Introduction
The incidence of thyroid cancer, which represents
the most common endocrine cancer, has increased over the past
decades (1). Thus, there is an
urgent need to further improve thyroid cancer diagnosis and
therapy, as is particularly the case for advanced iodine-refractory
thyroid cancer (2). A significant
contribution of the animal lectin, galectin-3, in the context of
thyroid tumors has been well-established (3,4). The
molecule belongs to the galectin family of soluble lectins that are
specific for β-galactosides. It is the only chimera type within the
galectin family and consists of 3 distinct domains: An
NH2-terminal domain, a proline-rich collagen-like domain
and a COOH-terminal carbohydrate recognition domain (CRD). As other
galectins, galectin-3 interacts with simple β-galactosides;
however, its affinity towards natural and more complex
polylactosamine structures is higher (5). Dependent on the cell type or cell
line, the molecule is expressed intra- (cytosol and nucleus) and
extracellularly (plasma membrane and extracellular matrix)
(6,7). It can interact with a variety of
other molecules, whereby, particularly in the intracellular
environment, also carbohydrate-independent interactions are
possible. The extracellular expression of galectin-3 has been
reported for numerous normal, as well as tumor cells, whereby it
can fulfil different functions. Galectin-3 secreted by tumor cells
binds both glycosylated IFNγ and glycoproteins of the tumor
extracellular matrix, thus avoiding IFNγ diffusion and the
formation of an IFNγ-induced chemokine gradient required for T cell
infiltration (8). In T-cells,
galectin-3 induces apoptosis, whereby both, the CRD as well as the
NH2-terminal tail are involved (9). Extracellular galectin-3 programs
multidrug resistance via Na+/K+-ATPase and
P-glycoprotein-dependent signaling mechanisms (10). Galectin-3 exhibits also
chemokinetic or chemotactic effects (11), whereby cell migration can be
induced in an ERK1/2-MAPK-dependent manner (12). Additionally, galectin-3 interacts
intracellularly with the Ras/ERK1/2 pathway, in that it augments
K-Ras, but attenuates ERK activity (13,14).
In comparison to normal thyroid or its benign
alterations, galectin-3 is expressed almost without exception in
differentiated thyroid carcinomas, as well as in fractions of
poorly differentiated and anaplastic thyroid carcinomas (15). As galectin-3 expression is
downregulated by wild-type (wt) p53, the high expression level of
galectin-3 in differentiated thyroid carcinomas appeared to be
paradoxical, until it could be demonstrated that in such types of
tumors, the p53 activator homeodomain interacting protein kinase-2
(HIPK2) is lacking, a kinase which is necessary for an adequate
function of p53. The loss of HIPK2 leads to galectin-3
overexpression and the inhibition of apoptosis (16).
Recently, the cell surface expression of galectin-3
has also been demonstrated for human thyroid tumor cell lines,
prompting D'Alessandria et al (2016) to verify whether
galectin-3 antibodies can be used in immunoPET studies (3). In murine xenograft models of human
thyroid cancer, a radiolabeled Fab-fragment of a galectin-3
specific antibody allowed the specific imaging of the thyroid
tumors (3,4). The present study focused primarily on
the functional significance of galectin-3 in the extracellular
environment and characterized its relevance in the context of
thyroid tumor cell adhesion and migration.
Materials and methods
Cell lines and culture conditions
The human de-differentiated thyroid carcinoma cell
line, B-CPAP, and the human follicular thyroid cancer cell line,
FTC-133, were provided by Professor G. Brabant (Department of
Endocrinology, Christie Hospital, Manchester, UK) and were
subjected to STR profiling and verification (Eurofins Genomics
Germany GmbH). The human anaplastic thyroid cancer cell line,
Cal-62, was obtained from Leibniz Institute DSMZ - German
Collection of Microorganisms and Cell Cultures. All cells were
tested as negative for mycoplasma prior to all experiments. The
cells lines were cultivated in DMEM supplemented with 10% fetal
calf serum (FCS), 100 U/ml penicillin-streptomycin and 2 mM
L-glutamine.
Antibodies and proteins
Recombinant galectin-3 was obtained from Abcam or
produced as previously described (17). Asialofetuin and fetuin were
obtained from Sigma-Aldrich; Merck KGaA, collagen type R (a mixture
of 90% collagen type I and 10% collagen type III) was from SERVA
Electrophoresis GmbH and EHS laminin from Roche Diagnostics GmbH.
The hybridoma cell line expressing a monoclonal antibody against
galectin-3 (M3/38) (18) was
obtained from the American Type Culture Collection (ATCC).
