Introduction
Mature B-cell neoplasms (MBNs) are the most common
lymphoproliferative disorders (LPDs) involving the peripheral blood
(PB) and bone marrow (BM). They represent >80% of all lymphoid
tumors. MBNs are a diverse group of diseases, including chronic
lymphocytic leukemia (CLL), mantle cell lymphoma (MCL), follicular
lymphoma (FL), splenic marginal zone lymphoma (SMZL),
lymphoplasmacytic lymphoma (LPL), B-cell prolymphocytic leukemia
(B-PLL) and hairy cell leukemia (HCL) (1). The accurate differential diagnosis
is critical for the initiation of an effective treatment
protocol.
The characterization of the cluster of
differentiation (CD) expression patterns in different MBNs has
permitted their utilization as molecular markers for the
development of a flow cytometry-based differential diagnostic
scheme for the different MBNs types. For example, the detection of
CD5 surface expression in clonal B-cells has served to narrow the
diagnostic possibilities among the several subtypes of B-cell
lymphomas. This is due to the fact that CD5+ B-cells are
mainly observed in CLL and MCL (2-4).
Assaying for CD23 expression in addition to CD5 greatly facilitates
the differential diagnosis of CLL from MCL (5), as the majority of cases of CLL are
CD23+, whereas the majority of cases of MCL are
CD23-.
The complete discrimination between the different
subtypes of MBNs is still far from being accomplished using the
currently identified CD markers. For example, the differential
diagnosis of CLL from MCL could not be achieved in all cases as
many CLL cases do not exhibit their known cell morphological
pattern together with a weak or even no expression of CD23.
Conversely, some MCL cases exhibit immunophenotypic characteristics
of CLL (6). In addition, other
subtypes of B-cell neoplasms may exhibit an atypical expression of
CD5, thus overlapping with MCL and CLL (7-10).
Further challenges arise when considering the differential
diagnosis of CD5- B-cell neoplasm subtypes due to the
observed immunophenotypic overlap, such as that observed between
the HCL and SMZL types (11,12). The imperfect discriminatory
performance of the currently standard CD markers in MBNs highlights
the need for use of multiple markers and/or additional markers in
order to improve the overall performance of multiparameter flow
cytometry-based differential diagnosis.
The CD200 marker, also known as OX-2, is a highly
conserved type IA transmembrane glycoprotein that is expressed in a
wide range of immunocytes, including myeloid cells, dendritic cells
and B and T lymphocytes. Several studies have demonstrated that
CD200 is upregulated in hematological malignancies, including
multiple myeloma and acute myeloid leukemia and that it is
associated with a poor prognosis (13-15).
Importantly, CD200 has been shown to exhibit a differential
expression pattern in different MBNs subtypes, being highly
expressed in CLL and HCL, but not in MCL (16,17). This expression pattern has made
CD200 a helpful marker in the differential diagnosis of the
different MBN subtypes. CD148 (DEP-1 or PTPRJ) is a receptor-type
protein tyrosine phosphatase that has been reported to be expressed
in the majority of mature T cells and subsets of B cells. CD148
processes a growth-stimulatory activity and has been found to be
differentially expressed in CD5+ MBN subtypes, being
overexpressed in MCL, but not in CLL (18). Thus, CD148 may be a useful marker
for the differential diagnosis of MCL and CLL. Another potentially
useful diagnostic marker is the CD160 marker that has been reported
to be highly expressed in the majority of CLL and HCL cases
(19). CD160 is an Ig-like
activating natural killer (NK) cell receptor that is expressed in
the majority of circulating NK cells and a subset of circulating
cytotoxic T cells, but is not normally expressed in B-cells
(19). However, the diagnostic
utility of CD160 among the different MBNs subtypes has not yet been
well established.
Receiver operating characteristic (ROC) analysis has
been the gold standard tool utilized to assess the discriminant
performance of a diagnostic test/classifier/biomarker between 2
classes. Its algorithm depends on assessing the true-positive rate
(sensitivity) and false-positive rate (1-specificity) at each
biomarker threshold (20). One
practical limitation of ROC analysis in hospital settings is the
requirement of a single biomarker as a classifier. In the case that
2 or more biomarkers are used to enhance the accuracy of
classification, methods are required to obtain a single composite
parameter before applying ROC analysis. This could be achieved, for
example, by using the ratio of 2 biomarkers or in the case of
multiple biomarkers, by using linear discriminant analysis or
logistic regression analysis (20). Support vector machine (SVM), on
the other hand, utilizes a completely different algorithm to
discriminate different classes by finding a decision hyperplane
with maximal distance to the nearest data points (support vectors)
(21). SVM can handle
classification by multiple biomarkers easily by transforming data
to a higher dimensional feature space using kernel function, where
a discriminatory hyperplane can be found (22). Discriminatory hyperplane in
p-dimensions is a p-1 dimensional subspace. In this regard, we
hypothesized that SVM may be more suitable for the analysis of the
complex data generated in the setting of multiparameter flow
cytometry. In addition, the SVM algorithm is amenable to
automation, rendering it a useful tool for the analysis of data
generated in settings of multiparameter flow cytometry. However,
SVM utilization in the analysis of flow cytometry data is still
uncommon and its discriminatory accuracy needs to be verified
against the well-established ROC analysis.
The first objective of the current study was to
capitalize on the differential expression patterns of CD200, CD148
and CD160 in the different MBN subtypes to characterize their
discriminatory performance, both as single markers or in
combination in the multiparameter flow cytometry-based diagnostic
setting. The second objective was to evaluate the performance of
the SVM classifier in the differential diagnosis of MBNs against
the well-established ROC analysis.
Subjects and methods
Study subjects
This study included 86 newly diagnosed patients with
B-cell non-Hodgkin lymphomas, 61 males and 25 females, and ranging
in age from 42 to 73 years. All patients presented to the Medical
Oncology Clinic, Zagazig University Hospital, during the period
from September, 2014 to June, 2017. Informed consents were obtained
from all patients. The study protocol conformed to the ethical
guidelines of the 1975 Declaration of Helsinki and was approved by
the Institutional Review Board, Zagazig University Hospital,
Faculty of Medicine. A medical history was collected from
participant patients who were then subjected to detailed clinical
examination. Fresh PB, and when needed BM specimens, were collected
and subjected to thorough morphological, cytogenetic and flow
cytometric immunophenotypic examination. Final diagnosis was
established according to the 2017 WHO guidelines (1).
