Introduction
Endometriosis is a common, estrogen-dependent
inflammatory disease characterized by the presence of endometrial
tissue outside the uterine cavity (1). Endometriosis includes superficial
peritoneal disease, ovarian endometrioma (OMA) and deep
endometriosis (DE) lesions as major phenotypes. The main symptoms
include pelvic pain, such as dysmenorrhea, dyspareunia and chronic
pelvic pain, and infertility. Statistically, 30-50% of females with
endometriosis experience infertility (2). Endometriosis-related pain symptoms
may be associated with the severity of diseases, such as adhesions
and posterior cul-de-sac and uterosacral lesions (3). Anatomical abnormalities with
adhesions and fibrosis lead to infertility, although even mild
endometriosis can cause infertility (4). The severity of symptoms, including
pain and infertility, is not always associated with the extent of
endometriosis.
Thus far, non-invasive diagnostic approaches, such
as transvaginal ultrasonography (TVS), magnetic resonance imaging
(MRI) and blood tests have exhibited considerable power in the
diagnosis of endometriosis (5).
TVS and MRI can help visualize OMA and DE, but not superficial
peritoneal disease. In addition, imaging is inadequate for the
prediction of endometriosis-associated infertility. Therefore, the
lack of non-invasive diagnostic tests that can predict infertility
leads to the long delay before treatment (6). The association between endometriosis
and infertility is well known; however, the mechanisms underlying
endometriosis-associated infertility have not yet been well
established. It has been pointed out that inflammatory cytokines
and oxidative stress may adversely affect eggs, sperm and
fertilized eggs, leading to impaired fertilization and
implantation, and decreased fertility (7,8).
Recently, Yoshimoto et al reported that the cyst fluid (CF)
concentrations of iron were elevated in patients with OMA (9). Excess labile and bioactive iron
concentrations can enhance toxic radical generation and induce
oxidative stress (10). A
preclinical study revealed that the CF concentration of iron was
associated with oxidative stress, and was involved in endometriosis
progression and malignant transformation (11). The present study measured iron
levels to assess whether iron-dependent oxidative stress is
involved in infertility, as it has previously been demonstrated
that oxidative stress is associated with infertility in
endometriosis (12).
The aim of the present study was to evaluate the
effects of the CF concentration of iron on infertility in patients
with OMA. The discovery of a sensitive, specific and non-invasive
biomarker for the prediction of endometriosis-associated
infertility may hold promise for the development of earlier
diagnosis and treatment strategies.
Materials and methods
Patients and study design
The present study was a single-center, retrospective
cohort study aimed at investigating the association between
infertility and the CF concentration of iron in patients with OMA.
Newly diagnosed patients were registered consecutively from
February, 2013 to May, 2019 at the Department of Obstetrics and
Gynecology, Nara Medical University Hospital, Kashihara, Japan.
Patients who visited the hospital complained of pelvic pain and/or
infertility. Participants were recommended to undergo routine TVS
and pre-operative MRI, performed as part of the clinical care
program. A sonography was performed by 2 experienced operators (MK
and SM) with a single ultrasound system (Voluson E8; GE Healthcare)
using a transvaginal transducer (5-7.5 MHz). MRI was obtained on a
3T system using T1W and T2W sequences (Magnetom Verio, Siemens
Healthcare). The TVS test and MRI were performed within 4 weeks
before surgery.
The patients were divided into 2 groups, namely
women experiencing current infertility (infertile group) and those
without complaints of infertility (non-infertile group). There were
2 types of patients in the infertility group: Patients who failed
to achieve a clinical pregnancy following ≥12 months of regular
unprotected sexual intercourse (13) and those who have already been
treated at fertility hospitals. Patients with OMA who received
laparotomy or laparoscopic surgery were enrolled in the present
study. Surgery was performed not only to treat infertility, but
also to improve pain. A histological diagnosis was confirmed by
surgical pathology. Exclusion criteria were any of the following:
No pathology, benign ovarian tumors other than OMA, OMA co-existing
with DIE, adenomyosis and malignant transformation, prior ovarian
surgery, unmarried women, women who did not wish to become
pregnant, menopausal women and women who had received hormonal
therapy within 3 months. A form of regional medical cooperation was
established and maintained with fertility hospitals, in order for
all potential patients to be referred to the appropriate
reproductive specialist following discharge. Therefore, detailed
information on the variables involved in the demographic and
clinical characteristics, such as the length of infertility,
ovarian function, semen status, or comorbidities of the infertile
women could not be obtained. The present study was conducted under
the guidelines that had been approved by the Medical Ethics
Committee of Nara Medical University. Written informed consent was
obtained from each patient.
