Introduction
There is an increasing need for the development of diverse prophylactic and therapeutic options against COVID-19. The majority of patients with COVID-19 have been treated with traditional herbal medicine in combination with standard pharmacotherapy in China since the outbreak (1-4). Several newly developed herbal formulas were encouraged for the management of COVID-19 in the latest version of the Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) released by the National Health Commission of China (5). One of these is Qingfei Paidu decoction (QFPD, the Chinese word for ‘lung cleansing and detoxifying decoction’). QFPD was formulated specifically for the treatment of patients with COVID-19 and has exhibited satisfactory therapeutic efficacy (6-10). QFPD administration combined with the standard of care has been shown to significantly enhance the cure rates and to prevent disease progression in mild to critical cases (6-8). The concomitant administration of QFPD and Western medicine to patients with mild to moderate disease has been shown to achieve greater improvements in blood outcome indicators, such as C-reactive protein, creatine kinase and lactate dehydrogenase (9). The early initiation of treatment with QFPD following symptom onset leads to favorable clinical outcomes, such as a more rapid recovery, earlier viral clearance and a shorter hospitalization (10).
QFPD contains 21 herbal components optimized for the symptoms of COVID-19. Recent network pharmacological studies identified a large number of active natural compounds contained in the herbal ingredients of QFPD, and predicted the comprehensive molecular, biological and functional networks underlying its pharmacological effects (11-15). The key active compounds include baicalin, glycyrrhizic acid, hesperidin, hyperoside, quercetin, glabridin, gallic acid, genistein and tectorigenin (11-15). These compounds interact with a wide variety of target proteins closely related to the symptoms of COVID-19, and thereby modulate the complex signaling networks involved in immune regulation, anti-inflammatory effects and multi-organ protection (11-15). Molecular docking analyses have revealed that the several active compounds have the potential to directly inhibit SARS-CoV-2 infection by interfering with host-virus protein interaction or downregulating the expression of angiotensin I converting enzyme 2, the viral entry receptor (11-15).
Although the network pharmacological approaches have provided in silico predictions on the possible molecular interaction networks targeted by active chemical compounds (11-15), there is limited information available on the in vivo immunological effects of QFPD. Furthermore, the feasibility of QFPD application to uninfected individuals for preventive purposes remains unconfirmed, despite its notable therapeutic benefits against COVID-19. In the present study, to obtain insight into these issues, a pilot study was conducted using uninfected individuals as subjects, and whether QFPD ingestion at a dose lower than that recommended for therapeutic use affects hematological and immunological parameters was examined.
Subjects and methods
Subjects
Participants were recruited through the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR) website, the website of our clinic, announcements in an e-mail newsletter and personal contacts. Individuals who met all of the following inclusion criteria were enrolled in the trial: Adults between the ages of 20 and 70, having negative PCR and IgM/IgG antibodies tests for SARS-CoV-2 at study entry. Individuals were excluded from the trial if they met any of the following exclusion criteria: Pregnancy; breastfeeding; duplicate enrollment in other clinical trials; history of infectious disease within the past 6 months; current or past history of chronic inflammatory diseases, immune-related diseases, or malignancy; history of drug use within the past 6 months; underlying conditions associated with a higher risk of infection with COVID-19, including hypertension, cardiovascular disease, cerebrovascular disease, diabetes, obesity [body mass index (BMI) ≥30], chronic obstructive pulmonary disease and chronic kidney disease.
The present study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures were reviewed and approved by the Ethics Committees of Takanawa Clinic (approval no. 2020-2). A signed informed consent form was obtained from each participant prior to inclusion in this study.
Preparation and administration of QFPD
A total of 21 types of traditional Chinese herbs for the QFPD formula were purchased from Shanghai Ruisha Comlat Co., Ltd. and mixed in accordance with the Chinese official guidelines (5). The dose of each herb was reduced to 1/30 on the basis of technical advice from herbal medicine specialists, considering possible adverse effects, such as palpitations in healthy subjects and the feasibility of the long-term daily use for prophylactic purposes. QFPD decoction was prepared immediately prior to the first administration. The mixed herbs were soaked in 600 ml of water for 30 min, simmered gently for 1 h, and strained through a tea strainer. The decoction was divided into 6 aliquots and stored at 4˚C during the trial. All procedures for the preparation and delivery of the decoction were performed by a specific pharmacy staff of Takanawa clinic to ensure quality control. The subjects were instructed to take an aliquot of the decoction orally 40 min after breakfast and dinner for 3 days in accordance with the administration protocol of the Chinese official guidelines (5).
