Introduction
The study of the therapeutic effects of medicinal plants over the past few decades has led to the identification of biologically active molecules. Phenolic acids are a subclass of plant phenolics widely distributed in the plant kingdom. They possess one or more hydroxyl groups attached to an aromatic ring, and at least one organic carboxylic acid (1). Diverse evidence indicates that these compounds have multiple health benefits (2). Cinnamic and coumaric acids are cinnamalglucoside polyphenols previously characterized from Carpolobia lutea with anti-nociceptive and anti-diarrheal effects associated with the reduction in the release or blocking of inflammatory mediators (3,4). 4-Hydroxycinnamic acid, also known as p-coumaric acid (p-CA), is a phenolic acid found in fruits, vegetables and mushrooms. Its biosynthesis is based on the transformation of tyrosine into p-CA by the action of tyrosine ammonia-lyase, and p-CA is subsequently converted to other phenolic acids, such as caffeic acid, ferulic acid or sinapic acid, as well as other secondary metabolites, including lignin and lignin precursors, feruloyl CoA and p-coumaryl CoA (5). In vitro and in vivo studies have demonstrated that p-CA possesses multiple bioactivities, such as antioxidant, anti-inflammatory, anti-platelet aggregation, analgesic, anticancer and neuroprotective properties (6,7).
However, despite its biological applications, studies investigating whether this compound can reduce, prevent or cure liver diseases are limited. The liver is composed of parenchymal and non-parenchymal cells, with a complex vasculature and biliary branches, thus conferring it a great metabolic capacity, and rendering it susceptible to damage by drugs and xenobiotics (8).
Moreover, amoebiasis results from a human gastrointestinal parasitic infection by Entamoeba histolytica (E. histolytica), which is treated with metronidazole (Mtz); however, there is evidence to suggest that, in addition to the undesirable treatment-related side-effects, resistance to Mtz has been identified clinically (9).
Therefore, the aim of the present study was to examine whether p-CA can prevent necrosis and cholestasis induced by two different types of liver damage, one induced by hepatotoxic carbon tetrachloride (CCl4) and the other by extrahepatic biliary obstruction induced by common bile duct ligation (BDL) in rats. Furthermore, the potential anti-amoebic activity of p-CA was examined.
Materials and methods
Reagents
p-CA, carboxymethylcellulose (CMC), ketamine, xylazine, p-nitrophenol, sodium hydroxide, glycine, magnesium chloride, p-nitrophenyl phosphate, disodium phosphate, monosodium phosphate, DL-alanine, α-ketoglutaric acid, 2,4-dinitrophenylhydrazine, hydrochloric acid, sodium pyruvate, p-nitroanilide, glycyl-glycine, gamma-glutamyl-p-nitroanilide, potassium hydroxide, anthrone, sulfuric acid, neutral formalin, acetone, 3-aminopropyl triethoxysilane, ethyl alcohol, Harris's hematoxylin, periodic acid Schiff reagent, eosin, resin, Mayer's hematoxylin, Mtz and Trypan blue were purchased from Sigma-Aldrich; Merck KGaA. Xylene was obtained from Supelco; Sigma-Aldrich; Merck KGaA. Formaldehyde and CCl4 were obtained from J.T. Baker.
p-CA administration
In the present study, a single dose of 100 mg/kg, of p-CA was used. This dose has been previously evaluated against oxidative stress induced by liver ischemia/reperfusion (10) and cisplatin (11) injuries in rats. The administration was per os (p.o.) as p-CA is absorbed in several sections of the rat gastrointestinal tract, including the stomach, jejunum, ileum and colon, with the highest absorption rate in the jejunum. In addition, p-CA has been shown to have a low toxicity in mice (LD50, 2,850 mg/kg body weight) (6).
Experimental animals
The present study used 40 male Wistar rats of 12 weeks of age, weighing 200-250 g, which were maintained on a standard diet, with free access to drinking water and under controlled conditions with a temperature at 24˚C, with 50-60% relative humidity and 12-h light/dark cycles. All experimental protocols using rats were approved by the Research Ethics Committee of the Faculty of Professional Studies Huasteca Zone of the Autonomous University of San Luis Potosí (FEPZH-2020-CEI-01), and were conducted according to the institutional guidelines for the care and use of experimental animals and to the national regulatory norm NOM-062-ZOO-1999.
Anti-necrotic and anti-cholestatic effects of p-CA
Acute liver injury induced by CCl4. CCl4 was used in the present study to induce severe acute liver injury, as previously described (12). The male Wistar rats were divided into four groups as follows: i) The control group (n=5), in which animals were treated with the vehicle compound as follows: CMC 0.5% (0.5 ml/100 g, p.o., p-CA vehicle) was administered three times, at 24 h and 1 h before mineral oil (0.5 ml/100 g, p.o., CCl4 vehicle); 1 h after mineral oil administration, one additional dose of CMC 0.5% was administered; ii) the CCl4 group (n=5), in which a single dose of CCl4 (4 g/kg, p.o.) was administered, and CMC 0.5% was administered in the same manner as in the control group; iii) the CCl4 + p-CA group (n=5), in which p-CA (100 mg/kg, p.o.) was administered three times, with the first two doses administered at 24 and 1 h prior to the CCl4 (4 g/kg, p.o.) administration, and the third dose was administered 1 h following CCl4 administration; and iv) the p-CA group (n=5), in which p-CA was administered in the same manner as in the CCl4 + p-CA group; however, instead of CCl4, mineral oil was administered orally (0.5 ml/100 g, p.o.) (Fig. 1). All animals were sacrificed 24 h following the CCl4 or mineral oil administrations.
