Introduction
Small double-stranded RNAs [synthetic small
interfering RNAs (siRNAs)] induce selective gene silencing by RNA
interference. siRNAs induce the sequence-specific degradation of
mRNAs with homologous sequences following the formation of an
RNA-induced silencing complex (RISC) when introduced into a cell
(1). siRNAs are designed to
specifically target a particular mRNA for degradation, resulting in
the effective knockdown of the expression of the corresponding
protein. Therefore, siRNA therapeutics are considered to be a
promising technology with which to treat various diseases by
inhibiting the production of target proteins (2,3).
However, siRNAs cannot diffuse passively into cells, as the cell
membrane is a negatively charged lipid bilayer consisting of
phospholipids, and siRNAs are also negatively charged due to the
phosphate backbone. In addition, the half-life of siRNAs in serum
is only approximately 20 min (4).
Therefore, for the clinical applications of siRNAs, systemic siRNA
therapy is hampered by barriers, such as low cellular uptake and
enzymatic instability of siRNAs in the blood circulation (5).
The effectiveness of siRNA therapy relies on an
siRNA delivery system that can protect siRNAs from serum nucleases
and deliver siRNAs efficiently into cells in a target tissue
(6). siRNAs can be delivered to
cells and tissues using siRNA carriers, such as cationic liposomes
and cationic polymers (6,7). In particular, cationic liposomes
have been widely investigated for siRNA delivery (8). However, for systemic siRNA delivery
with cationic liposomes, siRNA/cationic liposome complexes
(cationic lipoplexes) must be stabilized in the blood by avoiding
their interaction with blood components, such as erythrocytes
(9). Polyethylene glycol (PEG)
modification (PEGylation) on the surface of cationic lipoplexes can
protect lipoplexes from interaction with blood components and
macrophage capture, reduce protein absorption, and consequently
prolong retention in the blood circulation (10). However, the PEG coating of
cationic lipoplexes generally inhibits cellular association and/or
fusion with endosomal membranes, thus decreasing the gene silencing
or transfection efficiency, which is known as the PEG dilemma
(10,11). Lee and Ahn reported that the 2.5
mol% PEGylation of
3β[N-(N',N'-dimethylaminoethane)-carbamoyl]
cholesterol
(DC-Chol)/1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
(DOPE) lipoplexes exhibited gene silencing activity at tumor sites
following systemic injection (12). However, Zhang et al
demonstrated that DC-Chol/DOPE lipoplexes did not exhibit any siRNA
silencing activity by PEGylation with 1-5 mol%
PEG2000-DSPE (13).
Therefore, for the development of PEGylated cationic lipoplexes
without the loss of the gene silencing activity by
PEG-modification, an optimal amount of PEG-lipid must be included
in liposomal formulation.
Previously, we reported that 1 mol% PEGylated
cationic lipoplexes composed of cholesteryl
(2-((2-hydroxyethyl)amino)ethyl)carbamate (OH-C-Chol)/DOPE
significantly exerted gene silencing effects both in vitro
and in vivo, although they prevented cationic
lipoplex-induced agglutination with erythrocytes (14). In addition, the systemic injection
of 1 mol% PEGylated cationic lipoplexes (AtuFECT01) composed of
β-L-arginyl-2,3-L-diaminopropionic
acid-N-palmityl-N-oleyl-amide trihydrochloride as a
cationic lipid was shown to efficiently deliver siRNAs to the lung
endothelium and to suppress the expression of a target gene
(15,16). Therefore, in this study, in order
to examine the effects of cationic lipid in 1 mol% PEGylated
cationic liposomes on gene silencing effects following the systemic
injection of PEGylated cationic lipoplexes, we selected 4 types of
cationic cholesterol derivatives and 3 types of dialkyl or trialkyl
cationic lipids, and prepared 7 types of 1 mol% PEGylated cationic
liposomes composed of cationic lipid and DOPE for the evaluation of
siRNA biodistribution and the in vivo gene silencing effects
following intravenous injection. It was found that the siRNA
biodistribution and the in vivo knockdown efficiency were
strongly affected by the type of cationic lipid in PEGylated
cationic liposomes.
Materials and methods
Materials
N-(2-(2-Hydroxyethylamino)ethyl)cholesteryl-3-carboxamide
(OH-Chol) and OH-C-Chol were synthesized as previously described
(17). Cholesteryl
(3-((2-hydroxyethyl)amino)propyl)carbamate hydroiodide (HAPC-Chol)
and cholesteryl (3-((2-hydroxyethyl)(methyl)amino)propyl)carbamate
hydroiodide (MHAPC-Chol), were also synthesized as previously
described (18).
1,2-Dioleoyl-3-trimethyl-ammonium-propane methyl sulfate salt
(DOTAP) was obtained from Avanti Polar Lipids, Inc. (Alabaster, AL,
USA).
N,N-Dimethyl-N-octade-cyloctadecan-1-aminium
bromide [DC-1-18, also known as dimethyldioctadecylammonium bromide
(DDAB)] and 11-((1,3-bis
(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide (TC-1-12) were obtained from Sogo Pharmaceutical Co., Ltd.
(Tokyo, Japan). The lipid names of the DC- and TC-series are
product names of Sogo Pharmaceutical Co., Ltd. DOPE and
N-(methyl-poly-oxyethylene
oxycarbonyl)-1,2-distearoyl-sn-glycero-3-phosphoethanolamine,
sodium salt (PEG2000-DSPE, SUNBRIGHT®
DSPE-020CN) were obtained from NOF Co. Ltd. (Tokyo, Japan).
siRNAs
siRNAs targeting nucleotides of Firefly luciferase
(Luc siRNA), non-silencing siRNA [control (Cont) siRNA] as a
negative control for Luc siRNA, apolipoprotein B siRNA (ApoB
siRNA), luciferase siRNA (Cont siRNA) as a negative control for
ApoB siRNA, cyanine 5.5 (Cy5.5)-labeled pGL3 luciferase siRNA
(Cy5.5-siRNA) were synthesized by Sigma Genosys (Tokyo, Japan).
Mouse Tie2 siRNA and luciferase siRNA (Cont siRNA) as a negative
control for Tie2 siRNA were synthesized by Japan Bio Services Co.,
Ltd. (Saitama, Japan). The siRNA sequences of the Luc siRNA were as
reported previously (19).
Cy5.5-siRNA and Cont siRNA as a negative control for Luc siRNA were
as reported previously (20).
ApoB siRNA and Cont siRNA as a negative control for ApoB siRNA were
conjugated with cholesterol at the 3'-end of the sense strand, and
the siRNA sequences were as reported previously (21). Tie2 siRNA and Cont siRNA as a
negative control for Tie2 siRNA were blunt-ended, alternating
2'-O-methyl-modified siRNA, and the siRNA sequences were as
reported previously (16, 22).
Preparation of PEGylated cationic
liposomes and lipoplexes
The cationic cholesterol derivative-based liposomes
were prepared from OH-Chol/DOPE (composition designated as LP-OH),
OH-C-Chol/DOPE (composition designated as LP-OH-C), HAPC-Chol/DOPE
(composition designated as LP-HAPC) and MHAPC-Chol/DOPE
(composition designated as LP-MHAPC), at a molar ratio of 3:2.
Cationic liposomes, including dialkyl or trialkyl cationic lipids
were prepared from DOTAP/DOPE (composition designated as LP-DOTAP),
DC-1-18/DOPE (composition designated as LP-DC-1-18), and
TC-1-12/DOPE (composition designated as LP-TC-1-12), at a molar
ratio of 1:1. PEGylated cationic liposomes were incorporated with 1
mol% PEG2000-DSPE into each liposomal formulation.
For the preparation of cationic liposomes and
PEGylated cationic liposomes using a thin-film hydration method,
cationic lipid, DOPE and PEG2000-DSPE were dissolved in
chloroform, and chloroform was evaporated under vacuum in a rotary
evaporator at 60˚C to obtain a thin film. The thin film was
hydrated with water at 60˚C by vortex mixing. The liposomes were
sonicated in a bath-type sonicator (Bransonic®
2510J-MTH, 100W; Branson UL Trasonics Co., CT, USA) for 5-10 min at
room temperature.
To prepare cationic liposome/siRNA complexes
(lipoplexes), each cationic liposome was added to 50 pmol siRNA at
a charge ratio (+:-) of 7:1 for cationic liposomes composed of
cationic cholesterol derivatives and DOPE (17) or 4:1 for cationic liposomes
composed of dialkyl or trialkyl cationic lipids and DOPE (23) with vortex-mixing for 10 sec and
left at room temperature for 15 min. The charge ratio (+:-) of
liposomes:siRNA is expressed as the molar ratio of cationic lipid
to siRNA phosphate.
Size and ζ-potential of PEGylated
cationic liposomes and lipoplexes
The particle size distributions of non-PEGylated and
PEGylated cationic liposomes and lipoplexes were measured by the
cumulant method using a light-scattering photometer (ELS-Z2; Otsuka
Electronics Co., Ltd., Osaka, Japan) at 25˚C after diluting the
dispersion to an appropriate volume with water. The ζ-potentials
were measured using electrophoresis light-scattering methods with
the ELS-Z2 at 25˚C after diluting the dispersion with an
appropriate volume of water.
Cell culture
Human breast cancer MCF-7-Luc cells stably
expressing Firefly luciferase by the transfection of plasmid pcDNA3
containing the Firefly luciferase (hLuc) gene from plasmid
psiCHECK2 (Promega, Madison, WI, USA) were donated by Dr Kenji
Yamato (University of Tsukuba, Tsukuba, Japan). MCF-7-Luc cells
were grown in RPMI-1640 medium, supplemented with 10%
heat-inactivated fetal bovine serum (FBS) and 1.2 mg/ml G418 at
37˚C in a 5% CO2 humidified atmosphere.
