Introduction
Marjolin's ulcer (MU) is an ulcerating malignancy
that occurs in chronically inflamed or scarred tissues (1). It can be classified into acute or
chronic MU according to the latency period. No clearly defined
cause for MU has been described. However, it is considered that the
leading cause is mutations in focal cells due to chronic
inflammatory. Based on the medical history, lesion biopsy and
physical examination, MU can often be accurately diagnosed.
However, presently, there is no agreed standard for the definition,
classification, etiology, pathological type, location, treatment
and the prognosis of MU.
The present study reports a case of a 50-year-old
male with inguinal lymph node and bone metastases from a MU in a
burn scar on the left lower extremity. Furthermore, the relevant
literature is reviewed, in the hope that this may aid the
development of a preliminary clinical path for MU.
Case report
The present study was conducted in accordance with
the 1975 Declaration of Helsinki. The study protocol was approved
by the Ethics Committee of General Hospital of Southern Theater
Command, People's Liberation Army. The patient provided written
informed consent before investigations, screening, study and
treatment.
A 50-year-old male presented with a 49-year history
of a burn scar wound over the left lower extremity. When the
patient was 1 year old, the skin of his left lower limb was burnt
by fire. Following the change of the dressing, most of the wounds
healed. However, a wound with a size of approximately 3x2 cm behind
the knee did not heal for a long period of time. In August 2011,
the ulcer area had expanded, the wound edge had become red and
swollen, and was accompanied by pain and apparent colorless
exudation that occasionally turned red. Long-term antibiotic
therapy and dressing change failed to heal the ulcer.
Following a dermatological examination, the patient
was found to have an irregularly-shaped deep ulcer on the left
lower extremity dorsum, which was approximately 36x16 cm. The wound
granulation exhibited a cauliflower-like pattern, and a thick layer
of necrotic material was observed. The edge of the ulcer was hard
and everted, approximately 1 cm higher than the surrounding skin. A
large amount of exudate was observed on the wound, and a foul odor
was present. The left inguinal lymph nodes were enlarged. The size
of the lower lymph node group was approximately 3x5 cm, and the
upper group consisted of 5-6 lymph nodes of approximately 1x1 cm.
The enlarged lymph nodes were somewhat hard, with no apparent
tenderness, a low degree of activity, and no adhesion to the
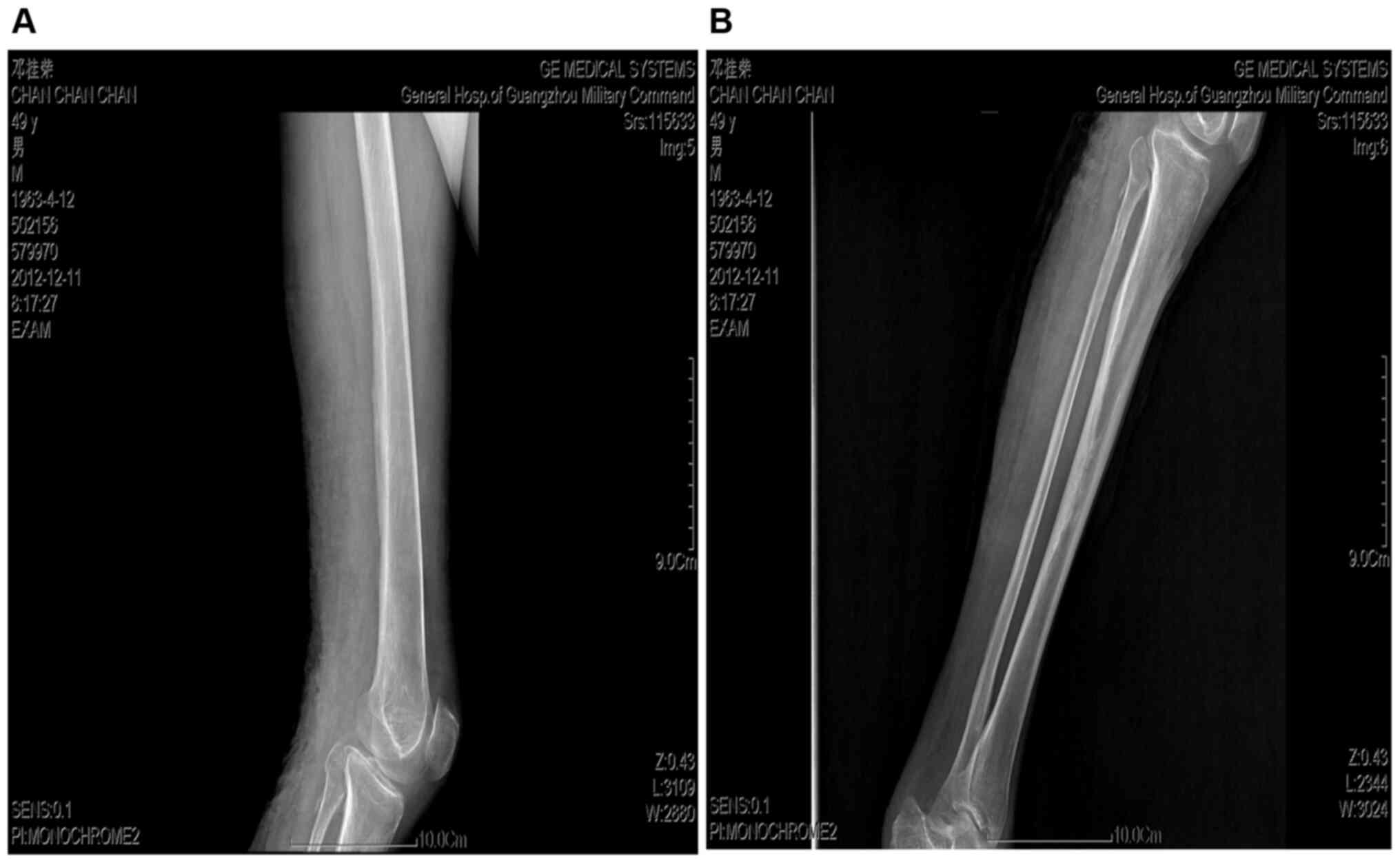
surrounding tissues. No bone tissue damage was detected on leg
radiographs (Fig. 1). A biopsy of
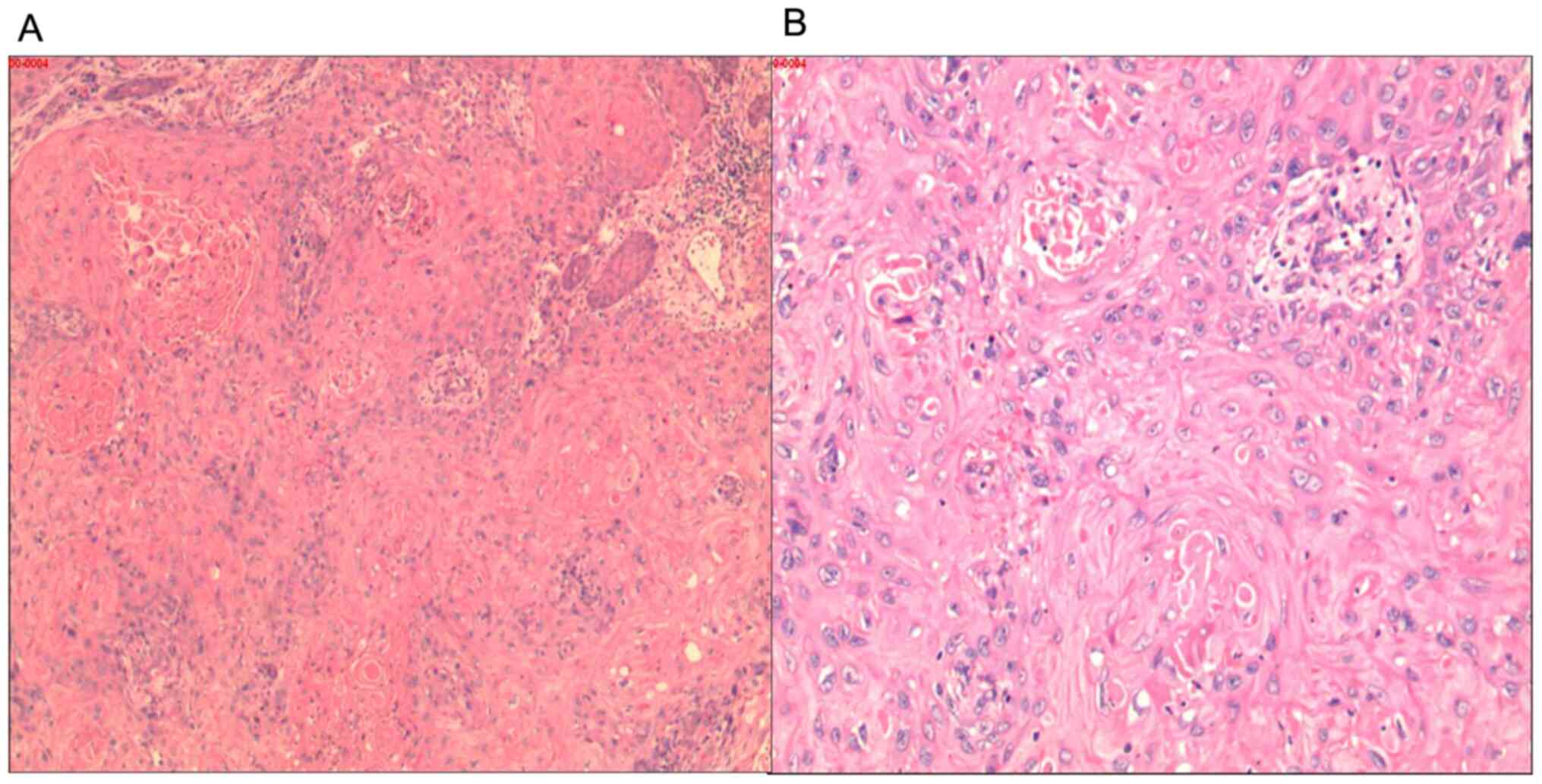
the ulcerative tissue (performed by a pathologist) revealed highly
differentiated squamous cell carcinoma (Fig. 2).
