Introduction
The optimal time interval between the end of
neoadjuvant chemotherapy and definitive surgery is unclear. Some
published large randomized clinical trials have revealed no
significant differences in disease-free and overall survival
between patients receiving chemotherapy in the adjuvant and the
neoadjuvant settings (1-4).
To date, to the best of our knowledge, no large
randomized clinical trials on neoadjuvant systemic therapy have
addressed the issue of delays in the time to surgery following the
completion of neoadjuvant chemotherapy and its effect on survival
outcomes. To the best of our knowledge, there are three
retrospective studies that have investigated the effects of the
time to surgery following neoadjuvant chemotherapy on survival
outcomes (5-7).
A prolonged time to surgery interval may theoretically increase the
risk of recurrence by allowing tumour neo-angiogenesis and tumour
growth.
The breast multidisciplinary team at Medway Hospital
(Gillingham, UK) agreed that upon an ideal time to surgery interval
of ≤28 days, which would allow a reasonable amount of time for
recovery from the side-effects of neoadjuvant chemotherapy without
compromising the survival outcomes.
The present retrospective clinical audit presents a
review of all breast cancer cases that received neoadjuvant
chemotherapy followed by surgery and examines the conformity to the
set audit standards.
Patients and methods
The present retrospective clinical audit presents a
review of all female breast cancer patients following neoadjuvant
chemotherapy followed by surgery to establish conformity to the set
audit standards. The exclusion criteria were male breast cancer
patients and metastatic breast cancer patients. A total of 59
breast cancer cases that received neoadjuvant chemotherapy that
satisfied the inclusion and exclusion criteria at Medway Hospital
between November 1, 2011 and October 31, 2016 were included in the
audit. The details of the patients included in the present audit
are listed in Table I.
 | Table IDetails of age distribution, type of
breast cancer, receptor positivity and type of surgery performed in
the patients included in the audit. |
Table I
Details of age distribution, type of
breast cancer, receptor positivity and type of surgery performed in
the patients included in the audit.
| Parameter | No. of patients |
|---|
| Age groups | |
|
31 to 50
years | 33 |
|
51 to 70
years | 24 |
|
71 to 80
years | 2 |
| Tumour type | |
|
Invasive
ductal carcinoma | 53 |
|
Invasive
lobular carcinoma | 5 |
|
Basal
type | 1 |
| Tumour grade | |
|
Grade 1 | 0 |
|
Grade 2 | 19 |
|
Grade 3 | 40 |
| Receptor status (ER,
PR, HER2) | |
|
Triple-negative
(ER-, PR- and HER2-) | 22 |
|
ER+,
PR+ and HER2- | 10 |
|
HER2-positive
only (ER-, PR- and HER2+) | 12 |
|
ER+,
PR- and HER2+ | 3 |
|
ER-positive
only (ER+, PR- and HER2-) | 8 |
|
Triple-positive
(ER+, PR+ and HER2+) | 4 |
| Type of surgery
performed | |
|
Mastectomy | 22 |
|
Wide local
excision with no wire localisation | 14 |
|
Wide local
excision with wire localisation | 23 |
Audit standards
In total, one primary audit standard and six
secondary audit standards were derived from the Medway hospital
Multidisciplinary Team (MDT) consensus agreement and the reasons
for non-conformity to these standards were audited.
Primary audit standard. All patients should
have a time to surgery within 28 days of completion of the last
cycle of neoadjuvant chemotherapy.
Secondary audit standards. i) All cases
should undergo MDT discussion prior to the completion of the last
cycle of neoadjuvant chemotherapy; ii) all cases should be added to
the surgical waiting list prior to the completion of the last cycle
of neoadjuvant chemotherapy; iii) all cases should have surgery
<4 weeks following the addition to the waiting list; iv) all
cases should have tumour coil insertion prior to the commencement
of the first cycle of neoadjuvant chemotherapy; v) all cases should
have mid-chemotherapy imaging to assess the tumour response to
neoadjuvant chemotherapy; vi) all cases should have
post-neoadjuvant chemotherapy imaging to aid in surgical
planning.
