1. Introduction
Every exposed human body surface, including the
skin, genitourinary, gastrointestinal and respiratory tracts, are
heavily colonized by as many as 10-100 trillion microorganisms,
including bacteria, fungi, archaea and viruses (1). In the recent years, commensal
microorganisms have been identified as key determinants of a host's
homeostasis and health (2). In
particular, among the human symbiotic microbial populations, the
gut microbiota is the most extensively populated, hosting up to 70%
of the microbes inhabiting the whole body (3). Gut microbiota is the name given to
the heterogeneous population of commensal microorganisms,
inhabiting the gastrointestinal tract, mostly the large intestine.
This population constitutes an agent to which we are constantly
exposed, at high doses, throughout an entire lifespan (4). The human gut is populated by 1,000
different bacterial species, prevalently belonging to the phyla of
Firmicutes and Bacteroidetes (5).
The intestine is the interface between the gut
commensal microbiota and the human body (6). On the one hand, the gastrointestinal
enteroendocrine cells secrete over 30 different peptide hormones
involved in key functions, including gastrointestinal motility,
food digestion and neuromodulation (7). It has been demonstrated that
gut-secreted hormones are able to modify the gut microbiome
composition, as during the response to stress (8-10).
On the other hand, the gut microbial population produces or
transforms active molecules, which may be sensed by the
gastrointestinal cells of the host (8). The derived functional effects range
from the modulation of the host's metabolism to the maintenance of
gut barrier integrity, xenobiotics metabolism, protection against
gastrointestinal pathogens and modulation of the host's immune
system (11-14).
Notably, certain commensal bacteria produce essential
micronutrients, including vitamin K and vitamin B. Additionally, a
number of gut commensals can transform amino acids into signaling
molecules, as for example glutamate into gamma-amino butyric acid
(GABA) or histidine to histamine. Finally, several Bacteroidetes
are able to catabolize phenolic compounds, as well as secondary
bile acids, moreover to synthetize the anti-diabetics linoleic acid
(15). Another class of
hormone-like metabolites produced by the human gut commensals is
represented by the short chain fatty acids (SCFAs), derived from
the bacterial fermentation of dietary fibers (16). The SCFAs, once synthetized in the
intestine, are transported to the liver where they are utilized as
a key source of energy. Additionally, SCFAs play a role in
controlling glucose and the lipid metabolism by affecting the gut
epithelial hormone peptide secretion (17).
Given the reported functional crosstalk between the
gastrointestinal microbiota and its host, the preservation of the
equilibrium in both composition and the relative abundance of the
gut microbial population is fundamental for the correct fulfilment
of pivotal host's metabolic, as well as immune functions (18-20).
Any disequilibrium in this delicate balance may lead to a defective
microbiota, a condition known as dysbiosis, mostly linked to
several human pathologies, including cancer (21).
The gut microbiome is defined as the whole genome of
the host's gut microbiota, and it encodes 100-fold more genes than
the human genome (22). Over the
past 10 years, classical fecal-derived microbe cultivation studies
have been strongly integrated with metagenomics approaches,
combining next-generation sequencing (NGS) with the computational
analysis of the 16S rRNA amplicons. Progresses in metagenomics
studies, together with many advancements in transcriptomics and
metabolomics, have allowed the characterization of both a diversity
and abundance of the gut microbiome, with the final goal of
determining the impact of each individual gut-populating species on
the health of the host (23,24). These novel approaches are
depicting the deep impact of the microbiome diversity and
composition on human health, as disclosed by the Human Microbiome
Project and the large number of originating publications (25-28).
A healthy gut microbiome is defined by a functional
core of metabolic and other molecular functions, which are not
necessarily performed by the same bacterial species in each
different individual (29). The
term ‘probiotic’ means pro-life. Probiotics are currently defined
by the Food and Agriculture Organization of the United Nations and
by the World Health Organization (FAO/WHO) as ‘live microorganisms,
which, when consumed in adequate amounts, confer a health effect on
the host’ (30). They are highly
present in fermented food and yoghurt. The vast majority of these
probiotics are lactic-acid producing, non-pathogenic bacteria, such
as Lactobacillus, Streptococcus,
Bifidobacterium, Propionibacterium and
Enterococcus or non-pathogenic yeasts including
Saccharomyces boulardii (30). Probiotics are administered orally
and arrive alive in the intestine (30). They are often administered in
combination with specific prebiotics (undigestible food
specifically metabolized by probiotics), to form synbiotic mixes
(31). Health benefits derived
from administering probiotics to healthy individuals include
improved digestion, immune defense mechanisms and nutrient
absorption. Importantly, probiotics have been proven to be able to
revert intestinal dysbiosis, which may play a role in the
development of several degenerative diseases, as well as chronic
diseases, including cancer (32).
A growing amount of clinical studies are currently
investigating the impact of probiotics on the treatment of
intestinal toxicity during chemotherapy, immunotherapy and
radiation, generating promising results. The present review aimed
to summarize the up-to-date clinical observations concerning the
role played by probiotics administered in association with
anticancer therapy.
2. Gut microbiota and cancer
The gut microbiota can be considered a factor to
which we are exposed throughout an entire lifespan, whereas
intestinal dysbiosis has been found to be linked to the
tumorigenesis of both local gastro-intestinal cancers and tumors
localized in distant sites of the body (33). Both environmental exposure (e.g.,
to cancerogenic substances or UV radiation) and lifestyle habits
significantly influence individual cancer risk (34-37).
This risk is associated with the dose, duration and the combination
of these exposures among each other, also depending on the
individual genetic background (38-43).
In fact, neoplasms bear an intrinsic complexity, as they are
derived from the stochastic acquisition of driver mutations within
genes involved in key processes (including DNA duplication, DNA
repair and oxidative stress response). Thanks to the accumulation
of mutations over time and space, cancerogenic cells adapt to the
hosting organism, therefore transforming from a normal cell into a
malignant one (44-47).
Moreover, given the stochastic gathering of mutations, together
with the intrinsic tumor cellular genomic instability, epigenetics
(including altered DNA methylation, as well as miRNA imbalance),
transcriptional and post-transcriptional intracellular changes,
from one original cancer can lead to the development of a
molecularly varied bulk tumor, made of multiple cancer cell clones,
each one presenting a differential sensitivity to the anticancer
therapies (48-60).
Anticancer therapies are designed with the final
goal of being effective in the eradication of the targeted
malignancy. As almost every available treatment is toxic towards
normal cells, their use may be coupled with toxic side-effects,
some of which can compromise the overall survival of the patients
(61). Importantly, the
intra-tumoral variety is tightly linked to the development of the
resistance to therapy, considered the first cause of failure of the
available treatments, as well as subsequent tumor relapses
(62). To fight the resistance,
integrated therapies and personalized approaches, based on the
specific genetic features of the malignancy, are in constant
development (62).
The host's immune system plays a fundamental role in
fighting and eliminating tumor cells (63-65).
On their side, malignant cells, thanks to their genetic
instability, constantly develop novel strategies with which to
escape from immunosurveillance (63,66). Targeted immunotherapy represents a
novel anticancer approach, able to boost the host anti-tumor immune
response, and, at the same time, help to ‘hit’ cancer resistance
and recurrence mechanisms (67,68).
Taken together, radiotherapy, chemotherapy and
immunotherapy, given their general toxicity, can compromise the gut
microbiome of patients. At the same time, modulating the gut
microbiome composition may deeply influence the outcome of patients
to therapies (69). It is
therefore of utmost importance to develop novel strategies with
which to manipulate the gut microbiome, with the main goal of
improving the therapeutic outcome of patients, without any
associated risk (70,71).