The patient refused to undergo computed tomography
(CT) or magnetic resonance imaging (MRI) in search of metastases
from the lesion and objected to lymph node biopsy or lymph node
resection. After correcting anemia, the left lower extremity lesion
was surgically removed with a wide excision. The resection included
a margin of 2-3 cm around the lesion and extended to the deep
fascia. Following a week of closed negative pressure suction, the
wound was transplanted with blade-thin skin taken from the
patient's back. The skin survived well after the surgery.
The MU recurred 3 months later. The tumor and the
inguinal lymph nodes were removed under general anesthesia. During
the surgery, the subcutaneous and muscular layers had a fish-like
appearance, and the bones were infiltrated approximately 5 cm below
the femoral head. Osteoporosis caused the bone to look appear
similar to a bean curd. The pathological diagnosis (performed by a
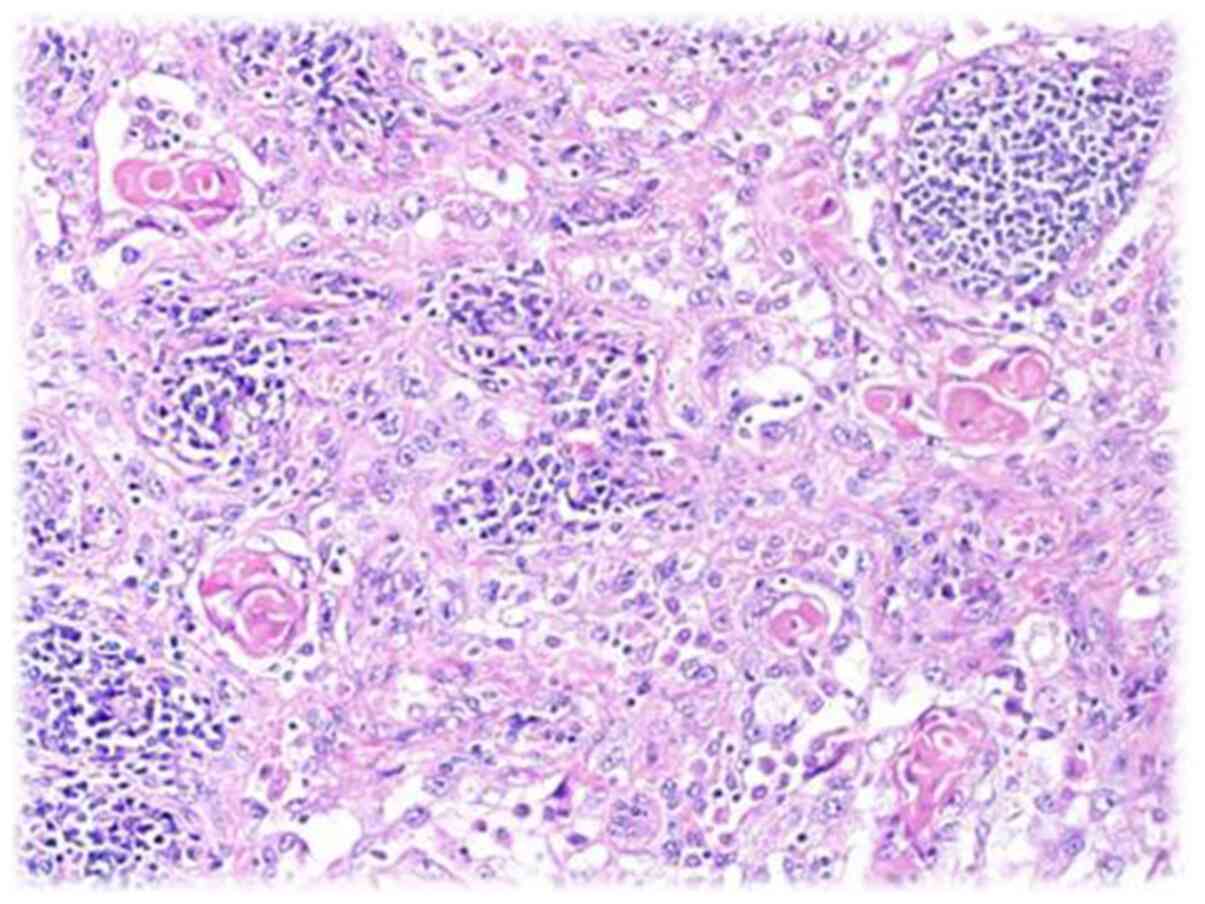
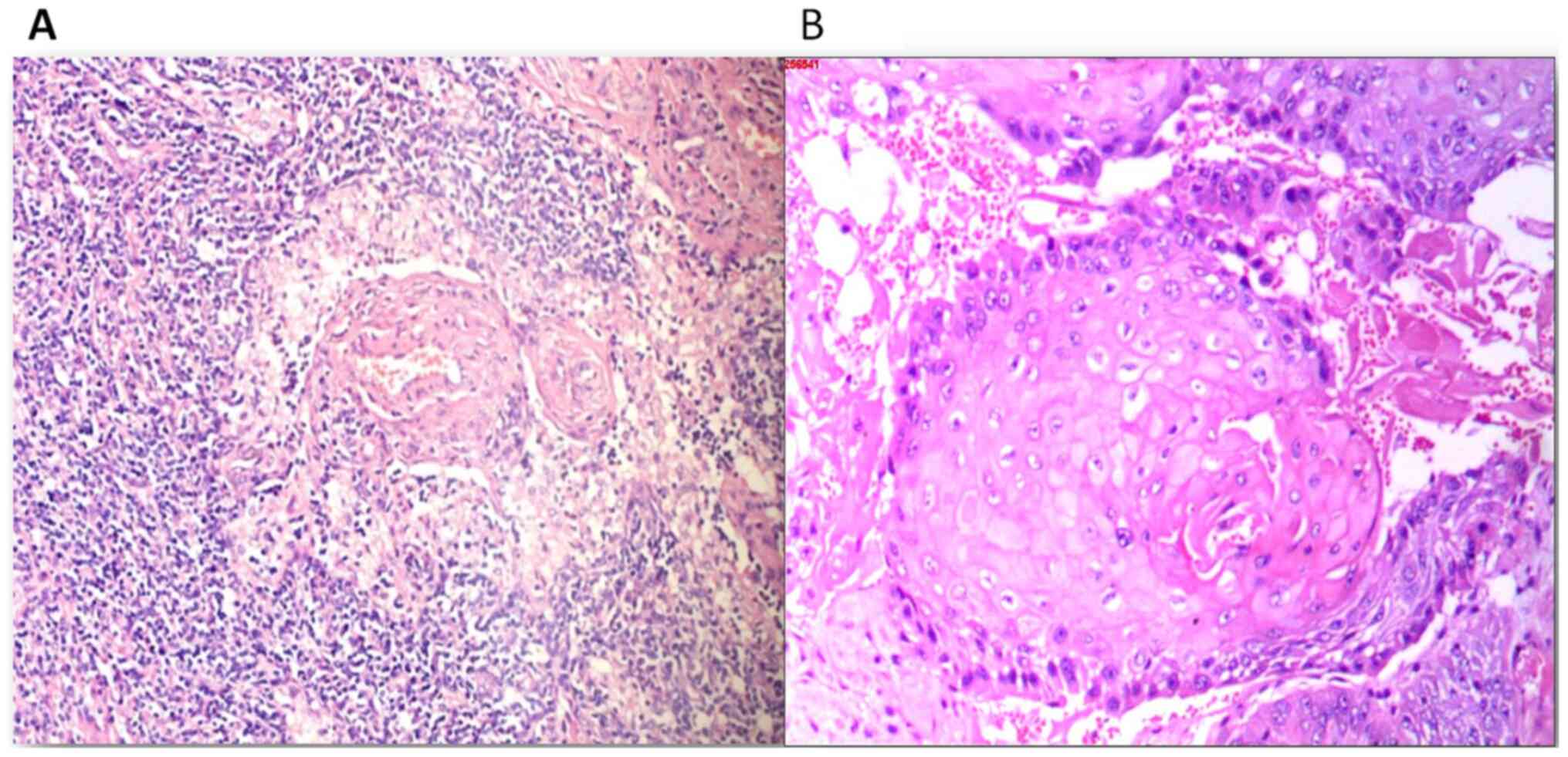
pathologist) of the lymphoid and ulcer tissues was squamous cell
carcinoma (Figs. 3 and 4). The wound was first treated with
negative pressure drainage, and was grafted with skin after the
granulation tissue filled the wound. The tumor did not recur during
the 1 year of follow-up.
As regards experiences and lessons learnt, he
principle of MU treatment is to extensively remove the tumor. In
order to achieve this, a thorough evaluation is required. This
should include CT or MRI and lymph node biopsies or resection in
search of metastases. The patient presented herein refused such
tests and the tumor recurred with bone metastasis. A more thorough
second surgical resection that included resection of the regional
lymph nodes was more effective as the tumor did not recur
again.
Discussion
MU is secondary to traumatized or chronically
inflamed skin, particularly following burns (1-3).
It is characterized as being aggressive, but has a low incidence
rate (1-3).
MU is often overlooked and inadequately treated (4-6).
Presently, information on MU is limited. The condition remains to
be defined by the medical community and guidelines for its
management are required.
Definition, classification and
characteristics of MU
In 1828, the French physician Jean NicholasMarjolin
first described the ‘warty ulcer' that occurred on scars and termed
it ‘cancroidal ulcer'. However, he did not know the warty ulcers he
described were malignant. In 1903, Da Costa reported 2 cases of
malignant ulcers on the lower extremities with a history of venous
ulcers and considered them to be consistent with the ulcers
described by Marjolin (7).
Subsequently, the malignant ulcers occurring on chronic wounds or
scars gradually began to be referred to ‘Marjolin ulcers'.
