Introduction
Adenomyosis is characterized by the presence of
ectopic endometrial glands and stroma located within the
hypertrophic and hyperplastic myometrium (1). Clinical manifestations are abnormal
uterine bleeding (AUB), dysmenorrhea and infertility; however,
approximately one third of these cases are completely asymptomatic
(2). Adenomyosis often co-exists
with endometriosis that causes dysmenorrhea and infertility
(3). Researchers have accumulated
clinical, histological and genetic, genomic, and proteomic data
supporting the pathogenesis of adenomyosis and endometriosis
(4). However, it remains
inconclusive as to whether adenomyosis and endometriosis are two
different diseases (2,5) or different phenotypes of a single
disease (3). The clinical
manifestations are similar between endometriosis and adenomyosis,
although adenomyosis is characterized by AUB (2,6).
Recently, some cases of life-threatening disseminated intravascular
coagulation (DIC) or thromboembolic events associated with
adenomyosis have been reported (7,8).
Empirically, giant adenomyosis is considered to cause massive
bleeding. On the other hand, such life-threatening events secondary
to endometriosis have not been observed to date, at least to the
best of the author's knowledge. Adenomyosis and endometriosis are
similar diseases; however, studying and elucidating the pathogenic
mechanisms of rare complications may provide insight into the
pathophysiology and etiology of adenomyosis. The purpose of the
present systematic review was to provide evidence of the clinical
features and risk factors of DIC or thromboembolism associated with
adenomyosis, and to explore the potential mechanisms of this rare
complication. The present systematic review is comprised of
sections focusing on ‘reviews of the existing literature on
adenomyosis-associated DIC or thromboembolism’ and ‘their
underlying mechanisms’. Finally, future directions on
diagnostic and treatment strategies based on the conclusions drawn
herein are discussed.
Data and methods
Search strategy and selection
criteria
A computerized literature search was performed to
identify relevant studies reported in the English language. The
study was conducted in accordance with the PRISMA guidelines
updated in 2020 (http://www.prisma-statement.org/) (9). The PubMed and Google Scholar
electronic databases were searched for studies published between
January, 2000 and November 2021, combining the following key words:
Adenomyosis, AUB, DIC and thromboembolism. As a result of the
PubMed search, there have been no reports of patients with
adenomyosis with DIC or thromboembolism prior to 2000; thus, the
search was performed for articles published after January, 2000.
The inclusion criteria were as follows: Publications of original
studies and review articles, and reference lists of the included
studies. The exclusion criteria were letters to the editor, poster
presentations, and literature unrelated to the research topic.
A two-step screening process was performed to obtain
eligible results. First, the PubMed search was conducted using
keywords with the following search combination: Group 1
(‘adenomyosis’ AND ‘abnormal uterine bleeding’), group 2
(‘adenomyosis’ AND ‘disseminated intravascular coagulation’) and
group 3 (‘adenomyosis’ AND ‘thromboembolism’). Second, the Google
Scholar search was performed using keywords with the following
search combination: (‘adenomyosis’, AND ‘thromboembolism’, OR
‘disseminated intravascular coagulation’). Given the heterogeneity
in the research theme, data from the studies were synthesized using
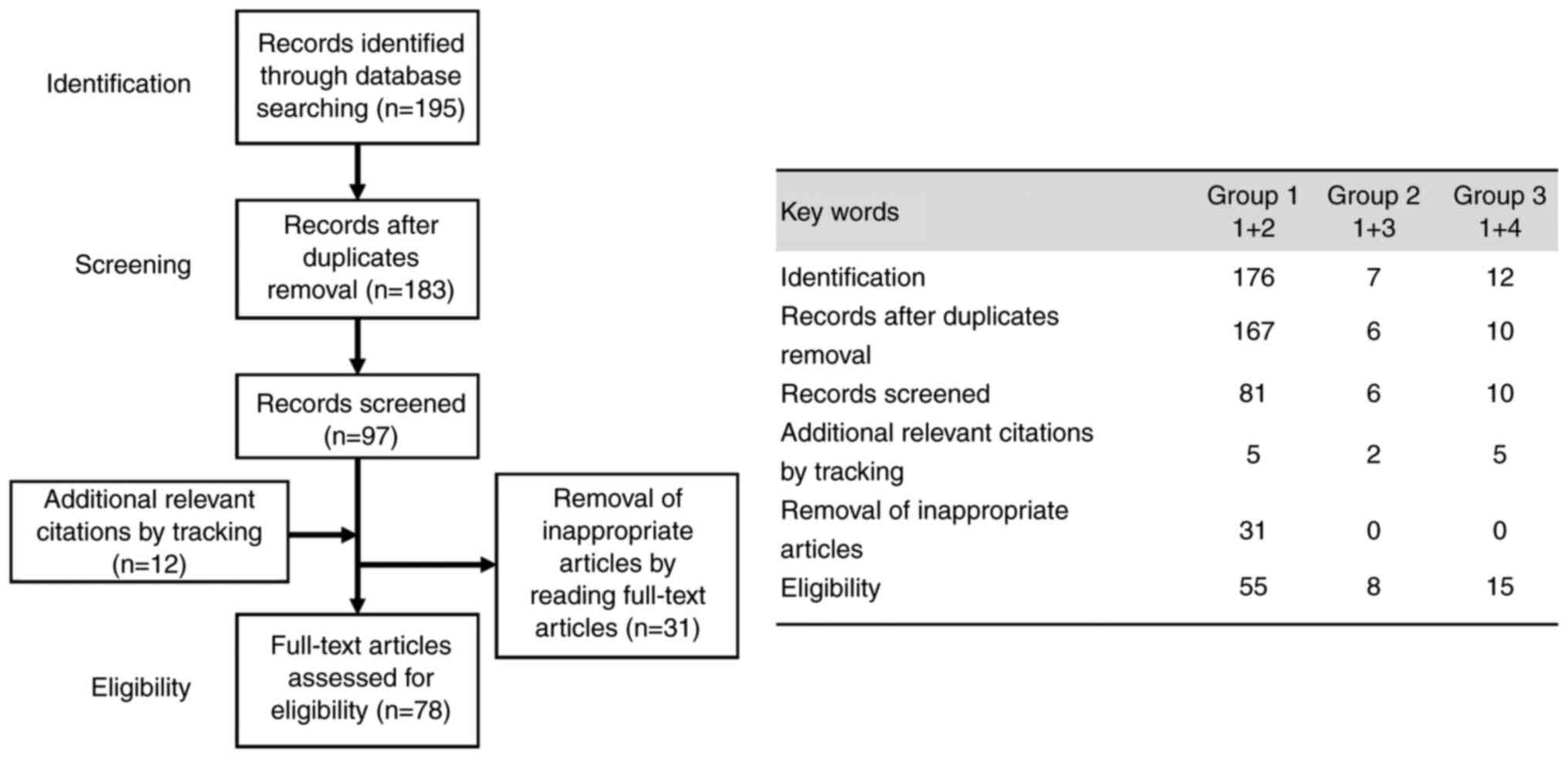
a descriptive review design with narrative methods. As illustrated
in Fig. 1, the first
identification phase included records identified through a database
search. Terms in the titles and abstracts were focused on in the
first screening stage. However, duplicates were removed during the
second screening phase, and titles, abstracts and full-text
articles were read to remove inappropriate articles. Citation
tracking was conducted to identify additional relevant citations.