3. Gut microbiota and anticancer
therapy
A dysbiotic gut microbiota deeply influences both
cancer pathogenesis and its therapeutic outcome, with the latter
tightly connected with the ability of the gut microbiota to
metabolize antitumoral compounds, as well as to modulate a host's
immune response and inflammation pathways (72). The combination of these two
effects explains the strong involvement of the patients' microbiome
composition in affecting their final outcome to treatments
(73).
As regards the effects of the gut microbiome on the
host's immune system, the past year witnessed the publication of
marking breakthrough, strongly coupling the patients' microbiome
composition with the efficacy of immune checkpoint inhibitors-based
immunotherapy (74-76).
Immune checkpoint inhibition consists of the administration of
therapeutic agents able to block the immune-inhibitory pathway,
thus modulating T cell activation against tumor target cells [i.e.,
monoclonal antibodies blocking cytotoxic T-lymphocyte-associated
antigen 4 (CTLA4), programmed cell death protein 1 (PD1) or
programmed death-ligand 1 (PD-L1) targets] (77,78).
In particular, Routy et al (74) observed that patients with melanoma
treated with antibiotics along with the anti-PD1/anti-PD-L1
immunotherapy had a lower survival rate. Following the metagenomic
fecal analysis, anti-PD1 responders were found enriched in two
phyla (Akkermansia and Alistipes). Performing Fecal
Microbiota Transplantation (FMT) from patients to germ-free mice,
the authors found that Akkermansia muciniphila
increased intra-tumoral cytotoxic T cell infiltrates, thus
ameliorating the PD-1 blockade response in mice (74). Similarly, Gopalakrishnan et
al (75) carried out the
metagenomic analysis on stool samples from patients with melanoma,
finding that the anti-PD1 responders' microbiome differed in
composition compared with that of non-responders. In fact, there
was an increase in the abundance of Clostridiales, Ruminococcaceae
and Faecalibacteriae. Functional studies performed with FMT in
germ-free mice have further demonstrated how the treatment of mice
with the identified bacteria, along with the anti-PD1 therapy,
significantly reduced the growth of melanoma (75). Likewise, Matson et al
(76), accomplishing the
metagenomic analysis of fecal samples from patients with melanoma
treated with immune checkpoint inhibitors, found that responders
had a different microbiome profile compared to not responders. They
identified and functionally proved in vivo the role played
by Bifidobacterium longum, Enterococcus faecium and
Collinsella aerofaciens in ameliorating anti-PD-L1 efficacy
(76).
Taken together, these results provide strong
evidence of the pivotal role of selected gut resident strains in
modulating the effects of both immunotherapy response and toxicity.
Nevertheless, several obstacles still interfere with the robust
translation of the described bench results to the bedside. In fact,
the gastrointestinal microbiome of each single patient can be
either detrimental or beneficial to tumor progression and therapy,
depending on the prevailing inhabiting species. Moreover, the fact
that often, cancer patients undergoing therapy are
immunocompromised, has to be taken into careful consideration, as
this delicate condition could lead to the development of defeating
infections, due to the proliferation of opportunistic bacterial
species. Consequently, it is necessary to carefully analyze both
the risks and benefits of probiotics treatments coupled with
anticancer therapy, with the final goal of pursuing only beneficial
effects, without any safety issues.
4. Probiotics as adjuvants of anticancer
therapy
Tremendous progress has been made over the past
century to improve anti-cancer therapies, significantly reducing
detrimental side-effects, with the final goal of improving the
compliance of patients (79).
Manipulating the intestinal microbiome through the oral delivery of
probiotics is used to improve the safety, as well as to reduce the
drastic gastrointestinal side-effects, which are often associated
with anticancer treatments, mainly diarrhea and mucositis. In fact,
probiotics have the great advantage of being inexpensive and are
broadly regarded as safe (80,81). Generally, the use of probiotics in
clinical practice has demonstrated that probiotics have a broad
spectrum of benefits, including the amelioration of antibiotic- and
Clostridium difficile-associated diarrhea, as well as
respiratory tract infections (82). Repopulating the gut microbiota
cancer of patients through the administration of probiotics,
re-establishes both the abundance and the functionality of the
commensal gut bacteria, which has been possibly depleted after the
therapies (83). The main issues
of administering probiotics to immunocompromised cancer patients
are both the risk of opportunistic infections, as well as the
potential transfer of antibiotics resistance (84,85). In spite of this, the
administration of probiotics in multiple trials has shown the
readjustment of a healthy intestinal microbiota composition, the
amelioration of diarrhea and other types of therapy-associated
damage to the gastrointestinal system, including mucositis
(80). Moreover, probiotics
containing the Lactobacillus species have been suggested as
food supplements for the prevention of diarrhea and for the relief
of mucositis in patients receiving chemotherapy and/or radiation
therapy for a pelvic malignancy (86,87).
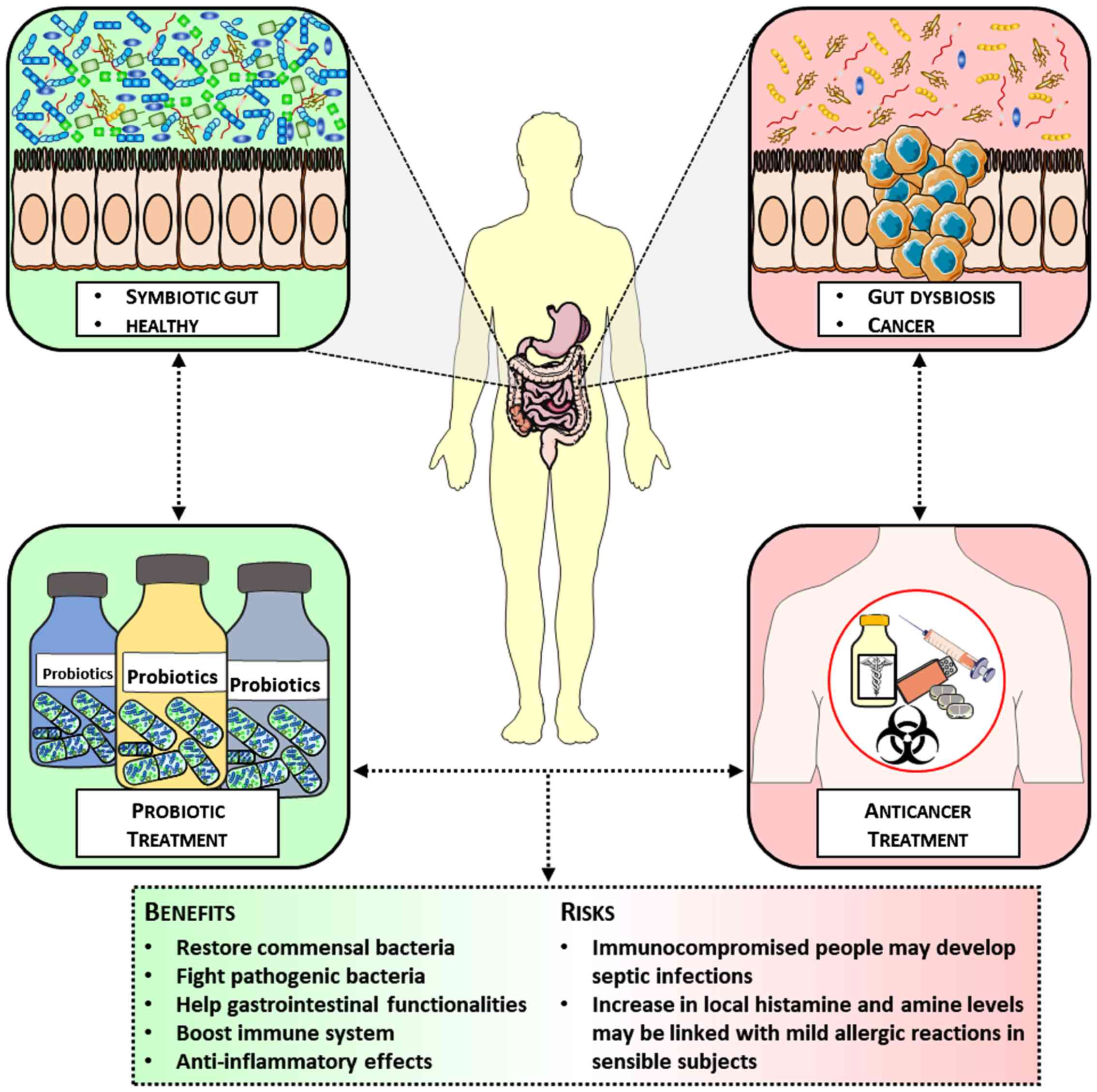
Fig. 1 summarizes
both the benefits and the risks potentially associated with the
administration of probiotics as adjuvants during anticancer
therapy, highlighting how probiotics may modulate the delicate gut
equilibrium, from a dysbiotic towards a healthy and functioning
microbiota.
