Upper tract urothelial carcinoma (UTUC) is a rare
yet clinically aggressive type of cancer, which arises from the
transitional epithelium lining the renal pelvis (RP), ureters and
proximal urethra (1). Distinct from
bladder urothelial carcinoma, UTUC is characterized by unique
biological behaviors in terms of clinical presentation, anatomical
localization and molecular alterations (2,3).
Accounting for 5–10% of all urothelial cancer cases, this disease
shows a high prevalence of nodal metastases at initial diagnosis,
with lymph node involvement being recorded in 20–30% of cases
(4). The frequent absence of early
symptoms is considered a marked challenge in the management of
UTUC, and commonly leads to late-stage diagnosis, with ~60% of
cases being diagnosed at an advanced disease stage (5). In high-grade or locally invasive
tumors, the lymph node metastasis (LNM) rate can increase to 30–40%
(6). Therefore, LNM is considered a
well-established predictor of poor survival outcomes (7).
The treatment approach for non-metastatic UTUC
depends on the risk level of the tumor. For patients with low-risk
(LR) UTUC, clinicians typically favor minimally invasive
approaches, including kidney-preserving endoscopic procedures that
maintain renal function. By contrast, high-risk (HR) UTUC typically
necessitates a conventional treatment approach, including radical
nephroureterectomy (RNU) combined with excision of the bladder
cuff. This strategy aids in local disease management and can
enhance cancer-specific survival (CSS) outcomes (1,8). It
has been reported that in UC, LNM can commonly spread to nearby
lymph nodes through the lymphatic drainage pathway (9). Therefore, systemic lymphadenectomy
could exert both therapeutic and diagnostic roles by providing more
accurate tumor staging, which in turn could improve treatment
planning after surgery (10,11).
This is particularly important for identifying patients who could
benefit from additional therapies (1,12).
However, there is still no consensus on the extent of lymph node
dissection (LND) in patients with UTUC.
The present review aims to provide an integrative
analysis of LND in the management of UTUC, highlighting its
evolving clinical applications. The clinical relevance of LND in
staging protocols and disease characterization, as well as its
prognostic significance in predicting therapeutic efficacy and
recurrence, are also discussed. Current controversies regarding
surgical strategies and the technical demands associated with LND
procedures are critically evaluated. Furthermore, the potential
survival benefits associated with the extent of lymph node
dissection are analyzed through both clinicopathological and
molecular perspectives.
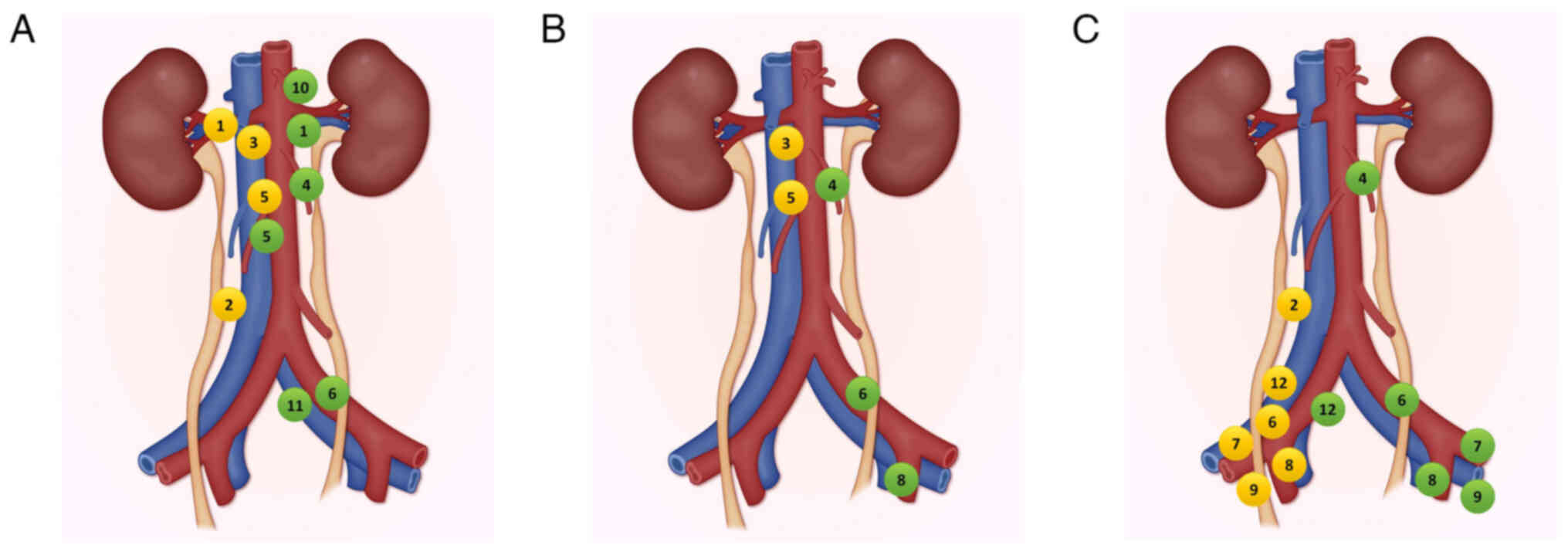
In UTUC, lymph nodes are the primary sites for
metastasis. Understanding lymphatic drainage patterns is crucial
for defining the extent of LND and improving the accuracy of
staging and prognosis (13). The
drainage variations by tumor location are illustrated in Fig. 1.
Although currently no standardized LND templates
have been established by major guidelines, such as those provided
by the European Association of Urology (EAU) and American
Urological Association (AUA) (14–16), a
foundational work by Akaza et al (17) was the first to propose a
Tumor-Node-Metastasis framework for UTUC. Later, Kondo and Tanabe
(9) refined this framework by
mapping eight anatomical zones associated with tumor location,
namely the RP, and the upper, middle or lower ureter, bilaterally.
Regional nodes were defined as those with a metastasis risk of
>10%. Proximal segments correspond to areas lying above the
inferior mesenteric artery (IMA), the mid-ureteral region spans
from the IMA to the common iliac (CI) artery and the distal ureter
(DU) extends to the ureteral meatus (9). This mapping laid the foundation for
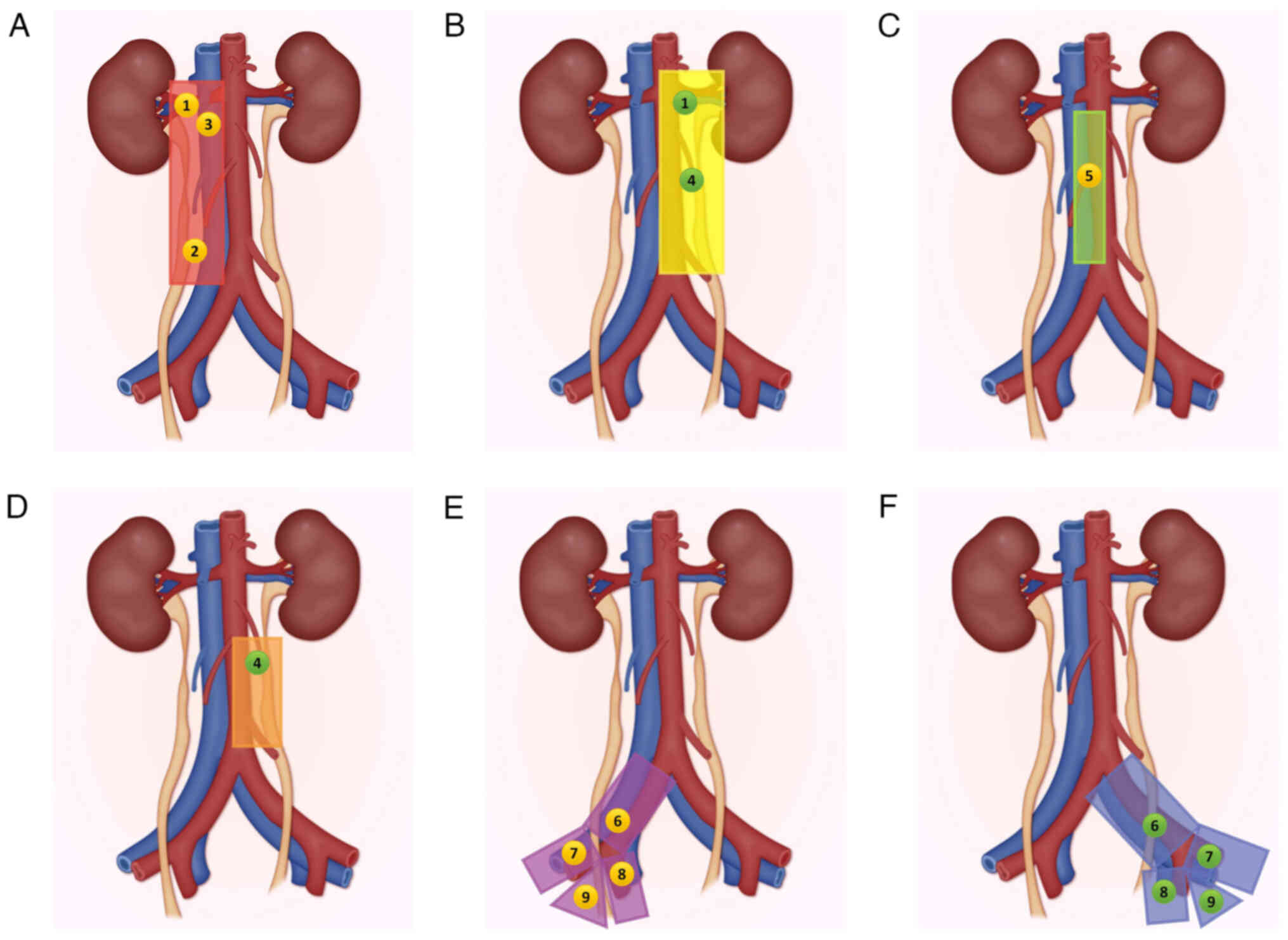
the widely referenced ‘Kondo and Matin templates’ (9,18,19).
