1. Introduction
Cholangiocarcinoma (CC), arising from the biliary
tract, is a rare malignant tumor accounting for almost 3% of all
gastrointestinal cancers (1). On
the basis of their location, two types of CCs have been
characterized, namely intrahepatic CC (ICC) or extrahepatic CC
(ECC) with clinical, pathological, epidemiological and molecular
differences between them (2). The
third one, which is considered as a sub-type of intrahepatic CC has
also been described. These hilar cancers (Klatskin tumors)
represents the most frequent category comprising 55-60% of CCs.
Non-hilar ECC comprises 20-30% and ICC 10% of the total CC cases.
More than 90% of CCs are adenocarcinomas (3). The common risk factors for CCs are
primary sclerosing cholangitis (PSC), liver fluke infestation,
congenital abnormalities, chronic hepatitis B virus infection,
chronic hepatitis C virus infection, and hepatolithiasis (4). Due to the typically clinically late
diagnosis and unresectable disease at presentation and the lack of
effective non-surgical therapeutic modalities, CCs are usually
fatal and patients usually succumb to the disease within 12 months.
Cancer cachexia, liver failure, recurrent sepsis secondary to
biliary obstruction and the subsequent rapid decline in performance
status mainly contribute to the high mortality rate associated with
this type of cancer. The overall survival rate, including in
patients who have undergone tumor resection, is poor, with <5%
of patients surviving 5 years. The poor survival rate has not
improved significantly over the past 30 years (5). Although CC is a relatively rare
tumor, the rising interest in this disease is due to the increasing
incidence and mortality rates for ICC worldwide and the cause
behind these remains unclear (4-9).
Early lymph node metastasis and perineural invasion account for the
poor outcome of patients with CC (10). Surgical liver resection with clear
margins has been considered as the standard treatment for
resectable CC; however, the survival rates are poor. Liver
transplant has yielded some recent excellent results in highly
selected patients with hilar CC; however, the major limitation is
the shortage of cadaveric donor organs (11).
2. Diagnostic snags
Despite the development of the diagnostic and
therapeutic modalities for various diseases and cancers, CC remains
difficult to diagnose and continues to be a highly lethal disease
with an extremely poor response to conventional anticancer
therapies and a poor survival rate (4,6).
The poor prognosis and survival rate associated with CC is mainly
due to the lack of early diagnosis. Although molecular markers,
including CA 19-9, carcinoembryonic antigen (CEA), CA-125,
platelet-derived growth factor and basic fibroblast growth factor
are being used for diagnosis, these markers lack the sensitivity
and specificity in early disease. Furthermore, CA19-9 has good
sensitivity and specificity for CC, but not for CC unassociated
with primary sclerosing cholangitis (12). Thus, there is a need for the
development of more effective and reliable markers for the early
detection of CC. The study of genetic and epigenetic alterations
mediating the molecular alterations and the malignant
transformation of biliary cells occurring in CC may foster novel
diagnostic, prognostic and therapeutic approaches (13). Developing interest in the
molecular medicine and molecular genetics in the context of
personalized, preventive, predictive and participatory medicine to
provide better medical care in order to decrease the incidence and
prevalence of the disease, as well as the study of the epigenetic
alterations for the identification of genes involved in the
tumorigenesis may prove to be beneficial (14). Since epigenetic alterations in
gene expression are associated with CC, genes that are
differentially methylated in CC may be useful in providing valuable
information on potential markers for the detection of early-stage
curable disease, markers prognostic of response to specific
treatments and overall prognosis and novel targets for the design
of rational therapies (4,6).
3. Mechanism of tumorigenesis
The accumulation of various defective cancerous or
mutated genes results in the activation of multistep processes to
induce tumorigenesis, resulting in CC, which is characterized by
the activation of growth-promoting genes and the silencing of tumor
growth suppressor genes mediating the uncontrolled proliferation by
altering the tissue homeostasis favorable for increasing the cell
proliferation rate, decreasing cell death rate, and creating a
growth-promoting environment (15). The main alterations in cancer gene
functions in cell physiology are self-sufficiency in growth
signals, insensitivity to growth-inhibitory signals, the evasion of
apoptosis, limitless replicative potential, sustained angiogenesis
and tissue invasion and metastasis (16). These changes may be due to genetic
alterations in oncogenes or due to epigenetic alterations. Two
major mechanisms, DNA methylation which adds methyl groups at CpG
sites, to convert cytosine to 5-methylcytosine, and the
post-translational histone modifications comprises the common
epigenetic mechanisms in cancer (4,6).
Epigenetics is an evolving area of research which
can provide deeper insight, and improved diagnostics and
theranostics for a number of human diseases. Epigenetic
modifications include both heritable and non-heritable changes that
regulate gene expression without altering the DNA sequence. Three
major epigenetic mechanisms have been identified which are mediated
through DNA methylation, histone modifications and non-coding RNAs.
In DNA methylation, the cytosine residue at the CG sequence in DNA
is specifically methylated, involving DNA methyltransferases
(DNMTs), which results in the formation of 5-methycytosine.
Subsequently, the methylated DNA fails to become transcribed and
this results in gene silencing (4,6).
More than 11 different types of post-translational modifications on
histone proteins have been described. Based on the type of
modification on specific residues of histone proteins, gene
expression becomes affected or induced, suggesting that histone
modifications epigenetically regulate gene expression. Non-coding
RNAs, which include microRNAs (miRNAs or miRs), siRNAs and long
non-coding RNAs (lncRNAs) have also been identified to
epigenetically affect gene expression. With the exception of
lncRNAs, these RNA species bind to the complementary sequence on
messenger RNA (mRNA) transcripts and thereby prevent protein
translation. In a number of cancer types and in different human
diseases, multiple different epigenetic mechanisms have been
identified as key regulators which can induce and enhance the
progression of disease. Therefore, targeting epigenetic mediators
is considered to be very promising in drug development for the
treatment of various human diseases (4,5,13,17,18).
4. Epigenetic alterations in
cholangiocarcinoma
DNA methylation
Studies have suggested that a number of genetic and
epigenetic alterations occur during the neoplastic transformation
of biliary epithelial cells that lead to the malignant progression
of CC (13,19,20). DNA methylation is the most
well-studied epigenetic mechanism in CC. In CC tumorigenesis, the
promoter regions of tumor suppressor genes are heavily methylated
(promoter hypermethylation), leading to gene silencing. Genomic DNA
is less methylated (global hypomethylation), resulting in increased
genomic instability and the reactivation of transposon elements
(4,21,22). In CC, the promoter
hypermethylation of genes involved in the cell cycle, cell
adhesion, DNA repair, apoptosis and carcinogen/drug metabolism have
been reported (4,6). The most common cancer-related genes
studied thus far in relation to CC are K-ras, p53, p14 alternate
reading frame gene (p14ARF), p16INK4a, SFRP1,
SFRP2 and β-catenin (4,6,22)
(Table I). The majority of K-ras
gene mutations occur in codon 12. Genetic alterations, such as
point mutations of K-ras and p53 have been frequently found in a
subset of CC cases (24-27);
however, the mutation or deletion of the cell cycle regulators,
p14ARF and p16INK4a, are not frequent
(28,29). Although β-catenin overexpression
is frequently encountered in CC, mutations in the β-catenin gene
have not been identified in ICC to date, at least to the best of
our knowledge (30). These
results suggest the crucial role of DNA methylation in the
tumorigenesis of CC and the potential of studying these epigenetic
alterations in order to identify and develop improved and more
effective therapeutic modalities in the future (22) (Table
I).
 | Table IDNA methylation in the genomic
sequences of specific genes that are associated with the
pathogenesis of cholangiocarcinoma. |
Table I
DNA methylation in the genomic
sequences of specific genes that are associated with the
pathogenesis of cholangiocarcinoma.
