Introduction
Wound anaplasis is a dynamic, complex process that
involves a number of cell types and a variety of biological
processes, such as proliferation, differentiation and migration
(1). The interactions between
these cells are initiated and mediated mostly by growth factors
(2-4).
One of these factors is insulin-like growth factor (IGF)-1. IGF-1
is a protein with a similar molecular structure to insulin, which
has been shown to play a crucial role during growth and exerts
several anabolic effects in humans (5). The main secretion of IGF-1 occurs in
the liver and is regulated by growth hormone. It is also produced
by other organs, such as the skeletal muscle, kidney and brain
(6-12).
The IGF-1 gene can produce different transcripts through
alternative splicing. This phenomenon results in three different
IGF-1 transcripts, namely IGF-1Ea, IGF-1Eb and IGF-1Ec, encoding
the IGF-1 protein isoforms (6-12).
Approximately 98% of IGF-1 is always bound to one of
six binding proteins (IGF-BP). IGF-BP3 accounts for 80% of all IGF
binding (13). IGF-1 exerts its
effects by binding to specific receptors on the cell surface
(10). There is increasing
evidence to indicate that IGF-1 plays a main role in the
regeneration of different tissues following injury (11,14-17).
Of note however, despite the escalating number of animal studies
(18-20),
there are fewer studies investigating the role of IGF-1 in the
wound healing process in humans.
In this context, the present study aimed to evaluate
the variations in the expression IGF-1 isoforms (IGF1-Ea, IGF1-Eb
and IGF1-Ec), as well as its binding protein and receptor (IGF-BP3
and IGF-1R) during wound healing.
Patients and methods
Patients
A total of 21 patients, presenting with the first
episode of sacrococcygeal pilonidal disease were enrolled in the
present study, from December, 2017 to December, 2018. The samples
were obtained at the Laiko University Hospital (Athens, Greece).
The present study population was selected due to the presence of an
open wound and the possibility of sampling on consecutive days. All
patients provided their written consent to participate in the
study, which followed the 1975 Helsinki guidelines and was approved
by the Bioethical Committee of the Medical School of the National
and Kapodistrian University of Athens (13-3-2017/Protocol no. 255).
Only adult patients were included in the present study and these
were patients with a first episode of pilonidal disease. Tissue
samples were obtained during surgery (time 0), as well as on day 2,
7 and 14 post-operatively. The days of sampling were selected based
on the phases of the healing process, always bearing in mind that
different healing phases are not mutually exclusive and tend to
overlap.
Tissue specimens
The size of the samples was 0.5 cm (depth),
consisting of full-thickness biopsies of the wound (skin and
subcutaneous tissue). Biopsy samples were immediately transferred
in Ambion RNAlater (Thermo Fisher Scientific Inc.) and rozen on
site at -80˚C.
RNA isolation and cDNA synthesis
Once all tissues were collected, RNA extraction was
performed. Total RNA was extracted from the tissue samples using
the TRItidy protocol (TRItidy G™ reagent, PanReac
AppliChem). According to this protocol, the total RNA was obtained
in the aqueous phase during the acidic extraction. Following RNA
isolation, reverse transcription reaction was performed using the
standard protocol of the Protoscript II First Strand cDNA synthesis
kit [ProtoScript® II First Strand cDNA Synthesis kit
(#E6560L; New England BioLabs, Inc.)].
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
In total, five sets of primers (Table I) were used to amplify five
different target mRNAs, IGF1-Ea, IGF1-Eb, IGF1-Ec, IGF-BP3 and
IGF-1R. 18S ribosomal RNA (rRNA) was used as the housekeeping gene.
The samples were amplified using a Thermal Cycler (Bio-Rad iCycler
Thermal Cycler IQ5 Multicolor Real-Time PCR Detection System). A
negative control with no template was used in each qPCR plate. The
samples were amplified using a Thermal Cycler (Bio-Rad iCycler
Thermal Cycler IQ5 Multicolor Real-Time PCR Detection System;
Bio-Rad Laboratories, Inc.) and each PCR reaction had a total
volume of 20 µl containing 12.5 µl iQ™ SYBR-Green Supermix (Bio-Rad
Laboratories, Inc.), 50 ng cDNA, 0.4 µΜ of each primer and
nuclease-free water. The following thermocycling conditions were
used: Initial denaturation at 95˚C for 4 min followed by 45 cycles
of 12 sec at 95˚C, 30 sec at 61˚C for annealing, and 30 sec at 72˚C
for extension. A final extension step was used at 72˚C for 5 min.