In 1990, Hahn et al (8) summarized the data of 19 patients with
MU and found that they were mainly 40-60-year-old males; the ulcers
were primarily in the lower limbs; the primary injuries were mostly
burns, followed by chronic osteomyelitis; the mean latent period
was 31.5 years; the rate of metastasis at the time of diagnosis was
32%; histopathological examination revealed that all were squamous
cell carcinoma; and the average time for local recurrence was 8.8
months. Copcu et al (1)
followed 264 patients with burns treated at the Izmir Ataturk
Hospital from 1994 to 2001. The average follow-up time was 3.8
years. Their survey found 31 patients with MU (11.7%) and 14
patients with benign ulcers (5.3%). Chalya et al (9) conducted a retrospective study of
patients with MU at the Bugando Medical Center, Tanzania, from 2001
to 2010. They found that MUs were not rare in their area. In 2005,
Kowal-Vern and Criswell (10)
found that the mean age at MU diagnosis in their study population
was 50 years, and males were more commonly affected than females,
with males accounting for approximately 62% of cases. Bazaliński
et al (11) reviewed the
history, etiopathogenesis, diagnosis and treatment of MU, and found
that the early diagnosis of these wounds could reduce tissue damage
and resection scope.
According to the available literature, MUs can be
divided into narrowly-defined scar cancers, which occur in
malignant tumors of scar ulcers (5,12)
caused by traumatic or chronically inflamed skin, burns in
particular (1,3). They are classified into acute and
chronic MU based on the latency period. The latency period is
#<1 year for acute MU and >1 year for chronic MU (11,13-15).
The lower extremities have the highest probability
of developing MU. However, MU can also occur in multiple other
parts of the body, including the neck, elbow, scalp, calvarial
bones, dura mater, brain, breast skin, nose and other sites
(16-21).
The pathological types of MU include squamous cell carcinoma, basal
cell carcinoma, malignant melanoma, sarcoma, squamous basal cell
carcinoma, squamous cell melanoma and other neoplasms (10,22,23).
Among these, squamous cell carcinoma is the most common
pathological type of MU (10).
Etiology
MU causative lesions include burns (70-90%), chronic
venous ulcers, chronic injuries, scars, chronic osteomyelitis,
radiation-induced wounds, diabetic foot, pressure ulcers, venous
stasis, hidradenitis and others (11,24-27).
There is currently no clear explanation as to the exact causes of
MU. MU is considered to arise primarily from long-term chronic
inflammatory stimulation that leads to mutations in focal cells.
Harland et al (28)
detected a homozygous deletion of the p53 gene in this
burn-related carcinoma. Lee et al (29) demonstrated that some burn
scar-related squamous cell carcinomas had Fas gene mutations
in regions important for the apoptosis function, and suggested
these to be involved in the pathogenesis of the disease. Sinha
et al (30) identified
transcriptional changes leading to malignancy by comparing
differentially expressed genes in squamous cells in squamous cell
carcinoma and MU.
Diagnosis
MUs can often be accurately diagnosed based on the
medical history and a physical examination. It should be suspected
that the ulcer is malignant when its recovery is prolonged and the
patient relapses, particularly when the secretions increase,
exhibit malodor and are prone to containing blood. All patients can
be further diagnosed by acquiring biopsies from the lesion.
Pathological biopsies should be taken from multiple locations in
the ulcer center and margin, and should go deep into the
subcutaneous tissue to avoid missed diagnosis. Ultrasonography can
be used as a primary modality to identify lymph node metastasis.
Radiography and CT can distinguish common imaging features of MU,
including bone destruction, soft tissue mass, and a periosteal
reaction (31). An MRI provides
excellent soft-tissue detail, such as tumor extent, depth, margins,
underlying bone cortical or marrow involvement, or the involvement
of adjacent neurovascular structures (32). Positron emission tomography-CT
(PET-CT) is useful in differentiating MU from benign inflammatory
conditions of chronic nonhealing ulcers. It reveals a relatively
good correlation with surgical or pathological results in
determining the invasion depth (33). PET-MRI is feasible and performs
equally well as PET-CT in the majority of cancers, with the benefit
that the human body is exposed to a far lower radiation dose
(34). However, there are no
reports on the use of PET-MRI for the diagnosis of MU.
Treatment and prognosis
There currently no standardized treatment protocol
available for MU. The extensive resection of the tumor is
necessary. Bang and Woo (35)
suggested that an aggressive excision and reconstruction with free
tissue transfer or regional flap transposition should be adopted
for adequate ablation and definitive coverage, rather than skin
graft and regular surveillance. It was recommended that the
smallest skin margin removed around the outer ulcer edge should be
at least 2.5 cm (11). There is no
consensus as to whether to perform sentinel node biopsy (36). If the MU is accompanied by local or
distant lymph node metastasis, lymph node resection is required.
Similarly, if the sentinel lymph node biopsy is positive, it is
recommended to perform routine lymph node resection. For MU of the
limb, amputation or hemipelvectomy (37) may be considered if the bone and
joint were invaded, rendering radical resection of the lesion
difficult to complete. Amputation or hemipelvectomy may also be
considered if the limb function is severely impaired following
radical resection. For the early stages of MU, an aggressive
combined approach that includes extensive excision, lymphatic
resection, postoperative radiotherapy and/or chemotherapy, and
amputation if needed, might increase the cure rate. However, there
is no consensus on advanced disease treatment, as it often produces
unsuccessful results. At present, it is not recommended to
routinely prescribe radiotherapy and chemotherapy to MU patients
because the sensitivity of MU to these treatments is low and
because of the risk of radiation-induced cell carcinogenesis.
