Introduction
A regimen using direct-acting antivirals (DAAs) without the use of interferon (IFN) was approved as a treatment in 2014(1). Compared to IFN-based regimens, DAAs have fewer side-effects and a much higher rate of achieving a sustained virological response (SVR) (2). Thus, DAAs can be used in older patients and patients with hepatic cirrhosis, with comparable therapeutic efficacy to IFN-based treatment.
However, a Spanish group reported a high short-term hepatocellular carcinoma (HCC) recurrence rate in patients with HCC treated with DAAs than those treated with IFN-based treatment (3). They pointed out a possibility that IFN and DAA treatment may significantly differ in viral suppression and related kinetics of inflammation. Moreover, they stated that hepatitis C virus (HCV) eradication may occur at an early stage following DAA treatment, while IFN treatment requires a longer period of time to eradicate the virus. Thus, DAA can reduce the expression of endogenous IFN in a short period of time, which has an antitumor effect, by reducing inflammation at an early stage. Furthermore, they hypothesized that IFN treatment is expected to exert a tumor suppressive effect due to the cancer immunity induced by IFN (3).
By contrast, French and Italian groups have reported that DAAs and IFN-based regimens are equally effective in suppressing the recurrence of HCC (4,5). Since DAA treatment without the use of IFN has only a short history, and given the lack of time for post-treatment follow-up, its long-term efficacy in suppressing the recurrence of HCC is unknown. A study using a database of patients who underwent DAA therapy at a Veterans Affairs Hospital in the USA investigated the recurrence of HCC in patients treated with IFN monotherapy, DAA therapy (including IFN) and DAA monotherapy. The results demonstrated that, following adjustment for background factors, there was no significant difference in the HCC recurrence rate between DAA monotherapy and other treatments (6). Furthermore, Japanese studies have found that when patients who received treatment with IFN-based regimens or DAAs were matched by background characteristics, there was no difference in the HCC recurrence between the two groups who achieved SVR (7-9).
The present study first confirmed whether the effect of DAA therapy in suppressing HCC recurrence in patients who achieved SVR was equivalent to that of an IFN-based regimen among different populations. Second, the possible predictive factors that may be associated with the cumulative HCC recurrence rates following DAA therapy were investigated.
Patients and methods
Patients
The present study was a multicenter study of patients in hospitals belonging to the Saiseikai Liver Study Group (SLSG) of the community hospitals affiliated with the Saiseikai Hospital Group. The study sites were Okayama Saiseikai General Hospital (Okayama, Japan), Fukui-ken Saiseikai Hospital (Fukui, Japan), Saiseikai Suita Hospital (Osaka, Japan), Saiseikai Karatsu Hospital (Karatsu, Japan), Saiseikai Wakayama Hospital (Wakayama, Japan), Saiseikai Utsunomiya Hospital (Utsunomiya, Japan), Saiseikai Imabari Hospital (Imabari, Japan), Saiseikai Niigata Daini Hospital (Niigata, Japan), Yokohamashi Nanbu Hospital (Yokohama, Japan), Tokyo Saiseikai Central Hospital (Tokyo, Japan), Saiseikai Kure Hospital (Kure, Japan), Kamisu Saiseikai Hospital (Kamisu, Japan) and Saiseikai Gotsu General Hospital (Gotsu, Japan). The present study was performed in compliance with the Declaration of Helsinki and approved by the Ethics Committee of Tokyo Saiseikai Central Hospital, where the principal investigator A.N. is employed, after which it was also approved by the ethics committees or equivalent committees of the participating study sites. Informed consent was obtained from the patients prior to enrolment.
The inclusion criteria for enrolment in the present study were patients with chronic hepatitis C who were treated with IFN or DAAs for HCC clinical stage I to III, aged >18 years, and confirmed to be completely cured. Treatment was administered in accordance with the Japan Society of Hepatology guidelines (10) by methods covered by regular health insurance, and results were evaluated in terms of virological response. Chronic hepatitis C was diagnosed based on serum HCV RNA positivity, and HCC was diagnosed by dynamic computed tomography (CT) and ethoxybenzyl magnetic resonance imaging (EOB-MRI). Complete cure was evaluated by resolution of the tumor on dynamic CT and EOB-MRI. The exclusion criteria were patients who were positive for serum hepatitis B; those with other liver injury causes, such as alcohol, autoimmune hepatitis and primary biliary hepatitis; and those who received a liver transplantation.
Following HCC treatment, the patients in the DAA group were treated with one of the following regimens: daclatasvir/asunaprevir; sofosbuvir/ledipasvir; ombitasvir/paritaprevir/ritonavir; elbasvir/grazoprevir; glecaprevir/pibrentasvir; or sofosbuvir/ribavirin. Patients in the IFN group were treated with an IFN-based regimen. The DAA group included 257 patients and the IFN group included 47 patients. The study period was from April 1, 2017 to January 31, 2020 with data collected data from patient records. The period (means ± SD) from treatment for HCC to DAA or IFN was 364±25 days or 369±372 days, respectively.
