Introduction
All living organisms need to maintain an internal
dynamic equilibrium for proper biological function. This complex
dynamic equilibrium is known as homeostasis and it is constantly
threatened by internal or external forces called stressors
(1). The state of threatened or
perceived as such homeostasis is known as stress, while the
response system organisms have developed to combat stress and
maintain or reinstate homeostasis is known as the stress system.
The stress system includes complex neuroendocrine responses and
functions through the activation of the
hypothalamic-pituitary-adrenal (HPA) axis and the locus coeruleus
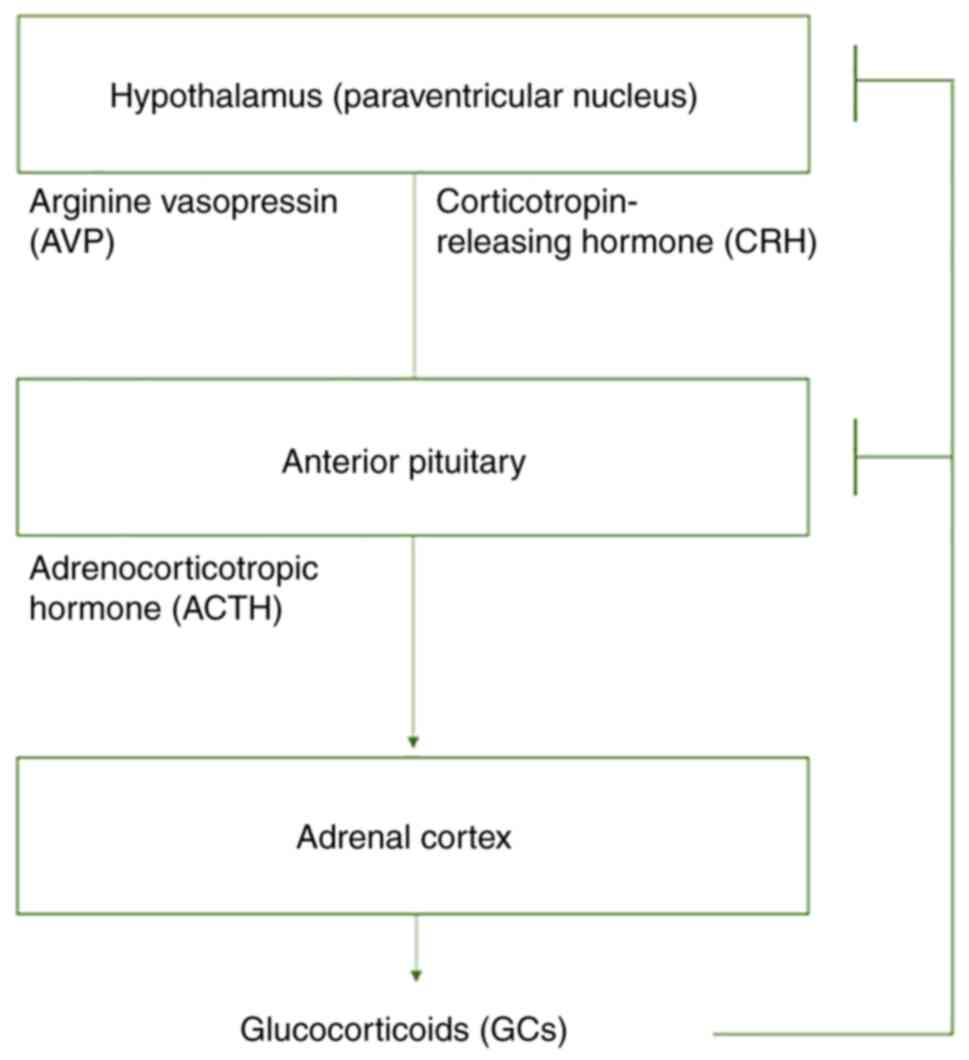
(LC)/norepinephrine (NE)-autonomic nervous system (2). The HPA axis consists of neurons
located in the paraventricular nucleus of the hypothalamus (PVN)
that secrete mainly corticotropin-releasing hormone (CRH) and
arginine vasopressin (AVP), endocrine cells in the anterior
pituitary that secrete adrenocorticotropic hormone (ACTH), and
endocrine cells in the adrenal cortex that secrete glucocorticoids
(GCs), specifically cortisol in humans and corticosterone in
rodents (Fig. 1). CRH and AVP
stimulate ACTH secretion in the anterior pituitary, and ACTH in
turn, stimulates GC secretion in the adrenal cortex. Lastly,
glucocorticoids can inhibit axis function by suppressing CRH and
ACTH secretion (Fig. 1) (3). The LC is a NE-producing nucleus that
is located in the posterior portion of the rostral pons. The LC is
characterized by numerous efferent NE projections to the entire
neuraxis and modulates neuronal function in both the sympathetic
and parasympathetic nervous systems (4). This neuromodulatory system has long
been associated with synaptic plasticity and is considered to help
local circuits dynamically adapt to new circumstances (5). Stress differs among individuals and,
based on the basal activity and time course of the neuroendocrine
responses, can either help an organism overcome certain challenges
or lead to an excessive or inadequate response to stressors with
pathological results (6,7).