However, radiotherapy may be considered if the MU pathology is of a
poorly differentiated type, the cancer has invaded the bone, there
is distant metastasis, or the patient cannot tolerate or refuses to
undergo surgery.
The prognosis is dictated by the time from injury to
malignancy development, size, location, degree of differentiation,
lymph node status, and metastasis at the time of diagnosis
(11,38,39).
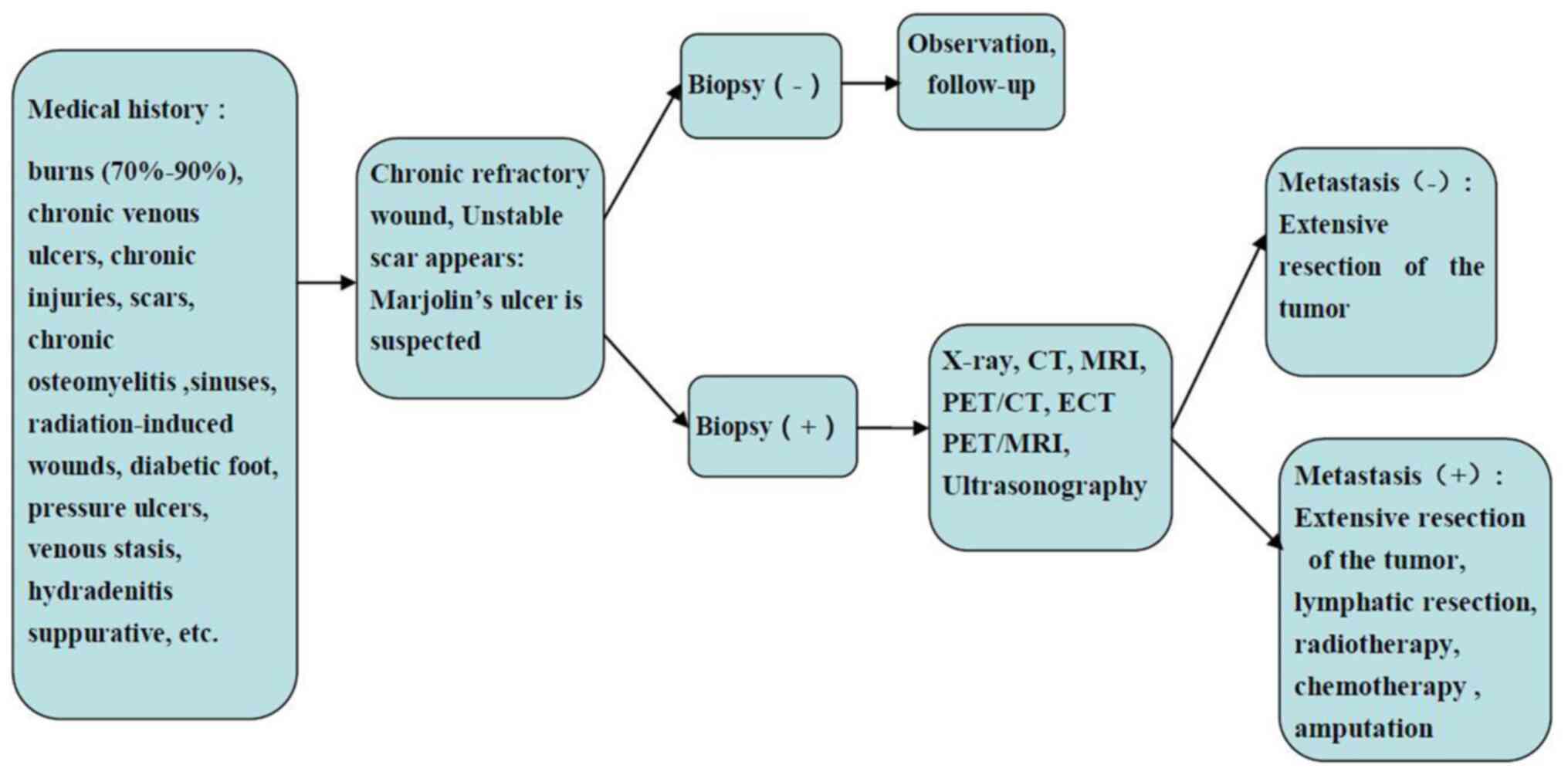
The current literature review suggests that the
diagnosis and treatment of MU should follow the flow chart
illustrated in Fig. 5. It is hoped
that the information provided herein will help standardize the
diagnosis and treatment of MU.
In conclusion, Mus may not be an uncommon health
issue with unique features. MU diagnosis and treatment guideline
development would aid in the early detection and proper management
of the disease, thus reducing the rates of missed diagnosis,
recurrence, and mortality due to MU. However, large-scale MU
clinical studies are limited; the staging criteria have not yet
been established, and the choice of treatment options is mostly
empirical or based on small sample observations rather than
evidence-based practice. Multicenter clinical collaborative
research is required to provide useful guidance for the precise
diagnosis and treatment of MU.
Acknowledgements
The authors are grateful to the staff of the
associated centers for providing assistance.