Cell extraction and affinity
purification of galectin-3
A total of 105 cells were seeded in 6-cm
Petri dishes and cultivated to >90% confluency. Monolayers were
washed with ice-cold phosphate-buffered saline (PBS) and then
solubilized for 10 min at 4˚C with PBS, 5 mM EDTA, 1% Triton X-100,
containing protease inhibitors (extraction buffer). The cell
extract was centrifuged for 10 min at 15,000 x g at 4˚C. A total of
500 µl each of the supernatant (derived from 1,000,000 cells) were
either mixed with 200 µl lactose-agarose (Sigma-Aldrich; Merck
KGaA) or agarose (Merck KGaA) and incubated at 4˚C for 90 min on a
shaker. The gel slurry was washed 3 times with extraction buffer
without Triton X-100. Bound proteins were eluted with 400 µl of
extraction buffer without Triton X-100 containing either 100 mM
lactose or 100 mM sucrose. A Bradford assay with Bio-Rad Protein
dye reagent was used for protein determination (Bio-Rad
Laboratories, Inc.).
Western blot analysis
Western blot analyses were performed essentially as
previously described (19).
Briefly, proteins (for the preparation of cell solubilisates please
see previous above) were separated by 10% SDS-PAGE and transferred
onto nitrocellulose filters. Filters were then blocked for 1 h in
Tris-buffered saline (TBS) containing 5% milk powder and 0.1%
Tween-20 (TBS-Tween). Filters were further incubated overnight at
4˚C with the M3/38 antibody (sc-23938; Santa Cruz Biotechnology,
Inc.) diluted 1:500 in TBS-Tween and then washed 3 times (10 min
each) in TBS-Tween at room temperature. Incubation with
HRP-conjugated secondary antibodies (goat anti-rat IgG coupled to
horseradish peroxidase from Jackson ImmunoResearch, cat. no.
112-035-003), diluted 1:2,000 in TBS-Tween, was performed for 1 h
at room temperature. Filters were then again washed in TBS-Tween
and antibody binding was visualized using the Pierce™ ECL Plus
Western Blotting Substrate (Thermo Fisher Scientific, Inc.) and the
ChemiDoc XRS system (Bio-Rad Laboratories, Inc.).
Cell adhesion assay
The assay was carried out essentially as previously
described (20). In brief,
proteins were diluted to final concentrations in PBS, spotted (3 µl
per spot) onto plastic Petri dishes (6 cm in diameter) and
incubated for 2 h in a humid atmosphere at room temperature. Dishes
were then washed once with PBS containing 5% heat-inactivated
bovine serum albumin (BSA) and incubated in the same solution for 1
h at room temperature. Dishes were subsequently washed 3 times with
PBS. Cell suspensions (1x106 cells/ml in serum-free
DMEM, 3 ml per dish) were then added and incubated at 37˚C with the
substrata for up to 4 h. Subsequently, non-adherent cells were
removed and the number of adherent cells monitored
microscopically.
For the analysis of the carbohydrate dependency of
cell adhesion to galectin-3, asialofetuin, fetuin (1 mg/ml each),
lactose, or sucrose (50 mM each) were present in the medium
throughout the assay.
Cell surface ELISA
Cells (10,000 cells per well) were seeded in 96-well
plates and allowed to adhere in complete culture medium for 5 h and
then incubated at room temperature in serum-free DMEM overnight.
The medium was then changed to DMEM with or without 50 mM lactose
or sucrose and cells incubated for 1 h at 37˚C. Following a washing
step with DMEM, cells were incubated with M3/38 antibody (sc-23938;
Santa Cruz Biotechnology, Inc.) diluted 1:500 in DMEM 1% BSA for 1
h at room temperature. Following another washing step, the cells
were fixed for 10 min at room temperature in 4% formaldehyde in
PBS, washed with PBS and blocked for 30 min with 5% BSA in PBS. The
cells were then incubated with secondary anti-rat IgG antibody
coupled to horseradish peroxidase (cat. no. 112-035-003; Jackson
ImmunoResearch) diluted 1:2,000 for 1 h at room temperature and
then washed with PBS. ABTS (from Roche Diagnostics GmbH) in acetate
buffer was used as enzyme substrate and optical density was
measured at 405 nm. In control experiments, cells were incubated
with secondary antibodies only.
Video time lapse analysis
Cells were seeded into pre-coated (20 µg/ml
galectin-3 or EHS laminin) 24-well plates (2,000 cells per well)
and allowed to adhere overnight. Video time lapse analyses were
then carried out as previously described (21). Briefly, the culture plates were
transferred to a pre-heated (37˚C), gassed (5% CO2/air)
and humidified chamber fitted onto an inverted microscope (Axiovert
200 M; Carl Zeiss AG) equipped with a motorized cross-stage. Images
were recorded with Axiovision (Rel. 4.8) every 10 min for 24 h with
the AxioCamMR3 (Carl Zeiss AG). Cell movements were tracked and
analysed in ImageJ (version 1.49n) using the ImageJ plugin
MTrackJ.
Statistical analysis
For statistical analysis, one-way-ANOVA with Tukey's
multiple comparisons post hoc test were carried out using GraphPad
Prism 6 Software (GraphPad Software Inc.). P-values <0.05 were
considered to indicate statistically significant differences
compared to the control experiments.
