Introduction
Gastrointestinal stromal tumors (GISTs) are a group
of tumors originating from the gastrointestinal mesenchymal tissue
and are the most common mesenchymal tumors of the digestive tract
(1); gastric stromal tumors
account for 60-70% of tumors of the digestive tract (1). GISTs occur in the fundus and stomach,
and exhibit malignant tendencies (2). Surgery, including open and
laparoscopic resection are the primary treatment options for GIST.
The laparoscopic treatment of GISTs is safe and effective, and is
suitable for the greater curvature or anterior wall lesions of the
stomach (3,4). However, tumors with intraluminal
growth and a small diameter, and lesions located in the cardia or
pylorus are difficult to locate. Excessive normal gastric tissues
may be removed, leading to gastric stenosis. Thus, the completely
effective application of this technique remains to be discussed
(4).
The endoscopic resection of GISTs has become the
mainstream treatment option, particularly for tumors with a
diameter of <3.5 cm (5).
Endoscopic mucosal resection (EMR) is simple, safe and effective
procedure, has few complications, and is easy to master. This
method can be used for the radical resection of small lesions
originating from the muscularis, particularly those without lymph
node and hematogenous metastasis. Intrinsic muscular layer GISTs
are treated with endoscopic mucosal dissection (ESD), endoscopic
submucosal excision (ESE) and endoscopic full-thickness resection
(EFR), all of which allow full tumor resection (6). However, given their origin from the
deep muscular or serosal layer, perforation is often difficult to
avoid, and endoscopic treatment is associated with the risk of
residual marginal tumor cells and the destruction of the tumor
capsule.
Natural orifice transluminal endoscopic surgery
(NOTES) was developed in 2007, and does not involve skin puncture,
but requires the insertion of a soft endoscopic device through the
body's natural orifices, such as the esophagus, stomach, vagina and
rectum into the abdominal cavity or in the body cavity for surgery
(7,8). This method has been applied for the
treatment of GISTs; however, the number of applied cases is limited
and the experience is not yet broad. For GISTs originating from the
serosal layer, there is no standard treatment plan which is being
followed at this stage. The present study describes a case of a
gastric stromal tumor treated with NOTES combined with ESD, and
provides a literature review in order to evaluate the safety and
feasibility of this technique.
Case report
General information and medical
history
The patient depicted in the present study was a
32-year-old married female admitted to the Department of
Gastroenterology, Taihe Hospital due togastric eminence. The
patient was first examined at the outpatient clinic due to
intermittent upper abdominal pain and bloating. An endoscopic
examination revealed chronic superficial gastritis with erosion,
and gastric antrum elevation and endoscopic ultrasound (EUS)
examination was recommended (Fig.
1A). Oral medications (unknown) were prescribed, and the
symptoms were alleviated, but recurred after the drug was stopped.
During the first hospital visit to the outpatient department, a
computed tomography (CT) scan of the upper abdomen was performed
(Fig. 2A). The greater curvature
of the gastric antrum showed small nodules, possibly enlarged lymph
nodes. The presence of a stromal tumor was excluded. Given that the
lesions were small, no further examinations and treatments were
performed. Following an EUS examination, a bulge in the gastric
antrum was still observed (Fig. 1B
and C). The patient's mucosa was
clear, and the possibility of stromal tumors was high. A relative
of the patient had passed way due to gastric cancer within the year
the lesion was discovered. The patient requested the lesion to be
removed and was admitted to the hospital. A physical examination
revealed a temperature of 36.6˚C, a pulse rate 70 beats/min,
respiratory rate (R) 18 breaths/min and a blood pressure of 118/65
mmHg. Epigastric tenderness was observed without other positive
signs. For the lesions, the combined results of EUS and the CT scan
suggested the presence of an exogenous stromal tumor possibly of
the serosal layer in origin. On the basis of minimal invasiveness,
surgical costs and post-operative cosmetic effects, the surgical
protocol was customized as ESD surgery combined with NOTES surgery.
A full communication with the patient and family members was
performed prior to the surgery. An informed written consent was
signed, and a hospital ethics committee certification was obtained.
The results of the pre-operative examination of the patient are
presented in Table I.
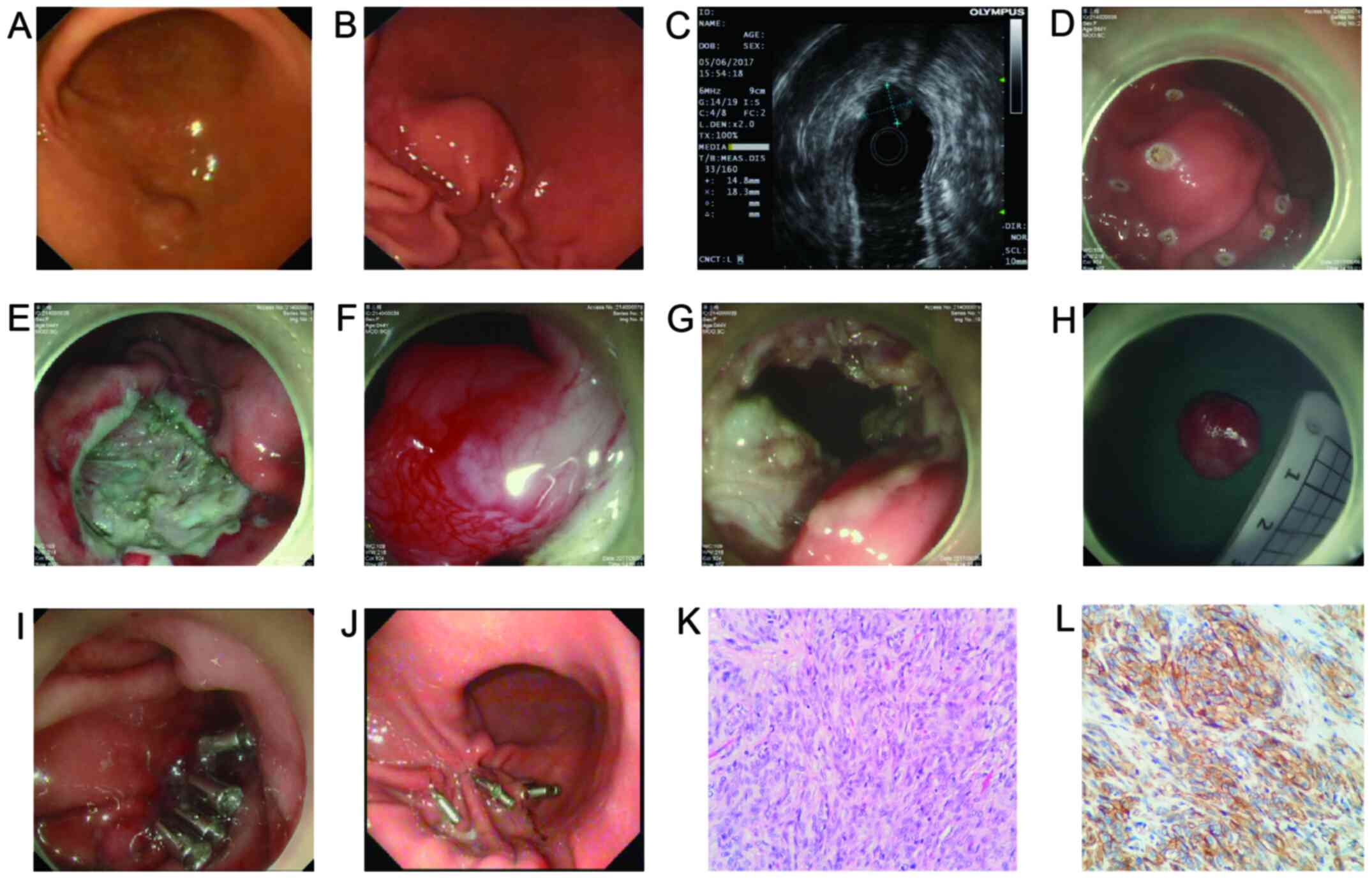 | Figure 1Endoscopy and surgery-related images.