The final eligibility phase included the full-text articles for
analysis after excluding those for which detailed data could not be
extracted. The last computerized literature search was conducted on
January 25, 2022.
Results
Selection of studies
The search in the PubMed database provided 195
literature citations (n=176 in group 1, n=7 in group 2, and n=12 in
group 3). Following the removal of overlaps, 183 records (n=167,
n=6 and n=10) were obtained, of which 86 were excluded, and 12
relevant articles (n=5, n=2 and n=5) were cited by the tracking of
references, and 78 (n=55, n=8 and n=15) met the inclusion and
exclusion criteria (Fig. 1).
Following a literature search on PubMed, eight records reporting 8
women with DIC and 13 records reporting 23 women with
thromboembolic complications were identified. Second, 64 records
met the eligibility criteria by key word searches on Google
Scholar. Of the 64 records, 47 records were excluded as their
content was not relevant to the study.
Reviews of the literature on
adenomyosis-associated massive AUB or DIC
Subsequently, reviews of the literature on patients
with adenomyosis with life-threatening massive AUB or DIC were
performed. The clinical manifestations, sites of bleeding events,
etiology, and risk factors are summarized in Table I. Some case reports on the topic
are presented below.
 | Table IReviews of the literature on patients
with adenomyosis-related massive abnormal uterine bleeding or
DIC. |
Table I
Reviews of the literature on patients
with adenomyosis-related massive abnormal uterine bleeding or
DIC.
| Authors | Yeara | Article title | Clinical
manifestations | The sites of
bleeding events | The etiology and
risk factors | (Refs.) |
|---|
| Nakamura et
al | 2002 | Acute disseminated
intravascular coagulation developed during menstruation in an
adenomyosis patient. | A case of
adenomyosis with acute DIC during menstruation. | Massive abnormal
uterine bleeding | Local hemorrhage,
blood vessel injury, and subsequent thrombosis in the adenomyosis
lesions may be associated with a rapid progression of DIC. | (14) |
| Son et
al | 2010 | Acute kidney injury
due. to menstruation-related disseminated intravascular coagulation
in an adenomyosis patient: A case report | A 40-year-old
woman. developed renal dysfunction associated with DIC after
receiving gonadotropins for ovulation induction therapy | Massive abnormal
uterine bleeding and DIC | The authors
reported a case of acute kidney injury resulting from
menstruation-related disseminated intravascular coagulation (DIC)
in a diffuse adenomyosis patients treated for primary infertility.
A 40-year-old woman who had received gonadotropin for ovulation
induction therapy developed renal dysfunction and DIC. DIC may be
triggered by the activation of the coagulation system due to
myometrial injury resulting from heavy intra-myometrial menstrual
flow by gonadotropins. In 2002, Nakamura et al (14) also reported that local hemorrhage,
blood vessel injury, and subsequent thrombosis in the myometrial
lesions of diffuse adenomyosis may play a crucial role in
pathophysiology of a rapid progression of DIC. | (13) |
| Ohashi et
al | 2011 | A case of anemia
with schistocytosis, thrombocytopenia, and acute renal failure
caused by adenomyosis. | After 6 months of
GnRH antagonist treatment for symptomatic adenomyosis, the patient
developed hemolytic anemia, DIC, and acute renal failure. DIC was
diagnosed by typical blood coagulation tests and clinical
information. | Massive abnormal
uterine bleeding. DIC was diagnosed by typical blood coagulation
tests and clinical information. Renal biopsy revealed DIC and
severe acute tubular necrosis. | Intramural bleeding
in diffuse adenomyosis lesions can cause vascular injury, TF
production, local microthrombosis, and ultimately DIC. | (15) |
| Yoo et
al | 2012 | Acute renal failure
induced by disseminated intravascular coagulopathy in a patient
with adenomyosis. | A case of diffuse
adenomyosis patient with DIC followed by acute renal failure. | Massive abnormal
uterine bleeding. | DIC is a major
contributing factor towards development of acute renal failure. The
patient was successfully treated with massive blood transfusion and
hysterectomy. | (11) |
| Zhang et
al | 2013 | Acute disseminated
intravascular coagulation developed after dilation and curettage in
an adenomyosis patient: A case report. | A rare case of
adenomyosis with acute DIC after dilation and curettage for missed
abortion. | Massive abnormal
uterine bleeding and DIC. Histological observation exhibited
hemorrhage, degeneration and necrosis in the myometrium of
adenomyosis lesions. | Uterus tissue
injury after curettage in adenomyosis patients accelerates
myometrium bleeding, degeneration, necrosis, microthrombus
formation, coagulation system activation, coagulation factor
depletion, and hyperfibrinolysis, which leads to severe
complications, such as DIC. | (12) |
| Nishino et
al | 2013 | Effective salvage
of acute massive uterine bleeding using intrauterine balloon
tamponade in a uterine adenomyosis patient on dienogest. | A case of a
37-year-old primigravida adenomyosis woman successfully treated
with an intrauterine balloon tamponade device to manage massive
uterine bleeding during dienogest therapy. | Massive abnormal
uterine bleeding. | The potential risk
of massive bleeding from the intramural fragile and permeable
vessels during dienogest treatment. Intrauterine balloon tamponade
is one of the options in managing severe uterine bleeding, avoiding
invasive surgical procedures, and maintaining fertility. | (16) |
| Yagi et
al | 2016 | Cardiac arrest due
to massive hemorrhage from uterine adenomyosis with leiomyoma
successfully treated with damage control resuscitation. | A case of
hemorrhagic shock and cardiopulmonary arrest occurred during
dienogest therapy for diffuse adenomyosis. | A life-threatening
massive abnormal uterine bleeding and DIC. | Treatment with
dienogest for giant adenomyosis resulted in DIC after heavy
menstrual bleeding. Surgical management can be recommended over
medical management for women with huge adenomyosis. | (18) |
| Yamanaka et
al | 2016 | Dysfunctional
coagulation and fibrinolysis systems due to adenomyosis is a
possible cause of thrombosis and menorrhagia. | Measurement of
specific biomarkers for coagulation and fibrinolysis in peripheral
blood of 8 patients with adenomyosis. | | Patients with
extensive adenomyosis with a uterus volume ≥100 cm3 are
at risk for hemorrhagic and thrombotic events during
menstruation. | (10) |
| Takamura et
al | 2020 | A case of
hemorrhagic shock occurred during dienogest therapy for uterine
adenomyosis. | A case of a
45-year-old woman required operative intervention for refractory
hemorrhagic shock occurred during dienogest therapy for
adenomyosis. | A life-threatening
massive abnormal uterine bleeding. | Imaging studies
revealed type I adenomyosis measuring 10 cm. The potential risk of
late bleeding from the intramural fragile and leaky vessels during
dienogest treatment in patients with subtype I adenomyosis. | (7) |
Yamanaka et al (10) examined the effects of adenomyosis
on the blood coagulation/fibrinolysis system during menstruation.
