Introduction
PD-1 is expressed on the surface of a variety of
immune cells, such as T cells, B cells and natural killer (NK)
cells, in which its ligand, programmed death-ligand (PD-L)1, is
widely expressed on the surface of various tissue cells, including
tumor cells, while the ligand PD-L2 is mainly expressed in
hematopoietic cells. PD-L1 is one of the numerous immune
checkpoints of T cells, and the PD-L1 signaling pathway inhibits
the activity of T cells at a later stage of the immune response
(1,2). PD1 monoclonal antibodies, including
pembrolizumab and nivolumab, block PD-1/PD-L1 association and
enhance the anti-tumor immune function of T cells.
Pembrolizumab is one of the immune checkpoint
inhibitors (ICIs), which is a humanized monoclonal IgG, applied in
the immune therapy of lung cancer (3), melanoma (4), renal cancer (5) and other malignant tumors (6). The survival benefit is evident
(7). Immune inhibition release
leads to immune-related side-effects, such as pembrolizumab, a PD-1
inhibitor with median toxicity of 77.1%. The incidence of toxicity
for above grade 3 is 20.8%. The overall immunotherapy is safe;
however, some of the fatal immunotoxicities, such as neurological
adverse events (nAEs) associated with ICIs should attract
sufficient attention, particularly when neurotoxicity is involved.
According to a previous study, the incidence of neurological
toxicity associated with the administration of PD-1 inhibitors is
6.1% (8), and neurotoxicity
accounts for approximately 15% of the deaths caused by PD-1/PD-L1
inhibitors (9).
Case report
A 44-year-old male was diagnosed with
extensive-stage small-cell lung cancer (ES-SCLC) accompanied by
bone, brain and mediastinal lymph node metastasis. Between March,
2019 to August, 2019, he received systemic treatment with 6-cycle
etoposide and cisplatin (EP) chemotherapy (etoposide 165
mg/m2 on days 1-3, and cisplatin 50 mg/m2 on
days 1-2) followed by 200 mg pembrolizumab (Keytruda®,