| Gene
(location) | Function
(Refs.) | Epigenetic
modification/effect (Refs.) | Outcome
(Refs.) |
|---|
| p16INK4A
or CDKN2A (9p21) | Tumor suppressor
gene Regulates cell proliferation and oncogenesis (31) | Promoter region
hypermethylation of the p16INK4A results in gene
inactivation | More frequent in
ECC cases (38) |
| | | Common event in
PSC-associated CC (28) | More commonly
observed in tumors with vascular invasion (38) |
| | | MF=17.7 to 83%
(20,32-35), 25% PSC-CC, 28.3%, liver fluke CC, and 100%
hepatolithiasis CC (28,36,37) | Poor clinical
outcome (20) |
| RASSF1A
(3p21.3) | Tumor suppressor
gene induces cell cycle arrest by inhibiting the accumulation of
cyclin D1 | Hypermethylation of
its CpG island promoter region results in inactivation MF=27-69%
(32,34,39) | Promoter
methylation is more common in ECC than ICC (32) |
| hMLH1 (3p21.3) | DNA mismatch repair
gene | Promoter
methylation/hypermethylation of the hMLH1 gene | Methylation
frequencies vary in sporadic CC, biliary papillary, neoplasms, and
liver fluke-related CC |
| | | MF=8.1 and 25%
sporadic CC (32,34), 0% biliary papillary CC (40), 44.6% Fluke-related CC (41) | Associated with
poorly differentiated subtype of CC with vascular invasion
(41) |
| FHIT (3p14.2) | Tumor suppressor
gene | Promoter
hypermethylation of the FHIT gene results in epigenetic silencing
of the FHIT promoter region MF=42% (42) | Development of
intrahepatic CCs |
| 14-3-3 | Tumor suppressor
gene. Regulates cell cycle and cell death | CpG island
hypermethylation cause inactivation of gene MF=59.5% CC (20) | Reported in ICC
(20) |
| TMS1/ASC
(16p11.2) | Tumor suppressor
gene | Aberrant
methylation of the TMS1/ASC cause inactivation of gene MF=36.1% CC
(43) | Associated with CC
(43) |
| APC (5q21–q22) | Tumor suppressor
gene Controls cell division, cell-cell interactions and cell
migration and invasion, and conservation of chromosomal number
during cell division | APC gene
hypermethylation MF=26.6 to 46% CC (20,32) | Worse clinical
outcome in CC (20,32) |
| DAPK (9q34.1) | Tumor suppressor
gene Positive mediator of interferon-γ (IFN-γ)-induced programmed
cell death | DAPK gene
hypermethylation MF=3 to 21.4% CC (32,34) | Associated with
poorly differentiated CCs and with a poor prognosis (32,34) |
| Epithelial (E)
cadherin gene (16q22.1) | Tumor suppressor
gene | Hypermethylation of
the promoter region of E gene Results in loss of function and
contribute to progression of cancer by increasing proliferation,
invasion and metastasis MF=21.5 to 43% CC (20,32,34,43,44) | Development of
intrahepatic CC |
| RAR-β (or HAP, RRB2
and NR1B2) (3p24) | Mediates cellular
signaling in embryonic morphogenesis, cell growth and
differentiation by regulating gene expression | Gene silencing by
promoter region hypermethylation Results in increased tumorigenesis
MF=14% of CCs (32) | Increased
tumorigenesis in CC |
| p73 gene
(1p36.3) | Tumor suppressor
gene and related to the p53 gene | Promoter region
hypermethylation increased tumorigenesis MF=36% CC (32) | Increased
tumorigenesis in CC |
| MGMT gene
(10q26) | Responsible for
repairing alkylation. DNA damage inhibits estrogen
receptor-mediated cell proliferation | Methylation of
discrete regions of the MGMT CpG island, results in
heterochromatinization of the MGMT transcription start site and
silencing of the gene MF=33% (32) and 49% CC (44) | Increased frequency
of GC to AT transitions in oncogenes and tumor suppressor genes and
a poor prognosis (44) |
| GSTP gene
(1q43) | Regulate drug and
xenobiotic. metabolism | Promoter region
hypermethylation (32) MF=<15%
CC (20) | Hypermethylation
more frequent in ICC than in ECC (32) |
| CHFR gene
(12q24.33) | Tumor suppressor
gene Delays the entry into the metaphase | Gene silencing by
promoter hypermethylation MF=16.2% in biliary tract carcinomas
(34) | Increased
tumorigenesis in CC |
| RUNX3 gene
(Ip36) | Tumor suppressor
gene Regulate proliferation of the biliary tract epithelium | Methylation of
RUNX3 results in gene silencing MF=56.8% in biliary tract cancer
(34) | Associated with
poorer survival (34) |
| TIMP3 gene
(22q12.3) | Plays a role in the
induction of apoptosis | CpG island
methylation of TIMP3 gene MF=8.9 and 9% CC (20,32) | Associated with
worse survival (20) |
| SEMA3B
(3p21.3) | Tumor suppressor
gene by inducing apoptosis. Plays a critical role in the guidance
of growth cones during neuronal development | Methylation of
SEMA3B gene MF=100% CC (45) | Increased
tumorigenesis in CC |
| BLU gene
(3p21.3) | Tumor suppressor
gene | Gene methylation
MF=20% CC (45) | Increased
tumorigenesis in CC |
| THBS1 gene
(15q15) | Mediates
cell-to-cell and cell-to-matrix interactions and play roles in
platelet aggregation, angiogenesis and tumorigenesis | Hypermethylation in
the promoter region of THBS1 gene MF=11% CC (20,45) | Increased
tumorigenesis in CC |
| p14ARF
(9p21) | Encoded by the β
transcript of CDKN2A (p16/CDKN2A) | Methylation of
p14ARF MF=38 and 25% (32,35); 40.2% liver fluke CC (37) | Increased
tumorigenesis in CC |
| p15INK4b
or p15 (9p21) | Effecter of
TGF-β-mediated cell cycle arrest | Promoter
hypermethylation of p15 gene, MF=50% of CC (32), 48.9% liver fluke CC (37) | Increased
tumorigenesis in CC |
| COX-2/PTGS2
(1q25.2–q25.3) | Acts both as a
dioxygenase and as a peroxidase | Methylation of
COX-2 gene, MF=5.1% of CC (20) | Increased
tumorigenesis in CC |
| OPCML | Tumor suppressor
gene | Hypermethylation of
OPCML, MF=72.5% Fluke CC (46) | Increased
tumorigenesis in CC |
| RIZ1 | Tumor suppressor
gene | Methylation of RIZ1
Results in chromatin compaction and gene silencing MF=38% liver
fluke CC (47) | Increased
proliferation and migration of CC cell line (48) |
Methylated CpG islands in tumor genes termed
methylated in tumor gene (MINT) are associated with carcinogenesis
of the biliary tract epithelium and other epithelial cancers. The
MINT loci associated with CC may be CpG island methylator phenotype
(CIMP)-positive or -negative, depending on the histological type of
CC (20). The methylation of
various genes presented in Table
I is associated with a poor survival and increased
tumorigenesis; however, the methylation of DcR1, the decoy
receptor, is associated with a significantly longer overall
survival. This suggests that the identification of specific
epigenetic alterations may serve as a prognostic marker in CC.
Furthermore, the positivity of epigenetic alterations in the less
differentiated CC, but not in normal adjacent tissue, suggests the
potential role of epigenetic biomarkers for prognosis and diagnosis
(46). Furthermore,
intraepithelial biliary neoplasms (IEBNs), the mucosal extension of
carcinoma and preinvasive neoplastic lesions in the bile ducts
around CC have been found to be associated with nodular-sclerosing
CC (NSCC), a common CC of the intrahepatic large, perihilar and
distal bile ducts. Immunohistochemical analysis with S100P,
vimentin, S100A4, E-cadherin, MUC1, MUC2, MUC5AC, MUC6, CDX2, CK7,
CK20, CDX2, CD10, p53 and Ki67 in NSCC has revealed a pre-invasive
and cancerous lesion zone (49).
Since, epigenetic alterations are found in less differentiated CC,
but not in normal adjacent tissue, studying the epigenetic and
proteomic alterations in pre-invasive compared to cancerous
lesions, may prove to be beneficial for the development of more
effective treatment strategies.
Histone modifications
Histones, complex with genomic DNA to form
nucleosomes, which consist of two turns of DNA wrapped around a
histone octamer composed of two subunits of each histone, H2A, H2B,
H3 and H4, with H1 as the linker histone between the core
nucleosomes (50).
Post-transcriptional modifications and gene expression through
histones are often regulated by histone acetylation, methylation
and phosphorylation (13).
Covalent modifications, such as the acetylation of lysines, the
methylation of lysines and arginines, the phosphorylation of
serines and threonines, and the ubiquitination of lysines occur at
the N-terminal tails of histone proteins, protruding out from the
core nucleosomes (51). Histone
can be mono-, di- or tri-methylated and H3 (lysines 4, 9 and 27)
and H4 (lysine 20) are the most frequently methylated histones
(52-53).
Histone acetylation and deacetylation catalyzed by histone
acetyltransferases (HATs) and histone deacetylases (HDACs) results
in transcriptional activation and repression, respectively
(54). However, depending on the
type of amino acid and its position in the histone tail, histone
methylation catalyzed by histone methyltransferases (HMTs) may
result in either transcriptional activation or repression (55). Similarly, H3K9, H3K27 and H4K20
methylation results in transcriptional repression, while H3K4
methylation results in transcriptional activation (56). The overexpression of HDAC1 is
associated with malignant behaviour and a poor prognosis of ICC
(57). Reduced survival and cell
growth arrest in the human CC cell lines with HDAC inhibitors, such
as MS-275, trichostatin A, NVPLAQ824 and NVPLBH589 in a
dose-dependent manner, suggests the possibility of suppressing CC
with HDAC inhibitors (58-60).