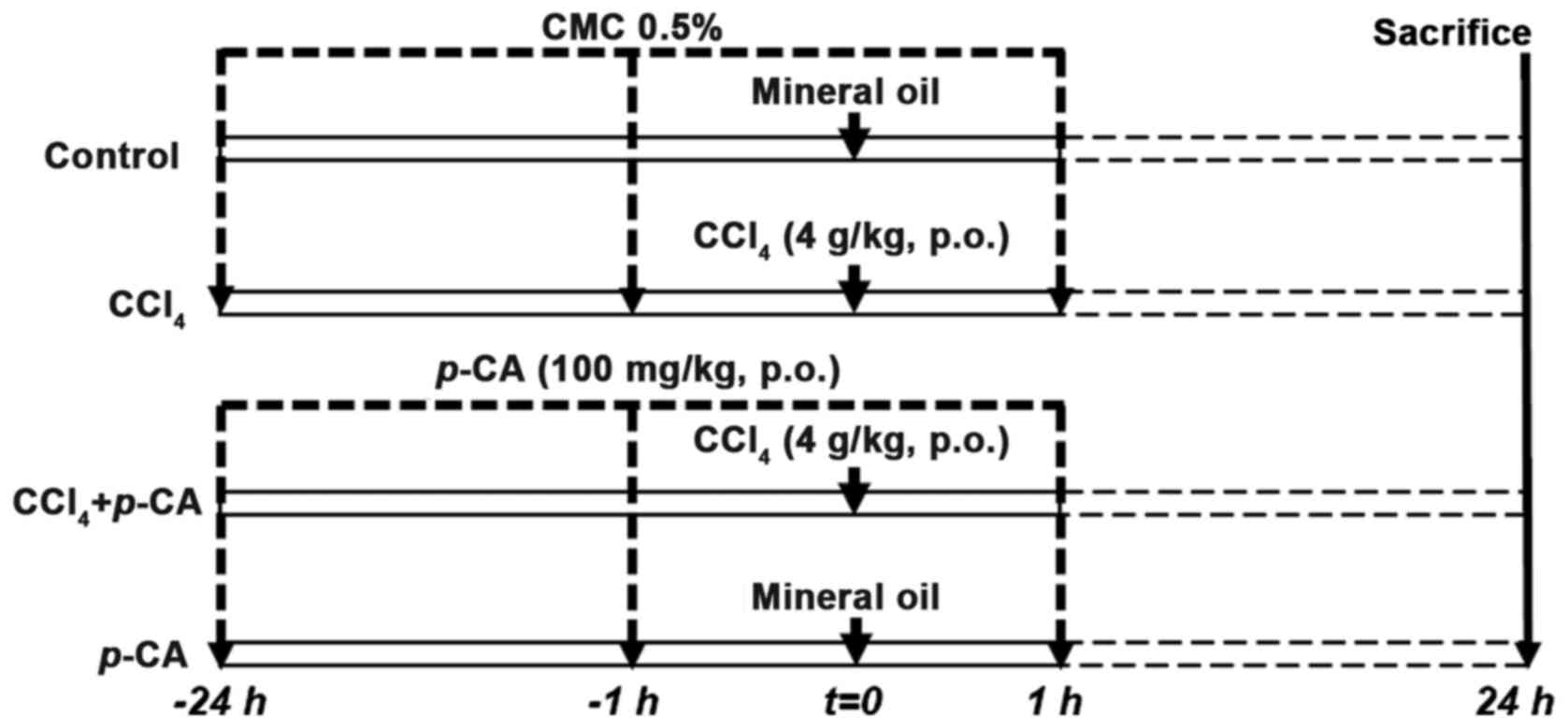 |
Figure 1
Experimental protocol to evaluate hepatoprotective effect of p-CA in the acute liver damage induced by CCl4. Rats were divided in four groups as follows: Control (n=5), CCl4 (n=5), CCl4 + p-CA (n=5) and p-CA (n=5). p-CA, p-coumaric acid; CCl4, carbon tetrachloride.
|
Acute liver injury induced by BDL. BDL was carried out following the specifications of previous publications (13,14). The rats were divided into the following groups: i) The sham-operated (sham) group (n=5), in which rats were administered CMC 0.5%, at 24 h and 1 h prior to sham surgery and at 1 h after this procedure; ii) the BDL group (n=5), in which rats were treated with CMC 0.5% in the same manner as in the sham group, although instead of sham surgery, a double ligation was performed in the bile duct and it was finally dissected; iii) the BDL + p-CA group (n=5), in which the animals were pre-treated with p-CA (100 mg/kg, p.o.) at 24 h and 1 h prior to BDL and at 1 h after surgery; and iv) the sham + p-CA group (n=5), in which the rats received the same treatment scheme as that of the p-CA group; however, instead of BDL, sham surgery was performed (Fig. 2). All animals were sacrificed at 48 h after surgery. The BDL and sham surgeries were performed under anesthesia with the combination of xylazine at 10 mg/kg and ketamine at 80 mg/kg (i.p.).
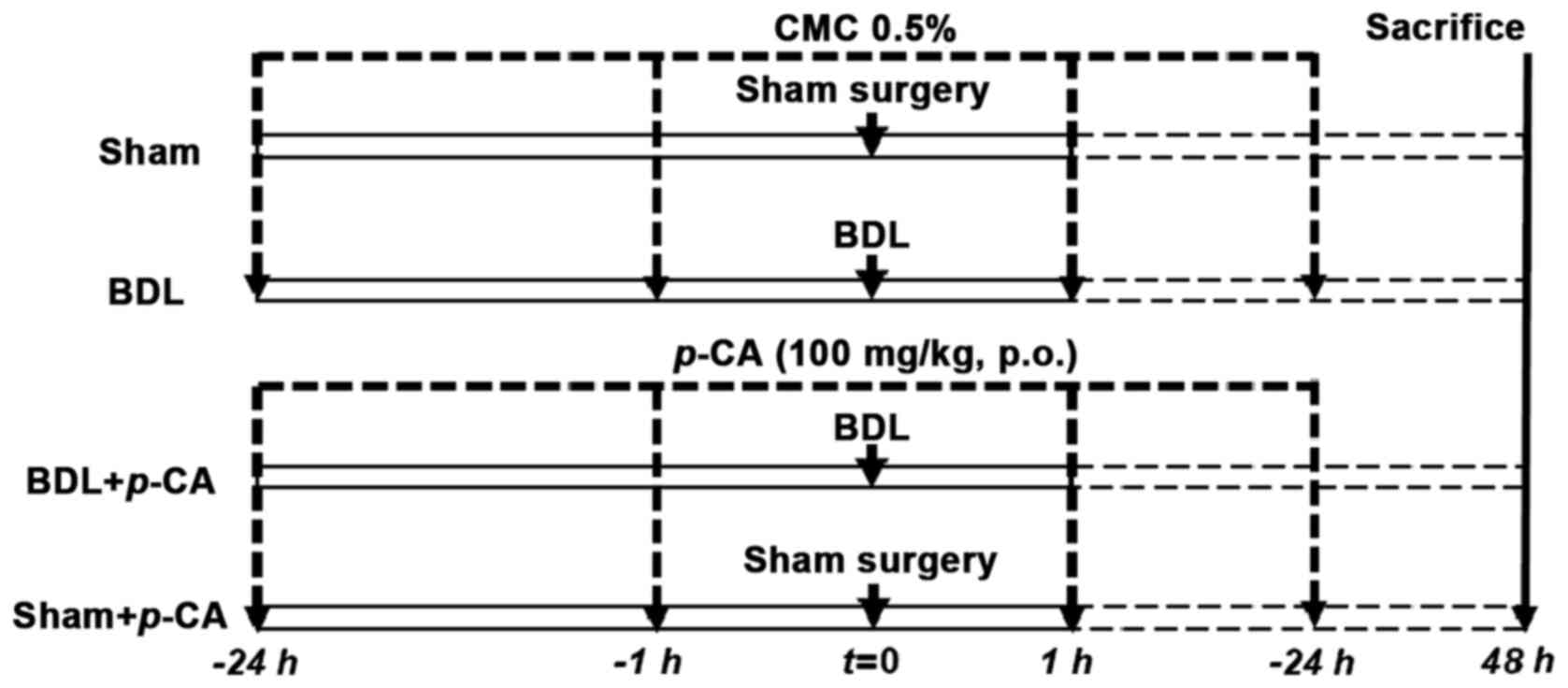 |
Figure 2
Experimental protocol to evaluate hepatoprotective effect of p-CA in the acute liver damage induced by common bile duct obstruction. Rats were divided in four groups as follows: Sham (n=5), BDL (n=5), BDL p-CA (n=5) and Sham + p-CA (n=5). p-CA, p-coumaric acid; BDL, bile duct ligation.
|
Animal sacrifice. At sacrifice, the rats were deeply anaesthetized with xylazine at 10 mg/kg and ketamine at 80 mg/kg i.p., mixed in the same syringe, and euthanized by cardiac puncture (4-6 ml of blood to obtain serum) and subsequent exsanguination (6-8 ml of blood) until cardiac and respiratory arrest and the absence of reflexes (in the hind legs) were confirmed. The collected blood was centrifuged (1,000 x g at 4˚C, for 10 min) to obtain serum. The livers were carefully dissected, freed from surrounding fatty and fibrous tissues and small samples were fixed in 4% p-formaldehyde.
