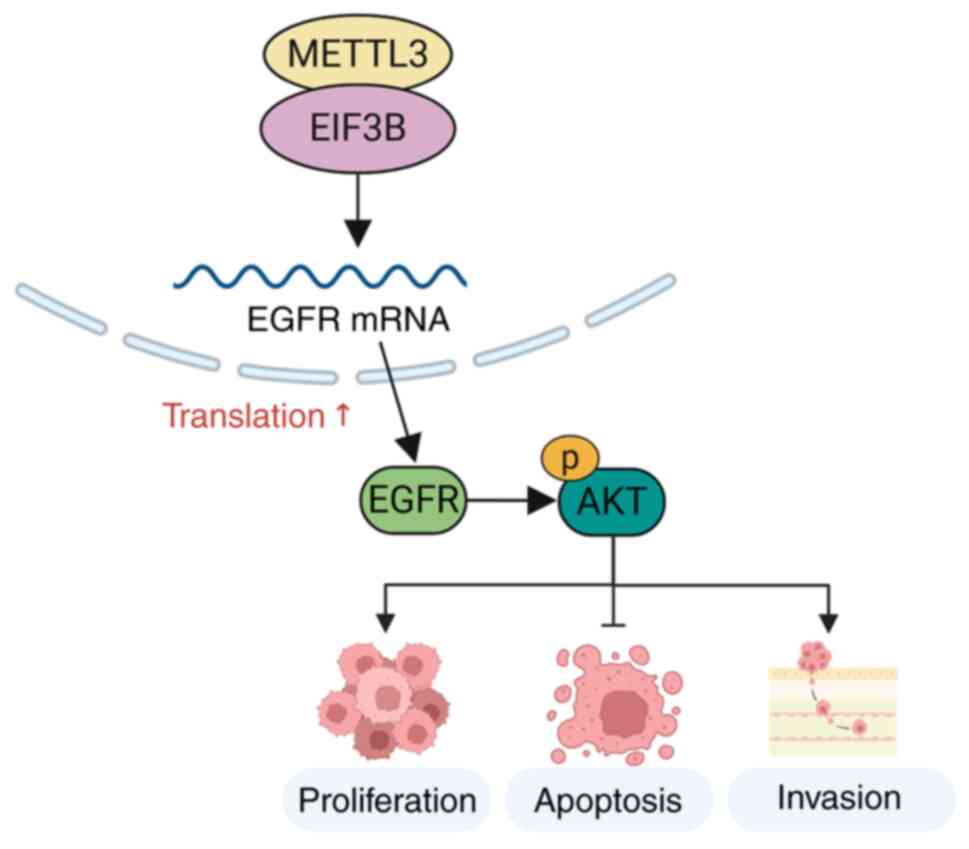
Schematic diagram
Combining the aforementioned data, it was
hypothesized that EIF3B and METTL3 form a complex to activate the
EGFR/AKT signaling pathway, and subsequently regulate cell
proliferation, apoptosis and invasion in CC. The details of this
interaction are illustrated in Fig.
7.
METTL3 expression in patients with
CESC from the TCGA database
The expression of METTL3 was highly expressed in the
tumor tissue compared with the non-tumor tissue in patients with
CESC (Fig. S3A). However, METTL3
did not associate with the survival in patients with CESC (Fig. S3B).
Discussion
EIF3, serving as a scaffold, encircles the 40S
ribosome and coordinates the actions of other EIFs during
translation (18). The capability
of EIF3 family members in facilitating gynecological cancer
progression has been well elucidated (18–21).
As demonstrated in a previous study, EIF3D promoted cervical
carcinoma growth via the Warburg effect both in vitro and
in vivo (19). Another study
reported that EIF3D overexpression induced stem cell properties and
the metastasis of CC cells (20).
Of note, another study found that the inhibition of EIF3B
compromised cell proliferation, but increased the apoptosis of
ovarian cancer cells, indicating that EIF3B may be a possible
target for ovarian cancer treatment (21).
The present study observed that EIF3B expression was
elevated in several CC cell lines compared with normal cervical
epithelial cells; moreover, EIF3B facilitated cell proliferation
and invasion, whereas it inhibited the apoptosis of CC cells. The
possible explanations for these effects are as follows: i) EIF3B
interfered with cell cycle arrest to enhance the malignant
anti-apoptotic properties (22);
ii) EIF3B, as a translational factor, induced EMT by the
preferential translation of Snail, and activated the EMT process,
promoting the migration and invasion of tumor cells (23–25).