Hematological and cytokine analyses
The primary outcome measure was changes in the plasma levels of inflammation-related cytokines after 3 days of low-dose QFPD ingestion compared with the baseline levels. The secondary outcome measure was changes in hematological parameters following low-dose QFPD ingestion for 3 days compared with baseline levels. Peripheral blood samples were obtained from each subject at 12 h prior to the first and after the final administration of QFPD. Hematological and blood biochemical tests were outsourced to SRL, Inc. The concentrations of plasma cytokines were measured using the V-PLEX Proinflammatory Panel 1 Human kit (K15049D-1, Meso Scale Diagnostics) and the human interleukin (IL)-18 ELISA kit (ab215539, Abcam) according to the manufacturers' protocols.
Negative control study
An additional negative control study (without QFPD ingestion) was conducted 9 months after the QFPD trial to exclude the influence of the circadian oscillation of blood cytokine levels and the effect of feeding on blood cytokine levels. Blood samples were re-collected with the same time schedule as that of the QFPD trial from 13 of the 18 subjects who had completed the previous QPPD trial, and plasma cytokines were measured as described above.
Statistical analysis
No outliers were taken into account, and all collected data (n=18) were subjected to statistical analysis. The normality of the data was tested using the normal quantile-quantile plots and the Shapiro-Wilk test. On the basis of the results from these normality tests, the two-tailed Wilcoxon signed-rank test at the significance level (α) of 0.05 was employed for the subsequent statistical analysis of the data. All statistical analyses were performed using EZR version 1.53 (Saitama Medical Center, Jichi Medical University, Saitama, Japan), which is a graphical user interface for R (The R Foundation for Statistical Computing, Vienna, Austria) (16). Post hoc power analysis was performed using G*Power version 3.1.9.2(17).
Results
Participant recruitment began in May 9, 2020 and was completed the following day. A total of 19 volunteers were screened for eligibility, found to be eligible, and enrolled in the trial (Fig. 1). QFPD was administered to all the enrolled participants from May 12 to 14, 2020, of which 1 participant was excluded from the main analysis due to an ingestion error. Consequently, 18 subjects [5 males and 13 females; aged, 22-58 years; mean age (SD), 33.8 (10.7) years] completed the intervention, and the data were subjected to statistical analysis.
 |
Figure 1
CONSORT flow diagram of the trial.
|
Marginal changes within reference intervals were found in mean corpuscular volume (Z=3.51, P=0.000454, r=0.827), mean corpuscular hemoglobin concentration (Z=3.07, P=0.00213, r=0.724) and blood urea nitrogen (Z=2.23, P=0.0260, r=0.525); however, there were no significant differences in other hematological and blood biochemical parameters between pre- and post-QFPD ingestion (Table I). Notably, low-dose QFPD ingestion induced significant increases in the plasma levels of the pro-inflammatory cytokines, tumor necrosis factor (TNF)-α (Z=3.46, P=0.000107, r=0.816), IL-1β (Z=3.30, P=0.000982, r=0.881), IL-18 (Z=3.07, P=0.00105, r=0.724), IL-2 (Z=1.98, P=0.0483, r=0.467) and IL-8 (Z=3.38, P=0.000191, r=0.796) (Table I). The levels of these cytokines were increased in 16 (88.9%), 14 (77.8%), 15 (83.3%), 13 (72.2%) and 17 (94.4%) out of the 18 subjects, respectively (Fig. 2). These significant changes in the plasma cytokine levels were not observed in the negative control study without QFPD ingestion (Table II), suggesting that the observed cytokine changes were due to the action of QFPD.