The LND templates based on the primary tumor location from the
studies by Matin et al (18), Kondo et al (19) are illustrated in Fig. 2.
Lymph node status remains a critical prognostic
indicator in UTUC. Therefore, previous studies indicated that
node-negative (pN0) patients exhibited significantly superior
5-year disease-free survival (DFS) (84.5 vs. 43.6%; P<0.001) and
CSS (78.3 vs. 33.2%; P<0.001) rates compared with node-positive
(pN+) patients (9,20,21).
These findings underscored the significant effect of nodal
involvement in the onset of adverse effects and highlighted the
importance of accurate nodal assessment in guiding adjuvant
treatment decision, as evidenced by clinical trials, such as POUT
(22).
Emerging evidence has also suggested that other
clinical factors, such as age and gender, and biomarkers, including
elevated fibrinogen, cystatin-C, C-reactive protein,
neutrophil-to-lymphocyte ratio of >2.7, albumin-to-globulin
ratio of <1.45, and preoperative anemia, can be also associated
with LNM risk (13,14,23–28).
Building on the anatomical principles established by
the ‘Kondo and Matin templates’ (9,18,19),
several clinical studies have evaluated the therapeutic outcomes of
LND. Kondo et al (29)
performed retrospective and prospective analyses via comparing the
efficacy of systematic complete LND (CLND), incomplete or limited
LND (ILND), and no LND (9,29–31).
The results demonstrated that in patients with ≥pT2 disease, CLND
could display superior CSS compared with ILND/no LND, and more
particularly in patients with pT3+ tumors (5-year CSS, 73.2 vs.
43.7 and 47.3%, respectively) (9).
For patients with ≥pT2 cN0M0 UTUC, CLND showed improved CSS
compared with ILND (86 vs. 71%; P=0.04). Furthermore, CLND could
independently predict both CSS and regional recurrence-free
survival (RFS) in RP tumors (31).
However, the survival benefits were less evident in distal ureteral
tumors, possibly due to variations in the extent of lymph node
dissection. By contrast, the population-based study by Lughezzani
et al (8), and a
multi-institutional study (32)
reported no significant therapeutic survival benefits for LND,
although in these studies the dissection templates were not
specified.
Extended LND is increasingly recognized for its
prognostic value. However, the majority of RNU procedures remove
<8 lymph nodes (33). It has
been reported that lymph node density, defined as the number of
positive nodes to that of total nodes, can provide higher
prognostic value compared with absolute lymph none counts.
Therefore, a nodal density ratio of >30% was associated with
higher recurrence [hazard ratio (HR), 1.8; P=0.021) and mortality
(HR,1.7; P=0.032) risks (34,35).
Consistently, lymph node density of >20% could predict worse RFS
(HR,1.94) and overall survival (OS; HR, 2.34) (20). The safety of LND has been also
well-documented. Therefore, operative duration, estimated blood
loss, and hospitalization period were comparable across
CLND/ILND/no LND cohorts, with only a minor increase in the
incidence of complications reported in CLND (9,36,37).
Due to limited data, the optimal extent of LND in
patients with UTUC remains still controversial. The widely adopted
‘Kondo and Matin template’ (9,18) is
based on small samples. Furthermore, the genomic differences in
UTUC between Chinese and Western populations suggests that the
application of LND templates should be region-specific. Notably, to
date no studies have directly compared the effect of different LND
extents on OS and PFS (38).
LNM is associated with adverse clinical outcomes and
substantially decreased survival (39). Although evidence remains conflicting
regarding the therapeutic value of LND, its potential in achieving
definitive pathological staging of UTUC has been well documented.
The majority of the available data has been derived from
retrospective, single-institution studies, with several of them
supporting the prognostic value of LND (12,29,40).
These reports demonstrated that pN0 patients exhibited a superior
CSS compared with pN+ patients, with 5-year CSS estimates of 56–85
and 0–39%, respectively. These findings indicated that accurate
nodal evaluation via LND during RNU could enhance the postoperative
risk classification and guide treatment decisions. Notably, two
studies identified pNx status as an independent survival risk
factor compared with pN0 status (29,41).
By contrast, another study did not detect nodal status-related
differences in survival endpoints (42). In addition, a population-based study
that included 2,824 subjects revealed marked disparities in 5-year
CSS, with rates of 34, 78, and 81% recorded for pN+, pNx and pN0
patients, respectively (P<0.001). However, no prognostic
significance was reported between the pN0 and pNx groups (43). Only two multicenter cohorts showed
differences in CSS rates between the pN0 and pNx groups (12,44).
Ouzzane et al (43) found no
independent association between CSS and nodal status in adjusted
models (pN+ vs. pN0: HR, 1.6; 95% CI, 0.8–3.4; P=0.104; and pNx vs.
pN0: HR, 1.14; 95% CI, 0.7–1.9; P=0.592). Subgroup analyses more
consistently supported the prognostic value of LND in staging
muscle-invasive or locally advanced UTUC (11,32,45).
However, the findings of the aforementioned studies have not been
verified by a multicenter study (43). Importantly, studies failing to
demonstrate staging benefits commonly lack dissection protocol
details, thus emphasizing the need for standardized LND
templates.
Recent advances in immunotherapy have further
underscored the critical role of LND in guiding systemic therapy
decisions. According to the 2023 EAU guidelines, adjuvant nivolumab
is now recommended for HR patients (≥pT3 or pN+) who are ineligible
for or decline platinum-based ChT, provided that their tumors
express programmed death-ligand 1 (PD-L1) ≥1% (15). This recommendation is based on
proven survival benefits observed in the CheckMate 274 trial in
patients with muscle-invasive UC (46). However, UTUC-specific data remain
limited. Notably, LND remains indispensable for identifying nodal
involvement (pN+ status), which directly determines eligibility for
both adjuvant ChT and immunotherapy. While immune checkpoint
inhibitors (ICIs) offer novel therapeutic insights, they do not
alter the fundamental indications or extent of LND, a
template-based dissection approach, which continues to be
prioritized for accurate pathological staging and local disease
control. Future studies should explore whether genomic differences,
such as fibroblast growth factor receptor (FGFR) alterations,
between Eastern and Western populations could affect immunotherapy
responsiveness and necessitate region-specific LND templates
(15).
For patients with cN+ UTUC commonly experiencing
lymphatic dissemination, neoadjuvant platinum-based ChT prior to
RNU/LND merits consideration. However, current guidelines lack
UTUC-specific evidence to endorse this strategy, compared with the
well-established protocols for bladder cancer (1,47).
Regarding ChT timing in operable cN+ disease, the multicenter study
by Shigeta et al (48)
compared neoadjuvant vs. adjuvant approaches and found that
preoperative ChT was associated with significantly improved 3-year
DFS (58.2 vs. 37.1%; P=0.004) and CSS (71.3 vs. 47.6%; P=0.002)
rates. Notably, selecting adjuvant therapy following RNU critically
depends on the pathological findings of LND (49). A previous study suggested that
initiating gemcitabine-cisplatin ChT within 90 days after surgery
could improve DFS rate (HR, 0.45; 95% CI, 0.30–0.70; P<0.0001)
in patients with locally advanced UTUC (22). In metastatic UC, cisplatin-based ChT
showed improved response rates compared with carboplatin-based ChT
(50). However, as non-responders
often switch to immunotherapy, selection bias persists (48). These findings reinforce managing cN+
disease as systemic malignancy requiring comprehensive treatment.
Importantly, the majority of studies evaluating RNU by LND excluded
cN+ patients, hindering natural history assessment and perpetuating
debates regarding the therapeutic value of LND. Although the
oncological effect of LND is modest in cN+ cases, accurate nodal
staging remains vital for systemic treatment planning (22,51).
The current and proposed staging classifications are summarized in
Table I (52,53).
Current urological guidelines show considerable
variability in their recommendations for LND in UTUC. According to
the 2022 EAU guidelines, template-based LND should be considered
over node-quantity-based approaches, thus emphasizing its potential
survival advantages. Template-based LND has been associated with
prolonged CSS time in muscle-invasive cases, reduced local
recurrence rates, and improved outcomes in patients without
clinical or pathological nodal involvement. Due to the difficulty
in preoperatively diagnosing Ta and T1 tumor stages, the EAU
strongly recommends concurrently performing template-based LND for
all patients undergoing RNU (15).
The 2023 AUA guidelines recommend LND during RNU or urethrectomy
for HR UTUC, while in patients with LR UTUC, it can be performed
under particular conditions, such as intraoperative detection of
suspicious nodes, mid/distal ureteral tumors requiring template
dissection, discordant biopsy/imaging features or when adjuvant
therapy eligibility requires pathological nodal staging (16). The 2023 edition of the National
Comprehensive Cancer Network (NCCN) guidelines recommends regional
LND for high-grade, large, renal pelvic cancers that invade the
renal parenchyma, and high-grade ureteral cancers (54). However, a more scientifically
grounded LND template should be developed based on studies of LNM
distribution and risk probability.
