Introduction
The human immunodeficiency virus (HIV) is a
lentivirus within the family of Retroviridae, subfamily
Orthoretrovirinae, that enters the body via mucous
membranes, injured skin or by parenteral inoculation. This
infection can progress with the marked reduction in CD4 cell
numbers (<300/µl) and the immune response to respiratory
microorganisms is suppressed (1).
The deleterious effects of HIV are controlled with
anti-retroviral drugs (2). Several
antiviral drugs, such as lopinavir, remdesivir and umifenovir, have
been tested without marked success for coronavirus disease 2019
(COVID-19) (3). COVID-19
infection, characterized by severe acute respiratory syndrome due
to coronavirus-2 (SARS-CoV-2), can lead to severe respiratory
illness, with neutrophilia and lymphopenia, and an increase in the
levels of inflammatory markers, such as C-reactive protein, urea,
creatinine and D-dimer (4).
Due to the ongoing COVID-19 pandemic, the
investigation of comorbidities (5-7)
or concomitant infection with COVID-19 are extremely relevant
(8,9). To date, there is no current standard
treatment available, and new treatments are currently under
investigation, particularly in higher-risk populations (10-12).
Therefore, in order to evaluate the influence of HIV infection on
COVID-19, in the present study, the cases of 2 female patients of a
similar age (48 and 49 years) are presented, who were followed-up
daily, from the time of hospitalization to SARS-CoV2 clearance.
Case reports
Patients were recruited at the Hospital das Clínicas
of the University of São Paulo (HCFMUSP), a public tertiary
university hospital with a special ward for COVID-19 patients.
Patients were selected on the bases of previous HIV infection and
recent SARS-CoV-2 infection. The diagnosis of SARS-CoV-2 infection
was confirmed by the nasopharyngeal detection of SARS-CoV-2 RNA by
reverse transcription-polymerase chain reaction (RT-PCR). Patients
tested negative for influenza and respiratory syncytial virus.
Informed consent was obtained from both patients. The present study
was approved by the Ethics Committee of HCFMUSP (no.
30800520.7.0000.0068-2020), and was performed out in conformity
with the 2013 revision of the Declaration of Helsinki.
Laboratory analysis was performed at the Central
Laboratory of HCFMUSP. Laboratory analysis included the following:
complete blood counts (CBCs), and alanine aminotransferase (ALT),
aspartate aminotransferase (AST), creatinine, glucose, C-reactive
protein, total protein (albumin and globulin) and urea levels,
prothrombin time and activated partial thromboplastin time.
Patient no
1. A female, non-pregnant, HIV-positive, 48-year-old
patient, weighing 75 kg, with blood glucose levels of 100 mg/dl,
attended the hospital on May 21, 2020, with a 5-day history of
COVID-19-associated symptoms that had begun on May 16, 2020. The
diagnosis of COVID-19 was confirmed on May 21, 2020. Patient no. 1
used Tenofovir, Lamivudi, Dolutegravir and Topotecan for the
control of HIV on a daily basis; HIV-infection date was not
available.
For COVID-19 infection, the patient received the
following treatment: Oseltamivir (75 mg; May 23 to May 26),
Ceftriaxone (2 g; May 23 to May 26), Clexane (40 mg; May 23),
Ceftriaxone (2 g; May 22), Prednisone (40 mg; May 26), Azythromycin
(500 mg; May 22 to May 26) and Levofloxacin (750 mg; May 26).
As depicted in Fig.
1 (black squares), patient no. 1 exhibited reduced
erythrocytes, hemoglobin, hematocrit and platelet levels on the
first day of hospitalization. Of note, the patient presented normal
leukocyte, lymphocyte and neutrophil numbers. The only increased
COVID-19-associated severity marker was C-reactive protein (73.7
mg/l), present at day 1(4).