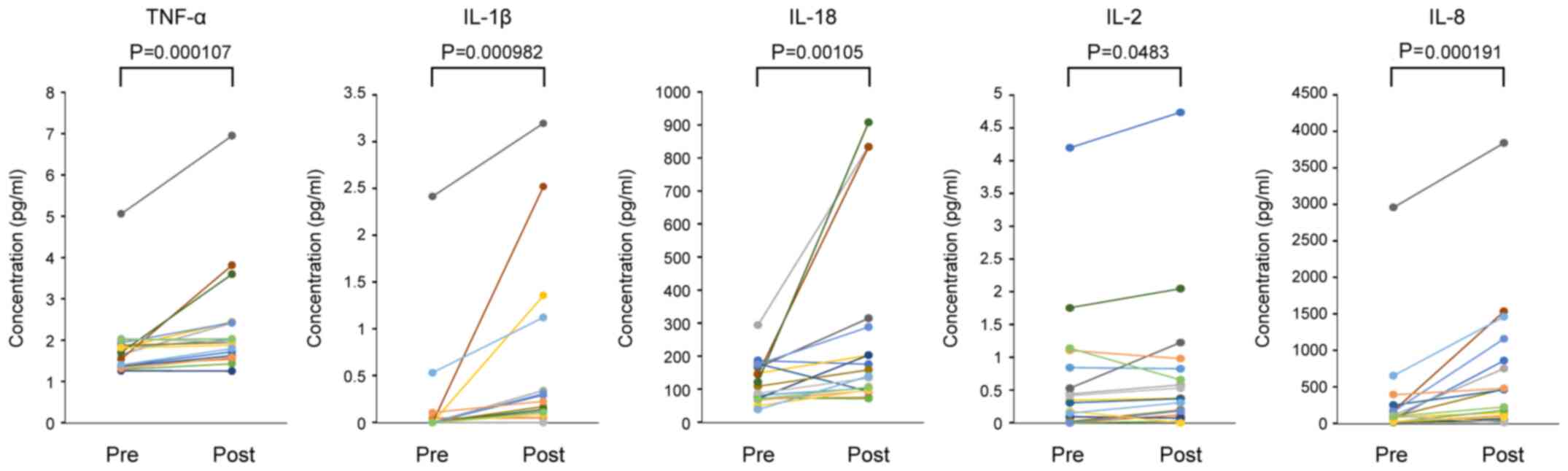 |
Figure 2
Changes in the plasma levels of TNF-α, IL-1β, IL-18, IL-2, and IL-8 before and after low-dose QFPD ingestion. Data (n=18) were analyzed using the two-tailed Wilcoxon signed-rank test at the significance level (α) of 0.05. Pre, pre-ingestion (baseline); Post, post-ingestion; QFPD, Qingfei Paidu decoction; TNF, tumor necrosis factor; IL, interleukin.
|
 |
Table I
Hematological and cytokine changes in subjects administered low-dose QFPD.
|
Table I
Hematological and cytokine changes in subjects administered low-dose QFPD.
| |
Pre |
Post |
|
| Test parameter |
Median |
(IQR) |
Median |
(IQR) |
Z |
P-value |
r |
| Complete blood count |
|
|
|
|
|
|
|
| Red blood cell count (x104/µl) |
459 |
(417-480) |
442 |
(417-482) |
0.610 |
0.542 |
0.144 |
| Hemoglobin (g/dl) |
13.7 |
(12.6-14.5) |
13.1 |
(12.6-14.6) |
0.0711 |
0.943 |
0.0167 |
| Hematocrit (%) |
41.4 |
(38.2-43.2) |
40.2 |
(38.8-44.6) |
1.26 |
0.207 |
0.298 |
| MCV (fl) |
89.7 |
(87.6-93.2) |
90.7 |
(88.7-98.0) |
3.51 |
0.000454c |
0.827 |
| MCH (pg) |
30.1 |
(29.6-31.5) |
30.0 |
(29.6-31.5) |
1.11 |
0.265 |
0.263 |
| MCHC (%) |
33.4 |
(32.8-33.8) |
32.7 |
(31.7-33.4) |
3.07 |
0.00213b |
0.724 |
| White blood cell count (/µl) |
5,850 |
(5600-6650) |
5,850 |
(5300-6180) |
1.39 |
0.163 |
0.329 |
| Platelet count (x104/µl) |
24.7 |
(22.3-27.1) |
24.2 |
(21.7-26.9) |
1.18 |
0.236 |
0.279 |
| White blood cell differential |
|
|
|
|
|
|
|
| Neutrophils (%) |
57.5 |
(54.5-63.8) |
56.8 |
(52.8–60.6) |
0.893 |
0.372 |
0.210 |
| Eosinophils (%) |
1.35 |
(0.950-2.43) |
1.20 |
(1.00-2.20) |
0.00 |
1.00 |
0.00 |
| Basophils (%) |
0.450 |
(0.325-0.500) |
0.500 |
(0.325-0.650) |
1.17 |
0.241 |
0.276 |
| Monocytes (%) |
4.65 |
(4.23-5.70) |
5.15 |
(4.13-6.10) |
0.129 |
0.897 |
0.0305 |
| Lymphocytes (%) |
36.5 |
(28.5-37.8) |
35.9 |
(32.3-38.4) |
0.479 |
0.632 |
0.113 |
| Blood biochemistry |
|
|
|
|
|
|
|
| AST (U/l) |
17.0 |
(16.0-20.0) |
18.0 |
(16.0-22.3) |
1.90 |
0.0574 |
0.448 |
| ALT (U/l) |
15.5 |
(11.0-22.5) |
15.0 |
(12.0-23.8) |
0.945 |
0.345 |
0.223 |
| γ-GT (U/l) |
19.5 |
(14.0-31.8) |
18.5 |
(13.3-32.8) |
0.699 |
0.485 |
0.165 |
| LDH (U/l) |
156 |
(137-163) |
155 |
(143-174) |
1.44 |
0.149 |
0.340 |
| Albumin (g/dl) |
4.75 |
(4.70-4.88) |
4.70 |
(4.60-4.88) |
1.17 |
0.243 |
0.275 |
| Urea nitrogen (mg/dl) |
13.2 |
(12.0-15.3) |
11.5 |
(10.9-13.6) |
2.23 |
0.0260a |
0.525 |
| HDL-cholesterol (mg/dl) |
70.0 |
(58.3-75.5) |
67.0 |
(60.8-76.3) |