Biochemical analyses. Serum samples were analyzed to determine liver damage, by quantifying the levels of alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT) and alkaline phosphatase (ALP) activity. The enzymatic activity quantification of ALT was carried out following the specifications described by Reitman and Frankel (15). Briefly, serum was reacted with the substrate (200 mM D/l alanine and 2 mM α-ketoglutaric acid) for 60 min at 37˚C; the mixture was then reacted for 15 min at 37˚C with the chromogenic reagent (1 mM 2,4-dinitrophenylhydrazine, in 1 N HCl), the reaction was terminated with 0.4 N NaOH and the optical density was measured at 515 nm using a Thermo Scientific™ GENESYS™ 10S UV-Vis Spectrophotometer (Thermo Fisher Scientific, Inc.). GGT activity was evaluated following the specifications described in the study by Glossmann and Neville (16). Briefly, serum samples were reacted for 30 min at 37˚C with 0.2 M Tris-HCl, 0.2 M MgCl2, 0.04 M glycyl-glycine and 10 mM γ-glutamyl-p-nitroanilide and this reaction was terminated with 1.5 M acetic acid, and the absorbance was measured at 410 nm. ALP activity was determined following the specifications described in the study by Berger and Rudolph (17). Briefly, serum samples were mixed with 0.1 M glycine and 1 mM MgCl2, and with p-nitrophenyl phosphate substrate, and incubated at 37˚C for 30 min; the reaction was terminated with 0.02 N NaOH, and the absorbance was measured at 410 nm. For each determination of enzymatic activity, a standard curve was used. Each serum sample was evaluated in triplicate for each quantification.
Histological analysis. Liver damage was also evaluated using hematoxylin and eosin (H&E) staining (18) in order to observe the morphological changes in the liver tissue. Briefly, the tissue sections previously fixed in 4% p-formaldehyde were dehydrated, diaphanized and immersed in paraffin. The sections were then cut into 4-µm-thick sections using a microtome and fixed on a slide coated with 2% 3-aminopropyl triethoxysilane in acetone. Subsequently, these slides were deparaffinized at 60˚C for 1 h, followed by xylol, and with etanol:xilol (1:1), absolute 96% alcohol, 80% alcohol, 70% alcohol, and finally with distilled water. Staining was carried out with Harris's hematoxylin (5 min; 25˚C), followed by rinsing with distilled water, acid alcohol and ammoniacal water, and eosin dye was then used (1 min; 25˚C) and the slides were dehydrated with 80% alcohol, 96% alcohol, absolute alcohol, ethanol:xylol (1:1) and then with xylol. Entellan was used as the mounting medium. The stained sections were visualized under a Zeiss Axioscope 40/40 FL microscope (Zeiss AG) and analyzed using ImageJ software version 1.53e (National Institutes of Health).
Anti-amoebic activity of p-CA
E. histolytica trophozoites. To evaluate the anti-amoebic capacity of p-CA, virulent trophozoites of the E. histolytica strain (HM-1:IMSS) were used, which were maintained according to the procedure described in the study by Diamond et al (19). Trophozoites HM-1:IMSS were donated by Dr Alfonso Olivos, from the Faculty of Medicine at the General Hospital of Mexico to JVJ.
Experimental in vitro assay. Under axenic conditions, culture media containing 1x105 trophozoites of E. histolytica were used for each assay, both at 12 and 24 h (Fig. 3). The positive control was only maintained with standard culture medium (TYI-S-33) at 35.9˚C, while the other three cultures were treated with p-CA (150, 300 or 500 µM). In addition, an internal control (ethanol 0.5% used as a p-CA diluent) and Mtz control (2 µM) were included. Cell viability was determined using a Trypan blue dye exclusion assay, which allowed the determination of the number of viable trophozoites in a cell suspension (20). Briefly, the amoeba culture tubes are cooled for 20 min at 4˚C, and were then centrifuged at 1,900 x g for 20 min; the culture medium was decanted and the pellet was diluted 1:2 with 0.4% trypan blue. Quantification is carried out using a hemocytometer, where the living trophozoites, with their cell membrane intact, exclude the dye, while the membrane-compromised trophozoites are stained. Each treatment was carried out by triplicate and repeated six times.
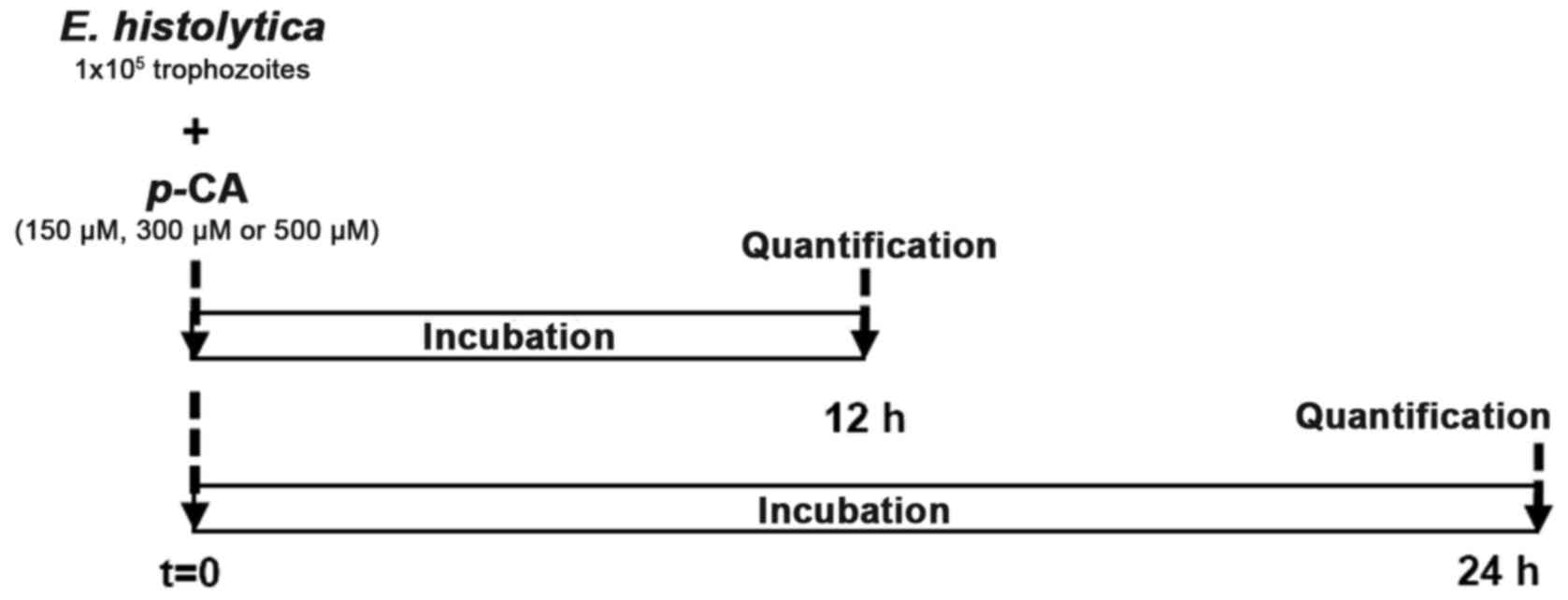 |
Figure 3
Experimental protocol to evaluate in vitro the anti-amoebic activity of p-CA. Trophozoites of E. histolytica (1x105) were incubated 12 or 24 h with various concentrations of p-CA (150, 300 or 500 µM). Cell viability quantification was carried out with the Trypan blue exclusion method. p-CA, p-coumaric acid; E. histolytica, Entamoeba histolytica.
|
Statistical analysis
Liver damage marker and in vitro analyses were evaluated statistically using GraphPad Prism 8.00 software (GraphPad Software, Inc.). The results are expressed as the mean values ± standard error (SEM) from each group and comparative analysis was carried out using analysis of one-way ANOVA followed by Tukey's post hoc test. P<0.05 was considered to indicate a statistically significant difference.