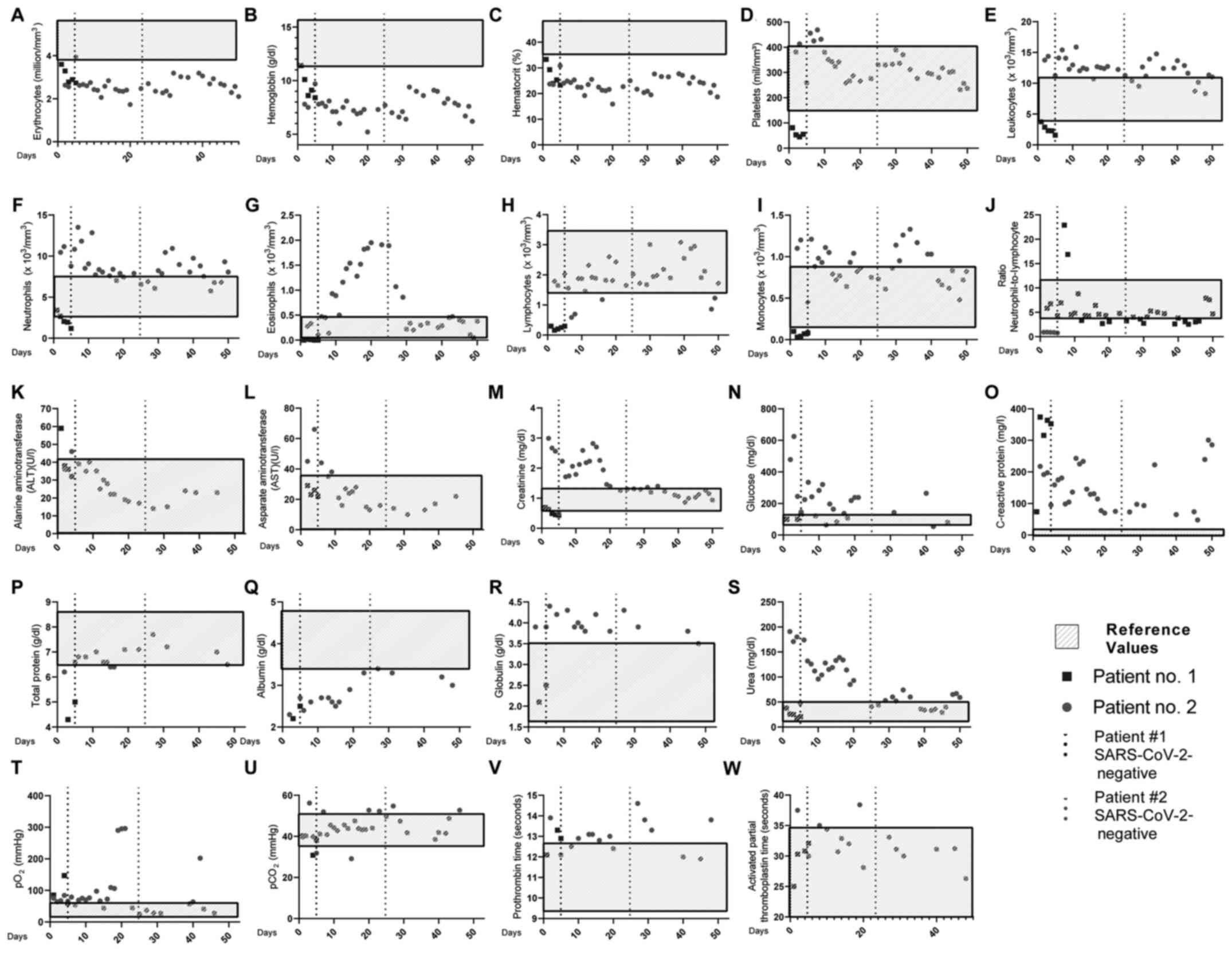 | Figure 1Daily clinical features of the 2
patients. Blood levels of (A) erythrocytes, (B) hemoglobin, (C)
hematocrit, (D) platelets, (E) leukocytes, (F) neutrophils, (G)
eosinophils, (H) lymphocytes, (I) monocytes, (J)
neutrophil-to-lymphocyte ratio, (K) ALT, (L) AST, (M) creatinine,
(N) glucose, (O) C-reactive protein, (P) total protein, (Q)
albumin, (R) globulin, (S) urea, (T) pO2, (U)
pCO2, (V) prothrombin time, (W) activated partial
thromboplastin time. The light grey rectangular boxes represent
reference values; black squares represent patient no. 1; closed
grey circles represent patient no. 2; dotted lines represent the
date the patients were cleared of SARS-CoV-2 infection. |
The patient was cleared of SARS-CoV-2 infection on
day 10 after the beginning of the symptoms (5 days after the
diagnosis of COVID-19). The patient was cured of COVID-19 and
released from the hospital on May 26, 2020.