The standardized LND boundaries recommended by the
EAU, NCCN and AUA guidelines are delineated in Table II (15,16,18,19).
The EAU criteria integrate findings from three foundational
studies, two of which provide precise anatomical resection
templates (18,31,55).
For malignancies involving the RP, upper ureter (UU) and mid-ureter
(MU), Kondo's protocol mandates resection of the renal hilar (RH),
paracaval (PC), retrocaval (RC) and IAC lymph nodes (31). By contrast, left-sided tumors
necessitate RH and periaortic (PA) lymph node clearance. Lesions
affecting the DU require the comprehensive dissection of
ipsilateral CI, external iliac (EI), internal iliac (II) and
obturator (Ob) nodal basins. Matin's approach differentiates
primary and extended dissection zones, particularly for MU tumors,
to optimize the detection of metastasis (18). For right RP and UU tumors, RH/PC/RC
dissections supplemented with IAC node dissection are recommended
to enhance sensitivity. For left counterparts, RH/PA nodes with IAC
inclusion are preferred. Right MU tumors need IAC, PC, and RC node
dissection for residual disease, while left-sided cases include PA,
CI and II nodes. Finally, for right-sided DU tumors, resection of
CI, EI, II, Ob and PC nodes is warranted, while for left-sided DU
tumors, the resection includes CI, EI, II, Ob and PA nodes
(18).
According to the NCCN guidelines, the surgical
management of the majority of MU neoplasms typically requires nodal
dissection extending from the renal hilum to the branching of the
inferior vena cava, including the precaval nodes, with concurrent
removal of the CI, EI, Ob and pelvic arterial lymph nodes. For PA
tumors, nodal resection should encompass the region between the
renal pedicle and aortic division, incorporating the CI, EI, Ob and
pelvic vascular nodes. In patients with DU tumors, complete
unilateral excision of the CI, EI, Ob and pelvic arterial lymph
nodes is required (54).
According to AUA recommendations, surgical
management of pelvicalyceal malignancies should include unilateral
dissection of the major vascular lymph node bundles spanning from
the RH to the origin of the IMA. Tumors located to the upper
two-thirds of the ureter warrant dissection of lymph nodes
extending from the RH to the aortic bifurcation. However, tumors of
the third distal ureter require removal of Ob and EI nodes, with
optional dissection of the II and CI nodes. Emerging evidence has
suggested that for cranial LNM metastasis, extended dissections
based on clinical assessment should be considered (18,29,36,37,45,56–63).
Given the ongoing debate regarding the efficacy of
LND in UTUC, defining a reasonable dissection range is urgently
needed. However, the current research on LND in UTUC remains
limited, and the field is still in an exploratory state. Notably,
no studies have directly compared the effects of different
dissection ranges on clinical outcomes, such as OS and PFS.
Although several anatomical templates for LND have been proposed,
standardizing LND indications and techniques is challenging due to
the retrospective and multicenter nature of the existing studies.
In the majority of cases, the decision to perform LND is based on
the judgment of the surgeon, considering different factors, such as
clinical presentation, tumor location and laterality. The patient
outcomes from the reviewed studies according to the lymph node
pattern are summarized in Table
III (11,14,20,30,32,40,41,45,56,59,61,65,66).
Radical RNU can be performed using open,
laparoscopic or robot-assisted techniques, with comparable
oncological and safety outcomes (67,68). A
study by Pearce et al (62)
showed that patients undergoing LND had a 30% higher risk of
postoperative complications compared with those without LND.
However, no significant differences were observed in intraoperative
complications among the different surgical approaches.
Robot-assisted NU has been associated with the lowest postoperative
complication rate, while open NU exhibits the highest rate,
particularly for gastrointestinal and hemorrhagic complications
(P<0.001).
Computed tomography (CT) imaging remains the
first-line diagnostic tool for pretherapeutic evaluation of UTUC,
with reported sensitivity and specificity rates of 92 and 95%,
respectively (73). Research by
Millán-Rodríguez et al (74), which included 93 upper tract
urothelial tumors from 82 consecutive UTUC patients scheduled for
nephroureterectomy, reported promising results for CT, with a
sensitivity and specificity of 87.5 and 98%, respectively.
Cross-sectional imaging, particularly CT, remains the cornerstone
of clinical nodal staging of UTUC. However, a previous multicenter
observational trial by Pallauf et al (75), encompassing 865 patients with UTUC
who underwent RNU with LND, revealed that conventional imaging
exhibited high specificity (91%) but low sensitivity (only 25%),
thus resulting in limited detection reliability [area under the
curve (AUC), 0.58]. Therefore, the it was proposed that CT imaging
should be primarily used to confirm, rather than to exclude,
LNM.
Conventional imaging techniques, such as CT and PET,
have limited precision in identifying the locations of LNM in UTUC
(2,75–77).
To enhance nodal staging accuracy, researchers are currently
investigating biomarker-based approaches and multimodal imaging
combinations. Among them, 18F-FDG/PET/CT imaging has shown emerging
utility in identifying metastatic nodal involvement in both UTUC
and bladder cancer (78–84). Although early studies indicated
comparable detection accuracy between 18F-FDG/PET/CT and
conventional magnetic resonance imaging (MRI) (82,84–86),
accumulating evidence has supported the superiority of this hybrid
modality over MRI, CT or PET alone. The strength of 18F-FDG/PET/CT
stems from its capacity to detect metabolically active
micrometastases (<2.0 mm), which can be indetectable by CT
alone, thus enhancing the sensitivity of nodal staging (82,86).
However, its limited specificity creates a challenge, particularly
in distinguishing cancerous from inflammatory nodes (81). Several retrospective studies have
assessed the utility of 18F-FDG/PET/CT for detecting preoperative
LNM in UTUC and bladder cancer (78,79). A
study published in 2020 reported a sensitivity rate of 82% and a
specificity rate of 84% for preoperative nodal staging in UTUC
using 18F-FDG/PET/CT (78).
In addition, a previous systematic review, which
included three retrospective studies on the detection of LNM in
UTUC, reported sensitivities of 82–95% and specificities of 84–91%,
thus highlighting the prognostic value of 18F-FDG/PET/CT (81). A comparative analysis in bladder
cancer demonstrated a higher sensitivity rate for 18F-FDG/PET/CT
(78%) compared with that for CT (44%) for nodal assessment
(87). A key limitation of
18F-FDG/PET/CT involves the interference from urinary excretion of
the radiotracer, which can reduce imaging clarity for nodal lesions
adjacent to urinary structures (88). Choline PET/CT, which uses (11)C-choline, has also been explored for
LNM detection in UTUC. A 2014 study showed high choline uptake in
affected lymph nodes in patients with UTUC (89). Additionally, Polom et al
(90) attempted to detect sentinel
lymph nodes using Technetium-99m injection during ureterorenoscopy
and single-photon emission-CT/CT lymphangiography. While
theoretically feasible, the method proved challenging due to
significant technical constrains during Technetium injection.
Ultrasmall superparamagnetic iron oxide (USPIO) has
gained increasing attention as a contrast agent in MRI. Metastatic
lymph nodes, lacking macrophages, fail to absorb USPIO, thus
resulting in minimal or absent T2-weighted imaging (WI) signal
loss. By contrast, benign lymph nodes, which retain the normal
activity of macrophages, absorb USPIO, eventually showing low T2WI
signals, which aids in distinguishing metastatic nodes from
non-metastatic nodes. Emerging evidence has suggested that
USPIO-MRI can promote the identification of small nodal metastases,
with reported sensitivity ranges of 65–92% and specificity levels
of 93–98% (91,92). In an innovative approach, Birkhäuser
et al (93) integrated
diffusion-weighted imaging sequences with USPIO-MRI, achieving
identification rates of 87 and 77% for lymph nodes of ≤8 and ≤3 mm
in the short-axis diameter, respectively. The reading time was
reduced from 32 to 9 min, with scanning performed 24–36 h
post-administration (94). A
previous meta-analysis also reported an overall sensitivity of 0.86
for USPIO-MRI in diagnosing pelvic LNM. However, its clinical
application remains limited due to safety concerns and high costs
(95).
Research into labeled monoclonal antibodies for
urothelial neoplasms is ongoing (77). Among them, girentuximab-labeled
PET/CT (89Zr-TLX250) has shown promising efficacy in staging renal
cell carcinoma and breast cancer. TLX250 targets carbonic anhydrase
IX, an enzyme which is significantly overexpressed in urothelial
cancer cells (96). A phase I
clinical trial is currently underway to assess the potential of
89Zr-TLX250 for imaging urothelial malignancies (87).
Imaging studies are increasingly integrated into
preoperative protocols for predicting lymph node metastases in
UTUC. A novel diagnostic model incorporating radiological
parameters and histopathological characteristics, including
pathological staging, lymphovascular invasion (LVI), lesion
dimensions and pretreatment nodal status, demonstrated a predictive
accuracy of 87.8% for detecting nodal metastasis (AUC=0.878;
adjusted concordance index=0.887) (97).