Results
Anti-necrotic and anti-cholestatic effects of p-CA
Acute liver injury induced by CCl4. Hepatic necrosis induced by acute intoxication with CCl4, allowed for the evaluation of the ability of p-CA to prevent this toxicity. The results of the analysis of liver damage markers are presented in Fig. 4. It was found that, in the CCl4 group, the levels of ALT, ALP and GGT were increased compared with those in the control group (Fig. 4). In the CCl4 + p-CA group, the increase in the ALT levels was partially, yet significantly prevented (Fig. 4A), while the increase in ALP and GGT activities was completely prevented by p-CA (Fig. 4B and C, respectively). The levels in the p-CA group did not differ significantly from those in the control group. The macroscopic and microscopic observations at x5 and x10 magnification with H&E staining (Fig. 5) were consistent with the results of biochemical analyses. The control and p-CA groups exhibited a healthy liver tissue appearance. In the CCl4 group, severe ballooning cell degeneration and necrosis were observed, while the CCl4 + p-CA group exhibited evidently less prominent cell changes compared with the CCl4 group.
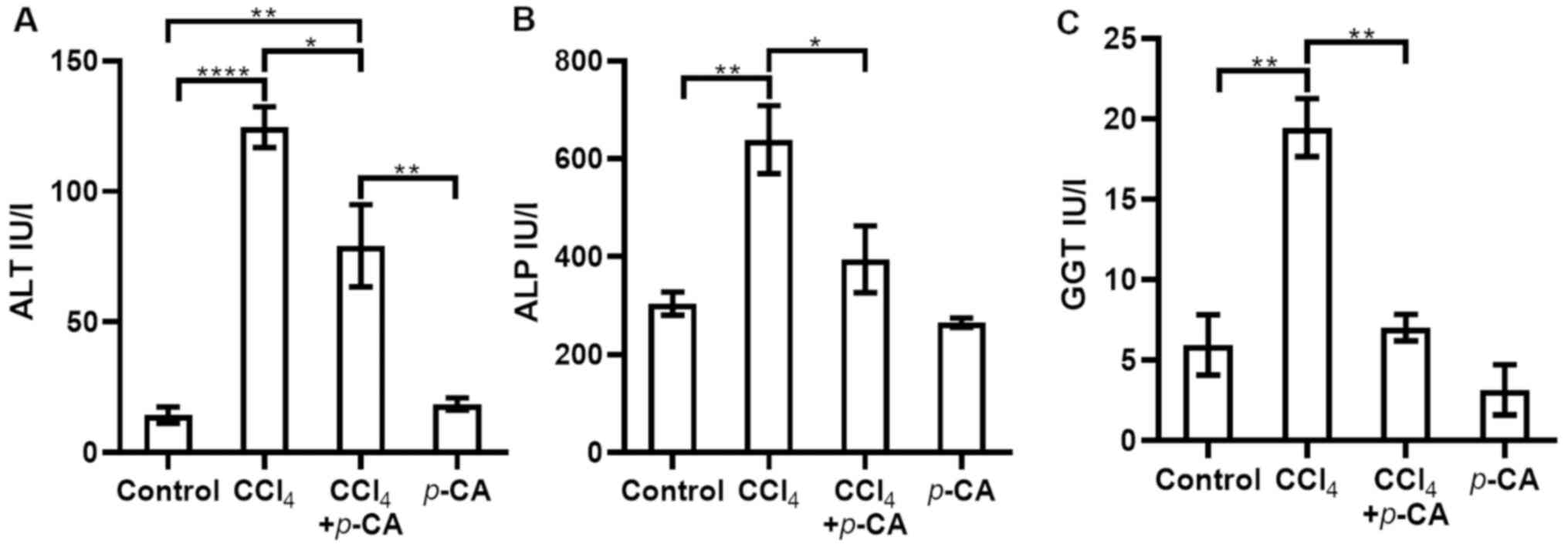 |
Figure 4
Evaluation of serum markers of liver damage in the CCl4 model. (A) ALT; (B) ALP; (C) GGT. The following groups were evaluated: Control, CCl4, CCl4 + p-CA and p-CA. Results are expressed as the mean ± SEM. Statistically significant differences are indicated as follows: *P<0.05, **P<0.01 and ****P<0.0001. p-CA, p-coumaric acid; CCl4, carbon tetrachloride; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase.
|
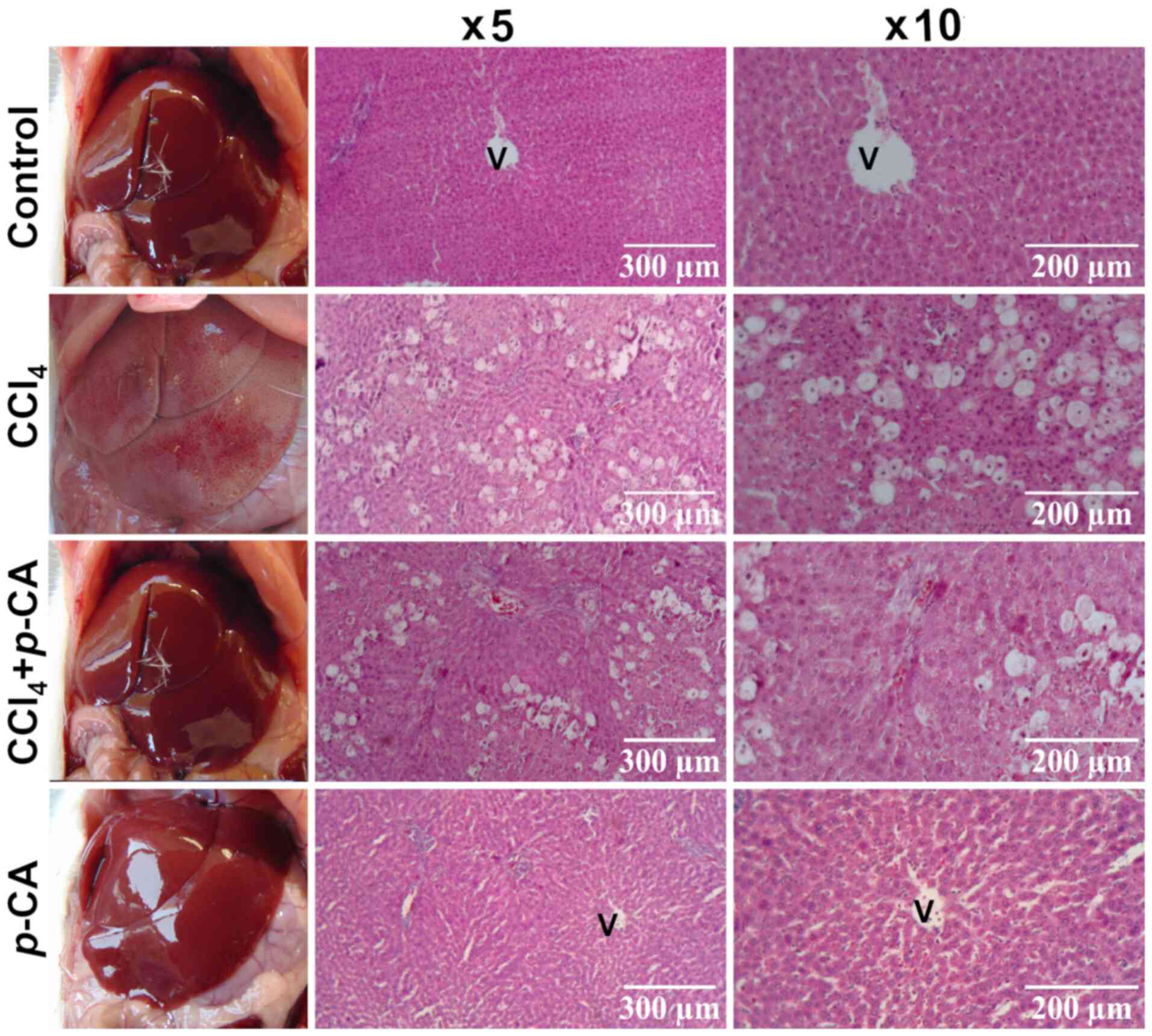 |
Figure 5
Liver in situ images and histological analysis of the liver sections of the CCl4 model stained with hematoxylin and eosin (H&E). Representative images of each experimental group: Control, CCl4, CCl4 + p-CA and p-CA. Micrographs were obtained at x5 and x10 magnification. V, centrilobular vein; p-CA, p-coumaric acid; CCl4, carbon tetrachloride.