Patient no
2. A female non-pregnant, 49-year-old patient,
weighing 90 kg, obese, with a body mass index (BMI) of 33.05 and
blood glucose levels of 478 mg/dl, attended the hospital on May 1,
2020 with a 4-day history of COVID-19-associated symptoms. COVID-19
was confirmed on May 1, 2020.
The patient's history included the following: Type 2
diabetes mellitus, obesity, 2 previous heart attacks, HIV
positivity since 2009, and the regular daily usage of quetiapine,
captopril and furosemide, omeprazole and enoxaparin, and darunavir
for the control of HIV. No HIV viral load was detectable.
During the COVID-19 infection, the patient underwent
a series of drug treatments: Ampicyllin (2 g; May 4 to May 12),
Polymyxin B (1000000 Units; May 12 to May 14), Meropenem (1 g; May
11 to May 19) and Vancomyicin (1 g; May 12 to May 19), Enoxaparin
(80 mg) and Ceftriaxone (2 g; May 21 to May 26), Azythromycin (500
mg; May 21 to May 25), Oseltamivir (75 mg; May 22).
As depicted in Fig.
1 (closed grey circles), patient no. 2 exhibited reduced
erythrocyte, hemoglobin and hematocrit levels. Of note, the patient
exhibited an increase in neutrophil numbers, but not in the
neutrophil-to-lymphocyte ratio (13). Other laboratory features which were
above the reference values were AST, creatinine, C-reactive protein
and urea (4).
The patient was cleared of SARS-CoV-2 infection on
day 29 after the beginning of the symptoms (25 days after the
diagnosis of COVID-19), but remained in the hospital due to
alterations in laboratory data. Notably, 10 days prior to the
SARS-CoV-2 infection, the eosinophil count began to increase,
peaking at 1 day prior to SARS-CoV-2 clearance, and abruptly
decreasing to regular levels over the following days. Blood glucose
and creatinine levels also reached normal levels almost
concomitantly to SARS-CoV-2 clearance (Fig. 1; closed grey circles).
Patient no. 2 was cured of COVID-19 and released
from the special ward for COVID-19 patients and being transferred
to the general ward on June 25 and later discharged on June 30,
2020.
Discussion
Both patients presented herein had a similar age and
were HIV-positive with no detectable viral load. Even though they
had SARS-CoV-2 infection, the general clinical features were
markedly distinct between them. Patient no. 1 had leucopenia and a
low neutrophil-to-lymphocyte ratio, a hallmark of severity in
COVID-19 (13,14). Patient no. 2 exhibited an increase
in leukocyte and neutrophil numbers, but maintained a normal level
of the neutrophil-to-lymphocyte ratio. A common feature between
both patients was the elevated c-reactive protein levels and low
levels of serum albumin, both previously associated with severity
in COVID-19(15).