Inclusion and exclusion criteria
Any patients newly diagnosed B-cell non-Hodgkin
lymphoma according to the WHO criteria and how had provided consent
to participate in the study were included. Cases with an uncertain
diagnosis, the existence of a definitive pathology, or previous
chemotherapy were excluded from the study.
Flow cytometry analysis
Multicolor flow cytometric analysis was performed on
fresh PB/BM specimens. All BM samples demonstrated a purity >80%
with no hemodilution problems. For combined antibody (AB) staining,
100 µl heparinized whole blood were incubated with 5 µl of
FITC-labeled mouse anti-human anti-CD19 AB (clone H1B19, cat. no.
555412, BD Biosciences) and 5 µl of either PE-labeled anti-CD200 AB
(clone MRC OX-104, cat. no. 552475, BD Biosciences), PE-labeled
anti-CD148 AB (clone A3, cat. no. 328708, BioLegend), or PE-labeled
anti-CD160 AB (clone BY55, cat. no. 562118, BD Biosciences) for 15
min in the dark at room temperature. Non-specific binding was
determined using PE-labeled mouse anti-human IgG isotypic AB (clone
G18-145, cat. no. 555787, BD Biosciences) and FITC-labeled mouse
antihuman IgG1 κ isotopic AB (Clone MOPC-21, cat. no.
555748, BD Biosciences). Following incubation, red blood cells
(RBCs) were lysed with BD PharmLyse (cat. no. 555899, BD
Biosciences), and lymphocytes were collected by centrifugation (250
x g for 5 min at room temperature), washed once with FACS buffer
[1X phosphate buffered saline (PBS, pH 7.4), 1% bovine serum
albumin (BSA), 1 mM EDTA, 0.1% sodium azide], and then re-suspended
in FACS buffer.
Lymphocytes were firstly identified using forward
scatter/side scatter (FSC/SSC) then gating was conducted to
identify CD19+/CD200+,
CD19+/CD160+, and
CD19+/CD148+ cells according to the threshold
established using the negative isotypic control antibodies. At
least 10,000 events from each sample were analyzed and the
intensity of positive expression was evaluated using the mean
fluorescence intensity (MFI). The MFI was defined as follows: A
weak expression when the MFI was <101, a moderate
expression when the MFI was >101 and
<102, and a strong expression when the MFI was
>102. Routine machine calibration and fluorescence
compensation was performed on a daily basis according to the
standard operating procedure of our clinical laboratory using
standard fluorescence beads (CaliBRITE beads; BD Biosciences).
Analysis was carried out using a BD FACSCalibur with CellQuest
software (BD Biosciences) by an investigator who was blinded to the
patients' clinical and laboratory data.
Statistical analysis
The distribution of MFI was examined using the
Kolmogorov-Smirnov and Shapiro-Wilk tests. The results are
expressed as medians ±95% confidence intervals. Comparisons between
groups were performed using Kruskal-Wallis one-way analysis
followed by the Dunn-Bonferroni post hoc test. To assess the
diagnostic cut-off value for the examined markers in discriminating
among the different types of MBNs, sensitivity and specificity
analysis was performed using the ROC curves. To assess the
diagnostic value of combining 2 markers, the MFI ratio of the 2
markers was used in ROC analysis. The area under ROC curve (AUC)
was used to compare the overall discriminatory performance of
different markers to assess their utility as diagnostic test. In
general, an AUC >0.97 is considered excellent, between 0.93 to
0.96 is very good, between 0.75 to 0.92 is good or moderate, and
<0.75 is poor and not clinically useful (23,24). Statistical comparisons of the ROC
curves were made and evaluated using the method described in the
study by DeLong et al (25).
To examine the discriminatory performance of SVM in
the setting of flow cytometry-based differential diagnosis of MBN
subtypes, the data were analyzed using SVM-optimized classification
models and compared to ROC results. The SVM best classifier models
were constructed by optimizing the C-parameter. The produced
classifier model could be used for class prediction in future
data.
A P-value <0.05 was considered to indicate a
statistically significant difference. GraphPad Prism 6 (GraphPad
Software Inc.), SPSS (Statistical package for social Scientists)
version 20 (IBM Inc.) and MedCalc version 14.8.1 (MedCalc Software
bvba), were used in the analysis. The freely available LIBSVM
(26) functions in the e1071
package version 1.6-8
(https://cran.r-project.org/web/packages/e1071/index.html) were
used in the open-source R statistical environment (http://www.r-project.org).
Results
Diagnosed MBN types
We identified 47 cases of CLL, 12 cases of MCL, 10
cases of LPL, 7 cases of SMZL, 4 cases of HCL, 3 cases of B-PLL and
3 cases of FL cases. Due to the few identified numbers, we did not
include B-PLL and FL in the statistical analysis. The
clinicopathological characteristics of the studied patients are
presented in Table SI.