The time to surgery following the completion of
neoadjuvant chemotherapy, the timing of surgical planning
multidisciplinary team meeting, the time to addition to the
surgical waiting list, tumour coil insertion timing, percentage of
cases who had mid-chemotherapy imaging and post-neoadjuvant
chemotherapy imaging were audited. The percentage of conformity to
the aforementioned standards was calculated and factors
contributing to non-conformity were investigated. Data were
extracted by the reviewing of existing medical records, clinical
letters, radiology reports, laboratory reports and the trust
oncology database. All data and records generated during the study
were kept secure and confidential in accordance with trust policies
on information governance and data protection.
Results
Primary standard
All cases should have surgery ≤28 days following the
completion of the last cycle of neoadjuvant chemotherapy. In the
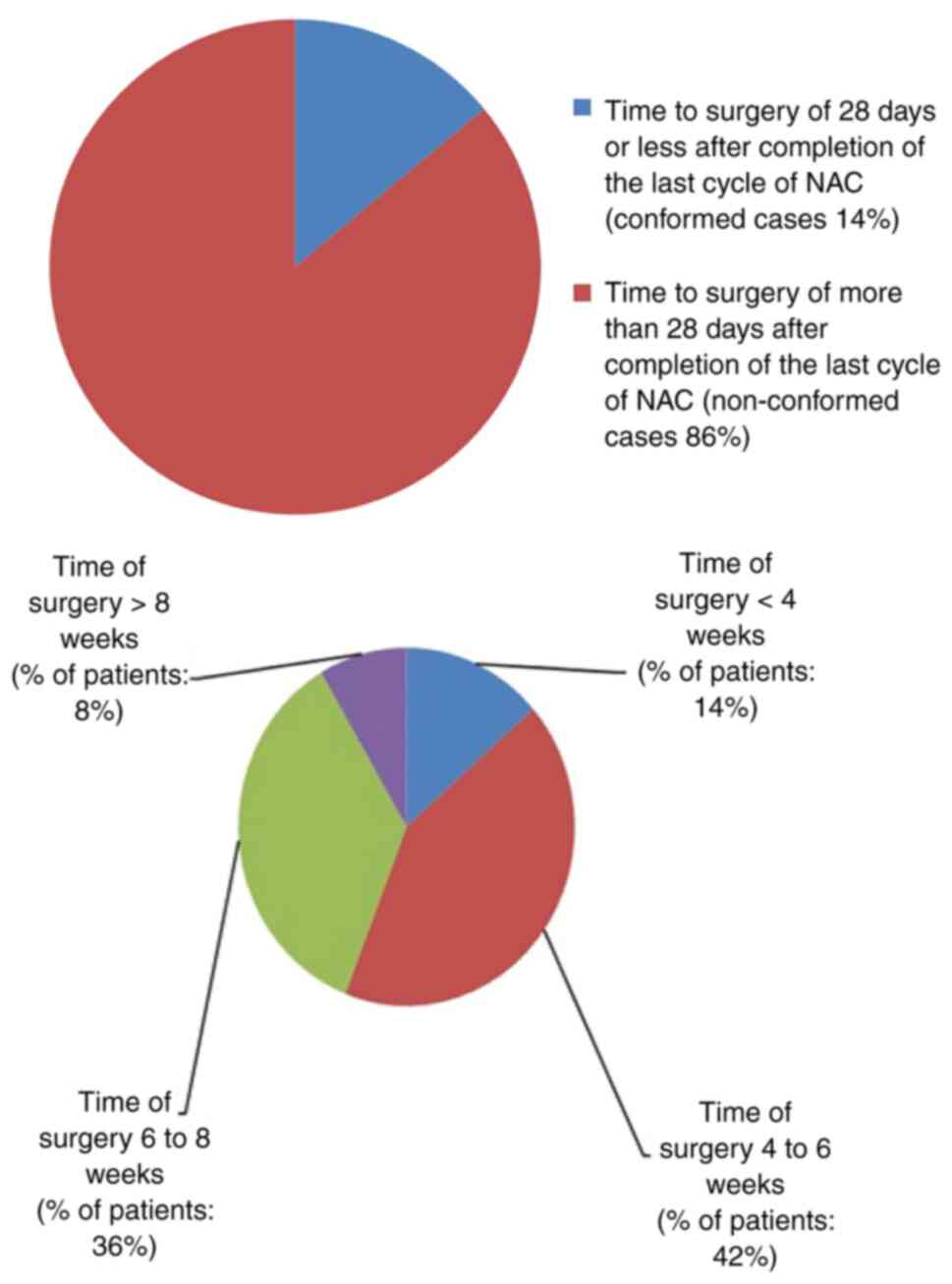
present audit, only 14% of patients conformed to this standard. The
details of non-conformed cases (86%) are illustrated in Fig. 1.