Quantification of iron concentrations
in CF
Cyst fluid samples were obtained during surgery.
Cyst fluids harvested were collected into a plastic tube without an
anticoagulant and centrifuged at 2,000 x g for 10 min at 4˚C.
Supernatants were immediately aliquoted and kept frozen at -20˚C
within 1 h of collection until the time of analysis. The amount of
iron was determined by inductively coupled plasma optical emission
spectrometry (ICP-OES) (Vista MPX, Varian, Inc.) with internal
standard method as described previously (9). The results of the assays were not
available to the clinicians and therefore did not influence
subsequent patient management.
Statistical analysis
SPSS 25.0 (SPSS Inc.) statistical software was used
for the statistical analysis. Comparison of categorical variables
between groups was performed using the Chi-squared test. The
Mann-Whitney U-test was used for the univariate analysis of
numerical data. The cut-off value of cyst fluid concentrations of
iron was calculated with the highest sensitivity and specificity
for predicting the two groups based on the receiver operator
characteristic (ROC) curve analysis. The potential factors for
predicting infertility were determined by logistic regression
analysis. The independent factors were identified by multivariate
regression analysis. Hazard ratios (HRs) were calculated using a
two-stage regression estimate with the infertility as the
instrumental variable to examine associations with age and iron
(Fig. 1) or parity and infertility
index (Fig. 2) by logistic
regression and Cox regression, respectively. Furthermore, the
infertility index, defined as iron level/age, was also subjected to
a multivariate analysis. Differences with P<0 .05 were
considered to be statistically significant.
Results
The present study included 77 patients who met the
inclusion and exclusion criteria during the period of the study.
Among these, 32 (41.6%) patients had infertility. Two women in the
infertile group became pregnant after receiving the assisted
reproductive technique (ART). The non-infertile group included 36
women who have previously given birth due to one or more
spontaneous pregnancies. In addition, 9 women in the non-infertile
group had one or more miscarriages, but failed to have live births.
The patient demographic factors and clinical characteristics of the
study population are summarized in Table I. The patients were significantly
younger in the infertile group than in the non-infertile group
(median age, 35 years; range, 24-47 years; vs. median age, 40
years; range, 21-53 years, respectively; P=0.003). Since the 2
groups of patients were not homogeneous, with a median age of 35
and 40 years for the infertile and non-infertile groups,
respectively, the association between age and the CF concentrations
of iron was analyzed. In the analyses of data from all the study
subjects, no significant correlation between patient age and the CF
concentration of iron was observed [y (CF concentration of
iron)=-1.63x (age) + 343.0, r2=0.004] (data not shown).
Not surprisingly, the infertile group exhibited a significantly
lower parity compared to the non-infertile group (P<0.001).
There were no significant differences among the 2 groups in
variables, such as pre-operative CA125 levels, pre-operative CA19-9
levels, cyst diameter and tumor localization. The CF concentrations
of iron were significantly higher in the infertile group compared
with the non-infertile group (median, 324.8 mg/l; range,
71.3-1046.3 mg/l; vs. median, 226.5; range, 65.3-737.5,
respectively; P=0.019) (Table
I).
 | Table IDemographic and clinical
characteristics of patients in the infertile and non-infertile
groups. |
Table I
Demographic and clinical
characteristics of patients in the infertile and non-infertile
groups.
| | Non-infertile group
(n=45) | Infertile group
(n=32) | P-value |
|---|
| Age, years; median
(range) | 40 (21-53) | 35 (24-47) | 0.003a |
| Parity | | | |
|
0 | 9 | 30 | |
|
≥1 | 36 | 2 |
<0.001b |
| CA125; median
(range) | 65.5
(10.0-1504.0) | 58.0
(16.0-1830.0) | 0.519a |
| CA19-9; median
(range) | 25.0 (1.0-252.0) | 27.5 (1.0-380.0) | 0.783a |
| Tumor diameter;
median (range) | 65.0
(27.0-193.0) | 70.0
(39.0-142.0) | 0.103a |
| Localization | | | |
|
Unilateral | 28 | 26 | |
|
Bilateral | 15 | 6 | 0.124a |
| CF concentration of
iron; median (range) | 226.5
(65.3-737.5) | 324.8
(71.3-1046.3) | 0.019a |
Since parity is the most important factor that
reflects the outcome of infertility, the addition of parity to the
multivariate analysis eliminated the other 2 variables (age and the
CF concentration of iron). First, the present study examined
whether age at diagnosis and the CF concentration of iron could
identify women experiencing current infertility (infertile group).