Results
Galectin-3 from thyroid carcinoma
cells harbors a functionally active CRD
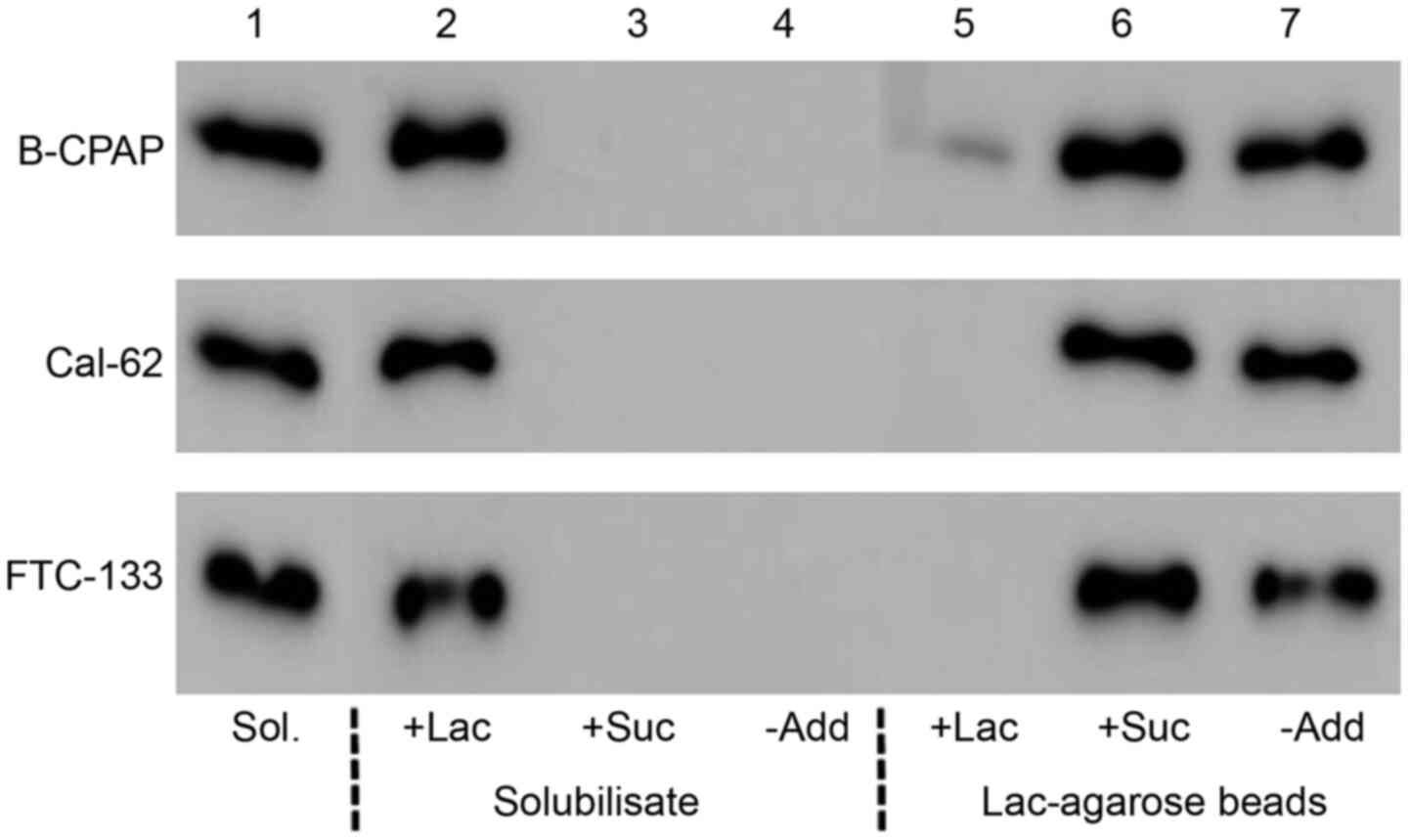
To analyze whether galectin-3 from thyroid carcinoma
cells is functionally intact, cell solubilisates from B-CPAP,
Cal-62 or FTC-133 cells were incubated with lactose-agarose beads
in the absence or presence of 50 mM lactose or sucrose. Bound
material was then eluted with a lactose- or sucrose-containing
buffer. Western blot analyses (Fig.
1) documented that galectin-3 present in cell solubilisates was
lost upon incubation with lactose agarose in the absence of
additives or in the presence of 50 mM sucrose, but not in the
presence of 50 mM lactose. Vice versa, galectin-3 was present in
the eluate (treatment with SDS sample buffer at 100˚C) of lactose
agarose beads incubated without additives or in the presence of 50
mM sucrose, but not in the presence of 50 mM lactose. Comparable
data were obtained for all 3 carcinoma cell lines, indicating that
the CRD of intrinsic galecin-3 is functionally active in this type
of tumor cells.