Information was obtained from each hospital using the same electrical format. The data collected included the date when HCC recurrence was confirmed; the date when the treatment was started; clinical laboratory data (before and after treatment); tumor marker levels [α-fetoprotein (AFP)/AFP L3/protein induced by vitamin K absence or antagonist-II (PIVKAII)]; hepatic fibrosis markers, such as type IV collagen 7S and Child-Pugh score; hepatic fibrosis scoring systems, such as fibrosis-4 (FIB-4), the Forns index (11) and aspartate aminotransferase (AST) to platelet ratio index (APRI); the number of previous HCC treatments; and the number of HCC tumors at the time of previous treatments.
Follow-up
At follow-up to identify HCC recurrence, blood tests, including tumor marker tests (AFP/PIVKAII), were performed at the start of administration and every at 24 weeks after the end of administration. In addition to blood tests, HCC was also examined basically every 24 weeks by abdominal ultrasonography, and confirmative diagnosis was performed by MRI and/or CT.
Statistical analysis
Differences in background characteristics between the DAA and IFN groups were compared using the t-test for continuous variables or Mann-Whitney U test for platelets, AST, alanine aminotransferase (ALT), ferritin, AFP and PIVKAII, and the χ2 test for categorical variables such as sex and Child-Pugh score. Data are presented as the mean ± SD or as medians (interquartile range). P<0.05 was considered to indicate a statistically significant difference.
Event analysis was conducted using the Kaplan-Meier method with HCC recurrence in the DAA and IFN groups as the outcome, and using the log-rank method to test for significance. The period was determined from the start of the viral treatment to the identification of HCC recurrence.
Since there were significant differences between the DAA and IFN groups in terms of the clinically important background factors [age, sex, weight, number of previous HCC treatments, baseline (before treatment) hemoglobin (Hb), baseline platelet count, baseline ALT, FIB-4 index, pre-treatment Forns index, and Child-Pugh score], Cox's proportional hazard model was first used to adjust for these as covariates.
Factors associated with HCC recurrence in the DAA group were then investigated. The associations with clinical laboratory data, tumor markers (AFP/AFP L3/PIVKAII), hepatic fibrosis markers, Child-Pugh score, FIB-4, Forns index, APRI and other fatty liver indicators, and hepatic fibrosis scoring systems were investigated by univariate analysis using Cox's model. The variables identified as significant in this univariate analysis were also clinically important factors, and as a result, multivariate analysis was performed with these factors entered in the Cox model.
To further control confounding factors, propensity scores were calculated for age, body mass index (BMI), baseline platelet count, albumin, AST, HbA1c, APRI, FIB-4, the number of previous HCC treatments, Forns index and Child-Pugh score, which differed significantly between the DAA and IFN groups. The concordance statistic (c statistic) was 0.672 [95% confidence interval (CI), 0.599-0.745]. Matching was then conducted using propensity scores. Kaplan-Meier analysis of the cumulative HCC recurrence rates in the DAA and IFN groups was conducted among the cases following matching. All statistical analyses were performed using IBM SPSS® Statistics ver. 25.0 (IBM Inc.).
Results
Characteristics of the two groups
A total of 304 patients in the 13 hospitals listed above were enrolled. The analysis population consisted of the DAA group, which included 257 patients, of whom 90 were treated with daclatasvir/asunaprevir, 89 with sofosbuvir/ledipasvir, 20 with ombitasvir/paritaprevir/ritonavir, 13 with elbasvir/grazoprevir, 10 with glecaprevir/pibrentasvir and 35 with sofosbuvir/ribavirin, and the IFN group, which included 47 patients treated with IFN-based regimens. The background characteristics of the patients in the two groups are presented in Table I. There were significant differences between patients with regards to age, platelets, ALT, triglycerides, AFP, PIVKAII, Child-Pugh score, FIB-4 index, Forns index and the number of HCC treatments.
 |
Table I
Baseline characteristics of the patients.
|
Table I
Baseline characteristics of the patients.