Funding
The present study was supported by grants from the
National Natural Science Foundation of China (nos. 81171812 and
81272105), the National Basic Science and Development Program (973
Program, 2012 CB518105), the National Key Research and Development
Plan of Chin (no. 2017YFC1103301), the Science and Technology Key
Project of Guangdong Province (no. 2014B020212010), the Science and
Technology Planning Project of Guangdong Province of China (no.
2015B020233012) and the Military Medical Innovation Special
Projects (no. 18CXZ029).
Availability of data and materials
Data sharing is not applicable to this article, as
no datasets were generated or analyzed during the current
study.
Authors' contributions
BC reviewed the literature, interpreted the
information, and drafted the review. JT and JPZ assisted in the
preparation of the figures, complied the reference list, and
revised the manuscript, and subsequently updated it as appropriate.
XFX and JBT provided recommendations and assisted in the drafting
and revising of the review. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
the 1975 Declaration of Helsinki. The study protocol was approved
by the Ethics Committee of General Hospital of Southern Theater
Command, People's Liberation Army. The patient provided written
informed consent before investigations, screening, study and
treatment.
Patient consent for publication
The patient signed an informed consent form to agree
that the data can be published.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Copcu E, Aktas A, Sişman N and Oztan Y:
Thirty-one cases of Marjolin's ulcer. Clin Exp Dermatol.
28:138–141. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Powell HB, Googe PB and Sayed CJ: Squamous
cell carcinoma arising in a chronic perineal wound in a patient
with long-standing cutaneous Crohn's disease. JAAD Case Rep.
4:346–348. 2018.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Saaiq M and Ashraf B: The menace of
self-immolation plaguing low income countries. Burns. 42:472–473.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pieptu D, Luchian S, Copăceanu M, Popa M,
Hriscu M and Stătescu C: Marjolin's ulcer on burn scar, a curable
but neglected disease. Rev Med Chir Soc Med Nat Iasi. 104:95–99.
2000.PubMed/NCBI(In Romanian).
|
|
5
|
Fleming MD, Hunt JL, Purdue GF and
Sandstad J: Marjolin's ulcer: a review and reevaluation of a
difficult problem. J Burn Care Rehabil. 11:460–469. 1990.PubMed/NCBI
|
|
6
|
Kerr-Valentic MA, Samimi K, Rohlen BH,
Agarwal JP and Rockwell WB: Marjolin's ulcer: Modern analysis of an
ancient problem. Plast Reconstr Surg. 123:184–191. 2009.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Da*Costa JC: III. Carcinomatous Changes in
an Area of Chronic Ulceration, or Marjolin's Ulcer. Ann Surg.
37:496–502. 1903.PubMed/NCBI
|
|
8
|
Hahn SB, Kim DJ and Jeon CH: Clinical
study of Marjolin's ulcer. Yonsei Med J. 31:234–241.
1990.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chalya PL, Mabula JB, Rambau P, Mchembe
MD, Kahima KJ, Chandika AB, Giiti G, Masalu N, Ssentongo R and
Gilyoma JM: Marjolin's ulcers at a university teaching hospital in
Northwestern Tanzania: A retrospective review of 56 cases. World J
Surg Oncol. 10(38)2012.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kowal-Vern A and Criswell BK: Burn scar
neoplasms: A literature review and statistical analysis. Burns.
31:403–413. 2005.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Bazaliński D, Przybek-Mita J, Barańska B
and Więch P: Marjolin's ulcer in chronic wounds - review of
available literature. Contemp Oncol (Pozn). 21:197–202.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Metwally IH, Roshdy A, Saleh SS and Ezzat
M: Epidemiology and predictors of recurrence of Marjolin's ulcer:
Experience from Mansoura Universityxs. Ann R Coll Surg Engl.
99:245–249. 2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Moonsamy P, Nazarian RM, Schulz JT and
Goverman J: Acute Marjolin's Ulcer Arising in a Split-Thickness
Skin Graft Postburn Injury. Eplasty. 16(ic31)2016.PubMed/NCBI
|
|
14
|
Baskara A, Sikka L, Khan F and Sapanara N:
Development of a Marjolin's ulcer within 9 months in a plantar
pressure ulcer. Eur J Dermatol. 20(225)2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Mohammadi AA, Foroutan A, Mohammadi S and
Anbardar MH: An odd very early marjolin's ulcer after minimal hand
burn. Ann Burns Fire Disasters. 30:218–219. 2017.PubMed/NCBI
|
|
16
|
Simmons MA, Edwards JM and Nigam A:
Marjolin's ulcer presenting in the neck. J Laryngol Otol.