The principal effectors of the stress system are
located in the HPA axis (8). GCs,
as the final product of the HPA axis, can be considered the most
critical stress-associated hormones. The action of GCs is mediated
by two receptors, the GC receptor (GR) and the mineralocorticoid
receptor (MR), with both receptors belonging to the nuclear
receptors (NRs) superfamily of transcription factors. Binding
assays have demonstrated that the MR has a 10-fold higher affinity
for GCs than the GR, indicating that the MR is activated at basal
levels, while the GR is activated during the circadian peak of GC
secretion or during stress (9).
Thus, GR signaling is of paramount importance in the stress
system.
GR structure and function are characteristic of its
NR status. NRs are relatively similar structure-wise, and apart
from the main functional domains, also feature regions which
interact with cofactors, such as activation function (AF)-1 and
AF-2 (Fig. 2) (10). Function-wise, NRs are
ligand-dependent transcription factors, with the majority of
mentioned receptors being regulated by small lipophilic ligands
with ligand-binding, leading to receptor conformational changes and
subsequent translocation to the nucleus and the binding of specific
DNA sequences. Once a NR is bound to its target DNA sequence,
various receptor cofactors are recruited to the site in order to
activate or repress target gene expression (11).
GR signaling is relatively similar to other nuclear
receptors, and more specifically, the steroid hormone receptor
subcategory of NRs (Fig. 3). In
the absence of GCs, the GR is located in the cytoplasm, where it is
bound to a number of cofactors, termed chaperone proteins, that
render it inactive. Specifically, following translation, heat-shock
protein (Hsp)70 binds the unfolded receptor in the cytoplasmic
matrix, a process accelerated by Hsp40, and promotes the folding of
the GR. A cofactor known as BAG family molecular chaperone
regulator 1 may inhibit the folding of the mentioned receptor,
either directly or by assisting the degradation of the unstable
folded GR complex with Hsp70 and Hsp40. The Hsp40/Hsp70-GR complex
is later recruited by the Hsp70-Hsp90 organizing protein (Hop) to
interact with Hsp90(12). Hsp90
binding of ATP leads to the dislodgement of Hop, Hsp40 and Hsp70
and sets in motion the subsequent interaction of the Hsp90-GR
complex with cochaperone proteins, such as FK506-binding protein
(FKBP)51 and prostaglandin E synthase 3, which gives rise to a
complex conformation with a high affinity for corticosteroids
(13,14). Ligand binding leads to
conformational changes in the ligand-binding domain (LBD) that
alter the proteins which comprise the heterocomplex, a prime
example being the replacement of FKBP51 by FKBP52, leading, mostly,
to GR dimerization and nuclear translocation, where the receptor
may now regulate transcription (14-16).
The nuclear import of the GR is a rapid and active process that
relies on GR association with the Hsp90, FKBP52 and importin-α. The
GR complex is transported into the nucleus along the cytoskeleton
and through the nuclear pore complex (NPC) with the help of dynein
(16). Once in the nucleus, the
activated GR can modulate gene transcription. Specifically,
transactivation can be achieved directly through GR homodimer
binding to distinct DNA sequences known as GC response elements
(GREs) or indirectly, where GR acts as a monomer and co-operates
with other transcription factors to induce transcription (17,18).
Transrepression can also be direct, via GR homodimer or,
preferably, monomer binding to a negative GRE, or indirect, where
GR acts as a monomer and binds to a pro-inflammatory transcription
factors, such as NF-κB (17-19).
It should also be mentioned that a large part of the receptor's
action is also exerted through protein-protein interactions
(20). The time length GR remains
bound to DNA depends on the bound ligand (21). Following ligand disengagement, GR
disconnects from DNA and is either degraded by the proteasome or
exported from the nucleus, an inactive process possibly occurring
through passive diffusion (16).