| Characteristic |
IFN (n=47) |
DAA (n=257) |
P-value |
| Age (years) |
71.5±9.0 |
75.2±7.4 |
0.003 |
| Sex (male/female) |
34/13 |
146/111 |
0.060 |
| BMI (kg/m2) |
23.4±3.2 |
22.8±3.2 |
0.235 |
| Platelets (x104/µl) |
13.5 (5.0) |
11.0 (6.6) |
<0.001 |
| Alb (g/dl) |
3.88±0.46 |
3.71±0.55 |
0.070 |
| AST (IU/l) |
45(46) |
50(35) |
0.748 |
| ATL (IU/l) |
49(54) |
39(34) |
0.020 |
| LDL-C (mg/dl) |
85.2±30.1 |
76.4±35.6 |
0.224 |
| TG (mg/dl) |
114.6±60.1 |
94.6±51.4 |
0.040 |
| BS (mg/dl) |
112.0±26.5 |
112.8±41.2 |
0.919 |
| Type IV collagen 7S (ng/ml) |
5.4±3.1 |
5.0±4.6 |
0.605 |
| M2BPGi (COI) |
7.92±4.75 |
4.6±5.1 |
0.120 |
| Ferritin (ng/ml) |
93.0 (208.3) |
25.2 (94.0) |
0.199 |
| AFP (ng/ml) |
7.1 (12.3) |
9.1 (15.3) |
0.169 |
| PIVKAII (mAU/ml) |
16.0 (8.8) |
18.0 (13.0) |
0.040 |
| FIB4 index |
3.85±1.94 |
6.09±3.47 |
<0.001 |
| Child-Pugh score |
5p; 27/≥6p; 12 |
5p; 103/6p; 88 |
<0.001 |
| HCVRNA (LogIU/ml) |
5.79±1.2 |
5.80±0.8 |
0.872 |
| Forns index |
8.1±1.5 |
9.1±1.7 |
<0.001 |
| Number of previous HCC treatments |
1.23±1.18 |
2.29±1.86 |
<0.001 |
Cumulative HCC recurrence rates between groups
The cumulative HCC recurrence rates in the DAA and IFN groups were analyzed by Kaplan-Meier analysis and the log-rank test. The results demonstrated that the rates were equivalent (Fig. 1A); Cox's proportional hazard model was then used to adjust for confounding background factors that differed significantly between the two groups; however, no significant differences were identified following adjustment (Fig. 1B).
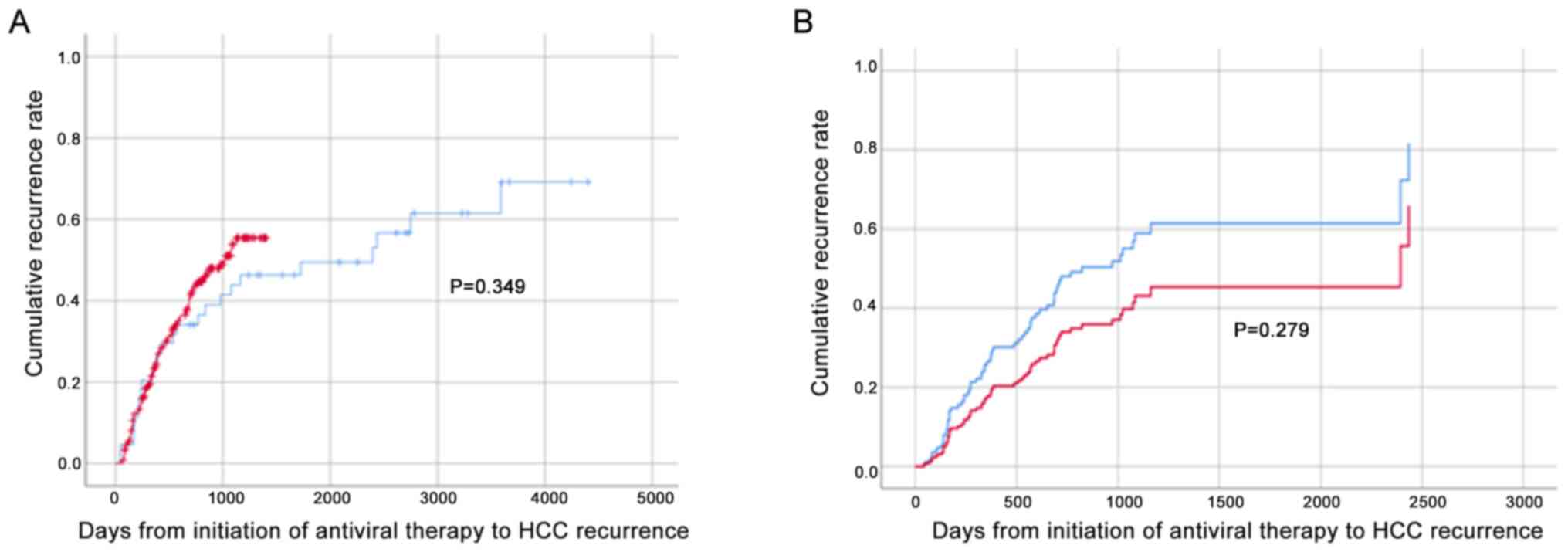 |
Figure 1
Cumulative HCC recurrence rate following DAA/IFN treatment. Red and blue lines represent DAA- and IFN-based treatment, respectively. (A) Kaplan-Meier analysis and log-rank test of the cumulative HCC recurrence rates in the DAA and IFN groups (DAA = 257, IFN = 47). (B) Cox’s proportional hazard model was used to adjust for confounding factors: Age, sex, baseline platelet counts, ALT, TG, AFP, PIVKAII, FIB-4, Forns index, Child-Pugh score and number of previous HCC treatments (DAA = 134, IFN = 23). HCC, hepatocellular carcinoma; DAA, direct-acting antiviral; IFN, interferon; ALT, alanine aminotransferase; TG, triglyceride; AFP, α-fetoprotein; PIVKAII, protein induced by vitamin K absence or antagonist-II; FIB-4, fibrosis-4.