Gene silencing effect by PEGylated
cationic lipoplexes in cultured cells
MCF-7-Luc cells were seeded in 6-well culture plate
at a density of 3x105 cells per well 24 h prior to
transfection. Non-PEGylated and PEGylated cationic lipoplexes were
formed by the addition of non-PEGylated and PEGylated cationic
liposomes, respectively, into 50 pmol Cont siRNA or Luc siRNA at
the indicated charge ratios (+:-) with vortex-mixing for 10 sec,
and left at room temperature for 15 min. For transfection, each
cationic lipoplex was diluted in 1 ml of medium supplemented with
10% FBS and the mixture was then added to the cells (50 pmol
siRNA/well). At 48 h following transfection, the luciferase
activity was measured as counts per sec (cps)/µg protein using the
luciferase assay system (Pica Gene; Toyo Ink Mfg. Co. Ltd., Tokyo,
Japan) and BCA reagent (Pierce, Rockford, IL, USA), as reported
previously (17). Luciferase
activity (%) was calculated as relative to the luciferase activity
(cps/µg protein) of untransfected cells.
Cytotoxicity by PEGylated cationic
lipoplex
MCF-7-Luc cells were seeded in 96-well plates 24 h
prior to transfection. Each cationic lipoplex with 50 pmol Cont
siRNA was diluted in 1 ml of medium supplemented with 10% FBS, and
the mixture (100 µl) was then added to the cells at 50% confluency
in the well (final 50 nM siRNA concentration). Following a 24-h
incubation period, cell numbers were determined using a Cell
Counting kit-8 (Dojindo Laboratories, Kumamoto, Japan). Cell
viability was expressed as relative to the absorbance at 450 nm of
untransfected cells.
Agglutination assay
One female BALB/c mouse (weighing 18-20 g, 8 weeks
of age; Sankyo Labo Service Corp., Tokyo, Japan) was housed in a
temperature- (24˚C) and humidity- (55%) controlled room with a 12 h
light/dark cycle (lights on at 8:00 a.m.) with ad libitum
access to food and water. Blood (0.5 ml) was collected from the
carotid artery of the mouse while under anesthesia by an
intraperitoneal injection of 50 mg/kg body weight of pentobarbital
(Nembutal; Dainippon Pharmaceutical Co., Ltd., Osaka, Japan).
Erythrocytes were collected from the whole blood at 4˚C by
centrifugation at 300 x g for 3 min and resuspended in PBS as a 2%
(v/v) suspension of erythrocytes. Non-PEGylated and PEGylated
cationic lipoplexes with 2 µg siRNA were added to 100 µl of 2%
(v/v) erythrocyte suspension. Following incubation for 15 min at
37˚C, the sample was placed on a glass plate and agglutination was
observed using an ECLIPSE TS100-F microscope (Nikon, Tokyo,
Japan).
Biodistribution of siRNA following the
intravenous injection of PEGylated cationic lipoplex to mice
All animal experiments were conducted in accordance
with the ‘Guide for the Care and Use of Laboratory Animals’ adopted
by the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan) (which is accredited by the Ministry of
Education, Culture, Sports, Science, and Technology, Japan).
Ethical approval for this study was obtained from the Institutional
Animal Care and Use Committee of Hoshi University (Permission no.
29-049).
Non-PEGylated and PEGylated cationic lipoplexes were
formed by the addition of non-PEGylated and PEGylated cationic
liposomes, respectively, into 20 µg of Cy5.5-siRNA with
vortex-mixing for 10 sec and left at room temperature for 15 min.
The non-PEGylated or PEGylated cationic lipoplexes with 20 µg of
Cy5.5-siRNA were administered intravenously via the lateral tail
vein into a total of 14 female BALB/c mice (weighing 18-20 g, 8
weeks of age; Sankyo Labo Service Corp.) (n=1 for each lipoplex).
At 1 h post-injection, the mice were sacrificed. Tissues were
frozen on dry ice and cut into 16-µm-thick slices. The localization
of Cy5.5-siRNA was examined using an Eclipse TS100-F
microscope.
For the observation of the biodistribution of siRNA
following the injection of PEGylated DOTAP/cholesterol lipoplexes,
PEGylated cationic liposomes were prepared by inclusion of 1, 2, 3
and 5 mol% PEG2000-DSPE into the formulations of
DOTAP/cholesterol liposomes (molar ratio of 1:1). The PEGylated
cationic lipoplexes with 20 µg of Cy5.5-siRNA were administered
intravenously via the lateral tail vein into a total of 4 female
BALB/c mice (weighing 18-20 g, 8 weeks of age; Sankyo Labo Service
Corp.) (n=1 for each lipoplex). At 1 h after the injection, the
mice were sacrificed, and Cy5.5 fluorescent imaging of the tissues
was performed using a NightOWL LB981 NC100 system (Berthold
Technologies, Bad Wildbad, Germany). In Cy5.5 fluorescent imaging,
the excitation and emission filters were set at 630/20 and 680/30
nm, respectively. The exposure time for fluorescence was 5 sec. A
grayscale body-surface reference image was collected using a
NightOWL LB981 CCD camera (Berthold Technologies). The images were
analyzed using IndiGo2 software (version 2.0.1.0; Berthold
Technologies) provided with the in vivo imaging system. The
tissues after fluorescent imaging were frozen on dry ice and sliced
at 16 µm. The localization of Cy5.5-siRNA was examined using an
Eclipse TS100-F microscope.
ApoB mRNA levels in the liver
following the intravenous injection of PEGylated cationic lipoplex
into mice
PEGylated cationic lipoplexes were formed by the
addition of PEGylated cationic liposomes into 50 µg Cont siRNA or
ApoB siRNA with vortex-mixing for 10 sec, and left at room
temperature for 15 min. The PEGylated cationic lipoplexes were
administered intravenously the via the lateral tail vein into
female BALB/c mice (8 weeks of age) (n=4 for each lipoplex).
For the expression level of ApoB mRNA in the liver,
the livers were excised from the mice at 48 h following the
injection of PEGylated cationic lipoplexes, and total RNA was then
isolated using Isogen II (Nippon Gene Co., Ltd., Tokyo, Japan).
cDNA was synthesized from total RNA of the liver and then
first-strand cDNA was synthesized from 2 µg of total RNA using
PrimeScript RTase (Takara Bio, Inc., Otsu, Japan). Reverse
transcription-quantitative PCR (RT-qPCR) was performed using a
Roche Light Cycler 96 system (Roche Diagnostics Ltd., Basel,
Switzerland) and TaqMan Gene expression assay (Apob: Mm01545150_m1,
gapdh: Mm99999915_g1; Applied Biosystems®, CA, USA). The
thermocycling conditions consisted of an initial denaturation at
95˚C for 600 sec, and 45 cycles of denaturation at 95˚C for 10 sec,
and primer annealing and extension at 60˚C for 30 sec (two step
amplification). The expression level of ApoB mRNA was normalized
using the amount of glyceraldehyde-3-phosphate dehydrogenase
(GAPDH) mRNA in the same sample, and analyzed using the comparative
Cq (2-ΔΔCq) method (24).
Determination of transaminase
activities in serum
PEGylated cationic lipoplexes were formed by the
addition of PEGylated cationic liposomes into 50 µg Cont siRNA or
ApoB siRNA with vortex-mixing for 10 sec and left at room
temperature for 15 min. PEGylated cationic lipoplexes were
administered intravenously via the lateral tail vein into female
BALB/c mice (8 weeks of age) (n=4 for each lipoplex). To measure
aspartate aminotransferase (AST/GOT) activity, serum was separated
from coagulated whole blood at 48 h after the injection. GOT levels
in the serum were determined using commercially available test
reagents (Transaminase CII-test kit; Wako Pure Chemicals, Osaka,
Japan). Normal values were determined using blood obtained from
age-matched, untreated female mice (n=4).
Tie2 mRNA levels in the lung following
the intravenous injection of PEGylated cationic lipoplexes into
mice
PEGylated cationic lipoplexes were formed by the
addition of PEGylated cationic liposomes into 50 µg Cont siRNA or
Tie2 siRNA with vortex-mixing for 10 sec and left at room
temperature for 15 min. The PEGylated cationic lipoplexes were
administered intravenously via the lateral tail vein into female
BALB/c mice (8 weeks of age) (n=3-4 for each lipoplex). For the
expression level of Tie2 mRNA in the lungs, the lungs were excised
from the mice at 48 h after the injection of PEGylated cationic
lipoplexes, and total RNA was then isolated using Isogen II. cDNA
was synthesized from total RNA of the lung, and RT-qPCR was
performed using a Roche Light Cycler 96 system and TaqMan Gene
expression assays [Tek (Tie-2): Mm00443243_m1, phosphatase and
tensin homolog (PTEN): Mm00477208_m1; Applied
Biosystems®]. The thermocycling conditions consisted of
an initial denaturation at 95˚C for 600 sec, and 45 cycles of
denaturation at 95˚C for 10 sec, and primer annealing and extension
at 60˚C for 30 sec (two step amplification). The expression levels
of Tie2 mRNA were normalized using the amount of PTEN mRNA in the
same sample as reported previously (16) and analyzed using the comparative
Cq (2-ΔΔCq) method.
Statistical analysis
The statistical significance of differences between
mean values was determined by a Student's t-test using GraphPad
Prism 4.0 (GraphPad Software Inc., La Jolla, CA, USA). A P-value
≤0.05 was considered to indicate a statistically significant
difference.
Results and Discussion
Characterization of cationic liposomes
and lipoplexes
Firstly, we examined whether the cationic lipid type
in cationic liposomes affected in vitro gene silencing.
Herein, we used OH-Chol, OH-C-Chol, HAPC-Chol and MHAPC-Chol as
cationic cholesterol derivatives; DOTAP and DC-1-18 as dialkyl
cationic lipids; and TC-1-12 as a trialkyl cationic lipid for the
preparation of cationic liposomes (Fig. 1). We have previously reported that
siRNA lipoplexes composed of OH-Chol/DOPE, OH-C-Chol/DOPE,
HAPC-Chol/DOPE, MHAPC-Chol/DOPE, DOTAP/DOPE, DC-1-18/DOPE and
TC-1-12/DOPE can strongly suppress the expression of a target gene
in MCF-7 cells (25). For
cationic liposomes with cationic cholesterol derivatives, LP-OH,
LP-OH-C, LP-HAPC and LP-MHAPC, were prepared from OH-Chol/DOPE,
OH-C-Chol/DOPE, HAPC-Chol/DOPE and MHAPC-Chol/DOPE, respectively,
at a molar ratio of 3:2 (Table I)
(25). By contrast, for cationic
liposomes with dialkyl or trialkyl cationic lipids, LP-DOTAP,
LP-DC-1-18, and LP-TC-1-12 were prepared from DOTAP/DOPE,
DC-1-18/DOPE and TC-1-12/DOPE, respectively, at a molar ratio of
1:1 (Table I) (25).