Cell surface-bound galectin-3 only
partially interacts via its CRD with the plasma membrane
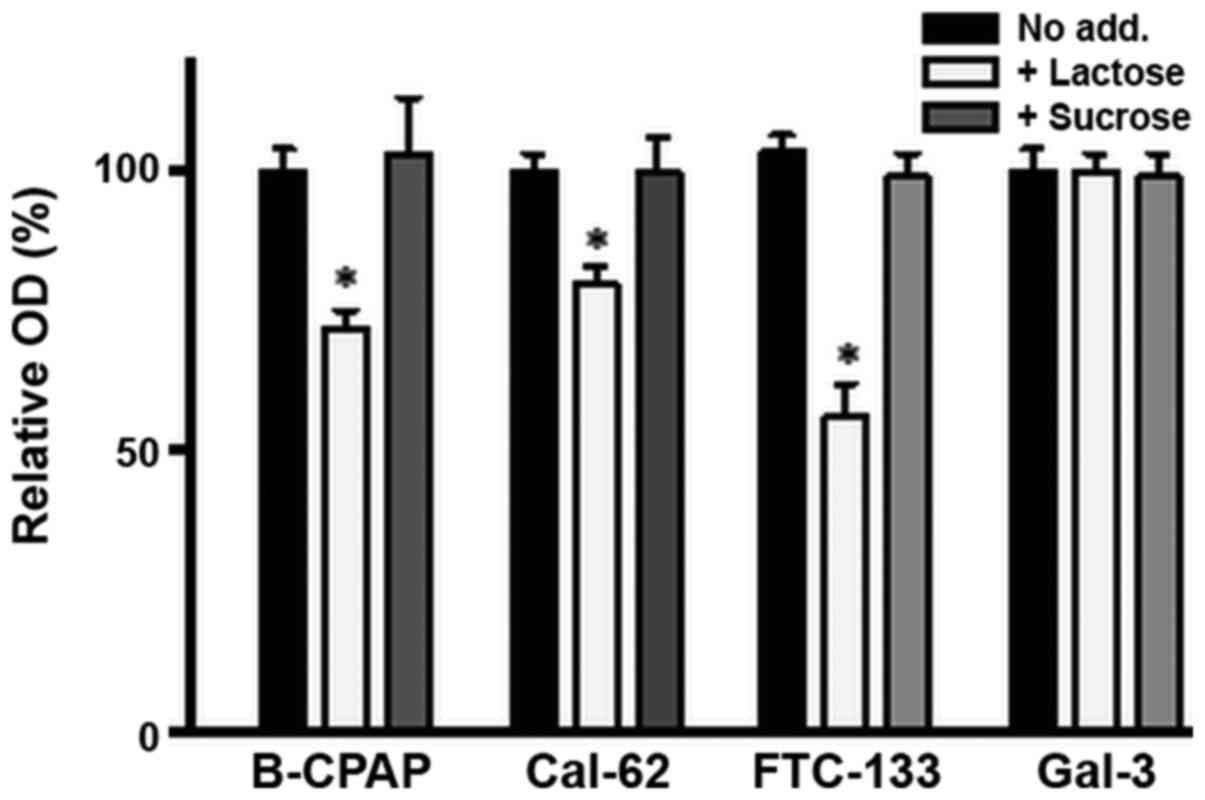
Thyroid carcinoma cells were seeded in 96-well
plates and cultivated to confluency. For the cell surface ELISA,
cells were incubated with DMEM medium with or without 50 mM lactose
or sucrose for 1 h, followed by incubation with a
galectin-3-specific antibody. In all cell lines, the amount of
galectin-3 expressed on the cell surface was substantially reduced
(approximately 25% in B-CPAP or Cal-62 and approximately 50% in
FTC-133 cells), but not completely abolished by lactose treatment,
whereas sucrose treatment did not exert any evident effects
(Fig. 2). Treatment with
galectin-3 immobilized on plastic with lactose or sucrose did not
exert any evident effects (Fig.
2). These data indicate that only a fraction of extracellular
galectin-3 interacts with the plasma membrane via the CRD. Notably,
treatment with asialofetuin or fetuin led to more strongly reduced
OD values in comparison to treatment with simple carbohydrates
(data not shown). However, such a reduction also occurred for
immobilized galectin-3, suggesting that asialofetuin or fetuin
bound to galectin-3 interfere with binding of the primary
antibody.