(A) A bulge of approximately 0.5x0.4 cm in the gastric antrum with
a smooth surface was found by endoscopy. (B) The gastric antrum
still had a lesion after 1 year. (C) The lesion was evaluated by
EUS: The lesion exhibited low to medium echo level, originate from
the intrinsic muscularis, and had a cross-sectional size of
approximately 1.8x1.4 cm. (D) The lesion margin was marked before
ESD surgery. (E) The mucosa to the submucosa was cut and the
submucosa and lamina propria were separated; and no obvious lesions
were observed. (F) Patient underwent NOTES, the endoscope entered
the abdominal cavity, and the gastric serosal surface was
approximately 1.5x1.8 cm with pedicle bulging, and the surface was
congested obviously. (G) Complete removal of the lesion along the
base of the bulge. (H) The size of the specimen was approximately
1.5x1.8 cm, and the whole specimen was sent for medical
examination. (I) Electrocoagulation treatment wounds after surgery;
metal clips were used to close the wounds. (J) The gastroscopy was
reviewed to observe the lesions, at 45 days after the surgery. (K)
Post-operative pathological images of the patient (a large number
of spindle cell tumors, H&E staining; x40 magnification). (L)
Immunohistochemical CD117-positive staining (x40 magnification).
EUS, endoscopic ultrasound; ESD, endoscopic submucosal dissection;
NOTES, natural orifice transluminal endoscopic surgery. |
 | Table ISummary of patient partial examination
results. |
Table I
Summary of patient partial examination
results.
| Item | Result |
|---|
| ECG | Sinus rhythm, ST
segment changes (ST IIIIIaVF horizontal type down 0.05 mV) |
| Hepatobiliary spleen
and pancreas | Calcification of the
right lobe of the liver, rough wall of the gallbladder, no obvious
abnormalities in the spleen and pancreas. |
| Endoscopy and
EUS | GF (July 16, 2016):
Greater curvature of the gastric antrum shows a 1.0x0.8 cm bulge,
with smooth surface. Diagnosis: gastric antrum bulge (recommended
ultrasound gastroscopy). EUS (June 5, 2017): The lesion is in the
middle and hypoechoic, of muscularis layer origin, the
cross-sectional size is ~1.8x1.4 cm, the remaining layer structure
is clear, no enlarged lymph nodes are seen around. Imaging
diagnosis: gastric submucosal mass. GF (July 20, 2017): Scars are
observed in the greater curvature of the stomach. 5 titanium clips
are observed, 3 titanium clips were removed. The stomach is normal,
and the peristalsis is good. Imaging diagnosis: chronic superficial
gastritis with erosion, duodenal inflammation, portal
inflammation. |
| Upper abdomen
enhanced CT | December 22, 2016:
The thick antrum shows the nodules, and it is close to the stomach
wall, and the boundary is clear. The size is ~1.3x1.5 cm.
Diagnosis: Greater curvature shows nodules of the antrum, large
lymph nodes, stromal tumors to be ruled out. July 19, 2017:
Post-operative gastric stromal tumor: The intensive nodules on the
greater curvature were removed, no obvious nodules and masses were
found locally, and no obvious enlarged lymph nodes were found in
the abdominal cavity and retroperitoneum. |
Instruments and methods used Surgical
instruments
A GIF-H260 electronic endoscope (Olympus
Corporation) was used to observe the lesion and complete the
surgery through the endoscope. Prior to the surgery, the front end
of the endoscope should be worn with a transparent cap. The UM-3R
ultrasound probe (frequency, 12 and 20 MHz; Olympus Corporation)
was used to complete the ultrasound examination to assess the size
and level of the lesion. During the surgery, an NM-200L-0421
injection needle (Olympus Corporation) was used to complete the
submucosal injection. A KD-650L dual knife (Olympus Corporation)
was used to remove the lesion, and the SD-5L-1 snare (Olympus
Corporation) was used to assist in the removal of the lesion.
During the surgery, FD-430L thermal biopsy forceps (Olympus
Corporation) and high-frequency electrocutting device (Erbe
Elektromedizin GmbH) were used to assist in the treatment of the
wound and complete hemostasis. After the lesion was removed, the
wound was sealed with the HX-610-135 metal titanium clip (Olympus
Corporation) during this period.