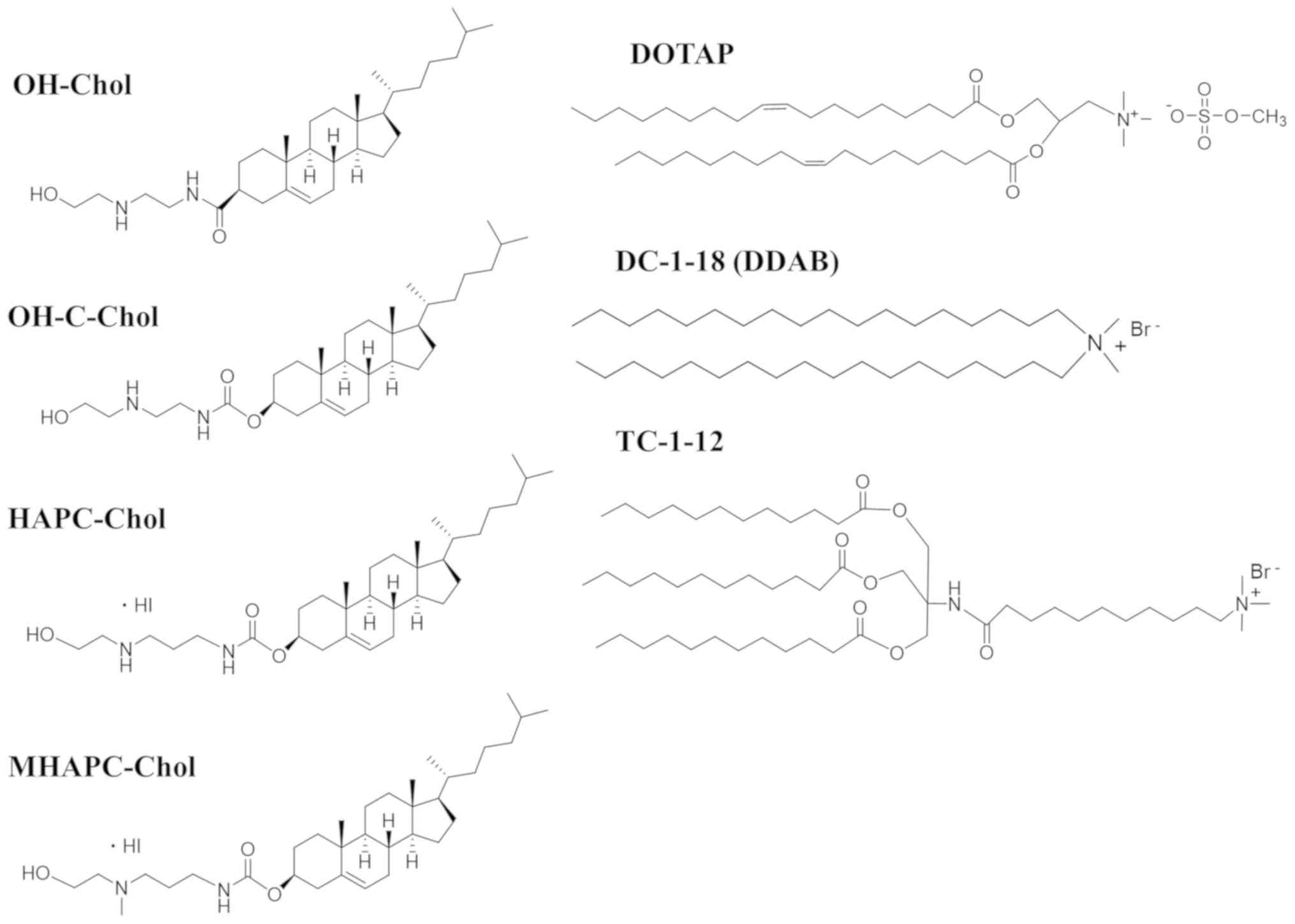 | Figure 1Structure of cationic cholesterol
derivatives and cationic lipids with dialkyl or trialkyl chains:
OH-Chol,
N-(2-(2-hydroxyethylamino)ethyl)cholesteryl-3-carboxamide;
OH-C-Chol, cholesteryl (2-((2-hydroxyethyl)amino)ethyl)carbamate;
HAPC-Chol, cholesteryl (3-((2-hydroxyethyl)amino)propyl)carbamate
hydroiodide; MHAPC-Chol, cholesteryl
(3-((2-hydroxyethyl)(methyl)amino)propyl)carbamate hydroiodide;
DOTAP, 1,2-dioleoyl-3-trimethylammonium-propane methyl sulfate
salt; DC-1-18,
N,N-dimethyl-N-octadecyloctadecan-1-aminium
bromide (DDAB; dimethyldioctadecylammonium bromide); TC-1-12,
11-((1,3-bis(dodecanoyloxy)-2-((dodecanoyloxy)methyl)propan-2-yl)amino)-N,N,N-trimethyl-11-oxoundecan-1-aminium
bromide. |
 | Table IParticle size and ζ-potential of
cationic liposomes and siRNA lipoplexes. |
Table I
Particle size and ζ-potential of
cationic liposomes and siRNA lipoplexes.
| | | Liposomes | Lipoplexes |
|---|
| Liposome | Formulation | Sizea (nm) |
ζ-potentiala (mV) | Charge ratio
(+:-) | Sizea (nm) |
ζ-potentiala (mV) |
|---|
| LP-OH | OH-Chol/DOPE | 86.3±6.5 | 51.1±0.4 | 1:1 | 105.8±1.6 | -42.2±1.8 |
| | | | | 3:1 | Aggregation | N.D. |
| | | | | 5:1 | Aggregation | N.D. |
| | | | | 7:1 | 150.5±6.3 | 42.6±0.5 |
| LP-OH-C | OH-C-Chol/DOPE | 82.6±1.0 | 49.7±1.4 | 1:1 | 139.4±1.1 | -38.7±2.7 |
| | | | | 3:1 | Aggregation | N.D. |
| | | | | 5:1 | Aggregation | N.D. |
| | | | | 7:1 | 112.3±1.1 | 41.4±2.9 |
| LP-HAPC | HAPC-Chol/DOPE | 99.5±2.6 | 41.7±0.7 | 1:1 | 156.0±0.9 | -24.9±1.3 |
| | | | | 3:1 | Aggregation | N.D. |
| | | | | 5:1 | 196.6±5.0 | 34.1±0.8 |
| | | | | 7:1 | 171.1±6.4 | 34.5±0.5 |
| LP-MHAPC |
MHAPC-Chol/DOPE | 115.7±1.4 | 55.4±0.5 | 1:1 | Aggregation | N.D. |
| | | | | 3:1 | 234.0±8.1 | 32.1±1.1 |
| | | | | 5:1 | 222.7±12.1 | 38.1±0.9 |
| | | | | 7:1 | 120.3±0.3 | 41.8±4.0 |
| LP-DOTAP | DOTAP/DOPE | 86.8±1.0 | 51.5±1.8 | 2:1 | 170.5±1.9 | 37.7±1.0 |
| | | | | 4:1 | 171.2±1.1 | 42.0±0.3 |
| | | | | 6:1 | 154.0±4.5 | 40.9±0.7 |
| LP-DC-1-18 | DC-1-18/DOPE | 86.6±1.8 | 52.8±0.4 | 2:1 | 179.5±6.7 | 35.8±0.3 |
| | | | | 4:1 | 154.5±3.1 | 45.9±2.6 |
| | | | | 6:1 | 111.8±0.7 | 45.1±1.6 |
| LP-TC-1-12 | TC-1-12/DOPE | 99.0±3.4 | 51.8±1.3 | 2:1 | 162.0±3.9 | 40.2±0.2 |
| | | | | 4:1 | 137.5±0.2 | 42.8±1.6 |
| | | | | 6:1 | 110.8±2.6 | 40.7±0.9 |
The sizes of the cationic liposomes were
approximately 80-120 nm, and the ζ-potentials were approximately
+42-55 mV (Table I). For the
preparation of cationic lipoplexes, cationic liposomes were mixed
with siRNA at charge ratios (+:-) of 1:1, 3:1, 5:1 and 7:1 for
LP-OH, LP-OH-C, LP-HAPC and LP-MHAPC, and 2:1, 4:1 and 6:1 for
LP-DOTAP, LP-DC-1-18 and LP-TC-1-12, respectively. As shown in
Table I, LP-OH and LP-OH-C
lipoplexes aggregated when they mixed with siRNA at charge ratios
(+:-) of 3:1-5:1. By contrast, LP-HAPC and LP-MHAPC lipoplexes
aggregated when they mixed with siRNA at charge ratios (+:-) of
approximately 3:1 and 1:1, respectively. These results suggested
that the cationic charge on the surface of the cationic lipoplexes
may be neutralized by the addition of siRNA, resulting in the
instability of the lipoplexes. By contrast, the LP-DOTAP,
LP-DC-1-18 and LP-TC-1-12 lipoplexes exhibited a positive charge in
ζ-potential beyond a charge ratio (+:-) of 2:1, and did not
aggregate by the addition of siRNA at any charge ratios (+:-).