In order to quantify and compare the expression levels of each gene
between the conditions, was used the automatically calculated
number of cycles required for the measured fluorescence to exceed
the threshold for detection (Cq). The relative analysis of gene
expression data and calculation of ΔΔCq, was performed according to
the well-established 2-ΔΔCt method, as described elsewhere
(21). Each sample was analyzed in
duplicate and relative quantification was performed using the
housekeeping gene glyceraldehyde 3-phosphate dehydrogenase (GAPDH)
as an internal control. The primers used are presented in Table I.
 | Table ISequences of the primers used for
RT-qPCR. |
Table I
Sequences of the primers used for
RT-qPCR.
| Gene | Primer sequence
(5'-3') |
|---|
| IGF1-Ea F |
GTGGAGACAGGGGCTTTTATTTC |
| IGF1-Ea R |
CTTGTTTCCTGCACTCCCTCTACT |
| IGF1-Eb F |
ATGTCCTCCTCGCATCTCT |
| IGF1-Eb R |
CCTCCTTCTGTTCCCCTC |
| IGF1-Ec F |
CGAAGTCTCAGAGAAGGAAAGG |
| IGF1-Ec R |
ACAGGTAACTCGTGCAGAGC |
| IGF-1R F |
ACCTCTTCCCCAACCTCAC |
| IGF-1R R |
CAGGCAGGCACACAGACAC |
| IGF-BP3 F |
AGTGAGTCGGAGGAGACCGCA |
| IGF-BP3 R |
CCTTGGTGGTGTAGCCTGGGAGA |
Statistical analysis
The statistical analysis of the results was
performed using GraphPad Prism software (GraphPad Prism version
8.0.0 for Windows, GraphPad Software, Inc.; www.graphpad.com). Statistical analysis of relative
quantification data (ΔΔCq) included a non-parametric Kruskal-Wallis
test with multiple comparisons of the three timepoints post-surgery
compared with baseline (day of surgery) and Dunn's method as a
post-hoc test. A P-value <0.05 was considered to indicate a
statistically significant difference.
Results
The median age of the study population was 25 years
old (range, 18-33 years), with almost 80% (17/21) being males. No
patient had received corticosteroids or any other immunosuppressive
medication. All patients had an ASA (American Society of
Anesthesiologists) (22) score of
I. No antibiotics or other medications were prescribed
post-operatively apart from paracetamol. The wound care was
performed on a daily basis-with mechanical irrigation with normal
saline and simple gauze dressings by the patient or their immediate
family members except for the days that they participated in the
protocol.
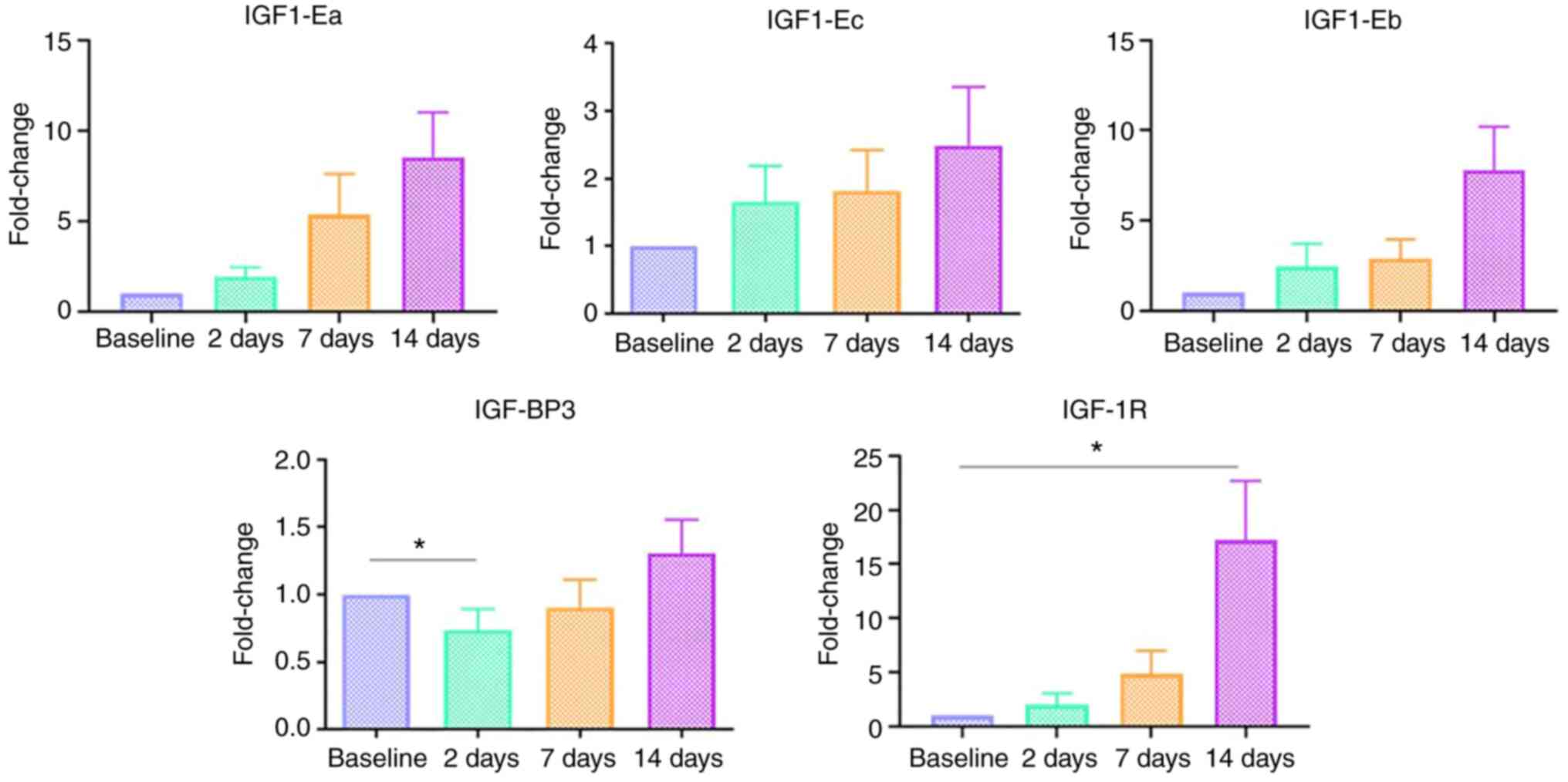
IGF-1 isoforms (IGF1-Ea, IGF1-Eb,
IGF1-Ec)
The expression of IGF-1 isoforms (IGF-1Ea, IGF-1Eb
and IGF-1Ec) was not significantly altered during the process of
wound healing in the present study population. All wounds were
clean and healing in an expected manner; despite that, no marked
differences were noted in the expression of these growth factors