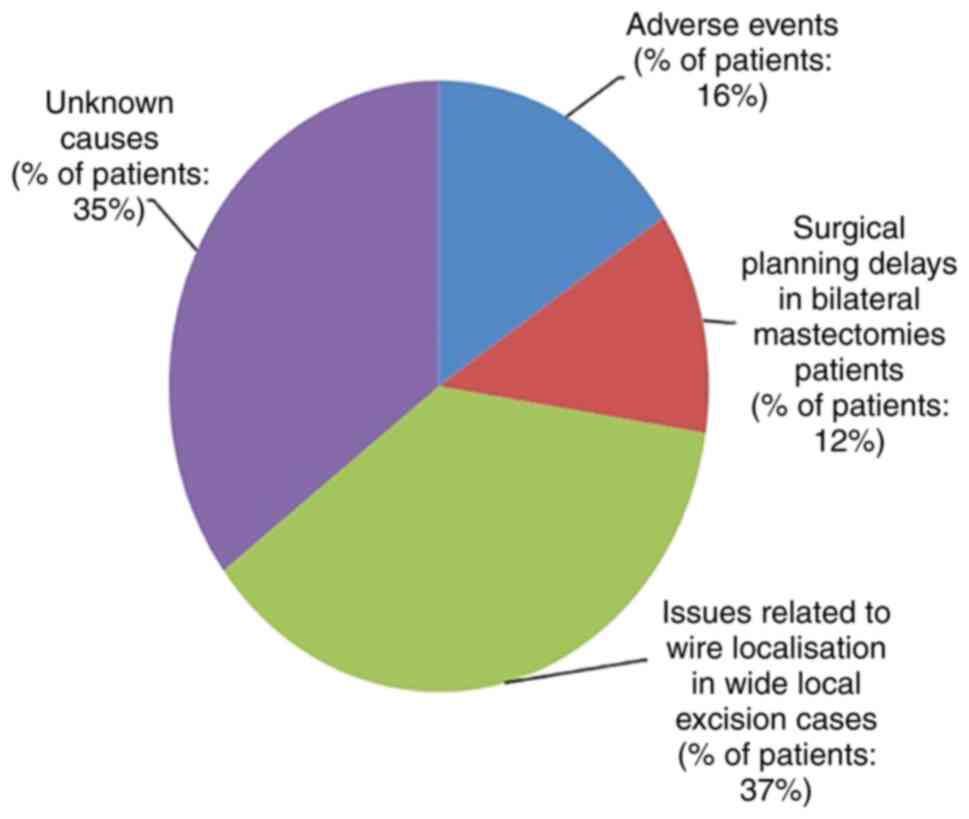
The causes for delays in time to surgery were
adverse events (16%), surgical planning delays in bilateral
mastectomy patients (12%), issues related to wire localization in
wide local excision cases (37%) and unknown causes (35%). The
adverse events noted were side-effects from chemotherapy and
persistent symptoms requiring additional imaging to rule out
metastasis. In total, three quarters of the non-conformed cases
with adverse events (75%) had surgery at ≥6 weeks following the
completion of the last cycle of neoadjuvant chemotherapy.
Furthermore, 37% of these cases waited >4 weeks on the waiting
list, resulting in further delays. The causes for surgical delays
in the non-conformed cases are illustrated in Fig. 2.
Time to surgery delays in bilateral mastectomy
patients were secondary to the need for multiple patient
consultations, such as genetic counselling, genetic testing and to
facilitate the patient's informed choice of reconstruction.
The cases with wire localised wide local excision
had surgery >6 weeks following the completion of their last
cycle of neoadjuvant chemotherapy in 53% of the cases and waited
>4 weeks after being added to the waiting list in 79% of cases,
resulting in further delays.
For cases undergoing wire guided wide local
excision, both a surgical theatre slot and a wire localisation
radiology appointment on the day of surgery is a necessity. This is
an important contributing factor for non-conformity in the present
cohort of patients. Wire localization procedures are only performed
by radiologists at Medway Hospital. This issue may have been
overcome by dedicated wire localization slots for these high-risk
patients. Some hospitals have trained radiographers taking over
these lists, offloading already strained consultant-led radiology
services.