0.0856 |
0.932 |
0.0202 |
| LDL-cholesterol (mg/dl) |
115 |
(75.5-135) |
111 |
(83.3-136) |
0.590 |
0.555 |
0.139 |
| Triglycerides (mg/dl) |
66.5 |
(48.5-122) |
78.5 |
(51.3-116) |
0.174 |
0.862 |
0.0411 |
| CRP (mg/dl) |
0.0400 |
(0.0225-0.0500) |
0.0400 |
(0.0300-0.0575) |
1.10 |
0.270 |
0.260 |
| Cytokines |
|
|
|
|
|
|
|
| IFN-γ (pg/ml) |
2.70 |
(1.39-4.64) |
3.78 |
(2.44-5.43) |
1.46 |
0.154 |
0.344 |
| IL-6 (pg/ml) |
0.443 |
(0.225-0.509) |
0.436 |
(0.355-1.10) |
1.59 |
0.113 |
0.385 |
| TNF-α (pg/ml) |
1.68 |
(1.39-1.87) |
1.95 |
(1.65-2.42) |
3.46 |
0.000107c |
0.816 |
| IL-1β (pg/ml) |
0.000 |
(0.000-0.000) |
0.156 |
(0.0583-0.333) |
3.30 |
0.000982c |
0.881 |
| IL-18 (pg/ml) |
98.2 |
(71.5-161) |
149 |
(98.8-267) |
3.07 |
0.00105b |
0.724 |
| IL-2 (pg/ml) |
0.326 |
(0.041-0.765) |
0.375 |
(0.185-0.785) |
1.98 |
0.0483a |
0.467 |
| IL-8 (pg/ml) |
92.3 |
(22.8-169) |
344 |
(89.8-834) |
3.38 |
0.000191c |
0.796 |
| IL-10 (pg/ml) |
0.175 |
(0.117-0.271) |
0.250 |
(0.144-0.318) |
1.76 |
0.0814 |
0.416 |
 |
Table II
Changes in cytokine levels observed in a negative control study (without QFPD administration).
|
Table II
Changes in cytokine levels observed in a negative control study (without QFPD administration).
| |
Pre |
Post |
|
| Test item |
Median |
(IQR) |
Median |
(IQR) |
Z |
P-value |
r |
| IFN-γ (pg/ml) |
1.73 |
(0.689-6.34) |
3.83 |
(1.29-7.16) |
0.454 |
0.685 |
0.126 |
| IL-6 (pg/ml) |
0.00294 |
(0.000949-1.90) |
1.79 |
(0.00943-1.92) |
1.01 |
0.340 |
0.281 |
| TNF-α (pg/ml) |
0.0119 |
(0.00653-1.04) |
0.580 |
(0.0165-0.971) |
0.734 |
0.497 |
0.204 |
| IL-1β (pg/ml) |
0.00 |
(0.00-0.0406) |
0.0251 |
(0.00-0.120) |
1.08 |
0.263 |
0.300 |
| IL-18 (pg/ml) |
126 |
(108-133) |
85.3 |
(78.6-102) |
1.85 |
0.068 |
0.514 |
| IL-2 (pg/ml) |
0.487 |
(0.0706-0.737) |
0.281 |
(0.182-0.595) |
0.943 |
0.376 |
0.262 |
| IL-8 (pg/ml) |
133 |
(12.7-263) |
113 |
(21.4-282) |
1.71 |
0.094 |
0.475 |
| IL-10 (pg/ml) |
0.134 |
(0.0383-0.275) |
0.143 |
(0.0101-0.345) |
0.734 |
0.497 |
0.204 |
An increase was also observed in the interquartile range of IL-6, an exacerbating factor for COVID-19 (18-20); however, the difference was not statistically significant (Z=1.59, P=0.113, r=0.385). Similarly, the median value and the interquartile range of the anti-inflammatory cytokine, IL-10, were increased, although the difference was not statistically significant (Z=1.76, P=0.0814, r=0.416) (Table I). No apparent adverse effects were observed during the trial.