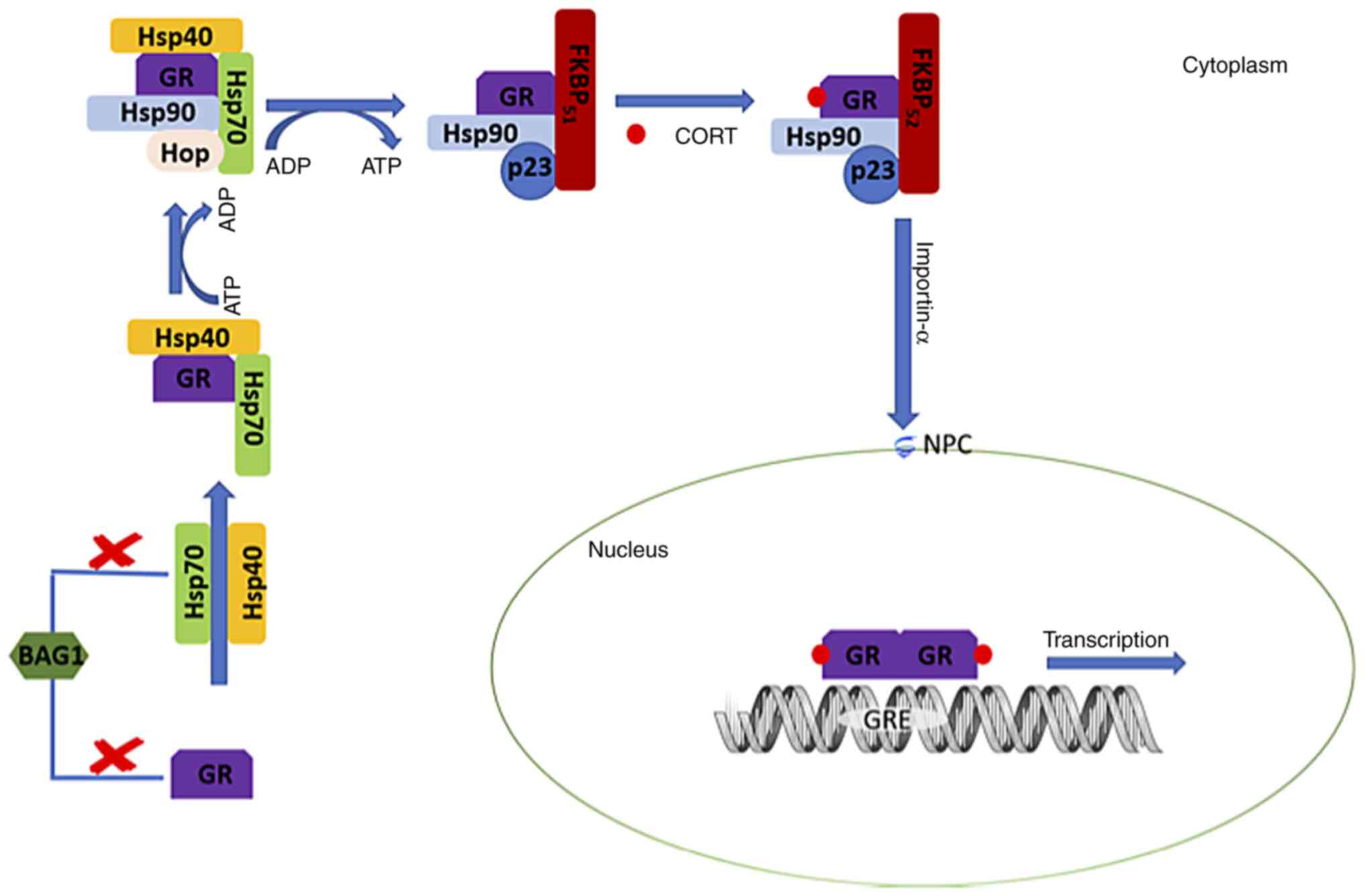 | Figure 3Schematic representation of GC
signaling resulting in GR homodimerization and transcription
initiation. Hsp70 binds the unfolded receptor in the cytosol, a
process accelerated by Hsp40 binding, and leads to the folding of
the GR. BAG-1 is a cofactor that may directly impair receptor
folding, while it may also aid in the degradation of the unstable
folded GR complex with Hsp70 and Hsp40. The interaction of GR with
the Hsp40/Hsp70-GR complex is then recruited by Hop, in an
ATP-dependent manner, to interact with Hsp90. Hop, Hsp40 and Hsp70
are dislodged from the Hsp90-GR complex upon Hsp90 binding ATP, and
the subsequent interaction of the Hsp90-GR complex with FKBP51 and
p23 give rise to a complex conformation with a high affinity for
corticosteroids. Ligand binding leads to the replacement of FKBP51
by FKBP52, mainly leading to GR dimerization and nuclear
translocation through the NPC with the aid of importin-α. Finally,
the GR homodimer binds to glucocorticoid response elements to
promote gene transcription. GC, glucocorticoid; GR, glucocorticoid
receptor; Hsp70, heat-shock protein70; Hsp40, heat shock protein
40; BAG1, BAG family molecular chaperone regulator 1; Hop,
Hsp70-Hsp90 organizing protein; p23, prostaglandin E synthase 3
protein; FKBP51, FK506-binding protein 51; CORT, cortisol; FKBP52,
FK506-binding protein 51; NPC, nuclear pore complex; GRE,
glucocorticoid response elements. |
As a main mediator of the stress response, the GR
also plays a role in numerous biological processes. Beginning from
the embryonic phase, the GR influences development and organ
maturation (22,23). The pulmonary and cardiovascular
systems are interconnected and are both connected to high-stress
levels and GR (24). The GR itself
has been shown to be associated with several cardiovascular
diseases (25). GCs are also known
to be essential for metabolism, influencing insulin signaling and