A total of 35% of the non-conformity cases were due
to unknown causes. Over-booked clinics and long surgical waiting
lists and communication delays between oncologists and surgeons may
have contributed to the delays in these cases.
Achieving the target time to surgery interval of ≤28
days is inter-dependent on other factors, including an efficient
multidisciplinary approach and effective waiting list management.
Further studies with larger sample sizes are required to provide
insight into the ideal time to surgery following the completion of
neoadjuvant chemotherapy and its effect on survival outcomes.
Secondary audit standard 1
All cases should be discussed in surgical planning
multidisciplinary meetings prior to the completion of the last
cycle of neoadjuvant chemotherapy. Multidisciplinary meetings for
surgical planning should be ideally timed close to the completion
of the last cycle of chemotherapy. This will facilitate the
addition of these cases promptly to the waiting list and reduce
time to surgery delays. Mid-treatment multidisciplinary meetings in
non-responsive cases provides an opportunity to determine whether
surgery is an option. These discussions were usually planned by the
oncologists to expedite surgery in these unresponsive cases. The
policy is to discuss all neoadjuvant chemotherapy cases in
multidisciplinary meetings for surgical planning prior to the
completion of the last dose of chemotherapy and mid-treatment
multidisciplinary meetings are held for unresponsive cases.
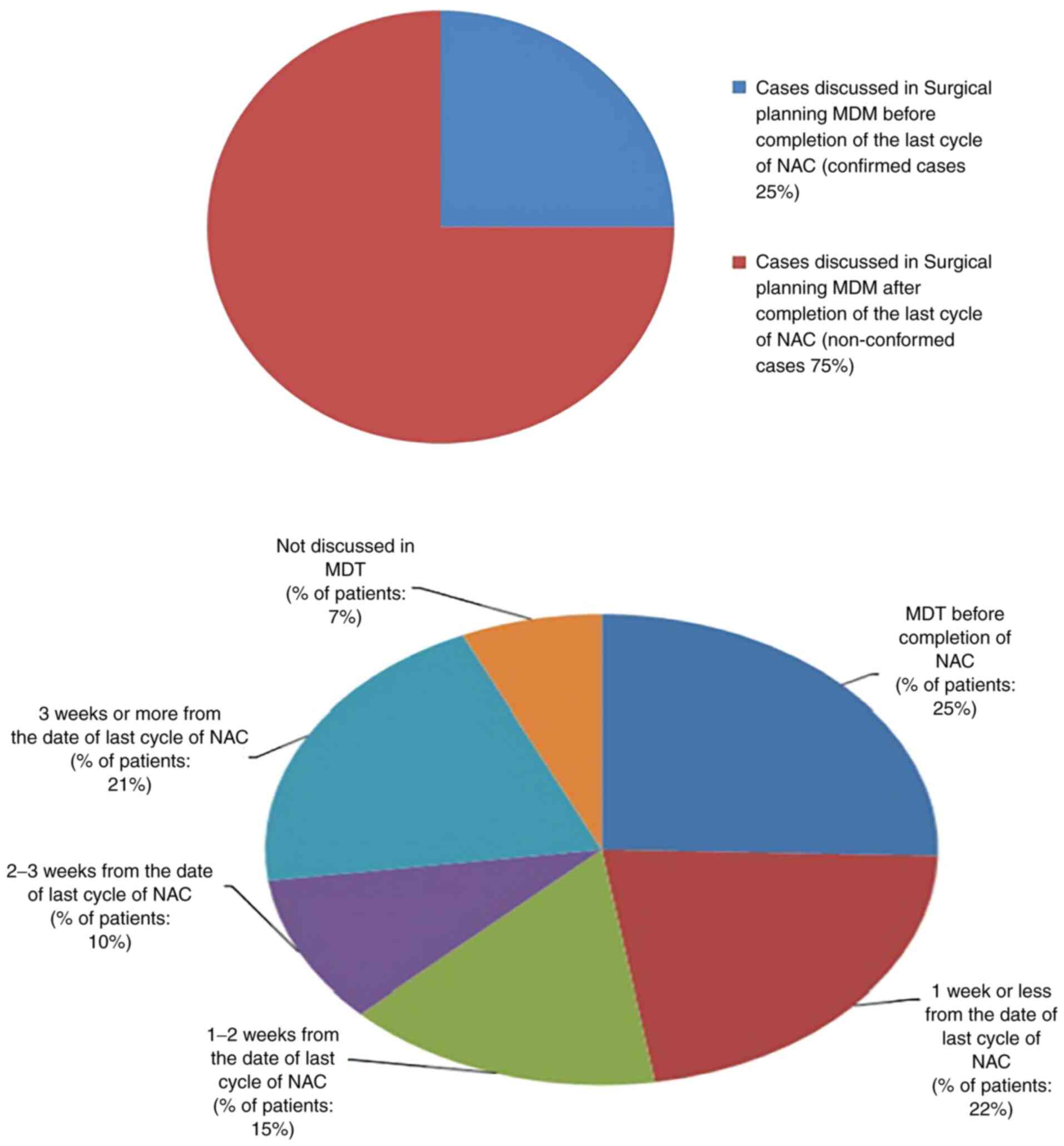
In the present audit, only 25% of the patients
conformed to this standard. Out of the 75% non-conforming cases, no
multidisciplinary meetings were held for 7% of the cases. These