|
Acute liver injury induced by BDL. Hepatic cholestatic damage allowed for the evaluation of the hepatoprotective ability of p-CA. The results of the analysis of enzymatic activities are presented in Fig. 6. The BDL group exhibited significantly increased levels of ALT, ALP and GGT compared with the control group (Fig. 6). In the BDL + p-CA group, the increase in the ALT levels was significantly reduced compared with the BDL group; however, the level was significant higher compared with the healthy group (sham + p-CA) (Fig. 6A). In addition, in the BDL + p-CA group, the values for ALP and GGT activities were significantly lower compared with those in the BDL group; however, the values did not differ significantly with respect to the healthy groups (Sham and Sham + p-CA) (Fig. 6B and C, respectively). These findings suggested that these increases were prevented by p-CA. The sham and sham + p-CA groups exhibited a similar trend in the levels of biochemical parameters examined, without significant differences between them (Fig. 6). Moreover, the macroscopic and microscopic observations at x5 and x10 magnification with H&E staining (Fig. 7) were consistent with the results of biochemical the markers. The sham and sham + p-CA groups exhibited healthy morphological characteristics in the liver. The BDL group exhibited necrosis and bile duct proliferation, while the BDL + p-CA group exhibited less necrosis and fewer bile ducts.
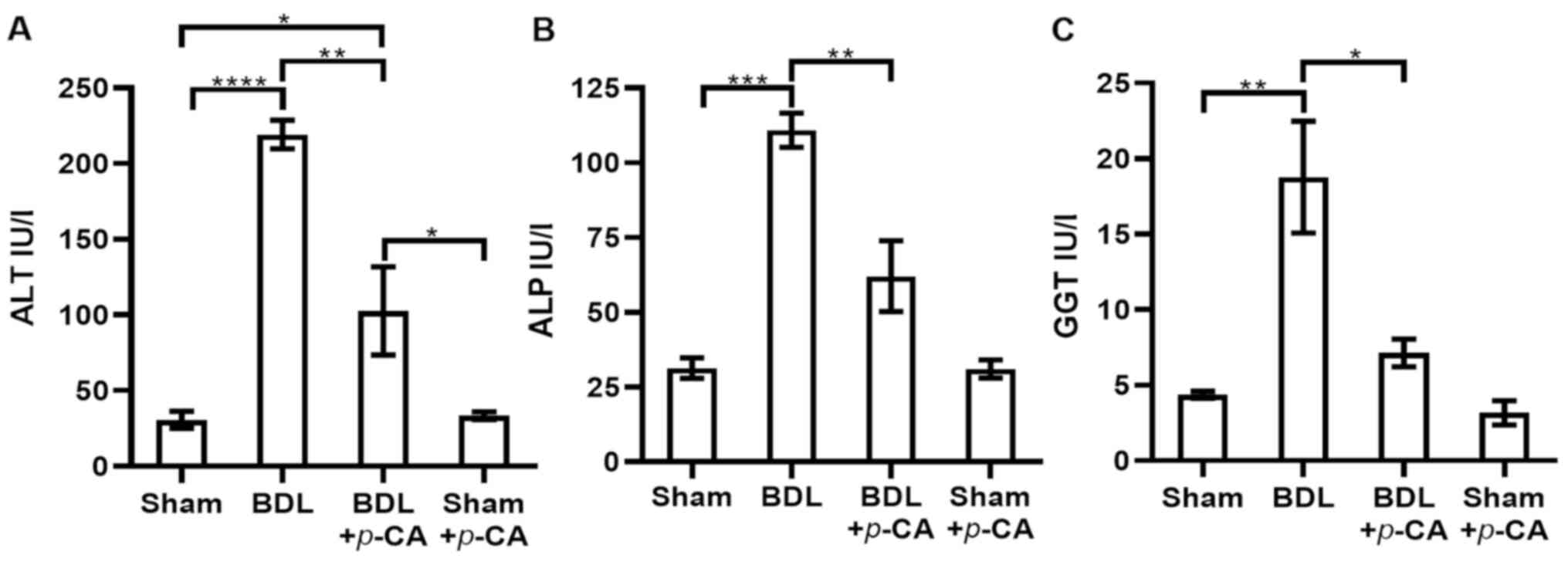 |
Figure 6
Evaluation of serum markers of liver damage in the BDL model. (A) ALT; (B) ALP; and (C) GGT. The following groups were evaluated: Sham, BDL, BDL + p-CA and Sham + p-CA. Results are expressed as the mean ± SEM. Statistically significant differences are indicated as follows: *P<0.05, **P<0.01, ***P<0.001 and ****P<0.0001. p-CA, p-coumaric acid; BDL, bile duct ligation; ALT, alanine aminotransferase; ALP, alkaline phosphatase; GGT, gamma-glutamyl transpeptidase.