(Fig. 1).
IGF-I-BP3
The expression of IGF-BP3 was significantly
increased during the post-operative period (P=0.014) compared with
the baseline. A pairwise post-hoc Dunn test indicated that it was
significantly decreased on day 2 post-operatively compared to the
day of surgery (Fig. 1).
IGF-1R
The expression of IGF-1R was also increased
post-operatively (P=0.018), with a significant increase observed on
the 14th post-operative day compared to the day of surgery
(Fig. 1).
Discussion
Wound healing is a complex process that occurs in
several phases and involves several factors. IGF-1 is a hormone
that plays a crucial role during growth and development and is
expressed in several tissues in humans where it exerts several
anabolic effects (23,24).
Several animal studies have been conducted to assess
the role of IGF-1 in the process of wound anaplasis, using both
local as well as systemic administration. Lynch et al
(3) studied the application of
recombinant IGF-I and platelet-derived growth factor-2 in partial
thickness wounds, which were surgically induced in the back and
thoracic areas of young white Yorkshire pigs. They reported a 132%
increase in the dermal thickness and a 300% increase in the number
of connective tissue cells within the wound site as well as in the
collagen content and maturity, following (3). Moreover, a placebo-controlled trial
demonstrated that IGF-1 depletion in hypophysectomized rats
resulted in a 50% reduction in wound protein levels and
hydroxyproline content, and that when IGF-1 was administered the
levels of these variables returned to normal levels (25).
Given the aforementioned findings in animal models,
further studies have been performed regarding the optimum means of
IGF-I delivery in the wound micro-environment. Jeschke et al
(26) concluded that wound
anaplasis can be accelerated by liposomal IGF-1 gene transfer.
Furthermore, another study reported an improved healing process in
collagenous tissue following the systemic administration of IGF-1
in rats (27).
On the other hand, in humans, there is limited
information available on the expression of IGF-1 or its different
isoforms following trauma and wound healing. Various responses in
IGF-1 transcriptional levels have been reported following
resistance exercise. As previously demonstrated, following 2.5 h of
resistance exercise, the IGF-1Ea isoform appears to be stable
(28), or to be downregulated
during the initial part of recovery (up to 2 days after exercise)
(29). In addition, the mRNA
levels of the IGF-1Eb and IGF-1Ec isoform have been found to be
unaffected up to 2 days following exercise (29).