cases were added to the waiting list directly from the clinic. No
apparent reason was found in these cases The details of
non-conformed cases (75%) are illustrated in Fig. 3
Secondary standard 2
All cases should be added to the waiting list prior
to the completion of last cycle of neoadjuvant chemotherapy. Adding
these high-risk patients to the waiting list soon after their
surgical planning multidisciplinary team meeting and effective
waiting list management will reduce the delays in time to surgery.
The unit policy is to add all neoadjuvant chemotherapy cases to the
waiting list prior to completion of the last cycle for effective
patient flow management. This is done in the next available breast
clinic by breast surgeons.
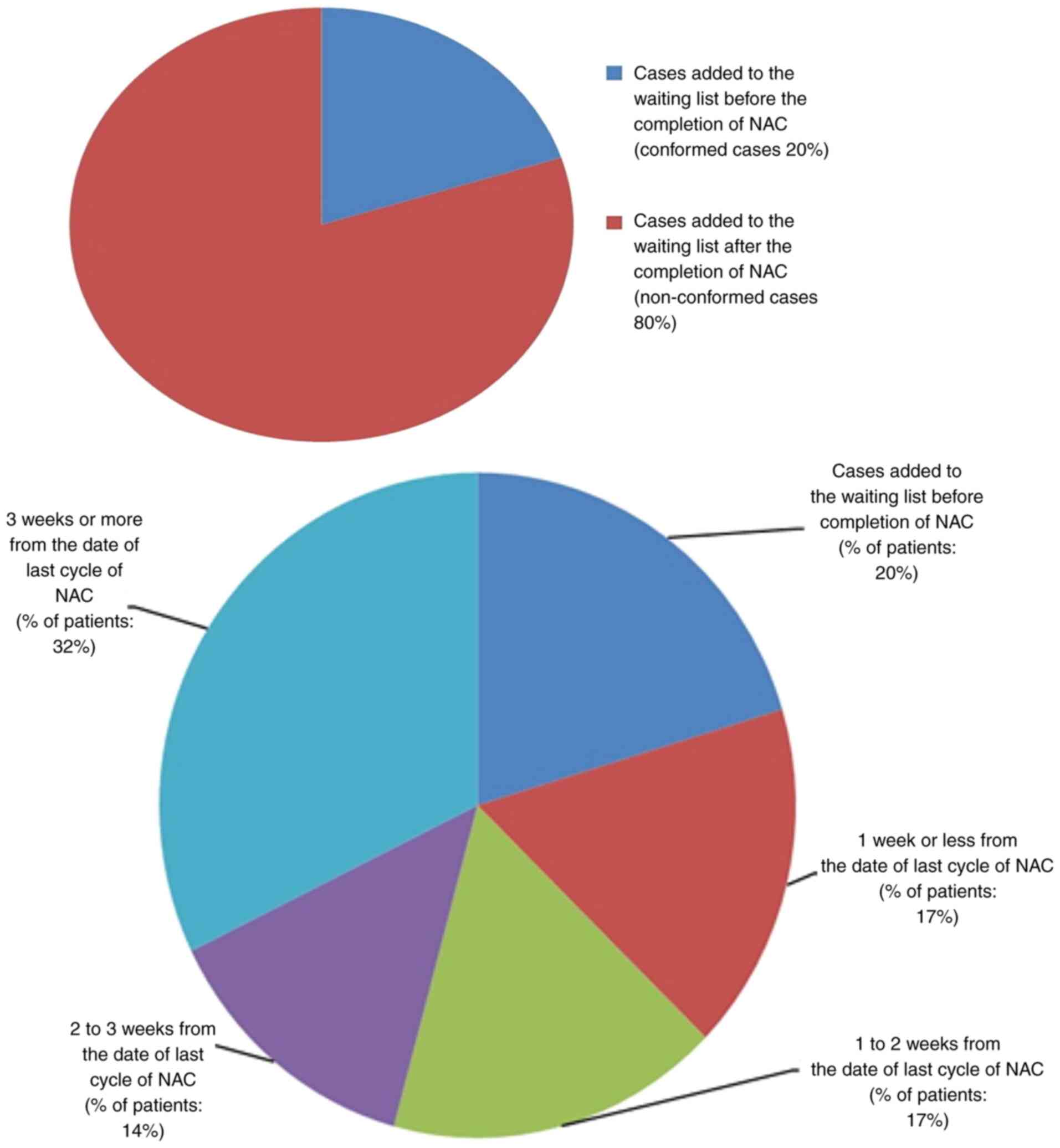
In the present audit, only 20% of patients conformed
to this standard. The details of non-conformed cases (80%) are
illustrated in Fig. 4.
Secondary standard 3
All cases should have surgery <4 weeks after
being added to the waiting list. The trust policy is to perform
surgery within 4 weeks after being added to the waiting list.
Effective waiting list management is a key element in reducing time
to surgery delays in this high-risk cohort of patients. This is
mainly performed by consultant secretaries and managerial staff.
Good communication is crucial for optimal outcomes. Maintaining
pooled surgical consultant waiting list and dedicated wire
localisation slots for neoadjuvant chemotherapy cases may reduce
time to surgery delays.
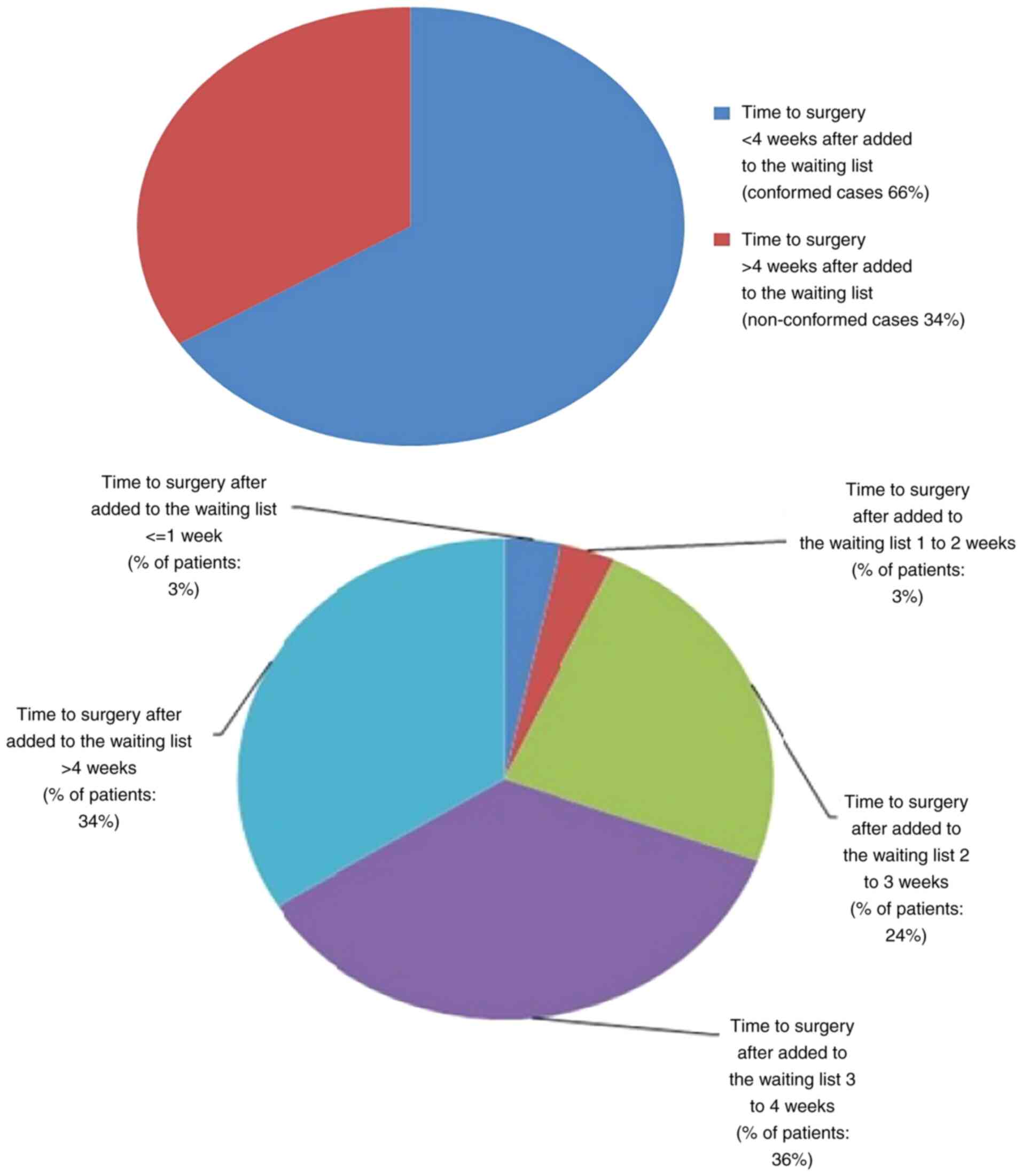
In the present audit, only 66% of patients conformed
to this standard; 34% of non-conformity cases were due to