|
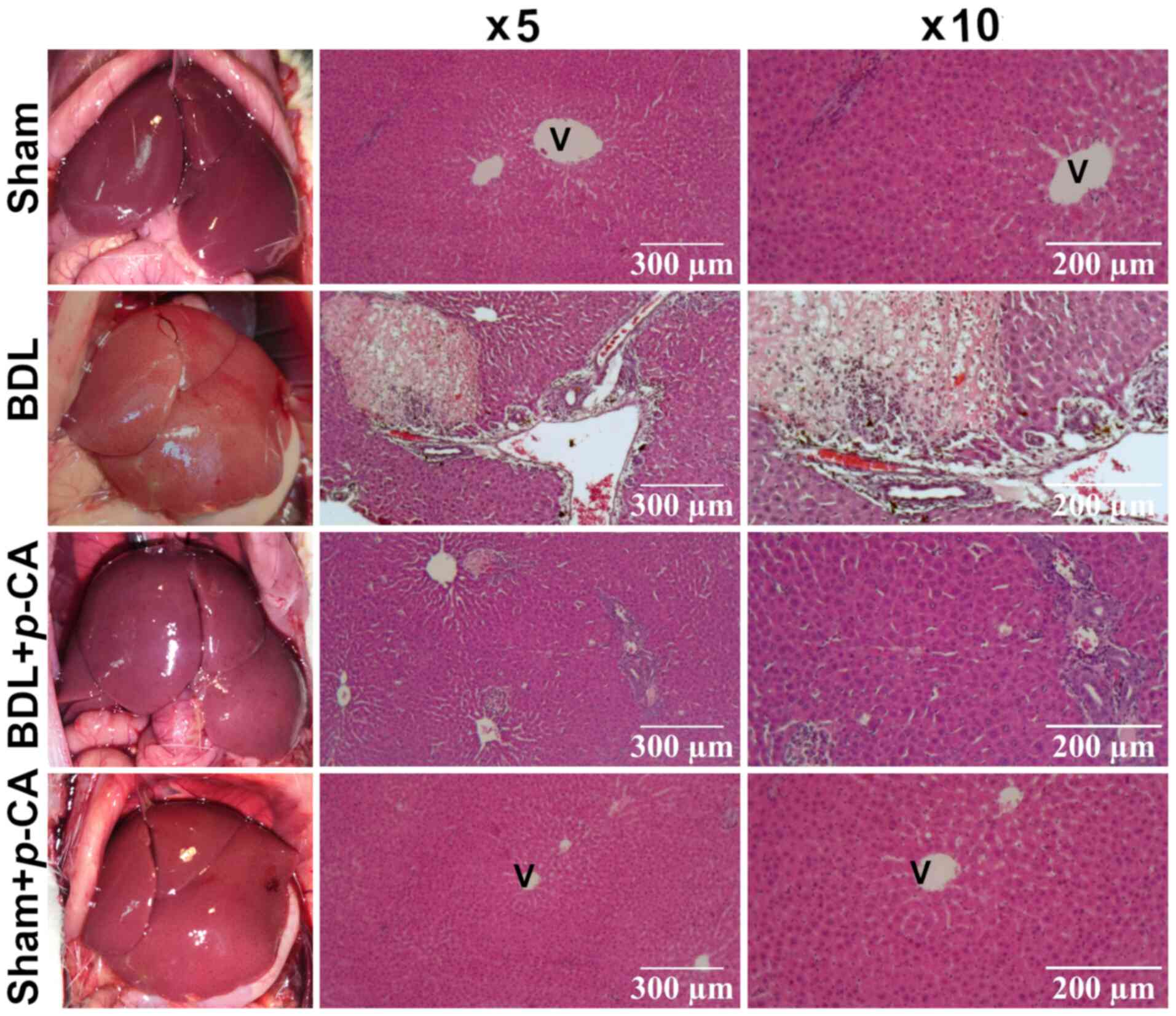 |
Figure 7
Liver in situ photograph and histological analysis of liver sections of the BDL model stained with hematoxylin and eosin (H&E). Representative images of each experimental group: Sham, BDL, BDL + p-CA and Sham + p-CA. H&E micrographs were obtained at x5 and x10 magnification. V, centrilobular vein; p-CA, p-coumaric acid; BDL, bile duct ligation.
|
Anti-amoebic activity of p-CA
Representative images of amoebic cultures that were incubated with various concentrations of p-CA are presented in Fig. 8A and B. The results of quantitative in vitro assays (Fig. 8C) revealed that, at 12 h, Mtz (4 µM) reduced the growth of E. histolytica by 46.6%, and this inhibition was 65.1% at 24 h. On the other hand, treatment with 150 and 300 µM p-CA exhibited a tendency to reduce cell viability; however, the most prominent effect was observed with 500 µM p-CA. At 12 h, a 26.5% growth inhibition was observed compared with the positive control; however, no significant difference was observed compared with the Mtz control. At 24 h, 500 µM p-CA decreased cell viability by 41.5% in comparison with the positive and internal controls.
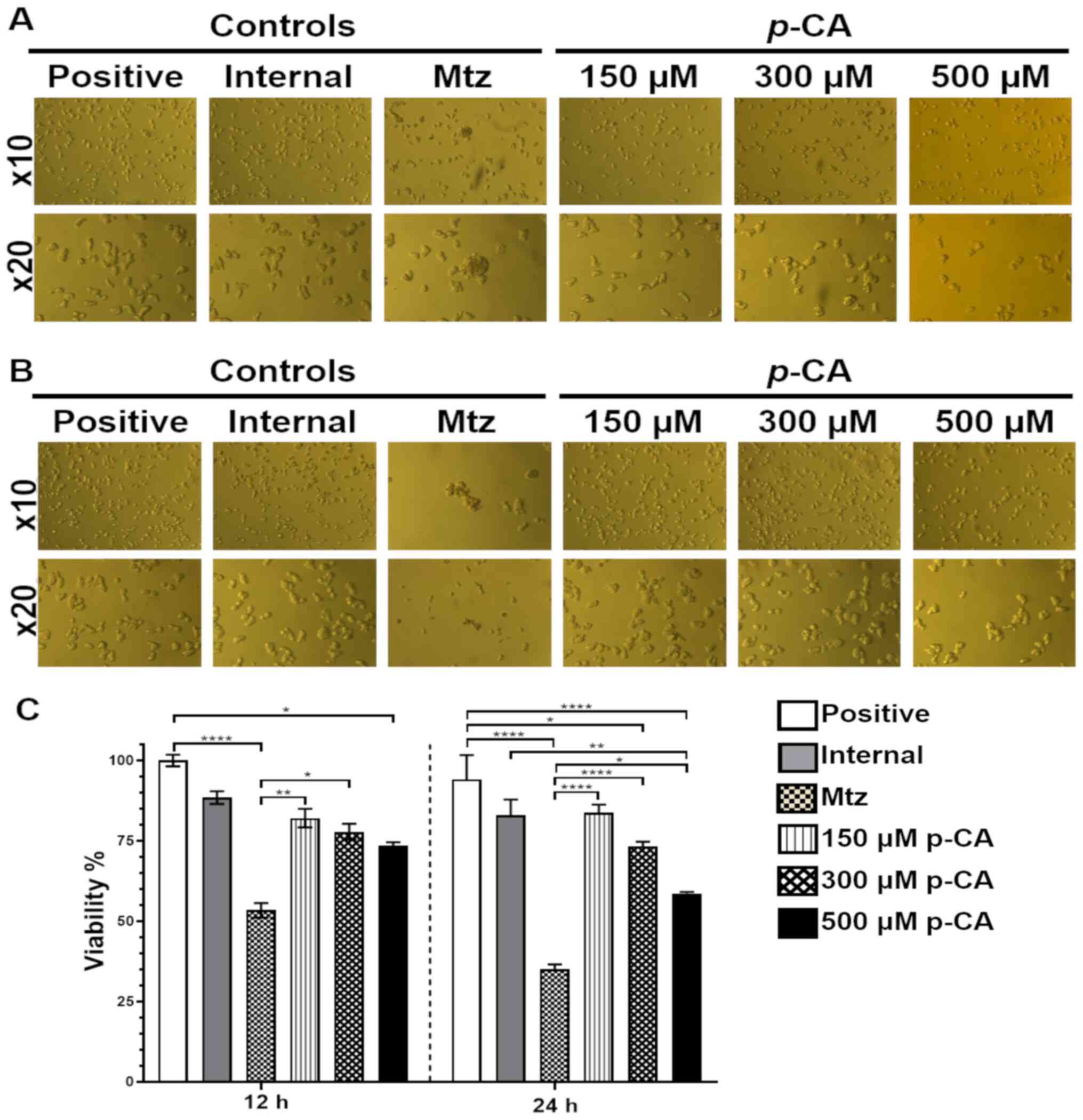 |
Figure 8
In vitro evaluation of the anti-amoebic activity of p-CA. Representative images were obtained using an inverted microscope at x10 and x20 magnification of trophozoites cultures with p-CA (150, 300 or 500 µM) at different time point: (A) 12 h; (B) 24 h. (C) Viability percentage at 12 and 24 h, with Mtz. Results are expressed as the mean ± SEM. Statistically significant differences are indicated as follows: *P<0.05, **P<0.01 and ****P<0.0001. p-CA, p-coumaric acid; Mtz, metronidazole.