|
The patients in the DAA group were then categorized into patients with or without HCC recurrence, and Cox's regression and univariate analyses of the clinical laboratory data following DAA therapy were performed. The results identified significant differences in the number of previous HCC treatments, Alb, AFP and PIVKAII (Table II). In addition, univariate analysis of hepatic fibrosis markers and scoring systems, such as the Child-Pugh score and Forns index also demonstrated significant differences (Table II).
 |
Table II
Univariate analysis of factors associated with HCC recurrence in the DAA group.
|
Table II
Univariate analysis of factors associated with HCC recurrence in the DAA group.
| Variable |
Hazard ratio |
95% CI |
|
P-value |
| BMI |
1.01 |
0.95 |
1.07 |
0.808 |
| Diabetes |
1.12 |
0.70 |
1.79 |
0.635 |
| Dyslipidemia |
1.17 |
0.98 |
1.23 |
0.079 |
| Number of previous HCC treatments |
1.33 |
1.21 |
1.46 |
<0.001 |
| Platelet count |
0.97 |
0.93 |
1.01 |
0.98 |
| Alb |
0.66 |
0.46 |
0.94 |
0.022 |
| ALT |
0.99 |
0.99 |
1.00 |
0.068 |
| LDL-C |
1.00 |
0.99 |
1.01 |
0.922 |
| TG |
1.00 |
1.00 |
1.00 |
0.841 |
| Ferritin |
1.00 |
1.00 |
1.00 |
0.875 |
| Type IV collagen 7S |
1.07 |
0.98 |
1.15 |
0.117 |
| M2BPGi |
1.00 |
0.95 |
1.05 |
0.912 |
| AFP |
1.00 |
1.00 |
1.01 |
0.050 |
| PIVKAII |
1.01 |
1.01 |
1.02 |
<0.001 |
| Forns index |
1.16 |
1.02 |
1.33 |
0.030 |
| APRI |
0.93 |
0.78 |
1.11 |
0.427 |
| FIB4 |
1.01 |
0.95 |
1.08 |
0.653 |
| Child-Pugh score |
1.35 |
1.12 |
1.62 |
0.001 |
Multivariate analysis, using the variables identified as significant on univariate analysis, revealed that PIVKA2, the number of previous HCC treatments (≥3) and the Forns index were significant independent variables (Table III).
 |
Table III
Multivariate analysis of patients with and without HCC recurrence in the DAA group.
|
Table III
Multivariate analysis of patients with and without HCC recurrence in the DAA group.
| Variable |
Hazard ratio |
95% CI |
P-value |
| Age |
0.97 |
0.93 |
1.01 |
0.145 |
| Sex |
1.31 |
0.74 |
2.32 |
0.351 |
| Platelet count |
1.07 |
0.98 |
1.17 |
0.129 |
| Alb |
0.77 |
0.42 |
1.42 |
0.403 |
| AFP |
1.00 |
1.00 |
1.01 |
0.134 |
| PIVKA2 |
1.02 |
1.01 |
1.03 |
<0.001 |
| Forns index |
|
|
|
|
| 1 |
1 |
Reference value |
|
| 2 |
4.58 |
1.40 |
14.98 |
0.012 |
| 3 |
5.27 |
1.52 |
18.29 |
0.009 |
| 4 |
9.12 |
2.51 |
33.07 |
0.001 |
| |
|
P-value for trend = 0.009 |
|
| Child-Pugh score |
|
|
|
|
| 4 |
1 |
Reference value |
|
| 5 |
1.75 |
0.58 |
5.28 |
0.323 |
| 6 |
2.04 |
0.64 |
6.52 |
0.229 |
| ≥7 |
2.37 |
0.70 |
8.06 |
0.166 |
| |
|
P-value for trend = 0.581 |
|
| Number of previous HCC treatments |
|
|
|
|
| 1 |
1 |
Reference value |
|
| 2 |
0.96 |
0.41 |
2.26 |
0.927 |
| 3 |
2.91 |
1.24 |
6.81 |
0.014 |
| ≥4 |
3.60 |
1.71 |
7.57 |
0.001 |
| |
|
P-value for trend <0.001 |
|
As a result of propensity score matching, 29 patients in each group were matched, and the background characteristics are presented in Table SI. The cumulative HCC recurrence rates for these 29 patients in the DAA and IFN groups were analyzed by the Kaplan-Meier method and the log-rank test; the results demonstrated no significant differences (Fig. 2), confirming that the HCC recurrence rate was equivalent in both groups.