gluconeogenesis (26,27), while an abnormal GR regulation has
been found to be associated with obesity and diabetes mellitus type
II (20). GR also plays a critical
role in the immune system, where it downregulates pro-inflammatory
transcription factors and cytokines (28-30).
It is also known that GCs produced under pathological circumstances
are capable of disrupting immune function, an effect that results
in susceptibility to infections from viruses and neoplasm
development (31). GCs also have
the ability to cross the blood-brain barrier, thus affecting
various aspects of the nervous system. GR regulates behavioral,
emotional and physical responses and can alter synapses (32) and appears to play a role in mood
disorder pathology (33).
The GR and its interactome can regulate numerous
pathways and systems in humans. Literature on these pathways and
systems has accumulated over the decades and since several of the
associated studies do not focus on the GR, lesser findings
associated with this receptor may have been overlooked by
researchers studying the GR. To the best of our knowledge, the
present study is novel in that it may provide further information
regarding the GR that has not been reported thus far, namely
crucial information on GR function and GR-related pathologies.
Specifically, a main aim of the present study was to identify the
most associated single nucleotide polymorphisms (SNPs) and their
genetic variants, gaining information that in the future can
potentially support a better understanding of the GR interactome.
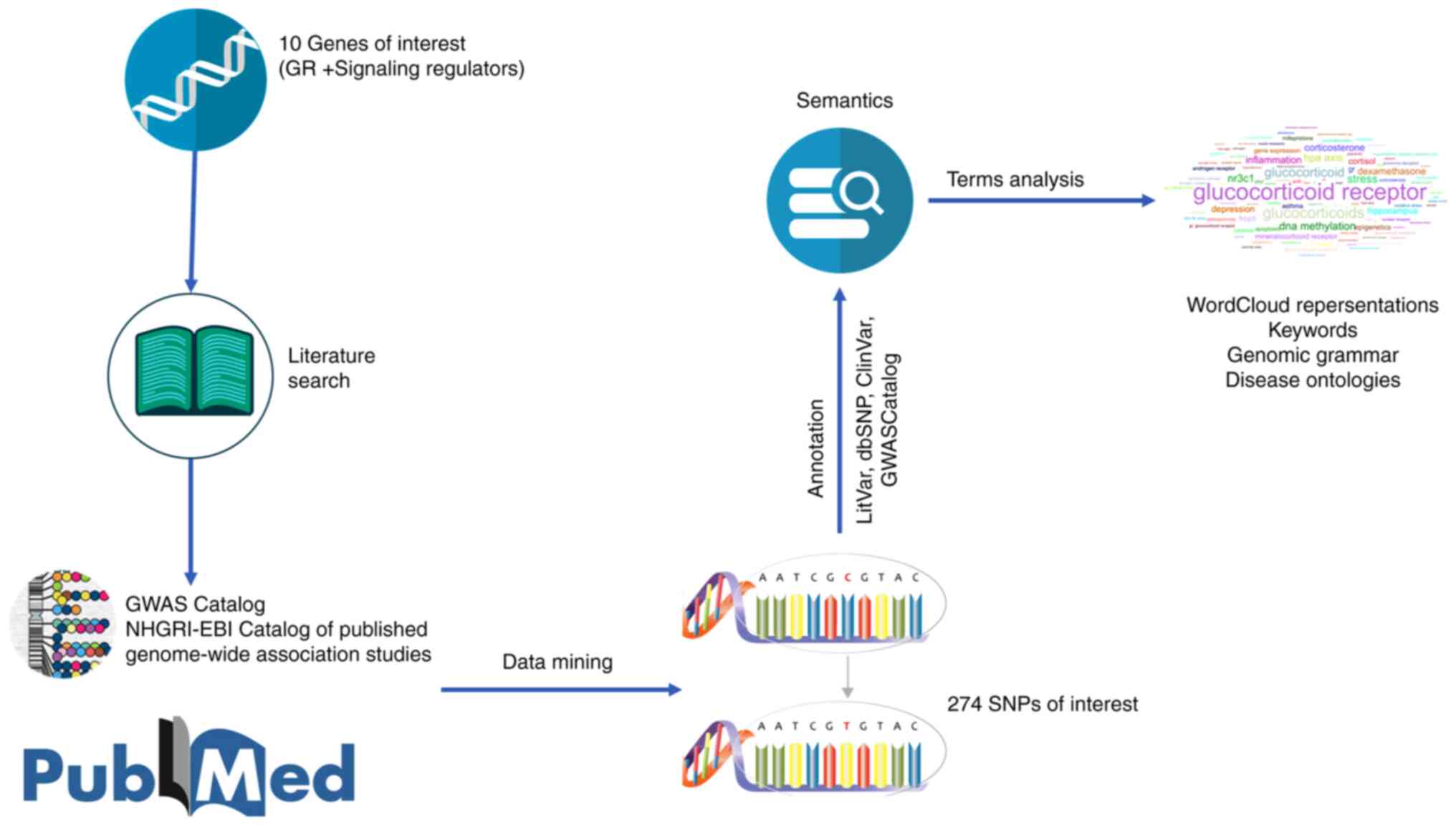
The general pipeline of the integrated bioinformatics approach is
presented in Fig. 4.
Data and methods
Data collection
The main regulators of GR signaling, as described in
the text above, along with the receptor itself, were the starting
point for a literature search. Specifically, 10 genes of interest