Furthermore, the synergistic growth inhibitory effect by the
induction of apoptosis and cell cycle arrest by HDAC inhibitors
with conventional cytostatic drugs, such as gemcitabine,
doxorubicin, sorafenib, or bortezomib supports the therapeutic role
of HDAC inhibitors in treating CC (58,60). However, the role of histone
modifications in the carcinogenesis and pathogenesis of CC is not
well documented; thus, further research to explore and unravel this
conundrum is required in order to develop more effective diagnostic
and therapeutic strategies for the treatment of CC.
miRNAs
miRNAs are small non-coding RNAs derived from
polyadenylated primary miRNAs (pri-miRNAs) and precursor miRNAs
(pre-miRNAs) involving RNA polymerase II and RNase III Drosha and
pasha/DGCR8(61). The maturation
of miRNAs is mediated by RNase III Dicer and binding with RISC
(RNA-induced silencing complex). Mature miRNAs regulate gene
expression at the post-transcriptional level by binding to the 3'
untranslated region (3'UTR) of target mRNAs, which leads to
degradation of mRNAs (62). The
upregulation (overexpression) or downregulation (underexpression)
of miRNAs regulates gene expression, thereby regulating
tumorigenesis (Table II). The
role of several miRNAs as oncogenes and tumor suppressor genes
(63) and as diagnostic and
prognostic markers (17) has been
documented. Recently, the presence of the increased expression of
oncogenic miR-24 and the decreased expression of the tumor
suppression gene, multiple endocrine neoplasia type 1 (also known
as menin 1 (MEN1)), and the role of miR-24 inhibition in
attenuating the progression of CC has been discussed (64). Since MEN1 overexpression is
associated with the decreased proliferation, angiogenesis,
migration and invasion of CC, the inhibition of miR-24 resulting in
an increased MEN1 protein expression may attenuate the
proliferation, angiogenesis, migration and invasion of CC. Thus,
targeting miR-24 may prove to be a novel therapeutic strategy.
 | Table IIUnique microRNAs that were identified
to promote the pathogenesis of cholangiocarcinoma. |
Table II
Unique microRNAs that were identified
to promote the pathogenesis of cholangiocarcinoma.
| Overexpressed or
upregulated miRNAs | Target gene | Correlation with CC
tumorigenesis | (Refs.) |
|---|
| miR-141 | CLOCK | Tumor suppressor
gene | (23) |
| miR-200b | PTPN12 | Tumor suppressor
gene | (23) |
| miR-21 | PTEN | Tumor suppressor
gene | (23) |
| let-7a | NF2 | Tumor suppressor
gene | (65) |
| miR-24 | MEN1(11q13) | Tumor suppressor
gene | (64) |
| miR-26a | GSK-3b | Tumor growth | (66) |
| miR-429 | CDH-6 | Tumor suppressor
gene | (67) |
| miR-21, miR-31, and
miR-223 | Multiple | No association with
clinic-pathological parameters of CC | (68) |
| Underexpressed or
downregulated miRNAs | Target gene | Correlation with CC
tumorigenesis | (Refs.) |
| miR-29b | MCL-1 | Tumor suppressor
gene | (65) |
| miR-370 | MAP3K8 | Tumor suppressor
gene | (65) |
| miR-148a | DNMT-1 | Regulate
methyltransferase | (69) |
| miR-152 | DNMT-1 | Regulate
methyltransferase | (69) |
| miR-124 | SMYD3 | Migration and
invasion of CC cells | (70) |
| miR-214 | Twist | Oncogene | (71) |
| miR-122, miR-145,
miR-200c, miR-221, and miR-222 | Multiple | Associated with
tumorigenesis of ICC | (68) |
DNA methylation, histone modification and
alterations in miRNA expression are involved in the tumorigenesis
of CC. Furthermore, the control of the transcription of miRNAs by
DNA methylation, histone modifications and the regulation of
epigenetic machinery by miRNAs suggest an association of these
mechanisms in CC tumorigenesis. This suggests that the study of
epigenetic alterations may provide novel and non-invasive
biomarkers, strong potential screening tools, and potentially
promising prognostic and diagnostic markers for CC in clinical
practice (18,72-74).
lncRNAs
lncRNAs pervasively transcribed in the genome, are
emerging as crucial regulators of cancer and play important roles
in almost every aspect of cell biology, including tumorigenesis.
lncRNAs regulate the malignant transformation of cells through
their interaction with DNA, proteins and RNA. Thus, lncRNA
molecular mechanisms involved in the tumorigenesis of CC may be
attractive targets for therapeutic intervention in the fight
against cancer (75-77).
Using lncRNAs, mRNA microarrays and RT-PCR, Wang et al
(78) examined the associations
between the expression levels of lncRNAs and target genes, and
found the upregulation of lncRNAs in ICC tissues and the
downregulation of lncRNAs in non-cancerous tissues. The majority of
upregulated genes are involved in carcinogenesis, hepatic system
diseases and signal transductions. Furthermore, the upregulation of
lncRNA CCAT1 and lncRNA AFAP1-AS1 in CC and their association with
the tumor growth promotion, aggressive malignant behavior and the
metastasis of CC suggest the role of lncRNAs in the pathogenesis of
CC (79-80). Competing
endogenous RNAs (ceRNAs) are a novel class of RNA species that can
regulate miRNAs, lncRNAs, and genes that play important roles in
the pathogenesis of CC (81). The
interaction between lncRNA MALAT1 and miR-204 has been shown to
modulate human hilar CC proliferation, migration and invasion by
targeting CXCR4(82). Wang et
al (76) found that cell
migration and invasion in CC, by targeting IL-6 and CXCR4 via
ceRNA, was regulated by lncRNA H19 and HULC, upregulated by
oxidative stress. Furthermore, the co-expression of the
carbamoyl-phosphate synthase 1 (CPS1) gene and its lncRNA has been
shown to be associated with a poor prognosis in CC (83). Hence, the increased expression of
lncRNAs in CC indicates that lncRNAs may be potential diagnostic
and prognostic biomarkers for ICC; the combined assessment of
lncRNA and mRNA expression levels may thus predict the survival of
patients with ICC (76-80,83).
Furthermore, the BRCA-1 associated protein-1 (BAP1)-dependent
expression of lncRNA NEAT-1 enhancing the sensitivity to
gemcitabine in CC, suggests the therapeutic role of lncRNAs
(84).
Protein modifications
Post-transcriptional modifications result in the
alteration of protein functions following protein expression and
are associated with carcinogenesis and a number of human diseases.
The wingless type (Wnt) signaling pathway plays a crucial role in
the tumorigenesis of CC. Davaadorj et al (85,86) found a negative correlation between
secreted frizzled-related protein-1 (SFRP1) expression and
β-catenin expression in ICC and suggested that the loss of the
negative regulator of the Wnt signaling pathway, SFRP1, located at
chromosome 8p12e11.1, was associated with a poor prognosis of
patients with ICC. Hence, the loss of SFRP1 may be a potential
prognostic biomarker for ICC. These data suggest that proteomics
analysis may be useful for the diagnosis and prognosis in CC.
Furthermore, the differential expression of proteins during
proteomics analysis may be used for the identification of the
transition of the infectious liver to CC, and may thus lead to the
early diagnosis and prevention of CC (87).
5. Diagnostic development
Immunohistochemistry
The immunostaining of formalin-fixed biopsied
tissues with various tumor-specific markers, including CD10, CEA,
CK7, CK20, CDX-2, TTF-1, ER, PR, BRST-2, ISHalbumin, Hep
Par 1, Ber-Ep4, chromogranin and PSA is being used to differentiate
and diagnose CC (88). However,
due to the close association of the anatomic sites in the embryonic
and the fetal development process of ICC from metastatic pancreatic
ductal adenocarcinomas or adenocarcinoma from the upper GI tract,
it has become difficult to differentiate due to the lack of
tissue-specific markers (88).
The inclusion of non-conventional markers (placental S100 (S100P),
von Hippel-Lindau tumor suppressor (pVHL), mucin 5AC (MUC5AC) and
CK17) with the existing markers may be beneficial (89). Despite the presence of various
diagnostic and prognostic markers (17), early diagnosis remains a challenge
and indicates thea need for more effective histological and
molecular diagnostics. Nakanuma et al (49) suggested the role of S100P
immunostaining in the differentiation of carcinomatous, perihilar
and normal tissue. Recently, Kanzawa et al (90) discussed the role of dual
immunostaining for maspin and p53 compared to S100P and p53 on cell
blocks in increasing the diagnostic value of biliary brushing
cytology. The role of tubulin β-III (TUBB٣) as a novel
immunohistochemical marker for intrahepatic peripheral CC has also
been discussed (91).