In addition, post hoc two-tailed power analysis (significance level, α=0.05; sample size, n=18) was performed and statistical powers (1 - β) were obtained of 0.836 (TNF-α), 0.655 (IL-1β), 0.648 (IL-18), 0.361 (IL-2) and 0.923 (IL-8) following the completion of the trial.
Discussion
Inflammation is a host defense mechanism against invading pathogens. Acute inflammatory responses to the infection of single-stranded RNA (ssRNA) viruses, such as SARS-CoV-2 are triggered by the foreign ssRNA sensors, Toll-like receptor (TLR)7, TLR8 and NLR family pyrin domain containing 3 (NLRP3) (21,22). TLR7 and TLR8 are expressed mainly in dendritic cells and macrophages, and induce the production of pro-inflammatory cytokines such as TNF-α and IL-6 in response to viral ssRNA in the endosome (21). NLRP3, when it recognizes viral ssRNA in the cytoplasm, activates the inflammasome-mediated processing of pro-IL-1β and pro-IL-18 to release active IL-1β and IL-18 (21,23). These ‘immediate-early’ cytokines initiate and coordinate a broad spectrum of downstream antiviral immune cascades (21-23). In the present study trial, low-dose QFPD ingestion induced significant increases in the levels of TNF-α, IL-1β and IL-18. It was therefore speculated that QFPD may partially mimic the blood cytokine environment produced by TLR7/8- and NLRP3-driven early immune responses to ssRNA viruses.
Since systemic hyperinflammation caused by complex immune dysregulation is a hallmark of COVID-19 (19,20,24), the pro-inflammatory activity of QFPD is apparently contradictory to its clinical benefits against COVID-19. However, recent metagenomics studies have highlighted the importance of the active TLR7/8- and NLRP3-mediated inflammatory pathways in anti- SARS-CoV-2 immunity (25,26). A whole-exome sequencing by van der Made et al identified rare loss-of-function variants of the TLR7 gene in severe COVID-19 patients: A 4-nucleotide deletion and a missense variant that cause impaired TLR7-dependent immune signaling (25). In addition, a comparative transcriptomics study by Mick et al demonstrated the suppressed expression of genes involved in innate immunity, including pattern recognition (TLR8), inflammasome activation (NLRP3 and CASP5) and inflammatory IL signaling (IL1A, IL1B, IL18RAP and IL1R2), in the upper airways of patients with COVID-19(26). Intriguingly, IL1B is the most strongly suppressed gene, and the NLRP3-inflammasome and IL-1 immune pathways are particularly non-responsive to SARS-CoV-2 infection early in the course of the disease (26). These transcriptional responses to SARS-CoV-2 lead to impaired neutrophil and macrophage recruitment to the upper airway (26). Collectively, these findings suggest that the TLR7/8- and NLRP3-driven inflammatory responses play important protective roles in the early stages of COVID-19. The QFPD-induced inflammatory tone may therefore be effective in preventing SARS-CoV-2 infection in uninfected individuals.
IL-2 is a type 1 helper T cytokine with diverse regulatory functions in cellular immunity (27). It stimulates CD8+ cytotoxic T lymphocytes, monocytes/macrophages and natural killer cells to eliminate virus-infected cells. IL-2 also promotes the differentiation, expansion and stability of CD4+CD25+Foxp3+ regulatory T cells that suppress excessive immune reactions. These opposite functions of IL-2 contribute to the maintenance of immune homeostasis. IL-8 is a potent neutrophil chemotactic factor produced by macrophages, epithelial cells, airway smooth muscle cells and vascular endothelial cells (28). It strongly induces the migration of neutrophils to the sites of infection and activates phagocytic elimination of invading pathogens and infected cells. The activity of low-dose QFPD to upregulate IL-2 and IL-8 may therefore be beneficial in terms of its prophylactic use. However, excessive IL-8 is known to be closely associated with acute lung injury and acute respiratory distress syndrome (29,30), and detailed dose-response trials are required to ensure the safety.