ROC curves were applied to assess the potential utility of these
indicators in discriminating between the infertile and
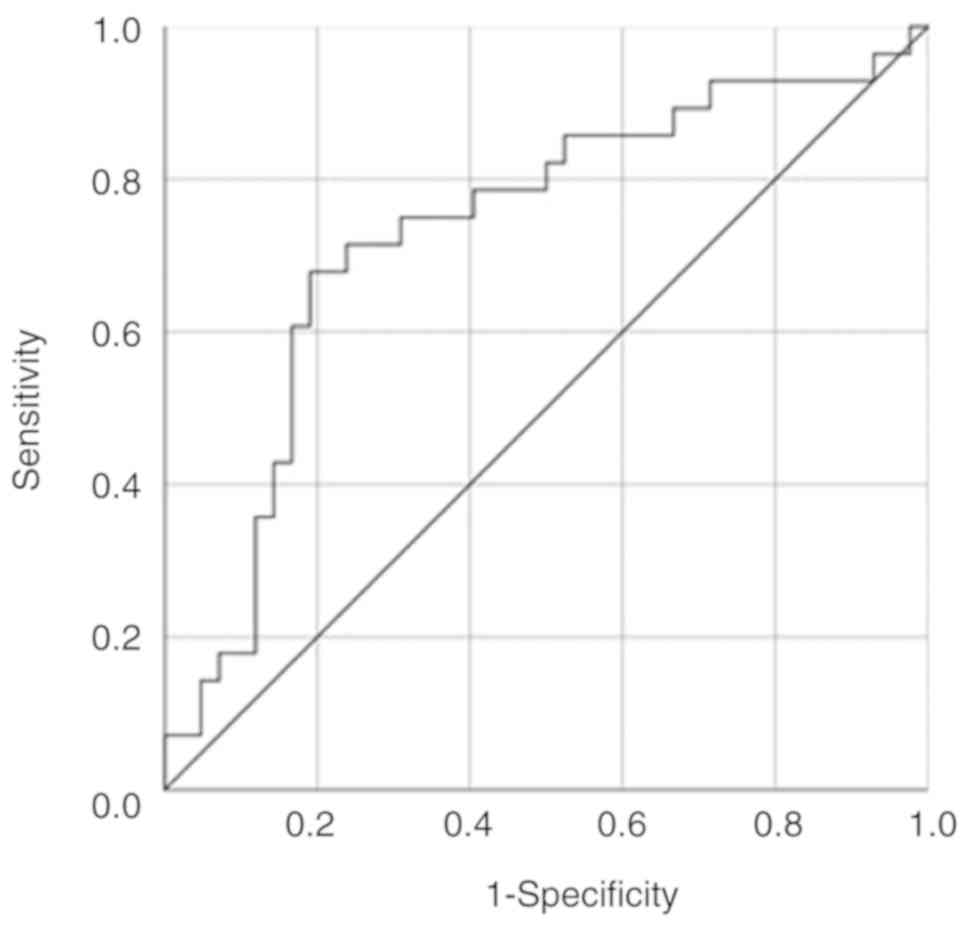
non-infertile groups (Fig. 1). ROC
curve analysis discriminated the infertile group from the
non-infertile group with an AUC value of 0.699 and an optimal
cut-off value of 37.5 years of age (sensitivity, 74.4%;
specificity, 62.5%) (Fig. 1A). It
was also found that the CF iron measurement successfully
discriminated between the 2 groups (AUC, 0.666) with an optimal
cut-off value of 326.6 mg/l (sensitivity, 50.0%; specificity,
81.0%) (Fig. 1B). Based on the
above-mentioned data, a multivariate logistic regression analysis
was conducted to identify independent variables associated with
infertility (Table II). The
results revealed that age at diagnosis (HR, 6.44; 95% CI,
2.06-20.12) and the CF concentration of iron (HR, 4.90; 95% CI,
1.48-16.22) were able to independently identify patients with OMA
experiencing current infertility.