Proteomics
The serum markers, CA19-9, CA125 and CEA, have been
used for the diagnosis of CC; however, their sensitivity and
specificity for all histological types of CC is unclear (12). Thus, there is a need for more
effective markers for early diagnosis. Patel et al (12) suggested that the addition of serum
CA19-9 may aid in the differentiation of CC in patients with PSC
and CC not associated with PSC. Including the proteomic-based
autoantibodies analysis against heat shock protein 70, enolase 1
and ribonuclease/angiogenin Inhibitor 1 as diagnostic markers may
increase the sensitivity and specificity in the early detection of
CC (92,93). Similarly, using matrix-assisted
laser desorption/ionization-imaging mass spectrometry (MALDI IMS)
to reveal tissue heterogeneity in hepatic CC may aid in revealing
novel relevant biomarkers for CC. Furthermore, these biomarkers may
be used for diagnostic and follow-up purposes in patients who are
at risk of developing CC if these are secreted and detectable in
blood (94). Further, the mass
spectrometry-based proteomics analysis of
formalin-fixed-paraffin-embedded extrahepatic CC and the
overexpression of proteins on immunohistochemical analysis with a
positive rate of S100P (84%), CEAM5 (75%), MUC5A (62%), OLFM4
(60%), OAT (42%), CAD17 (41%), FABPL (38%), AOFA (30%), K1C20 (25%)
and CPSM (22%) in extrahepatic CCs, but not in normal tissue,
suggest the potential role of proteomics analysis in elucidating
potential targets for future diagnostic biomarkers and therapy
(95). Stephenson et al
(96) also highlighted the role
of proteomics profiling for the quantitative assessment of cell
surface proteins to identify novel therapeutic targets in CC and to
distinguish between distal CC and pancreatic cancers. Additionally,
proteomics profiling for the identification of novel serum
biomarkers may aid in differentiating between CC from benign
biliary tract diseases (97). The
same reports also described FAM19A5, MAGED4B, KIAA0321, RBAK and
UPF3B as potential biomarkers of CC. These data suggest that
proteomics profiling may be used to elucidate the potentially novel
biomarker for the development of diagnosis, prognosis and therapies
(98-100).
Epigenetics
Tissue heterogeneity in carcinomas confers a
significant problem in early diagnosis. Although single-gene
predictive assays are available, there is a need for the analysis
of multiple gene loci, since the genetic, proteomic and miRNA
content may vary in the biopsied sample due to tissue heterogeneity
(14,49). Whole-genome sequencing and RNA
sequencing may provide a comprehensive analysis of the somatic
mutations and gene expression, and provide novel insights on the
use of genomic data for the treatment of individual patients
(14). Furthermore, genome-wide
expression patterns associated with oncogenesis and the sarcomatous
transdifferentiation of CC documented the up- and downregulation of
tumor-related and tumor suppressor genes and proteins in human CC,
including SPP1, EFNB2, E2F2, IRX3, PTTG1, PPARγ, KRT17, UCHL1,
IGFBP7 and SPARC (101). This
suggests that gene expression profiling for the deregulation of
oncogenes, tumor suppressor genes and methylation-related genes,
and their related proteins may be useful for the identification of
molecular targets for the diagnosis and prognosis of CC.
Furthermore, gene expression profiling may also be useful in
differentiating CC from other liver masses in addition to
subclassifying ICC, with better results compared to
histopathological findings. Furthermore, gene profiling can also be
helpful in predicting the outcome for various therapeutic
modalities and patient survival (101).
6. Epigenetic therapy of cancer
Epigenetic alterations result in the inactivation
and silencing of tumor suppressor genes and increased
tumorigenesis. Signal transducer and activator of transcription 3
(STAT3) overexpression is associated with metastasis and poor
post-surgical outcome in CC, and the inhibition of STAT3 may be a
novel therapeutic target (102).
Similarly, Braconi et al (103) discussed the potential of
targeting the IL-6 dependent phenotype through a computational
bioinformatics analysis of phenotype-based gene expression. The
Wnt/catenin pathway plays a crucial role in CC tumorigenesis and
the reversal of the silencing of genes involved in Wnt signaling,
including SOX17, WNT3A, DKK2, SFRP1, SFRP2 and SFRP4 with DNMT
inhibitor 5-aza-2'deoxycytidine in CC cells suggests the
significance of targeting epigenetic mediators in CC (22). Although 5-azacitidine and
5-aza-2'deoxycytidine are US Food and Drug Administration
(FDA)-approved drugs for the treatment of myelodysplastic syndrome,
the in vitro and in vivo toxicity of these drugs show
instability in neutral solutions (104,105). The role of the less toxic DNMT
inhibitor, zebularine, a novel DNA methyltransferase inhibitor,
alone or in combination with other DNMT inhibitors to enhance the
re-expression of epigenetically silenced genes in cancer cells and
as an inducer of cell death in CC, has been discussed (106-108).
Thus, the re-activation of the silenced gene may restore gene
function and its tumor-suppressing actions; thus, from this
perspective, demethylating agents and HDAC inhibitors may be useful
as drug candidates (22,57-60).
Cell growth arrest and cell death in cancer cells can be induced by
HDAC inhibitors. Another advantage with HDAC inhibitors is that
normal cells are relatively resistant to them. The HDAC inhibitor,
suberoylanilide hydroxamic acid (SAHA), has been approved by the
FDA for T-cell cutaneous lymphoma treatment. Furthermore, the role
of SAHA, valproic acid and the EZH2 inhibitor, 3-deazaneplanocin-A,
as therapeutic drugs in CC, has also been discussed (109-112).
Since molecular genetics, epigenetics and proteomics are evolving
and promising fields in research, the study of the epigenetics of
CC may enhance the effectiveness of CC therapeutics. Furthermore,
targeting the specific phenotype and pathways involved in the
carcinogenesis of CC, and the use of the computational
bioinformatics-driven approach to discover a novel drug may prove
to be beneficial (103). The key
proteins regulating the epigenetic mechanisms in the pathogenesis
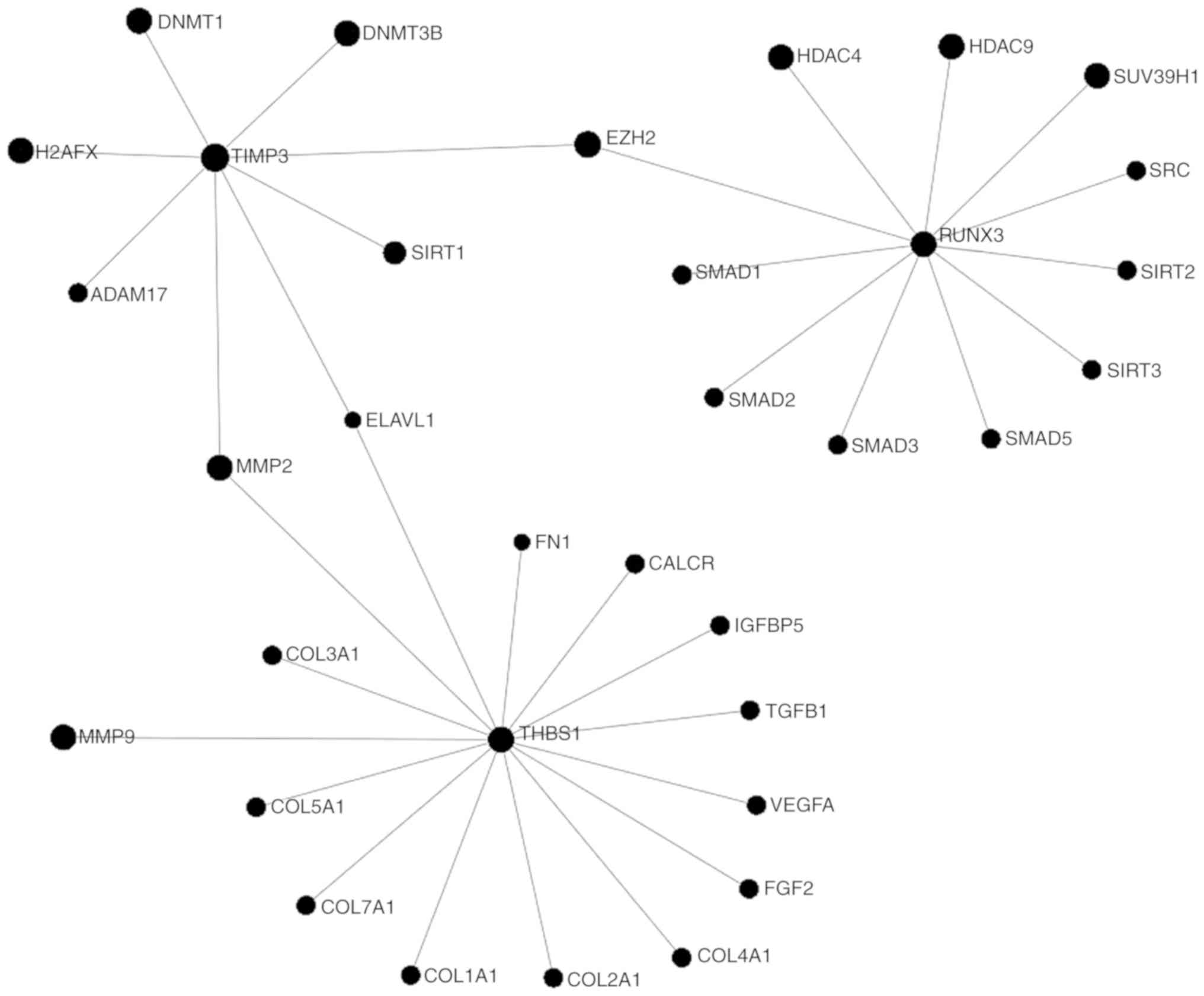
of CC are presented in Fig.
