Introduction
Gynecological malignancies represent a major threat
to women's health. According to data from the International Agency
for Research on Cancer in 2020, there are ~1.33 million new cases
and 540,000 deaths related to gynecological cancers worldwide each
year (1,2). Cervical, endometrial and ovarian
cancers are the three most common malignancies of the female
reproductive system and, together with breast cancer, constitute a
substantial global health burden for women, accounting for nearly
one-third of all cancer-related incidence and mortality among
female patients (3). Despite
significant progress in screening, diagnosis and treatment, these
malignancies remain leading causes of female cancer-related deaths
due to their biological heterogeneity, late-stage diagnosis and
therapeutic resistance (4,5). Breast cancer is the most prevalent
malignancy in women. Molecular subtyping-such as
estrogen/progesterone receptor (ER/PR)-positive, HER2-enriched, and
triple-negative breast cancer (TNBC)-has led to breakthroughs in
targeted therapies, including CDK4/6 inhibitors and anti-HER2
agents (6). However, the management
of metastatic disease and the emergence of drug resistance continue
to pose major challenges. Among gynecological cancers, ovarian
cancer has the highest mortality rate due to its asymptomatic
nature and frequent diagnosis at an advanced stage (7). Recent advances, such as PARP
inhibitors for BRCA-mutated tumors and immunotherapy, have
redefined treatment strategies, yet the high recurrence rate
underscores the urgent need for novel biomarkers (8). Endometrial cancer has traditionally
been associated with obesity and metabolic disorders, and molecular
subtypes identified through The Cancer Genome Atlas-including
POLE-ultra-mutated and microsatellite instability-high types-have
provided a basis for risk stratification and immunotherapy
application (9). While cervical
cancer is largely preventable through HPV vaccination and early
screening, it remains prevalent in low-resource regions. Emerging
therapies, such as PD-1/PD-L1 inhibitors and therapeutic vaccines,
offer new hope for patients with advanced disease (10).
Currently, surgical resection, radiotherapy,
chemotherapy, targeted therapy and immunotherapy are employed in
the treatment of gynecological malignancies. However, the incidence
and mortality rates of these cancers continue to rise annually
(11,12). Despite advances in modern medicine,
therapeutic efficacy remains limited, and challenges such as drug
resistance and adverse side effects persist (13). Increasing attention has therefore
been directed toward bioactive compounds from traditional Chinese
medicine as complementary or alternative therapeutic strategies in
breast and gynecological cancers (14). Natural products such as curcumin,
quercetin and tanshinone have demonstrated anti-proliferative,
pro-apoptotic, anti-metastatic and chemo-sensitizing activities via
regulation of multiple signaling pathways, including PI3K/AKT,
NF-κB and Wnt/β-catenin (15–17).
Among them, ursolic acid (UA), a pentacyclic triterpenoid widely
distributed in vegetables, fruits and medicinal herbs (18), has attracted increasing interest due
to its potent anticancer properties and relatively low toxicity. UA
exerts broad antitumor effects by modulating diverse cellular
processes, including proliferation, apoptosis, metastasis and
chemoresistance, while sparing normal cells (19,20).
Furthermore, UA exhibits neuroprotective effects such as analgesic,
anxiolytic, antidepressant and memory-enhancing properties,
underscoring its vast pharmacological potential and clinical
applicability (21,22). Recent studies suggest that UA holds
significant therapeutic potential in gynecological and breast
cancers, particularly through regulation of key oncogenic pathways
and enhancement of conventional treatment efficacy (23–25).
The present review summarizes current progress on the anticancer
mechanisms of UA in these malignancies, with emphasis on molecular
targets, the development of UA derivatives, and novel formulations
aimed at improving its bioavailability and clinical
applicability.
Structural properties, natural origins and
extraction methods of UA
UA, with the molecular formula
C30H48O3, is a pentacyclic
triterpenoid compound (26). It
exists either in its free acid form or as the aglycone component of
triterpenoid saponins, and is widely distributed in natural plants
such as Plantago asiatica, Cornus officinalis, Crataegus
pinnatifida, Hedyotis diffusa, Prunella vulgaris, rosemary,
loquat and apples (27). UA is a
structurally complex molecule featuring 10 chiral centers, making
it challenging to synthesize via conventional chemical methods. At
present, extraction from natural plant sources remains the primary
approach for obtaining UA.
Pure UA appears as white crystals that are soluble
in methanol, ethanol and butanol, sparingly soluble in ether and
chloroform, and insoluble in water and petroleum ether (28). Traditional extraction
techniques-such as Soxhlet extraction, pulverization extraction and
reflux extraction-often suffer from limitations including low
purity and extended processing time (29). With technological advancements,
novel extraction methods such as ultrasonic-assisted extraction,
supercritical fluid extraction and semi-bionic extraction have
gained attention (30). Among
these, the semi-bionic approach, which simulates gastrointestinal
digestion and absorption based on biopharmaceutics theory, has
shown improved efficiency in isolating target compounds. Studies
have demonstrated that parameters such as the solvent-to-material
ratio, ethanol concentration, ultrasonic duration and power
significantly influence the yield of UA during ultrasonic-assisted
extraction (21,31,32).
Due to its strong lipophilicity, UA is optimally extracted using
low-polarity solvents such as ethanol (33). Furthermore, with continued research,
the pharmacological potential of UA derivatives has been
increasingly recognized. Structural modifications at the C-3 and
C-28 positions have been found to enhance its bioactivity (19,34).
Some derivatives exhibit notable antitumor, antifungal and
antioxidant properties, providing a theoretical foundation and
experimental support for novel drug development (35,36).
Anticancer mechanisms of UA in gynecological
and breast cancers
UA, a natural pentacyclic triterpenoid compound, has
demonstrated broad-spectrum anticancer potential in various
gynecological malignancies, including ovarian, endometrial and
cervical cancers, as well as in breast cancer. Its multifaceted
mechanisms of action include induction of apoptosis, inhibition of
cell proliferation and metastasis, regulation of metabolic
reprogramming, suppression of cancer stem cell (CSC) properties,
and enhancement of chemosensitivity (Table SI).
UA and ovarian cancer
Induction of tumor cell apoptosis
UA can effectively inhibit the proliferation of
tumor cells by inducing apoptosis and causing cell cycle arrest.
Song et al (23)
systematically elucidated the pro-apoptotic mechanisms of UA and
found that it induces a classical apoptotic process in SKOV3
ovarian cancer cells, activates the caspase cascade, suppresses the
expression of oncogenic proteins such as c-Myc, and enhances its
antitumor effects by activating the GSK-3β/β-catenin signaling
pathway. Subsequently, Lin and Ye (37) further confirmed the inhibitory
effects of UA on ovarian cancer cells, demonstrating that it
suppresses cell proliferation in a dose-dependent manner with high
selectivity. The underlying mechanisms include the induction of
apoptosis, G2/M phase cell cycle arrest, elevation of intracellular
reactive oxygen species (ROS) levels, modulation of the Bax/Bcl-2
ratio, and inhibition of the PI3K/AKT signaling pathway.
Regulation of metabolic reprogramming
Beyond apoptosis, UA also disrupts ovarian cancer
metabolism. Glycolysis serves as a critical energy metabolism
pathway in ovarian cancer cells, and its dysregulation is closely
associated with tumor progression and chemoresistance (38). RNA sequencing and a series of in
vitro and in vivo experiments have demonstrated that UA
significantly inhibits ovarian cancer cell proliferation, induces
apoptosis, and reduces glycolytic activity (39). Mechanistically, UA binds to and
inhibits the transcription factor KLF5, thereby blocking the
transcriptional activation of the downstream PI3K/AKT signaling
pathway. This dual inhibition of glycolysis and cancer cell
survival highlights the therapeutic potential of UA in targeting
metabolic vulnerabilities in ovarian cancer.
Enhancing efficacy and reducing toxicity via
chemotherapy combination
In addition to direct tumor inhibition, UA also
plays an important role in improving chemotherapy outcomes.
Cisplatin (CDDP) is widely used in the treatment of various
cancers; however, its ototoxicity often leads to irreversible
hearing loss (40,41). A study by Di et al (42) demonstrated that UA significantly
alleviates CDDP-induced ototoxic damage and preserves auditory
function by inhibiting the TRPV1/Ca2+/calpain-oxidative
stress signaling pathway. Notably, UA also enhances the antitumor
efficacy of CDDP against ovarian cancer cells, exerting synergistic
and protective effects without compromising its anticancer potency.