 | Table IIThe univariate and multivariate
logistic regression analysis for identifying women experiencing
current infertility. |
Table II
The univariate and multivariate
logistic regression analysis for identifying women experiencing
current infertility.
| | Infertility |
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
≥38 | 1 | | | |
|
<38 | 5.03
(1.83-13.80) | 0.002 | 6.44
(2.06-20.12) | 0.001 |
| CF concentration of
iron |
|
≤326.6 | 1 | | | |
|
>326.6 | 4.25
(1.46-12.37) | 0.008 | 4.90
(1.48-16.22) | 0.009 |
Second, an age <37.5 years and a CF concentration
of iron >326.6 mg/l were predictors of infertility (Table II); thus, a combined analysis of
the 2 variables was performed. The iron level/age ratio was defined
as an infertility index. ROC curve analysis discriminated between
the infertile group from the non-infertile group with an optimal
iron level/age ratio cut-off value of 8.37 with an AUC value of
0.731 (sensitivity, 67.9%; specificity, 81.0%) (Fig. 2). Multivariate logistic regression
analysis revealed that both parity (HR, 0.012; 95% CI, 0.001-0.10;
P<0.001) and infertility index (HR, 4.85; 95% CI, 1.01-23.27;
P=0.049) significantly predicted infertility (Table III).
 | Table IIIUnivariate and multivariate logistic
regression analysis for the identification of women experiencing
current infertility. |
Table III
Univariate and multivariate logistic
regression analysis for the identification of women experiencing
current infertility.
| | Infertility |
|---|
| | Univariate
analysis | Multivariate
analysis |
|---|
| Variables | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Parity |
|
0 | 1 | | 1 | |
|
≥1 | 0.018
(0.004-0.09) | <0.001 | 0.012
(0.001-0.10) | <0.001 |
| Infertility
index |
|
≤8.37 | 1 | | 1 | |
|
>8.37 | 8.97
(2.97-27.10) | <0.001 | 4.85
(1.01-23.27) | 0.049 |
Discussion
In order to examine the role of iron in infertile
women, the present study analyzed the pre-operative CF
concentrations of iron in 77 patients with OMA who underwent
laparoscopic surgery. Women experiencing current infertility were 5
years younger than those without complaints of infertility (median
age, 35 vs. 40 years). The present study demonstrated for the first
time, at least to the best of our knowledge, that the CF
concentrations of total iron were significantly higher in infertile
patients than in non-infertile women (median, 324.8 mg/l vs. 226.5
mg/l; P=0.019). When the CF concentration of iron was ≥326.6 mg/l,
the patient was considered to be infertile, with a sensitivity and
specificity of 50.0 and 81.0%, respectively. In addition, the
combination of 2 variables (infertility index; iron level/age
ratio) exhibited a high sensitivity (67.9%) and specificity (81.0%)
in predicting current infertility in patients with OMA.
First, the severity of endometriosis is dependent on
anatomical factors and patient background. Age, the duration of
infertility, body mass index, the duration of the menstrual cycle,
history of abortion, dyspareunia, pelvic pain and a family history
of endometriosis are risk factors, and some may be independent
predictors of infertility associated with endometriosis (14). In addition as regards various risk
factors, some classifications have been reported to predict the
severity of endometriosis and infertility (15). Endometriosis has often been
classified by the size of its anatomical lesions; however, not only
the size of the lesions, but the location is also important
(15). The endometriosis fertility
index (EFI) has more predictive power for fecundity, in
vitro fertilization (IVF) outcomes, or post-operative pregnancy
in patients with endometriosis revised American than the Fertility
Society classification (r-AFS classification) (16-20).
Furthermore, endometriosis and its severity have
been reported to be dependent on multiple biochemical, genetic and
environmental factors, such as hormonal factors, altered immune
system, inflammation, growth factors, an imbalance between
pro-apoptosis and anti-apoptosis, increased neuroangiogenesis,
familial predisposition, genetic alterations, diet, environmental
factors and excessive oxidative stress (21-23).