In the present study trial, low-dose QFPD had no significant effects on the plasma levels of IL-6 and IL-10. IL-6 is known to be a critical driver of complex immune dysregulation and systemic hyperinflammation (18-20). There is a definite positive association between the blood IL-6 levels, and COVID-19 severity and mortality (31-33). Similarly, blood IL-10 levels are markedly elevated in patients with severe COVID-19 and are positively associated with disease severity and mortality (34-37). Although IL-10 has been commonly regarded as an immunosuppressive and anti-inflammatory cytokine, there is emerging evidence to indicate that IL-10 can act as an immunostimulatory and pro-inflammatory cytokine in some autoimmune diseases and cancers, which supports the hypothesis that IL-10 may contribute to COVID-19 progression (38). The ineffectiveness of low-dose QFPD on IL-6 and IL-10 may therefore be a suitable pharmacological property for its prophylactic administration to uninfected individuals.
The main limitations of the present study are the small number of participants and the use of uninfected individuals as subjects. Further trials with larger cohorts are essential to confirm the conclusion and determine generalizability. The present study employed uninfected individuals as subjects with the aim of testing the feasibility of the daily use for prophylactic purposes; however, additional studies are required to clarify whether full-dose QFPD induces similar changes in cytokine levels in patients with COVID-19. The safety of long-term, daily use of low-dose QFPD also needs to be confirmed in future longitudinal follow-up studies. QFPD was administered 40 min after breakfast and dinner for 3 days, and blood samples were collected in the evening, 12 h before the first administration of QFPD (shown as ‘Pre’ in Fig. 2), and in the morning, 12 h after the last administration of QFPD (shown as ‘Post’ in Fig. 2). Importantly, there are a large number of studies that have reported the circadian oscillation of serum cytokine levels (39,40), as well as the effects of feeding on serum cytokine levels (41,42). A negative control study without QFPD ingestion was also conducted (Table II); however, placebo-controlled trials are essential to adequately examine the immunological efficacy of QFPD.
In conclusion, the findings of the present study suggest that low-dose QFPD may partially mimic the blood inflammatory tone produced by TLR7/8- and NLRP3-dependent early defense responses to ssRNA viruses. Given that the TLR- and NLRP3-driven immune pathways are suppressed early in the course of COVID-19, it was hypothesized that QFPD may also be effective in reducing the risk of SARS-CoV-2 infection in uninfected individuals. The daily intake of low-dose QFPD may therefore be a possible option for the prevention of COVID-19 during the pandemic. Careful prescribing is required when used in uninfected individuals for preventive purposes until a better understanding of the in vivo pharmacological actions of QFPD is acquired.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request. The present study was prospectively registered at the University Hospital Medical Information Network-Clinical Trials Registry (UMIN-CTR) under the trial no. UMIN000040341 on May 9, 2020.
Authors' contributions
YK, TE, TA and TN were involved in the conceptualization of the study and in the study methodology. TN was responsible for formal analysis., YK, KA, KK, MM and TE were involved in the investigative aspects of the study and in the provision of resources. YK and TE were involved in data curation. TN was involved in the writing of the original draft and in visualization (figure preparation). YK, KA, KK, MM, TE and TA were involved in the writing, review and editing of the manuscript. YK, TA and TN were involved in study supervision. YK, KA and TE were involved in project administration. YK, TE and TN confirm the authenticity of all the raw data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was carried out in accordance with The Code of Ethics of the World Medical Association (Declaration of Helsinki). All procedures were reviewed and approved by the Ethics Committees of Takanawa Clinic (approval no. 2020-2). A signed informed consent form was obtained from each participant prior to inclusion in this study.
Patient consent for publication
Not applicable.
Competing interests
YK, KA, KK, MM and TE are employees of Takanawa Clinic. TA and TN serve as research advisers to Takanawa Clinic and receive advisory fees.
References
|
1
|
Yang Y, Islam MS, Wang J, Li Y and Chen X: Traditional Chinese Medicine in the Treatment of Patients Infected with 2019-New Coronavirus (SARS-CoV-2): A Review and Perspective. Int J Biol Sci. 16:1708–1717. 2020.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Zhang L, Yu J, Zhou Y, Shen M and Sun L: Becoming a Faithful Defender: Traditional Chinese Medicine against Coronavirus Disease 2019 (COVID-19). Am J Chin Med. 48:763–777. 2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Ren JL, Zhang AH and Wang XJ: Corrigendum to Traditional Chinese medicine for COVID-19 treatment. Pharmacol Res. 155(104768)2020.PubMed/NCBI View Article : Google Scholar : Erratum for: Pharmacol Res 155: 104743, 2020.