1.
Based on the above-mentioned study results, it is
imperative that despite the advancements in diagnostic tools and
treatment strategies, the early diagnosis and treatment of CC
remains challenging. Since the most common identified cause of CC
is PSC, the diagnosis of early-stage CC requires a high index of
suspicion in patients with PSC. Furthermore, due to the negative
results of endoscopic brush cytology, endoscopic biopsies and
imaging studies, a regular follow-up with magnetic resonance
cholangopancreatography (MRCP) and advanced imaging, such as
positron emission tomography (PET) scan should be carried out in
all patients with PSC. Along with the PET scan, a promising imaging
modality for the diagnosis of CC, namely the determination of serum
levels of CA19-9, CA125 and CEA, should be carried out in an annual
follow-up. Although the sensitivity and specificity of these
biomarkers for all histological types of CC is unclear, CA19-9
values >100 U/ml have a 75% sensitivity and 80% specificity for
CC (113). Since there are no
clinical surveillance guidelines for the early detection of
sporadic CC, the screening of patients with PSC with MRI, MRCP PET
scan, and the determination of CA19-9 levels, is the most effective
strategy for early detection. Since DNA hypermethylation is the
most common aberrant epigenetic alteration in CC, the early
detection of the CC can also be facilitated by DNA methylation
assay of the bile fluids, including a panel of CCND2, CDH13,
GRIN2B, Runt-related transcription factor 3 (RUNX3) and
Twist-related protein 1 (TWIST1). This method for the detection of
CC has a sensitivity of 83% and a specificity of 100% (13,114,115). Screening for the genetic and
epigenetic alterations in the precursor lesions, including
intraductal papillary neoplasm of the bile duct (IPNB) and biliary
intraepithelial neoplasia (BilIN), as discussed by Ettel et
al, may also be beneficial (116). The diagnostic and treatment
challenges are also due to the heterogeneity of clinical
presentations, which may be due to the origin of CC from
topographically heterogeneous cholangiocytes. In cases of ICC,
clinical presentation is highly heterogenous (mass-forming type,
infiltrative type, etc.) and genetic alterations in cases with
mass-forming type (ICC) have been shown to be similar to those in
cases with hepatocellular carcinoma, and in cases with infiltrative
type, genetic alterations have been shown to be similar with those
in cases with perihilar CC. The clinicopathological,
immunohistochemical and molecular profile similarity of Muc-ICCs
with hilar CCs (from mucin-producing cholangiocytes) and of
mixed-ICCs with CLCs (thought to be of HPC origin) and varying
degrees of biliary epithelial differentiation has been reported
(117,118). Due to the complexity of origin
and clinical presentation, the treatment of CC as a whole is
difficult, and there is a need to focus on the site of origin for
treatment. In other words, individualized treatment should be
preferred for the treatment of CC. In early-stage CC (perihilar
CCA), liver transplantation with pre-operative radiation and
chemotherapy and exploratory laparotomy needs to be performed to
ensure the absence of metastases as a viable therapeutic option,
whereas patients with ICC can be treated surgically (113,119).
7. Conclusions
CCs are rare notoriously malignant tumors with a
very poor survival rates even following surgical resection. The
delayed presentation of the tumor is the main reason for the
delayed diagnosis and poor survival. Currently, the available panel
of tissue and serum biomarkers can diagnose the tumor at a late
stage only, and is lacking any modalities for diagnosis at an early
stage. Thus, there is a need for the identification of more
effective diagnostic biomarkers for the early diagnosis of CC.
Recent advances in immunohistochemistry and molecular genetics
paved the way for improved diagnostics. The role of epigenetic and
proteomic alterations in the pathogenesis of CC has been
documented, and these alterations may serve as the early diagnostic
and prognostic markers for CC. Furthermore, the role of epigenetic
therapy with DNMT and HDAC inhibitors discussed in the literature
are in the early stages of clinical trials. The findings of various
studies discussed in this review suggest that epigenetic and
proteomic alterations may serve as more effective diagnostic
markers for CC in the early stages, and epigenetic therapy may be
beneficial for the treatmetn of CC. However, further research is
required to investigate the initial events occurring in CC.
Acknowledgements
Not applicable.
Funding
This study was supported by Creighton University
LB692 Clinical and Translational Research Grant to CSB.
Availability of data and materials
Not applicable.
Authors' contributions
VR, CSB and DKA were involved in the conception and
design of the study. Drafting of the article was performed by VR.
Critical revisions in the article were carried out by VR and CSB.
All authors have read complete manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vauthey JN and Blumgart LH: Recent
advances in the management of cholangiocarcinomas. Semin Liver Dis.
14:109–114. 1994.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lazaridis KN and Gores GJ:
Cholangiocarcinoma. Gastroenterology. 128:1655–1667.
2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Khan SA, Davidson BR, Goldin R, Pereira
SP, Rosenberg WM, Taylor-Robinson SD, Thillainayagam AV, Thomas HC,
Thursz MR and Wasan H: British Society of Gastroenterology:
Guidelines for the diagnosis and treatment of cholangiocarcinoma:
Consensus document. Gut ٦. (Suppl 51):VI1–9. 2002.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Sandhu DS, Shire AM and Roberts LR:
Epigenetic DNA hypermethylation in cholangiocarcinoma: Potential
roles in pathogenesis, diagnosis and identification of treatment
targets. Liver Int. 28:12–27. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Shaib Y and El-Serag HB: The epidemiology
of cholangiocarcinoma. Semin Liver Dis. 24:115–125. 2004.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Limpaiboon T: Epigenetic aberrations in
cholangiocarcinoma: Potential biomarkers and promising target for
novel therapeutic strategies. Asian Pac J Cancer Prev. 13
(Suppl):S41–S45. 2012.PubMed/NCBI
|
|
7
|
Khan SA, Taylor-Robinson SD, Toledano MB,
Beck A, Elliott P and Thomas HC: Changing international trends in
mortality rates for liver, biliary and pancreatic tumours. J
Hepatol. 37:806–813. 2002.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Patel T: Worldwide trends in mortality
from biliary tract malignancies. BMC Cancer. 2(10)2002.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Shaib YH, Davila JA, McGlynn K and
El-Serag HB: Rising incidence of intrahepatic cholangiocarcinoma in
the United States: A true increase? J Hepatol. 40:472–477.
2004.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Rosai J: Ackerman's Surgical Pathology.
Vol 2. 8th edition. Mosby. pp982–989. 1996.
|
|
11
|
Rea DJ, Heimbach JK, Rosen CB, Haddock MG,
Alberts SR, Kremers WK, Gores GJ and Nagorney DM: Liver
transplantation with neoadjuvant chemoradiation is more effective
than resection for hilar cholangiocarcinoma. Ann Surg. 242:451–458;
discussion 458-461. 2005.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Patel AH, Harnois DM, Klee GG, LaRusso NF
and Gores GJ: The utility of CA 19-9 in the diagnoses of
cholangiocarcinoma in patients without primary sclerosing
cholangitis. Am J Gastroenterol. 95:204–207. 2000.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Maroni L, Pierantonelli I, Banales JM,
Benedetti A and Marzioni M: The significance of genetics for
cholangiocarcinoma development. Ann Transl Med.
1(28)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Sheffield BS, Tessier-Cloutier B, Li-Chang
H, Shen Y, Pleasance E, Kasaian K, Li Y, Jones SJ, Lim HJ, Renouf
DJ, et al: Personalized oncogenomics in the management of
gastrointestinal carcinomas-early experiences from a pilot study.
Curr Oncol. 23:e571–e575. 2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ashkenazi R, Gentry SN and Jackson TL:
Pathways to tumorigenesis-modeling mutation acquisition in stem
cells and their progeny. Neoplasia. 10:1170–1182. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Berretta M, Cavaliere C, Alessandrini L,
Stanzione B, Facchini G, Balestreri L, Perin T and Canzonieri V:
Serum and tissue markers in hepatocellular carcinoma and
cholangiocarcinoma: Clinical and prognostic implications.
Oncotarget. 8:14192–14220. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Zhou J, Liu Z, Yang S and Li X:
Identification of microRNAs as biomarkers for cholangiocarcinoma
detection: A diagnostic meta-analysis. Clin Res Hepatol
Gastroenterol. 41:156–162. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Rashid A: Cellular and molecular biology
of biliary tract cancers. Surg Oncol Clin N Am. 11:995–1009.
2002.PubMed/NCBI
|
|
20
|
Lee S, Kim WH, Jung HY, Yang MH and Kang
GH: Aberrant CpG island methylation of multiple genes in
intrahepatic cholangiocarcinoma. Am J Pathol. 161:1015–1022.