Merck Sharp & Dohme Corp.) intravenously once every 3 weeks,
and the curative efficacy was evaluated as progressive disease
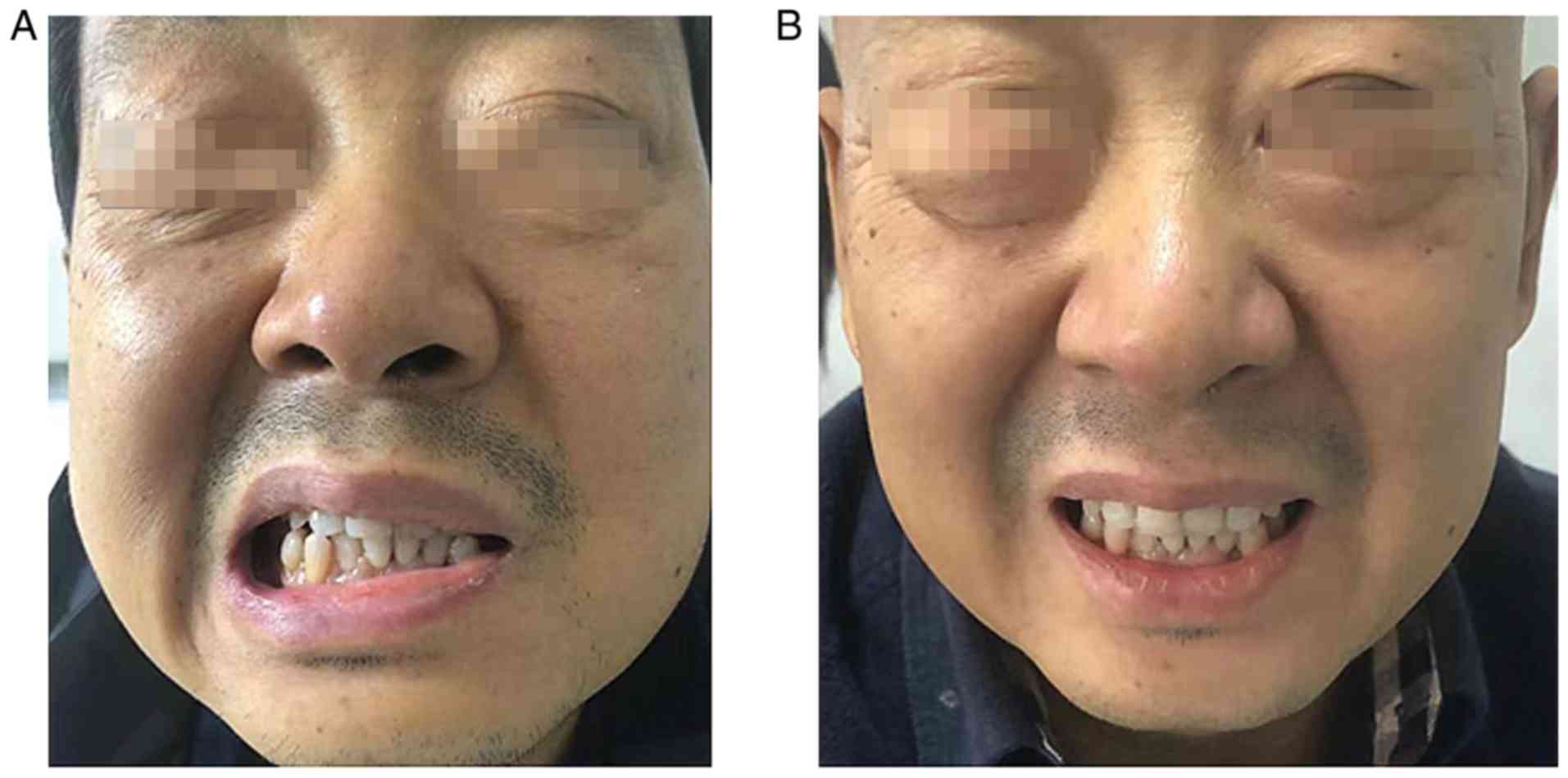
(PD). He arose with facial paralysis, facial numbness, pain,
tinnitus and limb joint pain on September 23, 2019 (Fig. 1A), although he had tendon reflexes.
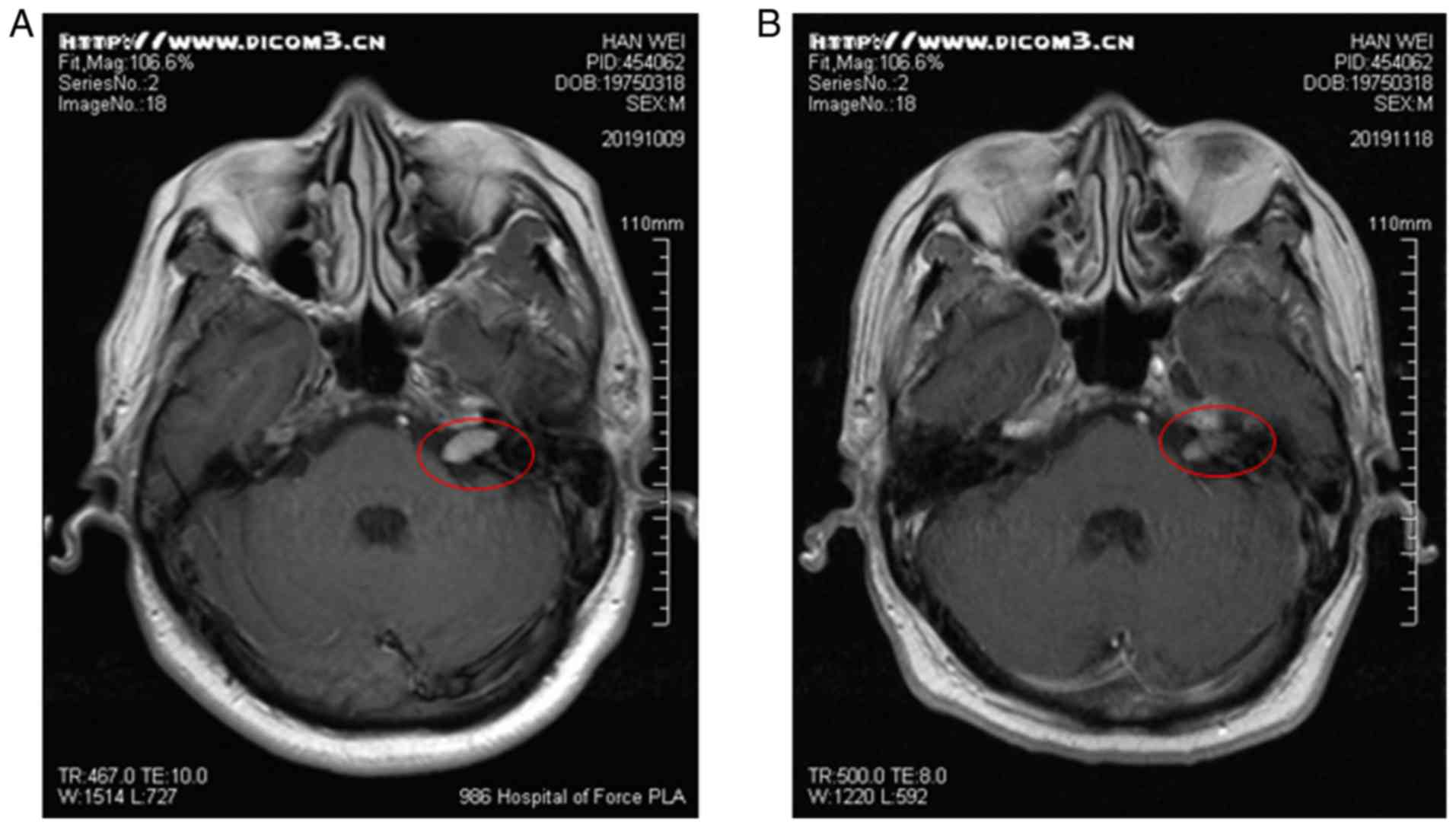
An enhanced cranial MRI examination revealed that there were
abnormal enhancement areas in the left pontine crus, with clear
boundaries (Fig. 2A). Facial nerve
thickening and swelling caused facial paralysis. According to the
toxicity grade, it was classified as grade 2. Thus, the use of
pembrolizumab was terminated, although EP chemotherapy continued
and he was treated with high-dose hormone therapy with the
resolution of neurological symptoms. Methylprednisolone at 40 mg
was administered for 5 days and he was then treated with oral
prednisone tablets (25 mg). After 1 week, his facial symptoms were
significantly alleviated (Fig.