They demonstrated that the blood levels of markers of coagulation
and fibrinolysis [thrombin-antithrombin complex (TAT), soluble
fibrin (SF), D-dimer and plasmin-alpha 2-plasmin inhibitor complex
(PIC)] could increase during menstruation. Patients with extensive
adenomyosis may be potentially associated with the risk of
activation of coagulation and fibrinolysis during menstruation
(10). Yoo et al (11) also presented a case of a patient
with diffuse adenomyosis with DIC followed by acute renal failure.
Massive blood transfusion and hysterectomy were necessary to
achieve successful hemostasis (11). Zhang et al (12) reported a rare case of adenomyosis
with acute DIC following dilation and curettage for missed
abortion. Uterine tissue injury following dilation and curettage
for missed abortion can lead to the development of DIC through
tissue damage, bleeding, degeneration, necrosis, thrombus
formation, coagulation system activation, coagulation factor
depletion and hyperfibrinolysis (12). It could be managed successfully
with tranexamic acid, blood transfusions and subtotal hysterectomy
(12). Son et al (13) reported a case of acute kidney
injury resulting from menstruation-related DIC in a patient with
diffuse adenomyosis treated for primary infertility. A 40-year-old
woman who had received gonadotropin for ovulation induction therapy
developed renal dysfunction and DIC (13). DIC may be triggered by the
activation of the coagulation system due to myometrial injury
resulting from heavy intra-myometrial menstrual flow by
gonadotropins (13). In 2002,
Nakamura et al (14) also
reported that local hemorrhage, blood vessel injury and subsequent
thrombosis in the myometrial lesions of diffuse adenomyosis may
play a crucial role in the pathophysiology of a rapid progression
of DIC. Ohashi et al (15)
presented a case of a 51-year-old woman with adenomyosis with
hemolytic anemia, DIC and acute renal failure after 6 months of
gonadotrophin-releasing hormone (GnRH) antagonist treatment.
Nishino et al (16)
presented a case of a 37-year-old woman with nulliparous
adenomyosis who developed massive AUB during dienogest therapy.
Hemostasis was successfully achieved using balloon tamponade to
prevent severe uterine bleeding (16). AUB caused by dienogest may occur
from fragile and leaky endometrial vessels (17). Yagi et al (18) also presented a case of a
57-year-old woman with hemorrhagic shock and cardiopulmonary arrest
caused by massive AUB from diffuse adenomyosis. She had very large
adenomyosis with a diameter of 22x20x16 cm and was treated with
dienogest therapy (18). Moreover,
Takamura et al (7)
presented a case of a 45-year-old woman who necessitated a surgical
management (i.e., emergency hysterectomy) due to refractory
hemorrhagic shock occurred during dienogest therapy for
adenomyosis. The patient was commenced on dienogest following 6
months of GnRH antagonist. At 9 months after commencing dienogest
therapy, it caused a life-threatening massive AUB. Surgical
intervention successfully controlled massive AUB (7).
Taken together, eight cases of adenomyosis with DIC
have been reported since 2002. Patients with extensive adenomyosis
are sometimes at a risk of developing a life-threatening massive
AUB or DIC. Surgical procedures (e.g., dilatation and curettage)
and pharmacological interventions (e.g., gonadotropins and
progestin-only pill) can cause vascular injury, local
microthrombosis, systemic coagulopathy and hemostastic
abnormalities, and ultimately, life-threatening DIC and further
massive AUB (15,19). Several studies have reported the
risk of developing massive AUB in patients with adenomyosis treated
with a dienogest-based regimen (7,16-18,20).
Of note, even women with diffuse adenomyosis, but without overt
clinical manifestations already bear high levels of blood markers
reflecting the activation of coagulation and fibrinolysis (10). Abnormal blood vessels in diffuse
adenomyosis create a rich uterine vasculature and enter the eutopic
endometrium (17). Adenomyosis
creates an abnormal vascular structure and a network characterized
by fragile and leaky blood vessels (4,17,21).
Therefore, some diffuse adenomyosis, consisting of a large number
of immature blood vessels, may have a high risk of developing
DIC.
Reviews of the literature on
adenomyosis-associated thromboembolism
A search of the association between thromboembolic
events and adenomyosis was then performed, and the sites of
thromboembolic events, etiology and risk factors are reported. The
clinical information is summarized in Table II. Some case reports on the topic
are presented below.
 | Table IIReviews of the literature on patients
with adenomyosis-associated thrombosis. |
Table II
Reviews of the literature on patients
with adenomyosis-associated thrombosis.
| Authors | Yeara | Article title | Clinical
manifestations | The sites of
thromboembolic events | The etiology and
risk factors | (Refs.) |
|---|
| Yamashiro et
al | 2012 | Cerebral infarcts
associated with adenomyosis among middle-aged women. | Four cases of
adenomyosis women with concomitant acute cerebral infarction. | Multiple
thromboembolisms in the cerebral infarction, fingers, kidneys,
brachiocephalic trunk, and left subclavian artery. | Risk factors
associated with a hyperviscosity and hypercoagulability, including
increased tissue factor expression levels, increased mucinous tumor
marker levels, and menstruation-related coagulopathy, may be
associated with the development of systemic thromboembolism. | (24) |
| Yamashiro et
al | 2012 | Cerebral infarction
developing in a patient without cancer with a markedly elevated
level of mucinous tumor marker. | The patient
developed motor aphasia due to cerebral infarction. | Cerebral infarction
in the left frontal lobe and right parietal lobe. | Increased levels of
CA125 (1,750 U/ml) and D-dimer (6.0 µg/ml). | (25) |
| Lee et
al | 2014 | Uterine infarction
in a patient with uterine adenomyosis following biochemical
pregnancy. | Focal uterine
infarction after in vitro fertilization and embryo transfer
(IVF-ET) in a patient with adenomyosis following biochemical
pregnancy. | Focal uterine
infarction. | Women experiencing
biochemical abortion after an IVF-ET procedure. | (45) |
| Hijikata et
al | 2016 | Multiple cerebral
infarctions in a patient with adenomyosis on hormone replacement
therapy. | A case of multiple
cerebral infarctions in a woman with adenomyosis on hormone
replacement therapy. | Multiple cerebral
infarctions. | Elevated levels of
mucinous tumor markers such as CA125 (334.8 U/ml) are associated
with a hyperviscosity state. | (26) |
| Yamanaka et
al | 2016 | Dysfunctional
coagulation and fibrinolysis systems due to adenomyosis is a
possible cause of thrombosis and menorrhagia. | Measurement of
fibrinolysis-related proteins in peripheral blood of patients with
adenomyosis. | A history of
cerebral infarction or pulmonary thromboembolism. | Elevated levels of
thrombin-antithrombin complex (TAT), soluble fibrin (SF), D-dimer
(DD), and plasmin-alpha 2-plasmin inhibitor complex (PIC).