In summary, EIF3B promoted the proliferation and invasion, and
suppressed the apoptosis of CC cells.
METTL3 is a catalytically active
N6-methyladenosine (m6A) writer that
comprises a zinc finger domain and a methyltransferase domain,
whose modulatory role in the progression of various cancers is
indispensable (12,26–29).
For example, a recent study demonstrated that the knockdown of
METTL3 inhibited cell proliferation, invasion, migration and
angiogenesis, whereas it promoted the apoptosis of esophageal
cancer (27). Another study
reported that METTL3 accelerated the cell viability, invasion,
migration and EMT of laryngeal squamous cell carcinoma cells by
upregulating WISP1 expression (28). Similarly, the present study
demonstrated that siMETTL3 suppressed the proliferation and
invasion, whereas it promoted the apoptosis of CC. The possible
reasons for these effects may be the following: i) METTL3
stimulated m6A modification, and the latter was required
for the tumor malignancy process (30); ii) METTL3 also exerted a catalytic
activity to trigger several oncogenes, thereby engaging in tumor
progression (31). As a result, the
suppression of METTL3 inhibited cell proliferation and invasion,
whereas it promoted apoptosis in CC.
Apart from its cancer-promoting function, METTL3
facilitates the translation due to its regulatory role of
m6A modification as well (32,33). A
recent study demonstrated that the METTL3-EIF3H interaction
participated in colorectal tumorigenesis (33). Notably, the present study found that
EIF3B mediated the malignant processes by specifically binding to
METTL3 in CC, which could be explained as follows: i) On the one
hand, METTL3 needed the interaction with translating-initiation
factors to stimulate its m6A RNA methyltransferase
activity and catalytic activity (32); on the other hand, METTL enhanced the
translation efficacy of EIF3B by stimulating the interplay of EIF3
complex and the 40S ribosomal subunit (14). Consequently, EIF3B and METTL3 formed
a complex in CC. ii) EIF3B was characterized by
translating-initiating feature, whose biological function required
the cooperation of METTL protein (14). Thus, EIF3B may play a CC-promoting
role by forming a complex with METTL3.
EGFR, a member of the receptor tyrosine kinase
family, relates to multiple tumor malignant phenotypes and has
become a target for the treatment of certain types of cancer,
including breast cancer, HNSCC and CC (34,35).
Furthermore, AKT is a target of phosphoinositide 3-kinase that
plays a regulatory role in epithelial cell motility and invasion by
phosphorylating a large number of substrates (36). In the present study, it was
demonstrated that EIF3B overexpression increased the levels EGFR
and p-AKT, while the silencing of METTL3 suppressed these levels in
CC, suggesting that EGFR/AKT signaling may serve as the downstream
pathway of EIF3B and METTL3. Furthermore, the present study also
revealed that EIF3B could not activate EGFR/AKT without METTL3. The
probable explanation for this is as follows: METTL3 has been
reported to promote the translation of EGFR in human cancer cells,
and the initiation of this translation process is mediated by EIF3B
(37). Apart from that, METTL3
could promote the translation of EGFR in various cancers, such as
the lung cancer cells and HCC, and it could be deduce that EGFR
might also be the downstream of METTL3 in CC (37,38).
Thus, the EIF3B-METTL3 complex facilitated EGFR/AKT signaling in
CC, and EIF3B alone could not exert the regulatory effect.
Besides, the present study has certain limitations.
First, the apoptosis data should be verified by flow cytometry
assay and TUNEL assay. However, this parameter was only detected by
the TUNEL assay. Further study could consider verifying this issue
by both methods. Second, METTL3 is a methyltransferase and its
interaction with EIF3B might be through multi-mechanism including
its effect on the methyltransferase of EIF3B. Third, according to
the analysis of METTL3 expression in the GEPIA database, there are
only 13 non-tumor tissues, which would largely decrease the
reliability of the results. Therefore, further larger sample size
study is needed to verify this finding (4). The m6A RIP-qPCR should be
conducted to confirm METTL3-dependent methylation of EGFR mRNA,
which would be a further study direction.