1B). Following hormone therapy, the re-examination of the head
MRI revealed that the lesion had shrunk and symptoms had improved
(Fig. 2B). Subsequently, he was
treated with etoposide/tenoposide alone, and he did not exhibit any
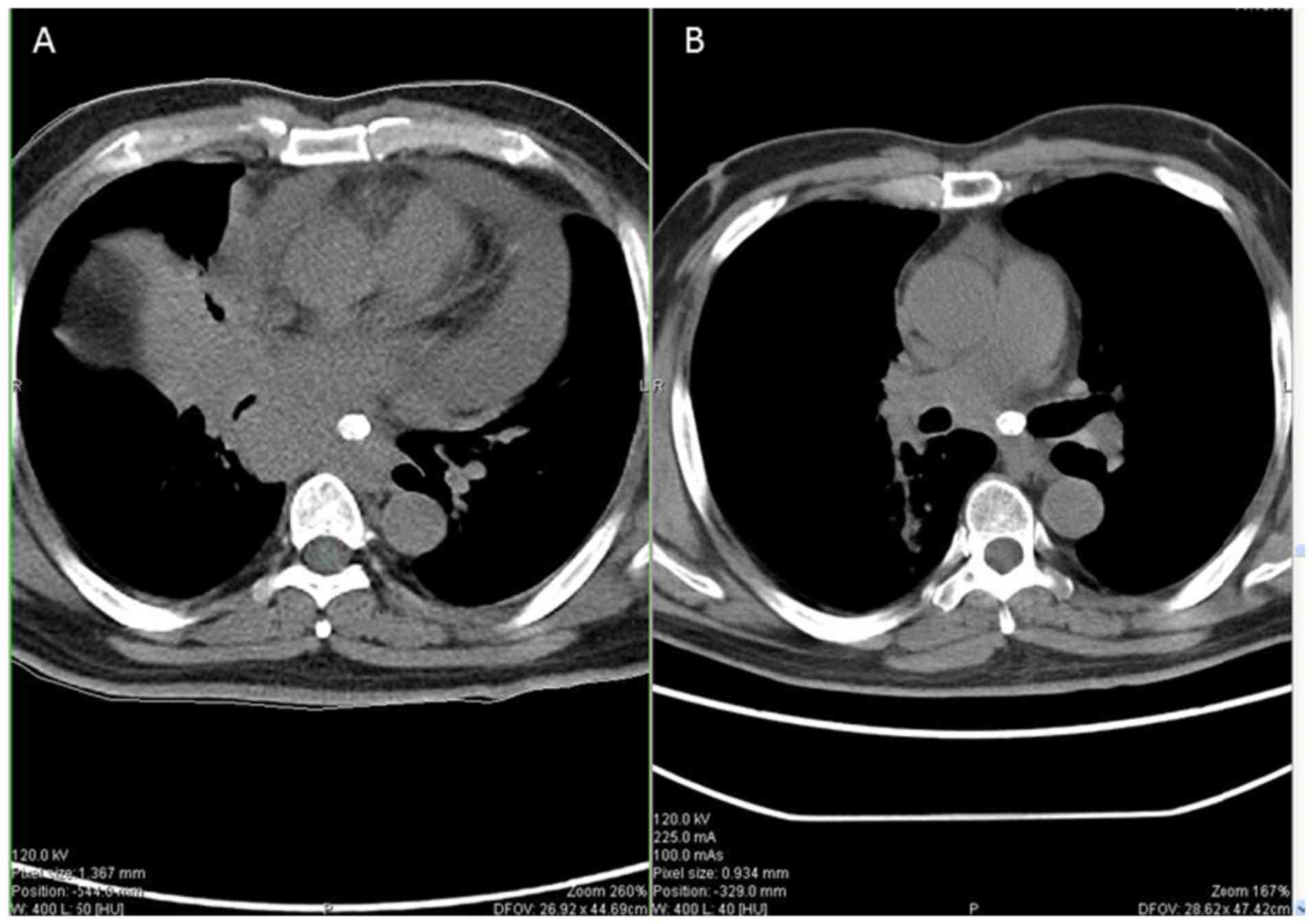
signs of neurotoxicity. After completing the whole chemotherapeutic
regimen, the primary tumor had markedly shrunk when comparing
pre-treatment (Fig. 3A) and
post-treatment (Fig. 3B).
Discussion
The common pembrolizumab-associated neurological
immune-related adverse effects (irAEs) span diverse entities,
including Guillain barre syndrome, transverse myelitis, myasthenia
gravis, peripheral neuropathy and aseptic meningitis (3). The presents study reports one case of
pembrolizumab-associated neurological irAEs manifesting as pain,
numbness and corner nakedness in the area of left facial nerve
innervation, no tinnitus and hearing loss. When enquiring about the
detailed history of the patient, a comprehensive detection of the
nervous system, brain MRI, a cerebrospinal fluid examination,
neurological analysis and consultation with a neurologist, tumor
progression, transfer, central nervous system infection, diabetic
neuropathy, or vitamin B12 deficiency-associated disease were
excluded. Given the specification of glucocorticoid treatment
underlying neurotoxicity, the symptoms and related image data
markedly improved, and the patient was considered to have suffered
from facial nerve toxicity caused by pembrolizumab. It has been
reported that pembrolizumab-associated neurological irAEs occur
both in peripheral neuropathy and Guillain barre syndrome (5), although facial nerve injury is rare.