Beyond CDDP, ovarian cancer is also prone to developing resistance
to chemotherapeutic agents such as doxorubicin, which remains a
major cause of treatment failure. Li et al (43) established a doxorubicin-resistant
SKOV3-Adr cell model and found that UA markedly increased the
sensitivity of these cells to doxorubicin. Furthermore, UA
synergistically enhanced the efficacy of doxorubicin in both
parental SKOV3 and A2780 cells. Mechanistically, although HuR mRNA
expression levels were comparable between SKOV3 and SKOV3-Adr
cells, HuR protein was predominantly accumulated in the cytoplasm
of resistant cells, where it stabilized MDR1 mRNA and promoted drug
resistance. UA facilitated the translocation of HuR from the
cytoplasm to the nucleus, thereby reducing MDR1 mRNA stability and
downregulating its expression, ultimately restoring sensitivity to
doxorubicin. The present study highlights a novel mechanism by
which UA reverses chemoresistance in ovarian cancer through
post-transcriptional regulation of mRNA stability, underscoring its
potential as an adjuvant to conventional chemotherapy.
Inhibition of CSC traits and drug-resistant
phenotype
In ovarian cancer, CSCs are recognized as key
contributors to tumor recurrence, metastasis and chemoresistance
(44,45). Emerging evidence suggests that UA
can enhance the sensitivity of ovarian cancer CSCs to CDDP,
significantly reversing the stemness and drug-resistant phenotypes
typically induced by the hypoxic tumor microenvironment (TME). This
effect is closely associated with inhibition of the PI3K/Akt
signaling pathway and downregulation of the HIF-1α/ABCG2 axis, with
further therapeutic enhancement observed when combined with HIF-1α
inhibitors (46). Moreover, UA
suppresses the self-renewal and migratory capacities of ovarian
CSCs by downregulating stem cell markers such as CD133 and ALDH1,
as well as epithelial-mesenchymal transition (EMT)-related factors
including N-cadherin and vimentin. These changes contribute to a
marked improvement in the efficacy of chemotherapeutic agents
(47). Collectively, these findings
highlight the promising potential of UA in targeting CSC-associated
stemness maintenance and overcoming chemoresistance in ovarian
cancer (Fig. 1).
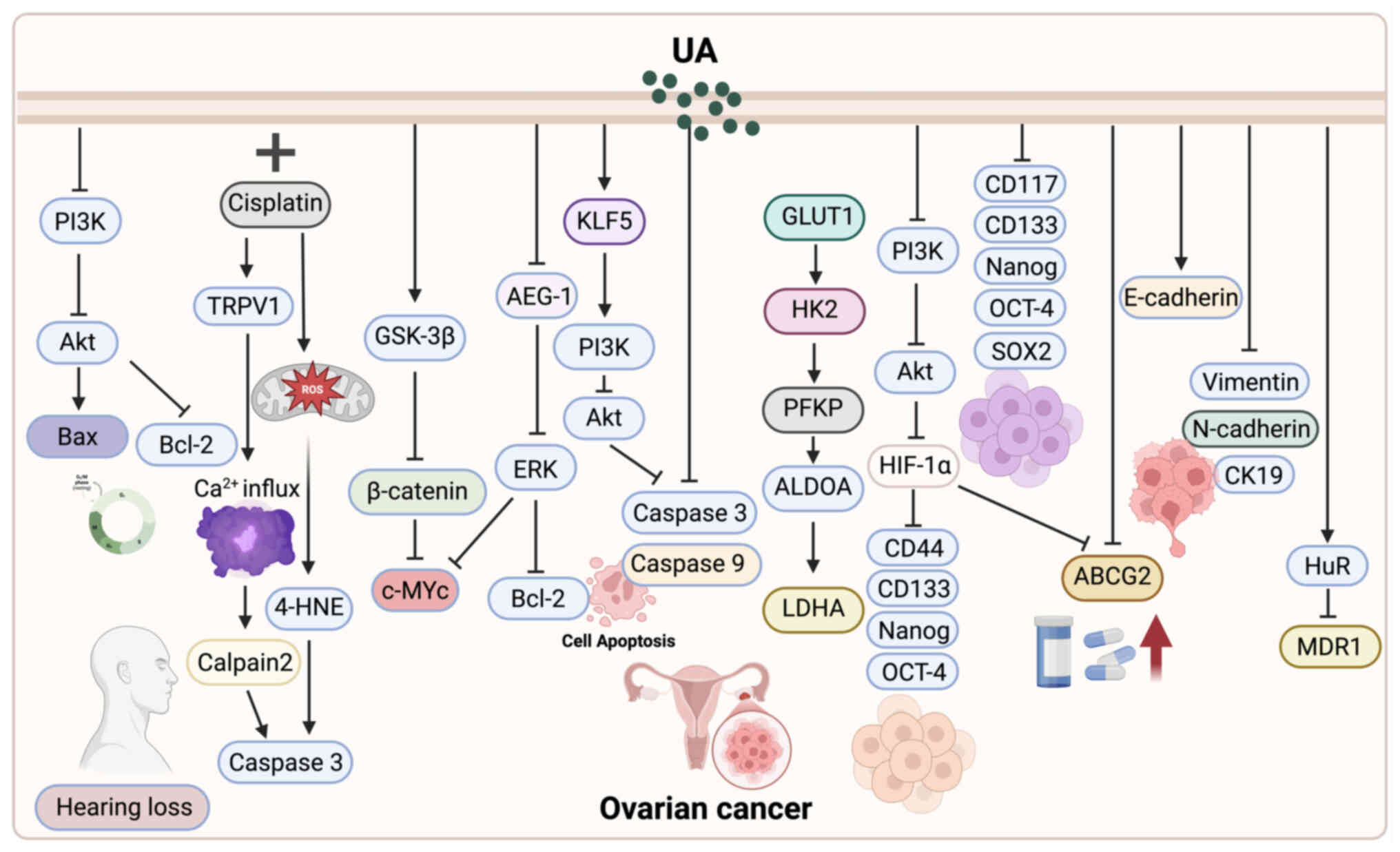 | Figure 1.UA exerts multifaceted antitumor
effects against ovarian cancer. It induces apoptosis and cell cycle
arrest by activating the caspase cascade, modulating the Bax/Bcl-2
ratio, and inhibiting the PI3K/AKT and GSK-3β/β-catenin pathways.
UA also suppresses glycolysis and metabolic reprogramming by
targeting KLF5 and downstream PI3K/AKT signaling. When combined
with chemotherapeutic agents such as cisplatin and doxorubicin, UA
enhances efficacy while reducing toxicity and reversing drug
resistance through regulation of HuR/MDR1 expression. Additionally,
UA targets ovarian cancer stem cells, inhibiting their
self-renewal, migration and stemness via suppression of the
PI3K/AKT and HIF-1α/ABCG2 pathways, and downregulation of CD133,
ALDH1, N-cadherin and vimentin. These mechanisms collectively
highlight UA's therapeutic potential in overcoming chemoresistance
and improving ovarian cancer treatment outcomes. UA, ursolic
acid. |
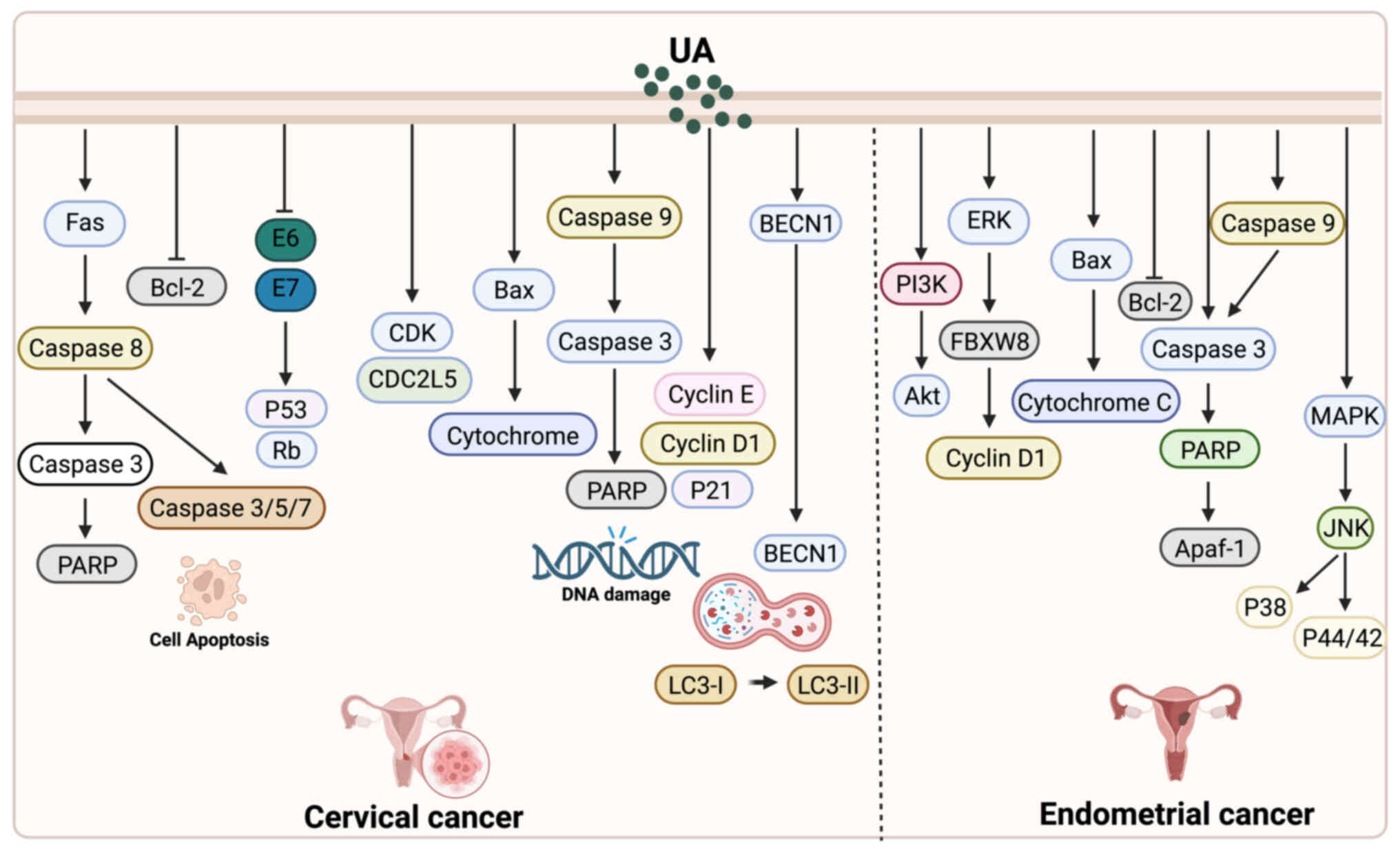
UA and cervical cancer
Antiviral and pro-apoptotic
effects
UA exhibits multiple biological effects in cervical
cancer cells, with particularly notable advantages in antiviral
activity and apoptosis induction. Using proteomic techniques
including 2DE/MALDI-TOF-MS and SELDI-TOF-MS, Yim et al
(25) analyzed HeLa cells treated
with UA and identified significant alterations in the expression of
25 proteins, most of which were associated with apoptotic
processes. Additionally, eight distinct peptide peaks were
observed. In a subsequent study, Yim et al (48) further demonstrated that UA exerts
potent antiproliferative and pro-apoptotic effects specifically in
HPV-positive cervical cancer cells, along with measurable antiviral
activity. Mechanistically, UA was shown to activate the
Fas/caspase-mediated apoptotic pathway and suppress the expression
of HPV oncogenes E6 and E7.
Autophagy-mediated cell death
Beyond apoptosis, UA also induces autophagy as an
alternative form of tumor cell death. Leng et al (49) reported that in mouse cervical cancer
TC-1 cells, UA predominantly induces Atg5-dependent autophagy
rather than classical apoptosis to exert its anticancer effects.
Inhibition of autophagy or knockdown of Atg5 significantly
increased cell viability, suggesting that UA promotes tumor cell
death primarily through autophagic pathways. These findings provide
new insights into autophagy as a complementary mechanism to
apoptosis in UA-mediated cancer therapy. Notably, however, in other
tumor types such as breast cancer, UA-induced autophagy may not
uniformly lead to cell death but instead exert context-dependent,