Redox-related changes in the peritoneal microenvironment are
closely associated with the pathogenesis of endometriosis, creating
favorable conditions for endometriotic cell proliferation and
survival. A number of researchers have investigated factors
associated with the disease severity using surgically resected
tissue, follicular fluid, peritoneal fluid, and blood. Wang et
al reported that inflammatory factors, such as interleukin
(IL)-6, IL-10, IL-13 and tumor necrosis factor (TNF)-α, could be
indicators for the diagnosis of endometriosis with infertility
(24). Cyclooxygenase-2 (COX-2), a
rate-limiting enzyme of prostaglandin (PG) synthesis, plays a
crucial role in the inflammation, proliferation and spread of
endometriotic lesions through the upregulation of certain growth
factors, such as transforming growth factor (TGF)-β expression
(25). In the peritoneal fluid of
infertile women with endometriosis, increased concentrations of PGs
have been shown to cause adverse effects on fertilization,
implantation and embryonic growth, which will lead to infertility
(26). Furthermore, an imbalance
between reactive oxygen species (ROS) and the antioxidant system in
the follicular fluid causes abnormal oocyte development and poor
egg quality through DNA, cytoskeleton and cell membrane damage
(27). Excessive oxidative stress
is regarded as a possible mechanism of endometriosis-related
infertility (27). Inflammatory
cytokines, ILs and oxidative stress may all be reliable markers for
diagnosing endometriosis and its severity (12). However, the methods for
non-invasively predicting or diagnosing infertility associated with
endometriosis are extremely limited, and no clinically available
markers have been reported thus far, at least to the best of our
knowledge.
The present study found that age at diagnosis and
the CF concentrations of iron could predict the risk of infertility
in patients with OMA. It is considered that women who presented
with complaints of infertility are more likely to be younger than
women without infertility as they often visit the clinic for
infertility counseling. The identification of risk factors can help
select populations that are prone to infertility.
Second, the present study aimed to determine whether
iron can induce infertility. When red blood cells are hemolyzed in
the peritoneal cavity or endometriotic cysts, hemoglobin releases
heme iron and free iron (28).
Hemoglobin undergoes autoxidation of the iron in its heme groups
and produces superoxide radicals through conversion to
methemoglobin (10). Bioactive
free iron also generates hydroxyl radicals, a potent ROS, through
the Fenton reaction (10). ROS,
such as superoxide anion, hydroxyl radical and hydrogen peroxide,
are inflammatory mediators known to exert deleterious effects by
causing DNA damage, methylation and epigenetic errors (29,30).
Therefore, iron-induced oxidative stress adversely affects female
and male gametes, sperm fertilizing ability, implantation, embryo
development, uterine receptivity, ART outcome and pregnancy rates
after IVF, resulting in infertility (12). Based on the above, it was thus
speculated that women with a high CF concentration of iron may be
more likely to become infertile. Women who wished to become
pregnant may require accurate counseling and an appropriate
conception plan, depending on iron levels.
Third, over the past decade, certain non-invasive
methods have been developed to quantify iron concentrations in
human organs. There are at least 2 different techniques for the
quantification of the iron concentration: T2 magnetic resonance
relaxometry methods and near infrared optical method. The hepatic
and cardiac iron content can be estimated on the effective
transverse relaxation rate (R2*) (31). Recently, T2 relaxometry has allowed
the non-invasive quantification of iron levels in CF and has
enabled the diagnosis of endometriosis (32). In addition, near infrared
spectroscopy is a validated method that allows for the
quantification of the CF concentration of iron non-invasively and
repeatedly (11). A new device,
consisting of transvaginal ultrasonography and near infrared
spectroscopy system (composite-type optical device), is currently
under development for the non-invasive quantification of iron
concentration in endometriotic CF (11,33).
Iron quantification may assist in the identification of patients at
high risk of infertility.
Finally, there are some limitations to the present
study. Patients with adenomyosis and/or DIE were excluded from the
study to evaluate the effects of OMA itself on infertility. As a
result, the small sample size limits the power of the present
study. It is necessary to accumulate data on CF concentrations of
iron in a large number of patients for clinical application.
Moreover, the cause of infertility was not clarified in the present
study. In addition, the present study does not mention that the CF
concentration of iron is a predictor of post-operative
infertility.
In conclusion, the present study demonstrated that
patients with OMA experiencing current infertility visited the Nara
Medical University Hospital at a younger age than those without
complaints of infertility, and that the CF concentration of iron
was significantly higher in the infertile group than in the
non-infertile group. If the CF concentration of iron is ≥326.6 mg/l
or the iron/age ratio is ≥8.37, the cause of infertility should be
evaluated for women who wish to become pregnant. CF iron may
provide useful information for comprehensive counseling and
treatment decisions regarding endometriosis-related infertility.