Galectin-3 promotes the adhesion of
thyroid carcinoma cells via the CRD
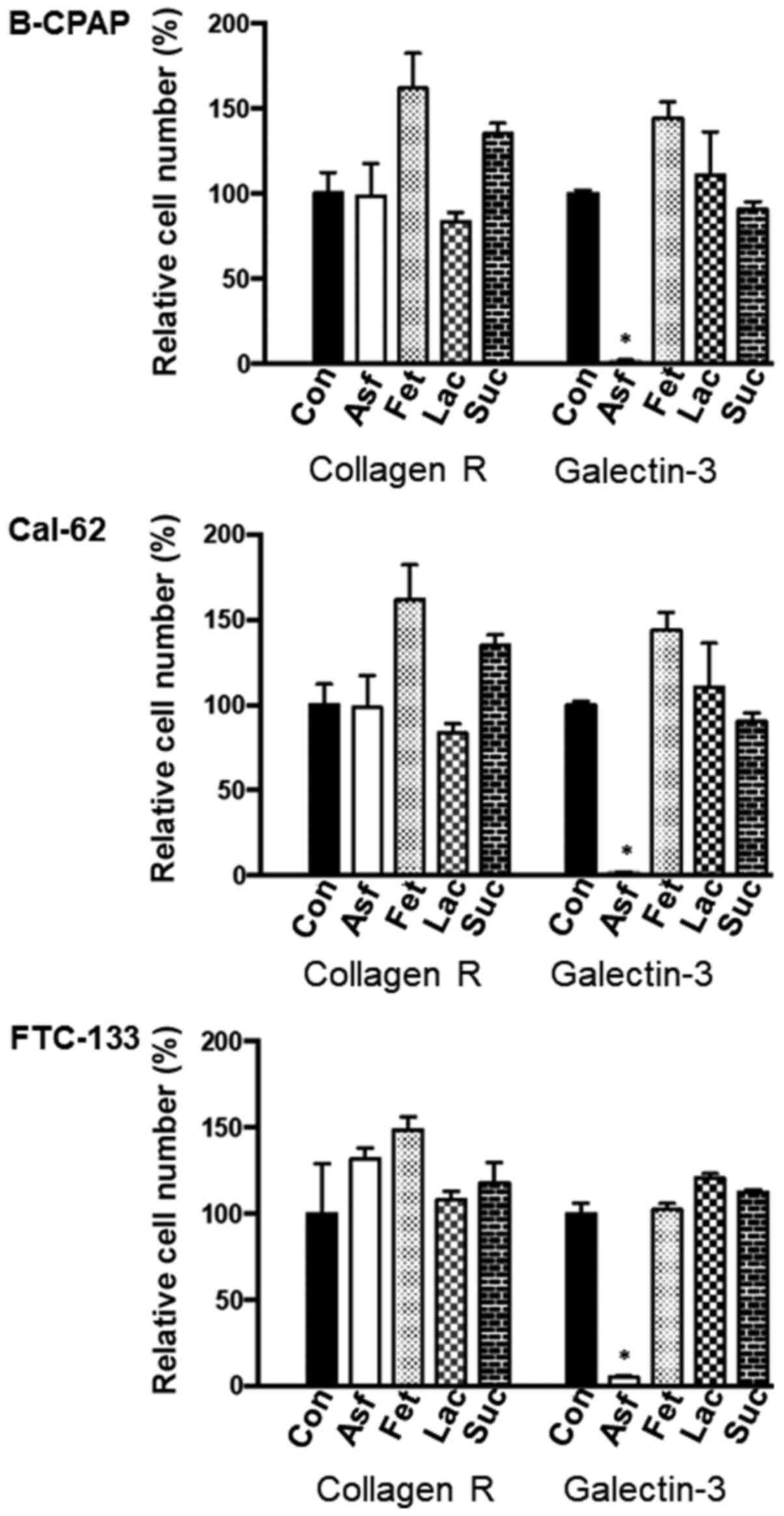
Galectin-3 or collagen R were immobilized on the
surface of Petri dishes in selected areas and single cell
suspensions of thyroid carcinoma cells were incubated with the
immobilized proteins either in the absence or in the presence of
asialofetuin (1 mg/ml), fetuin (1 mg/ml), lactose (50 mM) or
sucrose (50 mM) for 4 h. Following the removal of unbound cells,
adherent cells were counted microscopically in randomly selected
areas. All cell lines were able to adhere to galectin-3 or collagen
R (Fig. 3). Asialofetiun inhibited
the adhesion of carcinoma cells to galectin-3 by almost 100%, but
not to collagen R, whereas fetuin, lactose or sucrose were almost
ineffective. This finding is in accordance with previous data by
the authors demonstrating that cell adhesion to galectin occurs
predominantly in a carbohydrate-dependent manner (20). It should be emphasized that
asialofetuin is multivalent and carries terminal carbohydrate
structures with a higher affinity to galectin-3 in comparison to
fetuin or lactose (22). It is
also noteworthy to mention that cells adherent to galectin-3
continued to proliferate in normal cell culture medium for at least
further three days. Hereafter, a confluent cell monolayer has been
developed. During this time-period no significant signs of cell
death were observed.