and occasionally cytoprotective, effects.
Endoplasmic reticulum (ER) stress-related
apoptotic mechanism
Another important mechanism by which UA induces
cervical cancer cell death involves the activation of the ER stress
pathway. Guo et al (50)
treated HeLa cells with UA and/or the ER stress inhibitor
4-phenylbutyric acid (4-PBA), and found that UA inhibited cell
viability and induced apoptosis in a time- and dose-dependent
manner. Mechanistically, UA significantly upregulated the
expression of key ER stress markers such as GRP78 and CHOP, while
the addition of 4-PBA partially reversed its pro-apoptotic
effects.
Chemotherapy synergistic effect
In addition to its direct anticancer actions, UA
enhances the efficacy of conventional chemotherapy in cervical
cancer. Li et al (51)
reported that UA increases the sensitivity of cervical cancer cells
to commonly used chemotherapeutic agents such as paclitaxel and
CDDP by inhibiting the NF-κB signaling pathway. Combination
treatment activates the Fas/FasL-caspase-8-BID axis and enhances
the mitochondrial apoptotic pathway, selectively promoting
apoptosis in tumor cells without exerting toxicity on normal cells.
Furthermore, co-treatment with UA and CDDP significantly
downregulates the expression of NF-κB p65 and Bcl-2, while
upregulating the levels of Bax, cleaved caspase-3 and PARP cleavage
products, thereby markedly increasing the rate of apoptosis
(Fig. 2).
UA and endometrial cancer
UA has demonstrated multiple antitumor mechanisms in
endometrial cancer research (Fig.
2). Achiwa et al (52)
found that UA downregulates Cyclin D1 expression and interferes
with cell cycle regulation by inhibiting the MAPK-Cyclin D1
signaling pathway and the RING-type E3 ligase SCF complex, thereby
preventing the ubiquitin-dependent degradation of Cyclin D1.
Additionally, UA has been identified to exert its effects via the
CD36 membrane receptor, suggesting a potentially important role in
protein homeostasis and membrane receptor signaling regulation
(53). In terms of apoptosis'
induction, UA inhibits the proliferation of endometrial cancer
cells in a dose- and time-dependent manner. It triggers
mitochondrial-mediated apoptosis by activating caspases-3, −8, and
−9, promoting PARP cleavage, inducing DNA fragmentation, and
facilitating cytochrome c release. This is accompanied by
downregulation of the anti-apoptotic protein Bcl-2 and upregulation
of the pro-apoptotic protein Bax (54). Moreover, UA significantly suppresses
the PI3K-Akt and MAPK signaling pathways (including JNK, p38 and
ERK1/2), reducing the phosphorylation levels of key proteins and
thereby further enhancing its antiproliferative and pro-apoptotic
effects-particularly notable in SNG-II cells (55). Taken together, UA exerts synergistic
effects through multiple signaling pathways to regulate the cell
cycle, inhibit proliferation, and induce apoptosis, highlighting
its therapeutic potential in endometrial cancer.
UA and breast cancer
Inhibition of cell proliferation and
induction of apoptosis
One of the most extensively studied effects of UA in
breast cancer is its ability to suppress tumor cell proliferation
and trigger apoptosis. Zhang et al (24) found that UA suppresses the
proliferation of MDA-MB-231 breast cancer cells by modulating the
Keap1/Nrf2 and EGFR/Nrf2 pathways. It also upregulates Nrf2 and its
downstream target genes such as NQO1 and SOD1 via
post-translational regulation. De Angel et al (56) demonstrated that UA inhibits tumor
growth in breast cancer mouse models, potentially through
modulation of the Akt/mTOR pathway, induction of apoptosis, and
disruption of the cell cycle. Wang et al (57) reported that UA suppresses MCF-7 cell
proliferation by downregulating the transcription factor FoxM1 and
inhibiting the Cyclin D1/CDK4 signaling pathway. Additionally,
Kassi et al (58) observed
that UA induces intrinsic apoptosis via activation of the
mitochondrial pathway and may interact with the glucocorticoid
receptor (GR), suggesting a regulatory effect on GR function.
Mallepogu et al (59) found
that UA induces cell cycle arrest and apoptosis, and inhibits EMT
by downregulating EMT-related genes such as Snail and Slug.
Manouchehri and Kalafatis (60)
revealed that UA sensitizes triple-negative breast cancer cells to
rhTRAIL-induced apoptosis by upregulating death receptors DR4/5 and
downregulating the anti-apoptotic molecule c-FLIPL. Guo et
al (61) showed that UA
significantly inhibits the proliferation of MCF-7 cells by
suppressing both the RAF/ERK and IKK/NF-κB signaling pathways.
Furthermore, Kim (62) reported
that UA induces cell cycle arrest in breast cancer stem-like cells
by increasing the expression of p53 and p21 and decreasing the
expression of cyclins D and E, as well as CDK4 and CDK2. This is
accompanied by inhibition of the ERK and PI3K/AKT signaling
pathways, contributing to the anti-proliferative effects.