In conclusion, EIF3B binds to METTL3 to form a
complex, thereby promoting cell proliferation and invasion, and
activating EGFR/AKT signaling in CC. The findings suggest that
EIF3B may serve as a therapeutic target in CC; however, further
studies are warranted to validate these findings.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Top Talent of Changzhou ‘The
14th Five-Year Plan’ High-Level Health Talents Training Project
(No.2022CZBJ085), The third level of the sixth ‘333 High level
Talent Training Project’ in Jiangsu Province (No.2022 3-4-162), The
Application Foundation Project of Changzhou Science and Technology
Bureau (No. CJ20245041), The Science and Technology Development
Foundation Project of Nanjing Medical University (No.NMUB20240054),
Science and Technology Development Fund of Nanjing Medical
University (No.NMUB20230054) and Research project of Changzhou
Maternal and Child Health Hospital (No. YJ202408).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PZ contributed to the study conception and design.
CZ and XF performed data collection and analysis. JY was
responsible for the interpretation of data. PZ and CZ confirm the
authenticity of all the raw data. All authors contributed to
drafting of article and revising it critically for important
intellectual content. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Francoeur AA, Monk BJ and Tewari KS:
Treatment advances across the cervical cancer spectrum. Nat Rev
Clin Oncol. 22:182–199. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Xu M, Cao C, Wu P, Huang X and Ma D:
Advances in cervical cancer: Current insights and future
directions. Cancer Commun (Lond). 45:77–109. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tanigawa T, Takeshima N, Ishikawa H,
Nishio S, Usami T, Yamawaki T, Oishi T, Ihira K, Kato H, Goto M, et
al: Paclitaxel-carboplatin and bevacizumab combination with
maintenance bevacizumab therapy for metastatic, recurrent, and
persistent uterine cervical cancer: An open-label multicenter phase
II trial (JGOG1079). Gynecol Oncol. 165:413–419. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Muthusami S, Sabanayagam R, Periyasamy L,
Muruganantham B and Park WY: A review on the role of epidermal
growth factor signaling in the development, progression and
treatment of cervical cancer. Int J Biol Macromol. 194:179–187.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fontenot VE, Francoeur A and Tewari KS:
Review of emerging biological therapies for recurrent and advanced
metastatic cervical cancer. Expert Opin Biol Ther. 24:709–713.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Feng X, Li J and Liu P: The Biological
roles of translation initiation factor 3b. Int J Biol Sci.
14:1630–1635. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang R, Nie W, Mi L, Yao C and Zhu H:
EIF3B stabilizes PCNA by counteracting SYVN1-mediated
ubiquitination to serve as a promotor in cholangiocarcinoma. Aging.
16:7311–7330. 2024.PubMed/NCBI
|
|
10
|
Zhu H, Shan Y, Ge K, Lu J, Kong W and Jia
C: EIF3B promotes cancer progression in pancreatic cancer. Scand J
Gastroenterol. 56:281–288. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xu C, Shen Y, Shi Y, Zhang M and Zhou L:
Eukaryotic translation initiation factor 3 subunit B promotes head
and neck cancer via CEBPB translation. Cancer Cell Int. 22:1612022.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang H, Sun F, Jiang S, Yang F, Dong X,
Liu G, Wang M, Li Y, Su M, Wen Z, et al: METTL protein family:
Focusing on the occurrence, progression and treatment of cancer.
Biomark Res. 12:1052024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Qi YN, Liu Z, Hong LL, Li P and Ling ZQ:
Methyltransferase-like proteins in cancer biology and potential
therapeutic targeting. J Hematol Oncol. 16:892023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Su R, Dong L, Li Y, Gao M, He PC, Liu W,
Wei J, Zhao Z, Gao L, Han L, et al: METTL16 exerts an
m6A-independent function to facilitate translation and
tumorigenesis. Nat Cell Biol. 24:205–216. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu P, Tan Q, Jiang W, Ou Y, Xu P and Yuan
L: Eukaryotic translation initiation factor 3B is overexpressed and
correlates with deteriorated tumor features and unfavorable
survival profiles in cervical cancer patients. Cancer Biomark.
26:123–130. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandrashekar DS, Karthikeyan SK, Korla
PK, Patel H, Shovon AR, Athar M, Netto GJ, Qin ZS, Kumar S, Manne
U, et al: UALCAN: An update to the integrated cancer data analysis
platform. Neoplasia. 25:18–27. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zeman J, Itoh Y, Kukacka Z, Rosůlek M,
Kavan D, Kouba T, Jansen ME, Mohammad MP, Novák P and Valášek LS:
Binding of eIF3 in complex with eIF5 and eIF1 to the 40S ribosomal
subunit is accompanied by dramatic structural changes. Nucleic
Acids Res. 47:8282–8300. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu Q, Liu J, Zheng D, Zhang R, Xiang Y,
Xu F, Zhou X and Qin J: EIF3D promoted cervical carcinoma through
Warburg effect by interacting with GRP78. J Obstet Gynaecol.