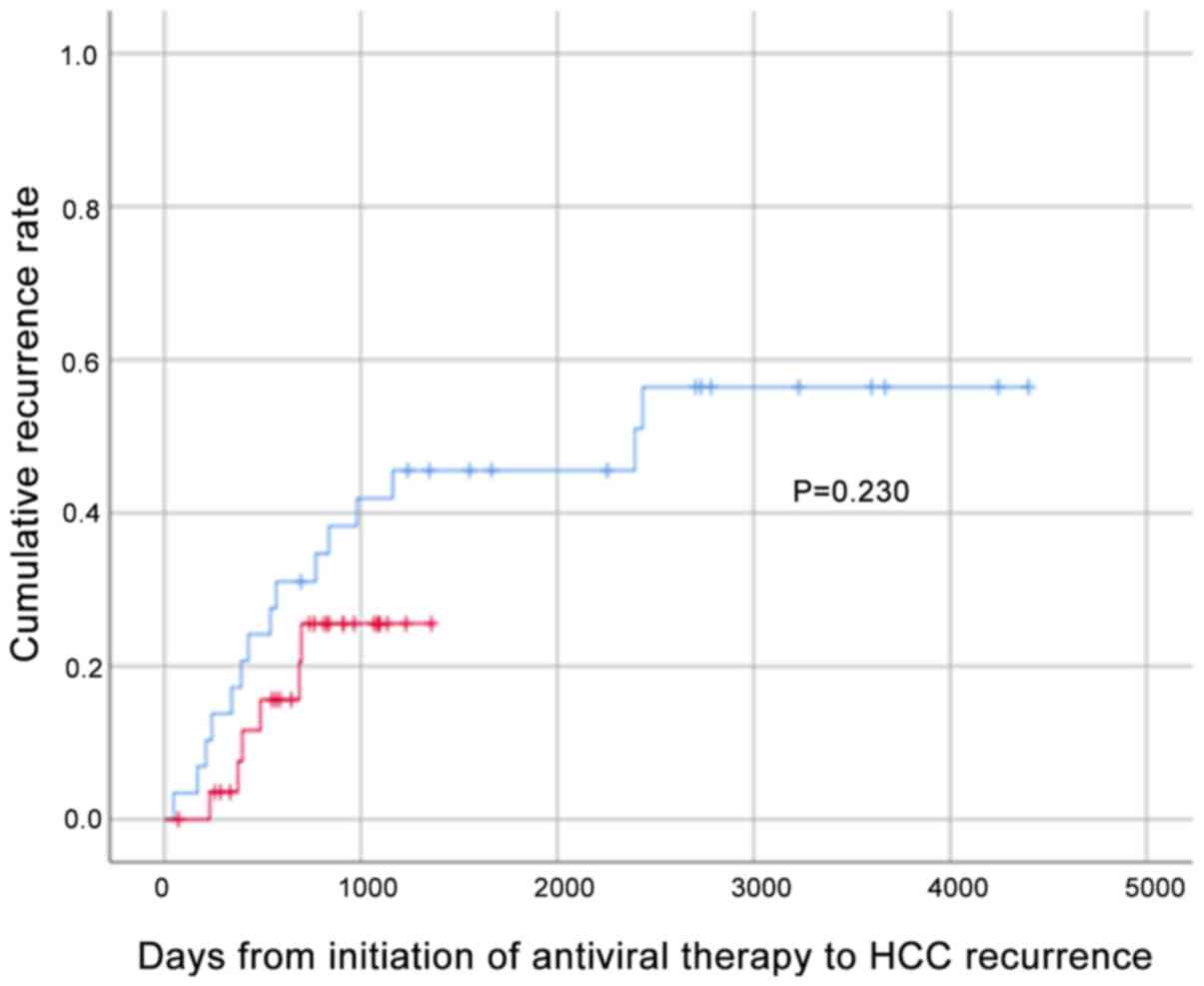 |
Figure 2
Cumulative recurrence rate by Kaplan-Meier analysis following propensity score matching. The cumulative HCC recurrence rate in the DAA and IFN groups was evaluated using Kaplan-Meier analysis and the log-rank test following propensity score matching by age, sex, weight, number of previous HCC treatments, baseline Hb, baseline platelet count, baseline ALT, baseline AFP, FIB-4, baseline Forns index and Child-Pugh score (DAA = 29, IFN = 29). Red and blue lines represent DAA- and IFN- based treatment, respectively. HCC, hepatocellular carcinoma; DAA, direct-acting antiviral; IFN, interferon; ALT, alanine aminotransferase; TG, triglyceride; AFP, α-fetoprotein; PIVKAII, protein induced by vitamin K absence or antagonist-II; FIB-4, fibrosis-4.