oversubscribed surgical waiting lists and issues related to
overbooked wire localisation lists. Some of these non-conformity
cases also had delays being added to the waiting list, which
further contributed to the delays in time to surgery with on
obvious reason. These results are illustrated in Fig. 5.
The results of the present audit revealed very poor
conformity with the audit standards. Multidisciplinary coordination
appears to be the means with which to improve the conformity to the
standards for better patient outcomes of these high-risk patients.
These results were discussed in the Medway hospital NHS trust audit
and clinical governance meeting and MDT recommendations were drawn
to achieve the target of time to surgery of ≤28 days.
Secondary audit standard 4
All cases should have tumour coil insertion before
the commencement of the first cycle of NAC. Conformity to this
standard was 36%.
Secondary audit standard 5
All cases should have mid-chemotherapy imaging to
assess the tumour response Conformity to this standard was 98%.
Secondary audit standard 6
All cases should have post-NAC imaging to aid
surgical planning. Conformity to this standard was 30%.
Discussion
The timing of surgery following neoadjuvant
chemotherapy in breast cancer patients is paramount. There is
published evidence in the form of retrospective studies which
investigated the impact of time to surgery after neoadjuvant
chemotherapy on survival outcomes.
Sanford et al (5) suggested that those patients with
neoadjuvant chemotherapy to surgery intervals of up to 8 weeks had
equivalent overall survival, recurrence-free survival and loco
regional recurrence-free survival rates. Omarini et al
(6) concluded that breast cancer