Following this perspective, a growing number of
clinical studies are currently ongoing, with the common intent of
investigating the therapeutic potential of gut microbiota
manipulation in cancer patients through the oral administration of
probiotics as food supplements, along with their anticancer
treatment. The results from the published clinical trials are
encouraging. In 2010, a double-blind clinical trial, performed on
cancer patients undergoing colorectal resection, demonstrated the
positive effects of probiotic administration on the gut microbiota
composition, as well as on the regulation of intestinal immune
functions (88). In particular,
Lactobacillus johnsonii, administered to patients, was able
to adhere to the colonic mucosa, thereby reducing the concentration
of gut pathogens and modulating the local immunity (88). In 2014, a double-blind controlled
trial demonstrated the beneficial role of the probiotics
Lactobacillus acidophilus and Bifidobacterium longum
in reducing radiation-induced diarrhea, when administered to cancer
patients receiving pelvic radiation therapy (89). Moreover, in 2015, a clinical trial
evaluated the safety and efficacy of a probiotic formula consisting
of 10 bacterial strains (including Lactobacilli and
Bifidobacteria), orally administered along with
irinotecan-based chemotherapy, to patients with colorectal cancer
(CRC). The authors successfully found an effective reduction of
diarrhea and gastrointestinal dysfunctions in patients receiving
the probiotics (90). In 2016,
another double-blind, randomized trial demonstrated that patients
subjected to CRC resection exhibited a decreased risk of developing
post-operatory irritable bowel syndrome (IBS), when co-treated with
a synbiotic mix of prebiotics and probiotics (91). Also in 2016, another randomized
trial performed in patients with colon-resected CRC came to the
conclusion that Saccaromices bulardii effectively
downregulated pro-inflammatory cytokines (92). In 2017, a randomized clinical
trial demonstrated how the perioperative administration of a
synbiotic mixture of probiotics and prebiotics significantly
reduced post-operative infection rates in patients affected by CRC
(93).
In addition to the described published findings, a
number of clinical trials are currently ongoing to evaluate the
safety and the efficacy of using probiotics with anticancer
therapy. In fact, regardless the observed beneficial effects, it is
of fundamental importance to truly establish the safety of
administering probiotics to patients with severe cancer conditions
in a larger cohort of cases. The complete list of the currently
registered clinical studies (clinicaltrials.gov) untangling the
effects of administering probiotics to cancer patients during their
therapy, is reported in Table
I.
 | Table IClinical studies registered at
clinicaltrials.gov involving the use of probiotics in combination
with anticancer therapy. |
Table I
Clinical studies registered at
clinicaltrials.gov involving the use of probiotics in combination
with anticancer therapy.
| Study ID | Title of the
study | Conditions | Interventions | Status | Ref. |
|---|
| NCT00936572 | Probiotics In
Colorectal Cancer Patients | CRC | Probiotics (B.
longum, L. johnsonii) | C | (88) |
| NCT01839721 | Impact of
Probiotics BIFILACT® on Diarrhea in Cancer Patients
Treated With Pelvic Radiation | CAN | BIFILACT (L.
acidophilus, B. longum) | C | (89) |
| NCT01410955 | Prevention of
Irinotecan Induced Diarrhea by Probiotics | CRC | Colon Dophilus
(Lactobacillus spp, Bifidobacterium spp) | C | (90) |
| NCT01479907 | Synbiotics and
Gastrointestinal Function Related Quality of Life After Colectomy
for Cancer | CRC | Synbiotic Forte
(Lactobacillus spp and prebiotics) | C | (91) |
| NCT01609660 | Impact of
Probiotics on the Intestinal Microbiota | CRC | S.
boulardii | C | (92) |
| NCT01468779 | Effect of
Probiotics in Patients Undergoing Surgery for Periampullary
Neoplasms | PC | Probiotic
formula | C | (93) |
| NCT03420443 | Action of
Synbiotics on Irradiated GI Mucosa in CRC Treatment (FIPIREX) | CRC | Synbiotics | C | |
| NCT01723592 | Orally Administered
Probiotics to Improve the Quality of the Vaginal Flora of Women
With Breast Cancer and Chemotherapy | BC | Probiotics
(Lactobacillus spp) | C | |
| NCT02771470 | Intestinal
Microflora in Lung Cancer After Chemotherapy | LC | C.
butyricum | C | |
| NCT01895530 | Impact of
Probiotics in Modulation of Intestinal Microbiota | CRC | S.
boulardii | C | |
| NCT02021253 | Influence of
Probiotics Administration Before Liver Resection in Liver
Disease | HC | Lactibiane (B.
lactis, L. acidophilus, L. plantarum, L.
salivarius) | C | |
| NCT03531606 | The Effects of
Mechnikov Probiotics on Symptom and Surgical Outcome | CRC | Probiotics | C | |
| NCT03782428 | An Evaluation of
Probiotic in the Clinical Course of Patients With Colorectal
Cancer | CRC | HEXBIO (L.
acidophilus, L. lactis, L. casei, B.
longum, B. bifidum, B. infantis) | C | |
| NCT03358511 | Engineering Gut
Microbiome to Target Breast Cancer | BC | Primal Defense
Ultra (Lactobacillus spp, Bifidobacterium spp) | O | |
| NCT03785938 | Mucositis and
Infection Reduction With Liquid Probiotics in Children With Cancer
(MaCROS) | PEDC | Symprove (L.
rhamnosus, E. faecium, L. acidophilus, L.
plantarum) | O | |
| NCT02944617 | Probiotic Yogurt
Supplement in Reducing Diarrhea in Patients With Metastatic Kidney
Cancer Being Treated With Vascular Endothelial Growth
Factor-Tyrosine Kinase Inhibitor | RCC | Yogurt | O | |
| NCT03704727 | The Effects of
Probiotics on Intestinal Permeability in Gastrointestinal Cancer
Patients in Chemotherapy | GIC | VSL3
(Lactobacillus spp, Bifidobacterium spp) | O | |
| NCT03742596 | The Effect of
Probiotics Supplementation on the Side Effects of Radiation Therapy
Among Colorectal Cancer Patients | CRC | Probiotic Formula
(Lactobacillus spp, Bifidobacterium spp) | O | |
| NCT03177681 | The Effect of
Yogurt in Cancer Patient With Moderate Gastrointestinal
Symptoms | CAN | Yogurt | O | |
| NCT02351089 | Probiotics in
Radiation-treated Gynecologic Cancer (ProRad) | GYC | Probiotics | O | |
| NCT03705442 | Probiotics as
Adjuvant Therapy in the Treatment of Metastatic Colorectal Cancer
(Probat-tmcc-17) | CRC | Omni-Biotic 10
(Lactobacillus spp, Bifidobacterium spp) | O | |
| NCT03552458 | Effects of
Probiotics in Preventing Oral Mucositis | HAN | L.