|
Discussion
Compared to IFN-based regimens, DAAs have fewer side-effects and provide a very high SVR achievement rate. However, DAA therapy without the use of IFN has a short history, and given the lack of sufficient time for post-treatment follow-up (2), its long-term efficacy in suppressing the recurrence of HCC remains unknown.
In the present study, the cumulative HCC recurrence rates in the DAA and IFN groups did not differ significantly, although the baseline potential status for HCC recurrence, such as fibrosis (i.e., the Fib-4 index and Child-Pugh score) and tumor makers (i.e., PIVKA2), were more progressive in patients who received DAAs than those who received IFN-based treatment. Regardless of this background bias, there was no significant difference in the HCC recurrence rate between the two groups. Furthermore, even following Cox's proportional model and propensity score matching to adjust for background factors, including those affecting HCC recurrence (despite them being in small numbers), there was no significant difference in the cumulative HCC recurrence rates between the DAA and IFN groups. These results confirm that the two treatment regimens had equivalent HCC recurrence.
A previous multicenter study of the HCC recurrence rates in patients treated with DAAs or an IFN-based regimen following treatment for HCC in hospitals belonging to the Japan Red Cross Hospitals group also found no significant difference in the HCC recurrence rate in patients who achieved SVR (8). Furthermore, other Japanese research groups have also performed studies involving patients extracted by propensity score matching, and, similar to the present results, they reported that there was no significant difference in the HCC recurrence rate between patients treated with DAAs and those treated with an IFN-based regimen (9,12).
IFN exerts both antiviral and antitumor effects; however, DAAs are HCV-specific antiviral agents that rapidly eliminate the virus, and the autoimmune response in the liver, including tumor immunity, is believed to decrease rapidly as a result. Furthermore, viral eradication by DAAs is cumulative HCC recurrence rates to result in a decrease in endogenous IFN and a consequent decline in tumor immunity, which has been considered to have the potential to encourage HCC recurrence. The high rate of HCC recurrence within a short period of time from the commencement of DAA therapy may be due to the presence of minute tumors at the commencement of DAA treatment. Very small tumors that are undetectable on diagnostic imaging may grow suddenly due to the rapid attenuation of the immune response after DAA therapy.
The rapid elimination of HCV is associated with a loss of intrahepatic activation of natural killer cell and immune system induced by IFN. It has been suggested that the elimination of HCV disrupts the host's innate immune response and probably promotes tumor cell growth (13).
Therefore, the most appropriate modality to use for assessment following HCC treatment remains unknown, since very small tumors may go undetected if only ultrasound is used. Ideally, EOB-MRI should be used for the assessment following HCC treatment, whenever possible. In addition, it is important to carry out this evaluation following HCC treatment at a time when the effect of very small tumors is no longer evident. A previous study found that the recurrence rate was higher among patients who began DAA treatment soon after HCC treatment (14), while another study demonstrated that the rate of recurrence was 3.8-fold higher if DAA treatment commenced within 1 year of HCC treatment (15).
Although viral eradication by DAAs reduces the risk of HCC development, it does not eliminate it completely. Patients with severe fibrosis are at a high risk, and even if SVR is achieved following DAA therapy, genomic alterations induced by HCV infection have a significant effect on HCC development (16). These findings indicate that careful screening by EOB-MRI should be performed following HCC treatment in patients with advanced fibrosis, such as hepatic cirrhosis, before starting DAA therapy. Conti et al (5) reported that 17 of 59 patients (28.8%) who began DAA treatment following HCC treatment developed recurrence, and multivariate analysis revealed that this was significantly associated with young age and hepatic fibrosis.
The authors have found the number of HCC treatments as a risk factor of recurrence in the DAA treatment group. That study included HCC cases in clinical stages I to III, which mainly depend on the number or size of the tumor. The results indicated no significant association of recurrence risk with tumor number or size (unpublished data). It was suggested that the number of HCC treatment sessions rather than the clinical stage was a more important risk factor for recurrence.
Studies have found that fat deposition in the liver does not improve in patients once the hepatitis virus has been eradicated, with one even reporting that although hepatic fibrosis improved following SVR, fat deposition in the liver actually worsened (17); however, further studies of its pathophysiology and prognosis are required to determine the underlying mechanisms. Furthermore, a Japanese study reported that the controlled attenuation parameter rose, and fat deposition in the liver increased, following DAA therapy in elderly patients with a median age of 71 years (18).