Galectin-3 does not promote the
migration of thyroid carcinoma cells
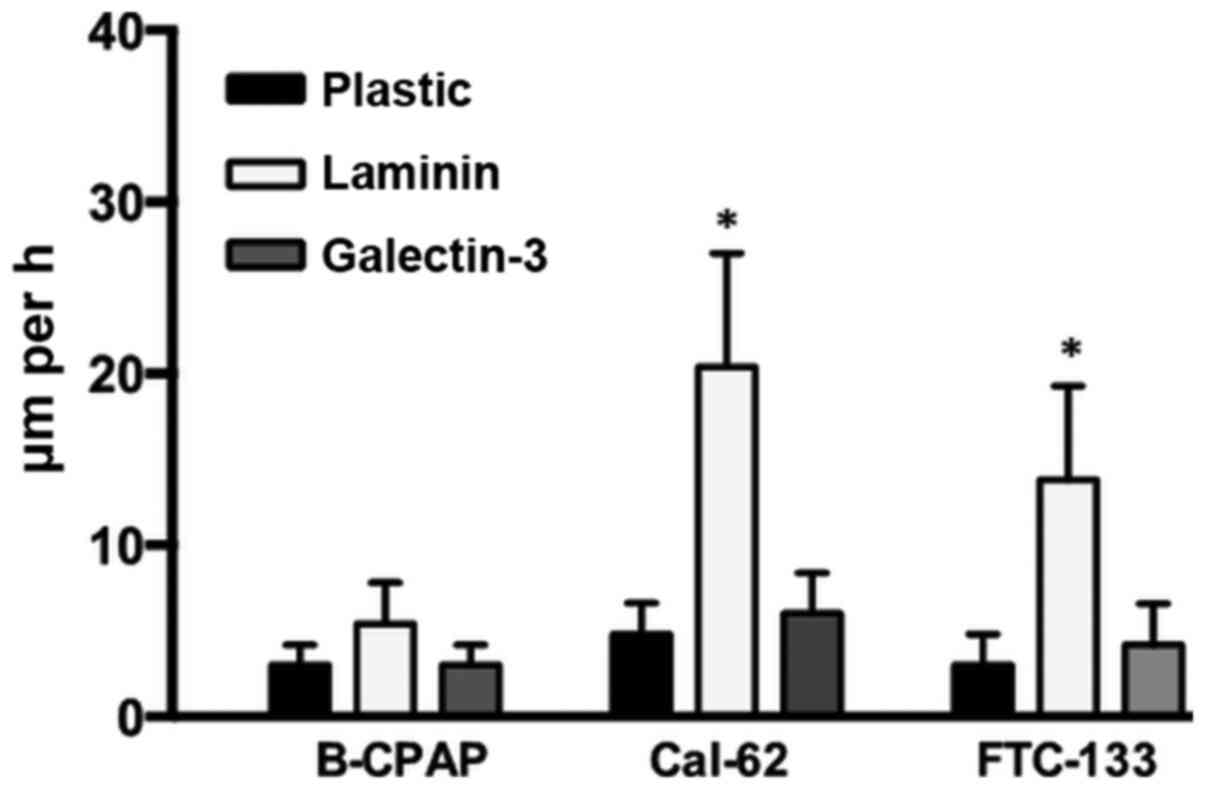
Thyroid carcinoma cells were seeded onto plastic,
galectin-3 or laminin substrata and the migration paths of
individual cells were recorded for 24 h as previously described
(21). Cells were mobile on a
laminin substrate to varying degrees: 5.4 µm/h for B-CPAP, 20.4
µm/h for Cal-62 and 13.8 µm/h for FTC-133 cells (Fig. 4). The velocity of cells on a
galectin-3 substrate was considerably lower (3.0 to 6.0 µm/h) and
comparable to the one on untreated cell culture plastic, indicating
that substrate-immobilized galectin-3 did not promote cell
migration.
Discussion
The present study provides evidence that intrinsic
galectin-3 of thyroid carcinoma cells harbors an active CRD that is
partially responsible for the molecular interaction with the plasma
membrane. When offered as a substrate, galectin-3 promotes adhesion
but not migration of thyroid carcinoma cells.
As also demonstrated for other cellular sources, the
functional activity of thyroid carcinoma-derived galectin-3 is not
dependent on the presence of reducing agents as necessary for other
types of galectins, such as galectin-1 (23,24);
i.e., the molecule could be efficiently affinity-purified with
lactose-agarose under oxidizing conditions. In principle, 3 types
of interactions of galectin-3 with its ligands are possible:
Interactions via the CRD with i) specific carbohydrate structures;
or ii) with molecules that mimic carbohydrate structures; or iii)
interactions that are independent of the CRD. Type ‘ii’ or ‘iii’
interactions are likely for interactions of galectin-3 in the
intracellular environment, for example with Bcl-2, Ras or synexin
(22). In addition, homophilic
interactions of galectin-3 can be type ‘ii’ or ‘iii’ interactions
(15). Moreover, specific peptides
can mimic carbohydrate structures and are recognized by galectin-3
via its CRD (25).
However, as only a fraction of the molecule could be
released from the plasma membrane with specific carbohydrates,
carbohydrate-independent molecular interactions must also take
place. The likelihood for the presence of such type of galectin-3
ligands has been documented for mast cells (26) and Sf9 insect cells (27). It has to be emphasized that the
extent to which the CRD-dependent interactions of galectin-3 with
the plasma membrane are realized via carbohydrates or
carbohydrate-mimicking structures cannot be distinguished.
Independently of their exact molecular nature, galectin-3 specific
ligands are expressed on the plasma membrane of thyroid carcinoma
cells in such an amount, that they allow stable cell adhesion. As
this adhesion process can be almost completely inhibited by
asialofetuin, the CRD of galectin-3 seems to be of predominant
importance under this aspect. This is in accordance with previous
data that suggest a more ubiquitous expression of such galectin-3
specific-ligands in a variety of different cells (20). However, as long as their precise
molecular nature is not identified, the valuation of the functional
relevance raised by these molecular interactions remains a
difficult task.