Reuteri | O | |
| NCT03518268 | Vivomixx for
Prevention of Bone Loss in Women With Breast Cancer Treated With an
Aromatase Inhibitor | BC | Vivomixx
(Lactobacillus spp, Bifidobacterium spp) | O | |
| NCT03574051 | Microbiota
Associated With Iodine-131 Therapy and Hypothyroidism | TC | Probiotics (B.
infantis, L. acidophilus, E. faecalis) | O | |
| NCT03642548 | Probiotics Combined
With Chemotherapy for Patients With Advanced NSCLC | NSCLC | Bifico (B.
Coagulans) | O | |
| NCT02751736 | The Effect Of
Probiotics On Bowel Function Restoration After Ileostomy Closure In
Patients With Rectal Cancer | CRC | L.
plantarum | O | |
| NCT01790035 | Probiotic LGG for
Prevention of Side Effects in Patients Undergoing Chemoradiation
for Gastrointestinal Cancer | GIC | L. rhamnosus
GG | O | (101) |
| NCT00197873 | Lactobacillus
rhamnosus in Prevention of Chemotherapy-related Diarrhoea | CRC | L. rhamnosus
GG | O | |
| NCT02544685 | Prevention of
Febrile Neutropenia by Synbiotics in Pediatric Cancer Patients | CAN | Probio-Fix Inum and
corn starch (L. rhamnosus GG, B. animalis) | O | |
| NCT02819960 | Prevention of
Irinotecan Induced Diarrhea by Probiotics | CAN | Probio-Fix Inum
(L. rhamnosus GG, B. animalis) | O | |
5. LGG, a model probiotic for use as an
anticancer adjuvant
The probiotic archetype Lactobacillus
rhamnosus GG (LGG) represents one of the first studied bacteria
in oncology (94). LGG is a
gut-resident bacterium which has the ability to restore gut
microbial balance, thanks to its anti-inflammatory properties
(95-99). The benefits of
administering LGG to cancer patients is supported by multiple in
vitro, in vivo and clinical studies, as recently
reviewed by our group (100).
Moreover, 70 trials are currently registered at clinicaltial.gov,
aiming to specifically determine the effects associated with the
administration of LGG in several different conditions (Table II).
 | Table IIClinical trials registered at
clinicaltrials.gov assessing the benefits of administering LGG in
association with a large number of different conditions. |
Table II
Clinical trials registered at
clinicaltrials.gov assessing the benefits of administering LGG in
association with a large number of different conditions.
| NCT No. | Status | Conditions |
|---|
| NCT01922895 | Active | Acute Alcoholic
Hepatitis |
| NCT03080818 | Active | Aging |
| NCT03449537 | Active | Allergy Milk |
| NCT03256708 | Active |
Antibiotic-Associated Diarrhea |
| NCT03449459 | Active | Chronic Obstructive
Pulmonary Disease |
| NCT03587545 | Active | Chronic
Rhinosinusitis |
| NCT03647995 | Active | Diarrhea,
Clostridium difficile |
| NCT02544685 | Active | Febrile
Neutropenia |
| NCT01790035 | Active | Gastrointestinal
Neoplasms |
| NCT02640625 | Active | Human
Immunodeficiency Virus |
| NCT02748317 | Active | Lower Urinary Tract
Symptoms |
| NCT02748356 | Active | Lower Urinary Tract
Symptoms |
| NCT03383874 | Active | Mania,
Neurotic |
| NCT03215784 | Active | Obesity, Pregnancy,
Inflammation |
| NCT03277820 | Active | Otitis Media |
| NCT03196453 | Active | Overweight,
Nutrition Disorder |
| NCT02462590 | Active | Pneumonia,
Infections, Diarrhea |
| NCT01454661 | Active | Premature
Infant |
| NCT00490425 | Completed | Allergic
Asthma |
| NCT01901380 | Completed | Allergy, Functional
Gastrointestinal Disorders |
| NCT00748748 | Completed |
Antibiotic-Associated Diarrhea |
| NCT02711800 | Completed | Anxiety, Abdominal
Pain |
| NCT00159523 | Completed | Asthma, Atopic
Dermatitis |
| NCT01148667 | Completed | Atopic
Dermatitis |
| NCT00325273 | Completed | Atopic Dermatitis,
Allergic Rhinitis, Asthma |
| NCT00224432 | Completed | Atopic Dermatitis,
Atopic Eczema |
| NCT03078179 | Completed | Caries, Dental |
| NCT01279265 | Completed | Colic,
Inflammation |
| NCT02466035 | Completed | Cow’s Milk
Allergy |
| NCT02779881 | Completed | Cow’s Milk
Allergy |
| NCT01956916 | Completed | Cystic
Fibrosis |
| NCT01961661 | Completed | Cystic
Fibrosis |
| NCT00318695 | Completed | Eczema, Asthma,
Allergic Rhinitis |
| NCT02642289 | Completed | Fibromyalgia |
| NCT01773967 | Completed |
Gastroenteritis |
| NCT02144701 | Completed | Graft Versus Host
Disease |
| NCT00620412 | Completed | Healthy |
| NCT00934453 | Completed | Healthy |
| NCT03168503 | Completed | Healthy |
| NCT01274598 | Completed | Healthy,
Elderly |
| NCT01368029 | Completed | Healthy,
Elderly |
| NCT01545349 | Completed | Healthy,
Influenza |
| NCT03427515 | Completed | Healthy,
Stress-related Problem, Anxiety |
| NCT01969331 | Completed | Helicobacter
pylori Infection |
| NCT03307772 | Completed | Herpes
Labialis |
| NCT03310294 | Completed | Herpes
Labialis |
| NCT01439841 | Completed | HIV-1
Infection |
| NCT01616693 | Completed | Immunity to Oral
Vaccines |
| NCT02046512 | Completed | Infection |
| NCT01551186 | Completed | Infectious Disease
of Digestive Tract |
| NCT01130792 | Completed | Infectious
Gastroenteritis |
| NCT No. | Status | Conditions |
| NCT02230345 | Completed | Inflammation,
Dyslipidemia |
| NCT01720329 | Completed | Influenza |
| NCT03100266 | Completed | Low Back Pain |
| NCT01164124 | Completed | Low Birth
Weight |
| NCT02288572 | Completed | Metabolic Syndrome
X |
| NCT01670916 | Completed | Necrotizing
Enterocolitis |
| NCT01868737 | Completed | Necrotizing
Enterocolitis |
| NCT02807246 | Completed | Neonatal
Hyperbilirubinemia |
| NCT02558192 | Completed | Nosocomial
Infection |
| NCT01870544 | Completed | Obesity |
| NCT02444182 | Completed | Periodontal Health,
Dental Plaque Accumulation |
| NCT00282113 | Completed | Premature
Infants |
| NCT02180581 | Completed | Respiratory
Infections, Gastrointestinal Infections |
| NCT01229917 | Completed | Respiratory Tract
Infections |
| NCT02110732 | Completed | Upper Respiratory
Infection, Acute Otitis Media |
| NCT01782755 | Completed | Ventilator
Associated Pneumonia |
| NCT00445120 | Completed | Vernal
Keratoconjunctivitis |
In line with these studies, a number of ongoing
clinical trials are currently testing both the effectiveness and
the safety of administering LGG to cancer patients subjected to
anticancer therapy (NCT01790035, NCT00197873, NCT02544685,
NCT02819960; Table I). Very
recently, pre-results in support of the ongoing clinical trial
NCT01790035 have been published. These results clearly show the
mechanisms through which LGG is able to selectively protect colon
normal cells during radiotherapy protocols, both in vitro
and in vivo. LGG functions as a ‘time-release capsule’, able
to deliver radioprotective lipoteichoic acid (LTA) within the
intestinal crypts, thereby selectively protecting from the
radiation-induced cell death the normal cells, but not the tumor
cells (101). Notably, the group
demonstrated that LGG-derived LTA activates peri-cryptal
macrophages, in turn protecting the epithelial stem cells from
radiation-induced apoptosis (101).