Fluorescence characteristics of the
examined markers in the studied MBN cases
The distributions of the cases according to the
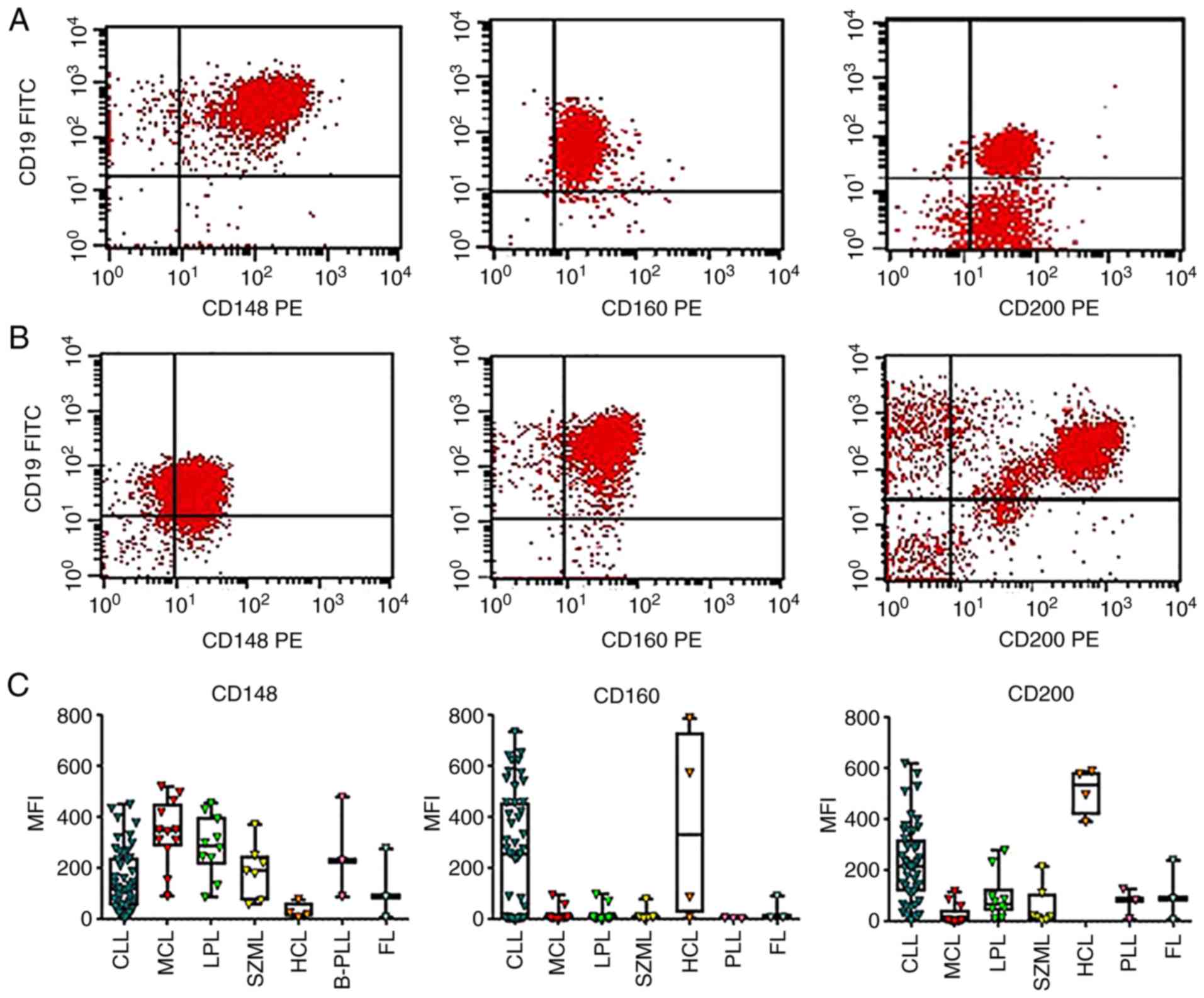
examined markers and MFI are presented in Table I and Fig. 1. Consistent with the findings of
previous research (18), the
expression of CD148 was higher in MCL vs. CLL (Fig. 1A and C). On the other hand, the expression
levels of CD200 and CD160 were higher in CLL compared to MCL
(Fig. 1B and C). A significantly greater percentage of
CLL cases exhibited a strong CD200 and CD160 expression compared to
the MCL cases (Table I). By
contrast, a significantly greater percentage of MCL cases exhibited
a higher CD148 expression compared to the CLL cases (Table I). As shown in Fig. 1C, the MFI was not normally
distributed in all MBN cases.
 | Table IDistribution of diagnosed mature
B-cell neoplasm cases according to the level of expression of the
three markers. |
Table I
Distribution of diagnosed mature
B-cell neoplasm cases according to the level of expression of the
three markers.
| Marker | Expression | CLL n (%) | MCL n (%) | LPL n (%) | SMZL n (%) | HCL n (%) | FLa n (%) | B-PLLa n (%) |
|---|
| CD200 | | | | | | | | |
| | Strong | 37 (78.7)b | 1 (8.3) | 3(30) | 2 (28.6) | 4(100) | 1 (33.3) | 1 (33.3) |
| | Weak | 9 (19.9) | 2 (16.7) | 6(60) | 3 (42.9) | 0 (0) | 1 (33.3) | 1 (33.3) |
| | Negative | 1 (2.1) | 9 (75.0) | 1(10) | 2 (28.6) | 0 (0) | 1 (33.3) | 1 (33.3) |
| CD160 | | | | | | | | |
| | Strong | 27
(57.4)b | 0 (0) | 0 (0) | 0 (0) | 2 (50.0) | 0 (0) | 0 (0) |
| | Weak | 3 (6.4) | 2 (16.7) | 2(20) | 1 (14.3) | 1 (25.0) | 1 (33.3) | 0 (0) |
| | Negative | 17 (36.2) | 10 (83.3) | 8(80) | 6 (85.7) | 1 (25.0) | 2(50) | 3(100) |
| CD148 | | | | | | | | |
| | Strong | 20 (42.6) | 11
(91.7)c | 9(90) | 5 (71.4) | 0 (0) | 1 (33.3) | 2 (66.6) |
| | Weak | 25 (53.2) | 1 (8.3) | 1(10) | 2 (28.6) | 3 (75.0) | 1 (33.3) | 1 (33.3) |
| | Negative | 2 (4.3) | 0 (0) | 0 (0) | 0 (0) | 1 (25.0) | 1 (33.3) | 0 (0) |
| Total | | 47 | 12 | 10 | 7 | 4 | 3 | 3 |
SMZL exhibited a similar expression pattern to that
of MCL, with a low expression of CD200 and CD160 compared to CD148
(Fig. 1C). The LPL cases
exhibited a higher expression of CD148 than CD200 and CD160
(Fig. 1C). Four cases with HCL
were identified in this study.