Substrate-bound galectin-3 did not the promote
migration in the type of assay used in the present study. However,
intracellular galectin-3 has recently been shown to promote the
migration of thyroid carcinoma cells, particularly under hypoxic
conditions through multiple signaling pathways (28). In colon cancer cells, it has been
demonstrated that soluble extracellular galectin-3 promotes cell
migration in a wound healing assay (29). Thus, it would be of interest to
reveal whether such effects are cell line-dependent or whether they
vary dependent on how the lectin is offered, i.e., in solution or
as immobilized substrate. It is noteworthy to mention that no
evidence that soluble galectin-3 provokes the migration of
carcinoma cells in Boyden chamber assays was found, even when used
at a concentration of 5.0 µg/ml that had been shown to induce the
migration of monocytes (30).
Thyroid carcinoma cells are per se able to migrate in such types of
assays, as revealed by their migration across laminin-coated porous
membranes, when 5% FCS was used as an attractant (unpublished
data). By contrast, Zheng et al (28) demonstrated that intrinsic
galectin-3 induced by hypoxia promoted the migration rate of
thyroid carcinoma cells in Boyden chamber assays, when serum was
used as an attractant. However, these studies did not allow the
discrimination between extra- and intracellular expressed
galectin-3.
At present, the relevance of galectin-3 in the
context of thyroid cancer is mainly focused on cancer diagnosis and
cancer imaging (15,31). However, a limited number of
experimental studies have suggested a contribution of galectin-3 in
the apoptosis (11), proliferation
(10,11) and migration (28) of thyroid tumor cells.
Unfortunately, these studies did not distinguish between extra- and
intracellular galectin-3, or focused on the intracellular
expression of galectin-3; i.e., galectin-3-Ras (11) or galectin-3-Bax interactions
(10).
In conclusion, the present study provides evidence
of the complex interactions of galectin-3 with a variety of
extracellular ligands in thyroid carcinoma cells. The precise
identification of such ligands, as well as conditions that allow an
upregulation of extracellular galectin-3 or its ligands is an
obvious challenge for the future. Such types of studies may
increase the relevance of galectin-3 in the treatment of thyroid
cancer, particularly in the context of antibody-based
therapies.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article or are available from the
corresponding author on reasonable request.
Authors' contributions
All authors (JW, AG, DK, SF, NV and RP) made
substantial contributions to the design of the study or the
acquisition, analysis, or interpretation of data for the study. All
authors (JW, AG, DK, SF, NV and RP) were involved in drafting the
work or revising it critically for important intellectual content.
All authors approved the final version to be published and agree to
be accountable for all aspects of the work in ensuring that
questions related to the accuracy or integrity of any part of the
work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kitahara CM and Sosa JA: Understanding the
ever-changing incidence of thyroid cancer. Nat Rev Endocrinol.
16:617–618. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Laha D, Nilubol N and Boufraqech M: New
therapies for advanced thyroid cancer. Front Endocrinol (Lausanne).
11(82)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
D'Alessandria C, Braesch-Andersen S, Bejo
K, Reder S, Blechert B, Schwaiger M and Bartolazzi A: Noninvasive
In vivo imaging and biologic characterization of thyroid tumors by
immunoPET targeting of galectin-3. Cancer Res. 76:3583–3592.
2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
De Rose F, Braeuer M, Braesch-Andersen S,
Otto AM, Steiger K, Reder S, Mall S, Nekolla S, Schwaiger M, Weber
WA, et al: Galectin-3 targeting in thyroid orthotopic tumors opens
new ways to characterize thyroid cancer. J Nucl Med. 60:770–776.
2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Cardoso AC, Andrade LN, Bustos SO and
Chammas R: Galectin-3 Determines tumor cell adaptive strategies in
stressed tumor microenvironments. Front Oncol.
6(127)2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Gordon-Alonso M, Bruger AM and van der
Bruggen P: Extracellular galectins as controllers of cytokines in
hematological cancer. Blood. 132:484–491. 2018.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Johannes L, Jacob R and Leffler H:
Galectins at a glance. J Cell Sci. 131(131)2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Gordon-Alonso M, Hirsch T, Wildmann C and
van der Bruggen P: Galectin-3 captures interferon-gamma in the
tumor matrix reducing chemokine gradient production and T-cell
tumor infiltration. Nat Commun. 8(793)2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xue H, Liu L, Zhao Z, Zhang Z, Guan Y,
Cheng H, Zhou Y and Tai G: The N-terminal tail coordinates with
carbohydrate recognition domain to mediate galectin-3 induced
apoptosis in T cells. Oncotarget. 8:49824–49838. 2017.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Harazono Y, Kho DH, Balan V, Nakajima K,
Hogan V and Raz A: Extracellular galectin-3 programs multidrug
resistance through Na+/K+-ATPase and
P-glycoprotein signaling. Oncotarget. 6:19592–19604.