In addition to the cited clinical trials, two
clinical trials designed by our group are currently opening and are
about to be registered at clinicaltrials.gov. The two studies,
entitled respectively: ‘Maintenance of normal gastrointestinal
function with dietary supplement containing Lactobacillus
rhamnosus GG in cancer patients treated with cytotoxic
chemotherapy and/or targeted therapy’ and ‘Maintenance of normal
gastrointestinal function with dietary supplement containing
Lactobacillus rhamnosus GG in patients treated with
abdominal or pelvic radiotherapy’, will assess the efficacy of LGG
daily oral administration in the maintenance of normal
gastrointestinal functions within cancer patients, treated either
with chemotherapy and/or targeted therapy or abdominal/pelvic
radiotherapy.
Once concluded, the currently ongoing clinical
studies, will shed light into the efficacy and safety of the use of
the promising probiotic, LGG, as an adjuvant in oncology. The
studies will assess whether LGG is truly able to protect cancer
patients from the detrimental gastrointestinal side-effects usually
associated with anticancer therapy.
6. Conclusions
The human gut microbiota composition consists of a
delicate balance, constantly modulated by several processes
affecting the host during the entire lifespan (including aging,
diet and lifetime exposure to heterogeneous environmental factors).
A healthy microbiota is able to perform core symbiotic functions
within his host, in a well-integrated host-microbiota
relationship.
Cancer is a condition which tremendously affects the
gut microbiota-host equilibrium, both during oncogenesis, as well
as concurrently with anticancer therapy. This unbalanced
equilibrium is often followed by the dysbiosis of the gut
microbiota. Consequently, current research is constantly aiming at
identifying methods with which to safely modulate a dysbiotic
microbiota, helping to heal detrimental conditions, such as the
gastrointestinal side-effects of chemotherapy, radiation therapy
and immunotherapy (including mucositis, diarrhea and opportunistic
infections).
The administration of probiotics during anticancer
therapy is yielding promising clinical results, as it improves gut
dysbiosis in cancer patients. Moreover, probiotics have been found
capable of significantly ameliorating patients' compliance to
treatments, as well as their overall quality of life. Among the
characterized probiotics, recent studies have suggested that LGG,
administered in vivo, is able to modulate the immune system,
reducing the detrimental toxic intestinal effects following pelvic
radiotherapy. This result is particularly promising and paves the
way towards the auspicious ongoing trials on cancer patients
undergoing anticancer treatments.
Despite the already published clinical results
reporting the beneficial role of probiotics in alleviating the
harmful side-effects of anticancer therapies, care needs to be
pursued, as patients are often immunocompromised; therefore, it is
important to evaluate the health risks possibly linked to the
administration of probiotics to sensitive individuals. In the
future, the design of novel experimental trials may undertake a
personalized approach, considering the specific clinical and
pathological background of each single patient to be enrolled, in
order to gain only the positive outcomes of probiotics
administration, possibly without any harmful side-effect.
Acknowledgements
The authors would like to thank the Italian League
Against Cancer (LILT) for its support.
Funding
No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SV and ML were involved in the conceptualization and
design of this review article. LF, MSB and CG were involved in
searching the literature for paragraphs 2 and 5. DN and MS were
involved in searching the relevant literature and databases for
paragraphs 3 and 4. SV and ML were involved in the preparation of
the original draft and in the preparation of the figure and tables.
SV, LF, MSB, DN, CG, ML and MS reviewed and edited the article. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
ML is the PI of a research grant founded by Dicofarm
Spa to his University Department. The other authors declare that
the research was conducted in the absence of any commercial or
financial relationships that could be construed as a potential
competing interest.
References
|
1
|
Ursell LK, Metcalf JL, Parfrey LW and
Knight R: Defining the human microbiome. Nutr Rev. 70 (Suppl
1):S38–S44. 2012.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang YJ, Li S, Gan RY, Zhou T, Xu DP and
Li HB: Impacts of gut bacteria on human health and diseases. Int J
Mol Sci. 16:7493–7519. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Feng Q, Chen WD and Wang YD: Gut
Microbiota: An Integral Moderator in Health and Disease. Front
Microbiol. 9(151)2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Lynch SV and Pedersen O: The Human
Intestinal Microbiome in Health and Disease. N Engl J Med.
375:2369–2379. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Greenhalgh K, Meyer KM, Aagaard KM and
Wilmes P: The human gut microbiome in health: Establishment and
resilience of microbiota over a lifetime. Environ Microbiol.
18:2103–2116. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Neuman H, Debelius JW, Knight R and Koren
O: Microbial endocrinology: The interplay between the microbiota
and the endocrine system. FEMS Microbiol Rev. 39:509–521.
2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Ceranowicz P, Warzecha Z and Dembinski A:
Peptidyl hormones of endocrine cells origin in the gut--their
discovery and physiological relevance. J Physiol Pharmacol.
66:11–27. 2015.PubMed/NCBI
|
|
8
|
Sandrini S, Aldriwesh M, Alruways M and
Freestone P: Microbial endocrinology: Host-bacteria communication
within the gut microbiome. J Endocrinol. 225:R21–R34.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ravussin Y, Koren O, Spor A, LeDuc C,
Gutman R, Stombaugh J, Knight R, Ley RE and Leibel RL: Responses of
gut microbiota to diet composition and weight loss in lean and
obese mice. Obesity (Silver Spring). 20:738–747. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Queipo-Ortuño MI, Seoane LM, Murri M,
Pardo M, Gomez-Zumaquero JM, Cardona F, Casanueva F and Tinahones
FJ: Gut microbiota composition in male rat models under different
nutritional status and physical activity and its association with
serum leptin and ghrelin levels. PLoS One. 8(e65465)2013.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Gensollen T, Iyer SS, Kasper DL and
Blumberg RS: How colonization by microbiota in early life shapes
the immune system. Science. 352:539–544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Schmidt TSB, Raes J and Bork P: The Human
Gut Microbiome: From Association to Modulation. Cell.