ROC analysis of the discriminatory
performance of CD200, CD148 and CD160 markers in the differential
diagnosis of different MBNs
ROC analysis was used to identify cut-off value,
sensitivity and specificity, and the AUC of each marker diagnostic
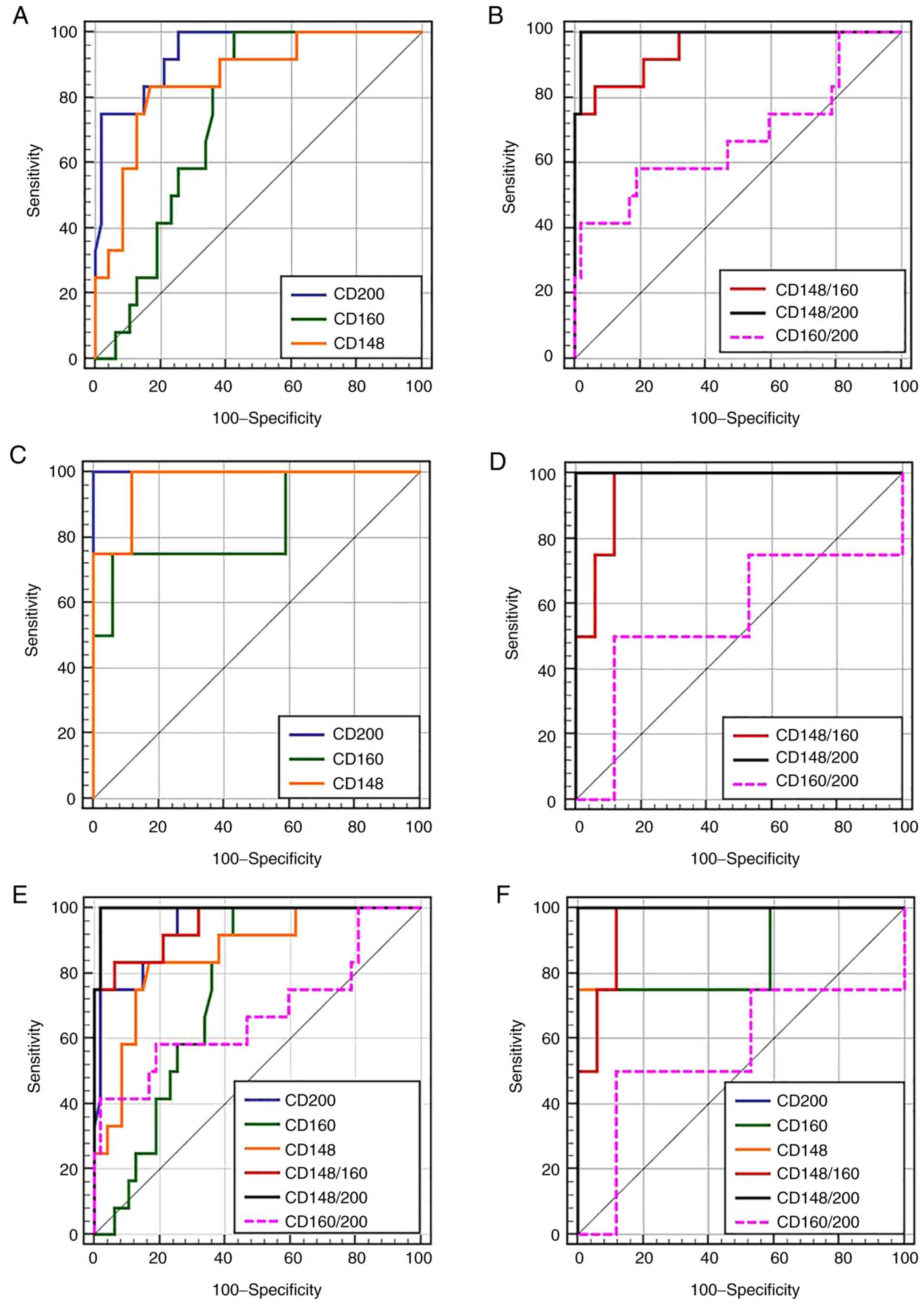
test in the differential diagnosis of MBNs (Table II and Fig. 2). CD200 yielded statistically
significant fluorescent signals that could potentially aid in
discriminating MCL from CLL (AUC=94%), HCL from LPL and SZML
(AUC=100%), and SZML from HCL and LPL (AUC=77.6%). However, it
failed to yield a statistically significant signal to discriminate
LPL from HCL and SZML (AUC=56%). CD148 yielded statistically
significant fluorescent signals in differentiating MCL from CLL
(AUC=85.7%), HCL from LPL and SZML (AUC=97.1%), and LPL from HCL
and SZML (AUC=84.5%), but failed to yield a significant signal to
differential SZML from HCL and LPL (AUC=56%). CD160 was successful
in differentiating MCL from CLL (AUC=74.7%) and HCL from LPL and
SZML (AUC=83.8%).
 | Table IIDiscriminative performance of single
marker flow cytometry in differential diagnosis of different
MBNs. |
Table II
Discriminative performance of single
marker flow cytometry in differential diagnosis of different
MBNs.
| Marker | MCL from CLL | HCL from LPL and
SMZL | LPL from HCL and
SMZL | SMZL from HCL and
LPL |
|---|
| CD200 | | | | |
|
Cut-off | <117.5 | >279 | ≤279 | <22.8 |
|
Sensitivity | 100 % (CI:
73.5-100.0) | 100% (CI:
39.8-100) | 100% (CI:
69.2-100) | 71.43 % (CI:
29-96.3) |
|
Specificity | 74.47% (CI:
59.7-86.1) | 100% (CI:
80.5-100) | 36.36% (CI:
10.9-69.2) | 85.71% (CI:
57.2-98.2) |
|
AUC | 94.1%a,b, P<0.001d (CI: 0.847-0.985) | 100%b, P<0.001d (CI: 0.839-1.000) | 56.4%, P=0.65 (CI:
0.333 to 0.776) | 77.6%,
P<0.05d (CI:
0.543-0.926) |
| CD160 | | | | |
|
Cut-off | <95.5 | >78.8 | ≤6.2 | >6.2 |
|
Sensitivity | 100% (CI:
73.5-100.0) | 5% (CI:
6.8-93.2) | 70% (CI:
34.8-93.3) | 85.7% (CI:
42.1-99.6) |
|
Specificity | 57.45 % (CI:
42.2-71.7) | 94.1% (CI:
71.3-99.9) | 81.8% (CI:
48.2-97.7) | 57.1% (CI:
28.9-82.3) |
|
AUC | 74.7%c, P<0.001d (CI: 0.617-0.851) | 83.8%b, P=0.02d (CI: 0.614-0.961) | 73.6%b, P<0.05 (CI:
0.501-0.902) | 53.1%, P 0.82 (CI:
0.304-0.749) |
| CD148 | | | | |
|
Cut-off | >270 | <77.6 | > 220 | ≤220 |
|
Sensitivity | 83.3% (CI:
51.6-97.9) | 100% (CI:
39.8-100) | 80% (CI:
44.4-97.5) | 71.4 (CI:
29-96.3) |
|
Specificity | 83.0% (CI:
69.2-92.4) | 88.24% (CI:
63.6-98.5) | 81.8% (CI:
48.2-97.7) | 57.1% (CI:
28.9-82.3) |
|
AUC | 85.7%b,c, P<0.001d (CI: 0.742-0.935) | 97.1%b, P<0.001d (CI: 0.789-1.000) | 84.5%b, P<0.001d (CI: 0.623-0.964) | 56.1%, P=0.639 (CI:
0.331-0.774) |
For the comparison of the discriminatory performance
of different markers, we compared the AUC of different markers
(Table II). CD200 was
significantly more superior than CD160 for discriminating MCL from
CLL. Although CD148 demonstrated a lower performance than CD200 in
discriminating MCL from CLL, it was not significantly different
from CD160. For the discrimination of HCL from LPL and SZML, CD200
demonstrated the highest performance (AUC=100%) followed by CD148
(AUC=97%) and lastly by CD160 (AUC=83.8%), albeit with no
significant difference between all of them (Table II). For the discrimination of LPL
from HCL and SZML, CD148 and CD160 yielded moderate discriminatory
performance that could negate their clinical utility (Table II). For the discrimination of
SZML from HCL and SZML, CD200 was the only marker that yielded a
significant signal, but with moderate discriminatory performance as
judged by an AUC of 77.6%, which questions its clinical utility for
this purpose.