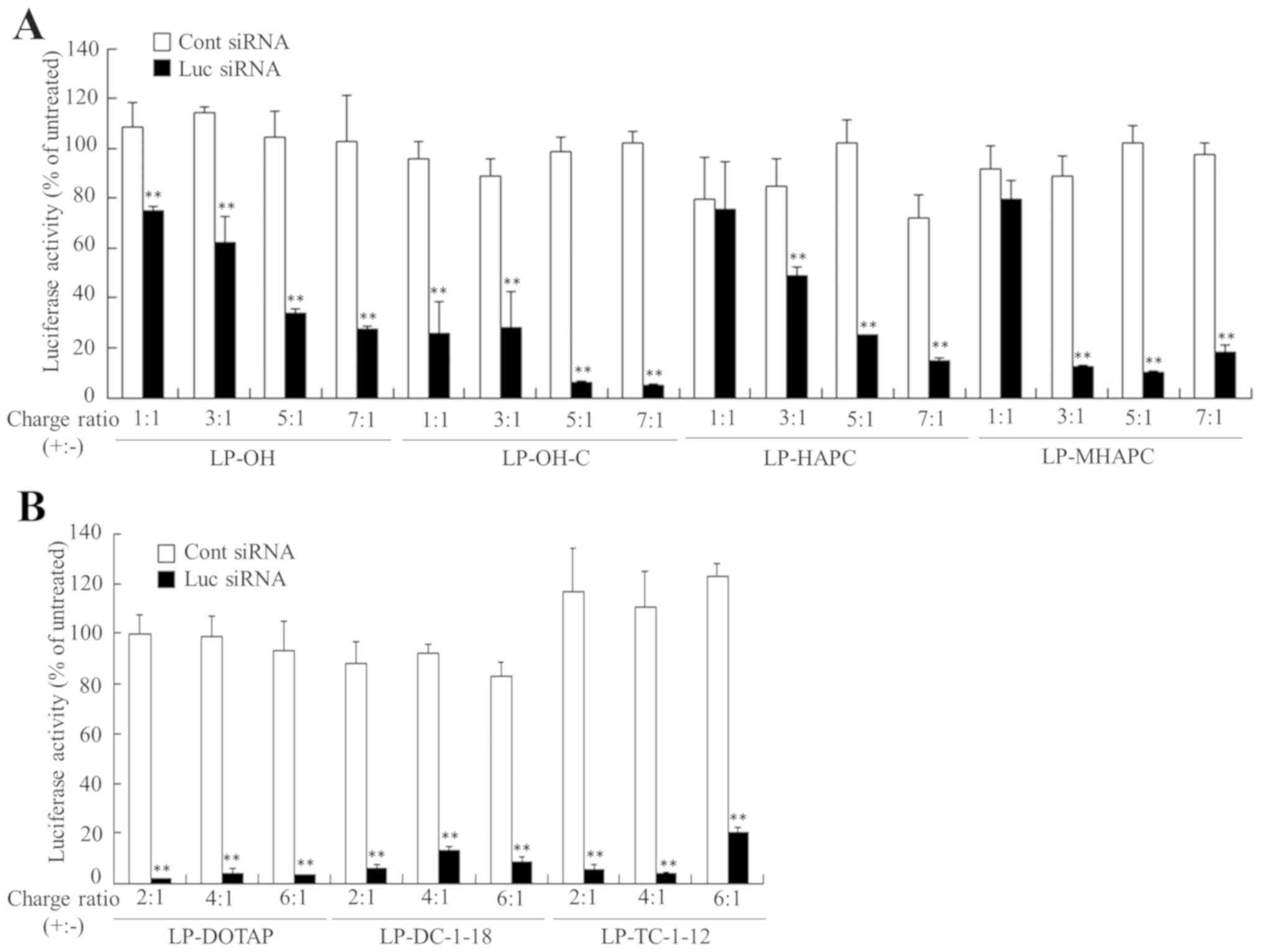
Effect of charge ratio (+:-) of
cationic lipoplexes on in vitro gene knockdown efficiency
To examine the effects of the charge ratio (+:-) of
cationic lipoplexes on gene knockdown, MCF-7-Luc cells were
incubated with cationic lipoplexes at a final concentration of 50
nM siRNA, and the gene-silencing effect was then assessed by
assaying the luciferase activity. LP-OH-C, LP-DOTAP, LP-DC-1-18 and
LP-TC-1-12 lipoplexes with Luc siRNA strongly suppressed luciferase
activity at any of the charge ratios (+:-) tested (Fig. 2). By contrast, the LP-OH, LP-HAPC
and LP-MHAPC lipoplexes with Luc siRNA increased the suppression
level of luciferase activity with an increase in the charge ratio
(+:-), and exhibited potent suppression of luciferase activity at
charge ratios (+:-) of approximately 5:1-7:1 (Fig. 2A). From the results of lipoplex
size (Table I) and the gene
silencing efficacy by Luc siRNA (Fig.
2), in subsequent experiments, we decided to use cationic
lipoplexes formed at charge ratios (+:-) of 7:1 for cationic
liposomes composed of cationic cholesterol derivatives and 4:1 for
those of dialkyl or trialkyl cationic lipids. Previously, we
examined the association of siRNA with each cationic liposomes
using an exclusion assay with SYBR®-Green I, and
confirmed that in all the cationic liposomes, the fluorescence of
SYBR®-Green I was markedly decreased by the addition of
cationic liposomes into siRNA solution at the above-mentioned
charge ratios (+:-) (25).
Characterization of PEGylated cationic
liposomes and lipoplexes
We have previously reported that PEGylated LP-OH-C
lipoplexes modified with 1 mol% PEG2000-DSPE exerted
portent in vitro gene silencing effects in MCF-7 cells, as
well as non-PEGylated LP-OH-C lipoplexes, and the injection of
PEGylated LP-OH-C lipoplexes with siRNA suppressed the expression
of a target gene in the liver (14). Furthermore, we found that the
PEGylation of LP-OH-C lipoplexes with 2-3 mol%
PEG2000-DSPE decreased the in vitro gene
silencing effect (unpublished data). Therefore, in this study, we
prepared PEGylated liposomes including 1 mol%
PEG2000-DSPE into the formulations of cationic
liposomes. LP-OH-PEG, LP-OH-C-PEG, LP-HAPC-PEG, LP-MHAPC-PEG,
LP-DOTAP-PEG, LP-DC-1-18-PEG and LP-TC-1-12-PEG included 1 mol%
PEG2000-DSPE in the formulations of LP-OH, LP-OH-C,
LP-HAPC, LP-MHAPC, LP-DOTAP, LP-DC-1-18 and LP-TC-1-12,
respectively. The sizes of the PEGylated cationic liposomes were
approximately 90-120 nm, and the ζ-potentials were approximately
+40-47 mV (Table II). When the
liposomes were mixed with siRNA, the lipoplex sizes were
approximately 120-170 nm and their ζ-potentials were approximately
+32-40 mV (Table II).
 | Table IIParticle size and ζ-potential of 1
mol% PEGylated cationic liposomes and siRNA lipoplexes. |
Table II
Particle size and ζ-potential of 1
mol% PEGylated cationic liposomes and siRNA lipoplexes.
| | | Liposomes |
Lipoplexesb |
|---|
| Liposome | Formulation | Sizea (nm) |
ζ-potentiala (mV) | Sizea (nm) |
ζ-potentiala (mV) |
|---|
| LP-OH-PEG |
OH-Chol/DOPE/PEG2000-DSPE | 91.8±1.5 | 39.9±1.6 | 166.9±1.8 | 38.0±2.2 |
| LP-OH-C-PEG |
OH-C-Chol/DOPE/PEG2000-DSPE | 89.6±0.1 | 45.1±1.5 | 121.3±1.1 | 38.9±5.7 |
| LP-HAPC-PEG |
HAPC-Chol/DOPE/PEG2000-DSPE | 99.5±1.1 | 39.7±1.0 | 150.3±0.8 | 31.5±0.1 |
| LP-MHAPC-PEG |
MHAPC-Chol/DOPE/PEG2000-DSPE | 96.9±1.0 | 46.5±0.6 | 160.0±1.4 | 36.8±1.4 |
| LP-DOTAP-PEG |
DOTAP/DOPE/PEG2000-DSPE | 122.5±1.0 | 40.2±0.5 | 172.3±2.0 | 37.8±0.4 |
| LP-DC-1-18-PEG |
DC-1-18/DOPE/PEG2000-DSPE | 109.6±1.1 | 46.5±4.1 | 163.1±1.2 | 40.3±1.2 |
| LP-TC-1-12-PEG |
TC-1-12/DOPE/PEG2000-DSPE | 101.9±0.9 | 45.5±0.4 | 123.0±0.5 | 31.8±0.4 |
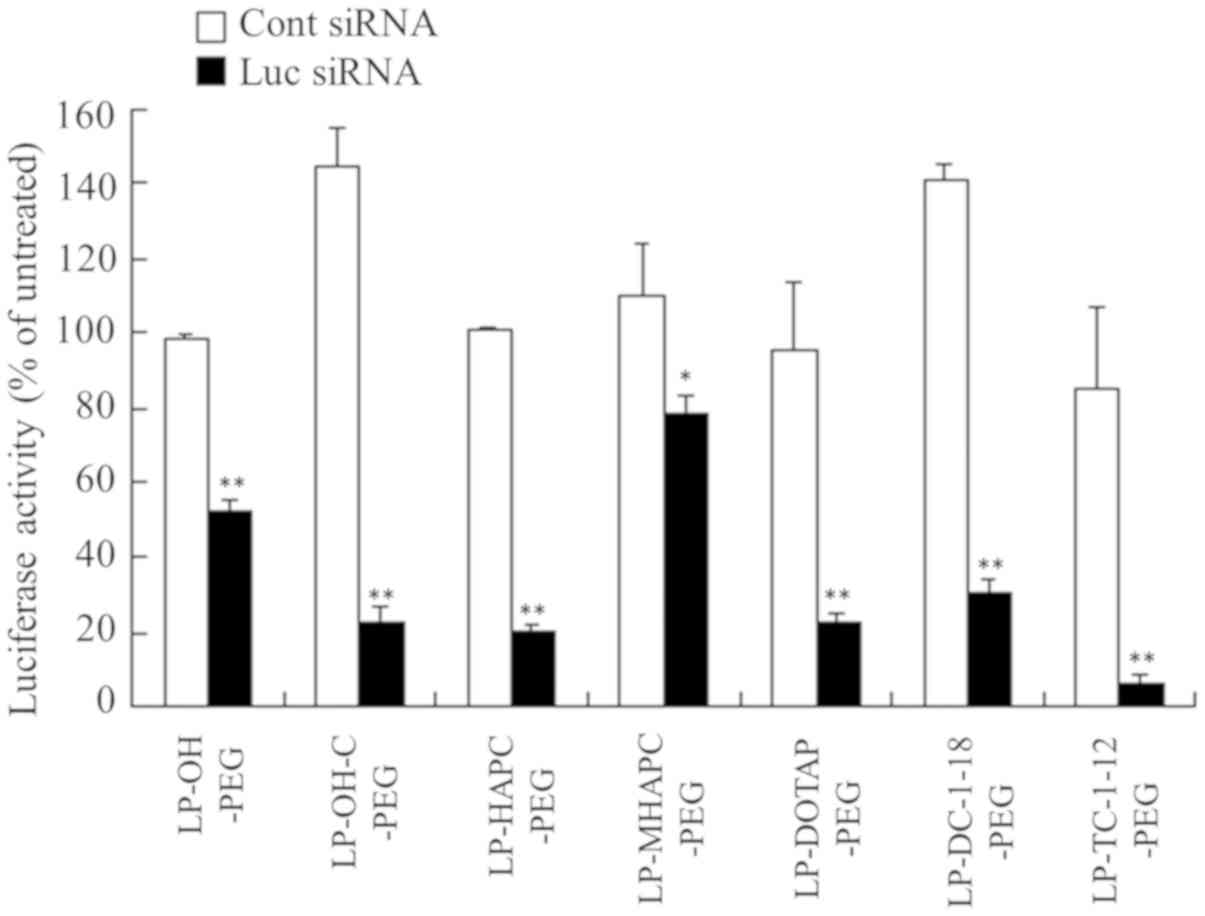
Effect of PEGylation of cationic
liposomes on in vitro gene knockdown efficiency
To examine the effects of the PEGylation of cationic
liposomes on gene knockdown by PEGylated cationic lipoplexes with
siRNA, MCF-7-Luc cells were incubated with PEGylated cationic
lipoplexes at a 50 nM final concentration of siRNA. As a result,
the LP-OH-C-PEG, LP-HAPC-PEG, LP-DOTAP-PEG, LP-DC-1-18-PEG and
LP-TC-1-12-PEG lipoplexes with Luc siRNA strongly suppressed
luciferase activity (>70% knockdown, compared with Cont siRNA)
(Fig. 3), suggesting that the
PEGylation did not largely affect the gene silencing effect of the
LP-OH-C, LP-HAPC, LP-DOTAP, LP-DC-1-18 and LP-TC-1-12 lipoplexes.