Surgical methods. The patient underwent
endoscopic surgery following tracheal intubation and general
anesthesia (sevoflurane 1%, penehyclidine hydrochloride 0.5 mg,
propofol 100 mg) (Fig. 1D-I). An
endoscopic examination revealed a proximal 1.5x1.8 cm bulge in the
stomach, and the mucosal surface was smooth. The edge of the lesion
was marked with a Dual knife. Approximately 3 ml of indigo carmine
and 1 ml of adrenaline were mixed with 100 ml of normal saline, and
a submucosal injection was then performed at the marked point.
After the lesion was fully lifted, the mucosa was excised from the
submucosa by Dual knife edge, and the submucosa and lamina propria
were separated. No lesions were observed. It was thus assessed that
the lesion was located in the serosal layer and growing out of the
cavity. NOTES was employed to enter the abdominal cavity.
Specifically, the muscularis and serosal layer were cut. The
endoscope was then inserted into the abdominal cavity. The
peritoneal serosal surface revealed a bulge of approximately
1.5x1.8 cm in size with a congested surface. The lesion was
completely removed along the base of the bulge. The wound was
treated by electrocoagulation and then closed with titanium clip. A
gastrointestinal decompression tube was implanted after the
surgery. The specimen had a size of approximately 1.5x1.8 cm and
was sent for histopathological examination. The surgery was
uneventful and adhered to the guidelines for tumor resection
(9). The duration of the surgery
110 min, and intraoperative blood loss was 5 ml.
Post-operative treatment
Post-operatively, for the patient, gastrointestinal
decompression was continued, and the post-operative feeding
protocol (9), hemostasis,
antibiotics (cefmetazole sodium, 2.0 g, b.i.d., for 2 days),
nutritional support and fluid replacement therapy were
administered. Vigilance was maintained for complications, such as
abdominal pain, peritonitis, bleeding and perforation.
Literature review search strategy
PubMed (https://pubmed.ncbi.nlm.nih.gov/), EMbase (https://www.embase.com), the Chinese National
Knowledge Infrastructure (https://www.cnki.net/) and Wanfang database
(http://www.wanfangdata.com.cn/index.html) were
searched to collect clinical research on the treatment of gastric
stromal tumors with NOTES and ESD. The search time was from the
establishment of the library until August, 2020. English and
Chinese studies were included, and the following search terms were
used: ‘Gastric submucosal tumor, gastric stromal tumor, serosal
layer, endoscopy, ESD, NOTES’. On this basis, the references
included in the preseant study were traced to obtain the relevant
information.
Inclusion and exclusion criteria
The inclusion criteria were as follows: The lesion
is a gastric stromal tumor. When the lesion originated from the
serosal layer or the muscularis, the modality of treatment is
NOTES, endoscopy (including ESD, EFR, ESE and STER) or laparoscopy,
or a combination of the two. All cases have complete clinical and
pathological data. The exclusion criteria were as follows: Repeated
or replicated publication of the literature, animal studies, or
studies of early or precancerous lesions; reviews, abstracts,
systematic reviews and letters from readers.
Patient treatment High power field
(HPF) findings
The macroscopic appearance must satisfy the
following: One polyp sample, no pedicle and a gray surface on the
section. Histopathological diagnosis includes the following:
Gastric stromal tumor, very low risk, tumor size of 1.5x1.5x1.3 cm,
mitotic count <50 HPF.
The report of the biopsy revealed the following:
Gastric stromal tumor, very low risk, the tumor size is 1.5x1.5x1.3
cm, and the mitotic image is <5/50 HPF. Immunohistochemistry
(performed by the hospital pathology department) revealed the
following: CD117 (+), CD34 (part +), Desmin (-), DOG1 (+), S-100
(-), SDHB (-), SMA (-) (Fig. 1K
and L).
Outpatient treatment and
follow-up
Post-operatively, the patient had no obvious
abdominal pain, abdominal distension or any other type of
discomfort. No bleeding, perforation or other symptoms occurred.
The patient recovered at 3 days after surgery and was discharged
after 5 days. Outside the hospital, the patient gradually
transitioned from liquid to semi-solid to normal diet and was
advised to pay attention to rest, avoid fatigue, and continue oral
acid suppression and mucoprotective medication. The patient was
under a weekly telephone follow-up. Apart from short-term abdominal
discomfort outside of the hospital, no adverse reactions occurred.
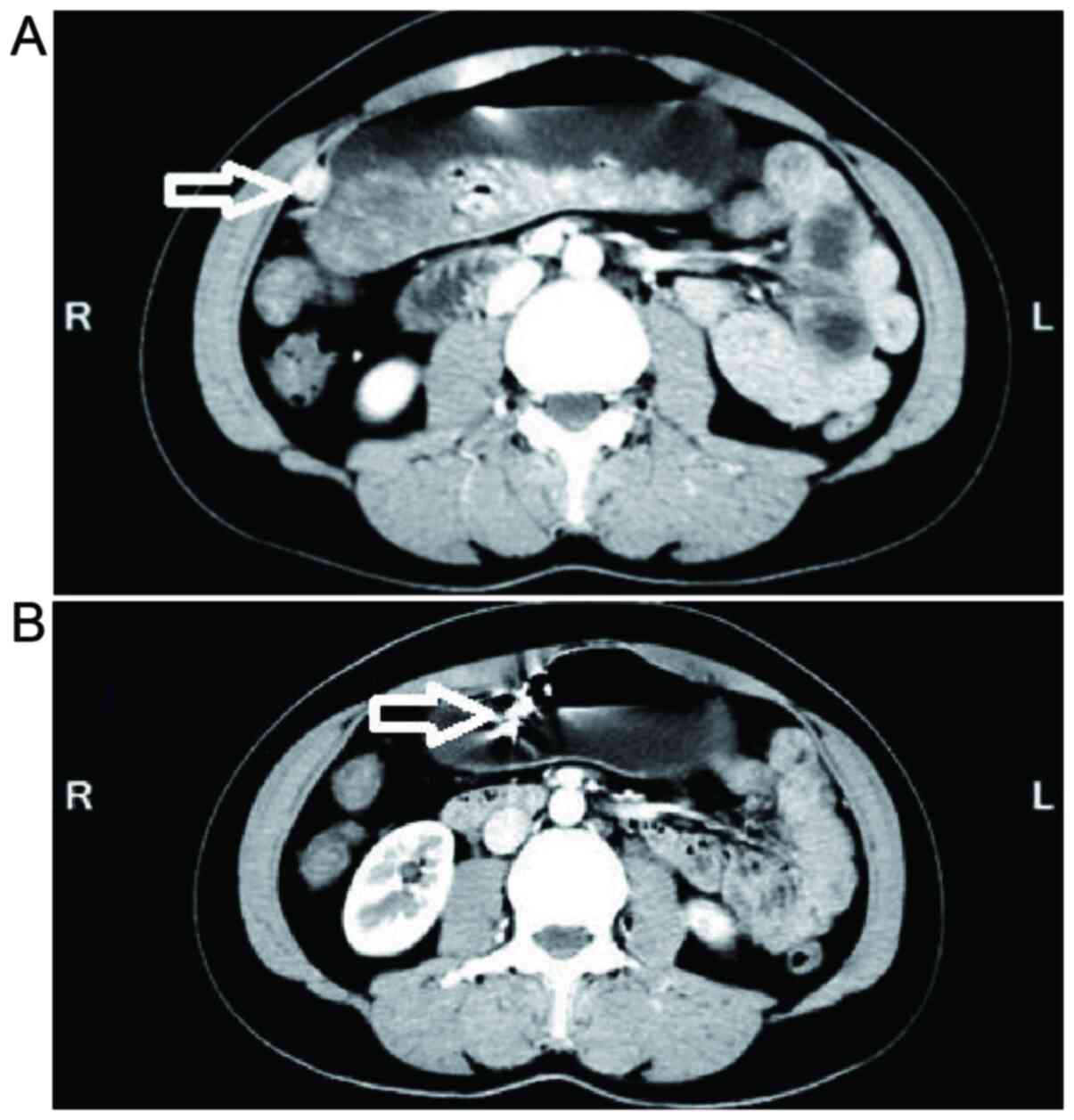
At 45 days following the surgery, the residual titanium clips were
observed by a CT scan (Fig. 2B).