2002.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Herman JG and Baylin SB: Gene silencing in
cancer in association with promoter hypermethylation. N Engl J Med.
349:2042–2054. 2003.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Goeppert B, Konermann C, Schmidt CR,
Bogatyrova O, Geiselhart L, Ernst C, Gu L, Becker N, Zucknick M,
Mehrabi A, et al: Global alterations of DNA methylation in
cholangiocarcinoma target the Wnt signaling pathway. Hepatology.
59:544–554. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Meng F, Henson R, Lang M, Wehbe H,
Maheshwari S, Mendell JT, Jiang J, Schmittgen TD and Patel T:
Involvement of human micro-RNA in growth and response to
chemotherapy in human cholangiocarcinoma cell lines.
Gastroenterology. 130:2113–2129. 2006.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kang YK, Kim WH, Lee HW, Lee HK and Kim
YI: Mutation of p53 and K-ras, and loss of heterozygosity of APC in
intrahepatic cholangiocarcinoma. Lab Invest. 79:477–483.
1999.PubMed/NCBI
|
|
25
|
Sturm PD, Baas IO, Clement MJ, Nakeeb A,
Johan G, Offerhaus A, Hruban RH and Pitt HA: Alterations of the p53
tumor-suppressor gene and K-ras oncogene in perihilar
cholangiocarcinomas from a high-incidence area. Int J Cancer.
78:695–698. 1998.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kiba T, Tsuda H, Pairojkul C, Inoue S,
Sugimura T and Hirohashi S: Mutations of the p53 tumor suppressor
gene and the ras gene family in intrahepatic cholangiocellular
carcinomas in Japan and Thailand. Mol Carcinog. 8:312–318.
1993.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wattanasirichaigoon S, Tasanakhajorn U and
Jesadapatarakul S: The incidence of K-ras codon 12 mutations in
cholangiocarcinoma detected by polymerase chain reaction technique.
J Med Assoc Thai. 81:316–323. 1998.PubMed/NCBI
|
|
28
|
Ahrendt SA, Eisenberger CF, Yip L, Rashid
A, Chow JT, Pitt HA and Sidransky D: Chromosome 9p21 loss and p16
inactivation in primary sclerosing cholangitis-associated
cholangiocarcinoma. J Surg Res. 84:88–93. 1999.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Tannapfel A, Benicke M, Katalinic A,
Uhlmann D, Köckerling F, Hauss J and Wittekind C: Frequency of
p16(INK4A) alterations and K-ras mutations in intrahepatic
cholangiocarcinoma of the liver. Gut. 47:721–727. 2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sugimachi K, Taguchi K, Aishima S, Tanaka
S, Shimada M, Kajiyama K, Sugimachi K and Tsuneyoshi M: Altered
expression of beta-catenin without genetic mutation in intrahepatic
cholangiocarcinoma. Mod Pathol. 14:900–905. 2001.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Serrano M, Hannon GJ and Beach D: A new
regulatory motif in cell-cycle control causing specific inhibition
of cyclin D/CDK4. Nature. 366:704–707. 1993.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yang B, House MG, Guo M, Herman JG and
Clark DP: Promoter methylation profiles of tumor suppressor genes
in intrahepatic and extrahepatic cholangiocarcinoma. Mod Pathol.
18:412–420. 2005.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ueki T, Hsing AW, Gao YT, Wang BS, Shen
MC, Cheng J, Deng J, Fraumeni JF Jr and Rashid A: Alterations of
p16 and prognosis in biliary tract cancers from a population-based
study in China. Clin Cancer Res. 10:1717–1725. 2004.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Tozawa T, Tamura G, Honda T, Nawata S,
Kimura W, Makino N, Kawata S, Sugai T, Suto T and Motoyama T:
Promoter hypermethylation of DAP-kinase is associated with poor
survival in primary biliary tract carcinoma patients. Cancer Sci.
95:736–740. 2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Tannapfel A, Sommerer F, Benicke M,
Weinans L, Katalinic A, Geissler F, Uhlmann D, Hauss J and
Wittekind C: Genetic and epigenetic alterations of the INK4a-ARF
pathway in cholangiocarcinoma. J Pathol. 197:624–631.
2002.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sasaki M, Yamaguchi J, Itatsu K, Ikeda H
and Nakanuma Y: Over-expression of polycomb group protein EZH2
relates to decreased expression of p16 INK4a in
cholangiocarcinogenesis in hepatolithiasis. J Pathol. 215:175–183.
2008.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Chinnasri P, Pairojkul C, Jearanaikoon P,
Sripa B, Bhudhisawasdi V, Tantimavanich S and Limpaiboon T:
Preferentially different mechanisms of inactivation of 9p21 gene
cluster in liver fluke-related cholangiocarcinoma. Hum Pathol.
40:817–826. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Hong SM, Choi J, Ryu K, Ro JY and Yu E:
Promoter hypermethylation of the p16 gene and loss of its protein
expression is correlated with tumor progression in extrahepatic
bile duct carcinomas. Arch Pathol Lab Med. 130:33–38.
2006.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Wong N, Li L, Tsang K, Lai PB, To KF and
Johnson PJ: Frequent loss of chromosome 3p and hypermethylation of
RASSF1A in cholangiocarcinoma. J Hepatol. 37:633–639.
2002.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Abraham SC, Lee JH, Boitnott JK, Argani P,
Furth EE and Wu TT: Microsatellite instability in intraductal
papillary neoplasms of the biliary tract. Mod Pathol. 15:1309–1317.
2002.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Limpaiboon T, Khaenam P, Chinnasri P,
Soonklang M, Jearanaikoon P, Sripa B, Pairojkul C and Bhudhisawasdi
V: Promoter hypermethylation is a major event of hMLH1 gene
inactivation in liver fluke related cholangiocarcinoma. Cancer
Lett. 217:213–219. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Foja S, Goldberg M, Schagdarsurengin U,
Dammann R, Tannapfel A and Ballhausen WG: Promoter methylation and
loss of coding exons of the fragile histidine triad (FHIT) gene in
intrahepatic cholangiocarcinomas. Liver Int. 25:1202–1208.
2005.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Liu XF, Zhu SG, Zhang H, Xu Z, Su HL, Li
SJ and Zhou XT: The methylation status of the TMS1/ASC gene in
cholangiocarcinoma and its clinical significance. Hepatobiliary
Pancreat Dis Int. 5:449–453. 2006.PubMed/NCBI
|
|
44
|
Koga Y, Kitajima Y, Miyoshi A, Sato K,
Kitahara K, Soejima H and Miyazaki K: Tumor progression through
epigenetic gene silencing of O(6)-methylguanine-DNA
methyltransferase in human biliary tract cancers. Ann Surg Oncol.
12:354–363. 2005.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Tischoff I, Markwarth A, Witzigmann H,
Uhlmann D, Hauss J, Mirmohammadsadegh A, Wittekind C, Hengge UR and
Tannapfel A: Allele loss and epigenetic inactivation of 3p21.3 in
malignant liver tumors. Int J Cancer. 115:684–689. 2005.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Sriraksa R, Zeller C, El-Bahrawy MA, Dai
W, Daduang J, Jearanaikoon P, Chau-In S, Brown R and Limpaiboon T:
CpG-island methylation study of liver fluke-related
cholangiocarcinoma. Br J Cancer. 104:1313–1318. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Khaenam P, Jearanaikoon P, Pairojkul C,
Bhudhisawasdi V and Limpaiboon T: Genetic and epigenetic
alterations of RIZ1 and the correlation to clinicopathological
parameters in liver fluke-related cholangiocarcinoma. Exp Ther Med.
1:385–390. 2010.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Khaenam P, Niibori A, Okada S,
Jearanaikoon P, Araki N and Limpaiboon T: Contribution of RIZ1 to
regulation of proliferation and migration of a liver fluke-related
cholangiocarcinoma cell. Asian Pac J Cancer Prev. 13:4007–4011.
2012.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Nakanuma Y, Uchida T, Sato Y and Uesaka K:
An S100P-positive biliary epithelial field is a preinvasive
intraepithelial neoplasm in nodular-sclerosing cholangiocarcinoma.
Hum Pathol. 60:46–57. 2017.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Khorasanizadeh S: The nucleosome: From
genomic organization to genomic regulation. Cell. 116:259–272.
2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Berger SL: Histone modifications in
transcriptional regulation. Curr Opin Genet Dev. 12:142–148.
2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Grant PA: A tale of histone modifications.
Genome Biol. 2(Reviews0003)2001.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Taby R and Issa JP: Cancer epigenetics. CA
Cancer J Clin. 60:376–392. 2010.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Shukla V, Vaissiere T and Herceg Z:
Histone acetylation and chromatin signature in stem cell identity
and cancer. Mutat Res. 637:1–15. 2008.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Esteller M: Epigenetics in cancer. N Engl
J Med. 358:1148–1159. 2008.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Cheung P and Lau P: Epigenetic regulation
by histone methylation and histone variants. Mol Endocrinol.