patients who underwent surgery within 21 days experienced maximal
benefit from previous treatment and this advantage is consistent
and maintained over time.
Prolonged time to surgery intervals may
theoretically increase the risk of recurrence by allowing tumour
neo-angiogenesis and tumour growth. Some studies have suggested
that significant reductions in Ki-67 expression following
neoadjuvant chemotherapy have been shown to be associated with
decreased recurrence rates. For example, Gabordi et al
reported that patients who underwent surgery >40 days following
neoadjuvant chemotherapy had lesser reductions in Ki-67 levels,
potentially indicating tumour regrowth and predicting a worse
oncologic outcome (7).
Our breast multidisciplinary team at Medway hospital
agreed that the ideal time to surgery interval was ≤28 days which
will allow a reasonable time for recovery from the side-effects of
chemotherapy without compromising the survival outcomes.
Hence, the primary standard of this audit was
derived as ‘surgery should be performed within 28 days of
completion of last cycle of neoadjuvant chemotherapy’. Only 14% of
cases conformed with this standard. The secondary standards data
analysis suggested that these surgical delays were mainly due to
chemotherapy side-effects, persistent symptoms requiring scans,
surgical planning MDM delays, clinic appointments delays, and long
waiting lists. Unnecessary delays can affect the survival outcomes
of those high-risk patients. Following the presentation of the
results of this audit at the Medway hospital NHS trust audit and
clinical governance meeting, recommendations were made by the
breast multidisciplinary team and an action plan was
formulated.