|
Discussion
The present study provides evidence that p-CA exerts anti-necrotic and anti-cholestatic effects against liver injury induced by CCl4 and BDL in rats. In addition, it possesses anti-amoebic activity in cultures of trophozoites of E. histolytica.
Liver diseases are a public health concern worldwide, as there is no effective treatment to prevent (21) or reverse liver damage (22). Necrosis and cholestasis occur during the development of almost all acute and chronic liver diseases, as a consequence of exposure to a wide variety of harmful agents, such as viruses, bacteria, parasites, toxins, metabolites and autoimmune disorders (23). Necrosis is an accidental and unregulated cell death, which is considered as a process difficult to prevent (24). The results of the present study demonstrated that p-CA prevented acute liver injury induced by two different etiologies. CCl4 is a hepatotoxic agent that induces oxidative stress, cell death and a significant increase in AST, ALT and GGT levels (25,26), while BDL induces liver damage as a consequence of impaired bile duct flow, thus leading to the accumulation of toxic bile acids that induce hepatocellular damage (27). In both models, necrosis and cholestasis are present, which can be evaluated using ALT, ALP and GGT as liver injury biomarkers. The basal concentration ALT in the bloodstream increases in the event of hepatocyte membrane rupture, indicating hepatocellular parenchymal damage (16). In the present study, the levels of the serum marker of hepatocyte necrosis, ALT, were significantly reduced following treatment with p-CA, regardless of the origin of hepatocellular damage, and this result was consistent with the morphological hepatic observations. Previous studies have demonstrated that p-CA prevents ischemia/reperfusion-, cisplatin- and acetaminophen-induced liver necrosis, by its ability to increase antioxidant enzyme levels and reduce oxidative stress (10,11,28); however, the anti-cholestatic effects of this compound have not been explored to date, at least to the best of our knowledge. Cholestasis is a pathological condition characteristic of chronic liver diseases and, in particular, cholangiopathies, which develops due to the obstruction of bile flow inducing cholangiocyte damage (29,30). In the early stage of cholangiopathies, bile duct injury is associated with cholangiocyte proliferation; progression to chronicity is associated with increased bile duct loss, biliary fibrosis and an increased incidence of cholangiocarcinoma (31). The BDL model is characterized by a well-established exuberant ductular reaction (30). In the present study, cholestatic damage was evidenced by the presence of necrosis and ductal biliary proliferation, and treatment with p-CA partly alleviated ductal proliferation and necrosis.
Intestinal amoebiasis is a parasitic human infection induced by the protozoan E. histolytica, which can disseminate to other organs, such as the liver, brain, heart, spleen and kidneys (32,33). This amoebic infection is one of the three parasitic causes of mortality worldwide (34). Mtz is the drug of choice for amoebiasis; however, it has been reported to eradicate only up to 50% of luminal infections, and for this reason, some researchers have considered that this level of efficacy suggests resistance (9). Resistance to Mtz in vitro has already been generated in E. histolytica strains HM-1:IMSS (35) and HTH-56:MUTM (9). Therefore, it is necessary to identify novel new anti-amoebic treatments that can be used as alternative agents in the treatment of amoebiasis. Over the years, research interests on p-CA and its antiviral, antifungal and anti-microbiological activities have increased (6). However, the individual anti-amoebic potential of p-CA has not yet been explored, at least to the best of our knowledge. In vitro analyses using methanol extracts of Peucedanum (P.) caucasicum, P. palimbioides, P. longibracteolatum and P. chryseum have demonstrated that these agents exhibit anti-amoebic activities, attributed to their phenolic acid composition (p-CA, gallic acid, p-hydroxybenzoic acid, o-coumaric acid, vanillic acid and rosmarinic acid) (36). In the present study, it was demonstrated that p-CA exerted anti-amoebic effects, limiting the proliferation of E. histolytica. However, further experimental studies using in vivo models, as well as quantitative structure-activity relationship (QSAR) studies, are warranted in order to identify the optimal anti-amoebic compound.
In conclusion, the present study demonstrated that p-CA treatment prevented liver injury progression induced by various mechanisms, suggesting that p-CA may be an alternative therapy for liver diseases. Furthermore, to the best of our knowledge, the present study was the first to demonstrate that p-CA exerted anti-amoebic effects, suggesting that this compound may be further evaluated as a potential anti-parasitic strategy.
Acknowledgements
The authors would like to thank to Dr Edgar Manuel Macías Pérez (General Hospital Loreto, Loreto, Zacatecas, Mexico) for providing his expertise in the anesthesia procedures.