Adenomyosis patients with a uterus volume ≥100 cm3 are
at risk of having an activated coagulation system. | (10) |
| Kim et
al | 2018 | Cerebral infarcts
by nonbacterial thrombotic endocarditis associated with
adenomyosis. | A rare case of
multiple infarctions due to nonbacterial thrombotic endocarditis
that occurred in the setting of adenomyosis-related hypercoagulable
state. | Bilateral
cerebellum and the right precentral gyrus associated with
nonbacterial thrombotic endocarditis. | Elevated levels of
D-dimer, CA19-9, and CA125. | (28) |
| Yin et
al | 2018 | Cerebral infarcts
associated with adenomyosis: a rare risk factor for stroke in
middle-aged women: A case series. | Cases with
adenomyosis who developed acute ischemic stroke during
menstruation. | Multiple
infarctions in the right cerebellum and left temporal lobe. | Mucin
protein-related hyperviscosity and hypercoagulability due to
increased levels of CA125, CA19-9, and D-dimer. | (29) |
| Uchino et
al | 2018 | Nonbacterial
thrombotic endocarditis complicated by cerebral infarction in a
patient with adenomyosis with high serum CA125 level. | A 48-year-old
adenomyosis woman with multiple cerebral infarctions caused by
NBTE. | Multiple cerebral
infarctions. | A hyperviscosity
nature and hypercoagulable state induced by elevated levels of
CA125 (901 U/ml). | (30) |
| Aso et
al | 2018 | Recurrent multiple
cerebral infarctions related to the progression of adenomyosis: A
case report. | A 44-year-old woman
presented with headache, left hand weakness, and gait disturbances
during the menstrual phase. Imaging studies revealed multiple
thrombotic lesions in cortical and subcortical areas in the
cerebrum and cerebellum. She had a history of adenomyosis-related
heavy menstrual bleeding and infertility. Hysterectomy prevented
recurrence of the multiple cerebral infarctions. | Imaging studies
revealed multiple thrombotic lesions in cortical and subcortical
areas in the cerebrum and cerebellum. | A history of
adenomyosis-related heavy menstrual bleeding and infertility. | (46) |
| Okazaki et
al | 2018 | Cerebral infarction
associated with benign mucin-producing adenomyosis: report of two
cases. | Women with
adenomyosis aged 42 and 50 years old developed right hemiparesis
and aphasia caused by cerebral infarctions in the left cerebral
hemisphere. | Cerebral
infarctions in the left cerebral hemisphere. | Elevated levels of
CA125. Mucin-producing malignant and benign tumors such as
adenomyosis may cause hypercoagulability and cerebral
infarction. | (31) |
| Zhao et
al | 2020 | Acute cerebral
infarction with adenomyosis in a patient with fever: a case
report. | A 34-year-old
adenomyosis woman presented with headache, fever, and left limb
weakness during the menstrual phase. Imaging studies revealed acute
cerebral infarction. | Imaging studies
revealed acute cerebral infarction in right basal ganglia and
subcortical region of right frontotemporal lobe. | Elevated CA125
(937.70 U/ml), elevated D-dimer (27.4 mg/l), anemia, menstruation,
and fever. | (32) |
| Hong et
al | 2020 | Venous
thromboembolism and adenomyosis: a retrospective review | Adenomyosis with
menorrhagia | Case reports of
five patients who developed pulmonary thromboembolism and/or deep
vein thrombosis with adenomyosis. | There were no
clinicopathological differences between VTE and non-VTE
patients. | (22) |
| Aiura et
al | 2021 | Systemic
thromboembolism including multiple cerebral infarctions with middle
cerebral artery occlusion caused by the progression of adenomyosis
with benign gynecological tumor. | Adenomyosis woman
with heavy uterine bleeding presented with neurological symptoms
such as impaired consciousness. | Middle cerebral
artery occlusion and recurrent cerebral infarction. | Elevated levels of
CA125 and D-dimer. Similar to Trousseau's syndrome, adenomyosis may
cause systemic thromboembolism. | (27) |
| Kimura et
al | 2021 | Case of adenomyosis
causing the activation of the coagulation system after a complete
loss of endometrium following microwave endometrial ablation. | A case of
adenomyosis with abnormal coagulation testing after microwave
endometrial ablation. | The formation of
microhemorrhage within adenomyotic lesions affected coagulation and
fibrinolysis function. | Elevated levels of
thrombin-antithrombin complex (TAT) and soluble fibrin. Localized
microhemorrhage, tissue injury, and necrosis within adenomyotic
lesions during menstruation may increase the generation of
thrombin, leading to a hypercoagulable state. | (23) |
| Yasuda et
al | 2021 | Recurrent cerebral
infarcts associated with uterine adenomyosis: successful prevention
by surgical removal. | A 47-year-old
Japanese woman with uterine adenomyosis who developed multiple
cerebral infarcts during menstruation. | Multiple cerebral
infarcts. | Hysterectomy
prevented recurrence of cerebral infarct and thrombosis in a
patient with adenomyosis. | (8) |
Hong et al (22) reported 5 patients with adenomyosis
who developed pulmonary thromboembolism and/or deep venous
thrombosis. However, there were no significant differences in the
clinicopathological characteristics between VTE and non-VTE
patients; thus, it was unclear whether there was a causal
association between adenomyosis and thromboembolism (22). Some patients with adenomyosis with
a history of cerebral infarction or pulmonary thromboembolism have
been shown to have elevated levels of plasma TAT, SF, D-dimer and
PIC, suggesting an increased predisposition to thromboembolic
events (10). Kimura et al
(23) also presented a rare case
of adenomyosis causing the activation of the coagulation system
following a complete loss of the endometrium following microwave
endometrial ablation. The authors of that study suggested that the
formation of microhemorrhage within adenomyotic lesions ws
associated with elevated serum levels of the TAT complex and SF,
reflecting a hypercoagulable state (23). Yamashiro et al (24) reported four cases of middle-aged
women with adenomyosis with concomitant acute cerebral infarction.
In addition to cerebral infarction, imaging analyses revealed
multiple thromboembolisms in the fingers, kidneys, brachiocephalic
trunk and left subclavian artery (24). Increased tissue factor (TF)
expression levels, increased mucinous tumor marker levels (e.g.,
CA125 and CA19-9), and/or menstruation-related coagulopathy were
commonly observed in these patients (24). Cancer-associated thrombotic events
are well known as Trousseau's syndrome. However, no malignant
tumors other than severe adenomyosis were found. Certain benign
adenomyotic cells are associated with a hyperviscosity nature and
hypercoagulable state due to upregulation of mucinous tumor markers
and TF (24). Yamashiro et
al (25) reported a
42-year-old woman with adenomyosis who presented with acute
cerebral infarction in the left frontal lobe and right parietal
lobe with motor aphasia. A hyperviscosity nature and
hypercoagulable state may be associated with diffuse adenomyosis,
accompanied by the production of mucinous tumor marker, such as
CA125 (1750 U/ml) (25). Hijikata
et al (26) presented a
case of a 59-year-old woman with adenomyosis with multiple cerebral
infarctions. She had long been maintained on combined
estrogen-progestin hormone replacement therapy for 10 years for the
treatment of menopausal symptoms (26). A laboratory examination revealed an
elevated serum CA125 level (334.8 U/ml) (26). Aiura et al (27) reported the case of a 48-year-old
woman with middle cerebral artery occlusion and recurrent cerebral
infarction caused by adenomyosis progression. She was successfully
treated with catheter-directed mechanical thrombectomy and by
hysterectomy (27). Kim et
al (28) reported a case of
multiple infarctions, including the bilateral cerebellum and the
right precentral gyrus associated with non-bacterial thrombotic
endocarditis (NBTE) in a patient with adenomyosis. NBTE is
associated with a hypercoagulable state and an inflammatory
response and often accompanies cancer (28). An adenomyosis-related
hypercoagulable state can lead to multiple infarctions with NBTE.