19:563–573. 2005.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Morine Y, Shimada M, Iwahashi S,
Utsunomiya T, Imura S, Ikemoto T, Mori H, Hanaoka J and Miyake H:
Role of histone deacetylase expression in intrahepatic
cholangiocarcinoma. Surgery. 151:412–419. 2012.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Baradari V, Höpfner M, Huether A, Schuppan
D and Scherübl H: Histone deacetylase inhibitor MS-275 alone or
combined with bortezomib or sorafenib exhibits strong
antiproliferative action in human cholangiocarcinoma cells. World J
Gastroenterol. 13:4458–4466. 2007.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Xu LN, Wang X and Zou SQ: Effect of
histone deacetylase inhibitor on proliferation of biliary tract
cancer cell lines. World J Gastroenterol. 14:2578–2581.
2008.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Bluethner T, Niederhagen M, Caca K, Serr
F, Witzigmann H, Moebius C, Mossner J and Wiedmann M: Inhibition of
histone deacetylase for the treatment of biliary tract cancer: A
new effective pharmacological approach. World J Gastroenterol.
13:4761–4770. 2007.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297.
2004.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Brennecke J, Stark A, Russell RB and Cohen
SM: Principles of microRNA-target recognition. PLoS Biol.
3(e85)2005.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Chuang JC and Jones PA: Epigenetics and
microRNAs. Pediatr Res. 61:24R–29R. 2007.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Ehrlich L, Hall C, Venter J, Dostal D,
Bernuzzi F, Invernizzi P, Meng F, Trzeciakowski JP, Zhou T,
Standeford H, et al: miR-24 inhibition increases menin expression
and decreases cholangiocarcinoma proliferation. Am J Pathol.
187:570–580. 2017.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Stutes M, Tran S and DeMorrow S: Genetic
and epigenetic changes associated with cholangiocarcinoma: From DNA
methylation to microRNAs. World J Gastroenterol. 13:6465–6469.
2007.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Zhang J, Han C and Wu T: MicroRNA-26a
promotes cholangiocarcinoma growth by activating β-catenin.
Gastroenterology. 143:246–256, e8. 2012.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Goeppert B, Ernst C, Baer C, Roessler S,
Renner M, Mehrabi A, Hafezi M, Pathil A, Warth A, Stenzinger A, et
al: Cadherin-6 is a putative tumor suppressor and target of
epigenetically dysregulated miR-429 in cholangiocarcinoma.
Epigenetics. 11:780–790. 2016.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Karakatsanis A, Papaconstantinou I,
Gazouli M, Lyberopoulou A, Polymeneas G and Voros D: Expression of
microRNAs, miR-21, miR-31, miR-122, miR-145, miR-146a, miR-200c,
miR-221, miR-222, and miR-223 in patients with hepatocellular
carcinoma or intrahepatic cholangiocarcinoma and its prognostic
significance. Mol Carcinog. 52:297–303. 2013.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Braconi C, Huang N and Patel T:
MicroRNA-dependent regulation of DNA methyltransferase-1 and tumor
suppressor gene expression by interleukin-6 in human malignant
cholangiocytes. Hepatology. 51:881–890. 2010.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Zeng B, Li Z, Chen R, Guo N, Zhou J, Zhou
Q, Lin Q, Cheng D, Liao Q, Zheng L and Gong Y: Epigenetic
regulation of miR-124 by hepatitis C virus core protein promotes
migration and invasion of intrahepatic cholangiocarcinoma cells by
targeting SMYD3. FEBS Lett. 586:3271–3278. 2012.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Li B, Han Q, Zhu Y, Yu Y, Wang J and Jiang
X: Down-regulation of miR-214 contributes to intrahepatic
cholangiocarcinoma metastasis by targeting Twist. FEBS J.
279:2393–2398. 2012.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Scott GK, Mattie MD, Berger CE, Benz SC
and Benz CC: Rapid alteration of microRNA levels by histone
deacetylase inhibition. Cancer Res. 66:1277–1281. 2006.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Valeri N, Vannini I, Fanini F, Calore F,
Adair B and Fabbri M: Epigenetics, miRNAs, and human cancer: A new
chapter in human gene regulation. Mamm Genome. 20:573–580.
2009.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Han L, Witmer PD, Casey E, Valle D and
Sukumar S: DNA methylation regulates MicroRNA expression. Cancer
Biol Ther. 6:1284–1288. 2007.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Schmitt AM and Chang HY: Long noncoding
RNAs in cancer pathways. Cancer Cell. 29:452–463. 2016.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Wang WT, Ye H, Wei PP, Han BW, He B, Chen
ZH and Chen YQ: LncRNAs H19 and HULC, activated by oxidative
stress, promote cell migration and invasion in cholangiocarcinoma
through a ceRNA manner. J Hematol Oncol. 9(117)2016.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yang W, Li Y, Song X, Xu J and Xie J:
Genome-wide analysis of long noncoding RNA and mRNA co-expression
profile in intrahepatic cholangiocarcinoma tissue by RNA
sequencing. Oncotarget. 8:26591–26599. 2017.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Wang J, Xie H, Ling Q, Lu D, Lv Z, Zhuang
R, Liu Z, Wei X, Zhou L, Xu X and Zheng S: Coding-noncoding gene
expression in intrahepatic cholangiocarcinoma. Transl Res.
168:107–121. 2016.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Jiang XM, Li ZL, Li JL, Zheng WY, Li XH,
Cui YF and Sun DJ: LncRNA CCAT1 as the unfavorable prognostic
biomarker for cholangiocarcinoma. Eur Rev Med Pharmacol Sci.
21:1242–1247. 2017.PubMed/NCBI
|
|
80
|
Shi X, Zhang H, Wang M, Xu X, Zhao Y, He
R, Zhang M, Zhou M, Li X, Peng F, et al: LncRNA AFAP1-AS1 promotes
growth and metastasis of cholangiocarcinoma cells. Oncotarget.
8:58394–58404. 2017.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Wan M, Zhang FM, Li ZL, Kang PC, Jiang PM,
Wang YM, Wang ZD, Zhong XY, Li CL, Wang H, et al: Identifying
survival-associated ceRNA clusters in cholangiocarcinoma. Oncol
Rep. 36:1542–1550. 2016.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Tan X, Huang Z and Li X: Long non-coding
RNA MALAT1 interacted with miR-204 to modulates human hilar
cholangiocarcinoma proliferation, migration and invasion by
targeting CXCR4. J Cell Biochem. 118:3643–3653. 2017.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Ma SL, Li AJ, Hu ZY, Shang FS and Wu MC:
Coexpression of the carbamoylphosphate synthase 1 gene and its long
noncoding RNA correlates with poor prognosis of patients with
intrahepatic cholangiocarcinoma. Mol Med Rep. 12:7915–7926.
2015.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Parasramka M, Yan IK, Wang X, Nguyen P,
Matsuda A, Maji S, Foye C, Asmann Y and Patel T: BAP1 dependent
expression of long non-coding RNA NEAT-1 contributes to sensitivity
to gemcitabine in cholangiocarcinoma. Mol Cancer.
16(22)2017.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Davaadorj M, Saito Y, Morine Y, Ikemoto T,
Imura S, Takasu C, Yamada S, Hiroki T, Yoshikawa M and Shimada M:
Loss of secreted frizzled-related protein-1 expression is
associated with poor prognosis in intrahepatic cholangiocarcinoma.
Eur J Surg Oncol. 43:344–350. 2017.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Davaadorj M, Imura S, Saito YU, Morine Y,
Ikemoto T, Yamada S, Takasu C, Hiroki T, Yoshikawa M and Shimada M:
Loss of SFRP1 expression is associated with poor prognosis in
hepatocellular carcinoma. Anticancer Res. 36:659–664.
2016.PubMed/NCBI
|
|
87
|
Khoontawad J, Pairojkul C, Rucksaken R,
Pinlaor P, Wongkham C, Yongvanit P, Pugkhem A, Jones A, Plieskatt
J, Potriquet J, et al: Differential protein expression marks the
transition from infection with Opisthorchis viverrini to
cholangiocarcinoma. Mol Cell Proteomics. 16:911–923.
2017.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Sempoux C, Jibara G, Ward SC, Fan C, Qin
L, Roayaie S, Fiel MI, Schwartz M and Thung SN: Intrahepatic
cholangiocarcinoma: New insights in pathology. Semin Liver Dis.