The present case report demonstrated that there were marked
individual differences in immune-related neurotoxic manifestations,
which increases the difficulty in the early identification of toxic
symptoms. The facial nerve is responsible for muscle movements in
the face, and pembrolizumab-associated neurological irAEs cause
facial deformation.
Although the compliance of ICI therapy with irAEs is
affected, it does not seem to affect the efficacy of immunotherapy
(6), and immune-related toxicity
may be an index for the reactivity of ICI treatment and survival
benefits (7,8). A previous study (9) demonstrated that ICI therapy for
patients with rheumatoid arthritis (RA) had a better response rate
(85.7 vs. 35.3%) than those who were negative for RA. Similarly, a
preliminary study revealed that ICI treatment with neurotoxicity
may have a better response rate (10). This may be one reason for the
better treatment response in the present case.
irAEs require early identification and timely and
effective drug intervention through NCCN guidelines (11). Glucocorticoids are the major drug
choices; however, it has been shown that patients with
immune-related neurotoxicity have different individual responses to
glucocorticoids (12), and
patients with resistance to glucocorticoids often need to be
replaced with (TNF-a) infliximab or (IVIG) immunoglobulin. The
patient, in this case (12), had a
good response to glucocorticoids, and the symptoms of facial
paralysis and pain in 4 joints were significantly improved
following 72 h of administration, without hormone resistance.
The mechanism of toxicity associated with ICIs has
not yet been fully clarified. It has been demonstrated that the
following main categories exist: First, T cells directly attack
normal tissues, and certain antigens can be expressed in both tumor
cells and normal cells; thus, the enhanced function of T cells can
cause damage to normal tissues (13). Another type of injury involves
inflammatory factors, such as pro-inflammatory factor IL-17, which
can be released with the recovery of T cell function (14,15).
Another type is that following treatment with ICI, the body's
pre-existing autoreactive antibodies can be activated, leading to
the occurrence of irAEs (16). In
addition, the mechanisms of irAEs occurring in different phases may
also differ. For example, the early side-effects are mainly
manifested by extensive epithelial cell damage caused by the
involvement of inflammatory cells, such as neutrophils, such as
skin rash, colitis, pneumonia and other symptoms. The late
side-effects are more likely to occur in the specific local organs,
such as nervous system toxicity, hypophysitis. In the present case
report, the patient facial nerve toxicity occurred following the
end of ICI therapy, which is an advanced toxic reaction, and the
specific mechanisms remain to be further elucidated.
In the present study, a case of a rare patient with
ES-SCLC treated with anti-PD-1 inhibitors (pembrolizumab) with
induced facial toxicity is reported, in order to provide some
references for the prediction and management of the side-effects
caused by ICIs.
In conclusion, the facial nerve injury caused by
ICIs is rare; thus, the neurotoxicity caused by ICIs should be a
matter of concern, and the related mechanisms underlying the injury
warrant further investigation. While chemotherapy does not cause
such nerve damage, immune-induced nerve damage is usually
alleviated with hormone therapy, which can distinguish metastatic
tumors from neuropathy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
YD and SH conceived the case report, wrote the
initial manuscript and reviewed the final manuscript. MW, WL, SH,
HG and TQ interpreted and created the radiographic images, and
reviewed the final manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the 986 Hospital
Affiliated to the Fourth Military Medical University Ethics Board
(approval no. KJ4-004-03; Xi'an, China). Consent to participate was
obtained from the patient.
Patient consent for publication
Informed written consent was obtained from the
patient for publication of this case.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Boussiotis VA: Molecular and biochemical
aspects of the PD-1 checkpoint pathway. N Engl J Med.