|
|
4
|
Li S, Liu C, Guo F, Taleb SJ, Tong M and Shang D: Traditional Chinese Medicine as Potential Therapy for COVID-19. Am J Chin Med. 48:1263–1277. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Wei RF (ed): Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J (Engl). 133:1087–1095. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao ZH, Zhou Y, Li WH, Huang QS, Tang ZH and Li H: Analysis of Traditional Chinese Medicine Diagnosis and Treatment Strategies for COVID-19 Based on ‘The Diagnosis and Treatment Program for Coronavirus Disease-2019’ from Chinese Authority. Am J Chin Med. 48:1035–1049. 2020.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Zhong LLD, Lam WC, Yang W, Chan KW, Sze SCW, Miao J, Yung KKL, Bian Z and Wong VT: Potential Targets for Treatment of Coronavirus Disease 2019 (COVID-19): A Review of Qing-Fei-Pai-Du-Tang and Its Major Herbs. Am J Chin Med. 48:1051–1071. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Cao P, Wu S, Wu T, Deng Y, Zhang Q, Wang K and Zhang Y: The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohydr Polym. 240(116346)2020.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Xin S, Cheng X, Zhu B, Liao X, Yang F, Song L, Shi Y, Guan X, Su R, Wang J, et al: Clinical retrospective study on the efficacy of Qingfei Paidu decoction combined with Western medicine for COVID-19 treatment. Biomed Pharmacother. 129(110500)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Shi N, Liu B, Liang N, Ma Y, Ge Y, Yi H, Wo H, Gu H, Kuang Y, Tang S, et al: Association between early treatment with Qingfei Paidu decoction and favorable clinical outcomes in patients with COVID-19: A retrospective multicenter cohort study. Pharmacol Res. 161(105290)2020.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yang R, Liu H, Bai C, Wang Y, Zhang X, Guo R, Wu S, Wang J, Leung E, Chang H, et al: Chemical composition and pharmacological mechanism of Qingfei Paidu Decoction and Ma Xing Shi Gan Decoction against Coronavirus Disease 2019 (COVID-19): In silico and experimental study. Pharmacol Res. 157(104820)2020.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhao J, Tian S, Lu D, Yang J, Zeng H, Zhang F, Tu D, Ge G, Zheng Y, Shi T, et al: Systems pharmacological study illustrates the immune regulation, anti-infection, anti-inflammation, and multi-organ protection mechanism of Qing-Fei-Pai-Du decoction in the treatment of COVID-19. Phytomedicine: Sep 9, 2020 (Epub ahead of print).
|
|
13
|
Niu W, Wu F, Cui H, Cao W, Chao Y, Wu Z, Fan M and Liang C: Network Pharmacology Analysis to Identify Phytochemicals in Traditional Chinese Medicines That May Regulate ACE2 for the Treatment of COVID-19. Evid Based Complement Alternat Med. 2020(7493281)2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Zhang DH, Zhang X, Peng B, Deng SQ, Wang YF, Yang L, Zhang KZ, Ling CQ and Wu KL: Network pharmacology suggests biochemical rationale for treating COVID-19 symptoms with a Traditional Chinese Medicine. Commun Biol. 3(466)2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Chen J, Wang YK, Gao Y, Hu LS, Yang JW, Wang JR, Sun WJ, Liang ZQ, Cao YM and Cao YB: Protection against COVID-19 injury by qingfei paidu decoction via anti-viral, anti-inflammatory activity and metabolic programming. Biomed Pharmacother. 129(110281)2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Kanda Y: Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant. 48:452–458. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Faul F, Erdfelder E, Lang AG and Buchner A: G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 39:175–191. 2007.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Aziz M, Fatima R and Assaly R: Elevated interleukin-6 and severe COVID-19: A meta-analysis. J Med Virol. 92:2283–2285. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, Jordan TX, Oishi K, Panis M, Sachs D, et al: Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 181:1036–1045.e9. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Giamarellos-Bourboulis EJ, Netea MG, Rovina N, Akinosoglou K, Antoniadou A, Antonakos N, Damoraki G, Gkavogianni T, Adami ME, Katsaounou P, et al: Complex immune dysregulation in COVID-19 patients with severe respiratory failure. Cell Host Microbe. 27:992–1000.e3. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Chen N, Xia P, Li S, Zhang T, Wang TT and Zhu J: RNA sensors of the innate immune system and their detection of pathogens. IUBMB Life. 69:297–304. 2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Moreno-Eutimio MA, López-Macías C and Pastelin-Palacios R: Bioinformatic analysis and identification of single-stranded RNA sequences recognized by TLR7/8 in the SARS-CoV-2, SARS-CoV, and MERS-CoV genomes. Microbes Infect. 22:226–229. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Slaats J, Ten Oever J, van de Veerdonk FL and Netea MG: IL-1β/IL-6/CRP and IL-18/ferritin: distinct inflammatory programs in infections. PLoS Pathog. 12(e1005973)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gustine JN and Jones D: Immunopathology of hyperinflammation in COVID-19. Am J Pathol. 191:4–17. 2021.PubMed/NCBI View Article : Google Scholar