A distinguishing feature between both patients was
blood glucose levels: patient no. 1 evolved with normal levels
during hospitalization, while patient no. 2 exhibited elevated
blood glucose levels during SARS-CoV-2-infection, which and
returned to close to normal ranges following the clearance of
SARS-CoV-2. A recent study described that high glucose levels may
facilitate SARS-CoV-2 replication, and therefore promote and
exacerbate the inflammatory response by monocytes (16). Of note, patient no. 2 also
presented an increase in circulating monocyte numbers. Monocytes
have been also implicated in the production of IL-6 and the
induction of the cytokine storm in COVID-19, further increasing
IL-6 production in intensive care unit patients (17).
Although previous research has not identified the
mechanisms through which eosinophils may contribute to COVID-19
resolution, it has been hypothesized that an increase in eosinophil
numbers may be an indicator of COVID-19 improvement (18). These findings are in accordance
with the present findings, revealing an increase in the eosinophil
count previous to SARS-CoV-2 clearance in patient no. 2.
A striking difference between the 2 patients was the
SARS-CoV-2 clearance time: This was 10 days for patient no. 1 (5
days after hospitalization) and 29 days for patient no. 2 (25 days
after hospitalization). A previous case report on a patient with
HIV identified a delayed immune response against SARS-CoV-2, with
detectable blood IgM identified 60 days following infection
(19). A larger cohort study also
verified that almost one-third of HIV-positive patients remained
positive for SARS-CoV-2 after 40 days of the initiation of symptoms
(20).
Härter et al identified that among
HIV-positive fatalities, other comorbidities than HIV infection may
present a larger role in SARS-CoV-2 infection (2). In the present case report, this
appears to be true, as both patients exhibited an undetectable HIV
viral load and a similar CD4/CD8 cell count. A hallmark difference
between the 2 patients was the presence of comorbidities other than
HIV. Patient no. 1 had no comorbidities, while patient no. 2 had
systemic arterial hypertension (SAH), type 2 diabetes mellitus and
obesity (body mass index, 33.05).
HIV infection also predisposes individuals to the
development of type 2 diabetes mellitus (21), via alterations in adipocyte and
T-cell metabolism, increased lipolysis, hyperglycemia,
hypertriglyceridemia and hepatic steatosis (21). Obesity also increases T-cell
activation and pro-inflammatory cytokine production in consequence
of the caloric surplus and an increase in adipose tissue
inflammation. Therefore, HIV and obesity may play a synergic role,
impairing the anti-SARS-CoV-2 immune response (22).
Patient no. 2 also presented SAH, a risk factor for
mortality in COVID-19 (4,23). It is noteworthy that there is no
consensus as to whether HIV or anti-viral treatment may influence
SAH development; however, obesity, type 2 diabetes mellitus and SAH
are associated, as approximately 80% of patients with type 2
diabetes develop SAH (24).
A recent retrospective study identified no
differences in the infection rate and severity on SARS-CoV-2
infection regarding HIV infection (25); nevertheless, the effects of
COVID-19 on patients with acquired immunodeficiency syndrome (AIDS)
warrant further in-depth investigations (26). Overall, the present case report
study reinforces a potential role for other comorbidities, rather
than HIV infection as regards SARS-CoV-2 infection outcomes.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Fundação de
Amparo à Pesquisa do Estado de São Paulo (FAPESP; grant nos.
19/02679-7 and 17/18199-9).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
RWA conceived and designed the study, and performed
the data analysis and interpretation. VA conceived and designed the
study, and performed the data analysis and interpretation. MNS
performed the data analysis and interpretation. All the authors
contributed to the writing and final approval of the manuscript and
agree to be accountable for all aspects of the research in ensuring
that the accuracy or integrity of any part of the work are
appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Hospital das Clínicas from the University of São Paulo
(HCFMUSP; no. 30800520.7.0000.0068-2020), and was performed out in
conformity with the 2013 revision of the Declaration of Helsinki.
Informed consent was obtained from both patients.