Of note, the present study has several limitations.
Firstly, there was no control group, and the intended sample size
(n=30) was not reached. In addition, there was no standardized
method of estimating the healing process of the wound, which
severely restricted the translation of the results. Furthermore,
additional experiments, such as western blot analysis and
immunohistochemistry were not employed.
In conclusion, IGF-1 is a hormone with profound
anabolic activities and a crucial role in wound anaplasis. The
IGF-1-induced stimulation of wound healing has been demonstrated in
several animal studies. A recent systematic review (30) demonstrated a potentially promising,
evidence-based practice favoring the use of IGF-I in addressing
patients with large burn wounds, chronic diabetic ulcers, and
patients with impaired wound healing. Studying the variations of
IGF-1 expression may help in the wound healing process by detecting
the solely responsible binding protein and receptor on the cell
surface, resulting in most targeted future therapies. Thus, further
consistent clinical trials are warranted, focusing on the medical
use of recombinant IGF-1 in patients whose healing process has been
compromised.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZG contributed to the experiments and the drafting
of the manuscript. APh and APa contributed to the statistical
analysis. APa, DV and EK contributed to the experimental process.
APh, GT, GK and DM were involved in the conception and design of
the study. ZG and DM confirm the authenticity of all raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided their written consent to
participate in the study, which followed the 1975 Helsinki
guidelines and was approved by the Bioethical Committee of the
Medical School of the National and Kapodistrian University of
Athens (13-3-2017/Protocol no. 255).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Clark RA: Cutaneous tissue repair: Basic
biologic considerations. I. J Am Acad Dermatol. 13:701–725.
1985.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Ksander GA: Chapter 24. Exogenous growth
factors in dermal wound healing. Annu Rep Med Chem. 24:223–232.
1989.
|
|
3
|
Lynch SE, Colvin RB and Antoniades HN:
Growth factors in wound healing. Single and synergistic effects on
partial thickness porcine skin wounds. J Clin Invest. 84:640–646.
1989.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wahl SM, Wong H and Mc-Cartney-Francis N:
Role of growth factors in inflammation and repair. J Cell Biochem.
40:193–199. 1989.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rinderknecht E and Humbel RE: The amino
acid sequence of human insulin-like growth factor I and its
structural homology with proinsulin. J Biol Chem. 253:2769–2776.
1978.PubMed/NCBI
|
|
6
|
Moschos MM, Armakolas A, Philippou A,
Pissimissis N, Panteleakou Z, Nezos A, Kaparelou M and Koutsilieris
M: Expression of the insulin-like growth factor 1 (IGF-1) and type
I IGF receptor mRNAs in human HLE-B3 lens epithelial cells. In
Vivo. 25:179–184. 2011.PubMed/NCBI
|
|
7
|
Milingos DS, Philippou A, Armakolas A,
Papageorgiou E, Sourla A, Protopapas A, Liapi A, Antsaklis A,
Mastrominas M and Koutsilieris M: Insulinlike growth factor-1Ec
(MGF) expression in eutopic and ectopic endometrium:
characterization of the MGF E-peptide actions in vitro. Mol Med.
17:21–28. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Stavropoulou A, Halapas A, Sourla A,
Philippou A, Papageorgiou E, Papalois A and Koutsilieris M: IGF-1
expression in infarcted myocardium and MGF E peptide actions in rat
cardiomyocytes in vitro. Mol Med. 15:127–135. 2009.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Durzyńska J, Philippou A, Brisson BK,
Nguyen-McCarty M and Barton ER: The pro-forms of insulin-like
growth factor I (IGF-I) are predominant in skeletal muscle and
alter IGF-I receptor activation. Endocrinology. 154:1215–1224.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Philippou A, Maridaki M, Pneumaticos S and
Koutsilieris M: The complexity of the IGF1 gene splicing,
posttranslational modification and bioactivity. Mol Med.
20:202–214. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Thomas CG, Psarros C, Gekas A, Vandoros
GP, Philippou A and Koutsilieris M: Alternative splicing of IGF1
gene as a potential factor in the pathogenesis of Peyronie's
diseas. In Vivo. 30:251–256. 2016.PubMed/NCBI
|
|
12
|
Philippou A, Maridaki M, Halapas A and
Koutsilieris M: The role of the insulin-like growth factor 1
(IGF-1) in skeletal muscle physiology. In Vivo. 21:45–54.