|
|
25
|
van der Made CI, Simons A, Schuurs-Hoeijmakers J, van den Heuvel G, Mantere T, Kersten S, van Deuren RC, Steehouwer M, van Reijmersdal SV, Jaeger M, et al: Presence of genetic variants among young men with severe COVID-19. JAMA. 324:1–11. 2020.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Mick E, Kamm J, Pisco AO, Ratnasiri K, Babik JM, Castañeda G, DeRisi JL, Detweiler AM, Hao SL, Kangelaris KN, et al: Upper airway gene expression reveals suppressed immune responses to SARS-CoV-2 compared with other respiratory viruses. Nat Commun. 11(5854)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Spolski R, Li P and Leonard WJ: Biology and regulation of IL-2: From molecular mechanisms to human therapy. Nat Rev Immunol. 18:648–659. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Remick DG: Interleukin-8. Crit Care Med. 33 (Suppl 12):S466–S467. 2005.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Allen TC and Kurdowska A: Interleukin 8 and acute lung injury. Arch Pathol Lab Med. 138:266–269. 2014.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shen Y, Wang D and Wang X: Role of CCR2 and IL-8 in acute lung injury: A new mechanism and therapeutic target. Expert Rev Respir Med. 5:107–114. 2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Zhang X, Tan Y, Ling Y, Lu G, Liu F, Yi Z, Jia X, Wu M, Shi B, Xu S, et al: Viral and host factors related to the clinical outcome of COVID-19. Nature. 583:437–440. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Elshazli RM, Toraih EA, Elgaml A, El-Mowafy M, El-Mesery M, Amin MN, Hussein MH, Killackey MT, Fawzy MS and Kandil E: Diagnostic and prognostic value of hematological and immunological markers in COVID-19 infection: A meta-analysis of 6320 patients. PLoS One. 15(e0238160)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mesas AE, Cavero-Redondo I, Álvarez-Bueno C, Sarriá Cabrera MA, Maffei de Andrade S, Sequí-Dominguez I and Martínez-Vizcaíno V: Predictors of in-hospital COVID-19 mortality: A comprehensive systematic review and meta-analysis exploring differences by age, sex and health conditions. PLoS One. 15(e0241742)2020.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Neumann J, Prezzemolo T, Vanderbeke L, Roca CP, Gerbaux M, Janssens S, Willemsen M, Burton O, Van Mol P, Van Herck Y, et al: CONTAGIOUS co-authors: Increased IL-10-producing regulatory T cells are characteristic of severe cases of COVID-19. Clin Transl Immunology. 9(e1204)2020.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Udomsinprasert W, Jittikoon J, Sangroongruangsri S and Chaikledkaew U: Circulating levels of interleukin-6 and interleukin-10, but not tumor necrosis factor-alpha, as potential biomarkers of severity and mortality for COVID-19: systematic review with meta-analysis. J Clin Immunol. 41:11–22. 2021.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Zhao Y, Qin L, Zhang P, Li K, Liang L, Sun J, Xu B, Dai Y, Li X, Zhang C, et al: Longitudinal COVID-19 profiling associates IL-1RA and IL-10 with disease severity and RANTES with mild disease. JCI Insight. 5(e139834)2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Han H, Ma Q, Li C, Liu R, Zhao L, Wang W, Zhang P, Liu X, Gao G, Liu F, et al: Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 9:1123–1130. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Lu L, Zhang H, Dauphars DJ and He YW: A Potential role of interleukin 10 in COVID-19 pathogenesis. Trends Immunol. 42:3–5. 2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Labrecque N and Cermakian N: Circadian Clocks in the Immune System. J Biol Rhythms. 30:277–290. 2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Waggoner SN: Circadian Rhythms in Immunity. Curr Allergy Asthma Rep. 20(2)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Abella V, Scotece M, Conde J, Pino J, Gonzalez-Gay MA, Gómez-Reino JJ, Mera A, Lago F, Gómez R and Gualillo O: Leptin in the interplay of inflammation, metabolism and immune system disorders. Nat Rev Rheumatol. 13:100–109. 2017.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pérez-Pérez A, Vilariño-García T, Fernández-Riejos P, Martín-González J, Segura-Egea JJ and Sánchez-Margalet V: Role of leptin as a link between metabolism and the immune system. Cytokine Growth Factor Rev. 35:71–84. 2017.PubMed/NCBI View Article : Google Scholar
|
