Induction of autophagy and improvement
of metabolism
UA exhibits significant potential in inducing
autophagy and modulating cellular metabolism in breast cancer
cells, but its effects appear to be context-dependent. Regarding
autophagy, Zhao et al (63)
reported that UA upregulates MCL1 via the MAPK1/3 signaling
pathway, which partially counteracts UA-induced apoptosis,
indicating a cytoprotective role of autophagy. By contrast, Gupta
et al (64) demonstrated
that UA, in combination with oleanolic acid, synergistically
induces cytotoxic autophagy, thereby enhancing its antitumor
efficacy. Lewinska et al (65) further showed that UA promotes
oxidative stress and DNA damage by inhibiting the AKT pathway and
activating the AMPK pathway, ultimately triggering both autophagy
and apoptosis, while Fogde et al (66) found that UA induces apoptosis by
disrupting lysosomal function and indirectly suppressing autophagy.
Collectively, these findings suggest that UA-induced autophagy in
breast cancer may function as a ‘double-edged sword’, either
supporting tumor cell survival or promoting cell death depending on
the cellular and signaling context.
In addition to its role in autophagy, UA also
exhibits pronounced metabolic regulatory effects. Guerra et
al (67) revealed that UA
influences key metabolic processes, including glycolysis the
tricarboxylic acid cycle and lipid biosynthesis, in both breast
cancer cells and normal mammary epithelial cells, suggesting a
broader cellular detoxification response. Similarly, Wang et
al (68) demonstrated that UA
activates the SP1/Caveolin-1 pathway to suppress glycolysis and
impair mitochondrial function, leading to mitochondria-dependent
apoptosis. These findings highlight that UA not only reprograms
cancer cell metabolism but also couples metabolic stress with cell
death pathways. Taken together, UA enhances its antitumor activity
in breast cancer through the dual regulation of autophagy and
metabolic reprogramming.
Importantly, the context-dependent nature of
UA-induced autophagy also carries therapeutic implications. When
autophagy functions as a pro-death mechanism, UA may synergize with
apoptosis to maximize anticancer efficacy. Conversely, when
autophagy plays a pro-survival role, it could undermine UA's
therapeutic effects, suggesting that combination with autophagy
inhibitors (for example, chloroquine or 3-methyladenine) may
enhance treatment outcomes. This dualistic role underscores the
necessity of identifying tumor-specific contexts and potential
biomarkers to predict autophagy responses. Future studies should
therefore not only clarify the upstream and downstream signaling
pathways of UA-mediated autophagy but also evaluate combined
therapeutic strategies that either exploit or counteract autophagy,
thereby optimizing the clinical benefit of UA-based
interventions.
Targeting breast CSCs
CSCs play a crucial role in therapy resistance and
disease relapse. Accumulating evidence suggests that UA effectively
targets breast CSCs (BCSCs). Mandal et al (69) found that UA downregulates the
oncogenic microRNA miR-499a-5p and upregulates the Wnt inhibitor
sFRP4, thereby inhibiting the Wnt/β-catenin signaling pathway and
attenuating the proliferation, migration and stem-like traits of
BCSCs. Similarly, Liao et al (70) demonstrated that UA suppresses the
expression of Argonaute 2, leading to reduced levels of miR-9 and
miR-221, which in turn decreases the expression of stemness markers
and EMT-related proteins. This results in the inhibition of CSC
characteristics, as well as diminished migratory and invasive
capacities. Moreover, Yang et al (71) reported that UA stabilizes KEAP1 and
inhibits NRF2 pathway activation, inducing ferroptosis in
triple-negative BCSCs. This effectively impairs their stemness and
proliferative abilities and has been validated in vivo using
animal models. Collectively, these findings highlight the potential
of UA in targeting BCSCs, offering novel insights into its
application as an anticancer therapeutic agent.
Interfering with metastasis and
invasion
Metastasis is the leading cause of breast cancer
mortality, and UA has been shown to effectively inhibit this
process. Yeh et al (72)
reported that UA, at non-cytotoxic concentrations, markedly
suppresses the migration and invasion of TNBC cells. This effect is
mediated by the downregulation of MMP-2 and u-PA expression, along
with the upregulation of endogenous inhibitors, thereby disrupting
metastatic signaling pathways. Luo et al (73) further confirmed that UA inhibits the
PI3K/Akt signaling pathway, activates GSK and caspase-3, and
downregulates Cyclin D1 and Bcl-2 expression. These molecular
events collectively induce autophagy and apoptosis while
suppressing NF-κB signaling, contributing to both anti-inflammatory
effects and reduced cellular invasiveness. Additionally, Zhang
et al (74) demonstrated
that UA inhibits TNBC cell proliferation, migration and invasion in
a dose-dependent manner by inducing cell cycle arrest and
apoptosis. Together, these studies highlight the potential of UA as
a promising agent for targeting metastasis and invasion in breast
cancer therapy.
Enhancing drug sensitivity and
reversing drug resistance
Chemoresistance is a major obstacle in breast cancer
treatment, and UA has shown significant promise in overcoming this
challenge. Lu et al (75)
found that UA reverses doxorubicin (DOX) resistance in TNBC by
downregulating ZEB1-AS1, thereby releasing its sponging effect on
miR-186-5p and ultimately suppressing ABCC1 expression. Similarly,
Wang et al (76) reported
that UA significantly increases the sensitivity of breast cancer
cells to epirubicin, partly by modulating the PI3K/Akt/mTOR
signaling pathway to enhance chemotherapeutic efficacy. Zong et
al (77) demonstrated that UA,
when co-administered with DOX, markedly increases DOX accumulation
in multidrug-resistant breast cancer cells and promotes its nuclear
translocation, thereby enhancing its antitumor activity. Further
studies by the same group revealed that UA also inhibits the
Erk-VEGF/MMP-9 signaling pathway, thereby reducing cell invasion
and migration while concurrently increasing intracellular DOX
accumulation (78). Luo et
al (79) showed that the
combination of UA and DOX significantly suppresses the
proliferation and migration of drug-resistant breast cancer cells.
This effect is mediated through modulation of the AMPK/mTOR/PGC-1α
axis, resulting in elevated ROS production and inhibition of
aerobic glycolysis, ultimately enhancing DOX efficacy.
Additionally, UA has been shown to reverse paclitaxel resistance in
breast cancer by upregulating miR-149-5p, inhibiting MyD88, and
downregulating the Akt signaling pathway (80). Collectively, these findings provide
robust theoretical and experimental support for the use of UA as an
adjuvant agent in combination chemotherapy to overcome drug
resistance (Fig. 3).
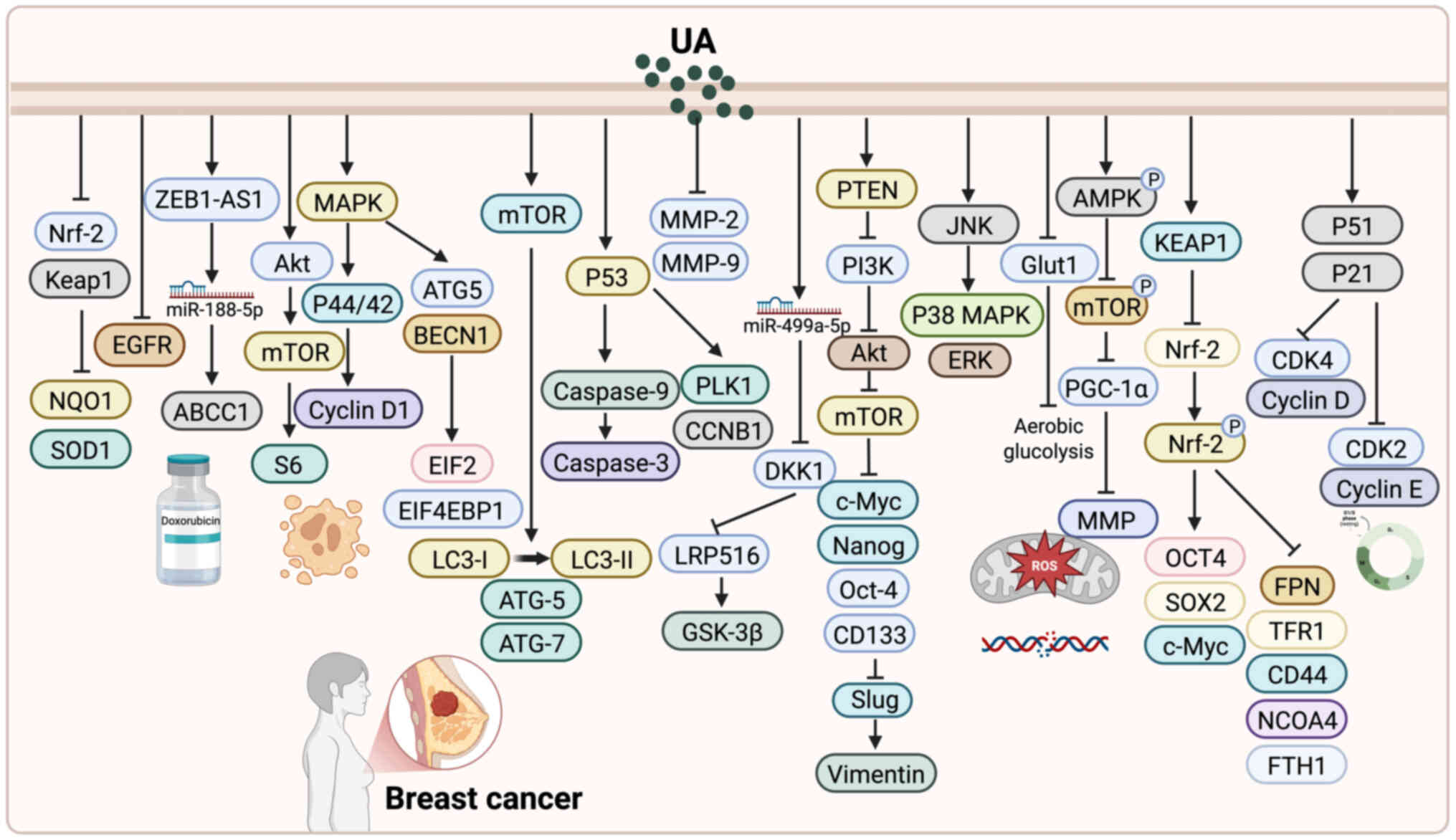 | Figure 3.Antitumor mechanisms of UA in breast
cancer. UA inhibits proliferation and induces apoptosis in breast
cancer cells via pathways such as Keap1/Nrf2, Akt/mTOR, FoxM1 and
mitochondrial caspase signaling. It induces autophagy and metabolic
disruption through modulation of MAPK, SP1/CAV1, AMPK and AKT
pathways, and regulates glycolysis and mitochondrial function. UA
also targets breast cancer stem cells by suppressing Wnt/β-catenin,
AGO2/miR-9/miR-221 and NRF2 signaling, impairing stemness and
promoting ferroptosis. Additionally, UA inhibits invasion and
metastasis by downregulating MMP-2, u-PA, and PI3K/Akt, while
enhancing autophagy, apoptosis, and anti-inflammatory responses.