By contrast, the LP-OH-PEG and LP-MHAPC-PEG lipoplexes decreased
the gene silencing activity by 1 mol% PEGylation (47 and 28%
knockdown, respectively, compared with Cont siRNA) (Fig. 3). These results indicated that the
inhibition of the gene silencing effect by PEGylation was affected
by cationic lipid type in the PEGylated cationic liposomes.
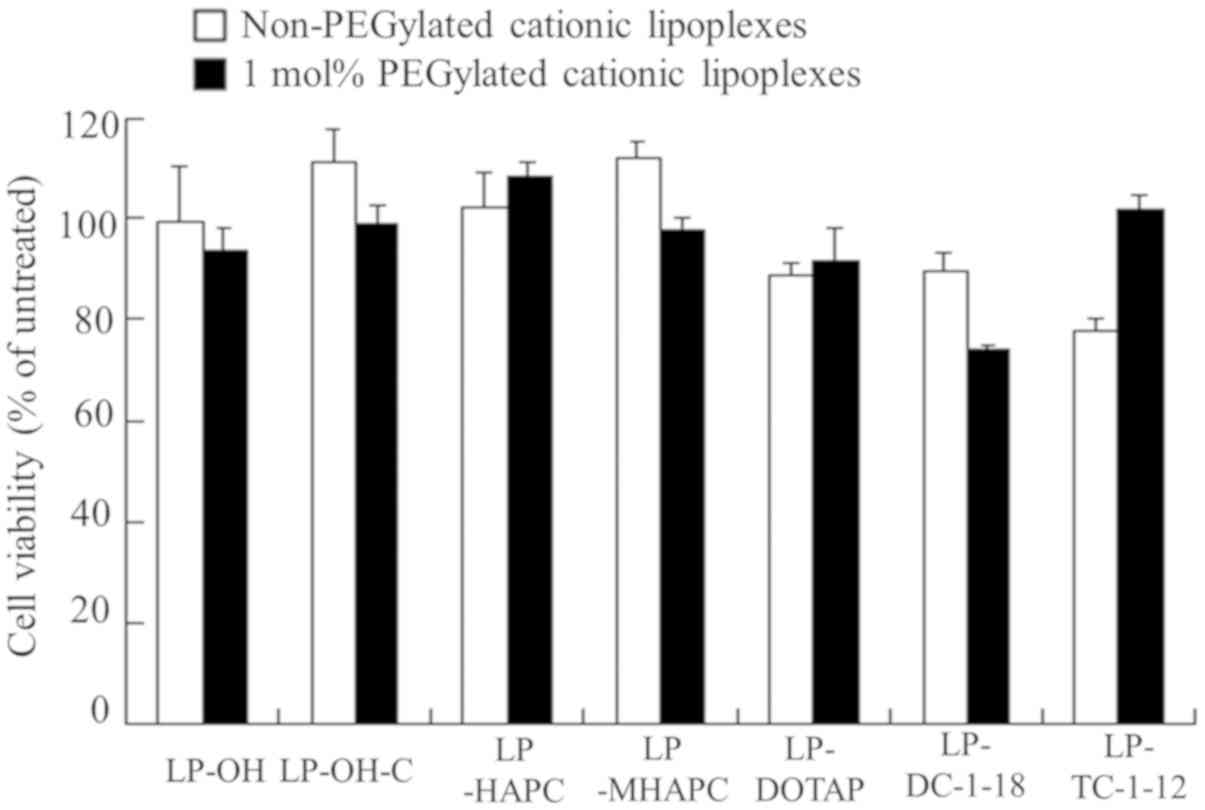
Cytotoxicity by PEGylated cationic
lipoplexes
To examine the effects of the type of cationic lipid
on cytotoxicity of non-PEGylated and PEGylated cationic lipoplexes,
we investigated cell viabilities at 24 h following transfection in
the MCF-7 cells with non-PEGylated and PEGylated cationic
lipoplexes. The LP-DC-1-18-PEG and non-PEGylated LP-TC-1-12
lipoplexes exhibited slight cytotoxicity (74 and 78%, respectively,
in cell viability); however, the other lipoplexes did not exhibit
cytotoxicity (Fig. 4).
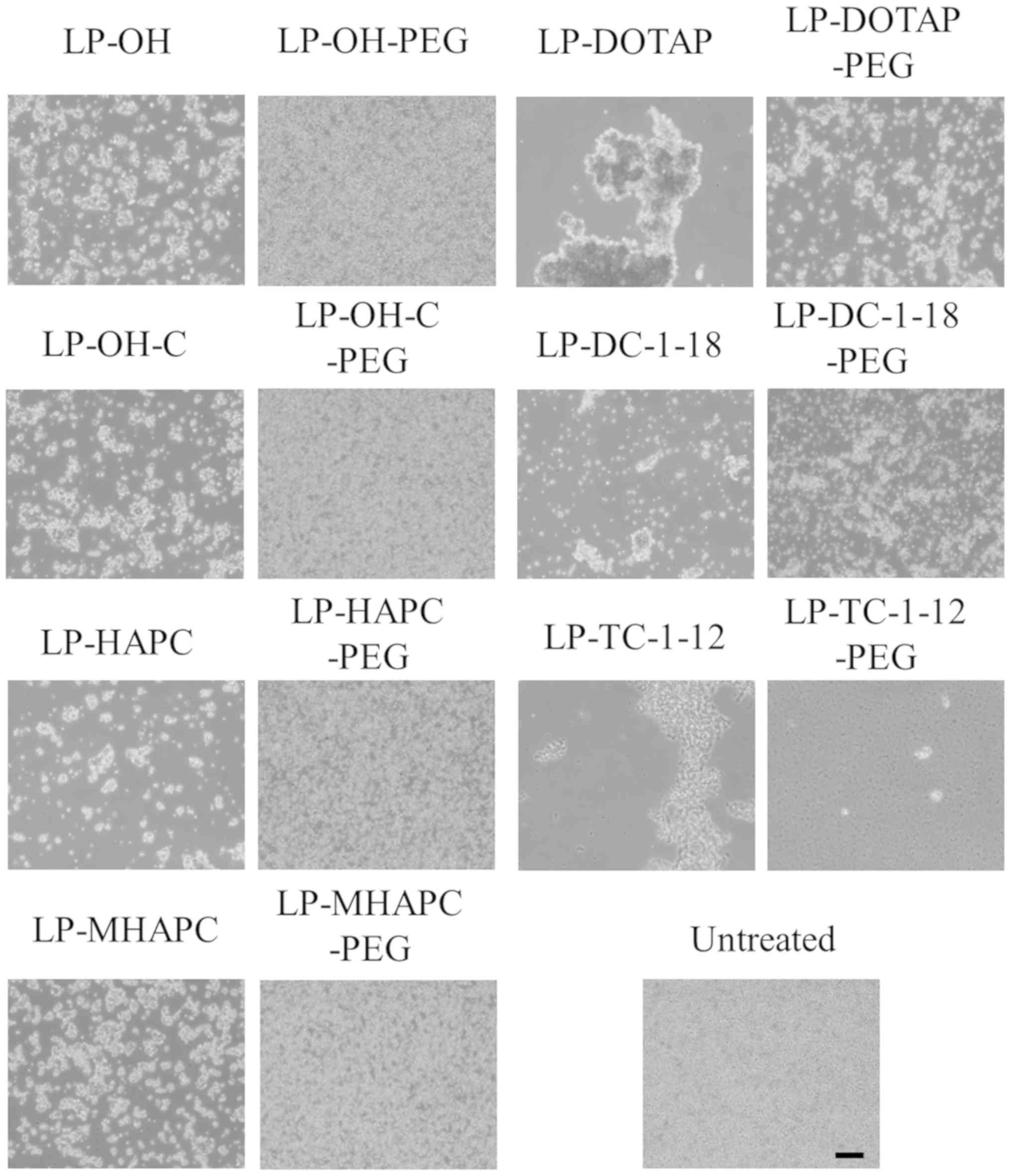
Interaction with erythrocytes and
PEGylated cationic lipoplexes
To prevent the aggregation of cationic lipoplexes
with blood components, such as erythrocytes following systemic
injection, the modification of the liposome surface with PEG has
generally been used. Previously, we reported that 1 mol% PEGylation
of LP-OH or LP-OH-C lipoplexes prevented cationic lipoplex-induced
agglutination (14). In this
study, to examine this effect, non-PEGylated and PEGylated cationic
lipoplexes were added into erythrocyte suspensions. Of the
non-PEGylated cationic liposomes, all the cationic lipoplexes
induced agglutination after mixing with the erythrocyte suspension,
regardless of the type of cationic lipid in the liposomal
formulation (Fig. 5). In
particular, the formation of large-sized aggregates was observed
after mixing LP-DOTAP or LP-TC-1-12 lipoplexes with erythrocyte
suspensions. In the PEGylated cationic liposomes with cationic
cholesterol derivatives, PEG on the surface of cationic lipoplexes
prevented agglutination with erythrocyte (Fig. 5). However, in the LP-DOTAP-PEG and
LP-DC-1-18-PEG lipoplexes, the formation of small-sized aggregates
was observed after the addition of their lipoplexes, indicating
that PEGylation could not completely prevent the interaction
between cationic lipoplexes and erythrocytes. By contrast, in the
LP-TC-1-12-PEG lipoplexes, decreased numbers of erythrocytes were
observed following the addition of LP-TC-1-12-PEG lipoplexes into
the erythrocyte suspension. LP-TC-1-12-PEG, with short-length
trialkyl chains (C12), might induce hemolysis by altering the
erythrocyte membrane (26). From
this result, LP-TC-1-12-PEG lipoplexes may not be suitable for
in vivo transfection.