The wounds were completely healed, as shown by the endoscopic
examination, and residual titanium clips remained (Fig. 1J).
Literature search results
Literature explicitly describing ESD combined with
NOTES was not retrieved. Different methods of minimally invasive
treatment of GIST was classified into single endoscopic treatment,
endoscopic combined with laparoscopic treatment, and NOTES
treatment of GIST. The data were summarized and analyzed.
Individual endoscopic treatments of GIST from different studies are
presented in Table II. In
addition, studies using NOTES for the treatment of GIST are
presented in Table III.
 | Table IICommon endoscopic procedures combined
with laparoscopic treatment procedures for GISTs. |
Table II
Common endoscopic procedures combined
with laparoscopic treatment procedures for GISTs.
| Author/(Refs.) | Year | Procedure name | Abbreviation | Procedure | Duration of surgery
(min) | Peri-operative
bleeding (ml) | Tumor diameter
(cm) | Complication |
Indications/advantages | Limitation |
|---|
| Cai et al
(10) | 2015 | Laparoscopic wedge
resection. | LWR | According to the
location of tumor, wedge resection or local gastrectomy should be
performed. The resected tumor is taken out through abdominal
incision. | 106±40.1 | 67.3±80.5 | 3.5±1.9 | 3 Cases of delayed
gastric emptying, 1 case of lung infection. | Suitable for excision
of GIST, particularly <5 cm lesions. | It is difficult to
identify the edge of the tumor from the serosalsurface.
Unintentional removal of healthy gastric tissue may occur. Surgery
near the esophagogastric junction or pylorus may lead to stricture
or obstruction of the stomach inlet or outlet. |
| Matsuda et al
(11) | 2017 | Laparoscopic
endoscopic cooperative surgery. | LECS | The lesions are
located by endoscopy, and wedge resection or local gastrectomy is
performed according to the location of the tumor. The resected
tumor is taken out through abdominal incision. | 190.2±66.8 | 15.1±38.6 | NA | NA | Suitable for all
sizes and location of GIST, combined with endo- scopic techniques
and laparoscopic gastrectomy to prevent excessive gastric resection
and postoperative gastric deformation. | Advanced endoscopic
and laparoscopic techniques and skilled collaboration between
internal and external surgeons are required; long operation time
may increase digestive fluid spillover and abdominal infection
risk. |
| Nunobe et al
(12) | 2012 | Inverted
laparoscopic endoscopic cooperative surgery. | Inverted LECS | The process from
marking to artificial perforation is similar to classic LECS. In
order to prevent the tumor from contacting visceral tissue, the
gastric wall is lifted in the tangential direction by suture. Thus,
the serosal layer is opened around the submucosal incision with ESD
or laparoscope equipment and the tumor is removed. After removing
the specimen, the gastric wall defect is sutured using a
laparoscope. | N/A | N/A | N/A | N/A | The procedure was
developed to prevent the contents of the stomach from spilling into
the clean abdominal cavity. | Repeated
intervention to the tumor may cause tumor recurrence and there is
still a risk of gastric contents entering the abdominal
cavity. |
| Ye et al
(13) | 2018 | Laparoscopic
exogastric wedge resection. | LEWR | The gastroscope
passes through the cardia, identifies the site in gastric cavity to
avoid stenosis. This procedure prevents excessive normal gastric
tissue resection and uses a laparoscope to perform a wedge-shaped
resection of the stomach wall from the distal end. | 108.35±47.23 | 31.83±38.85 | 2.97±2.02 | 2 Cases of fever,
symptomatic relief. | First choice of
treatment of gastric submucosal tumor. | Need to open the
anterior wall of the stomach. The duration of the surgery is
prolonged, blood loss is increased and risks are amplified. Post-
operative recovery time is prolonged, gastric juice enters the
abdominal cavity and the risk of abdominal infection
increases. |
| Ye et al
(13) | 2018 | Laparoscopic
transgastric wedge resection. | LTWR | If the location of
the GIST is found to be unsuitable for LEWR, including posterior
wall lesions or incomplete exposure after gastric rotation, the
LTWR method is used to locate the lesion via endoscope and the
gastrectomy is performed on the anterior wall of the tumor. After
the tumor is removed, a wedge- shaped resection is performed
sequentially along the normal stomach wall with a laparoscope and
the gastric incision is sutured with a laparoscopic linear suturing
device | 149.44±49.78 | 82.22±119.87 | 3.27±1.36 | 1 Case of fever,
symptomatic relief. | LTWR should be
considered for growths in gastric posterior wall, intraluminal
growths and lesions close to EGJ. | |
| Mahawongkajit et
al (14) | 2020 | Non- exposed | NEWS endoscopic
wall- inversion surgery | After the tumor is
located under the endoscope, the seromuscular layer is opened
underlaparoscopic surgery, and the tumor is sutured along the
incision line, and then the tumor is inwardly turned into the
gastric cavity which is then removed by endoscopy. | 207.5±30.7 | 1.5±0.8 | 2.1±0.5 | N/A | The resection line
can be determined with high accuracy without causing peritoneal
contamination, and the tumor can be prevented from being exposed to
the peritoneal cavity, and SETs with a maximum diameter of <3 cm
are feasible. Removal of the tumor in the mouth by total removal of
stomach wall, thus avoiding the risk of intraperitoneal
seeding. | If the size of the
lesion is <3 cm, NEWS is preferred, and the patient sample size
is small. |
| Hajer et al
(15) | 2018 | Non- exposure
technique | CLEAN- NET | Endoscopic
examination of gastrointestinal stromal tumors which is then marked
with electrocautery, followed by injection of methylene blue dye
into the lesion, surrounding the serosal muscle incision. Stapling
is done around the wound, elevation of submucosa away from the
gastric wall. Resection of the tumor with a stapling device, then
suturing the mucosal surface of the stomach wall eventually
suturing the serosal surface of the stomach wall. | 120-180 (average,
150) | N/A | 3.0-4.5 (average,
3.75) | N/A | Applicable to