ROC analysis of the discriminatory
performance of combined markers in the differential diagnosis of
different MBNs
We used the MFI ratio of any 2 markers to assess
their discriminatory performance (Table III). The comparison of
sensitivity, specificity and the AUC (Table III and Fig. 2) revealed that the CD148/CD200
ratio outperformed single CD200, CD148 and CD160 markers, and
CD160/CD200 and CD148/CD160 ratios in differentiating MCL from CLL
and HCL from LPL plus SMZL. Although the CD148/CD160 ratio
exhibited a lower performance than the CD148/CD200 ratio, the
differences were not statistically significant. In addition,
CD148/CD160 was the only combination that yielded a significant AUC
(82.7%) when discriminating LPL from HCL and SZML, but with
moderate performance that negated its clinical utility.
 | Table IIIDiscriminative performance of
combined markers in the differential diagnosis of different
MBNs. |
Table III
Discriminative performance of
combined markers in the differential diagnosis of different
MBNs.
| Combined
marker | MCL from CLL | HCL from LPL and
SMZL | LPL from HCL and
SMZL | SMZL from HCL and
LPL |
|---|
| CD148/CD200 | | | | |
|
Cut-off | >2.63 | <0.157 | >0.1565 | >6.6107 |
|
Sensitivity | 100% (CI:
73.5-100.0) | 100% (CI:
39.8-100) | 100% (CI:
69.2-100) | 71.43% (CI:
29-96.3) |
|
Specificity | 100% (CI:
88.7-99.9) | 100% (CI:
80.5-100) | 36.36% (CI:
10.9-69.2) | 85.71% (CI:
57.2-98.2) |
|
AUC | 99.5%a,b, P<0.001c (CI: 0.929-1.00) | 100%a,b, P<0.001c (CI: 0.839-1.00) | 60%, P<0.466
(CI: 0.37-0.804) | 73.5%, P=0.514 (CI:
0.499-0.90) |
| CD160/CD200 | | | | |
|
Cut-off | >2.798 | >1.1779 | <0.495 | >0.4206 |
|
Sensitivity | 97.87% (CI:
15.2-72.3) | 50% (CI:
6.8-93.2) | 90% (CI:
55.5-99.7) | 71.43% (CI:
29-96.3) |
|
Specificity | 41.67% (CI:
88.7-99.9) | 88.24% (CI:
63.6-98.5) | 54.55% (CI:
23.4-83.3) | 71.43% (CI:
41.9-91.6) |
|
AUC | 67.7%, P=0.088 (CI:
0.543-0.793) | 55.9%, P=0.787 (CI:
0.329-0.772) | 70.9%, P=0.838 (CI:
0.473-0.88) | 69.4%, P=0.1454
(CI: 0.457-0.87) |
| CD148/CD160 | | | | |
|
Cut-off | >14.0 | <4.777 | >39.05 | <39.05 |
|
Sensitivity | 93.62 (CI:
82.5-98.7) | 100% (CI:
39.8-100) | 70% (CI:
34.8-93.3) | 100 (CI:
59-100) |
|
Specificity | 83.33 (CI:
51.6-97.9) | 88.24% (CI:
63.6-98.5) | 100% (CI:
71.5-100) | 50% (CI:
23-77) |
|
AUC | 95%a,b, P<0.001c (CI: 0.860-0.990) | 95.6%a,b, P<0.001c (CI: 0.767-0.999) | 82.7%,
P<0.05c (CI:
0.60-0.955) | 55.1%, P=0.697 (CI:
0.32-0.765) |
Support vector machine analysis of the
discriminatory performance of combined CD148 and CD200 in MBN
cases
As CD148/CD200 demonstrated the best performance, we
analyzed the expression pattern of the 2 markers, rather than their
MFI ratio, using SVM. We aimed to find an optimal linear hyperplane
(decision boundary) that could be compared with the finding of ROC
analysis of the MFI ratio of the two markers. As shown in Fig. 3A, the decision boundary that best
discriminated between MCL from CLL had a slope of 2.2, which agrees
well with the cut-off ratio of 2.6 identified in ROC analysis. SVM
discriminated MCL from CLL cases with 100% sensitivity and 97.9%
specificity, which was very close to ROC analysis. As shown in
Fig. 3B, SVM discriminated HCL
cases from LPL and SZML with 100% sensitivity and a specificity
similar to that of ROC analysis. As shown in Fig. 3B, there was difficulty in finding
a decision boundary between LPL and SZML due to their overlapping
expression pattern. SVM discriminated LPL from SZML and HCL with a
sensitivity of 90%, but with a specificity of 57%. These results
closely matched those of ROC analysis.