UA, ursolic acid. |
Advances in drug delivery strategies and
derivatives of UA
In recent years, various drug delivery strategies
and structural derivatives have been developed to address the
clinical limitations of UA, including poor water solubility, low
bioavailability and limited targeting capacity. In ovarian cancer,
Wang et al (81) designed a
reduction-responsive amphiphilic prodrug, Pt(IV)-UA-PEG, which
self-assembles into nanoparticles [Pt(IV)-UA NPs] with high
drug-loading efficiency. These nanoparticles enable efficient drug
release in the reductive and acidic TME, effectively overcoming
cisplatin resistance and significantly suppressing the
proliferation of resistant ovarian cancer cells. In breast cancer,
Jin et al (82) reported
folic acid-modified chitosan nanoparticles that enhance targeted
uptake and mitochondrial localization, inducing apoptosis via the
mitochondrial pathway. Similarly, Fu et al (83) fabricated UA nanofibers for topical
therapy in advanced breast cancer with skin ulceration, achieving
improved transdermal penetration, inhibition of STAT3 and ERK1/2
signaling, and caspase-3-mediated apoptosis. For TNBC, Sharma et
al (84) developed
enzyme-responsive HA-UA/PTX nanoparticles, which enhanced
CD44-mediated cellular uptake, apoptosis, and tumor suppression
in vivo. Additionally, Liu et al (85) combined UA with a Nectin-4-targeted
oncolytic measles virus, demonstrating synergistic apoptosis
induction and enhanced autophagic flux, while UA-loaded
nanoparticles further reinforced antitumor effects across breast
cancer cell lines. In cervical cancer, Wang et al (86) constructed UA-loaded gold/PLGA
nanocomposites for intranasal delivery, which inhibited
proliferation, invasion and migration, while activating p53 and
caspase signaling to induce apoptosis in vitro and in
vivo.
Beyond delivery systems, structural optimization of
UA has yielded derivatives with improved anticancer activity and
pharmacological properties. The derivative FZU3010 demonstrated
stronger anticancer activity than UA, particularly in renal cancer
and TNBC cells, where it induced G1-phase arrest and apoptosis by
inhibiting STAT3 and upregulating p21/p27 (87,88).
The same group also reported an Asp-UA co-drug that effectively
inhibited breast cancer cell adhesion, migration and invasion,
highlighting its potential against metastasis (89). Pattnaik et al (90) synthesized novel UA derivatives with
selective activity against MDA-MB-231 cells, among which compound
17 showed superior efficacy compared with doxorubicin. UA232,
another optimized derivative, inhibited breast and cervical cancer
cell proliferation by inducing apoptosis, cell cycle arrest, ER
stress and lysosomal dysfunction, and exhibited robust antitumor
effects in vivo (91). Other
derivatives, such as water-soluble UAOS-Na with improved
pharmacokinetics (92) and UA312
with radioprotective effects in zebrafish models (93), further demonstrate the versatility
of rational structural modifications. Substitutions at key
positions (for example, C-3 or C-28) have also generated potent
derivatives with selective activity against leukemia and glioma
cells (94).
Collectively, nanoparticle-based strategies,
including polymeric nanoparticles and gold/PLGA nanocomposites,
have proven most effective in improving water solubility, tumor
targeting and controlled release, thereby enhancing intra-tumoral
accumulation and antitumor efficacy. Liposomal and nanofiber-based
formulations provide additional advantages in topical delivery and
tissue penetration, making them particularly promising for breast
cancer with skin involvement. By contrast, structural derivatives
such as FZU3010 and UA232 demonstrate superior pharmacological
potency and a broader range of mechanisms, including apoptosis,
ferroptosis and ER stress, although their pharmacokinetic
advantages remain less established compared with nanoparticle
systems. Taken together, these complementary approaches indicate
that while nanoparticles effectively overcome bioavailability
limitations, rational structural modifications expand UA's
mechanistic spectrum and therapeutic potential. Future
translational studies could benefit from combining both
strategies-for example, encapsulating potent derivatives into
advanced nanocarriers-to maximize bioavailability, therapeutic
efficacy and clinical applicability.
Challenges and future perspectives
UA has demonstrated broad-spectrum antitumor
potential in gynecologic malignancies, including ovarian
endometrial cervical and breast cancers. Its anticancer mechanisms
are multifaceted, involving the induction of apoptosis, cell cycle
arrest, regulation of metabolic reprogramming, inhibition of CSC
properties and enhancement of chemotherapeutic efficacy (95). Studies have shown that UA induces
apoptosis in ovarian cancer cells through the activation of caspase
cascades, upregulation of the Bax/Bcl-2 ratio and inhibition of the
PI3K/AKT signaling pathway, thereby effectively suppressing tumor
cell proliferation. Notably, when used in combination with
chemotherapeutic agents, UA not only enhances their antitumor
effects but also alleviates associated toxicities, such as
CDDP-induced ototoxicity. Moreover, UA has been reported to exert
antitumor effects in ovarian cancer by inhibiting glycolysis and
modulating the KLF5/PI3K/AKT signaling axis, offering a novel
strategy to overcome chemoresistance. In endometrial and cervical
cancers, UA also exhibits potent antiproliferative and
pro-apoptotic activities. Particularly in cervical cancer, UA
activates the Fas/caspase-mediated apoptotic pathway and suppresses
HPV-related oncogene expression, demonstrating both antiviral and
antitumor effects. Regarding its synergistic role in cervical
cancer chemotherapy, UA has been found to enhance the efficacy of
paclitaxel and CDDP by inhibiting the NF-κB signaling pathway.
Importantly, it exerts minimal toxicity on normal cells,
highlighting its potential for clinical application as an adjuvant
therapeutic agent (Table SII).
Although UA demonstrates promising antitumor
activity in vitro and in animal models, its clinical
application is significantly hindered by poor oral bioavailability
and complex metabolic processing. As a Biopharmaceutics
Classification System Class IV compound, UA suffers from low
aqueous solubility and limited intestinal permeability, resulting
in poor absorption and diminished therapeutic efficacy (96). Following oral administration, UA is
primarily distributed to the lungs, spleen and liver, but undergoes
extensive first-pass metabolism-mainly through cytochrome P450
enzymes CYP3A4 and CYP2C9 in the liver and intestines-thereby
reducing its systemic exposure and bioactivity (90,92).
To overcome these limitations, various advanced drug delivery
systems-such as chitosan-modified nanoparticles, liposomes and
co-amorphous formulations-have been developed to improve UA's
solubility, bioavailability and tumor-targeting capacity (87–91).
These strategies have shown encouraging results in preclinical
models, including enhanced oral absorption and increased
accumulation at tumor sites. Nonetheless, their clinical utility
remains uncertain and requires further optimization and validation
(97). In addition, the safety and
long-term toxicity profile of UA are not yet fully understood.