tumors >4 cm in diameter, extraluminal growth tumors, fully
protect gastric function. | The sample size is
small, only 2 cases. |
| Kikuchi et
al (16) | 2017 | Closed laparoscopic
and endoscopic cooperative surgery. | Closed LECS | Endoscopic
submucosal dissection around the lesion and the serosal surface was
marked by laparoscopy. Laparoscopic suture of the stomach wall was
made along the marked line and the lesion was enclosed in the
stomach. Endoscopic dissection was continued and the tumor was
taken out through the mouth. | 253±45 | 18±55 | 2.41±0.76 | Abdominal abscess
in one patient | Without
contamination and tumor cells spreading into the abdominal
cavity. | The number of cases
is small, the follow-up time is short, and the long-term effect is
unknown. |
| Okumura et
al (17) | 2017 | Lift-and-cut
method | N/A | First, the
seromuscular layer around the tumor was removed. As the mucosa and
submucosa are stretchable, the tumor was elevated into the
abdominal cavity. Following elevation, the gastric tissue under the
tumor was cut in the submucosa with a linear stapler. | 65-302 (average,
126) | 0-200 (average,
10) | 1.0-7.7 (average,
3.3) | N/A | Minimize gastric
tissue resection and reduce the chance of contaminating the
abdominal cavity. | Single-center study
biased, long follow-up time, no prospective study compared to other
surgical methods. |
| Dong et al
(18) | 2014 | Modified
laparoscopic intragastric surgery. | MLIGS | The position of the
tumor was confirmed under endoscopy. The laparoscopic lamp was
placed in the cavity using the trocar at the navel, and the other
two trocars were inserted into the abdominal wall and the stomach
wall. The gastroscope was monitored by a laparoscope, and the
operation was performed in the gastric cavity. The tumor tissue was
taken out with the forceps and the wound was closed by titanium
clip. | 85±25.77 | 20±10.35 | 2.75±1.07 | Abdominal pain for
1.875±1.46 days | MLIGS and EFR are
effective in treating the muscularis propria gastric stromal tumor,
and they are non-invasive. | N/A |
| Moriyama et
al (19) | 2012 | Robot- assisted
laparoscopic resection. | N/A | The Da Vinci
surgical system (da Vinci; Intuitive Surgical Inc.) was used to
make an incision 30 mm above the umbilicus and introduced an Alexis
wound retractor to establish a pneumoperitoneum. The 3-arm Da Vinci
was used to control the surgical instruments with the right and
left arms, and the endoscope was attached to the central arm. The
omentum was dissected and the stomach was elevated to the abdominal
wall. Using an electric knife the tumor was dissected, the tumor
was removed, and the gastric and abdominal wall was closed. | 190 | <50 | 3.4 | N/A | Simplify the
process of laparoscopic resection, making it easier to perform
gastric incision and suture. | The sample size is
small, and some institutions do not have this equipment. |
 | Table IIISummary of NOTES treatment for
GISTs. |
Table III
Summary of NOTES treatment for
GISTs.
| Author/(Refs.) | Year | Procedure | Abbreviation | n | Procedure | Surgery time
(min) | Peri-operative
bleeding | Post-operative
examination | Complication | Technique
advantages |
|---|
| Lee et al
(20) | 2013 | Transgastric NOTE
speritoneoscopy. | TGP | 5 | Mucosa is marked
and a submucosal tunnel is established. The distal end is opened,
balloon is dilated. Biopsy forceps is used for abdominal nodule
biopsy and the incision is closed. | 10.44±2.42 | N/A | 4 Cases of
peritoneal cancer and 1 case of tuberculosis. | N/A | Simple technology,
short operating time, less complications, low sedation
requirements, no need for general anesthesia. |
| | | Endoscopic
full-thickness resection | EFTR | 5 | Mark the lesions,
establish tunnels, remove lesions in tunnels, close mucosal
incisions. | 18.80±9.41
Resection time | N/A | 3 Cases of GIST and
2 cases of schwannomas. | 1 Case of abdominal
pain, symptomatic treatment. | |
| Mori et al
(21) | 2011 | Hybrid natural
orifice transluminal endoscopic surgery. | Hybrid NOTES | 6 | Under the
endoscope, balloon was fixed at the bulb of the duodenum, and it
was fixed with 60-70 ml of gas to make the stomach filled. The ESD
method was then used to peel off the muscle layer, causing active
perforation ~1 cm outside the tumor, and the endoscope entered the
abdominal cavity. EFR after inversion; laparoscopic observation of
lesions, tumor removal was done from the mouth, laparoscopic suture
of the stomach wall was performed. | 180-360 (average
288) | N/A | GIST | N/A | Operation is not
affected by the disease, can adjust the position; reduce the
economic burden of the patient, mental and psychological pressure,
shortcomings and operation time. |
| Nakajima et
al (22) | 2009 | Hybrid transvaginal
NOTES | NA | 2 | Under the
assistance of laparoscope, endoscopic treatment was performed
following the establishment of a channel through the vagina. | 365 and 170 | Almost no
bleeding | 1 Case of
hemorrhagic lipoma, 1 case of GIST | N/A | Abdominal incision
is avoided, the disadvantage is that the operation is
cumbersome. |
Discussion
Submucosal tumors (SMTs) can originate from the
mucosal muscle layer, submucosa or muscularis propria (23). With the popularity of endoscopy,
the diagnostic rate is gradually increasing. Common types of SMT
include lipoma, leiomyoma and stromal tumor. SMTs originating from
the muscularis propria are mostly GISTs. SMTs have different
biological characteristics and the treatment options include
follow-up, endoscopic resection, laparoscopic resection and
surgical treatment (24). GISTs
have malignant potential, and the malignancy accounts for
approximately 25% (25). The need
for treatment is dependent on the location and size of the tumor
and clinical manifestations and the risk stratification of clinical
malignancies (26). GISTs which
are <2 cm in diameter do not require treatment, but must be
closely monitored to ensure that the lesion does not increase in
size (4). Moreover, GISTs with a
diameter >5 cm or lesions causing obstruction and bleeding
require surgery (27). Surgery or
endoscopic resection is the primary treatment option (28).