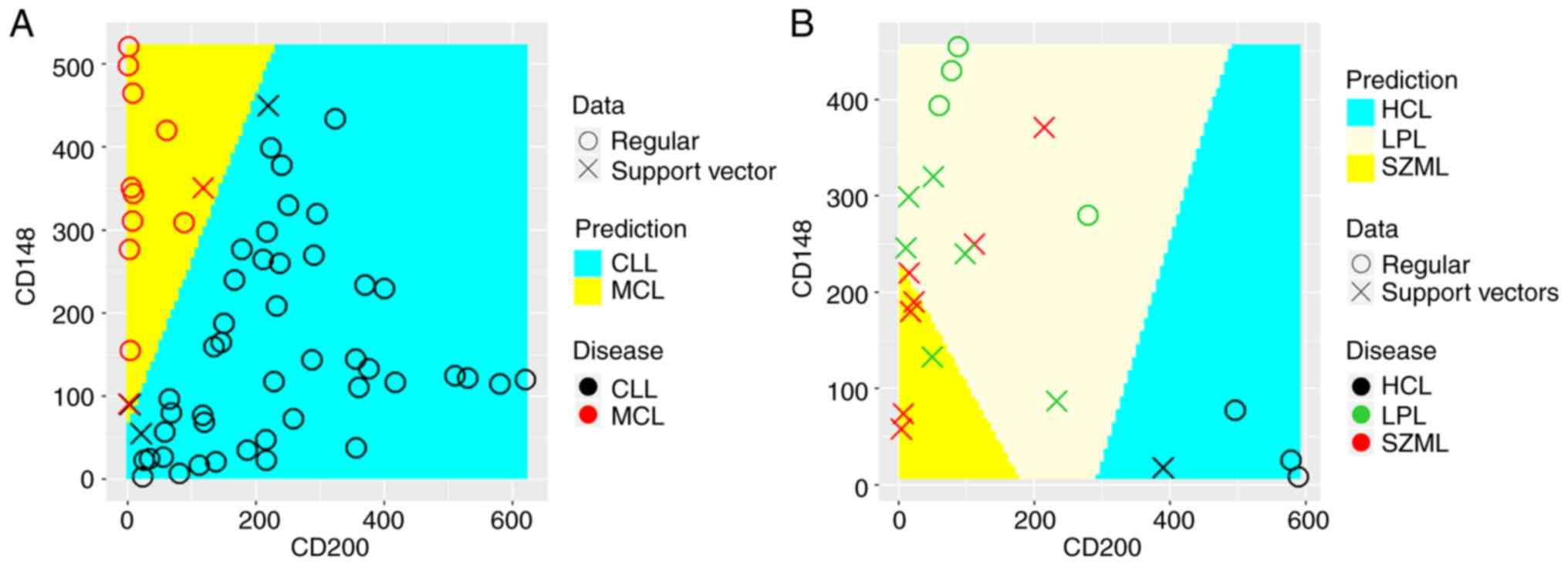 | Figure 3.Support vector machine analysis of the
discriminatory performance of CD148 and CD200 in MBN cases. The ‘x’
symbol represents data points used as support vectors to construct
the discriminatory boundary between diagnosed classes, whereas the
circular symbol ‘o’ represents the remaining data points. True
classes are highlighted using symbol color (red for MCL and black
for CLL or red for LPL, green for SZML and black for HCL), whereas
the predicted class was highlighted using the colored background
identified through the optimization process. (A) Linear SVM could
accurately discriminate MCL from CLL cases with an accuracy of
98.3%. The discriminatory boundary has a slope of 2.2, which
reflects the ratio of CD148/CD200. This results agrees well with
that obtained by ROC analysis. (B) Multiclass classification with
linear SVM using one-against-one classification strategy could
accurately distinguish HCL from LPL and SZML but could not
accurately distinguish LPL from SZML. This result recapitulates the
results of ROC analysis. In all analyses, the tune function in
package e1071 was used to optimize the cost function to provide the
highest classification with least error. MBN, mature B-cell
neoplasm; MCL, mantle cell lymphoma; CLL, chronic lymphocytic
leukemia; HCL, hairy cell leukemia; LPL, lymphoplasmacytic
lymphoma; SMZL, splenic marginal zone lymphoma. |
Discussion
Immunophenotyping by flow cytometry constitutes an
important pillar of the diagnostic makeup of leukemia and lymphomas
because of its simplicity, cost-effectiveness and capability of
characterizing multiple cellular characteristics simultaneously.
The characterization of the differential cellular expression
pattern of different antigenic markers in lymphoid disorders could
enhance the accuracy of flow cytometry-based diagnosis. In the
current study, we evaluated the utility of using single and dual
combination of CD148, CD160 and CD200 in the differential diagnosis
of different types of mature B-cell neoplasms.
CD200 and CD148 exhibited reciprocal expression
patterns in CLL, whereas CD160 exhibited a high expression pattern
in CLL. The differential expression patterns of the 3 markers
suggested that combination of 2 markers could enhance the flow
cytometric-based differential diagnosis of MBNs. In this study, we
first evaluated the performance of single markers in
differentiating CLL from MCL. Both neoplasms are morphologically
indistinguishable in many cases which complicates clinical
management as these two diseases require drastically different
lines of treatments. Single marker analysis confirmed the
previously reported a high expression of CD200 in CLL (16,27,28). Fluorescence intensity less than
the cut-off value (117.5) determined by ROC curve analysis
differentiated MCL from CLL with a sensitivity of 100% and a
specificity of 74.5%. CD148, in contrast to CD200, exhibited a low
expression pattern in MCL compared to CLL. A fluorescence intensity
higher than the cut-off value (270) determined by ROC curve
analysis differentiated MCL from CLL with a sensitivity and
specificity of 83.3 and 83.0%, respectively. This pattern of CD148
expression was previously reported (18,29).
Using the combined markers CD148/CD200 improved the
distinction between MCL from CLL. A cut-off value of a ratio
>2.6 was successful in discriminating MCL from CLL with 100%
sensitivity and specificity. Similar results were obtained with
SVM, which utilizes a different statistical algorithm than ROC
analysis. A decision boundary slope of 2.3 was found to
discriminate MCL from CLL with an accuracy of 98.3% (Fig. 3A). The diagnostic utility of
CD148/CD200 could be of value to the differential diagnosis of
cases of CLL with trisomy 12, in which many cases were reported to
exhibit a dim CD200 expression. In such cases, the high expression
of CD148, as demonstrated by the high ratio of CD148/CD200, could
enhance their diagnosis. The expression pattern of CD160 was
parallel to that of CD200 with a high expression in CLL and a low
expression in MCL. A cut-off value of <95.5 demonstrated a
perfect sensitivity of 100% in differentiating MCL from CLL, but
with a very low specificity of approximately (57.5%). This result
supports the finding of Lesesve et al (30) who recommended that CDs160/200
expression levels should be included in a multiparameter approach
as a second step in ambiguous cases. The combined use of
CD148/CD160 significantly improved the diagnostic performance
(AUC=95% vs. 74.7% in CD160 alone), but it was still lower than
that of CD148/CD200 (AUC=99.5%). The combined use of CD160/CD200
demonstrated the lowest discriminative performance of MCL from CLL
(AUC=67.7%; Fig. 2).
Subsequently, we evaluated the performance of each
marker in the differential diagnosis of HCL from SZML and LPL.
These neoplasms are difficult to be distinguished in many cases
either morphologically or immunophenotypically with the standard
marker panel. We observed a high CD200 MFI in HCL compared to LPL
and SMZL, which was consistent with previous reports (16,17). Using ROC curve analysis of CD200
MFI, we identified a threshold value of 279 to maximally
differentiate HCL from SZML and LPL. CD200 MFI >279 in our
sample could perfectly discriminate HCL from SZML and LPL with 100%
sensitivity and specificity. We could not establish a CD200 cut-off
value to discriminate SZL and LPL. This could be explained by a
similarly low CD200 expression in both SZML and LPL. Similar
results were observed for CD148, in which we established a cut-off
value of 77.6 to maximally differentiate HCL from LPL and SZML.