Although current data suggest minimal toxicity toward normal cells
in short-term studies, potential off-target effects,
bioaccumulation and organ-specific toxicity following prolonged
administration cannot be excluded. Most available evidence is
limited to preclinical research, highlighting the urgent need for
systematic and long-term toxicological evaluations to support
future clinical development.
UA has shown promise as an adjuvant agent in
gynecological cancers, where it can enhance the efficacy of
conventional chemotherapeutic drugs such as CDDP and paclitaxel
while reversing drug resistance. Its therapeutic potential in
breast cancer, particularly TNBC, also merits further
investigation. However, most current evidence derives from in
vitro studies and small animal models, and robust clinical
trial data are still lacking. Future research should therefore
prioritize clinical evaluation of UA to clarify its
pharmacodynamics, safety and potential synergistic effects with
standard treatments. In addition, the development of UA derivatives
and novel combinatory regimens with other anticancer agents,
including immunotherapies, represents an important direction.
Notably, UA's anti-inflammatory and immunomodulatory properties
suggest that it may improve the tumor immune microenvironment and
enhance responsiveness to immune checkpoint inhibitors such as
PD-1/PD-L1 blockade, although this hypothesis requires validation
in preclinical and clinical studies.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
TC drafted the manuscript and prepared the figures.
SS conducted the literature search and data collection. ZZ revised
and proofread the manuscript. XZ supervised the overall study
design, guided the research direction, and was responsible for
manuscript drafting and final approval of the submitted version.
All authors read and approved the final version of the manuscript.
Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Hanvic B and Ray-Coquard I: Gynecological
sarcomas: Literature review of 2020. Curr Opin Oncol. 33:345–350.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Korenaga TK and Tewari KS: Gynecologic
cancer in pregnancy. Gynecol Oncol. 157:799–809. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marcolin JC, Lichtenfels M, da Silva CA
and de Farias CB: Gynecologic and breast cancers: What's new in
chemoresistance and chemosensitivity tests? Curr Probl Cancer.
47:1009962023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Alizadeh H, Akbarabadi P, Dadfar A, Tareh
MR and Soltani B: A comprehensive overview of ovarian cancer stem
cells: Correlation with high recurrence rate, underlying
mechanisms, and therapeutic opportunities. Mol Cancer. 24:1352025.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang M, Liu C, Tu J, Tang M, Ashrafizadeh
M, Nabavi N, Sethi G, Zhao P and Liu S: Advances in cancer
immunotherapy: Historical perspectives, current developments, and
future directions. Mol Cancer. 24:1362025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rosenbaum SR, Hughes CJ, Fields KM, Purdy
SC, Gustafson AL, Wolin A, Hampton D, Shrivastava NM, Turner N,
Danis E, et al: EYA3 regulation of NF-κB and CCL2 suppresses
cytotoxic NK cells in the premetastatic niche to promote TNBC
metastasis. Sci Adv. 11:eadt05042025. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Konstantinopoulos PA and Matulonis UA:
Clinical and translational advances in ovarian cancer therapy. Nat
Cancer. 4:1239–1257. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Gupta R, Kumar R, Penn CA and Wajapeyee N:
Immune evasion in ovarian cancer: Implications for immunotherapy
and emerging treatments. Trends Immunol. 46:166–181. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Makker V, MacKay H, Ray-Coquard I, Levine
DA, Westin SN, Aoki D and Oaknin A: Endometrial cancer. Nat Rev Dis
Primers. 7:882021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Burd EM: Human papillomavirus and cervical
cancer. Clin Microbiol Rev. 16:1–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Qin SY, Cheng YJ, Lei Q, Zhang AQ and
Zhang XZ: Combinational strategy for high-performance cancer
chemotherapy. Biomaterials. 171:178–197. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zemek RM, Anagnostou V, Pires da Silva I,
Long GV and Lesterhuis WJ: Exploiting temporal aspects of cancer
immunotherapy. Nat Rev Cancer. 24:480–497. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stewart MD, Keane A, Butterfield LH,
Levine BL, Thompson B, Xu Y, Ramsborg C, Lee A, Kalos M, Koerner C,
et al: Accelerating the development of innovative cellular therapy
products for the treatment of cancer. Cytotherapy. 22:239–246.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang X, Wei X, Shi L, Jiang H, Ma F, Li
Y, Li C and Ma Y and Ma Y: The latest research progress: Active
components of traditional Chinese medicine as promising candidates
for ovarian cancer therapy. J Ethnopharmacol. 337:1188112025.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Shafabakhsh R and Asemi Z: Quercetin: A
natural compound for ovarian cancer treatment. J Ovarian Res.
12:552019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu Z, Zhu W, Kong X, Chen X, Sun X, Zhang
W and Zhang R: Tanshinone IIA inhibits glucose metabolism leading
to apoptosis in cervical cancer. Oncol Rep. 42:1893–1903.
2019.PubMed/NCBI
|
|
17
|
Zhang X, Zhu L, Wang X, Zhang H, Wang L
and Xia L: Basic research on curcumin in cervical cancer: Progress
and perspectives. Biomed Pharmacother. 162:1145902023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sandhu SS, Rouz SK, Kumar S, Swamy N,
Deshmukh L, Hussain A, Haque S and Tuli HS: Ursolic acid: A
pentacyclic triterpenoid that exhibits anticancer therapeutic
potential by modulating multiple oncogenic targets. Biotechnol
Genet Eng Rev. 39:729–759. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kashyap D, Tuli HS and Sharma AK: Ursolic
acid (UA): A metabolite with promising therapeutic potential. Life
Sci. 146:201–213. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sun Q, He M, Zhang M, Zeng S, Chen L, Zhou
L and Xu H: Ursolic acid: A systematic review of its pharmacology,
toxicity and rethink on its pharmacokinetics based on PK-PD model.
Fitoterapia. 147:1047352020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Cargnin ST and Gnoatto SB: Ursolic acid
from apple pomace and traditional plants: A valuable triterpenoid
with functional properties. Food Chem. 220:477–489. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ramos-Hryb AB, Pazini FL, Kaster MP and
Rodrigues ALS: Therapeutic potential of ursolic acid to manage
neurodegenerative and psychiatric diseases. CNS Drugs.
31:1029–1041. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Song YH, Jeong SJ, Kwon HY, Kim B, Kim SH
and Yoo DY: Ursolic acid from Oldenlandia diffusa induces apoptosis
via activation of caspases and phosphorylation of glycogen synthase
kinase 3 beta in SK-OV-3 ovarian cancer cells. Biol Pharm Bull.
35:1022–1028. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang X, Li T, Gong ES and Liu RH:
Antiproliferative activity of ursolic acid in MDA-MB-231 human
breast cancer cells through Nrf2 pathway regulation. J Agric Food
Chem. 68:7404–7415. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yim EK, Lee KH, Namkoong SE, Um SJ and
Park JS: Proteomic analysis of ursolic acid-induced apoptosis in
cervical carcinoma cells. Cancer Lett. 235:209–220. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Khwaza V and Aderibigbe BA: Potential
pharmacological properties of triterpene derivatives of ursolic
acid. Molecules. 29:38842024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shi Y, Leng Y, Liu D, Liu X, Ren Y, Zhang
J and Chen F: Research advances in protective effects of ursolic
acid and oleanolic acid against gastrointestinal diseases. Am J
Chin Med. 49:413–435. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu HR, Ahmad N, Lv B and Li C: Advances
in production and structural derivatization of the promising
molecule ursolic acid. Biotechnol J. 16:e20006572021. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Giacinti G, Raynaud C, Capblancq S and
Simon V: Evaluation and prevention of the negative matrix effect of
terpenoids on pesticides in apples quantification by gas
chromatography-tandem mass spectrometry. J Chromatogr A. 1483:8–19.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li H, Liu Y, Guo S, Shi M, Qin S and Zeng
C: Extraction of ursolic acid from apple peel with hydrophobic deep
eutectic solvents: comparison between response surface methodology
and artificial neural networks. Foods. 12:3102023. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xia EQ, Yu YY, Xu XR, Deng GF, Guo YJ and
Li HB: Ultrasound-assisted extraction of oleanolic acid and ursolic
acid from Ligustrum lucidum Ait. Ultrason Sonochem. 19:772–776.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Xia EQ, Wang BW, Xu XR, Zhu L, Song Y and
Li HB: Microwave-assisted extraction of oleanolic acid and ursolic
acid from Ligustrum lucidum Ait. Int J Mol Sci. 12:5319–5329. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
K V, L S, N V K, R P, M P DR, Suneetha C,
Palpandi Raja R and Muthusamy S: Promising approaches in the
extraction, characterization, and biotechnological applications of
ursolic acid: A review. Prep Biochem Biotechnol. 55:973–984. 2025.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mlala S, Oyedeji AO, Gondwe M and Oyedeji
OO: Ursolic acid and its derivatives as bioactive agents.