Among the endoscopic techniques for the clinical
treatment of GISTs, ESD is the most widely used and can achieve
complete resection (29). EFR is a
derivative of ESD that can completely remove lesions from all
layers, including the serosal layer (30). Furthermore, this method is
recommended for lesions derived from the muscularis propria and the
serosal layer (31). However, EFR
has a number of complications, such as perforation and bleeding,
and needs to be performed by highly skilled surgeons. ESE is also a
derivative of ESD and is widely used in the treatment of GISTs
derived from the muscularis propria, particularly for intraluminal
lesions (32). This technique can
maintain the physiological integrity of the digestive tract and
reduce complications, but it also requires a highly skilled
surgeon. During the surgery, the tumor must be excised from the
submucosa or inner muscle layer along the external edge of the
tumor, and the capsule of the tumor must remain intact. STER
technology is close to endoscopic myotomy (POEM) (33), which involves a tunnel to treat
multiple lesions (34). However,
the space inside of the tunnel is limited, and the operation and
removal of large lesions are difficult. This condition also
increases the risk of perforation, and the possibility of tunnel
wall damage during operation is high. STER is recommended for
treating tumors originating from intrinsic muscle layer, but not
for those arising from muscularis propria. EMD can better expose
the lesions, which is beneficial to the examination of disease
compared with STER.
Longitudinal incision is used to simplify the
surgical procedure and reduce the chances of perforation for
lesions originating from the muscularis propria. Furthermore, this
technique is rarely used, and the follow-up time is short.
Long-term effects of this procedure are unknown. Suction treatment
of exogenous GIST has the advantage of reducing the risk of
perforation. However, this technique cannot be used when the
lesions do not move towards the endoscope during the extraction,
thereby limiting its clinical promotion. In conclusion, endoscopic
GIST treatment has many advantages, including postoperative gastric
structural integrity, short hospital stay, relatively simple
sedation, low operating cost, and low manpower requirement.
However, when the lesion is large, the tumor complete resection
rate is low, the risk of perforation is increased, and a risk of
tumor peritoneal implantation occurs when the perforation is
large.
On the bases of the characteristics of laparoscopic
and endoscopic techniques, scholars have proposed laparoscopic and
endoscopic combined surgery (LECS) for the treatment of GISTs.
Various surgical techniques have also been developed. Classical
LECS includes endoscopic mucosal resection and laparoscopic
surgery. The technique uses ESD or EMR to perform mucosal resection
under the guidance of an endoscope and uses a laparoscopic incision
for the closure of the surgical site, thus achieving minimally
invasive treatment (35). LECS can
be used to avoid the excessive resection of the stomach wall and
maintain its structural and physiological integrity. However, a
risk of peritoneal spread of tumoris possible. This technique is
currently used for gastric GISTs with a diameter ≤5 cm and is not
recommended for large and/or ulcerated GISTs with a diameter >5
cm (36). However, this procedure
is suggested for larger-sized GISTs (37).
Domestic and foreign scholars have improved classic
LECS, including laparoscopic-assisted endoscopic resection,
endoscopic assisted wedge resection, reverse LECS, non-exposure
technique (CLEAN-NET), non-exposure endoscopic gastric wall
inversion (NEWS), laparoscopic-assisted endoscopic total resection
(LAEFR) and clean non-exposure techniques. The above-mentioned
techniques are mainly performed to avoid the spread of tumor cells
following gastric wall incision and the contamination of the
abdominal cavity by gastric contents (38). Laparoscopic and endoscopic
techniques have expanded the indications for GIST surgery and have
reduced the chances of contamination and tumor spread.
NOTES technology enters the body cavity through the
natural cavity for exploration, biopsy and various surgical
operations. This method proposes a novel direction for GIST
treatment. At present, technology faces various difficulties,
including surgical indications, choice of surgical pathway,
incision closure, infection control during treatment, and patient
acceptance, prior to their clinical application. For GIST, scholars
have proposed mixed NOTES; however, most of these techniques are
limited to small-sample clinical studies and lack large-sample,
multicenter, prospective randomized controlled trials.
Although the future of NOTES remains unknown,
surgeons have made a positive attempt for this method. Cases have
been rigorously screened, the location and origin of the lesions
have been fully evaluated, and reasonable treatment plans and
countermeasures have been formulated. Full communication must be
ensured before surgery to obtain the informed consent of patients
and their families.
As the tumor of the patient in the present study was
small, traction technology was not used upon entry of the
gastroscope into the abdominal cavity. Instead, the endoscope was
used to pull the tumor directly into the stomach and ESD was then
used to remove it. After the tumor was removed, metal clips were
used to seal the wound under the endoscope as the wound was fairly
small. From past experience, it was learnt that closing with metal
clips alone is often difficult when the tumor exceeds 3.5 cm. In
this case, suturing the wound with nylon rope or combining the
procedure with laparoscopy to remove the tumor may be
necessary.