2015.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nangia-Makker P, Balan V and Raz A:
Regulation of tumor progression by extracellular galectin-3. Cancer
Microenviron. 1:43–51. 2008.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Gao X, Balan V, Tai G and Raz A:
Galectin-3 induces cell migration via a calcium-sensitive
MAPK/ERK1/2 pathway. Oncotarget. 5:2077–2084. 2014.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Elad-Sfadia G, Haklai R, Balan E and Kloog
Y: Galectin-3 augments K-Ras activation and triggers a Ras signal
that attenuates ERK but not phosphoinositide 3-kinase activity. J
Biol Chem. 279:34922–34930. 2004.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Levy R, Grafi-Cohen M, Kraiem Z and Kloog
Y: Galectin-3 promotes chronic activation of K-Ras and
differentiation block in malignant thyroid carcinomas. Mol Cancer
Ther. 9:2208–2219. 2010.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Bartolazzi A, Sciacchitano S and
D'Alessandria C: Galectin-3: The impact on the clinical management
of patients with thyroid nodules and future perspectives. Int J Mol
Sci. 19(E445)2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Lavra L, Rinaldo C, Ulivieri A, Luciani E,
Fidanza P, Giacomelli L, Bellotti C, Ricci A, Trovato M, Soddu S,
et al: The loss of the p53 activator HIPK2 is responsible for
galectin-3 overexpression in well differentiated thyroid
carcinomas. PLoS One. 6(e20665)2011.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kuklinski S and Probstmeier R: Homophilic
binding properties of galectin-3: Involvement of the carbohydrate
recognition domain. J Neurochem. 70:814–823. 1998.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ho MK and Springer TA: Mac-2, a novel
32,000 Mr mouse macrophage subpopulation-specific antigen defined
by monoclonal antibodies. J Immunol. 128:1221–1228. 1982.PubMed/NCBI
|
|
19
|
Pesheva P, Urschel S, Frei K and
Probstmeier R: Murine microglial cells express functionally active
galectin-3 in vitro. J Neurosci Res. 51:49–57. 1998a.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Pesheva P, Kuklinski S, Schmitz B and
Probstmeier R: Galectin-3 promotes neural cell adhesion and neurite
growth. J Neurosci Res. 54:639–654. 1998b.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lobastova L, Kraus D, Glassmann A, Khan D,
Steinhäuser C, Wolff C, Veit N, Winter J and Probstmeier R:
Collective cell migration of thyroid carcinoma cells: A beneficial
ability to override unfavourable substrates. Cell Oncol (Dordr).
40:63–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Knibbs RN, Agrwal N, Wang JL and Goldstein
IJ: Carbohydrate-binding protein 35. II. Analysis of the
interaction of the recombinant polypeptide with saccharides. J Biol
Chem. 268:14940–14947. 1993.PubMed/NCBI
|
|
23
|
Fettis MM and Hudalla GA: Engineering
reactive oxygen species-resistant galectin-1 dimers with enhanced
lectin activity. Bioconjug Chem. 29:2489–2496. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cedeno-Laurent F and Dimitroff CJ:
Galectin-1 research in T cell immunity: Past, present and future.
Clin Immunol. 142:107–116. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Anananuchatkul T, Chang IV, Miki T,
Tsutsumi H and Mihara H: Construction of a stapled α-helix peptide
library displayed on phage for the screening of galectin-3-binding
peptide ligands. ACS Omega. 5:5666–5674. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Frigeri LG and Liu FT: Surface expression
of functional IgE binding protein, an endogenous lectin, on mast
cells and macrophages. J Immunol. 148:861–867. 1992.PubMed/NCBI
|
|
27
|
Inohara H and Raz A: Functional evidence
that cell surface galectin-3 mediates homotypic cell adhesion.
Cancer Res. 55:3267–3271. 1995.PubMed/NCBI
|
|
28
|
Zheng J, Lu W, Wang C, Xing Y, Chen X and
Ai Z: Galectin-3 induced by hypoxia promotes cell migration in
thyroid cancer cells. Oncotarget. 8:101475–101488. 2017.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Wu KL, Kuo CM, Huang EY, Pan HM, Huang CC,
Chen YF, Hsiao CC and Yang KD: Extracellular galectin-3 facilitates
colon cancer cell migration and is related to the epidermal growth
factor receptor. Am J Transl Res. 10:2402–2412. 2018.PubMed/NCBI
|
|
30
|
Sano H, Hsu DK, Yu L, Apgar JR, Kuwabara
I, Yamanaka T, Hirashima M and Liu FT: Human galectin-3 is a novel
chemoattractant for monocytes and macrophages. J Immunol.
165:2156–2164. 2000.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Peplau E, De Rose F, Reder S, Mittelhäuser
M, Scafetta G, Schwaiger M, Weber WA, Bartolazzi A, Skerra A and
D'Alessandria C: Development of a chimeric antigen-binding fragment
directed against human galectin-3 and validation as an
immuno-positron emission tomography tracer for the sensitive in
vivo imaging of thyroid cancer. Thyroid. 30:1314–1326.
2020.PubMed/NCBI View Article : Google Scholar
|