Novel molecular classification frameworks for UTUC
increasingly integrate genomic signatures and transcriptome
analysis. Fibulin-2 (FBLN), a significant prognostic indicator, is
strongly associated with diminished CSS rates and increased risk of
metastasis in urothelial malignancies (P<0.001). It has been
reported that FBLN is upregulated in advanced-stage tumors, also
being associated with LNM and enhanced cell proliferation activity
(93,98–106).
The pyruvate dehydrogenase kinase (PDK) family, which is involved
in mediating chemotherapeutic resistance and disease advancement in
patients with bladder carcinomas (105–107), exhibits parallel pathobiological
significance in UTUC. A previous study by Kuo et al
(108) indicated that PDK3
upregulation was significantly associated with aggressive
clinicopathological features, including metastatic nodal disease,
elevated tumor grade and unfavorable survival prognosis
(P<0.0001).
Furthermore, other studies demonstrated that
cartilage oligomeric matrix protein, another prognostic indicator,
was associated with advanced T-stage, LNM, LVI, perineural
infiltration, high-grade histology and increased mitotic rates
(109–113). Metallothionein 2A was also
associated with aggressive behaviors and advanced staging
parameters in urothelial malignancies (114).
Laboratory-based biomarkers are also gaining
increasing attention. Therefore, the de Ritis ratio (alanine
aminotransferase/aspartate aminotransferase) showed prognostic
potential in a previous study, since analysis of 135 cases of
patients with a de Ritis index of ≥1.3 was strongly predictive of
LNM (P=0.0096) (115,116). It has been reported that the
systemic inflammatory status can affect metastasis, thus being
considered as an additional prognostic tool (115,116). The systemic inflammation index
(SII), defined as the number of neutrophils × platelet/lymphocyte
count, serves as a low-cost prognostic tool (117–122). A study demonstrated that elevated
SII values were associated with LVI positivity and poorer OS rates
in UTUC (122). Kobayashi et
al (123) incorporated SII
>520, Eastern Cooperative Oncology Group Performance Status
>0 and clinical stage ≥T3 into a preoperative risk model to
guide therapeutic decision-making.
The 2023 EAU guidelines recognize several emerging
molecular biomarkers in UTUC. However, their integration into
surgical decision-making remains limited. For example, DNA mismatch
repair (MMR) deficiency, and particularly germline
MSH2/MSH6 mutations associated with Lynch syndrome
(LS), were recorded in 9% of patients with UTUC, which was a
markedly higher rate compared with that observed in bladder cancer
(1%). Although LS screening is recommended for patients <60
years or with suggestive family history, its effect typically comes
into practice for surveillance of metachronous cancers rather than
in tailoring surgical approaches (124–130). PD-L1 expression (≥1%) serves as a
predictive biomarker for ICIs in metastatic UTUC, with
pembrolizumab or atezolizumab recommended for cisplatin-ineligible
PD-L1-positive patients. Adjuvant nivolumab can be considered for
patients with HR (≥pT3/pN+) cisplatin-ineligible UTUC post RNU.
However, the aforementioned UTUC-specific survival benefits have
not been fully investigated (51,131–133). Alterations in FGFR2/3 guide
later-line targeted therapy for platinum-refractory disease
(erdafitinib). However, they do affect primary surgical approaches
(134). While molecular subtyping
(luminal and basal subtypes) holds prognostic potential, its
clinical application in surgical planning is limited due to lack of
validated treatment response associations (135).
Significant gaps remain in the integration of
biomarkers into personalized surgery strategies. Kidney-sparing
surgery (KSS) eligibility relies solely on clinical factors, such
as tumor size, grade and focality, with no molecular predictors
currently used to assess ipsilateral recurrence risk or progression
to refine patient selection (136,137). LND templates are determined by
tumor location and risk, without biomarkers predictive of occult
micrometastasis. Furthermore, neoadjuvant ChT recommendations lack
biomarkers, such as ERCC2 mutations for platinum
sensitivity, to identify non-responders, which account for ~40% of
all cases, risking delayed surgery without therapeutic benefits
(138). For patients with
LS-associated UTUC, molecular risk stratification, including
particular MMR mutations (e.g., MLH1, MSH2, MSH6 and PMS2), does
not guide prophylactic surgical interventions. Future efforts
should be made to prospectively validate biomarkers in surgical
trials, to develop integrated clinical-molecular prognostic models
and to investigate biomarker-driven neoadjuvant therapies, such as
FGFR inhibitors, to expand KSS eligibility. In summary, while
biomarkers increasingly guide systemic therapy and genetic
screening, their application in optimizing surgical management,
including decisions on KSS candidacy, LND extent and utilization of
neoadjuvant ChT, remains an unmet need in the evolution of UTUC
precision medicine (55,136–138).
UTUC remains as a challenging oncological entity
profoundly affecting a patient's survival and quality of life. The
clinical utility of LND during RNU continues to be a subject of
debate among urological specialists. While existing evidence
supports the diagnostic value and possible therapeutic effects of
LND, and particularly muscle-invasive and locally extensive
disease, the optimal extent of dissection and its definitive effect
on patient survival need further clarification. Although major
guidelines vary between LND protocols, accumulating evidence
increasingly supports the adoption of standardized anatomical
templates to enhance diagnostic accuracy. Therefore, further
high-quality studies are necessitated to establish consensus-driven
practices. Advancements in functional imaging modalities, such as
PET-CT/MRI, and molecular biomarker development, can promote
tailored surgical planning. Ultimately, clarifying the therapeutic
role of LND in the management of UTUC requires prospective
multicenter trials to strengthen evidence-based protocols for
optimizing outcomes.
Not applicable.
This study was supported by the National Natural Science
Foundation of China (grant no. 82360603), the Yunnan Fundamental
Research Projects (grant nos. 202001AY070001-163, 202201AU070220,
202201AY070001-113 and 202401AU070010) and the Yunnan Provincial
Department of Education Project (grant no. 2024J0225).
The data generated in the present study may be
requested from the corresponding author.
HW, MD and ZT were responsible for
conceptualization. ZT, CY, JW, YH and SF designed the literature
search strategy, screening criteria and evidence synthesis
framework.. Formal analysis and data interpretation were performed
by YH, SF, HL, DL, CG and JW. ZT, CY and JW helped write the
original draft. HW, MD, ZT, CY, JW, YH, SF, HL, DL and CG reviewed
and edited the manuscript. Visualization of figures/tables was
performed by HL, DL, CG and JW. HW and MD supervised the study. HW
and MD acquired funding. All authors have read and approved the
manuscript. All authors have made substantial intellectual
contributions, critically revised the manuscript for important
content, and agree to be accountable for all aspects of the work.
Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Rouprêt M, Babjuk M, Burger M, Capoun O,
Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P,
Hugh Mostafid A, et al: European association of urology guidelines
on upper urinary tract urothelial carcinoma: 2020 update. Eur Urol.
79:62–79. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Green DA, Rink M, Xylinas E, Matin SF,
Stenzl A, Roupret M, Karakiewicz PI, Scherr DS and Shariat SF:
Urothelial carcinoma of the bladder and the upper tract: Disparate
twins. J Urol. 189:1214–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peyrottes A, Ouzaid I, Califano G, Hermieu
JF and Xylinas E: Neoadjuvant immunotherapy for muscle-invasive
bladder cancer. Medicina (Kaunas). 57:7692021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giudici N, Bonne F, Blarer J, Minoli M,
Krentel F and Seiler R: Characteristics of upper urinary tract
urothelial carcinoma in the context of bladder cancer: A narrative
review. Transl Androl Urol. 10:4036–4050. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG;
Upper Tract Urothelial Carcinoma Collaboration The Upper Tract
Urothelial Carcinoma Collaboration, : Outcomes of radical
nephroureterectomy: A series from the upper tract urothelial
carcinoma collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Batata MA, Whitmore WF, Hilaris BS, Tokita
N and Grabstald H: Primary carcinoma of the ureter: A prognostic
study. Cancer. 35:1626–1632. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Collà Ruvolo C, Nocera L, Stolzenbach LF,
Wenzel M, Cucchiara V, Tian Z, Shariat SF, Saad F, Longo N,
Montorsi F, et al: Incidence and survival rates of contemporary
patients with invasive upper tract urothelial carcinoma. Eur Urol
Oncol. 4:792–801. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lughezzani G, Jeldres C, Isbarn H, Sun M,
Shariat SF, Alasker A, Pharand D, Widmer H, Arjane P, Graefen M, et
al: Nephroureterectomy and segmental ureterectomy in the treatment
of invasive upper tract urothelial carcinoma: A population-based
study of 2299 patients. Eur J Cancer. 45:3291–3297. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kondo T and Tanabe K: Role of
lymphadenectomy in the management of urothelial carcinoma of the
bladder and the upper urinary tract. Int J Urol. 19:710–721. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chappidi MR, Kates M, Johnson MH, Hahn NM,
Bivalacqua TJ and Pierorazio PM: Lymph node yield and tumor
location in patients with upper tract urothelial carcinoma
undergoing nephroureterectomy affects survival: A U.S.
population-based analysis (2004–2012). Urol Oncol.
34:531.e15–531.e24. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lughezzani G, Jeldres C, Isbarn H, Shariat
SF, Sun M, Pharand D, Widmer H, Arjane P, Graefen M, Montorsi F, et
al: A critical appraisal of the value of lymph node dissection at
nephroureterectomy for upper tract urothelial carcinoma. Urology.