Yin et al (29) summarized
three cases with adenomyosis who developed acute ischemic stroke
during menstruation. These patients were also accompanied by NBTE
(29). Elevated levels of CA125,
CA19-9 and D-dimer have been observed only during menstruation
(29). Mechanisms, such as mucin
protein-related hyperviscosity and hypercoagulability increase the
risk of thrombosis formation (29).
Taken together, 18 cases of adenomyosis with
thromboembolisms have been reported since 2012. The number of
reported cases is limited (24);
however, adenomyosis causes serious thromboembolism, including
multiple cerebral infarctions. Diffuse adenomyosis, elevated levels
of mucinous tumor markers, CA125 and CA19-9, and coexistence of
NBTE may be at increased risk of developing thromboembolism
(23-32).
Mechanisms underlying the pathogenesis
of DIC and thromboembolism in adenomyosis
Heavy menstrual bleeding is one of the most common
clinicopathological characteristics of women with adenomyosis
(33). Although adenomyosis and
endometriosis are both characterized by ectopic endometrial glands
and share a similar clinical presentation, patients with
adenomyosis have a unique symptom, such as AUB and
hypercoagulability. Common patterns of aberrant gene expression,
including KRAS mutations, increased estrogen biosynthesis,
progesterone resistance and inflammation, have been reported both
in adenomyosis and endometriosis (34), suggesting that gene expression
profiles are similar in the early stages of disease onset. However,
endometrial cells in adenomyosis markedly alter the gene expression
patterns during adapting to ever-changing host environments, such
as tissue injury, repair, and remodeling. Xiaoyu et al
(5) identified the patterns of
differentially expressed proteins between adenomyosis and
endometriosis using a proteomic approach coupled using mass
spectrometry. The proteomics analysis revealed that the most
significantly enriched protein pathway in adenomyosis was
coagulation cascades, while endometriosis was tightly associated
with chronic inflammation (5).
This indicates that the coagulation system is closely involved in
the pathophysiology of established adenomyosis. As evidence,
patients with adenomyosis during menstruation may have laboratory
abnormalities associated with hypercoagulability and excessive
hyperfibrinolysis (10,23). Elevated TAT, SF, D-dimer and PIC
levels in patients with adenomyosis reflect a hypercoagulable and
hyperfibrinolytic condition (10,23).
In addition, patients with adenomyosis had prolonged activated
partial thromboplastin time and a shortened thrombin time than
those with uterine fibroids, indicating that adenomyosis affects
the hemostatic system (19). These
hemostasis abnormalities suggest a potential anti- or
pro-thrombotic state in this pathology and predispose patients to
bleeding and thrombotic complications.
In addition, adenomyosis is a progressive disease
involving pathological angiogenesis and, unlike normal uterine
vessels, increases uterine vascularity due to the abundant blood
vessels penetrating within the myometrium (6,35).
Recently, Stratopoulou et al (36) reported that the total number of
adenomyotic vessels was significantly higher in lesions than in the
healthy endometrium, and fewer vessels were surrounded by α-smooth
muscle actin. This suggests structural abnormalities of the normal
vasculature that is composed of endothelial cells, smooth muscle
cells and fibroblasts. Therefore, the vasculature of adenomyosis is
morphologically abnormal and characterized by the development of
leaky, fragile and easily rupturing new vessels (6). Similarly, the tumor vasculature is
also characterized by immature, leaky, tortuous, dilated and
fragile vessels, and the loss of hierarchical architecture and is
known to cause spontaneous hemorrhages (8). These data indicate that the blood
vessel formation of adenomyosis may be regulated through a
mechanism similar to the carcinogenesis theory, affecting
angiogenesis and vasculogenesis (6). Indeed, increased microvascular
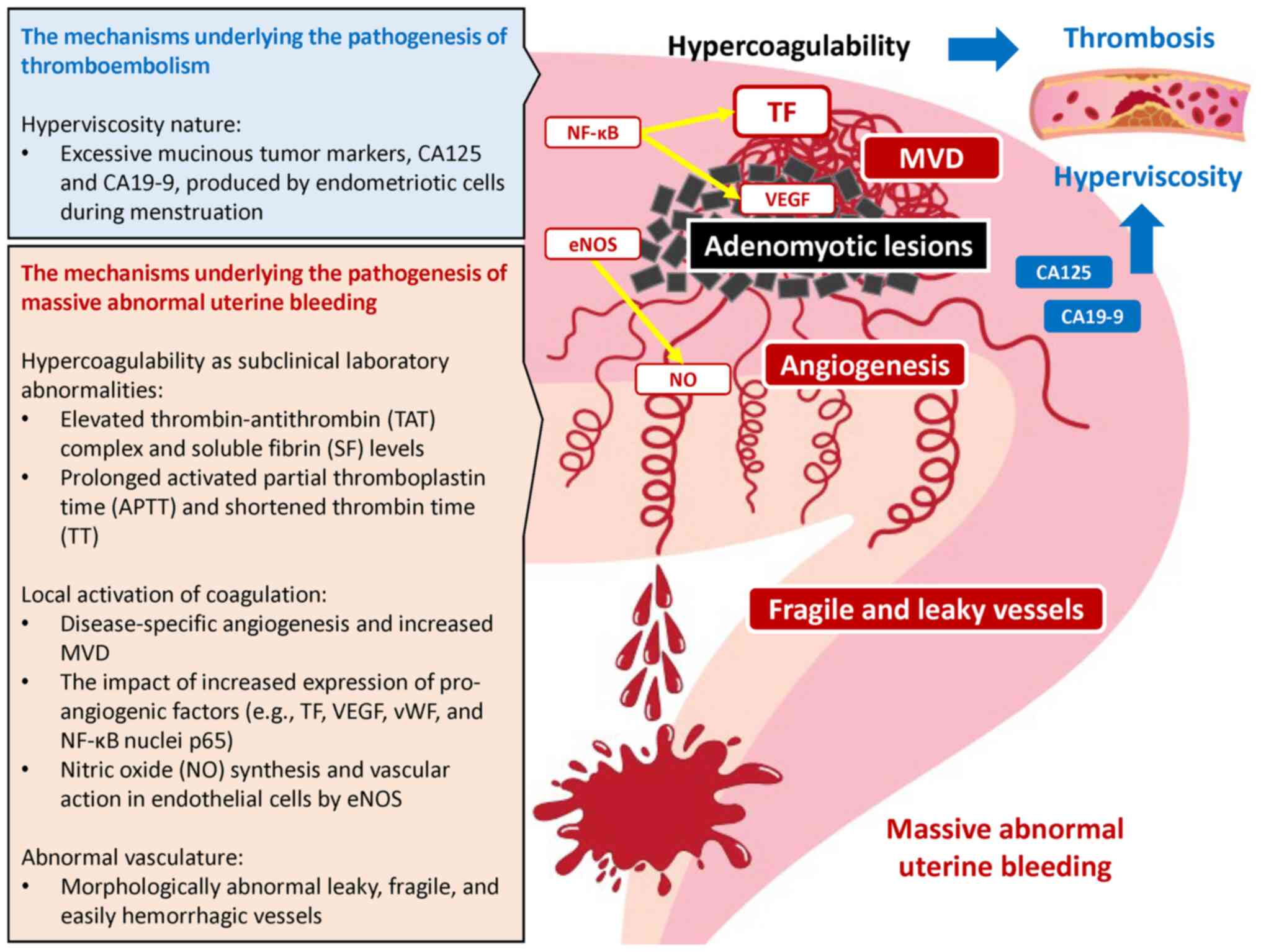
density (MVD) and higher vascular endothelial growth factor (VEGF)
expression have been shown in the endometrium of patients with
adenomyosis as compared with the normal endometrium of women
without disease (6) (Fig. 2). Pro-angiogenic markers [e.g., TF,
VEGF, von Willebrand factor and nuclear factor-κB (NF-κB)] have
also been shown to be higher in the adenomyosis than the control
group, and to be positively associated with the amount of bleeding
(6,37). The angiogenesis-related genes and
their multiple signal pathways, such as VEGF, TF, matrix
metalloproteinase (MMP)-2, MMP-9 and cyclooxygenase-2 are
downstream targets for NF-κB in adenomyosis (6,38).