Molecules. 24:27512019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Khwaza V, Oyedeji OO and Aderibigbe BA:
Ursolic acid-based derivatives as potential anti-cancer agents: An
update. Int J Mol Sci. 21:59202020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Erdmann J, Kujaciński M and Wiciński M:
Beneficial effects of ursolic acid and its derivatives-focus on
potential biochemical mechanisms in cardiovascular conditions.
Nutrient. 13:39002021. View Article : Google Scholar
|
|
37
|
Lin W and Ye H: Anticancer activity of
ursolic acid on human ovarian cancer cells via ROS and MMP mediated
apoptosis, cell cycle arrest and downregulation of PI3K/AKT
pathway. J BUON. 25:750–756. 2020.PubMed/NCBI
|
|
38
|
Ganapathy-Kanniappan S and Geschwind JF:
Tumor glycolysis as a target for cancer therapy: Progress and
prospects. Mol Cancer. 12:1522013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xu M, Li X, Yuan C, Zhu T, Wang M, Zhu Y,
Duan Y, Yao J, Luo B, Wang Z, et al: Ursolic acid inhibits
glycolysis of ovarian cancer via KLF5/PI3K/AKT signaling pathway.
Am J Chin Med. 52:2211–2231. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liu H, Zou J, Li X, Ge Y and He W: Drug
delivery for platinum therapeutics. J Control Release. 380:503–523.
2025. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Talach T, Rottenberg J, Gal B, Kostrica R,
Jurajda M, Kocak I, Lakomy R and Vogazianos E: Genetic risk factors
of cisplatin induced ototoxicity in adult patients. Neoplasma.
63:263–268. 2016.PubMed/NCBI
|
|
42
|
Di Y, Xu T, Tian Y, Ma T, Qu D, Wang Y,
Lin Y, Bao D, Yu L, Liu S and Wang A: Ursolic acid protects against
cisplatin-induced ototoxicity by inhibiting oxidative stress and
TRPV1-mediated Ca2+-signaling. Int J Mol Med. 46:806–816. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Li W, Luo L, Shi W, Yin Y and Gao S:
Ursolic acid reduces Adriamycin resistance of human ovarian cancer
cells through promoting the HuR translocation from cytoplasm to
nucleus. Environ Toxicol. 36:267–275. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Batlle E and Clevers H: Cancer stem cells
revisited. Nat Med. 23:1124–1134. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Saw PE, Liu Q, Wong PP and Song E: Cancer
stem cell mimicry for immune evasion and therapeutic resistance.
Cell Stem Cell. 31:1101–1112. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wang WJ, Sui H, Qi C, Li Q, Zhang J, Wu
SF, Mei MZ, Lu YY, Wan YT, Chang H and Guo PT: Ursolic acid
inhibits proliferation and reverses drug resistance of ovarian
cancer stem cells by downregulating ABCG2 through suppressing the
expression of hypoxia-inducible factor-1α in vitro. Oncol Rep.
36:428–440. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang J, Wang W, Qian L, Zhang Q, Lai D
and Qi C: Ursolic acid inhibits the proliferation of human ovarian
cancer stem-like cells through epithelial-mesenchymal transition.
Oncol Rep. 34:2375–2384. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yim EK, Lee MJ, Lee KH, Um SJ and Park JS:
Antiproliferative and antiviral mechanisms of ursolic acid and
dexamethasone in cervical carcinoma cell lines. Int J Gynecol
Cancer. 16:2023–2031. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Leng S, Hao Y, Du D, Xie S, Hong L, Gu H,
Zhu X, Zhang J, Fan D and Kung HF: Ursolic acid promotes cancer
cell death by inducing Atg5-dependent autophagy. Int J Cancer.
133:2781–2790. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Guo JL, Han T, Bao L, Li XM, Ma JQ and
Tang LP: Ursolic acid promotes the apoptosis of cervical cancer
cells by regulating endoplasmic reticulum stress. J Obstet Gynaecol
Res. 45:877–881. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Li Y, Xing D, Chen Q and Chen WR:
Enhancement of chemotherapeutic agent-induced apoptosis by
inhibition of NF-kappaB using ursolic acid. Int J Cancer.
127:462–473. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Achiwa Y, Hasegawa K and Udagawa Y: Effect
of ursolic acid on MAPK in cyclin D1 signaling and RING-type E3
ligase (SCF E3s) in two endometrial cancer cell lines. Nutr Cancer.
65:1026–1033. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Achiwa Y, Hasegawa K and Udagawa Y:
Molecular mechanism of ursolic acid induced apoptosis in poorly
differentiated endometrial cancer HEC108 cells. Oncol Rep.
14:507–512. 2005.PubMed/NCBI
|
|
54
|
Achiwa Y, Hasegawa K and Udagawa Y:
Regulation of the phosphatidylinositol 3-kinase-Akt and the
mitogen-activated protein kinase pathways by ursolic acid in human
endometrial cancer cells. Biosci Biotechnol Biochem. 71:31–37.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Achiwa Y, Hasegawa K, Komiya T and Udagawa
Y: Ursolic acid induces Bax-dependent apoptosis through the
caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep.
13:51–57. 2005.PubMed/NCBI
|
|
56
|
De Angel RE, Smith SM, Glickman RD,
Perkins SN and Hursting SD: Antitumor effects of ursolic acid in a
mouse model of postmenopausal breast cancer. Nutr Cancer.
62:1074–1086. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wang JS, Ren TN and Xi T: Ursolic acid
induces apoptosis by suppressing the expression of FoxM1 in MCF-7
human breast cancer cells. Med Oncol. 29:10–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kassi E, Sourlingas TG, Spiliotaki M,
Papoutsi Z, Pratsinis H, Aligiannis N and Moutsatsou P: Ursolic
acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast
cancer cells. Cancer Invest. 27:723–733. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Mallepogu V, Sankaran KR, Pasala C, Bandi
LR, Maram R, Amineni UM and Meriga B: Ursolic acid regulates key
EMT transcription factors, induces cell cycle arrest and apoptosis
in MDA-MB-231 and MCF-7 breast cancer cells, an in-vitro and in
silico studies. J Cell Biochem. 124:1900–1918. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Manouchehri JM and Kalafatis M: Ursolic
acid promotes the sensitization of rhTRAIL-resistant
triple-negative breast cancer. Anticancer Res. 38:6789–6795. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Guo W, Xu B, Wang X, Zheng B, Du J and Liu
S: The analysis of the anti-tumor mechanism of ursolic acid using
connectively map approach in breast cancer cells line MCF-7. Cancer
Manag Res. 12:3469–3476. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kim GD: Ursolic acid decreases the
proliferation of MCF-7 cell-derived breast cancer stem-like cells
by modulating the ERK and PI3K/AKT signaling pathways. Prev Nutr
Food Sci. 26:434–444. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhao C, Yin S, Dong Y, Guo X, Fan L, Ye M
and Hu H: Autophagy-dependent EIF2AK3 activation compromises
ursolic acid-induced apoptosis through upregulation of MCL1 in
MCF-7 human breast cancer cells. Autophagy. 9:196–207. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Gupta KB, Gao J, Li X, Thangaraju M, Panda
SS and Lokeshwar BL: Cytotoxic autophagy: A novel treatment
paradigm against breast cancer using oleanolic acid and ursolic
acid. Cancers (Basel). 16:33672024. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lewinska A, Adamczyk-Grochala J,
Kwasniewicz E, Deregowska A and Wnuk M: Ursolic acid-mediated
changes in glycolytic pathway promote cytotoxic autophagy and
apoptosis in phenotypically different breast cancer cells.
Apoptosis. 22:800–815. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Fogde DL, Xavier CPR, Balnytė K, Holland
LKK, Stahl-Meyer K, Dinant C, Corcelle-Termeau E, Pereira-Wilson C,
Maeda K and Jäättelä M: Ursolic acid impairs cellular lipid
homeostasis and lysosomal membrane integrity in breast carcinoma
cells. Cells. 11:40782022. View Article : Google Scholar
|
|
67
|
Guerra ÂR, Paulino AF, Castro MM, Oliveira
H, Duarte MF and Duarte IF: Triple negative breast cancer and
breast epithelial cells differentially reprogram glucose and lipid
metabolism upon treatment with triterpenic acids. Biomolecules.
10:11632020. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang S, Chang X, Zhang J, Li J, Wang N,
Yang B, Pan B, Zheng Y, Wang X, Ou H and Wang Z: Ursolic acid
inhibits breast cancer metastasis by suppressing glycolytic
metabolism via activating SP1/caveolin-1 signaling. Front Oncol.