31:49–60. 2011.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Lok T, Chen L, Lin F and Wang HL:
Immunohistochemical distinction between intrahepatic
cholangiocarcinoma and pancreatic ductal adenocarcinoma. Hum
Pathol. 45:394–400. 2014.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Kanzawa M, Sanuki T, Onodera M, Fujikura
K, Itoh T and Zen Y: Double immunostaining for maspin and p53 on
cell blocks increases the diagnostic value of biliary brushing
cytology. Pathol Int. 67:91–98. 2017.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Zen Y, Britton D, Mitra V, Pike I, Sarker
D, Itoh T, Heaton N and Quaglia A: Tubulin β-III: A novel
immunohistochemical marker for intrahepatic peripheral
cholangiocarcinoma. Histopathology. 65:784–792. 2014.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Mustafa MZ, Nguyen VH, Le Naour F, De
Martin E, Beleoken E, Guettier C, Johanet C, Samuel D,
Duclos-Vallee JC and Ballot E: Autoantibody signatures defined by
serological proteome analysis in sera from patients with
cholangiocarcinoma. J Transl Med. 14(17)2016.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Rucksaken R, Pairojkul C, Pinlaor P,
Khuntikeo N, Roytrakul S, Selmi C and Pinlaor S: Plasma
autoantibodies against heat shock protein 70, enolase 1 and
ribonuclease/angiogenin inhibitor 1 as potential biomarkers for
cholangiocarcinoma. PLoS One. 9(e103259)2014.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Le Faouder J, Laouirem S, Alexandrov T,
Ben-Harzallah S, Léger T, Albuquerque M, Bedossa P and Paradis V:
Tumoral heterogeneity of hepatic cholangiocarcinomas revealed by
MALDI imaging mass spectrometry. Proteomics. 14:965–972.
2014.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Maeda S, Morikawa T, Takadate T, Suzuki T,
Minowa T, Hanagata N, Onogawa T, Motoi F, Nishimura T and Unno M:
Mass spectrometry-based proteomic analysis of formalin-fixed
paraffin-embedded extrahepatic cholangiocarcinoma. J Hepatobiliary
Pancreat Sci. 22:683–691. 2015.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Stephenson B, Shimwell N, Humphreys E,
Ward D, Adams D, Martin A and Afford S: Quantitative assessment of
the cell surface proteome to identify novel therapeutic targets in
cholangiocarcinoma. Lancet. ١ (Suppl 385)(S94)2015.PubMed/NCBI View Article : Google Scholar
|
|
97
|
Janvilisri T, Leelawat K, Roytrakul S,
Paemanee A and Tohtong R: Novel serum biomarkers to differentiate
cholangiocarcinoma from benign biliary tract diseases using a
proteomic approach. Dis Markers. 2015(105358)2015.PubMed/NCBI View Article : Google Scholar
|
|
98
|
Adisakwattana P, Suwandittakul N, Petmitr
S, Wongkham S, Sangvanich P and Reamtong O: ALCAM is a novel
cytoplasmic membrane protein in TNF-α stimulated invasive
cholangiocarcinoma cells. Asian Pac J Cancer Prev. 16:3849–3856.
2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Wasuworawong K, Roytrakul S, Paemanee A,
Jindapornprasert K and Komyod W: Comparative proteomic analysis of
human cholangiocarcinoma cell lines: S100A2 as a potential
candidate protein inducer of invasion. Dis Markers.
2015(629367)2015.PubMed/NCBI View Article : Google Scholar
|
|
100
|
Haonon O, Rucksaken R, Pinlaor P,
Pairojkul C, Chamgramol Y, Intuyod K, Onsurathum S, Khuntikeo N and
Pinlaor S: Upregulation of 14-3-3 eta in chronic liver fluke
infection is a potential diagnostic marker of cholangiocarcinoma.
Proteomics Clin Appl. 10:248–256. 2016.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Seol MA, Chu IS, Lee MJ, Yu GR, Cui XD,
Cho BH, Ahn EK, Leem SH, Kim IH and Kim DG: Genome-wide expression
patterns associated with oncogenesis and sarcomatous
transdifferentation of cholangiocarcinoma. BMC Cancer.
11(78)2011.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Yang XW, Li L, Hou GJ, Yan XZ, Xu QG, Chen
L, Zhang BH and Shen F: STAT3 overexpression promotes metastasis in
intrahepatic cholangiocarcinoma and correlates negatively with
surgical outcome. Oncotarget. 8:7710–7721. 2017.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Braconi C, Swenson E, Kogure T, Huang N
and Patel T: Targeting the IL-6 dependent phenotype can identify
novel therapies for cholangiocarcinoma. PLoS One.
5(e15195)2010.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Yoo CB and Jones PA: Epigenetic therapy of
cancer: Past, present and future. Nat Rev Drug Discov. 5:37–50.
2006.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Beisler JA: Isolation, characterization,
and properties of a labile hydrolysis product of the antitumor
nucleoside, 5-azacytidine. J Med Chem. 21:204–208. 1978.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Cheng JC, Weisenberger DJ, Gonzales FA,
Liang G, Xu GL, Hu YG, Marquez VE and Jones PA: Continuous
zebularine treatment effectively sustains demethylation in human
bladder cancer cells. Mol Cell Biol. 24:1270–1278. 2004.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Marquez VE, Barchi JJ Jr, Kelley JA, Rao
KV, Agbaria R, Ben-Kasus T, Cheng JC, Yoo CB and Jones PA:
Zebularine: A unique molecule for an epigenetically based strategy
in cancer chemotherapy. The magic of its chemistry and biology.
Nucleosides Nucleotides Nucleic Acids. 24:305–318. 2005.PubMed/NCBI
|
|
108
|
Nakamura K, Nakabayashi K, Htet Aung K,
Aizawa K, Hori N, Yamauchi J, Hata K and Tanoue A: DNA
methyltransferase inhibitor zebularine induces human
cholangiocarcinoma cell death through alteration of DNA methylation
status. PLoS One. 10(e0120545)2015.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kelly WK and Marks PA: Drug insight:
Histone deacetylase inhibitors-development of the new targeted
anticancer agent suberoylanilide hydroxamic acid. Nat Clin Pract
Oncol. 2:150–157. 2005.PubMed/NCBI View Article : Google Scholar
|
|
110
|
Sharma S, Kelly TK and Jones PA:
Epigenetics in cancer. Carcinogenesis. 31:27–36. 2010.PubMed/NCBI View Article : Google Scholar
|
|
111
|
Sriraksa R and Limpaiboon T: Histone
deacetylases and their inhibitors as potential therapeutic drugs
for cholangiocarcinoma-cell line findings. Asian Pac J Cancer Prev.
14:2503–2508. 2013.PubMed/NCBI View Article : Google Scholar
|
|
112
|
Nakagawa S, Sakamoto Y, Okabe H, Hayashi
H, Hashimoto D, Yokoyama N, Tokunaga R, Sakamoto K, Kuroki H, Mima
K, et al: Epigenetic therapy with the histone methyltransferase
EZH2 inhibitor 3-deazaneplanocin A inhibits the growth of
cholangiocarcinoma cells. Oncol Rep. 31:983–988. 2014.PubMed/NCBI View Article : Google Scholar
|
|
113
|
Gores GJ: Early detection and treatment of
cholangiocarcinoma. Liver Transpl. 6 (6 Suppl 2):S30–S34.
2000.PubMed/NCBI View Article : Google Scholar
|
|
114
|
Nakaoka T, Saito Y and Saito H: Aberrant
DNA methylation as a biomarker and a therapeutic target of
cholangiocarcinoma. Int J Mol Sci. 18(E1111)2017.PubMed/NCBI View Article : Google Scholar
|
|
115
|
Jusakul A, Cutcutache I, Yong CH, Lim JQ,
Huang MN, Padmanabhan N, Nellore V, Kongpetch S, Ng AWT, Ng LM, et
al: Whole-genome and epigenomic landscapes of etiologically
distinct subtypes of cholangiocarcinoma. Cancer Discov.
7:1116–1135. 2017.PubMed/NCBI View Article : Google Scholar
|
|
116
|
Ettel M, Eze O and Xu R: Clinical and
biological significance of precursor lesions of intrahepatic
cholangiocarcinoma. World J Hepatol. 7:2563–2570. 2015.PubMed/NCBI View Article : Google Scholar
|
|
117
|
Fujimoto A, Furuta M, Shiraishi Y, Gotoh
K, Kawakami Y, Arihiro K, Nakamura T, Ueno M, Ariizumi S, Nguyen
HH, et al: Whole-genome mutational landscape of liver cancers
displaying biliary phenotype reveals hepatitis impact and molecular
diversity. Nat Commun. 6(6120)2015.PubMed/NCBI View Article : Google Scholar
|
|
118
|
Komuta M, Govaere O, Vandecaveye V, Akiba
J, Van Steenbergen W, Verslype C, Laleman W, Pirenne J, Aerts R,
Yano H, et al: Histological diversity in cholangiocellular
carcinoma reflects the different cholangiocyte phenotypes.
Hepatology. 55:1876–1888. 2012.PubMed/NCBI View Article : Google Scholar
|
|
119
|
Rizvi S and Gores GJ: Pathogenesis,
diagnosis, and management of cholangiocarcinoma. Gastroenterology.
145:1215–1229. 2013.PubMed/NCBI View Article : Google Scholar
|