Basing on the above experience, the surgeon believes
that NOTES combined with ESD is safe and feasible. The current
endoscopic resection diameter of <3.5 cm GIST has become the
norm. With the experience of flat ESD, NOTES combined with ESD is
safe and feasible treatment for plasma membrane-originating GIST,
particularly for exogenous lesions <3.5 cm in diameter. Relative
laparoscopic surgery has the following advantages: Small trauma, no
influence on the appearance of the patient (cosmetic), a low
treatment cost, short hospital stay, quick recovery after surgery,
and few complications, and thus is worthy of clinical promotion.
The procedure may also have the following disadvantages: The lesion
is difficult to locate, the technique is difficult, and high
technical requirements from the surgeon are necessary. Larger
sample, multicenter, prospective, randomized controlled trials are
thus required to further assess the safety and feasibility of this
technique.
Acknowledgements
Not applicable.
Funding
The present study was funded by Shiyan City Science
and Technology Bureau Guiding Research Project (19Y44) and the
Foundation of Taihe Hospital (2019JJXM032).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
XBL, SBL and SJ conceived and designed the study.
ZYG, CTS and XBL searched and collected the data. XBL, SP and BZS
contributed to data extraction and data analysis. SJ and PL
provided surgical and pathological information. XBL and ZYG wrote
the manuscript. CTS, SJ and SBL reviewed and revised the
manuscript. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The study protocol was reviewed and approved by the
Taihe Hospital Ethics Committee. Informed consent was obtained from
the patient.
Patient consent for publication
Written informed consent for publication was
obtained from the participant.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Menge F, Jakob J, Kasper B, Smakic A,
Gaiser T and Hohenberger P: Clinical presentation of
gastrointestinal stromal tumors. Visc Med. 34:335–340.
2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chun SY, Kim KO, Park DS, Lee IJ, Park JW,
Moon SH, Baek IH, Kim JH, Park CK and Kwon MJ: Endoscopic
submucosal dissection as a treatment for gastric subepithelial
tumors that originate from the muscularis propria layer: A
preliminary analysis of appropriate indications. Surg Endosc.
27:3271–3279. 2013.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Koo DH, Ryu MH, Kim KM, Yang HK, Sawaki A,
Hirota S, Zheng J, Zhang B, Tzen CY, Yeh CN, et al: Asian consensus
guidelines for the diagnosis and management of gastrointestinal
stromal tumor. Cancer Res Treat. 48:1155–1166. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Xu C, Chen T, Hu Y, Balde AI, Liu H, Yu J,
Zhen L and Li G: Retrospective study of laparoscopic versus open
gastric resection for gastric gastrointestinal stromal tumors based
on the propensity score matching method. Surg Endosc. 31:374–381.
2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Zhang Y, Ye LP and Mao XL: Endoscopic
treatments for small gastric subepithelial tumors originating from
muscularis propria layer. World J Gastroenterol. 21:9503–9511.
2015.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Wang W, Shi X, Jin Z, et al: Key issues
about the endoscopic treatment for upper gastrointestinal
submucosal tumors. Chin J Dig Endosc. 34:764–768. 2017.DOI:
10.3760/cma.j.issn.1007-5232.2017.11.002.
|
|
7
|
Bernhardt J, Sasse S, Ludwig K and Meier
PN: Update in Natural Orifice Translumenal Endoscopic Surgery
(NOTES). Curr Opin Gastroenterol. 33:346–351. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Shiroshita H, Etoh T, Yasuda K, Inomata M
and Kitano S: Natural orifice translumenal endoscopic surgery.
Nihon Geka Gakkai Zasshi. 117:376–380. 2016.PubMed/NCBI(In Japanese).
|
|
9
|
Zhou PH, Cai MY and Yao LQ: Chinese
Consensus on Endoscopic Diagnosis and Management of
Gastrointestinal Submucosal Tumor (Version 2018). Zhonghua Wei
Chang Wai Ke Za Zhi. 21:841–852. 2018.PubMed/NCBI(In Chinese).
|
|
10
|
Cai JQ, Chen K, Mou YP, Pan Y, Xu XW, Zhou
YC and Huang CJ: Laparoscopic versus open wedge resection for
gastrointestinal stromal tumors of the stomach: A single-center
8-year retrospective cohort study of 156 patients with long-term
follow-up. BMC Surg. 15(58)2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Matsuda T, Nunobe S, Kosuga T, Kawahira H,
Inaki N, Kitashiro S, Abe N, Miyashiro I, Nagao S, Nishizaki M, et
al: Society for the Study of Laparoscopy and Endoscopy Cooperative
Surgery. Laparoscopic and luminal endoscopic cooperative surgery
can be a standard treatment for submucosal tumors of the stomach: A
retrospective multicenter study. Endoscopy. 49:476–483.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nunobe S, Hiki N, Gotoda T, Murao T,
Haruma K, Matsumoto H, Hirai T, Tanimura S, Sano T and Yamaguchi T:
Successful application of laparoscopic and endoscopic cooperative
surgery (LECS) for a lateral-spreading mucosal gastric cancer.
Gastric Cancer. 15:338–342. 2012.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ye X, Yu J, Kang W, Ma Z and Xue Z: Short-
and long-term outcomes of endoscope-assisted laparoscopic wedge
resection for gastric submucosal tumors adjacent to esophagogastric
junction. J Gastrointest Surg. 22:402–413. 2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Mahawongkajit P and Chanswangphuvana P:
Laparoscopy-assisted endoscopic full-thickness resection of upper
gastrointestinal subepithelial tumors: A single-center early
experience. Mol Clin Oncol. 12:461–467. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Hajer J, Havlůj L, Whitley A and Gürlich
R: Non-exposure endoscopic-laparoscopic cooperative surgery for
stomach tumors: First experience from the Czech republic. Clin
Endosc. 51:167–173. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kikuchi S, Nishizaki M, Kuroda S, Tanabe
S, Noma K, Kagawa S, Shirakawa Y, Kato H, Okada H and Fujiwara T:
Nonexposure laparoscopic and endoscopic cooperative surgery (closed
laparoscopic and endoscopic cooperative surgery) for gastric
submucosal tumor. Gastric Cancer. 20:553–557. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Okumura S, Kanaya S, Hosogi H, Ito T,
Miura S, Okada T, Shimoike N, Akagawa S, Kawada H and Arimoto A:
Our experience with laparoscopic partial gastrectomy by the
‘lift-and-cut method’ for gastric gastrointestinal stromal tumor
with maximal preservation of the remnant stomach. Surg Endosc.