11:7455842021. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Mandal S, Gamit N, Varier L, Dharmarajan A
and Warrier S: Inhibition of breast cancer stem-like cells by a
triterpenoid, ursolic acid, via activation of Wnt antagonist, sFRP4
and suppression of miRNA-499a-5p. Life Sci. 265:1188542021.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Liao WL, Liu YF, Ying TH, Shieh JC, Hung
YT, Lee HJ, Shen CY and Cheng CW: Inhibitory effects of ursolic
acid on the stemness and progression of human breast cancer cells
by modulating argonaute-2. Int J Mol Sci. 24:3662022. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Yang X, Liang B, Zhang L, Zhang M, Ma M,
Qing L, Yang H, Huang G and Zhao J: Ursolic acid inhibits the
proliferation of triple-negative breast cancer stem-like cells
through NRF2-mediated ferroptosis. Oncol Rep. 52:942024. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Yeh CT, Wu CH and Yen GC: Ursolic acid, a
naturally occurring triterpenoid, suppresses migration and invasion
of human breast cancer cells by modulating c-Jun N-terminal kinase,
Akt and mammalian target of rapamycin signaling. Mol Nutr Food Res.
54:1285–1295. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Luo J, Hu YL and Wang H: Ursolic acid
inhibits breast cancer growth by inhibiting proliferation, inducing
autophagy and apoptosis, and suppressing inflammatory responses via
the PI3K/AKT and NF-κB signaling pathways in vitro. Exp Ther Med.
14:3623–3631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhang Y, Ma X, Li H, Zhuang J, Feng F, Liu
L, Liu C and Sun C: Identifying the effect of ursolic acid against
triple-negative breast cancer: Coupling network pharmacology with
experiments verification. Front Pharmacol. 12:6857732021.
View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lu Q, Chen W, Ji Y, Liu Y and Xue X:
Ursolic acid enhances cytotoxicity of doxorubicin-resistant
triple-negative breast cancer cells via ZEB1-AS1/miR-186-5p/ABCC1
axis. Cancer Biother Radiopharm. 37:673–683. 2022.PubMed/NCBI
|
|
76
|
Wang Z, Zhang P, Jiang H, Sun B, Luo H and
Jia A: Ursolic acid enhances the sensitivity of MCF-7 and
MDA-MB-231 cells to epirubicin by modulating the autophagy pathway.
Molecules. 27:33992022. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zong L, Cheng G, Liu S, Pi Z, Liu Z and
Song F: Reversal of multidrug resistance in breast cancer cells by
a combination of ursolic acid with doxorubicin. J Pharm Biomed
Anal. 165:268–275. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Zong L, Cheng G, Zhao J, Zhuang X, Zheng
Z, Liu Z and Song F: Inhibitory effect of ursolic acid on the
migration and invasion of doxorubicin-resistant breast cancer.
Molecules. 27:12822022. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Luo F, Zhao J, Liu S, Xue Y, Tang D, Yang
J, Mei Y, Li G and Xie Y: Ursolic acid augments the
chemosensitivity of drug-resistant breast cancer cells to
doxorubicin by AMPK-mediated mitochondrial dysfunction. Biochem
Pharmacol. 205:1152782022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Xiang F, Fan Y, Ni Z, Liu Q, Zhu Z, Chen
Z, Hao W, Yue H, Wu R and Kang X: Ursolic acid reverses the
chemoresistance of breast cancer cells to paclitaxel by targeting
MiRNA-149-5p/MyD88. Front Oncol. 9:5012019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wang Y, Luo Z, Zhou D, Wang X, Chen J,
Gong S and Yu Z: Nano-assembly of ursolic acid with platinum
prodrug overcomes multiple deactivation pathways in
platinum-resistant ovarian cancer. Biomater Sci. 9:4110–4119. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jin H, Pi J, Yang F, Jiang J, Wang X, Bai
H, Shao M, Huang L, Zhu H, Yang P, et al: Folate-chitosan
nanoparticles loaded with ursolic acid confer anti-breast cancer
activities in vitro and in vivo. Sci Rep. 6:307822016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Fu H, Wu TH, Ma CP and Yen FL: Improving
water solubility and skin penetration of ursolic acid through a
nanofiber process to achieve better in vitro anti-breast cancer
activity. Pharmaceutics. 16:11472024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Sharma R, Yadav V, Jha S, Dighe S and Jain
S: Unveiling the potential of ursolic acid modified hyaluronate
nanoparticles for combination drug therapy in triple negative
breast cancer. Carbohydr Polym. 338:1221962024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Liu CH, Wong SH, Tai CJ, Tai CJ, Pan YC,
Hsu HY, Richardson CD and Lin LT: Ursolic acid and its
nanoparticles are potentiators of oncolytic measles virotherapy
against breast cancer cells. Cancers (Basel). 13:1362021.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Wang S, Meng X and Dong Y: Ursolic acid
nanoparticles inhibit cervical cancer growth in vitro and in vivo
via apoptosis induction. Int J Oncol. 50:1330–1340. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Li W, Zhang H, Nie M, Tian Y, Chen X, Chen
C, Chen H and Liu R: Ursolic acid derivative FZU-03,010 inhibits
STAT3 and induces cell cycle arrest and apoptosis in renal and
breast cancer cells. Acta Biochim Biophys Sin (Shanghai).
49:367–373. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Li W, Zhang H, Nie M, Wang W, Liu Z, Chen
C, Chen H, Liu R, Baloch Z and Ma K: A novel synthetic ursolic acid
derivative inhibits growth and induces apoptosis in breast cancer
cell lines. Oncol Lett. 15:2323–2329. 2018.PubMed/NCBI
|
|
89
|
Tang Q, Liu Y, Li T, Yang X, Zheng G, Chen
H, Jia L and Shao J: A novel co-drug of aspirin and ursolic acid
interrupts adhesion, invasion and migration of cancer cells to
vascular endothelium via regulating EMT and EGFR-mediated signaling
pathways: Multiple targets for cancer metastasis prevention and
treatment. Oncotarget. 7:73114–73129. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Pattnaik B, Lakshmi JK, Kavitha R,
Jagadeesh B, Bhattacharjee D, Jain N and Mallavadhani UV:
Synthesis, structural studies, and cytotoxic evaluation of novel
ursolic acid hybrids with capabilities to arrest breast cancer
cells in mitosis. J Asian Nat Prod Res. 19:260–271. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gou W, Luo N, Yu B, Wu H, Wu S, Tian C,
Guo J, Ning H, Bi C, Wei H, et al: Ursolic acid derivative UA232
promotes tumor cell apoptosis by inducing endoplasmic reticulum
stress and lysosomal dysfunction. Int J Biol Sci. 18:2639–2651.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Wang M, Gu C, Yang Y, Chen L, Chen K, Du
J, Wu H and Li Y: Ursolic acid derivative UAOS-Na treats
experimental autoimmune encephalomyelitis by immunoregulation and
protecting myelin. Front Neurol. 14:12698622023. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Xu FF, Shang Y, Wei HQ, Zhang WY, Wang LX,
Hu T, Zhang SQ, Li YL, Shang HH, Hou WB, et al: Ursolic acid
derivative UA312 ameliorates ionizing radiation-induced
cardiotoxicity and neurodevelopmental toxicity in zebrafish via
targeting chrna3 and grik5. Acta Pharmacol Sin. April 28–2025.(Epub
ahead of print). View Article : Google Scholar
|
|
94
|
da Silva EF, de Vargas AS, Willig JB, de
Oliveira CB, Zimmer AR, Pilger DA, Buffon A and Gnoatto SCB:
Synthesis and antileukemic activity of an ursolic acid derivative:
A potential co-drug in combination with imatinib. Chem Biol
Interact. 344:1095352021. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Shanmugam MK, Dai X, Kumar AP, Tan BK,
Sethi G and Bishayee A: Ursolic acid in cancer prevention and
treatment: molecular targets, pharmacokinetics and clinical
studies. Biochem Pharmacol. 85:1579–1587. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Antonio E, Dos Reis Antunes Junior O,
Marcano RGDJV, Diedrich C, da Silva Santos J, Machado CS, Khalil NM
and Mainardes RM: Chitosan modified poly (lactic acid)
nanoparticles increased the ursolic acid oral bioavailability. Int
J Biol Macromol. 172:133–142. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Namdeo P, Gidwani B, Tiwari S, Jain V,
Joshi V, Shukla SS, Pandey RK and Vyas A: Therapeutic potential and
novel formulations of ursolic acid and its derivatives: An updated
review. J Sci Food Agric. 103:4275–4292. 2023. View Article : Google Scholar : PubMed/NCBI
|