43:21302002023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhong Y and Lan J: Overexpression of
Eukaryotic translation initiation factor 3D induces stem cell-like
properties and metastasis in cervix cancer by activating FAK
through inhibiting degradation of GRP78. Bioengineered.
13:1952–1961. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang L and Ouyang L: Effects of EIF3B gene
downregulation on apoptosis and proliferation of human ovarian
cancer SKOV3 and HO-8910 cells. Biomed Pharmacother. 109:831–837.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ren H, Mai G, Liu Y, Xiang R, Yang C and
Su W: Eukaryotic Translation initiation factor 3 Subunit B is a
promoter in the development and progression of pancreatic cancer.
Front Oncol. 11:6441562021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sobocan M, Smolle MA, Schatz C and
Haybaeck J: The interplay of tumor stroma and translational factors
in endometrial cancer. Cancers (Basel). 12:20742020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liaghat M, Ferdousmakan S, Mortazavi SH,
Yahyazadeh S, Irani A, Banihashemi S, Seyedi Asl FS, Akbari A,
Farzam F, Aziziyan F, et al: The impact of epithelial-mesenchymal
transition (EMT) induced by metabolic processes and intracellular
signaling pathways on chemo-resistance, metastasis, and recurrence
in solid tumors. Cell Commun Signal. 22:5752024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li N, Wei X, Dai J, Yang J and Xiong S:
METTL3: A multifunctional regulator in diseases. Mol Cell Biochem.
480:3429–3454. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo X, Huang A, Qi Y, Chen J, Yang M and
Jin M: METTL3/IGF2BP2 promotes the malignant progression of
esophageal cancer by activating the PIK3CA/AKT pathway. Thorac
Cancer. 16:e700222025. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang W, Peng Z, Mingchu Z and Deshui Y:
METTL3 mediated WISP1 m6A modification promotes
epithelial-mesenchymal transition and tumorigenesis in laryngeal
squamous cell carcinoma via m6A reader IGF2BP1. Gene.
941:1492222025. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang H, Sun F, Cao H, Yang L, Yang F,
Chen R, Jiang S, Wang R, Yu X, Li B and Chu X: UBA protein family:
An emerging set of E1 ubiquitin ligases in cancer-A review. Int J
Biol Macromol. 308:1422772025. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Singh S, Gupta S, Abhishek R and Sachan M:
Regulation of m6A (N6-Methyladenosine) methylation modifiers in
solid cancers. Funct Integr Genomics. 24:1932024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zeng C, Huang W, Li Y and Weng H: Roles of
METTL3 in cancer: Mechanisms and therapeutic targeting. J Hematol
Oncol. 13:1172020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choe J, Lin S, Zhang W, Liu Q, Wang L,
Ramirez-Moya J, Du P, Kim W, Tang S, Sliz P, et al: mRNA
circularization by METTL3-eIF3h enhances translation and promotes
oncogenesis. Nature. 561:556–560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zheng R, Zhang K, Tan S, Gao F, Zhang Y,
Xu W, Wang H, Gu D, Zhu L, Li S, et al: Exosomal circLPAR1
functions in colorectal cancer diagnosis and tumorigenesis through
suppressing BRD4 via METTL3-eIF3h interaction. Mol Cancer.
21:492022. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dickerson H, Diab A and Al Musaimi O:
Epidermal growth factor receptor tyrosine kinase inhibitors in
cancer: Current use and future prospects. Int J Mol Sci.
25:100082024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hamid MB, Serafin AM and Akudugu JM:
Selective therapeutic benefit of X-rays and inhibitors of EGFR,
PI3K/mTOR, and Bcl-2 in breast, lung, and cervical cancer cells.
Eur J Pharmacol. 912:1746122021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hua H, Zhang H, Chen J, Wang J, Liu J and
Jiang Y: Targeting Akt in cancer for precision therapy. J Hematol
Oncol. 14:1282021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin S, Choe J, Du P, Triboulet R and
Gregory RI: The m(6)A methyltransferase METTL3 promotes translation
in human cancer cells. Mol Cell. 62:335–345. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang L, Yang Q, Zhou Q, Fang F, Lei K, Liu
Z, Zheng G, Zhu L, Huo J, Li X, et al:
METTL3-m6A-EGFR-axis drives lenvatinib resistance in
hepatocellular carcinoma. Cancer Lett. 559:2161222023. View Article : Google Scholar : PubMed/NCBI
|