Biodistribution of siRNA following the
intravenous injection of PEGylated cationic lipoplexes
To examine the effects of PEGylation of cationic
liposomes on the biodistribution of siRNA following the intravenous
injection of PEGylated cationic lipoplexes, we injected
non-PEGylated or PEGylated cationic lipoplexes with Cy5.5-siRNA
intravenously into mice and observed the biodistribution of siRNA
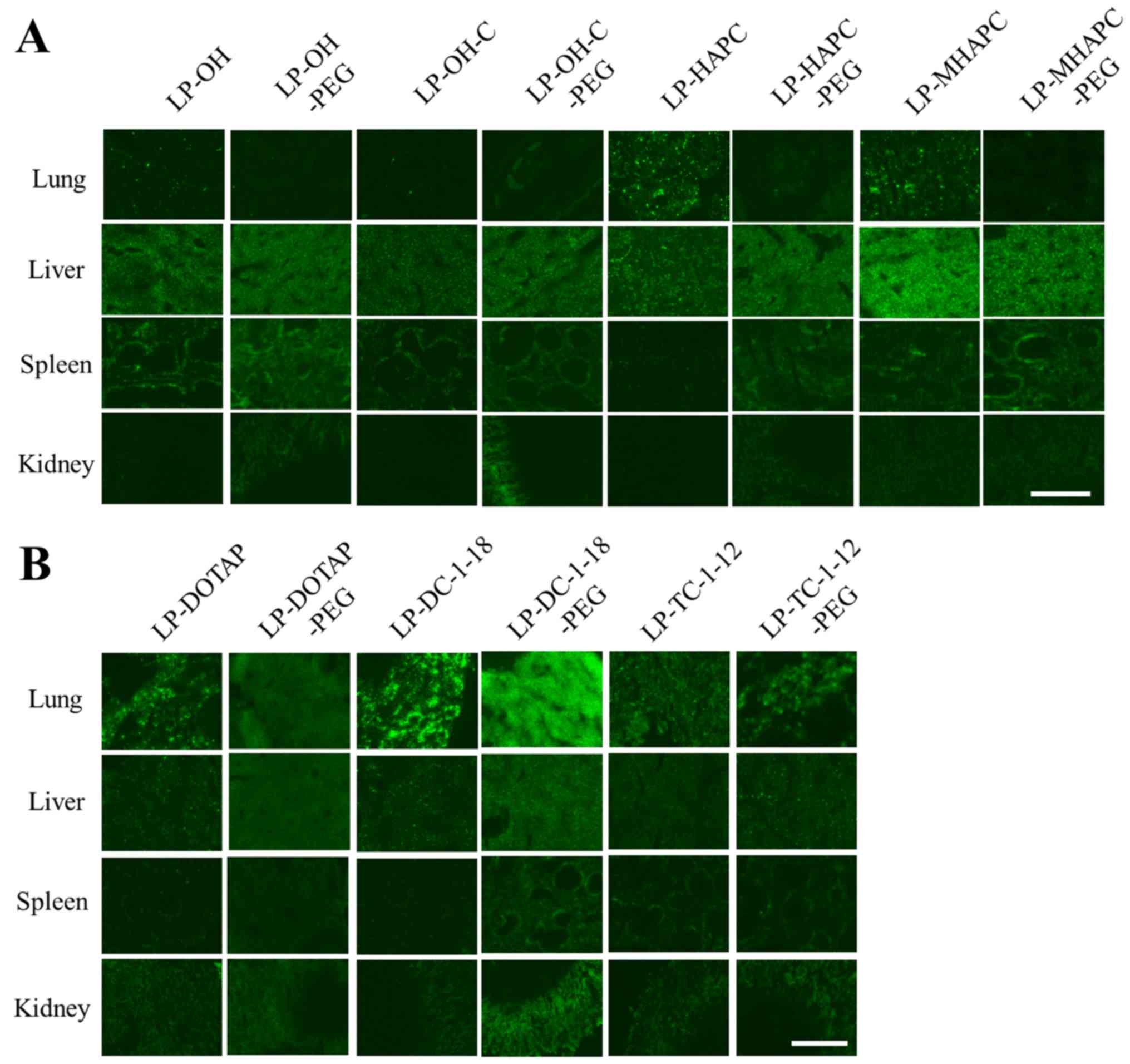
at 1 h after the injection. In non-PEGylated cationic lipoplexes,
the LP-HAPC, LP-DOTAP, LP-DC-1-18 and LP-TC-1-12 lipoplexes largely
accumulated mainly in the lungs (Fig.
6), as previously reported (25). By contrast, the LP-OH, LP-OH-C and
LP-MHAPC lipoplexes largely accumulated mainly in the liver
(25). We have previously
reported that differences in the hydrophobic anchor or the length
of the alkyl chains of cationic lipids strongly influenced the
biodistribution of siRNA after the injection of cationic lipoplexes
(25). The injection of cationic
lipoplexes with cationic cholesterol derivatives or dialkyl
cationic lipids with short dialkyl chains (C12-C14) tended to
induce siRNA accumulation in the liver, and those with long dialkyl
chains (C16-C18) or with short trialkyl chains (C12) induced siRNA
accumulation in the lungs. These findings indicated that siRNA
biodistribution after intravenous injection of cationic lipoplexes
was strongly affected by the type of cationic lipid in the cationic
liposomes. In the PEGylated cationic lipoplexes, the PEGylation of
LP-OH, LP-OH-C, LP-HAPC, and LP-MHAPC lipoplexes increased the
accumulation of siRNA in the liver (Fig. 6A). Blood components, such as
erythrocytes and fibronectin are bound to positively charged
lipoplexes in the blood circulation (27). The agglutinates of cationic
lipoplexes with erythrocytes contribute to the high entrapment of
lipoplexes in highly extended lung capillaries (28). These findings indicated that the
PEGylation of cationic lipoplexes could prevent agglutination with
erythrocytes in the blood circulation, and lead to an increase in
the accumulation of siRNA in the liver. However, in the
LP-DOTAP-PEG, LP-DC-1-18-PEG and LP-TC-1-12-PEG lipoplexes, siRNA
accumulated mainly in the lungs, suggesting that 1 mol% PEGylation
did not largely affect the siRNA biodistribution (Fig. 6B). In the LP-DOTAP-PEG and
LP-DC-1-18-PEG lipoplexes, small-sized aggregates of erythrocytes
(Fig. 5) may be entrapped in lung
capillaries.
In a preliminary experiment, for PEGylated cationic
liposomes with dialkyl cationic lipid, we prepared 1, 2, 3 and 5
mol% PEGylated cationic liposomes composed of DOTAP/cholesterol
(Chol). We found that 1 mol% PEGylation of cationic lipoplexes
prevented the agglutination of erythrocytes by cationic lipoplexes;
however, 5 mol% PEGylation was required for cationic liposomes to
prevent accumulation of siRNA in the lungs (Fig. S1). This
indicated that PEGylated cationic lipoplexes with 1-3 mol%
PEG2000-DSPE may accumulate in the lungs without
agglutination with erythrocytes. It has been previously reported
that 1 mol% PEGylated cationic lipoplexes (AtuFECT01) with
asymmetric dialkyl cationic lipids (C16 and C18) selectively target
the vasculature in the lungs after systemic injection into mice
(15). The PEGylated cationic
lipoplexes with dialkyl or trialkyl cationic lipids used in the
present study may induce the accumulation of siRNA in the lungs by
the direct interaction of the lipoplexes with pulmonary endothelial
cells.