75:118–124. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roscigno M, Brausi M, Heidenreich A, Lotan
Y, Margulis V, Shariat SF, Van Poppel H and Zigeuner R:
Lymphadenectomy at the time of nephroureterectomy for upper tract
urothelial cancer. Eur Urol. 60:776–783. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Deuker M, Rosiello G, Stolzenbach LF,
Martin T, Collà Ruvolo C, Nocera L, Tian Z, Roos FC, Becker A,
Kluth LA, et al: Sex- and age-related differences in the
distribution of metastases in patients with upper urinary tract
urothelial carcinoma. J Natl Compr Canc Netw. 19:534–540. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Inokuchi J, Kuroiwa K, Kakehi Y, Sugimoto
M, Tanigawa T, Fujimoto H, Gotoh M, Masumori N, Ogawa O, Eto M, et
al: Role of lymph node dissection during radical nephroureterectomy
for upper urinary tract urothelial cancer: Multi-institutional
large retrospective study JCOG1110A. World J Urol. 35:1737–1744.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rouprêt M, Seisen T, Birtle AJ, Capoun O,
Compérat EM, Dominguez-Escrig JL, Gürses Andersson I, Liedberg F,
Mariappan P, Hugh Mostafid A, et al: European association of
urology guidelines on upper urinary tract urothelial carcinoma:
2023 update. Eur Urol. 84:49–64. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Coleman JA, Clark PE, Bixler BR, Buckley
DI, Chang SS, Chou R, Hoffman-Censits J, Kulkarni GS, Matin SF,
Pierorazio PM, et al: Diagnosis and management of non-metastatic
upper tract urothelial carcinoma: AUA/SUO guideline. J Urol.
209:1071–1081. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akaza H, Koiso K and Niijima T: Clinical
evaluation of urothelial tumors of the renal pelvis and ureter
based on a new classification system. Cancer. 59:1369–1375. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Matin SF, Sfakianos JP, Espiritu PN,
Coleman JA and Spiess PE: Patterns of lymphatic metastases in upper
tract urothelial carcinoma and proposed dissection templates. J
Urol. 194:1567–1574. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kondo T, Hara I, Takagi T, Kodama Y,
Hashimoto Y, Kobayashi H, Iizuka J, Omae K, Yoshida K and Tanabe K:
Template-based lymphadenectomy in urothelial carcinoma of the renal
pelvis: A prospective study. Int J Urol. 21:453–459. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mason RJ, Kassouf W, Bell DG, Lacombe L,
Kapoor A, Jacobsen N, Fairey A, Izawa J, Black P, Tanguay S, et al:
The contemporary role of lymph node dissection during
nephroureterectomy in the management of upper urinary tract
urothelial carcinoma: the Canadian experience. Urology. 79:840–845.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhai TS, Jin L, Zhou Z, Liu X, Liu H, Chen
W, Lu JY, Yao XD, Feng LM and Ye L: Effect of lymph node dissection
on stage-specific survival in patients with upper urinary tract
urothelial carcinoma treated with nephroureterectomy. BMC cancer.
19:12072019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Birtle A, Johnson M, Chester J, Jones R,
Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto
JWF, et al: Adjuvant chemotherapy in upper tract urothelial
carcinoma (the POUT trial): A phase 3, open-label, randomised
controlled trial. Lancet. 395:1268–1277. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu H, Ai JZ, Tan P, Lin TH, Jin X, Gong
LN, Lei HR, Yang L and Wei Q: Pretreatment elevated fibrinogen
level predicts worse oncologic outcomes in upper tract urothelial
carcinoma. Asian J Androl. 22:177–183. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tan P, Shi M, Chen J, Xu H, Xie N, Xu H,
Jiang Y, Ai JZ, Liu LR, Yang L and Wei Q: The preoperative serum
cystatin-C as an independent prognostic factor for survival in
upper tract urothelial carcinoma. Asian J Androl. 21:163–169. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Saito K, Kawakami S, Ohtsuka Y, Fujii Y,
Masuda H, Kumagai J, Kobayashi T, Kageyama Y and Kihara K: The
impact of preoperative serum C-reactive protein on the prognosis of
patients with upper urinary tract urothelial carcinoma treated
surgically. BJU Int. 100:269–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xu H, Tan P, Ai J, Huang Y, Lin T, Yang L
and Wei Q: Prognostic impact of preoperative albumin-globulin ratio
on oncologic outcomes in upper tract urothelial carcinoma treated
with radical nephroureterectomy. Clin Genitourin Cancer.
16:e1059–e1068. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rink M, Sharifi N, Fritsche HM, Aziz A,
Miller F, Kluth LA, Ngamsri T, Dahlem R, Chun FK, Shariat SF, et
al: Impact of preoperative anemia on oncologic outcomes of upper
tract urothelial carcinoma treated with radical nephroureterectomy.
J Urol. 191:316–322. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vartolomei MD, Mathieu R, Margulis V,
Karam JA, Rouprêt M, Lucca I, Mbeutcha A, Seitz C, Karakiewicz PI,
Fajkovic H, et al: Promising role of preoperative
neutrophil-to-lymphocyte ratio in patients treated with radical
nephroureterectomy. World J Urol. 35:121–130. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kondo T, Hashimoto Y, Kobayashi H, Iizuka
J, Nakazawa H, Ito F and Tanabe K: Template-based lymphadenectomy
in urothelial carcinoma of the upper urinary tract: Impact on
patient survival. Int J Urol. 17:848–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kondo T, Nakazawa H, Ito F, Hashimoto Y,
Toma H and Tanabe K: Impact of the extent of regional
lymphadenectomy on the survival of patients with urothelial
carcinoma of the upper urinary tract. J Urol. 178:1212–1217. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kondo T, Hara I, Takagi T, Kodama Y,
Hashimoto Y, Kobayashi H, Iizuka J, Omae K, Ikezawa E, Yoshida K
and Tanabe K: Possible role of template-based lymphadenectomy in
reducing the risk of regional node recurrence after
nephroureterectomy in patients with renal pelvic cancer. Jpn J Clin
Oncol. 44:1233–1238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Burger M, Shariat SF, Fritsche HM,
Martinez-Salamanca JI, Matsumoto K, Chromecki TF, Ficarra V,
Kassouf W, Seitz C, Pycha A, et al: No overt influence of
lymphadenectomy on cancer-specific survival in organ-confined
versus locally advanced upper urinary tract urothelial carcinoma
undergoing radical nephroureterectomy: A retrospective
international, multi-institutional study. World J Urol. 29:465–472.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Duquesne I, Ouzaid I, Loriot Y, Moschini M
and Xylinas E: Lymphadenectomy for upper tract urothelial
carcinoma: A systematic review. J Clin Med. 8:11902019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Stein JP, Cai J, Groshen S and Skinner DG:
Risk factors for patients with pelvic lymph node metastases
following radical cystectomy with en bloc pelvic lymphadenectomy:
Concept of lymph node density. J Urol. 170:35–41. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bolenz C, Shariat SF, Fernández MI,
Margulis V, Lotan Y, Karakiewicz P, Remzi M, Kikuchi E, Zigeuner R,
Weizer A, et al: Risk stratification of patients with nodal
involvement in upper tract urothelial carcinoma: Value of
lymph-node density. BJU Int. 103:302–306. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Rao SR, Correa JJ, Sexton WJ, Pow-Sang JM,
Dickinson SI, Lin HY and Spiess PE: Prospective clinical trial of
the feasibility and safety of modified retroperitoneal lymph node
dissection at time of nephroureterectomy for upper tract urothelial
carcinoma. BJU Int. 110:E475–E480. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Abe T, Takada N, Matsumoto R, Osawa T,
Sazawa A, Maruyama S, Tsuchiya K, Harabayashi T, Minami K, Nagamori
S, et al: Outcome of regional lymphadenectomy in accordance with
primary tumor location on laparoscopic nephroureterectomy for
urothelial carcinoma of the upper urinary tract: A prospective
study. J Endourol. 29:304–309. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lu H, Liang Y, Guan B, Shi Y, Gong Y, Li
J, Kong W, Liu J, Fang D, Liu L, et al: Aristolochic acid
mutational signature defines the low-risk subtype in upper tract
urothelial carcinoma. Theranostics. 10:4323–4333. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Novara G, De Marco V, Gottardo F, Dalpiaz
O, Bouygues V, Galfano A, Martignoni G, Patard JJ, Artibani W and
Ficarra V: Independent predictors of cancer-specific survival in
transitional cell carcinoma of the upper urinary tract:
multi-institutional dataset from 3 European centers. Cancer.