Recommendations and Action plan
Medical oncologists need to ensure that coil
insertion is performed prior to the commencement of chemotherapy,
mid-chemotherapy imaging is performed to assess the tumour response
and imaging is performed following the penultimate cycle of
neoadjuvant chemotherapy; multidisciplinary meetings should also be
booked following the treatment scan results in order to aid
surgical planning in collaboration with radiologists and breast
surgeons.
Breast surgeons need to ensure the addition to the
waiting list following surgical planning multidisciplinary
meetings. Pooled waiting lists for all consultants may be helpful
for reducing the surgical waiting time to <28 days. Radiologists
also need to ensure dedicated slots on wire localization lists to
reduce further delays. The results of the present audit were
discouraging. Recommendations were drawn with the aim of
re-auditing following the implementation of the changes agreed upon
by the MDT.
In conclusion, achieving a time to surgery of <28
days following the completion of neoadjuvant chemotherapy is a
complex and challenging process, which is inter-dependent on a
variety of factors. An effective multidisciplinary approach with
efficient patient flow management and prioritising high-risk
patients is necessary to achieve this target. The team efforts of
oncologists, radiologists and breast surgeons will help to overcome
the challenges involved and to provide improved patient care.
Acknowledgements
The authors would like to extend their special
gratitude to Dr Maher Hadaki, Consultant medical oncologist, Medway
Hospital for providing access to the Trust oncology database and
for his valuable assistance during the data collection phase.
Funding
Funding: No funding was received.
Availability of data and materials
The availability of datasets used and/or analysed
during the current study are available only from the corresponding
author on reasonable request subjected to prior approval by the
Medway Hospital Ethics and clinical Governance Committee.
Authors' contributions
BSP was involved in the conception and design of the
audit, in data collection and analysis, and in the writing,
revising and reviewing of manuscript. DH, AK, CA and IA were
involved in the conception and design of the audit, and in the
revising and reviewing of the manuscript. BSP and IA confirm the
authenticity of all the raw data. All authors have read and
approved the final manuscript.
Ethics approval and consent to
participate
The present clinical audit was registered with the
Medway Hospitals Audit and Clinical Effectiveness Committee.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Deo SV, Bhutani M, Shukla NK, Raina V,
Rath GK and Purkayasth J: Randomized trial comparing neo-adjuvant
versus adjuvant chemotherapy in operable locally advanced breast
cancer (T4b N0-2 M0). J Surg Oncol. 84:192–197. 2003.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mauri D, Pavlidis N and Loannidis JP:
Neoadjuvant versus adjuvant systemic treatment in breast cancer: A
meta-analysis. J Natl Cancer Inst. 97:188–194. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Kupstas AR, Hoskin TL, Day CN, Habermann
EB and Boughey JC: Effect of surgery type on time to adjuvant
chemotherapy and impact of delay on breast cancer survival: A
National cancer database analysis. Ann Surg Oncol. 26:3240–3249.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Cai L, Tong Y, Zhu X, Shen K, Zhu J and
Chen X: Prolonged time to adjuvant chemotherapy initiation was
associated with worse disease outcome in triple negative breast
cancer patients. Sci Rep. 10(7029)2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Sanford RA, Lei X, Barcenas CH, Mittendorf
EA, Caudle AS, Valero V, Tripathy D, Giordano SH and
Chavez-MacGregor M: Impact of time from completion of neoadjuvant
chemotherapy to surgery on survival outcomes in breast cancer
patients. Ann Surg Oncol. 23:1515–1521. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Omarini C, Guaitoli G, Noventa S,
Andreotti A, Gambini A, Palma E, Papi S, Tazzioli G, Balduzzi S,
Dominici M, et al: Impact of time to surgery after neoadjuvant
chemotherapy in operable breast cancer patients. Eur J Surg Oncol.
43:613–618. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Gabordi RC, Huth J, Rivers A, Hendrix AA,
Wooldridge R, Leitch AM and Rao R: Optimal time interval to surgery
after neoadjuvant chemotherapy in breast cancer. Annual Meeting,
The American Society of Breast Surgeons, Las Vegas, NV, USA, April
30 - May 4, 2014. https://www.breastsurgeons.org/docs/resources/old_meetings/2014_Official_Proceedings_ASBrS.pdf.
|