CD148 MFI levels <77.6 were successful in discriminating HCL
from LPL plus SZML (sensitivity 100% and specificity of 88.2%).
This could be explained by the low CD148 expression in HCL, but a
high expression in SZML and LPL. We could not establish a cut-off
value to discriminate between LPL and SZML. The CD160 demonstrated
a pattern of expression similar to CD200 with high MFI in HCL but
low MFI in SZML and LPL. This pattern was previously reported by
others (30,31). We established a cut-off value of
78.8 for CD160 to maximally discriminate between HCL from SZML and
LPL. This resulted in moderate discriminatory performance with an
AUC of 83.8%. Thus, although the CD160 expression pattern was
similar to that of CD200, it demonstrated a lower performance in
discriminating HCL from SZML plus LPL. The similar expression
pattern in SZML and LPL did not allow establishing any cut-off
value to differentiate between them.
We also assessed whether the utilization of two
combined markers would improve the discrimination between HCL, LPL
and SZML. ROC analysis identified a CD148/CD200 cut-off ratio of
<0.15 that achieved 100% sensitivity and specificity in
discriminating HCL from both LPL and SMZL. However, discrimination
between LPL from SMZL was unfeasible. CD148/CD160 demonstrated a
lower performance, but not with a significant difference, from
CD148/CD200 in discriminating HCL from LPL and SMZL (Table III). Ratio values below the
established cut-off value of approximately 4.8 demonstrated a good
discriminatory performance of HCL from LPL plus SMZL (AUC=95.6%)
with a sensitivity and specificity of 100 and 88%, respectively. In
contrast CD160/CD200 demonstrated poor performance in
differentiating any of HCL from LPL plus SMZL or LPL from SMZL.
We evaluated the discriminatory performance of a
linear SVM classifier using the CD148 and CD200 data. Given the
2-dimensional nature of our data, the discriminatory hyperplane was
just a line. The SVM discriminatory accuracy of MCL from CLL cases
achieved 100% sensitivity and 97.9% specificity (Fig. 3A), whereas it achieved 100%
sensitivity and specificity in discriminating HCL from LPL and SZML
cases (Fig. 3B). It was difficult
to find a decision boundary between LPL and SZML, where the SVM
discriminatory accuracy of LPL from SZML and HCL achieved a
sensitivity of 90% and a low specificity of 57%. These results
demonstrate that SVM classification accuracy matched that of ROC
analysis.
One limitation of our study was the limited sample
size. The results need to be validated in independent and larger
samples. Obviously the cut-off ratios of combined markers that
achieve best discriminatory performance need to be established
operationally in each laboratory. In conclusion, we provided
operational diagnostic criteria for the use of single or combined
CD200, CD148, and CD160 markers in the differential diagnosis of
different MBNs subtypes in flow cytometry setting. The combined use
of CD148/CD200 has outperformed CD160/CD200, CD148/CD160, and all
single markers in discriminating MCL from CLL. CD200 was as
efficient as CD148/CD200 in discriminating HCL from SMZL plus LPL.
Distinction between SMZL and LPL was unfeasible with the used
markers and still need further research to identify suitable
markers. Both SVM and ROC classifiers yielded similar
discrimination accuracy in the differential diagnosis of different
MBN subtypes. This result suggests that SVM may be a suitable
alternative for ROC analysis given its capability of classifying
multidimensional data without intermediary calculations and its
amenability for automation.
Supplementary Material
Clinicopathological characteristics of
the studied MBN cases.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
AFE contributed to the study design, flow cytometry,
data analysis, table and graph presentation, the writing of the
manuscript and preparing the final revision. AAO contributed to
patient recruitment, sample collection, flow cytometry, and to the
writing of the manuscript. HEA contributed to patient recruitment,
sample collection and flow cytometry. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
Informed consents were obtained from willing
participants after explaining the study purpose and design. The
study protocol conformed to the ethical guidelines of the 1975
Declaration of Helsinki and was approved by Institutional Review
Board, Zagazig University Hospital, Faculty of Medicine.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Swerdlow SH, Campo E, Harris NL, Jaffe ES,
Pileri SA, Stein H, Thiele J and Vardiman JW (eds): Introduction
and overview of the classification of the lymphoid neoplasms. In:
WHO Classification of Tumours of Haematopoietic and Lymphoid
Tissues. 4th edition. IARC, Lyon. pp190–198. 2017.
|
|
2
|
Craig FE: Flow cytometric evaluation of
B-cell lymphoid neoplasms. Clin Lab Med. 27:487–512, vi.
2007.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Craig FE and Foon KA: Flow cytometric
immunophenotyping for hematologic neoplasms. Blood. 111:3941–3967.
2008.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Stetler-Stevenson M: Flow cytometry in
lymphoma diagnosis and prognosis: Useful? Best Pract Res Clin
Haematol. 16:583–597. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Matutes E, Owusu-Ankomah K, Morilla R,
Garcia Marco J, Houlihan A, Que TH and Catovsky D: The
immunological profile of B-cell disorders and proposal of a scoring
system for the diagnosis of CLL. Leukemia. 8:1640–1645.
1994.PubMed/NCBI
|
|
6
|
Kroft SH: Uncovering clinically relevant
phenotypic variations in malignancies: CD23 in mantle cell
lymphoma. Am J Clin Pathol. 130:159–161. 2008.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sánchez ML, Almeida J, Vidriales B,
López-Berges MC, García-Marcos MA, Moro MJ, Corrales A, Calmuntia
MJ, San Miguel JF and Orfao A: Incidence of phenotypic aberrations
in a series of 467 patients with B chronic lymphoproliferative
disorders: Basis for the design of specific four-color staining to
be used for minimal residual disease investigation. Leukemia.
16:1460–1469. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Baseggio L, Traverse-Glehen A, Petinataud
F, Callet-Bauchu E, Berger F, Ffrench M, Couris CM, Thieblemont C,
Morel D, Coiffier B, et al: CD5 expression identifies a subset of
splenic marginal zone lymphomas with higher lymphocytosis: A
clinico-pathological, cytogenetic and molecular study of 24 cases.