NF-κB regulates the expression of a number of molecules and
pathways responsible for angiogenesis, cell invasion,
proliferation, anti-apoptosis and impaired cytokine expression
(6). The abnormal dysregulation of
NF-kB is considered to be a hallmark of adenomyosis (6). Furthermore, endothelial nitric oxide
synthase (eNOS) is highly expressed in the endometrial and
myometrial tissues of women with adenomyosis-related heavy
menstrual bleeding (39,40). Nitric oxide (NO) synthesized in
endothelial cells by eNOS may cause AUB possibly through the
vascular relaxation and platelet aggregation inhibition (39,40).
Therefore, repeated microbleeding episodes from fragile and more
permeable vessels within the adenomyosis lesions may trigger
activation of the TF and VEGF-dependent coagulation pathways
through tissue damage (10,14,29).
Furthermore, the persistent activation of the coagulation cascade
can lead to massive AUB and life-threatening DIC.
On the other hand, endometriotic cells that are
abundant in diffuse adenomyosis produce excessive CA125 and CA19-9
during menstruation (10,25,29).
Indeed, markedly elevated levels of CA125 and CA19-9 hare detected
most often in patients with adenomyosis who develop multiple
thromboembolisms during menstruation (10,25,29).
Members of the mucin family glycoproteins, CA125 and CA19-9, are
relatively large molecules that can increase blood viscosity. The
entry of CA125 and CA19-9 into the systemic circulation leads to
blood hyperviscosity (10,25,29).
The hyperviscosity is a risk factor for hypercoagulability and
predisposes patients to thrombosis. Therefore, elevated levels of
these tumor markers may be associated with an increased risk of the
development of thrombosis. Furthermore, the hypercoagulable state
and the hyperviscosity nature are likely to be associated with a
risk of the development of NBTE (41). The ability of diagnosis and
management of adenomyosis-related hemostasis abnormalities is
important in reducing life-threatening complications, such as DIC
and thromboembolism.
Discussion
The present systematic review summarizes the
clinical features, risk factors and potential mechanisms of severe
hemorrhagic and thrombotic events associated with adenomyosis. DIC
and thromboembolism are rare, yet life-threatening complications in
adenomyosis. The clinical characteristics and risk factors for
adenomyosis-associated DIC and thromboembolism are summarized in
Table III. That is, clinical
features, such as adenomyosis phenotypes (e.g., diffuse or type 1)
may increase the risk of developing severe hemorrhagic events,
while a marked increase in mucinous tumor markers may confer the
risk of developing severe thrombotic events. In addition,
adenomyosis-specific abnormal blood vessels can cause
thrombo-hemorrhagic events, but they are clinically undetectable
until surgical procedures are performed. More specifically, the
pathophysiology of adenomyosis-associated thrombo-hemorrhagic
events could be related to disease-specific endogenous risk factors
and exogenous factors such as current therapeutic strategies.
Examples of the former include changes in the gene and protein
expression patterns related to the coagulation and fibrinolysis
systems, abnormal vascular distribution and network formation, a
marked elevation in serum mucinous tumor markers, and subtypes of
adenomyosis, while examples of the latter include treatment with
progestin-only pill or dilation and curettage for abortion. In
particular, the extent and subtype of adenomyosis lesions are the
most clinically influential factors in predicting the onset.
Adenomyosis is composed of multiple heterogeneous subtypes. Types I
(intrinsic) and II (extrinsic) consist of adenomyosis that occurs
in the uterine inner and outer layer, respectively (42). Immature and fragile blood vessels
penetrate the endometrial-myometrial barrier in type I adenomyosis
(17). Moreover, Turner et
al (43) suggested that
‘impaired venous drainage and endometrial vascular ectasia,
secondary to increased intramural pressure’ can cause AUB in
diffuse adenomyosis. Therefore, adenomyosis-specific angiogenesis,
increased MVD and morphologically abnormal blood vessels with leaky
and fragile features are considered to cause vascular damage,
leading to the extravasation of blood, damage to surrounding
tissue, and subsequently, to thrombo-hemorrhagic events (43). In patients with diffuse or type I
adenomyosis, surgical procedures, such as dilatation and curettage
for abortion and pharmacological interventions, such as
gonadotropins and progestin-only pill, may be a potential risk
factor for massive hemorrhage and life-threatening DIC.
Additionally, markedly elevated levels of mucinous tumor markers,
CA125 and CA19-9 during menstruation may pose as risk factors for
thromboembolism. The fragile blood vessels in adenomyosis can lead
to massive uterine bleeding during menstruation, while locally
produced pro-angiogenic factors, coagulation-related factors and
mucins may cause hypercoagulability and hyperviscosity, leading to
thrombosis. The clinical manifestations are secondary to different
conditions, such as structural vascular abnormality, a
hyperviscosity state, or a hypercoagulable state, and range from
asymptomatic and sub-clinical illness to severe, life-threatening
DIC and thromboembolism.
 | Table IIIThe clinical characteristics and risk
factors for adenomyosis-associated DIC and thromboembolism. |
Table III
The clinical characteristics and risk
factors for adenomyosis-associated DIC and thromboembolism.