Gene knockdown in liver following the
injection of PEGylated cationic lipoplexes in mice
To examine the effects of cationic lipid type in
PEGylated cationic liposomes on the gene silencing effect of siRNA
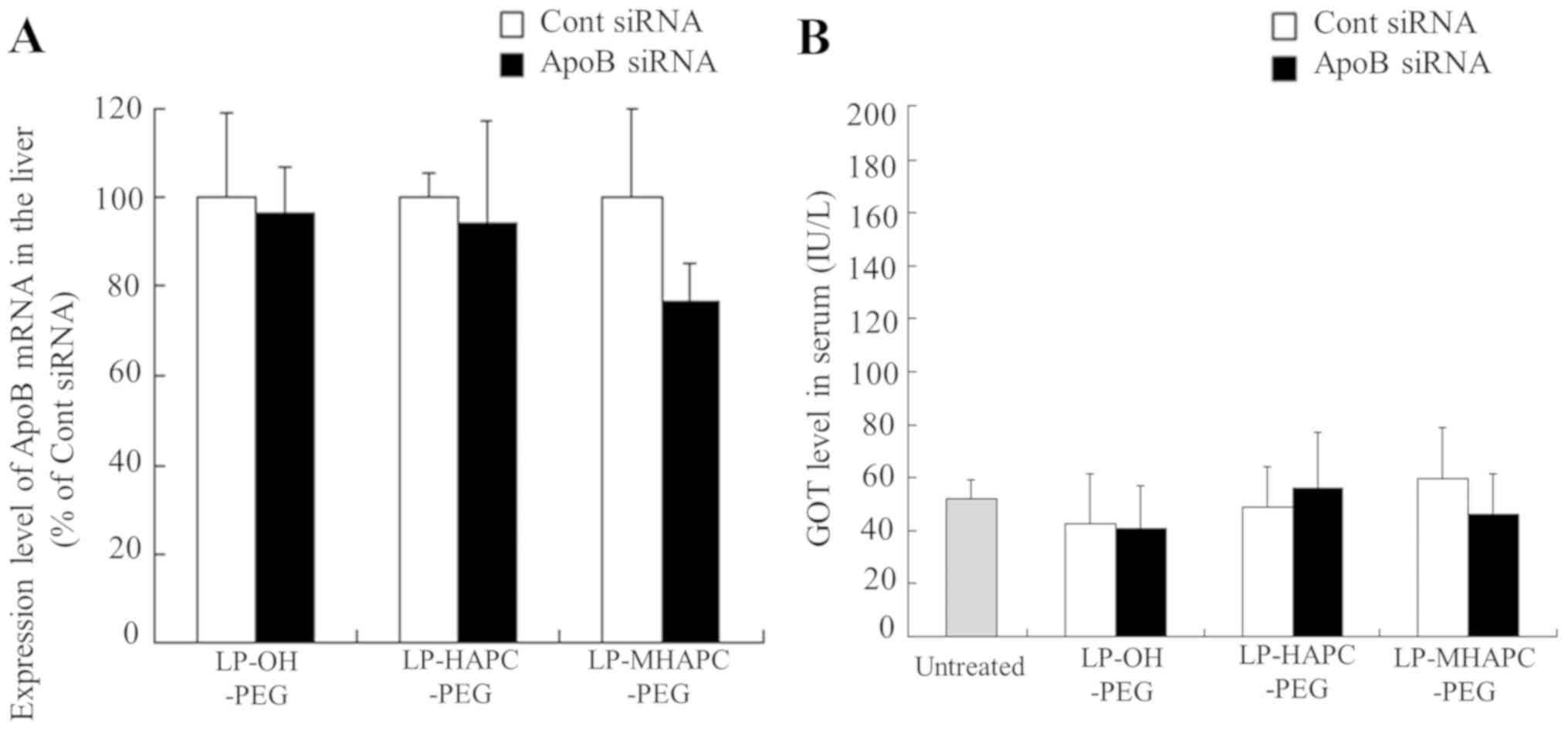
in mouse liver, we injected PEGylated cationic lipoplexes with ApoB
siRNA intravenously into mice to evaluate the knockdown efficiency
in the liver. The ApoB gene is a hepatocyte-expressed gene involved
in cholesterol transport. Among the PEGylated cationic lipoplexes,
PEGylated cationic lipoplexes with cationic cholesterol derivatives
induced the accumulation of siRNA in the liver after intravenous
injection (Fig. 6A). Previously,
we reported that LP-OH-C-PEG lipoplexes containing 1 mol%
PEG2000-DSPE significantly suppressed ApoB mRNA levels
in the liver, compared with Cont siRNA (approximately 59%
knockdown) (14). Therefore, in
this study, we evaluated the knockdown efficiency of ApoB mRNA in
the liver following the intravenous injection of LP-OH-PEG,
LP-HAPC-PEG and LP-MHAPC-PEG lipoplexes with ApoB siRNA. However,
the injection of these lipoplexes with ApoB siRNA did not affect
the ApoB mRNA levels in the liver (Fig. 7A). Furthermore, in order to
evaluate liver toxicity in mice, we assessed the GOT levels in
serum at 48 h after the injection of PEGylated cationic lipoplexes.
The injection of any of the tested lipoplexes did not significantly
elevate the GOT levels in serum (Fig.
7B), indicating that injection of PEGylated cationic lipoplexes
had no side-effects with regard to hepatotoxicity, regardless of
the type of cationic lipid in the liposomal formulation.
The injection of LP-OH-PEG, LP-HAPC-PEG and
LP-MHAPC-PEG lipoplexes with ApoB siRNA did not affect the ApoB
mRNA levels in the liver (Fig.
7A), although their lipoplexes accumulated mainly in the liver
(Fig. 6A). We speculated that PEG
may not be able to completely protect LP-OH-PEG, LP-HAPC-PEG and
LP-MHAPC-PEG lipoplexes from protein absorption in the blood
circulation, resulting in their capture by Kupffer cells in the
liver. However, the mechanisms through which LP-OH-C-PEG lipoplexes
suppress the expression of a target gene in the liver are unclear
(14). Further studies are
required to examine the effects of a cationic cholesterol
derivative in PEGylated cationic liposomes on the gene silencing
effect of siRNA in the liver following intravenous injection. From
the results in this and previous studies (14), LP-OH-C-PEG may have potential as a
gene vector for siRNA delivery into the liver.
Gene knockdown in the lungs following
the intravenous injection of PEGylated cationic lipoplexes
For evaluation of the gene silencing effect in mouse
lungs by PEGylated cationic lipoplexes, we injected PEGylated
cationic lipoplexes with Tie2 siRNA intravenously into mice. The
Tie2 gene has been used as a marker for vascular endothelium
(16). Among the PEGylated
cationic lipoplexes, PEGylated cationic lipoplexes with dialkyl or
trialkyl cationic lipid induced the accumulation of siRNA in the
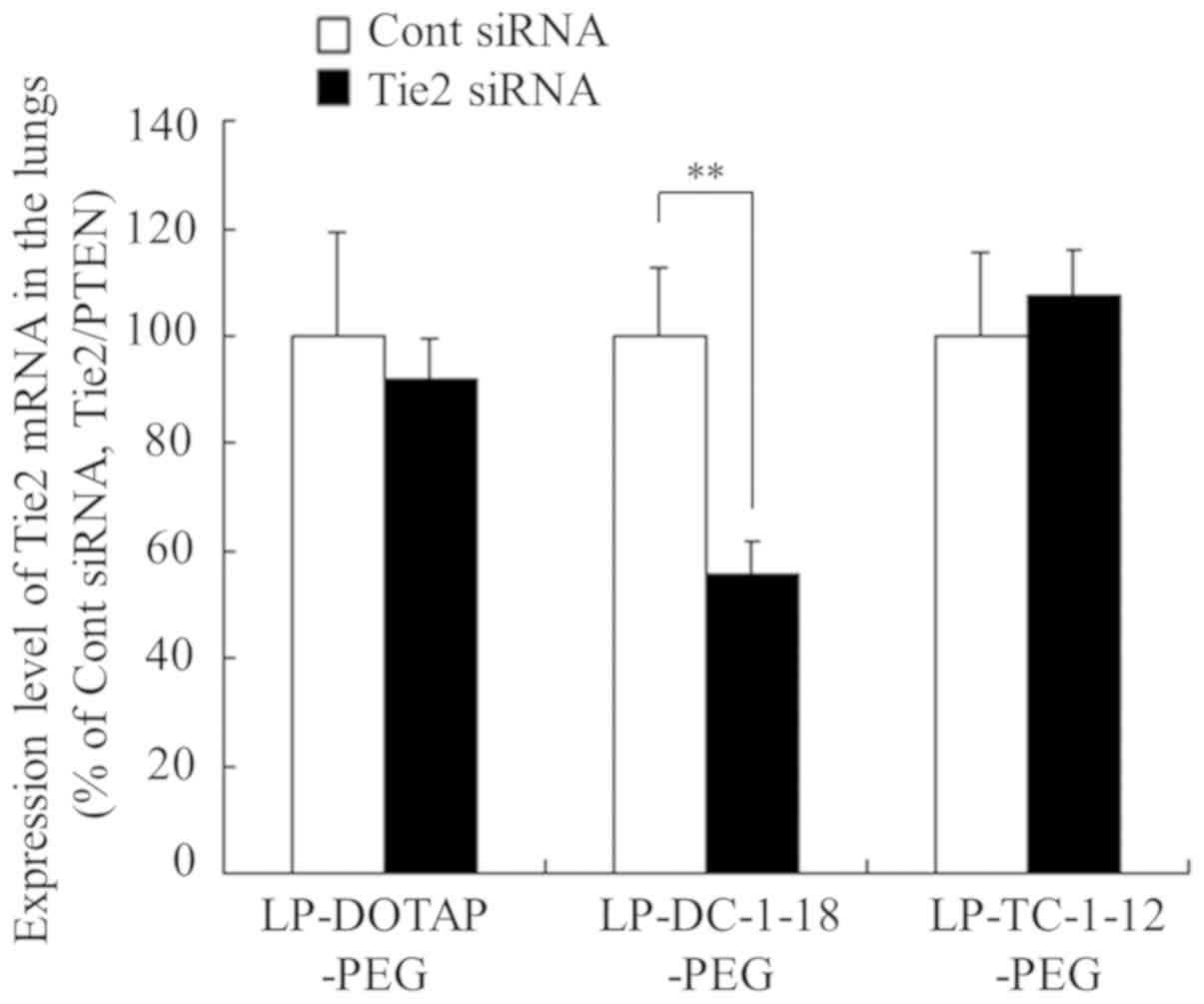
lungs following intravenous injection (Fig. 6B). Therefore, we used
LP-DOTAP-PEG, LP-DC-1-18-PEG and LP-TC-1-12-PEG for the evaluation
of the knockdown efficiency in the lungs. As a result, the
injection of LP-DC-1-18-PEG lipoplexes with Tie2 siRNA
significantly suppressed the Tie2 mRNA levels in the pulmonary
endothelium (approximately 45% knockdown), compared with Cont siRNA
(Fig. 8). By contrast, the
injection of LP-DOTAP-PEG and LP-TC-1-12-PEG lipoplexes with Tie2
siRNA did not affect the mRNA levels compared with Cont siRNA.