Haematologica. 95:604–612. 2010.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Asplund SL, McKenna RW, Doolittle JE and
Kroft SH: CD5-positive B-cell neoplasms of indeterminate
immunophenotype: A clinicopathologic analysis of 26 cases. Appl
Immunohistochem Mol Morphol. 13:311–317. 2005.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Dronca RS, Jevremovic D, Hanson CA, Rabe
KG, Shanafelt TD, Morice WG, Call TG, Kay NE, Collins CS, Schwager
SM, et al: CD5- positive chronic B-cell lymphoproliferative
disorders: Diagnosis and prognosis of a heterogeneous disease
entity. Cytometry B Clin Cytom. 78 (Suppl 1):S35–S41.
2010.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Del Giudice I, Matutes E, Morilla R,
Morilla A, Owusu-Ankomah K, Rafiq F, A'Hern R, Delgado J,
Bazerbashi MB and Catovsky D: The diagnostic value of CD123 in
B-cell disorders with hairy or villous lymphocytes. Haematologica.
89:303–308. 2004.PubMed/NCBI
|
|
12
|
Stetler-Stevenson M and Tembhare PR:
Diagnosis of hairy cell leukemia by flow cytometry. Leuk Lymphoma.
52 (Suppl 2):S11–S13. 2011.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Brunetti L, Di Noto R, Abate G, Gorrese M,
Gravetti A, Raia M, Scalia G, Pascariello C, Camera A and Del
Vecchio L: CD 200/OX2, a cell surface molecule with
immune-regulatory function, is consistently expressed on hairy cell
leukemia neoplastic cells. Br J Haematol. 145:665–678.
2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tonks A, Hills R, Whitw P, Roise B, Mils
KI, Birnett AK and Darley RL: CD200 as a prognostic factor in acute
myeloid leukemia. Leukemia. 21:566–568. 2007.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Moreaux J, Hose D, Reme T, Jourdan E,
Hyndemer M, Legouffe E, Moine P, Bourin P, Moos M, Corre J, et al:
CD 200 is a new prognostic factor in multiple myeloma. Blood.
108:4194–4197. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sandes AF, de Lourdes Chauffaille M,
Oliveria CR, Maekawa Y, Tamashro N, Takao TT, Ritter EC and
Rizzatti EG: CD 200 has an important role in the differential
diagnosis of mature B-cell neoplasms by multiparameter flow
cytometry. Cytometry B Clin Cytom. 86:98–105. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Pillai V, Pozdnyakova O, Charest K, Li B,
Shahsafaei A and Dorfman DM: CD200 flow cytometric assessment and
semiquantitative immunohistochemical staining distinguishes hairy
cell leukemia from hairy cell leukemia-variant and other B-cell
lymphoproliferative disorders. Am J Clin Pathol. 140:536–543.
2013.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Miguet L, Béchade G, Fornecker L, Zink E,
Felden C, Gervais C, Herbrecht R, Van Dorsselaer A, Mauvieux L and
Sanglier-Cianferani S: Proteomic analysis of malignant B-cell
derived microparticles reveals CD148 as a potentially useful
antigenic biomarker for mantle cell lymphoma diagnosis. J Proteome
Res. 8:3346–3354. 2009.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Liu FT, Giustiniani J, Farren T, Jia L,
Bensussan A, Gribben JG and Agrawal SG: CD160 signaling mediates
PI3K-dependent survival and growth signals in chronic lymphocytic
leukemia. Blood. 115:3079–3088. 2010.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Shultz EK: Multivariate receiver-operating
characteristic curve analysis: Prostate cancer screening as an
example. Clin Chem. 41:1248–1255. 1995.PubMed/NCBI
|
|
21
|
Sommer C and Girlish DW: Machine learning
in cell biology-teaching computers to recognize phenotypes. J Cell
Sci. 126:5529–5539. 2013.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nayak J, Naik B and Behera HS: A
comprehensive survey on support vector machine in data mining
tasks: Applications & challenges. Int J Database Theory Appl.
8:169–186. 2015. View Article : Google Scholar
|
|
23
|
Jones CM and Athanasiou T: Summary
receiver operating characteristic curve analysis techniques in the
evaluation of diagnostic tests. Ann Thorac Surg. 79:16–20.
2005.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fan J, Upadhye S and Worster A:
Understanding receiver operating characteristic (ROC) curves. CJEM.
8:19–20. 2006.PubMed/NCBI
|
|
25
|
DeLong ER, DeLong DM and Clarke-Pearson
DL: Comparing the areas under two or more correlated receiver
operating characteristic curves: A nonparametric approach.
Biometrics. 44:837–845. 1988.PubMed/NCBI
|
|
26
|
Chang CC and Lin CJ: LIBSVM: A Library for
Support Vector Machines. ACM Transactions on Intelligent Systems
and Technology. 2(27): 1–27, 27. 2011. View Article : Google Scholar : http://www.csie.ntu.edu.tw/~cjlin/libsvm.
|
|
27
|
Palumbo GA, Parrinello N, Fargione G,
Csrdillo K, Chiarenza A, Berretta S, Conticello C, Villari L and Di
Raimondo F: CD200 expression may help in differential diagnosis
between mantle cell lymphoma and B-cell chronic lymphocytic
leukemia. Leuk Res. 33:1212–1216. 2009.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Challagundla P, Medeiros LJ,
Kanagal-Shamanna R, Miranda RN and Jorgensen JL: Differential
expression of CD 200 in B-cell neoplasms by flow cytometry can
assist in diagnosis, sub classification and bone marrow staging. Am
J Clin Pathol. 142:837–844. 2014.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Fan L, Miao Y, Wu YJ, Wang Y, Guo R, Wang
L, Shen AL, Chen YY, Xu W and Li JY: Expression patterns of CD200
and CD148 in leukemic B-cell chronic lymphoproliferative disorders
and their potential value in differential diagnosis. Leuk Lymphoma.
56:3329–3335. 2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Lesesve JF, Tardy S, Frotscher B,
Latger-Cannard V, Feugier P and De Carvalho Bittencourt M:
Combination of CD160 and CD200 as a useful tool for differential
diagnosis between chronic lymphocytic leukemia and other mature
B-cell neoplasms. Int J Lab Hematol. 37:486–494. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Farren TW, Giustiniani J, Liu FT,
Tsitsikas DA, Macey MG, Cavenagh JD, Oakervee HE, Taussig D,
Newland AC, Calaminici M, et al: Differential and tumor-specific
expression of CD160 in B-cell malignancies. Blood. 118:2174–2183.
2011.PubMed/NCBI View Article : Google Scholar
|