110:1715–1722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Secin FP, Koppie TM, Salamanca JI, Bokhari
S, Raj GV, Olgac S, Martignoni G, Patard JJ, Artibani W and Ficarra
V: Evaluation of regional lymph node dissection in patients with
upper urinary tract urothelial cancer. Int J Urol. 14:26–32. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Roscigno M, Cozzarini C, Bertini R,
Scattoni V, Freschi M, Da Pozzo LF, Briganti A, Gallina A,
Capitanio U, Colombo R, et al: Prognostic value of lymph node
dissection in patients with muscle-invasive transitional cell
carcinoma of the upper urinary tract. Eur Urol. 53:794–802. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Cho KS, Choi HM, Koo K, Park SJ, Rha KH,
Choi YD, Chung BH, Cho NH, Yang SC and Hong SJ: Clinical
significance of lymph node dissection in patients with
muscle-invasive upper urinary tract transitional cell carcinoma
treated with nephroureterectomy. J Korean Med Sci. 24:674–678.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Ouzzane A, Colin P, Ghoneim TP, Zerbib M,
De La Taille A, Audenet F, Saint F, Hoarau N, Adam E, Azemar MD, et
al: The impact of lymph node status and features on oncological
outcomes in urothelial carcinoma of the upper urinary tract (UTUC)
treated by nephroureterectomy. World J Urol. 31:189–197. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Abe T, Shinohara N, Muranaka M, Sazawa A,
Maruyama S, Osawa T, Harabayashi T, Kubota K, Matsuno Y, Shibata T,
et al: Role of lymph node dissection in the treatment of urothelial
carcinoma of the upper urinary tract: multi-institutional relapse
analysis and immunohistochemical re-evaluation of negative lymph
nodes. Eur J Surg Oncol. 36:1085–1091. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Roscigno M, Shariat SF, Margulis V,
Karakiewicz P, Remzi M, Kikuchi E, Langner C, Lotan Y, Weizer A,
Bensalah K, et al: Impact of lymph node dissection on cancer
specific survival in patients with upper tract urothelial carcinoma
treated with radical nephroureterectomy. J Urol. 181:2482–2489.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Galsky MD, Witjes JA, Gschwend JE,
Milowsky MI, Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret
T, Shariat SF, et al: Adjuvant nivolumab in high-risk
muscle-invasive urothelial carcinoma: Expanded efficacy from
checkmate 274. J Clin Oncol. 43:15–21. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Abufaraj M, Li R, Meeks J and Shariat SF:
Cytoreductive surgery in patients with urothelial bladder cancer.
Eur Urol Focus. 9:278–279. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shigeta K, Matsumoto K, Ogihara K,
Murakami T, Anno T, Umeda K, Izawa M, Baba Y, Sanjo T, Shojo K, et
al: Does neoadjuvant chemotherapy have therapeutic benefit for
node-positive upper tract urothelial carcinoma? Results of a
multi-center cohort study. Urol Oncol. 40:105.e19–105.e26. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Jung H, Giusti G, Fajkovic H, Herrmann T,
Jones R, Straub M, Baard J, Osther PJS and Brehmer M: Consultation
on UTUC, Stockholm 2018: Aspects of treatment. World J Urol.
37:2279–2287. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Galsky MD, Chen GJ, Oh WK, Bellmunt J,
Roth BJ, Petrioli R, Dogliotti L, Dreicer R and Sonpavde G:
Comparative effectiveness of cisplatin-based and carboplatin-based
chemotherapy for treatment of advanced urothelial carcinoma. Ann
Oncol. 23:406–410. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Bajorin DF, Witjes JA, Gschwend JE,
Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat
SF, Park SH, et al: Adjuvant nivolumab versus placebo in
muscle-invasive urothelial carcinoma. N Engl J Med. 384:2102–2114.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li Z, Li X, Liu Y, Fang J, Zhang X and
Xiao K: Can American Joint Committee on Cancer prognostic groups be
individualized in patients undergoing surgery for Stage IV invasive
upper tract Urothelial Carcinoma? J Cancer. 12:2023–2029. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Abdel-Rahman O: Revisiting the prognostic
heterogeneity of AJCC Stage IV carcinomas of the upper urinary
tract. Clin Genitourin Cancer. 16:e859–e865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Flaig TW, Spiess PE, Chair V, Abern M,
Agarwal N and Buyyounouski MK: NCCN Guidelines Version 3.2023
Bladder Cancer. National Comprehensive Cancer Network. Available
from:. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1417
|
|
55
|
Dominguez-Escrig JL, Peyronnet B, Seisen
T, Bruins HM, Yuan CY, Babjuk M, Böhle A, Burger M, Compérat EM,
Gontero P, et al: Potential benefit of lymph node dissection during
radical nephroureterectomy for upper tract urothelial carcinoma: A
systematic review by the European association of urology guidelines
panel on non-muscle-invasive bladder cancer. Eur Urol Focus.
5:224–241. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Brausi MA, Gavioli M, De Luca G, Verrini
G, Peracchia G, Simonini G and Viola M: Retroperitoneal lymph node
dissection (RPLD) in conjunction with nephroureterectomy in the
treatment of infiltrative transitional cell carcinoma (TCC) of the
upper urinary tract: Impact on survival. Eur Urol. 52:1414–1418.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Matsumoto R, Abe T, Takada N, Minami K,
Harabayashi T, Nagamori S, Hatanaka KC, Yamashiro K, Kikuchi H,
Osawa T, et al: Oncologic outcomes of laparoscopic radical
nephroureterectomy in conjunction with template-based lymph node
dissection: An extended follow-up study. Urol Oncol.
38:933.e13–933.e18. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Furuse H, Matsushita Y, Yajima T, Kato T,
Suzuki T, Matsumoto R, Motoyama D, Sugiyama T, Otsuka A and Ozono
S: Systematic regional lymph node dissection for upper tract
urothelial carcinoma improves patient survival. Jpn J Clin Oncol.
47:239–246. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ikeda M, Matsumoto K, Sakaguchi K, Ishii
D, Tabata KI, Kurosawa K, Urakami S, Okaneya T and Iwamura M:
Effect of lymphadenectomy during radical nephroureterectomy in
locally advanced upper tract urothelial carcinoma. Clin Genitourin
Cancer. 15:556–562. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Yoo S, You D, Jeong IG, Hong B, Hong JH,
Ahn H and Kim CS: Does lymph node dissection during
nephroureterectomy affect oncological outcomes in upper tract
urothelial carcinoma patients without suspicious lymph node
metastasis on preoperative imaging studies? World J Urol.
35:665–673. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Inokuchi J, Eto M, Hara T, Fujimoto H,
Nishiyama H, Miyazaki J, Kikuchi E, Hinotsu S, Koie T and Ohyama C;
Cancer Registration Committee of the Japanese Urological
Association, : Impact of lymph node dissection on clinical outcomes
during nephroureterectomy in patients with clinically node-negative
upper urinary tract urothelial cancer: Subanalysis of a
multi-institutional nationwide case series of the Japanese
Urological Association. Jpn J Clin Oncol. 47:652–659. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Pearce SM, Pariser JJ, Patel SG, Steinberg
GD, Shalhav AL and Smith ND: The effect of surgical approach on
performance of lymphadenectomy and perioperative morbidity for
radical nephroureterectomy. Urol Oncol. 34:121.e15–21. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Winer AG, Vertosick EA, Ghanaat M, Corradi
RB, Carlsson S, Sjoberg DD, Sankin AI, Sfakianos JP, Cha EK,
Dalbagni G and Coleman JA: Prognostic value of lymph node yield
during nephroureterectomy for upper tract urothelial carcinoma.
Urol Oncol. 35:151.e9–151.e15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Dong F, Xu T, Wang X, Shen Y, Zhang X,
Chen S, Zhong S, Zhang M and Ding Q: Lymph node dissection could
bring survival benefits to patients diagnosed with clinically
node-negative upper urinary tract urothelial cancer: A
population-based, propensity score-matched study. Int J Clin Oncol.
24:296–305. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Brown GA, Busby JE, Wood CG, Pisters LL,
Dinney CP, Swanson DA, Grossman HB, Pettaway CA, Munsell MF, Kamat
AM and Matin SF: Nephroureterectomy for treating upper urinary
tract transitional cell carcinoma: Time to change the treatment
paradigm? BJU Int. 98:1176–1180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Li CC, Chang CH, Huang CP, Hong JH, Huang
CY, Chen IA, Lin JT, Lo CW, Yu CC, Tseng JS, et al: Comparing
oncological outcomes and surgical complications of hand-assisted,
laparoscopic and robotic nephroureterectomy for upper tract
urothelial carcinoma. Front Oncol. 11:7314602021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Clements MB, Krupski TL and Culp SH:
Robotic-assisted surgery for upper tract urothelial carcinoma: A
comparative survival analysis. Ann Surg Oncol. 25:2550–2562. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Peyronnet B, Seisen T, Dominguez-Escrig
JL, Bruins HM, Yuan CY, Lam T, Maclennan S, N'dow J, Babjuk M,
Comperat E, et al: Oncological outcomes of laparoscopic
nephroureterectomy versus open radical nephroureterectomy for upper
tract urothelial carcinoma: An European association of urology
guidelines systematic review. Eur Urol Focus. 5:205–223. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Ishiyama Y, Kondo T, Kubota S, Shimada K,
Yoshida K, Takagi T, Iizuka J and Tanabe K: Therapeutic benefit of
lymphadenectomy for older patients with urothelial carcinoma of the
upper urinary tract: A propensity score matching study. Jpn J Clin
Oncol. 51:802–809. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Blom JH, van Poppel H, Maréchal JM,
Jacqmin D, Schröder FH, de Prijck L and Sylvester R; EORTC
Genitourinary Tract Cancer Group, : Radical nephrectomy with and
without lymph-node dissection: final results of European
Organization for Research and Treatment of Cancer (EORTC)
randomized phase 3 trial 30881. Eur Urol. 55:28–34. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Kondo T, Takagi T and Tanabe K:
Therapeutic role of template-based lymphadenectomy in urothelial
carcinoma of the upper urinary tract. World J Clin Oncol.