| Clinical
characteristics | Risk factors and
pathophysiology |
|---|
|
Adenomyosis-associated DIC |
| Massive AUB (from
heavy menstrual bleeding to life-threatening DIC) | Diffuse adenomyosis
or subtype I adenomyosis |
| | Treatment with
dienogest or gonadotropins |
| | Dilation and
curettage for missed abortion |
| | Elevated
pro-angiogenic markers (e.g., TF, VEGF, vWF, and NF-κB) |
| | Leaky, fragile, and
easily hemorrhagic vessels |
|
Adenomyosis-associated
thromboembolism |
| Similar to
Trousseau's syndrome | Diffuse
adenomyosis |
| More often
associated with multiple cerebral infarctions | A hyperviscosity
nature due to elevated levels of mucinous tumor markers, CA125 and
CA19-9 |
| | Local hemorrhage in
the adenomyosis lesions followed by a hypercoagulable state through
activation of the TF and |
| | VEGF-dependent
coagulation pathways |
| | The coexistence of
non-bacterial thrombotic endocarditis (NBTE) |
Finally, based on the present systematic review,
future directions of diagnostic and therapeutic strategies for the
field are explored. As a first step towards clinical diagnosis, it
is crucial to distinguish focal and diffuse adenomyosis or type I
and type II adenomyosis through the assessment of transvaginal
ultrasound. In patients with type I adenomyosis, the damaged
microvessels are contiguous with endometrial stromal cells at the
inner myometrium and endometrium (17). Blood vessels in patients with
diffuse adenomyosis demonstrate a morphologically and functionally
abnormal phenotype that includes leaky, fragile and easily
rupturing vessels (6). Therefore,
type I and diffuse adenomyosis may be associated with severe
unpredictable bleeding (17).
Microvascular damage induced by surgical procedures or hormonal
treatment in these patients can contribute to excessive bleeding.
Moreover, CA125 and CA19-9 are mucin glycoproteins produced by
endometrial cells. Diffuse adenomyosis is particularly rich in
endometrial cells and is considered to be more likely to produce
these mucins. Elevated levels of mucin protein (CA125 and
CA19-9)-related hyperviscosity and hypercoagulability increases the
risk of thromboembolism in women affected by extensive adenomyosis
(24,29). Additionally, estrogen is generally
considered to induce the activation of coagulation (e.g., factors
II, VII, IX, X and XII, and protein C) and fibrinolysis (e.g.,
plasminogen) genes that play roles in coagulation, fibrinolysis and
inflammation (44). However, it is
currently unknown when and how these genes and proteins are
regulated in adenomyosis lesions. Collectively, patients with
diffuse or type I adenomyosis may develop AUB/DIC or
thromboembolism; thus, specific attention should be paid to
surgical and hormonal therapy.
Acknowledgements
The author would like to thank Mrs. Toyomi Kobayashi
(Clef Co., Ltd., Nara, Japan) for generating all the figures.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Author's contributions
HK performed all of the following regarding the
preparation of this manuscript: Conception and design, acquisition
of data, analysis and interpretation of data, and writing the
manuscript. HK confirms the authenticity of all the raw data. The
author has read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Senturk LM and Imamoglu M: Adenomyosis:
What is new? Womens Health (Lond). 11:717–724. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Lacheta J: Uterine adenomyosis:
Pathogenesis, diagnostics, symptomatology and treatment. Ceska
Gynekol. 84:240–246. 2019.PubMed/NCBI
|
|
3
|
Maruyama S, Imanaka S, Nagayasu M, Kimura
M and Kobayashi H: Relationship between adenomyosis and
endometriosis; Different phenotypes of a single disease? Eur J
Obstet Gynecol Reprod Biol. 253:191–197. 2020.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Kobayashi H, Matsubara S and Imanaka S:
Relationship between magnetic resonance imaging-based
classification of adenomyosis and disease severity. J Obstet
Gynaecol Res. 47:2251–2260. 2021.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Xiaoyu L, Weiyuan Z, Ping J, Anxia W and
Liane Z: Serum differential proteomic analysis of endometriosis and
adenomyosis by iTRAQ technique. Eur J Obstet Gynecol Reprod Biol.
182:62–65. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Harmsen MJ, Wong CFC, Mijatovic V,
Griffioen AW, Groenman F, Hehenkamp WJK and Huirne JAF: Role of
angiogenesis in adenomyosis-associated abnormal uterine bleeding
and subfertility: A systematic review. Hum Reprod Update.
25:647–671. 2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Takamura M, Koga K, Harada M, Hirota Y,
Fujii T and Osuga Y: A case of hemorrhagic shock occurred during
dienogest therapy for uterine adenomyosis. J Obstet Gynaecol Res:
Oct 8, 2020 doi: 10.1111/jog.14519 (Epub ahead of print).
|
|
8
|
Yasuda M, Yamanaka Y, Kano H, Araki N,
Ishikawa H, Ikeda JI and Kuwabara S: recurrent cerebral infarcts
associated with uterine adenomyosis: Successful prevention by
surgical removal. Intern Med. 61:735–738. 2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Page MJ, Moher D, Bossuyt PM, Boutron I,
Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: PRISMA 2020 explanation and elaboration: Updated
guidance and exemplars for reporting systematic reviews. BMJ.
372(n160)2021.PubMed/NCBI View
Article : Google Scholar
|
|
10
|
Yamanaka A, Kimura F, Yoshida T, Kita N,
Takahashi K, Kushima R and Murakmai T: Dysfunctional coagulation
and fibrinolysis systems due to adenomyosis is a possible cause of
thrombosis and menorrhagia. Eur J Obstet Gynecol Reprod Biol.
204:99–103. 2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Yoo HJ, Chang DS and Lee KH: Acute renal
failure induced by disseminated intravascular coagulopathy in a
patient with adenomyosis. J Obstet Gynaecol Res. 38:593–596.
2012.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Zhang J, Xiao X, Luo F, Shi G, He Y, Yao Y
and Xu L: Acute disseminated intravascular coagulation developed
after dilation and curettage in an adenomyosis patient: A case
report. Blood Coagul Fibrinolysis. 24:771–773. 2013.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Son J, Lee DW, Seong EY, Song SH, Lee SB,
Kang J, Yang BY, Lee SJ, Choi JR, Lee KS and Kwak IS: Acute kidney
injury due to menstruation-related disseminated intravascular
coagulation in an adenomyosis patient: A case report. J Korean Med
Sci. 25:1372–1374. 2010.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Nakamura Y, Kawamura N, Ishiko O and Ogita
S: Acute disseminated intravascular coagulation developed during
menstruation in an adenomyosis patient. Arch Gynecol Obstet.
267:110–112. 2002.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Ohashi N, Aoki R, Shinozaki S, Naito N and
Ohyama K: A case of anemia with schistocytosis, thrombocytopenia,
and acute renal failure caused by adenomyosis. Intern Med.
50:2347–2350. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nishino K, Hayashi K, Chaya J, Kato N and
Yamamuro O: Effective salvage of acute massive uterine bleeding
using intrauterine balloon tamponade in a uterine adenomyosis
patient on dienogest. J Obstet Gynaecol Res. 39:738–741.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Matsubara S, Kawaguchi R, Akinishi M,
Nagayasu M, Iwai K, Niiro E, Yamada Y, Tanase Y and Kobayashi H:
Subtype I (intrinsic) adenomyosis is an independent risk factor for
dienogest-related serious unpredictable bleeding in patients with
symptomatic adenomyosis. Sci Rep. 9(17654)2019.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Yagi T, Fujita M, Inoue T, Otsuji M, Koga
Y, Nakahara T, Miyauchi T, Kaneda K, Oda Y and Tsuruta R: Cardiac
arrest due to massive hemorrhage from uterine adenomyosis with
leiomyoma successfully treated with damage control resuscitation.