In the present study, the significantly higher Forns index in those with HCC recurrence than in those without HCC recurrence among patients in the DAA group suggested the Forns index as a possible predictive marker for recurrence. The other liver fibrosis markers are the FIB-4 index and the APRI, which are based on the following respective formulae (19,20): FIB-4 index = AST (IU/l) x age (years)/platelet count (109/l) xALT (IU/l) 1/2; APRI = [AST (IU/l)/upper limit of normal AST (IU/l)] x100/platelet count (109/l). On the other hand, the formula for the Forns index is as follows (11): Forns index = 7.811-3.131(lnplatelet count) + 0.781.lnGGT + 3.467.lnage - 0.014.cholesterol.
The FIB-4 and APRI are calculated based on only platelet and liver function, whereas the Forns index includes total cholesterol level. According to this formula, serum cholesterol is negatively associated with liver fibrosis. Regarding this point, Forns mentioned reduction in cholesterol synthesis secondary to advanced liver dysfunction and alterations in lipid profile secondary to different cytokine profiles induced by viral infections. Moreover, lipid metabolism has been suggested to be associated with cancer risk. A meta-analysis of 24 large randomized controlled trials on lipid-modifying therapy (21) concluded that the risk of incident cancer had a significant inverse association with HDL-C, independent of low-density lipoprotein cholesterol (LDL-C), age, BMI, diabetes, sex and smoking. That study found an inverse association between serum high-density lipoprotein cholesterol (HDL-C) and cancer risk in the general population. Recently, however, Carr et al (22) reported an association between serum lipid parameters and the indices of HCC growth, invasion, aggressiveness and survival; surprisingly, HDL-C was positively associated with a poor prognosis of HCC, whereas LDL was not included in their parameters. In that study, the authors mentioned that lipid profile could be associated with tumor lipid droplets, mitochondria, and membranes. The dataset in this study revealed that the hazard ratio (HR) of total cholesterol was 0.995 (95% CI, 0.99-0.999, P=0.02), whereas the HR values of LDL-C and HDL-C were not significant (unpublished data). The cholesterol level in the Forns index comprises a combination of LDL-C and HDL-C. However, whether HDL-C is a risk marker or causative factor remains a topic of discussion. Therefore, further studies are required to determine the importance of HDL-C, LDL-C, and total cholesterol levels as predictive markers for HCC recurrence.
In conclusion, the present study demonstrated that the HCC recurrence rate following curative treatment for HCC was equivalent in patients treated with DAAs and those treated with an IFN-based regimen. The present study supports previous results (7-9) showing that DAA treatment did not accelerate HCC recurrence. In addition, multivariate analysis of factors affecting HCC recurrence in the DAA group identified the number of previous HCC treatments (≥3) and the Forns index as significant independent risk factors.
Supplementary Material
Background characteristics following matching with the propensity score.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Saiseikai Medical and Welfare Joint Research Grant no. (2017‑2020).
Availability of data and materials
The datasets generated and/or analyzed during the current study are not publicly available due to patient privacy concerns, but are available from the corresponding author on reasonable request.
Authors' contributions
All authors (AN, SF, KN, TS, KiY, MK, TT, MO, TI, IK, NT, SF, HI, TN, YH, KaY and TO), except for MT, conceived and designed the study and, carried out the study and collected data. AN and MT analyzed the data and wrote the manuscript. TS and NT supervised the study. AN and MT confirm the authenticity of all the raw data. All authors reviewed the manuscript, and all authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Tokyo Saiseikai Central Hospital (no. R28-64) where the principal investigator, AN, is employed, after which it was also approved by the ethics committees or equivalent committees of the participating study sites. Informed consent was obtained from the patients prior to enrolment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Kumada H, Suzuki Y, Ikeda K, Toyota J, Karino Y, Chayama K, Kawakami Y, Ido A, Yamamoto K, Takaguchi K, et al: Daclatasvir plus asunaprevir for chronic HCV genotype 1b infection. Hepatology. 59:2083–2091. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Toshikuni N: Therapy with direct-acting antiviral agents for hepatitis C-related liver cirrhosis. Gut Liver. 11:335–348. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Reig M, Mariño Z, Perelló C, Iñarrairaegui M, Ribeiro A, Lens S, Díaz A, Vilana R, Darnell A, Varela M, et al: Unexpected high rate of early tumor recurrence in patients with HCV-related HCC undergoing interferon-free therapy. J Hepatol. 65:719–726. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