31:3398–3404. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Dong HY, Wang YL, Jia XY, Li J, Li GD and
Li YQ: Modified laparoscopic intragastric surgery and endoscopic
full-thickness resection for gastric stromal tumor originating from
the muscularis propria. Surg Endosc. 28:1447–1453. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Moriyama H, Ishikawa N, Kawaguchi M,
Hirose K and Watanabe G: Robot-assisted laparoscopic resection for
gastric gastrointestinal stromal tumor. Surg Laparosc Endosc
Percutan Tech. 22:e155–e156. 2012.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Lee SH, Kim SJ, Lee TH, Chung IK, Park SH,
Kim EO, Lee HJ and Cho HD: Human applications of submucosal
endoscopy under conscious sedation for pure natural orifice
transluminal endoscopic surgery. Surg Endosc. 27:3016–3020.
2013.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Mori H, Kobara H, Kobayashi M, Muramatsu
A, Nomura T, Hagiike M, Izuishi K, Suzuki Y and Masaki T:
Establishment of pure NOTES procedure using a conventional flexible
endoscope: Review of six cases of gastric gastrointestinal stromal
tumors. Endoscopy. 43:631–634. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Nakajima K, Nishida T, Takahashi T, Souma
Y, Hara J, Yamada T, Yoshio T, Tsutsui T, Yokoi T, Mori M, et al:
Partial gastrectomy using natural orifice translumenal endoscopic
surgery (NOTES) for gastric submucosal tumors: Early experience in
humans. Surg Endosc. 23:2650–2655. 2009.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Zhou P, Zhong Y and Li Q: Chinese
consensus on endoscopic diagnosis and management of
gastrointestinal submucosal tumor (version 2018). Zhonghua Wei
Chang Wai Ke Za Zhi. 21:841–852. 2018.PubMed/NCBI(In Chinese).
|
|
24
|
Ponsaing LG and Hansen MB: Therapeutic
procedures for submucosal tumors in the gastrointestinal tract.
World J Gastroenterol. 13:3316–3322. 2007.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Jumniensuk C and Charoenpitakchai M:
Gastrointestinal stromal tumor: Clinicopathological characteristics
and pathologic prognostic analysis. World J Surg Oncol.
16(231)2018.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim HH: Endoscopic treatment for
gastrointestinal stromal tumor: Advantages and hurdles. World J
Gastrointest Endosc. 7:192–205. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Liu J, Huang C, Peng C, Xu F, Li Y, Yutaka
Y, Xiong B and Yang X: Stromal fibroblast activation protein alpha
promotes gastric cancer progression via epithelial-mesenchymal
transition through Wnt/β-catenin pathway. BMC Cancer.
18(1099)2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Yang Z, Feng X, Zhang P, Chen T, Qiu H,
Zhou Z, Li G, Tao KX and Li Y: Clinicopathological features and
prognosis of 276 cases of primary small (≤2 cm) gastric
gastrointestinal stromal tumors: A multicenter data review. Surg
Endosc. 33:2982–2990. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Iwamuro M, Tsuzuki T, Ohya S, Okada H,
Tanaka T, Hori K, Kita M, Kawano S, Kawahara Y and Yamamoto K:
Ectopic pancreas in the stomach successfully resected by endoscopic
submucosal dissection. Case Rep Med. 2015(147927)2015.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Li J, Meng Y, Ye S, Wang P and Liu F:
Usefulness of the thread-traction method in endoscopic
full-thickness resection for gastric submucosal tumor: A
comparative study. Surg Endosc. 33:2880–2885. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Huang J, Xian X S, Huang LY, Zhang B, Wu
CR and Jun Cui J: Endoscopic full-thickness resection for gastric
gastrointestinal stromal tumor originating from the muscularis
propria. Rev Assoc Med Bras (1992). 64:1002–1006. 2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wang Y, Li Y, Luo H and Yu H: Efficacy
analysis of endoscopic submucosal excavation for gastric
gastrointestinal stromal tumors. Zhonghua Wei Chang Wai Ke Za Zhi.
17:352–355. 2014.PubMed/NCBI(In Chinese).
|
|
33
|
Tan Y, Tan L, Lu J, Huo J and Liu D:
Endoscopic resection of gastric gastrointestinal stromal tumors.
Transl Gastroenterol Hepatol. 2(115)2017.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Wang H, Tan Y, Huo J and Liu D: Submucosal
1-tunnel endoscopic resection for treating upper gastrointestinal
multiple submucosal tumor originating from the muscularis propria
layer: A report of 12 cases. Medicine (Baltimore).
98(e14484)2019.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Gluzman MI, Kashchenko VA, Karachun AM,
Orlova RV, Nakatis IA, Pelipas IV, Vasiukova EL, Rykov IV, Petrova
VV, Nepomniashchaia SL, et al: Technical success and short-term
results of surgical treatment of gastrointestinal stromal tumors:
An experience of three centers. Transl Gastroenterol Hepatol.
2(56)2017.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Tsuji R, Komatsu S, Kumano T, Ohta A,
Furuke H, Tanaka S, Imura K, Shimomura K, Ikeda J, Taniguchi F, et
al: Laparoscopy and Endoscopy Cooperative Surgery (LECS)-Assisted
open partial gastrectomy for a high-risk gastrointestinal stromal
tumor. Gan To Kagaku Ryoho. 46:172–174. 2019.PubMed/NCBI(In Japanese).
|
|
37
|
Aisu Y, Yasukawa D, Kimura Y and Hori T:
Laparoscopic and endoscopic cooperative surgery for gastric tumors:
Perspective for actual practice and oncological benefits. World J
Gastrointest Oncol. 10:381–397. 2018.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ntourakis D and Mavrogenis G: Cooperative
laparoscopic endoscopic and hybrid laparoscopic surgery for upper
gastrointestinal tumors: Current status. World J Gastroenterol.
21:12482–12497. 2015.PubMed/NCBI View Article : Google Scholar
|