172:1198–1215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Bultman SJ: Emerging roles of the
microbiome in cancer. Carcinogenesis. 35:249–255. 2014.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Cani PD: Human gut microbiome: Hopes,
threats and promises. Gut. 67:1716–1725. 2018.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mohajeri MH, Brummer RJM, Rastall RA,
Weersma RK, Harmsen HJM, Faas M and Eggersdorfer M: The role of the
microbiome for human health: From basic science to clinical
applications. Eur J Nutr. 57 (Suppl 1):1–14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fukui H, Xu X and Miwa H: Role of Gut
Microbiota-Gut Hormone Axis in the Pathophysiology of Functional
Gastrointestinal Disorders. J Neurogastroenterol Motil. 24:367–386.
2018.PubMed/NCBI View
Article : Google Scholar
|
|
17
|
Clarke G, Stilling RM, Kennedy PJ, Stanton
C, Cryan JF and Dinan TG: Minireview: Gut microbiota: the neglected
endocrine organ. Mol Endocrinol. 28:1221–1238. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vaishnava S, Behrendt CL, Ismail AS,
Eckmann L and Hooper LV: Paneth cells directly sense gut commensals
and maintain homeostasis at the intestinal host-microbial
interface. Proc Natl Acad Sci USA. 105:20858–20863. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Belkaid Y and Naik S: Compartmentalized
and systemic control of tissue immunity by commensals. Nat Immunol.
14:646–653. 2013.PubMed/NCBI View
Article : Google Scholar
|
|
20
|
Magnúsdóttir S, Ravcheev D, de
Crécy-Lagard V and Thiele I: Systematic genome assessment of
B-vitamin biosynthesis suggests co-operation among gut microbes.
Front Genet. 6(148)2015.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Carding S, Verbeke K, Vipond DT, Corfe BM
and Owen LJ: Dysbiosis of the gut microbiota in disease. Microb
Ecol Health Dis. 26(26191)2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Grice EA and Segre JA: The human
microbiome: Our second genome. Annu Rev Genomics Hum Genet.
13:151–170. 2012.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Geva-Zatorsky N, Sefik E, Kua L, Pasman L,
Tan TG Ortiz-Lopez A, Yanortsang TB, Yang L, Jupp R, Mathis D, et
al: Mining the Human Gut Microbiota for Immunomodulatory Organisms.
Cell. 168:928–943.e11. 2017.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Haber AL, Biton M, Rogel N, Herbst RH,
Shekhar K, Smillie C, Burgin G, Delorey TM, Howitt MR, Katz Y, et
al: A single-cell survey of the small intestinal epithelium.
Nature. 551:333–339. 2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Nash AK, Auchtung TA, Wong MC, Smith DP,
Gesell JR, Ross MC, Stewart CJ, Metcalf GA, Muzny DM, Gibbs RA, et
al: The gut mycobiome of the Human Microbiome Project healthy
cohort. Microbiome. 5(153)2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Lloyd-Price J, Mahurkar A, Rahnavard G,
Crabtree J, Orvis J, Hall AB, Brady A, Creasy HH, McCracken C,
Giglio MG, et al: Strains, functions and dynamics in the expanded
Human Microbiome Project. Nature. 550:61–66. 2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Rothschild D, Weissbrod O, Barkan E,
Kurilshikov A, Korem T, Zeevi D, Costea PI, Godneva A, Kalka IN,
Bar N, et al: Environment dominates over host genetics in shaping
human gut microbiota. Nature. 555:210–215. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Korem T, Zeevi D, Suez J, Weinberger A,
Avnit-Sagi T, Pompan-Lotan M, Matot E, Jona G, Harmelin A, Cohen N,
et al: Growth dynamics of gut microbiota in health and disease
inferred from single metagenomic samples. Science. 349:1101–1106.
2015.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lloyd-Price J, Abu-Ali G and Huttenhower
C: The healthy human microbiome. Genome Med. 8(51)2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Hill C, Guarner F, Reid G, Gibson GR,
Merenstein DJ, Pot B, Morelli L, Canani RB, Flint HJ, Salminen S,
et al: Expert consensus document. The International Scientific
Association for Probiotics and Prebiotics consensus statement on
the scope and appropriate use of the term probiotic. Nat Rev
Gastroenterol Hepatol. 11:506–514. 2014.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Pandey KR, Naik SR and Vakil BV:
Probiotics, prebiotics and synbiotics- a review. J Food Sci
Technol. 52:7577–7587. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Tsai YL, Lin TL, Chang CJ, Wu TR, Lai WF,
Lu CC and Lai HC: Probiotics, prebiotics and amelioration of
diseases. J Biomed Sci. 26(3)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Goodman B and Gardner H: The microbiome
and cancer. J Pathol. 244:667–676. 2018.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tomasetti C and Vogelstein B: Cancer
etiology. Variation in cancer risk among tissues can be explained
by the number of stem cell divisions. Science. 347:78–81.
2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ashford NA, Bauman P, Brown HS, Clapp RW,
Finkel AM, Gee D, Hattis DB, Martuzzi M, Sasco AJ and Sass JB:
Cancer risk: Role of environment. Science. 347(727)2015.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Polo A, Crispo A, Cerino P, Falzone L,
Candido S, Giudice A, De Petro G, Ciliberto G, Montella M, Budillon
A, et al: Environment and bladder cancer: Molecular analysis by
interaction networks. Oncotarget. 8:65240–65252. 2017.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Falzone L, Marconi A, Loreto C, Franco S,
Spandidos DA and Libra M: Occupational exposure to carcinogens:
Benzene, pesticides and fibers (Review). Mol Med Rep. 14:4467–4474.
2016.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Anand P, Kunnumakkara AB, Sundaram C,
Harikumar KB, Tharakan ST, Lai OS, Sung B and Aggarwal BB: Cancer
is a preventable disease that requires major lifestyle changes.
Pharm Res. 25:2097–2116. 2008.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Global Burden of Disease Cancer
Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L,
Bhutta ZA, Brenner H, Dicker DJ, Chimed-Orchir O, Dandona R, et al:
Global, Regional, and National Cancer Incidence, Mortality, Years
of Life Lost, Years Lived With Disability, and Disability-Adjusted
Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic
Analysis for the Global Burden of Disease Study. JAMA Oncol.
3:524–548. 2017.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Wang H, Naghavi M, Allen C, Barber RM,
Bhutta ZA, Carter A, Casey DC, Charlson FJ, Chen AZ, Coates MM, et
al: GBD 2015 Mortality and Causes of Death Collaborators: Global,
regional, and national life expectancy, all-cause mortality, and
cause-specific mortality for 249 causes of death, 1980-2015: A
systematic analysis for the Global Burden of Disease Study 2015.
Lancet. 388:1459–1544. 2016.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Rapisarda V, Salemi R, Marconi A, Loreto
C, Graziano AC, Cardile V, Basile MS, Candido S, Falzone L,
Spandidos DA, et al: Fluoro-edenite induces fibulin-3
overexpression in non-malignant human mesothelial cells. Oncol
Lett. 12:3363–3367. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Rapisarda V, Ledda C, Matera S, Fago L,
Arrabito G, Falzone L, Marconi A, Libra M and Loreto C: Absence of
t(14;18) chromosome translocation in agricultural workers after
short-term exposure to pesticides. Mol Med Rep. 15:3379–3382.
2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Fenga C, Gangemi S, Di Salvatore V,
Falzone L and Libra M: Immunological effects of occupational
exposure to lead (Review). Mol Med Rep. 15:3355–3360.
2017.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Vogelstein B, Papadopoulos N, Velculescu
VE, Zhou S, Diaz LA Jr and Kinzler KW: Cancer genome landscapes.