Future studies are required however, to elucidate the key
mechanisms that link iron with infertility.
Acknowledgements
Not applicable.
Funding
The present was supported by JSPS KAKENHI (grant
nos. 20K09604, 20K09647 and 20K09648).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SM, SI, MK and MN performed the literature search
and collected data using the PubMed database. MN and HK made
substantial contribution to the conception of the study. SI
contributed to the study design and interpretation of the included
research studies. NK performed the statistical analysis. The final
version of the manuscript has been read and approved by all
authors.
Ethics approval and consent to
participate
The present study was conducted under the guidelines
that had been approved by the medical ethics committee of the Nara
Medical University. Written informed consent was obtained from each
patient.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Koninckx PR, Ussia A, Adamyan L, Wattiez
A, Gomel V and Martin DC: Pathogenesis of endometriosis: The
genetic/epigenetic theory. Fertil Steril. 111:327–340.
2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Meuleman C, Vandenabeele B, Fieuws S,
Spiessens C, Timmerman D and D'Hooghe T: High prevalence of
endometriosis in infertile women with normal ovulation and
normospermic partners. Fertil Steril. 92:68–74. 2009.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hsu AL, Sinaii N, Segars J, Nieman LK and
Stratton P: Relating pelvic pain location to surgical findings of
endometriosis. Obstet Gynecol. 118:223–230. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Tamburro S, Canis M, Albuisson E,
Dechelotte P, Darcha C and Mage G: Expression of transforming
growth factor beta1 in nerve fibers is related to dysmenorrhea and
laparoscopic appearance of endometriotic implants. Fertil Steril.
80:1131–1136. 2003.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Nisenblat V, Bossuyt PM, Farquhar C,
Johnson N and Hull ML: Imaging modalities for the non-invasive
diagnosis of endometriosis. Cochrane Database Syst Rev.
2(CD009591)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Agarwal SK, Chapron C, Giudice LC, Laufer
MR, Leyland N, Missmer SA, Singh SS and Taylor HS: Clinical
diagnosis of endometriosis: A call to action. Am J Obstet Gynecol.
220:354.e1–354.e12. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Sharma I, Dhaliwal LK, Saha SC, Sangwan S
and Dhawan V: Role of 8-iso-prostaglandin F2alpha and
25-hydroxycholesterol in the pathophysiology of endometriosis.
Fertil Steril. 94:63–70. 2010.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Choi YS, Cho S, Seo SK, Park JH, Kim SH
and Lee BS: Alteration in the intrafollicular thiol-redox system in
infertile women with endometriosis. Reproduction. 149:155–162.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Yoshimoto C, Iwabuchi T, Shigetomi H and
Kobayashi H: Cyst fluid iron-related compounds as useful markers to
distinguish malignant transformation from benign endometriotic
cysts. Cancer Biomark. 15:493–499. 2015.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Iwabuchi T, Yoshimoto C, Shigetomi H and
Kobayashi H: Oxidative stress and antioxidant defense in
endometriosis and its malignant transformation. Oxid Med Cell
Longev. 2015(848595)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kobayashi H, Yamada Y, Kawahara N, Ogawa K
and Yoshimoto C: Modern approaches to noninvasive diagnosis of
malignant transformation of endometriosis. Oncol Lett.
17:1196–1202. 2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gupta S, Goldberg JM, Aziz N, Goldberg E,
Krajcir N and Agarwal A: Pathogenic mechanisms in
endometriosis-associated infertility. Fertil Steril. 90:247–257.
2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Zegers-Hochschild F, Adamson GD, de Mouzon
J, Ishihara O, Mansour R, Nygren K, Sullivan E and Vanderpoel S:
International Committee for Monitoring Assisted Reproductive
Technology and World Health Organization. International Committee
for monitoring assisted reproductive technology (ICMART) and the
World Health Organization (WHO) revised glossary of ART
terminology, 2009. Fertil Steril. 92:1520–1524. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Ashrafi M, Sadatmahalleh SJ, Akhoond MR
and Talebi M: Evaluation of risk factors associated with
endometriosis in infertile women. Int J Fertil Steril. 10:11–21.
2016.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bouquet de Joliniere J, Major A, Ayoubi
JM, Cabry R, Khomsi F, Lesec G, Frydman R and Feki A: It is
necessary to purpose an add-on to the american classification of
endometriosis? This disease can be compared to a malignant
proliferation while remaining benign in most cases. Endogram® is a
new profile witness of its evolutionary potential. Front Surg.