6:237–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Janisch F, Shariat SF, Baltzer P, Fajkovic
H, Kimura S, Iwata T, Korn P, Yang L, Glybochko PV, Rink M and
Abufaraj M: Diagnostic performance of multidetector computed
tomographic (MDCTU) in upper tract urothelial carcinoma (UTUC): A
systematic review and meta-analysis. World J Urol. 38:1165–1175.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Millán-Rodríguez F, Palou J, de la
Torre-Holguera P, Vayreda-Martija JM, Villavicencio-Mavrich H and
Vicente Rodríguez J: Conventional CT signs in staging transitional
cell tumors of the upper urinary tract. Eur Urol. 35:318–322. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Pallauf M, D'Andrea D, König F, Laukhtina
E, Yanagisawa T, Rouprêt M, Daneshmand S, Djaladat H, Ghoreifi A,
Soria F, et al: Diagnostic accuracy of clinical lymph node staging
for upper tract urothelial cancer patients: A multicenter,
retrospective, observational study. J Urol. 209:515–524. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Rink M, Ehdaie B, Cha EK, Green DA,
Karakiewicz PI, Babjuk M, Margulis V, Raman JD, Svatek RS, Fajkovic
H, et al: Stage-specific impact of tumor location on oncologic
outcomes in patients with upper and lower tract urothelial
carcinoma following radical surgery. Eur Urol. 62:677–684. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Al-Zubaidi M, Viswambaram P, McCombie S,
Liow E, Lenzo N, Ferguson T, Redfern AD, Gauci R and Hayne D:
89Zirconium-labelled girentuximab (89Zr-TLX250) PET in Urothelial
Cancer Patients (ZiPUP): Protocol for a phase I trial of a novel
staging modality for urothelial carcinoma. BMJ Open.
12:e0604782022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Voskuilen CS, Schweitzer D, Jensen JB,
Nielsen AM, Joniau S, Muilwijk T, Necchi A, Azizi M, Spiess PE,
Briganti A, et al: Diagnostic Value of 18F-fluorodeoxyglucose
positron emission tomography with computed tomography for lymph
node staging in patients with upper tract urothelial carcinoma. Eur
Urol Oncol. 3:73–79. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zattoni F, Incerti E, Colicchia M,
Castellucci P, Panareo S, Picchio M, Fallanca F, Briganti A,
Moschini M, Gallina A, et al: Comparison between the diagnostic
accuracies of 18F-fluorodeoxyglucose positron emission
tomography/computed tomography and conventional imaging in
recurrent urothelial carcinomas: A retrospective, multicenter
study. Abdom Radiol (NY). 43:2391–2399. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Soubra A, Hayward D, Dahm P, Goldfarb R,
Froehlich J, Jha G and Konety BR: The diagnostic accuracy of
18F-fluorodeoxyglucose positron emission tomography and computed
tomography in staging bladder cancer: A single-institution study
and a systematic review with meta-analysis. World J Urol.
34:1229–1237. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Aydh A, Abufaraj M, Mori K, Quhal F,
Pradere B, Motlagh RS, Mostafaei H, Karakiewicz PI and Shariat SF:
Performance of fluoro-2-deoxy-D-glucose positron emission
tomography-computed tomography imaging for lymph node staging in
bladder and upper tract urothelial carcinoma: A systematic review.
Arab J Urol. 19:59–66. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jensen TK, Holt P, Gerke O, Riehmann M,
Svolgaard B, Marcussen N and Bouchelouche K: Preoperative
lymph-node staging of invasive urothelial bladder cancer with
18F-fluorodeoxyglucose positron emission tomography/computed axial
tomography and magnetic resonance imaging: Correlation with
histopathology. Scand J Urol Nephrol. 45:122–128. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Asai S, Fukumoto T, Tanji N, Miura N,
Miyagawa M, Nishimura K, Yanagihara Y, Shirato A, Miyauchi Y,
Kikugawa T and Yokoyama M: Fluorodeoxyglucose positron emission
tomography/computed tomography for diagnosis of upper urinary tract
urothelial carcinoma. Int J Clin Oncol. 20:1042–1047. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Tanaka H, Yoshida S, Komai Y, Sakai Y,
Urakami S, Yuasa T, Yamamoto S, Masuda H, Koizumi M, Kohno A, et
al: Clinical value of 18F-fluorodeoxyglucose positron emission
tomography/computed tomography in upper tract urothelial carcinoma:
Impact on detection of metastases and patient management. Urol Int.
96:65–72. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Takayanagi A, Takahashi A, Yorozuya W,
Okabe K, Kaji T and Takagi Y: The usefulness of positron emission
tomography/computed tomography in the diagnosis of metastasis in
patients with urothelial carcinoma. Nihon Hinyokika Gakkai Zasshi.
113:51–55. 2022.(In Japanese). PubMed/NCBI
|
|
86
|
Galili U: Cancer immunotherapy by
anti-gal-mediated in situ conversion of tumors into autologous
vaccines. The Natural Anti-gal Antibody as Foe Turned Friend in
Medicine, . 1st edition. Elsevier; Amsterdam: pp. 171–198. 2018
|
|
87
|
Nayak B, Dogra PN, Naswa N and Kumar R:
Diuretic 18F-FDG PET/CT imaging for detection and locoregional
staging of urinary bladder cancer: Prospective evaluation of a
novel technique. Eur J Nucl Med Mol Imaging. 40:386–393. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Jana S and Blaufox MD: Nuclear medicine
studies of the prostate, testes, and bladder. Semin Nucl Med.
36:51–72. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Sassa N, Kato K, Abe S, Iwano S, Ito S,
Ikeda M, Shimamoto K, Yamamoto S, Yamamoto T, Gotoh M and Naganawa
S: Evaluation of 11C-choline PET/CT for primary diagnosis and
staging of urothelial carcinoma of the upper urinary tract: A pilot
study. Eur J Nucl Med Mol Imaging. 41:2232–2241. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Polom W, Cytawa W, Polom A, Frankiewicz M,
Szurowska E, Lass P and Matuszewski M: Challenging visualization of
sentinel lymph nodes in upper urinary tract urothelial carcinoma. J
Clin Med. 10:54652021. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Lebastchi AH, Gupta N, DiBianco JM, Piert
M, Davenport MS, Ahdoot MA, Gurram S, Bloom JB, Gomella PT,
Mehralivand S, et al: Comparison of cross-sectional imaging
techniques for the detection of prostate cancer lymph node
metastasis: A critical review. Transl Androl Urol. 9:1415–1427.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Fortuin AS, Smeenk RJ, Meijer HJ, Witjes
AJ and Barentsz JO: Lymphotropic nanoparticle-enhanced MRI in
prostate cancer: Value and therapeutic potential. Curr Urol Rep.
15:3892014. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Birkhäuser FD, Studer UE, Froehlich JM,
Triantafyllou M, Bains LJ, Petralia G, Vermathen P, Fleischmann A
and Thoeny HC: Combined ultrasmall superparamagnetic particles of
iron oxide-enhanced and diffusion-weighted magnetic resonance
imaging facilitates detection of metastases in normal-sized pelvic
lymph nodes of patients with bladder and prostate cancer. Eur Urol.
64:953–960. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Caglic I and Barrett T: Diffusion-weighted
imaging (DWI) in lymph node staging for prostate cancer. Transl
Androl Urol. 7:814–823. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Woo S, Suh CH, Kim SY, Cho JY and Kim SH:
The diagnostic performance of MRI for detection of lymph node
metastasis in bladder and prostate cancer: An updated systematic
review and diagnostic meta-analysis. AJR Am J Roentgenol.
210:W95–W109. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shamis SAK, Edwards J and McMillan DC: The
relationship between carbonic anhydrase IX (CAIX) and patient
survival in breast cancer: Systematic review and meta-analysis.
Diagn Pathol. 18:462023. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Venkat S, Khan AI, Lewicki PJ, Borregales
L and Scherr DS: Novel nomograms to predict muscle invasion and
lymph node metastasis in upper tract urothelial carcinoma. Urol
Oncol. 40:108.e11–108.e17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Li WM, Chan TC, Huang SK, Wu WJ, Ke HL,
Liang PI, Wei YC, Shiue YL and Li CF: Prognostic utility of FBLN2
expression in patients with urothelial carcinoma. Front Oncol.
10:5703402020. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Ibrahim AM, Sabet S, El-Ghor AA, Kamel N,
Anis SE, Morris JS and Stein T: Fibulin-2 is required for basement
membrane integrity of mammary epithelium. Sci Rep. 8:141392018.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
de Vega S, Iwamoto T and Yamada Y:
Fibulins: Multiple roles in matrix structures and tissue functions.
Cell Mol Life Sci. 66:1890–1902. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Argraves WS, Greene LM, Cooley MA and
Gallagher WM: Fibulins: Physiological and disease perspectives.