Patient consent for publication
Informed consent was obtained from both
patients.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jambo KC, Sepako E, Fullerton DG, Mzinza
D, Glennie S, Wright AK, Heyderman RS and Gordon SB:
Bronchoalveolar CD4+ T cell responses to respiratory
antigens are impaired in HIV-infected adults. Thorax. 66:375–382.
2011.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Härter G, Spinner CD, Roider J, Bickel M,
Krznaric I, Grunwald S, Schabaz F, Gillor D, Postel N, Mueller MC,
et al: COVID-19 in people living with human immunodeficiency virus:
A case series of 33 patients. Infection. 48:681–686.
2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Hussain N, Yoganathan A, Hewage S, Alom S
and Harky A: The effect of antivirals on COVID-19: A systematic
review. Expert Rev Anti Infect Ther: Oct 20, 2020 (Epub ahead of
print).
|
|
4
|
Chen G, Wu D, Guo W, Cao Y, Huang D, Wang
H, Wang T, Zhang X, Chen H, Yu H, et al: Clinical and immunological
features of severe and moderate coronavirus disease 2019. J Clin
Invest. 130:2620–2629. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Branco ACCC, Sato MN and Alberca RW: The
possible dual role of the ACE2 receptor in asthma and coronavirus
(SARS-CoV2) infection. Front Cell Infect Microbiol.
10(550571)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Alberca RW, Pereira NZ, Oliveira LMDS,
Gozzi-Silva SC and Sato MN: Pregnancy, viral infection, and
COVID-19. Front Immunol. 11(1672)2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Alberca RW: Asthma endotypes and COVID-19.
J Asthma: Oct 1, 2020 (Epub ahead of print).
|
|
8
|
Mirzaei H, McFarland W, Karamouzian M and
Sharifi H: COVID-19 among people living with HIV: a systematic
review. AIDS Behav: Jul 30, 2020 (Epub ahead of print).
|
|
9
|
Alberca RW, Yendo TM, Leuzzi Ramos YÁ,
Fernandes IG, Oliveira LM, Teixeira FME, Beserra DR, de Oliveira
EA, Gozzi-Silva SC, Andrade MMS, et al: Case report: COVID-19 and
Chagas disease in Two coinfected patients. Am J Trop Med Hyg: Oct
6, 2020 (Epub ahead of print).
|
|
10
|
Marino A, Pampaloni A, Scuderi D,
Cosentino F, Moscatt V, Ceccarelli M, Gussio M, Celesia BM, Bruno
R, Borraccino S, Borraccino S, et al: High-flow nasal cannula
oxygenation and tocilizumab administration in patients critically
ill with COVID-19: A report of three cases and a literature review.
World Acad Sci J. 2(23)2020.
|
|
11
|
Chen H, Zhang L, He Z, Wang D, Liu L,
Xhang W, Chen T, Dai Z, Han Z and Chen M: Systemic administration
of human umbilical cord-derived mesenchymal stem cells effectively
ameliorates the outcomes of a critically ill elderly patient with
COVID-19 with multiple comorbidities: A case report. World Acad Sci
J. 2(29)2020.
|
|
12
|
Alberca RW, Teixeira FME, Beserra DR, de
Oliveira EA, Andrade MMS, Pietrobon AJ and Sato MN: Perspective:
The Potential Effects of Naringenin in COVID-19. Front Immunol.