6(27)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Zhou Y, Lin L, Chen Z, Wang Y, Chen C, Li
E and Wu R: Fertility performance and the predictive value of the
endometriosis fertility index staging system in women with
recurrent endometriosis: A retrospective study. Medicine
(Baltimore). 98(e16965)2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Negi N, Roy KK, Kumar S, Nair VG and
Vanamail P: Clinical outcome analysis and correlation of
reproductive outcome with endometriosis fertility index in
laparoscopically managed endometriosis patients: A retrospective
cohort study. J Hum Reprod Sci. 12:98–103. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Li X, Zeng C, Zhou YF, Yang HX, Shang J,
Zhu SN and Xue Q: Endometriosis fertility index for predicting
pregnancy after endometriosis surgery. Chin Med J (Engl).
130:1932–1937. 2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang W, Li R, Fang T, Huang L, Ouyang N,
Wang L, Zhang Q and Yang D: Endometriosis fertility index score
maybe more accurate for predicting the outcomes of in vitro
fertilisation than r-AFS classification in women with
endometriosis. Reprod Biol Endocrinol. 11(112)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Revised American society for reproductive
medicine classification of endometriosis: 1996. Fertil Steril 67:
817-821, 1997.
|
|
21
|
Tosti C, Pinzauti S, Santulli P, Chapron C
and Petraglia F: Pathogenetic mechanisms of deep infiltrating
endometriosis. Reprod Sci. 22:1053–1059. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Ahn SH, Monsanto SP, Miller C, Singh SS,
Thomas R and Tayade C: Pathophysiology and immune dysfunction in
endometriosis. Biomed Res Int. 2015(795976)2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
McKinnon B, Mueller M and Montgomery G:
Progesterone resistance in endometriosis: An acquired property?
Trends Endocrinol Metab. 29:535–548. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Wang XM, Ma ZY and Song N: Inflammatory
cytokines IL-6, IL-10, IL-13, TNF-α and peritoneal fluid
flora were associated with infertility in patients with
endometriosis. Eur Rev Med Pharmacol Sci. 22:2513–2518.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yuan L, Shen F, Lu Y, Liu X and Guo SW:
Cyclooxygenase-2 overexpression in ovarian endometriomas is
associated with higher risk of recurrence. Fertil Steril. 91 (Suppl
4):S1303–S1306. 2009.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Sales KJ and Jabbour HN: Cyclooxygenase
enzymes and prostaglandins in pathology of the endometrium.
Reproduction. 126:559–567. 2003.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Prieto L, Quesada JF, Cambero O, Pacheco
A, Pellicer A, Codoceo R and Garcia-Velasco JA: Analysis of
follicular fluid and serum markers of oxidative stress in women
with infertility related to endometriosis. Fertil Steril.
98:126–130. 2012.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kobayashi H, Yamada Y, Kanayama S,
Furukawa N, Noguchi T, Haruta S, Yoshida S, Sakata M, Sado T and Oi
H: The role of iron in the pathogenesis of endometriosis. Gynecol
Endocrinol. 25:39–52. 2009.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Menezo YJ, Silvestris E, Dale B and Elder
K: Oxidative stress and alterations in DNA methylation: Two sides
of the same coin in reproduction. Reprod Biomed Online. 33:668–683.
2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jomova K and Valko M: Advances in
metal-induced oxidative stress and human disease. Toxicology.
283:65–87. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Verlhac S, Morel M, Bernaudin F, Béchet S,
Jung C and Vasile M: Liver iron overload assessment by MRI R2*
relaxometry in highly transfused pediatric patients: An agreement
and reproducibility study. Diagn Interv Imaging. 96:259–264.
2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Yoshimoto C, Takahama J, Iwabuchi T,
Uchikoshi M, Shigetomi H and Kobayashi H: Transverse relaxation
rate of cyst fluid can predict malignant transformation of ovarian
endometriosis. Magn Reson Med Sci. 16:137–145. 2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kawahara N, Yamada Y, Ito F, Hojo W,
Iwabuchi T and Kobayashi H: Discrimination of malignant
transformation from benign endometriosis using a near-infrared
approach. Exp Ther Med. 15:3000–3005. 2018.PubMed/NCBI View Article : Google Scholar
|