Acute Med Surg. 3:388–391. 2016.PubMed/NCBI View
Article : Google Scholar
|
|
19
|
Zhang DY, Peng C, Zhou YF, Huang Y and
Song H: Changes of coagulation function in patients with
adenomyosis and its clinical significance. Zhonghua Fu Chan Ke Za
Zhi. 55:749–753. 2020.PubMed/NCBI View Article : Google Scholar : (In Chinese).
|
|
20
|
Nagata C, Yanagida S, Okamoto A, Morikawa
A, Sugimoto K, Okamoto S, Ochiai K and Tanaka T: Risk factors of
treatment discontinuation due to uterine bleeding in adenomyosis
patients treated with dienogest. J Obstet Gynaecol Res. 38:639–644.
2012.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Kobayashi H, Matsubara S and Imanaka S:
Clinicopathological features of different subtypes in adenomyosis:
Focus on early lesions. PLoS One. 16(e0254147)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Hong EY, Lin HZ and Fong YF: Venous
thromboembolism and adenomyosis: A retrospective review. Gynecol
Minim Invasive Ther. 9:64–68. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kimura F, Hanada T, Nakamura A, Amano T,
Tsuji S, Kita N and Murakami T: Case of adenomyosis causing the
activation of the coagulation system after a complete loss of
endometrium following microwave endometrial ablation. J Obstet
Gynaecol Res. 47:3385–3391. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Yamashiro K, Tanaka R, Nishioka K, Ueno Y,
Shimura H, Okuma Y, Hattori N and Urabe T: Cerebral infarcts
associated with adenomyosis among middle-aged women. J Stroke
Cerebrovasc Dis. 21:910.e1–e5. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yamashiro K, Furuya T, Noda K, Urabe T,
Hattori N and Okuma Y: Cerebral infarction developing in a patient
without cancer with a markedly elevated level of mucinous tumor
marker. J Stroke Cerebrovasc Dis. 21:619.e1–e2. 2012.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Hijikata N, Sakamoto Y, Nito C, Matsumoto
N, Abe A, Nogami A, Sato T, Hokama H, Okubo S and Kimura K:
Multiple cerebral infarctions in a patient with adenomyosis on
hormone replacement therapy: A case report. J Stroke Cerebrovasc
Dis. 25:e183–e184. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Aiura R, Nakayama S, Yamaga H, Kato Y and
Fujishima H: Systemic thromboembolism including multiple cerebral
infarctions with middle cerebral artery occlusion caused by the
progression of adenomyosis with benign gynecological tumor: A case
report. BMC Neurol. 21(14)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Kim B, Kim SH and Kim T: Cerebral infarcts
by nonbacterial thrombotic endocarditis associated with
adenomyosis: A case report. J Stroke Cerebrovasc Dis. 27:e50–e53.
2018.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Yin X, Wu J, Song S, Zhang B and Chen Y:
Cerebral infarcts associated with adenomyosis: A rare risk factor
for stroke in middle-aged women: A case series. BMC Neurol.
18(213)2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Uchino K, Shimizu T, Mizukami H, Isahaya
K, Ogura H, Shinohara K and Hasegawa Y: Nonbacterial thrombotic
endocarditis complicated by cerebral infarction in a patient with
adenomyosis with high serum CA125 level; A case report. J Stroke
Cerebrovasc Dis. 27:e42–e45. 2018.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Okazaki K, Oka F, Ishihara H and Suzuki M:
Cerebral infarction associated with benign mucin-producing
adenomyosis: Report of two cases. BMC Neurol.
18(166)2018.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhao Y, Zhang Y and Yang Y: Acute cerebral
infarction with adenomyosis in a patient with fever: A case report.
BMC Neurol. 20(210)2020.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Davies J and Kadir RA: Endometrial
haemostasis and menstruation. Rev Endocr Metab Disord. 13:289–299.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Bulun SE, Yildiz S, Adli M and Wei JJ:
Adenomyosis pathogenesis: Insights from next-generation sequencing.
Hum Reprod Update. 27:1086–1097. 2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Cunningham RK, Horrow MM, Smith RJ and
Springer J: Adenomyosis: A Sonographic diagnosis. Radiographics.
38:1576–1589. 2018.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Stratopoulou CA, Camboni A, Donnez J and
Dolmans MM: Identifying common pathogenic features in deep
endometriotic nodules and uterine adenomyosis. J Clin Med.
10(4585)2021.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liu X, Nie J and Guo SW: Elevated
immunoreactivity to tissue factor and its association with
dysmenorrhea severity and the amount of menses in adenomyosis. Hum
Reprod. 26:337–345. 2011.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yi KW, Kim SH, Ihm HJ, Oh YS, Chae HD, Kim
CH and Kang BM: Increased expression of p21-activated kinase 4 in
adenomyosis and its regulation of matrix metalloproteinase-2 and -9
in endometrial cells. Fertil Steril. 103:1089–1097.e2.
2015.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Oh NJ, Ryu KY, Jung CN, Yi SY and Kim SR:
Expression of endothelial nitric oxide synthase in the uterus of
patients with leiomyoma or adenomyosis. J Obstet Gynaecol Res.
39:536–542. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Oh SJ, Shin JH, Kim TH, Lee HS, Yoo JY,
Ahn JY, Broaddus RR, Taketo MM, Lydon JP, Leach RE, et al:
β-Catenin activation contributes to the pathogenesis of adenomyosis
through epithelial-mesenchymal transition. J Pathol. 231:210–222.
2013.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Jovin TG, Boosupalli V, Zivkovic SA,
Wechsler LR and Gebel JM: High titers of CA-125 may be associated
with recurrent ischemic strokes in patients with cancer. Neurology.
64:1944–1945. 2005.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Kishi Y, Suginami H, Kuramori R, Yabuta M,
Suginami R and Taniguchi F: Four subtypes of adenomyosis assessed
by magnetic resonance imaging and their specification. Am J Obstet
Gynecol. 207:114.e1–e17. 2012.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Turner BM, Cramer SF and Heller DS: The
pathogenesis of abnormal uterine bleeding in myopathic uteri. Ann
Diagn Pathol. 52(151726)2021.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Wessler S: Estrogen-associated
thromboembolism. Ann Epidemiol. 2:439–443. 1992.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Lee JY, Hwang KR, Won KH, Lee DY, Jeon HW
and Moon MH: Uterine infarction in a patient with uterine
adenomyosis following biochemical pregnancy. Clin Exp Reprod Med.
41:174–177. 2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Aso Y, Chikazawa R, Kimura Y, Kimura N and
Matsubara E: Recurrent multiple cerebral infarctions related to the
progression of adenomyosis: A case report. BMC Neurol.
18(119)2018.PubMed/NCBI View Article : Google Scholar
|