11(570919)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Liu Y, Du X, Chen J, Jin Y, Peng L, Wang
HHX, Luo M, Chen L and Zhao Y: Neutrophil-to-lymphocyte ratio as an
independent risk factor for mortality in hospitalized patients with
COVID-19. J Infect. 81:e6–e12. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alberca RW, Andrade MMS, Branco ACCC,
Pietrobon AJ, Pereira NZ, Fernandes IG, Oliveira LM, Teixeira FME,
Beserra DR, de Oliveira EA, et al: Frequencies of CD33+CD11b+
HLA-DR-CD14-CD66b+ and CD33+CD11b+HLA-DR-CD14+ CD66b- cells in
peripheral blood as severity immune biomarkers in COVID-19. Front
Med (Lausanne). 7(580677)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
de la Rica R, Borges M, Aranda M, Del
Castillo A, Socias A, Payeras A, Rialp G, Socias L, Masmiquel L and
Gonzalez-Freire M: Low albumin levels are associated with poorer
outcomes in a case series of COVID-19 patients in Spain: a
retrospective cohort study. Microorganisms. 8(1106)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Codo AC, Davanzo GG, Monteiro LB, de Souza
GF, Muraro SP, Virgilio-da-Silva JV, Prodonoff JS, Carregari VC, de
Biagi Junior CAO, Crunfli F, et al: Elevated glucose levels favor
SARS-CoV-2 infection and monocyte response through a
HIF-1α/glycolysis dependent axis. Cell Metab. 32:498–499.
2020.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhou Y, Fu B, Zheng X, Wang D, Zhao C, Qi
Y, Sun R, Tian Z, Xu X and Wei H: Pathogenic T cells and
inflammatory monocytes incite inflammatory storm in severe COVID-19
patients. Natl Sci Rev. 7:998–1002. 2020.
|
|
18
|
Liu F, Xu A, Zhang Y, Xuan W, Yan T, Pan
K, Yu W and Zhang J: Patients of COVID-19 may benefit from
sustained Lopinavir-combined regimen and the increase of Eosinophil
may predict the outcome of COVID-19 progression. Int J Infect Dis.
95:183–191. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Wang M, Luo L, Bu H and Xia H: One case of
coronavirus disease 2019 (COVID-19) in a patient co-infected by HIV
with a low CD4+ T-cell count. Int J Infect Dis. 96:148–150.
2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vizcarra P, Pérez-Elías MJ, Quereda C,
Moreno A, Vivancos MJ, Dronda F, Casado JL, Moreno S, Pérez-Elías
MJ, Fortún J, et al: COVID-19 ID Team: Description of COVID-19 in
HIV-infected individuals: A single-centre, prospective cohort.
Lancet HIV. 7:e554–e564. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Agarwal N, Iyer D, Gabbi C, Saha P, Patel
SG, Mo Q, Chang B, Goswami B, Schubert U, Kopp JB, et al: HIV-1
viral protein R (Vpr) induces fatty liver in mice via LXRα and
PPARα dysregulation: Implications for HIV-specific pathogenesis of
NAFLD. Sci Rep. 7(7)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Wanjalla CN, McDonnell WJ and Koethe JR:
Adipose tissue T Cells in HIV/SIV infection. Front Immunol.
9(2730)2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alberca RW, Oliveira LM, Branco ACCC,
Pereira NZ and Sato MN: Obesity as a risk factor for COVID-19: An
overview. Crit Rev Food Sci Nutr: Jun 15, 2020 (Epub ahead of
print).
|
|
24
|
Lukic L, Lalic NM, Rajkovic N, Jotic A,
Lalic K, Milicic T, Seferovic JP, Macesic M and Gajovic JS:
Hypertension in obese type 2 diabetes patients is associated with
increases in insulin resistance and IL-6 cytokine levels: Potential
targets for an efficient preventive intervention. Int J Environ Res
Public Health. 11:3586–3598. 2014.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Gervasoni C, Meraviglia P, Riva A,
Giacomelli A, Oreni L, Minisci D, Atzori C, Ridolfo A and Cattaneo
D: Clinical features and outcomes of patients with human
immunodeficiency virus with COVID-19. Clin Infect Dis.
71:2276–2278. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Cooper TJ, Woodward BL, Alom S and Harky
A: Coronavirus disease 2019 (COVID-19) outcomes in HIV/AIDS
patients: A systematic review. HIV Med. 21:567–577. 2020.PubMed/NCBI View Article : Google Scholar
|