Science. 339:1546–1558. 2013.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Guarneri C, Bevelacqua V, Polesel J,
Falzone L, Cannavò PS, Spandidos DA, Malaponte G and Libra M: NF-κB
inhibition is associated with OPN/MMP-9 downregulation in cutaneous
melanoma. Oncol Rep. 37:737–746. 2017.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Salemi R, Falzone L, Madonna G, Polesel J,
Cinà D, Mallardo D, Ascierto PA, Libra M and Candido S: MMP-9 as a
Candidate Marker of Response to BRAF Inhibitors in Melanoma
Patients With BRAFV600E Mutation Detected in Circulating-Free DNA.
Front Pharmacol. 9(856)2018.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Leonardi GC, Falzone L, Salemi R, Zanghì
A, Spandidos DA, Mccubrey JA, Candido S and Libra M: Cutaneous
melanoma: From pathogenesis to therapy (Review). Int J Oncol.
52:1071–1080. 2018.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Bhang HE, Ruddy DA, Krishnamurthy
Radhakrishna V, Caushi JX, Zhao R, Hims MM, Singh AP, Kao I, Rakiec
D, Shaw P, et al: Studying clonal dynamics in response to cancer
therapy using high-complexity barcoding. Nat Med. 21:440–448.
2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Kloor M and von Knebel Doeberitz M: The
Immune Biology of Microsatellite-Unstable Cancer. Trends Cancer.
2:121–133. 2016.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Carter SL, Eklund AC, Kohane IS, Harris LN
and Szallasi Z: A signature of chromosomal instability inferred
from gene expression profiles predicts clinical outcome in multiple
human cancers. Nat Genet. 38:1043–1048. 2006.PubMed/NCBI View
Article : Google Scholar
|
|
51
|
Dagogo-Jack I and Shaw AT: Tumour
heterogeneity and resistance to cancer therapies. Nat Rev Clin
Oncol. 15:81–94. 2018.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Falzone L, Romano GL, Salemi R, Bucolo C,
Tomasello B, Lupo G, Anfuso CD, Spandidos DA, Libra M and Candido
S: Prognostic significance of deregulated microRNAs in uveal
melanomas. Mol Med Rep. 19:2599–2610. 2019.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Battaglia R, Palini S, Vento ME, La
Ferlita A, Lo Faro MJ, Caroppo E, Borzì P, Falzone L, Barbagallo D,
Ragusa M, et al: Identification of extracellular vesicles and
characterization of miRNA expression profiles in human blastocoel
fluid. Sci Rep. 9(84)2019.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Basile MS, Fagone P, Mangano K, Mammana S,
Magro G, Salvatorelli L, Li Destri G, La Greca G, Nicoletti F,
Puleo S and Pesce A: KCNMA1 Expression is Downregulated in
Colorectal Cancer via Epigenetic Mechanisms. Cancers (Basel).
11pii. (E245)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Falzone L, Scola L, Zanghì A, Biondi A, Di
Cataldo A, Libra M and Candido S: Integrated analysis of colorectal
cancer microRNA datasets: Identification of microRNAs associated
with tumor development. Aging (Albany NY). 10:1000–1014.
2018.PubMed/NCBI View Article : Google Scholar
|
|
56
|
McCubrey JA, Fitzgerald TL, Yang LV,
Lertpiriyapong K, Steelman LS, Abrams SL, Montalto G, Cervello M,
Neri LM, Cocco L, et al: Roles of GSK-3 and microRNAs on epithelial
mesenchymal transition and cancer stem cells. Oncotarget.
8:14221–14250. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Falzone L, Candido S, Salemi R, Basile MS,
Scalisi A, McCubrey JA, Torino F, Signorelli SS, Montella M and
Libra M: Computational identification of microRNAs associated to
both epithelial to mesenchymal transition and NGAL/MMP-9 pathways
in bladder cancer. Oncotarget. 7:72758–72766. 2016.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Hafsi S, Candido S, Maestro R, Falzone L,
Soua Z, Bonavida B, Spandidos DA and Libra M: Correlation between
the overexpression of Yin Yang 1 and the expression levels of
miRNAs in Burkitt's lymphoma: A computational study. Oncol Lett.
11:1021–1025. 2016.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Falzone L, Lupo G, Rosa GRM, Crimi S,
Anfuso CD, Salemi R, Rapisarda E, Libra M and Candido S:
Identification of Novel MicroRNAs and Their Diagnostic and
Prognostic Significance in Oral Cancer. Cancers (Basel). 11pii.
(E610)2019.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Falzone L, Salemi R, Travali S, Scalisi A,
McCubrey JA, Candido S and Libra M: MMP-9 overexpression is
associated with intragenic hypermethylation of MMP9 gene in
melanoma. Aging (Albany NY). 8:933–944. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Dy GK and Adjei AA: Understanding,
recognizing, and managing toxicities of targeted anticancer
therapies. CA Cancer J Clin. 63:249–279. 2013.PubMed/NCBI View Article : Google Scholar
|
|
62
|
McGranahan N and Swanton C: Biological and
therapeutic impact of intratumor heterogeneity in cancer evolution.
Cancer Cell. 27:15–26. 2015.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Thorsson V, Gibbs DL, Brown SD, Wolf D,
Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy
JA, et al: Cancer Genome Atlas Research Network: The Immune
Landscape of Cancer. Immunity. 48:812–830.e14. 2018.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Presti M, Mazzon E, Basile MS, Petralia
MC, Bramanti A, Colletti G, Bramanti P, Nicoletti F and Fagone P:
Overexpression of macrophage migration inhibitory factor and
functionally-related genes, D-DT, CD74, CD44, CXCR2 and CXCR4, in
glioblastoma. Oncol Lett. 16:2881–2886. 2018.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Mangano K, Mazzon E, Basile MS, Di Marco
R, Bramanti P, Mammana S, Petralia MC, Fagone P and Nicoletti F:
Pathogenic role for macrophage migration inhibitory factor in
glioblastoma and its targeting with specific inhibitors as novel
tailored therapeutic approach. Oncotarget. 9:17951–17970.
2018.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Basile MS, Mazzon E, Russo A, Mammana S,
Longo A, Bonfiglio V, Fallico M, Caltabiano R, Fagone P, Nicoletti
F, et al: Differential modulation and prognostic values of
immune-escape genes in uveal melanoma. PLoS One.
14(e0210276)2019.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Emens LA, Ascierto PA, Darcy PK, Demaria
S, Eggermont AMM, Redmond WL, Seliger B and Marincola FM: Cancer
immunotherapy: Opportunities and challenges in the rapidly evolving
clinical landscape. Eur J Cancer. 81:116–129. 2017.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Toh HC: Cancer immunotherapy-the end of
the beginning. Linchuang Zhongliuxue Zazhi. 7(12)2018.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Roy S and Trinchieri G: Microbiota: A key
orchestrator of cancer therapy. Nat Rev Cancer. 17:271–285.
2017.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Nayak RR and Turnbaugh PJ: Mirror, mirror
on the wall: Which microbiomes will help heal them all? BMC Med.
14(72)2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Fessler JL and Gajewski TF: The
Microbiota: A New Variable Impacting Cancer Treatment Outcomes.
Clin Cancer Res. 23:3229–3231. 2017.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Schwabe RF and Jobin C: The microbiome and
cancer. Nat Rev Cancer. 13:800–812. 2013.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Gopalakrishnan V, Helmink BA, Spencer CN,
Reuben A and Wargo JA: The Influence of the Gut Microbiome on
Cancer, Immunity, and Cancer Immunotherapy. Cancer Cell.
33:570–580. 2018.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Routy B, Le Chatelier E, Derosa L, Duong
CPM, Alou MT, Daillère R, Fluckiger A, Messaoudene M, Rauber C,
Roberti MP, et al: Gut microbiome influences efficacy of PD-1-based
immunotherapy against epithelial tumors. Science. 359:91–97.