2007.PubMed/NCBI
|
|
13
|
Jansen M, van Schaik FM, Ricker AT,
Bullock B, Woods DE, Gabbay KH, Nussbaum AL, Sussenbach JS and Van
den Brande JL: Sequence of cDNA encoding human insulin-like growth
factor I precursor. Nature. 306:609–611. 1983.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Karalaki M, Fili S, Philippou A and
Koutsilieris M: Muscle regeneration: Cellular and molecular events.
In Vivo. 23:779–796. 2009.PubMed/NCBI
|
|
15
|
Philippou A, Papageorgiou E, Bogdanis G,
Halapas A, Sourla A, Maridaki M, Pissimissis N and Koutsilieris M:
Expression of IGF-1 isoforms after exercise-induced muscle damage
in humans: Characterization of the MGF E peptide actions in vitro.
In Vivo. 23:567–575. 2009.PubMed/NCBI
|
|
16
|
Philippou A, Halapas A, Maridaki M and
Koutsilieris M: Type I insulin-like growth factor receptor
signaling in skeletal muscle regeneration and hypertrophy. J
Musculoskelet Neuronal Interact. 7:208–218. 2007.PubMed/NCBI
|
|
17
|
Bhora FY, Dunkin BJ, Batzri S, Aly HM,
Bass BL, Sidawy AN and Harmon JW: Effect of growth factors on cell
proliferation and epithelialization in human skin. J Surg Res.
59:236–244. 1995.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kanje M, Skottner A, Sjöberg J and
Lundborg G: Insulin-like growth factor I (IGF-I) stimulates
regeneration of the rat sciatic nerve. Brain Res. 486:396–398.
1989.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Sjöberg J and Kanje M: Insulin-like growth
factor (IGF-1) as a stimulator of regeneration in the
freeze-injured rat sciatic nerve. Brain Res. 485:102–108.
1989.PubMed/NCBI View Article : Google Scholar
|
|
20
|
LeRoith D: Clinical relevance of systemic
and local IGF-I: Lessons from animal models. Pediatr Endocrinol
Rev. 5 (Suppl 2):S739–S743. 2008.PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
22
|
American Society of Anesthesiologists
ASA): ASA Physical Status Classification System. ASA, Washington,
DC, 2020. https://www.asahq.org/standards-and-guidelines/asa-physical-status-classification-system.
Accessed February 22, 2022.
|
|
23
|
Baker J, Liu JP, Robertson EJ and
Efstratiadis A: Role of insulin-like growth factors in embryonic
and postnatal growth. Cell. 75:73–82. 1993.PubMed/NCBI
|
|
24
|
Daughaday WH and Rotwein P: Insulin-like
growth factors I and II. Peptide, messenger ribonucleic acid and
gene structures, serum, and tissue concentrations. Endocr Rev.
10:68–91. 1989.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Mueller RV, Hunt TK, Tokunaga A and
Spencer EM: The effect of insulinlike growth factor I on wound
healing variables and macrophages in rats. Arch Surg. 129:262–265.
1994.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Jeschke MG, Schubert T, Krickhahn M,
Polykandriotis E, Klein D, Perez-Polo JR, Przkora R and Herndon DN:
Interaction of exogenous liposomal insulin-like growth factor-I
cDNA gene transfer with growth factors on collagen expression in
acute wounds. Wound Repair Regen. 13:269–277. 2005.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Provenzano PP, Alejandro-Osorio AL, Grorud
KW, Martinez DA, Vailas AC, Grindeland RE and Vanderby R Jr:
Systemic administration of IGF-I enhances healing in collagenous
extracellular matrices: Evaluation of loaded and unloaded
ligaments. BMC Physiol. 7(2)2007.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Hameed M, Orrell RW, Cobbold M, Goldspink
G and Harridge SD: Expression of IGF-I splice variants in young and
old human skeletal muscle after high resistance exercise. J
Physiol. 547:247–254. 2003.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Psilander N, Damsgaard R and Pilegaard H:
Resistance exercise alters MRF and IGF-I mRNA content in human
skeletal muscle. J Appl Physiol (1985). 95:1038–1044.
2003.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Garoufalia Z, Papadopetraki A, Karatza E,
Vardakostas D, Philippou A, Kouraklis G and Mantas D: Insulin-like
growth factor-I and wound healing, a potential answer to
non-healing wounds: A systematic review of the literature and
future perspectives. Biomed Rep. 15(66)2021.PubMed/NCBI View Article : Google Scholar
|