Previously, we reported that the injection of non-PEGylated
LP-DOTAP, LP-DC-1-18, and LP-TC-1-12 lipoplexes with Tie2 siRNA
suppressed the Tie2 mRNA levels in the pulmonary endothelium
(approximately 19, 74 and 60% knockdown, respectively), compared
with those with Cont siRNA (25).
LP-TC-1-12-PEG lipoplexes may abolish in vivo gene silencing
activity by the PEGylation of cationic liposomes. Among the
PEGylated cationic liposomes, LP-DC-1-18-PEG may have potential for
use as a gene vector for siRNA delivery into the lungs. From the
results of this study, the siRNA biodistribution and in vivo
knockdown efficiency following the intravenous injection of
PEGylated cationic lipoplexes were strongly affected by the type of
cationic lipid in PEGylated cationic liposomes. The selection of
cationic lipids in the liposomal formulations may be important for
the successful in vivo delivery of siRNA by PEGylated
cationic liposomes.
In conclusion, the PEGylation of cationic liposomes
generally lowers the efficiency of siRNA-mediated gene silencing
in vitro and in vivo. Therefore, in this study, we
evaluated whether 1 mol% PEGylation of cationic liposomes could
improve the stability of cationic lipoplexes following systemic
injection and suppress the expression of a target gene. From the
results of this study, the in vivo silencing effects by
PEGylated cationic lipoplexes did not closely correspond to the
in vitro silencing effects. The siRNA biodistribution and
in vivo knockdown efficiency following the intravenous
injection of PEGylated cationic lipoplexes were strongly affected
by the type of cationic lipid in PEGylated cationic liposomes. This
study provides valuable information about the PEGylation of
cationic lipoplex for efficient siRNA delivery in vivo.
Acknowledgements
The authors would like to thank Dr Kumi Kawano, Ms.
Yui Asami, Ms. Haruka Honma, Ms. Ruri Miura, Mr. Yuki Yoshida, Ms.
Mana Kubo, Ms. Hitomi Hasegawa, Ms. Ai Murata, Mr. Kodai Horiuchi
and Mr. Yuki Yoshiike (Department of Drug Delivery Research, Hoshi
University, Tokyo, Japan) for providing assistance with the
experimental work. The authors would also like to thank Professor
Hiroaki Ohno and Mr. Masamitsu Taguchi (Graduate School of
Pharmaceutical Sciences, Kyoto University, Kyoto, Japan) for
supplying OH-C-Chol.
Funding
This study was funded only by the resources of our
department.
Availability of data and materials
All data analyzed during this study are available
from the corresponding author on reasonable request.
Authors' contributions
YH conceived and designed the study. YH, MN, NT, KT,
KO and HO performed the experiments. YH wrote the manuscript. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in accordance
with the 'Guide for the Care and Use of Laboratory Animals' adopted
by the Institutional Animal Care and Use Committee of Hoshi
University (Tokyo, Japan) (which is accredited by the Ministry of
Education, Culture, Sports, Science, and Technology, Japan).
Ethical approval for this study was obtained from the Institutional
Animal Care and Use Committee of Hoshi University (Permission no.
29-049).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson RC and Doudna JA: Molecular
mechanisms of RNA interference. Annu Rev Biophys. 42:217–239.
2013.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Bumcrot D, Manoharan M, Koteliansky V and
Sah DW: RNAi therapeutics: A potential new class of pharmaceutical
drugs. Nat Chem Biol. 2:711–719. 2006.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Castanotto D and Rossi JJ: The promises
and pitfalls of RNA-interference-based therapeutics. Nature.
457:426–433. 2009.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Hickerson RP, Vlassov AV, Wang Q, Leake D,
Ilves H, Gonzalez-Gonzalez E, Contag CH, Johnston BH and Kaspar RL:
Stability study of unmodified siRNA and relevance to clinical use.
Oligonucleotides. 18:345–354. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wang J, Lu Z, Wientjes MG and Au JL:
Delivery of siRNA therapeutics: Barriers and carriers. AAPS J.
12:492–503. 2010.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen X, Mangala LS, Rodriguez-Aguayo C,
Kong X, Lopez-Berestein G and Sood AK: RNA interference-based
therapy and its delivery systems. Cancer Metastasis Rev.
37:107–124. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
de Fougerolles AR: Delivery vehicles for
small interfering RNA in vivo. Hum Gene Ther. 19:125–132.
2008.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Zhang S, Zhi D and Huang L: Lipid-based
vectors for siRNA delivery. J Drug Target. 20:724–735.
2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Satterlee A and Huang L: In vivo
gene delivery by nonviral vectors: Overcoming hurdles? Mol Ther.
20:1298–1304. 2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xia Y, Tian J and Chen X: Effect of
surface properties on liposomal siRNA delivery. Biomaterials.
79:56–68. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Hatakeyama H, Akita H and Harashima H: The
polyethyleneglycol dilemma: Advantage and disadvantage of
PEGylation of liposomes for systemic genes and nucleic acids
delivery to tumors. Biol Pharm Bull. 36:892–899. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Lee J and Ahn HJ: PEGylated DC-Chol/DOPE
cationic liposomes containing KSP siRNA as a systemic siRNA
delivery Carrier for ovarian cancer therapy. Biochem Biophys Res
Commun. 503:1716–1722. 2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zhang Y, Li H, Sun J, Gao J, Liu W, Li B,
Guo Y and Chen J: DC-Chol/DOPE cationic liposomes: A comparative
study of the influence factors on plasmid pDNA and siRNA gene
delivery. Int J Pharm. 390:198–207. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hattori Y, Machida Y, Honda M, Takeuchi N,
Yoshiike Y, Ohno H and Onishi H: Small interfering RNA delivery
into the liver by cationic cholesterol derivative-based liposomes.
J Liposome Res. 27:264–273. 2017.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Santel A, Aleku M, Keil O, Endruschat J,
Esche V, Fisch G, Dames S, Löffler K, Fechtner M, Arnold W, et al:
A novel siRNA-lipoplex technology for RNA interference in the mouse
vascular endothelium. Gene Ther. 13:1222–1234. 2006.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fehring V, Schaeper U, Ahrens K, Santel A,
Keil O, Eisermann M, Giese K and Kaufmann J: Delivery of
therapeutic siRNA to the lung endothelium via novel Lipoplex
formulation DACC. Mol Ther. 22:811–820. 2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Hattori Y, Hara E, Shingu Y, Minamiguchi
D, Nakamura A, Arai S, Ohno H, Kawano K, Fujii N and Yonemochi E:
siRNA delivery into tumor cells by cationic cholesterol
derivative-based nanoparticles and liposomes. Biol Pharm Bull.
38:30–38. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ding W, Hattori Y, Higashiyama K and
Maitani Y: Hydroxyethylated cationic cholesterol derivatives in
liposome vectors promote gene expression in the lung. Int J Pharm.
354:196–203. 2008.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hattori Y, Nakamura T, Ohno H, Fujii N and
Maitani Y: siRNA delivery into tumor cells by lipid-based
nanoparticles composed of hydroxyethylated cholesteryl triamine.
Int J Pharm. 443:221–229. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Hattori Y, Arai S, Kikuchi T, Ozaki KI,
Kawano K and Yonemochi E: Therapeutic effect for liver-metastasized
tumor by sequential intravenous injection of anionic polymer and
cationic lipoplex of siRNA. J Drug Target. 24:309–317.
2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Hattori Y, Arai S, Okamoto R, Hamada M,
Kawano K and Yonemochi E: Sequential intravenous injection of
anionic polymer and cationic lipoplex of siRNA could effectively
deliver siRNA to the liver. Int J Pharm. 476:289–298.
2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Aleku M, Schulz P, Keil O, Santel A,
Schaeper U, Dieckhoff B, Janke O, Endruschat J, Durieux B, Röder N,
et al: Atu027, a liposomal small interfering RNA formulation
targeting protein kinase N3, inhibits cancer progression. Cancer
Res. 68:9788–9798. 2008.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Hattori Y, Nakamura A, Arai S, Kawano K,
Maitani Y and Yonemochi E: siRNA delivery to lung-metastasized
tumor by systemic injection with cationic liposomes. J Liposome
Res. 25:279–286. 2015.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-ΔΔC(T)) method. Methods. 25:402–408. 2001.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Hattori Y, Nakamura M, Takeuchi N, Tamaki
K, Shimizu S, Yoshiike Y, Taguchi M, Ohno H, Ozaki KI and Onishi H:
Effect of cationic lipid in cationic liposomes on siRNA delivery
into the lung by intravenous injection of cationic lipoplex. J Drug
Target. 27:217–227. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hattori Y, Takeuchi N, Nakamura M,
Yoshiike Y, Taguchi M, Ohno H, Ozaki K and Onishi H: Effect of
cationic lipid type in cationic liposomes for siRNA delivery into
the liver by sequential injection of chondroitin sulfate and
cationic lipoplex. J Drug Deliv Sci Technol. 48:235–244. 2018.
View Article : Google Scholar
|
|
27
|
Yoshikawa N, Fumoto S, Nakashima M,
Shimokawa K, Miyamoto H and Nishida K: The role of fibronectin in
pulmonary gene transfer following intravenous administration of
lipoplex in mice. Biol Pharm Bull. 36:1807–1813. 2013.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Simberg D, Weisman S, Talmon Y, Faerman A,
Shoshani T and Barenholz Y: The role of organ vascularization and
lipoplex-serum initial contact in intravenous murine lipofection. J
Biol Chem. 278:39858–39865. 2003.PubMed/NCBI View Article : Google Scholar
|