EMBO Rep. 4:1127–1131. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Timpl R, Sasaki T, Kostka G and Chu ML:
Fibulins: A versatile family of extracellular matrix proteins. Nat
Rev Mol Cell Biol. 4:479–489. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Baird BN, Schliekelman MJ, Ahn YH, Chen Y,
Roybal JD, Gill BJ, Mishra DK, Erez B, O'Reilly M, Yang Y, et al:
Fibulin-2 is a driver of malignant progression in lung
adenocarcinoma. PLoS One. 8:e670542013. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Senapati S, Gnanapragassam VS, Moniaux N,
Momi N and Batra SK: Role of MUC4-NIDO domain in the MUC4-mediated
metastasis of pancreatic cancer cells. Oncogene. 31:3346–3356.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mohammad T, Arif K, Alajmi MF, Hussain A,
Islam A, Rehman MT and Hassan I: Identification of high-affinity
inhibitors of pyruvate dehydrogenase kinase-3: Towards therapeutic
management of cancer. J Biomol Struct Dyn. 39:586–594. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Zhu J, Zheng G, Xu H, Jin X, Tang T and
Wang X: Expression and prognostic significance of pyruvate
dehydrogenase kinase 1 in bladder urothelial carcinoma. Virchows
Arch. 477:637–649. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Woolbright BL, Choudhary D, Mikhalyuk A,
Trammel C, Shanmugam S, Abbott E, Pilbeam CC and Taylor JA III: The
role of pyruvate dehydrogenase kinase-4 (PDK4) in bladder cancer
and chemoresistance. Mol Cancer Ther. 17:2004–2012. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Kuo YH, Chan TC, Lai HY, Chen TJ, Wu LC,
Hsing CH and Li CF: Overexpression of pyruvate dehydrogenase
kinase-3 predicts poor prognosis in urothelial carcinoma. Front
Oncol. 11:7491422021. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Posey KL, Coustry F and Hecht JT:
Cartilage oligomeric matrix protein: COMPopathies and beyond.
Matrix Biol. 71-72:161–173. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Englund E, Bartoschek M, Reitsma B,
Jacobsson L, Escudero-Esparza A, Orimo A, Leandersson K, Hagerling
C, Aspberg A, Storm P, et al: Cartilage oligomeric matrix protein
contributes to the development and metastasis of breast cancer.
Oncogene. 35:5585–5596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Liu TT, Liu XS, Zhang M, Liu XN, Zhu FX,
Zhu FM, Ouyang SW, Li SB, Song CL, Sun HM, et al: Cartilage
oligomeric matrix protein is a prognostic factor and biomarker of
colon cancer and promotes cell proliferation by activating the Akt
pathway. J Cancer Res Clin Oncol. 144:1049–1063. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Norman GL, Gatselis NK, Shums Z, Liaskos
C, Bogdanos DP, Koukoulis GK and Dalekos GN: Cartilage oligomeric
matrix protein: A novel non-invasive marker for assessing cirrhosis
and risk of hepatocellular carcinoma. World J Hepatol. 7:1875–1883.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Kuo YH, Lai HY, Chan TC, Hsing CH, Huang
SK, Hsieh KL, Chen TJ, Li WS, Lu JC and Li CF: Upregulation of
cartilage oligomeric matrix protein predicts poor prognosis in
urothelial carcinoma. Onco Targets Ther. 15:727–740. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Li WM, Ke HL, Kuo YH, Lai HY, Chan TC,
Hsing CH, Hsieh KL, Li WS, Chen TJ, Wei YC, et al: High MT2A
expression predicts worse prognosis in patients with urothelial
carcinoma. Oncology. 100:485–497. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Nishikawa M, Miyake H, Kurahashi T and
Fujisawa M: Significance of multiple preoperative laboratory
abnormalities as prognostic indicators in patients with urothelial
carcinoma of the upper urinary tract following radical
nephroureterectomy. Int J Clin Oncol. 23:151–157. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Mori K, Janisch F, Mostafaei H, Kimura S,
Lysenko I, Karakiewicz PI, Briganti A, Enikeev DV, Rouprêt M,
Margulis V, et al: Prognostic role of preoperative De Ritis ratio
in upper tract urothelial carcinoma treated with
nephroureterectomy. Urol Oncol. 38:601.e17–601.e24. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Coussens LM and Werb Z: Inflammation and
cancer. Nature. 420:860–867. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Wang K, Diao F, Ye Z, Zhang X, Zhai E, Ren
H, Li T, Wu H, He Y, Cai S and Chen J: Prognostic value of systemic
immune-inflammation index in patients with gastric cancer. Chin J
Cancer. 36:752017. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Zhang Y, Chen B, Wang L, Wang R and Yang
X: Systemic immune-inflammation index is a promising noninvasive
marker to predict survival of lung cancer: A meta-analysis.
Medicine (Baltimore). 98:e137882019. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Wang L, Wang C, Wang J, Huang X and Cheng
Y: A novel systemic immune-inflammation index predicts survival and
quality of life of patients after curative resection for esophageal
squamous cell carcinoma. J Cancer Res Clin Oncol. 143:2077–2086.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Jan HC, Wu KY, Tai TY, Weng HY, Yang WH,
Ou CH and Hu CY: The systemic immune-inflammation index (SII)
increases the prognostic significance of lymphovascular invasion in
upper tract urothelial carcinoma after radical nephroureterectomy.
Cancer Manag Res. 14:3139–3149. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Kobayashi S, Ito M, Takemura K, Suzuki H,
Yonese I and Koga F: Preoperative models incorporating the systemic
immune-inflammation index for predicting prognosis and muscle
invasion in patients with non-metastatic upper tract urothelial
carcinoma. Int J Clin Oncol. 27:574–584. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33.
2021.PubMed/NCBI
|
|
125
|
Therkildsen C, Eriksson P, Höglund M,
Jönsson M, Sjödahl G, Nilbert M and Liedberg F: Molecular subtype
classification of urothelial carcinoma in Lynch syndrome. Mol
Oncol. 12:1286–1295. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Pradere B, Lotan Y and Roupret M: Lynch
syndrome in upper tract urothelial carcinoma: Significance,
screening, and surveillance. Curr Opin Urol. 27:48–55. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Audenet F, Colin P, Yates DR, Ouzzane A,
Pignot G, Long JA, Soulie M, Phé V, Bensadoun H, Guy L, et al: A
proportion of hereditary upper urinary tract urothelial carcinomas
are misclassified as sporadic according to a multi-institutional
database analysis: Proposal of patient-specific risk identification
tool. BJU Int. 110:E583–E589. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Ju JY, Mills AM, Mahadevan MS, Fan J, Culp
SH, Thomas MH and Cathro HP: Universal lynch syndrome screening
should be performed in all upper tract urothelial carcinomas. Am J
Surg Pathol. 42:1549–1555. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Gayhart MG, Johnson N, Paul A, Quillin JM,
Hampton LJ, Idowu MO and Smith SC: Universal mismatch repair
protein screening in upper tract urothelial carcinoma. Am J Clin
Pathol. 154:792–801. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Rasmussen M, Madsen MG and Therkildsen C:
Immunohistochemical screening of upper tract urothelial carcinomas
for lynch syndrome diagnostics: A systematic review. Urology.
165:44–53. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Vuky J, Balar AV, Castellano D, O'Donnell
PH, Grivas P, Bellmunt J, Powles T, Bajorin D, Hahn NM, Savage MJ,
et al: Long-term outcomes in KEYNOTE-052: Phase II study
investigating first-line pembrolizumab in cisplatin-ineligible
patients with locally advanced or metastatic urothelial cancer. J
Clin Oncol. 38:2658–2666. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Balar AV, Galsky MD, Rosenberg JE, Powles
T, Petrylak DP, Bellmunt J, Loriot Y, Necchi A, Hoffman-Censits J,
Perez-Gracia JL, et al: Atezolizumab as first-line treatment in
cisplatin-ineligible patients with locally advanced and metastatic
urothelial carcinoma: A single-arm, multicentre, phase 2 trial.
Lancet. 389:67–76. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Bellmunt J, de Wit R, Vaughn DJ, Fradet Y,
Lee JL, Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK,
et al: Pembrolizumab as second-line therapy for advanced urothelial
carcinoma. N Engl J Med. 376:1015–1026. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Loriot Y, Necchi A, Park SH, Garcia-Donas
J, Huddart R, Burgess E, Fleming M, Rezazadeh A, Mellado B,
Varlamov S, et al: Erdafitinib in locally advanced or metastatic
urothelial carcinoma. N Engl J Med. 381:338–348. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Fujii Y, Sato Y, Suzuki H, Kakiuchi N,
Yoshizato T, Lenis AT, Maekawa S, Yokoyama A, Takeuchi Y, Inoue Y,
et al: Molecular classification and diagnostics of upper urinary
tract urothelial carcinoma. Cancer Cell. 39:793–809.e8. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Seisen T, Colin P, Hupertan V, Yates DR,
Xylinas E, Nison L, Cussenot O, Neuzillet Y, Bensalah K, Novara G,
et al: Postoperative nomogram to predict cancer-specific survival
after radical nephroureterectomy in patients with localised and/or
locally advanced upper tract urothelial carcinoma without
metastasis. BJU Int. 114:733–740. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Seisen T, Peyronnet B, Dominguez-Escrig
JL, Bruins HM, Yuan CY, Babjuk M, Böhle A, Burger M, Compérat EM,
Cowan NC, et al: Oncologic outcomes of kidney-sparing surgery
versus radical nephroureterectomy for upper tract urothelial
carcinoma: A systematic review by the EAU non-muscle invasive
bladder cancer guidelines panel. Eur Urol. 70:1052–1068. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Leow JJ, Chong YL, Chang SL, Valderrama
BP, Powles T and Bellmunt J: Neoadjuvant and adjuvant chemotherapy
for upper tract urothelial carcinoma: A 2020 systematic review and
meta-analysis, and future perspectives on systemic therapy. Eur
Urol. 79:635–654. 2021. View Article : Google Scholar : PubMed/NCBI
|