Funding
The authors would like to acknowledge the funding received by ‘Support for Teachers with Desirable Profile’ (Folio: 162896; Agreement: 511‑6/2019.‑9704) and the ‘Science Immersion Program 2019‑UASLP’ (Funding entity: PROFEXCE; Agreement: C20‑PROFEXCE‑10‑18.18.).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LRAM and JRMP participated in the conception and design of the in vivo experiments. MHMO, SLMH and BAS participated in the analysis and interpretation of serum markers of liver damage. JVJ contributed to the morphological and histopathological analysis of the livers. JVJ, AHM and AMPH participated in the design and analysis of the in vitro experiments on amoebiasis. AHM, MHMO and BAS confirm the authenticity of all the raw data. All authors participated in the writing and revision of the manuscript. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All experimental protocols using the rats were approved by the Research Ethics Committee of the Faculty of Professional Studies Huasteca Zone of the Autonomous University of San Luis Potosí (FEPZH-2020-CEI-01), and were conducted under institutional guidelines for the care and use of experimental animals and with the national regulatory norm NOM-062-ZOO-1999.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Białecka-Florjańczyk E, Fabiszewska A and Zieniuk B: Phenolic acids derivatives - biotechnological methods of synthesis and bioactivity. Curr Pharm Biotechnol. 19:1098–1113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Kumar N and Goel N: Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol Rep (Amst). 24(pe00370)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Nwidu LL, Nwafor PA, da Silva VC, Rodrigues CM, dos Santos LC, Vilegas W and Nunes-de-Souza RL: Anti-nociceptive effects of Carpolobia lutea G. Don (Polygalaceae) leaf fractions in animal models. Inflammopharmacology. 19:215–225. 2011.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Nwidu LL, Essien GE, Nwafor PA and Vilegas W: Antidiarrheal mechanism of Carpolobia lutea leaf fractions in rats. Pharm Biol. 49:1249–1256. 2011.PubMed/NCBI View Article : Google Scholar
|
|
5
|
El-Seedi HR, El-Said AM, Khalifa SA, Göransson U, Bohlin L, Borg-Karlson AK and Verpoorte R: Biosynthesis, natural sources, dietary intake, pharmacokinetic properties, and biological activities of hydroxycinnamic acids. J Agric Food Chem. 60:10877–10895. 2012.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Pei K, Ou J, Huang J and Ou S: p-Coumaric acid and its conjugates: Dietary sources, pharmacokinetic properties and biological activities. J Sci Food Agric. 96:2952–2962. 2016.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Hong SY, Jeong WS and Jun M: Protective effects of the key compounds isolated from Corni fructus against β-amyloid-induced neurotoxicity in PC12 cells. Molecules. 17:10831–10845. 2012.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Piñeiro-Carrero VM and Piñeiro EO: Liver. Pediatrics. 113 (Suppl 4):1097–1106. 2004.PubMed/NCBI
|
|
9
|
Upcroft P and Upcroft JA: Drug targets and mechanisms of resistance in the anaerobic protozoa. Clin Microbiol Rev. 14:150–164. 2001.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Parvizi F, Yaghmaei P, Haeri Rohani SA and Mard SA: Hepatoprotective properties of p-coumaric acid in a rat model of ischemia-reperfusion. Avicenna J Phytomed. 10:633–640. 2020.PubMed/NCBI
|
|
11
|
Ekinci Akdemir FN, Albayrak M, Çalik M, Bayir Y and Gülçin İ: The protective effects of p-coumaric acid on acute liver and kidney damages induced by cisplatin. Biomedicines. 5(E18)2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Frank D, Savir S, Gruenbaum BF, Melamed I, Grinshpun J, Kuts R, Knyazer B, Zlotnik A, Vinokur M and Boyko M: Inducing acute liver injury in rats via carbon tetrachloride (CCl4) exposure through an orogastric tube. J Vis Exp: Apr 28, 2020 (Epub ahead of print). doi: 10.3791/60695.
|
|
13
|
Van Campenhout S, Van Vlierberghe H and Devisscher L: Common bile duct ligation as model for secondary biliary cirrhosis. Methods Mol Biol. 1981:237–247. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Georgiev P, Jochum W, Heinrich S, Jang JH, Nocito A, Dahm F and Clavien PA: Characterization of time-related changes after experimental bile duct ligation. Br J Surg. 95:646–656. 2008.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Reitman S and Frankel S: A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am J Clin Pathol. 28:56–63. 1957.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Glossmann H and Neville DM: gamma-glutamyltransferase in kidney brush border membranes. FEBS Lett. 19:340–344. 1972.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Bergmeyer HU, Grabl M and Walter HE: Enzymes. In: Methods of Enzymatic Analysis, a.M.G.V.-C.W. Bergmeyer J (ed), pp269-270, 1983.
|
|
18
|
Luna LG: Manual of Histologic Staining Methods of the Arme Forces Institute of Pathology. 3rd edition. McGraw-Hill, New York, NY, 1968.
|
|
19
|
Diamond LS, Harlow DR and Cunnick CC: A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans R Soc Trop Med Hyg. 72:431–432. 1978.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Strober W: Trypan blue exclusion test of cell viability. Curr Protoc Immunol: May 1, 2015 (Epub ahead of print). doi: 10.1002/0471142735.ima03bs21.
|
|
21
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW and Feng Y: The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 16:26087–26124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Sun M and Kisseleva T: Reversibility of liver fibrosis. Clin Res Hepatol Gastroenterol. 39 (Suppl 1):S60–S63. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luedde T, Kaplowitz N and Schwabe RF: Cell death and cell death responses in liver disease: Mechanisms and clinical relevance. Gastroenterology. 147:765–783.e4. 2014.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Kanduc D, Mittelman A, Serpico R, Sinigaglia E, Sinha AA, Natale C, Santacroce R, Di Corcia MG, Lucchese A, Dini L, et al: Cell death: Apoptosis versus necrosis (Review). Int J Oncol. 21:165–170. 2002.PubMed/NCBI
|
|
25
|
Boll M, Weber LW, Becker E and Stampfl A: Mechanism of carbon tetrachloride-induced hepatotoxicity. Hepatocellular damage by reactive carbon tetrachloride metabolites. Z Naturforsch C J Biosci. 56:649–659. 2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Deniz GY, Laloglu E, Koc K, Nadaroglu H and Geyikoglu F: The effect of black mulberry (Morus nigra) extract on carbon tetrachloride-induced liver damage. Arch Biol Sci. 70:371–378. 2018.
|
|
27
|
Sokol RJ, Devereaux M, Dahl R and Gumpricht E: ‘Let there be bile’ - understanding hepatic injury in cholestasis. J Pediatr Gastroenterol Nutr. 43 (Suppl 1):S4–S9. 2006.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Cha H, Lee S, Lee JH and Park JW: Protective effects of p-coumaric acid against acetaminophen-induced hepatotoxicity in mice. Food Chem Toxicol. 121:131–139. 2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Li M, Cai SY and Boyer JL: Mechanisms of bile acid mediated inflammation in the liver. Mol Aspects Med. 56:45–53. 2017.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Mariotti V, Cadamuro M, Spirli C, Fiorotto R, Strazzabosco M and Fabris L: Animal models of cholestasis: An update on inflammatory cholangiopathies. Biochim Biophys Acta Mol Basis Dis. 1865:954–964. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Glaser SS, Onori P, Wise C, Yang F, Marzioni M, Alvaro D, Franchitto A, Mancinelli R, Alpini G, Munshi MK, et al: Recent advances in the regulation of cholangiocyte proliferation and function during extrahepatic cholestasis. Dig Liver Dis. 42:245–252. 2010.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Chou A and Austin RL: Entamoeba Histolytica. StatPearl, Treasure Island, FL, 2021.
|
|
33
|
Zulfiqar H, Mathew G and Horrall S: Amebiasis. StatPearls, Treasure Island, FL, 2021.
|
|
34
|
Kantor M, Abrantes A, Estevez A, Schiller A, Torrent J, Gascon J, Hernandez R and Ochner C: Entamoeba Histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can J Gastroenterol Hepatol. 2018(4601420)2018.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Wassmann C, Hellberg A, Tannich E and Bruchhaus I: Metronidazole resistance in the protozoan parasite Entamoeba histolytica is associated with increased expression of iron-containing superoxide dismutase and peroxiredoxin and decreased expression of ferredoxin 1 and flavin reductase. J Biol Chem. 274:26051–26056. 1999.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Degerli S and Tepe B: Phenolic acid composition and anti-parasitic effects of four peucedanum species on Entamoeba histolytica Trophozoites. Iran J Parasitol. 10:420–431. 2015.PubMed/NCBI
|






