2018.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gopalakrishnan V, Spencer CN, Nezi L,
Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman
K, Wei SC, et al: Gut microbiome modulates response to anti-PD-1
immunotherapy in melanoma patients. Science. 359:97–103.
2018.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Matson V, Fessler J, Bao R, Chongsuwat T,
Zha Y, Alegre ML, Luke JJ and Gajewski TF: The commensal microbiome
is associated with anti-PD-1 efficacy in metastatic melanoma
patients. Science. 359:104–108. 2018.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Chen Q, Wang C, Chen G, Hu Q and Gu Z:
Delivery Strategies for Immune Checkpoint Blockade. Adv Healthc
Mater. 7(e1800424)2018.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Buchbinder EI and Desai A: CTLA-4 and PD-1
Pathways: Similarities, Differences, and Implications of Their
Inhibition. Am J Clin Oncol. 39:98–106. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Falzone L, Salomone S and Libra M:
Evolution of Cancer Pharmacological Treatments at the Turn of the
Third Millennium. Front Pharmacol. 9(1300)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Mego M, Holec V, Drgona L, Hainova K,
Ciernikova S and Zajac V: Probiotic bacteria in cancer patients
undergoing chemotherapy and radiation therapy. Complement Ther Med.
21:712–723. 2013.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Maria-Aggeliki KS, Nikolaos KL, Kyrias GM
and Vassilis KE: The potential clinical impact of probiotic
treatment for the prevention and/or anti-inflammatory therapeutic
effect against radiation induced intestinal mucositis. A review.
Recent Pat Inflamm Allergy Drug Discov. 3:195–200. 2009. View Article : Google Scholar
|
|
82
|
Rondanelli M, Faliva MA, Perna S, Giacosa
A, Peroni G and Castellazzi AM: Using probiotics in clinical
practice: Where are we now? A review of existing meta-analyses. Gut
Microbes. 8:521–543. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Zitvogel L, Ma Y, Raoult D, Kroemer G and
Gajewski TF: The microbiome in cancer immunotherapy: Diagnostic
tools and therapeutic strategies. Science. 359:1366–1370.
2018.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Vanderhoof JA and Young R: Probiotics in
the United States. Clin Infect Dis. 46 (Suppl 2):S67–S72;
discussion S144-S151. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
85
|
Redman MG, Ward EJ and Phillips RS: The
efficacy and safety of probiotics in people with cancer: A
systematic review. Ann Oncol. 25:1919–1929. 2014.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Peterson DE, Boers-Doets CB, Bensadoun RJ,
Herrstedt J and Committee EG: ESMO Guidelines Committee Management
of oral and gastrointestinal mucosal injury: ESMO Clinical Practice
Guidelines for diagnosis, treatment, and follow-up. Ann Oncol. 26
(Suppl 5):v139–v151. 2015. View Article : Google Scholar
|
|
87
|
Lalla RV, Bowen J, Barasch A, Elting L,
Epstein J, Keefe DM, McGuire DB, Migliorati C, Nicolatou-Galitis O,
Peterson DE, et al: Mucositis Guidelines Leadership Group of the
Multinational Association of Supportive Care in Cancer and
International Society of Oral Oncology (MASCC/ISOO): MASCC/ISOO
clinical practice guidelines for the management of mucositis
secondary to cancer therapy. Cancer. 120:1453–1461. 2014.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Gianotti L, Morelli L, Galbiati F,
Rocchetti S, Coppola S, Beneduce A, Gilardini C, Zonenschain D,
Nespoli A and Braga M: A randomized double-blind trial on
perioperative administration of probiotics in colorectal cancer
patients. World J Gastroenterol. 16:167–175. 2010.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Demers M, Dagnault A and Desjardins J: A
randomized double-blind controlled trial: Impact of probiotics on
diarrhea in patients treated with pelvic radiation. Clin Nutr.
33:761–767. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Mego M, Chovanec J, Vochyanova-Andrezalova
I, Konkolovsky P, Mikulova M, Reckova M, Miskovska V, Bystricky B,
Beniak J, Medvecova L, et al: Prevention of irinotecan induced
diarrhea by probiotics: A randomized double blind, placebo
controlled pilot study. Complement Ther Med. 23:356–362.
2015.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Theodoropoulos GE, Memos NA, Peitsidou K,
Karantanos T, Spyropoulos BG and Zografos G: Synbiotics and
gastrointestinal function-related quality of life after elective
colorectal cancer resection. Ann Gastroenterol. 29:56–62.
2016.PubMed/NCBI
|
|
92
|
Consoli ML, da Silva RS, Nicoli JR,
Bruña-Romero O, da Silva RG, de Vasconcelos Generoso S and Correia
MI: Randomized Clinical Trial: Impact of Oral Administration of
Saccharomyces boulardii on Gene Expression of Intestinal Cytokines
in Patients Undergoing Colon Resection. JPEN J Parenter Enteral
Nutr. 40:1114–1121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Flesch AT, Tonial ST, Contu PC and Damin
DC: Perioperative synbiotics administration decreases postoperative
infections in patients with colorectal cancer: A randomized,
double-blind clinical trial. Rev Col Bras Cir. 44:567–573.
2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Goldin BR, Gualtieri LJ and Moore RP: The
effect of Lactobacillus GG on the initiation and promotion
of DMH-induced intestinal tumors in the rat. Nutr Cancer.
25:197–204. 1996.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Banna GL, Torino F, Marletta F, Santagati
M, Salemi R, Cannarozzo E, Falzone L, Ferraù F and Libra M:
Lactobacillus rhamnosus GG: An Overview to Explore the
Rationale of Its Use in Cancer. Front Pharmacol.
8(603)2017.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Lee CS, Ryan EJ and Doherty GA:
Gastro-intestinal toxicity of chemotherapeutics in colorectal
cancer: The role of inflammation. World J Gastroenterol.
20:3751–3761. 2014.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Khailova L, Baird CH, Rush AA, Barnes C
and Wischmeyer PE: Lactobacillus rhamnosus GG treatment
improves intestinal permeability and modulates inflammatory
response and homeostasis of spleen and colon in experimental model
of Pseudomonas aeruginosa pneumonia. Clin Nutr. 36:1549–1557.
2017.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Wang Y, Liu L, Moore DJ, Shen X, Peek RM,
Acra SA, Li H, Ren X, Polk DB and Yan F: An LGG-derived protein
promotes IgA production through upregulation of APRIL expression in
intestinal epithelial cells. Mucosal Immunol. 10:373–384.
2017.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Fong FLY, Kirjavainen PV and El-Nezami H:
Immunomodulation of Lactobacillus rhamnosus GG (LGG)-derived
soluble factors on antigen-presenting cells of healthy blood
donors. Sci Rep. 6(22845)2016.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Vivarelli S, Salemi R, Candido S, Falzone
L, Santagati M, Stefani S, Torino F, Banna GL, Tonini G and Libra
M: Gut Microbiota and Cancer: From Pathogenesis to Therapy. Cancers
(Basel). 11pii. (E38)2019.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Riehl TE, Alvarado D, Ee X, Zuckerman A,
Foster L, Kapoor V, Thotala D, Ciorba MA and Stenson WF: GG
protects the intestinal epithelium from radiation injury through
release of lipoteichoic acid, macrophage activation and the
migration of mesenchymal stem cells. Gut. 68:1003–1013.
2018.PubMed/NCBI View Article : Google Scholar
|