375:1767–1778. 2016.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Coles SJ, Gilmour MN, Reid R, Knapper S,
Burnett AK, Man S, Tonks A and Darley RL: The immunosuppressive
ligands PD-L1 and CD200 are linked in AML T-cell immunosuppression:
Identification of a new immunotherapeutic synapse. Leukemia.
29:1952–1954. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Lim SH, Sun JM, Lee SH, Ahn JS, Park K and
Ahn MJ: Pembrolizumab for the treatment of non-small cell lung
cancer. Expert Opin Biol Ther. 16:397–406. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Robert C, Ribas A, Schachter J, Arance A,
Grob JJ, Mortier L, Daud A, Carlino MS, McNeil CM, Lotem M, et al:
Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006):
Post-hoc 5-year results from an open-label, multicentre,
randomised, controlled, phase 3 study. Lancet Oncol. 20:1239–1251.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rini BI, Plimack ER, Stus V, Gafanov R,
Hawkins R, Nosov D, Pouliot F, Alekseev B, Soulières D, Melichar B,
et al: Pembrolizumab plus axitinib versus sunitinib for advanced
renal-cell carcinoma. N Engl J Med. 380:1116–1127. 2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Fradet Y, Bellmunt J, Vaughn DJ, Lee JL,
Fong L, Vogelzang NJ, Climent MA, Petrylak DP, Choueiri TK, Necchi
A, et al: Randomized phase III KEYNOTE-045 trial of pembrolizumab
versus paclitaxel, docetaxel, or vinflunine in recurrent advanced
urothelial cancer: Results of >2 years of follow-up. Ann Oncol.
30:970–976. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gao HX, Huang SG, Du JF, Zhang XC, Jiang
N, Kang WX, Mao J and Zhao Q: Comparison of prognostic indices in
NSCLC patients with brain metastases after radiosurgery. Int J Biol
Sci. 14:2065–2072. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cuzzubbo S, Javeri F, Tissier M, Roumi A,
Barlog C, Doridam J, Lebbe C, Belin C, Ursu R and Carpentier AF:
Neurological adverse events associated with immune checkpoint
inhibitors: Review of the literature. Eur J Cancer. 73:1–8.
2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors: A
systematic review and meta-analysis. JAMA Oncol. 4:1721–1728.
2018.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Feng S, Coward J, McCaffrey E, Coucher J,
Kalokerinos P and O'Byrne K: Pembrolizumab-induced encephalopathy:
A review of neurological toxicities with immune checkpoint
inhibitors. J Thorac Oncol. 12:1626–1635. 2017.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thompson JA, Schneider BJ, Brahmer J,
Andrews S, Armand P, Bhatia S, Budde LE, Costa L, Davies M,
Dunnington D, et al: Management of immunotherapy-related
toxicities, version 1.2019. J Natl Compr Canc Netw. 17:255–289.
2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Garcia CA, El-Ali A, Rath TJ, Contis LC,
Gorantla V, Drappatz J and Davar D: Neurologic immune-related
adverse events associated with adjuvant ipilimumab: Report of two
cases. J Immunother Cancer. 6(83)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Johnson DB, Balko JM, Compton ML, Chalkias
S, Gorham J, Xu Y, Hicks M, Puzanov I, Alexander MR, Bloomer TL, et
al: Fulminant myocarditis with combination immune checkpoint
blockade. N Engl J Med. 375:1749–1755. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Tarhini AA, Zahoor H, Lin Y, Malhotra U,
Sander C, Butterfield LH and Kirkwood JM: Baseline circulating
IL-17 predicts toxicity while TGF-β1 and IL-10 are prognostic of
relapse in ipilimumab neoadjuvant therapy of melanoma. J Immunother
Cancer. 3(39)2015.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Feng T, Qin H, Wang L, Benveniste EN,
Elson CO and Cong Y: Th17 cells induce colitis and promote Th1 cell
responses through IL-17 induction of innate IL-12 and IL-23
production. J Immunol. 186:6313–6318. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017.PubMed/NCBI View Article : Google Scholar
|