114:980–982. 2000.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Ogawa B, Chen M, Margolis J, Schiller FJ
and Schnall SB: Marjolin's ulcer arising at the elbow: A case
report and literature review. Hand (N Y). 1:89–93. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Gupta SK, Sandhir RK, Jaiswal AK and Kumar
S: Marjolin's ulcer of the scalp invading calvarial bone, dura and
brain. J Clin Neurosci. 12:693–696. 2005.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Gatto A, Sebastiani S, Falvo L,
Giustiniani C and La*Rovere C: A rare case of aggressive
squamous-cell carcinoma of the breast skin: Marjolin's ulcer. A
case report. Chir Ital. 60:577–582. 2008.PubMed/NCBI
|
|
20
|
Mishra SS, Behera SK, Panigrahi S and
Senapati SB: Penetrating Marjolin's ulcer of scalp with
intracranial extension: A multidisciplinary experience. Asian J
Neurosurg. 9(240)2014.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Bhasme V, Agrawal M, Poonia NC, Bagaria H
and Chakotiya P: Post-burn spontaneous brain fungation caused by
infiltrating Marjolin's ulcer of scalp. Asian J Neurosurg.
12:256–258. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Koh SH, Oh SJ, Chun H and Kim SG:
Pseudoangiosarcomatous squamous cell carcinoma developing on a burn
scar: A case report and review of the literature. Burns.
40:e47–e52. 2014.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Noori VJ, Trehan K, Savetamal A and Carter
DW: New onset squamous cell carcinoma in previous split-thickness
skin graft donor site. Int J Surg. 52:16–19. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nthumba PM: Marjolin's ulcers: Theories,
prognostic factors and their peculiarities in spina bifida
patients. World J Surg Oncol. 8(108)2010.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Eliassen A, Vandy F, McHugh J and Henke
PK: Marjolin's ulcer in a patient with chronic venous stasis. Ann
Vasc Surg. 27:1182.e5–1182.e8. 2013.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Yon JR, Son JD, Fredericks C, Morton M,
Kingsley S, Gupta S, Poulakidas S and Bokhari F: Marjolin's Ulcer
in Chronic Hidradenitis Suppurativa: A Rare Complication of an
Often Neglected Disease. J Burn Care Res. 38:121–124.
2017.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Garcia-Marín JA, de Alcala Martinez-Gomez
D, Campillo-Soto A and Aguayo-Albasini JL: Marjolin's ulcer. A 10
year experience in a diabetic foot unit. Cir Cir. 84:340–343.
2016.PubMed/NCBI View Article : Google Scholar : (In Spanish).
|
|
28
|
Harland DL, Robinson WA and Franklin WA:
Deletion of the p53 gene in a patient with aggressive burn scar
carcinoma. J Trauma. 42:104–107. 1997.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lee SH, Shin MS, Kim HS, Park WS, Kim SY,
Jang JJ, Rhim KJ, Jang J, Lee HK, Park JY, et al: Somatic mutations
of Fas (Apo-1/CD95) gene in cutaneous squamous cell carcinoma
arising from a burn scar. J Invest Dermatol. 114:122–126.
2000.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Sinha S, Su S, Workentine M, Agabalyan N,
Cheng M, Gabriel V and Biernaskie J: Transcriptional analysis
reveals evidence of chronically impeded ECM turnover and epithelium
to mesenchyme transition in scar tissue giving rise to Marjolin's
ulcer. J Burn Care Res. 38:e14–e22. 2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Mohamed S, Abdullah B, Singh DA and Heng
KS: CT appearances of Marjolin's ulcer in the left gluteal region
of a young man. Biomed Imaging Interv J. 2(e26)2006.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Sawhney S, Jain R, Kakaria A and Chopra P:
Marjolin's Ulcer: Radiographic and magnetic resonance appearances
in two cases. Sultan Qaboos Univ Med J. 9:162–166. 2009.PubMed/NCBI
|
|
33
|
Ko Y, Han YM, Hwang HS, Kang IW, Hwang DH,
Lee ES and Lee GK: Role of 18F-FDG PET/CT in the
diagnosis of clinically suspected Marjolin ulcer. AJR Am J
Roentgenol. 199:1375–1379. 2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Lee MS, Cho JY, Kim SY, Cheon GJ, Moon MH,
Oh S, Lee J, Lee S, Woo S and Kim SH: Diagnostic value of
integrated PET/MRI for detection and localization of prostate
cancer: Comparative study of multiparametric MRI and PET/CT. J Magn
Reson Imaging. 45:597–609. 2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Bang CY and Woo SH: The Fate of Chronic
Burn Wounds Suspected as Marjolin's Ulcers. J Burn Care Res.
39:148–153. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Motamedolshariati M, Rezaei E,
Beiraghi-Toosi A, Jahani A, Tayyebi Meibodi N, Fattahi A and
Sadeghi R: Sentinel node mapping in Marjolin's ulcers: Is it.
feasible? Wounds. 27:54–62. 2015.PubMed/NCBI
|
|
37
|
Carlesimo B, Monarca C, Rizzo MI,
Tariciotti F and Staccioli S: Hemipelvectomy and reconstruction in
a patient with advanced Marjolin's ulcer: A case report. Int J Low
Extrem Wounds. 8:162–164. 2009.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Aydoğdu E, Yildirim S and Aköz T: Is
surgery an effective and adequate treatment in advanced Marjolin's.
ulcer? Burns. 31:421–431. 2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Pekarek B, Buck S and Osher L: A
Comprehensive Review on Marjolin's Ulcers: Diagnosis and Treatment.
J Am Col Certif Wound Spec. 3:60–64. 2011.PubMed/NCBI View Article : Google Scholar
|