ANRS collaborative study group on hepatocellular carcinoma (ANRS CO22 HEPATHER, CO12 CirVir and CO23 CUPILT cohorts). Electronic address: simplestanislas.pol@aphp.fr. Lack of evidence of an effect of direct-acting antivirals on the recurrence of hepatocellular carcinoma: Data from three ANRS cohorts. J Hepatol. 65:734–740. 2016.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, et al: Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 65:727–733. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y and El-Serag HB: Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 153:996–1005.e1. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Nagata H, Nakagawa M, Asahina Y, Sato A, Asano Y, Tsunoda T, Miyoshi M, Kaneko S, Otani S, Kawai-Kitahata F, et al: Ochanomizu Liver Conference Study Group: Effect of interferon-based and -free therapy on early occurrence and recurrence of hepatocellular carcinoma in chronic hepatitis C. J Hepatol. 67:933–939. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Mashiba T, Joko K, Kurosaki M, Ochi H, Osaki Y, Kojima Y, Nakata R, Goto T, Takehiro A, Kimura H, et al: Does interferon-free direct-acting antiviral therapy for hepatitis C after curative treatment for hepatocellular carcinoma lead to unexpected recurrences of HCC? A multicenter study by the Japanese Red Cross Hospital Liver Study Group. PLoS One. 13(e0194704)2018.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Nagaoki Y, Imamura M, Teraoka Y, Morio K, Fujino H, Ono A, Nakahara T, Murakami E, Yamauchi M, Kawaoka T, et al: Impact of viral eradication by direct-acting antivirals on the risk of hepatocellular carcinoma development, prognosis, and portal hypertension in hepatitis C virus-related compensated cirrhosis patients. Hepatol Res. 50:1222–1233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Asahina Y, Izumi N, Hiromitsu K, Kurosaki M, Koike K, Suzuki F, Takikawa H, Tanaka A, Tanaka E, Tanaka Y, et al: JSH Guidelines for the management of hepatitis C virus infection: A 2016 update for genotype 1 and 2. Hepatol Res. 46:129–165. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM and Rodés J: Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 36:986–992. 2002.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Nagaoki Y, Imamura M, Nishida Y, Daijo K, Teraoka Y, Honda F, Nakamura Y, Morio K, Fujino H, Nakahara T, et al: The impact of interferon-free direct-acting antivirals on clinical outcome after curative treatment for hepatitis C virus-associated hepatocellular carcinoma: Comparison with interferon-based therapy. J Med Virol. 91:650–658. 2019.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Llovet JM and Villanueva A: Liver cancer: Effect of HCV clearance with direct-acting antiviral agents on HCC. Nat Rev Gastroenterol Hepatol. 13:561–562. 2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nishibatake Kinoshita M, Minami T, Tateishi R, Wake T, Nakagomi R, Fujiwara N, Sato M, Uchino K, Enooku K, Nakagawa H, et al: Impact of direct-acting antivirals on early recurrence of HCV-related HCC: Comparison with interferon-based therapy. J Hepatol. 70:78–86. 2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et al: Kyushu University Liver Disease Study (KULDS) Group: Short-term risk of hepatocellular carcinoma after hepatitis C virus eradication following direct-acting anti-viral treatment. Aliment Pharmacol Ther. 47:104–113. 2018.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Hamdane N, Jühling F, Crouchet E, El Saghire H, Thumann C, Oudot MA, Bandiera S, Saviano A, Ponsolles C, Roca Suarez AA, et al: HCV-induced epigenetic changes associated with liver cancer risk persist after sustained virologic response. Gastroenterology. 156:2313–2329.e7. 2019.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Rout G, Nayak B, Patel AH, Gunjan D, Singh V and Kedia S: Shalimar. Therapy with oral directly acting agents in hepatitis C infection is associated with reduction in fibrosis and increase in hepatic steatosis on transient elastography. J Clin Exp Hepatol. 9:207–214. 2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ogasawara N, Kobayashi M, Akuta N, Kominami Y, Fujiyama S, Kawamura Y, Sezaki H, Hosaka T, Suzuki F, Saitoh S, et al: Serial changes in liver stiffness and controlled attenuation parameter following direct-acting antiviral therapy against hepatitis C virus genotype 1b. J Med Virol. 90:313–319. 2018.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al: APRICOT Clinical Investigators: Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 43:1317–1325. 2006.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS and Lok AS: A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 38:518–526. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jafri H, Alsheikh-Ali AA and Karas RH: Baseline and on-treatment high-density lipoprotein cholesterol and the risk of cancer in randomized controlled trials of lipid-altering therapy. J Am Coll Cardiol. 55:2846–2854. 2010.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Carr BI, Giannelli G, Guerra V, Giannini EG, Farinati F, Rapaccini GL, Marco MD, Zoli M, Caturelli E, Masotto A, et al: Plasma cholesterol and lipoprotein levels in relation to tumor aggressiveness and survival in HCC patients. Int J Biol Markers. 33:423–431. 2018.PubMed/NCBI View Article : Google Scholar
|
