were used as key words for an in-depth search on the PubMed
database (Table I) (34,35).
After using a filtering algorithm, duplicates were removed, and the
study focused on the identification of SNPs that have been found to
be associated with the GR and the main regulators of its signaling.
Information regarding GR-related SNPs and the other genes of
interest was also extracted and merged from the genome-wide
association studies (GWAS) Catalog database. Last, but not least,
all the identified SNP terms were stored in a structured database
and all the available entries were associated with the SNP ID
number as referred to in the dbSNP database (36).
 | Table IGenes used and the corresponding PMID
of articles reporting their interaction with the GR. |
Table I
Genes used and the corresponding PMID
of articles reporting their interaction with the GR.
| Serial no. | Gene name | PMID: Interaction
with GR |
|---|
| 1 | GR | - |
| 2 | FKBP5 | 19560279 |
| 3 | FKBP4 | 32557257 |
| 4 | HSP90 (AA1) | 28224564 |
| 5 | PTGES3 (p23) | 24345775 |
| 6 | STIP1 (HOP) | 32612187 |
| 7 | HSP70 | 32612187 |
| 8 | HSP40 | 30585227 |
| 9 | NR3C2 | 28686058 |
| 10 | BAG1 | 30585227 |
Data mining
All identified SNPs of interest were annotated with
relevant information from the ClinVar (37), LitVar (38), dbSNP (36) and GWAS Catalog (34) databases. Specifically, the dbSNP
database was used to find the genomic location of the SNP and their
position in a gene, the ClinVar database to find potential
associations with human pathological conditions, the GWASCatalog
database to find associations with specific traits, and the LitVar
database to find the most co-occurred entities in a sentence
featuring the aforementioned designated key words, with a focus on
diseases, chemicals and genomic variants.
Semantics and terms analyses
Semantics and terms analyses were conducted towards
extracting beneficial knowledge, including the genomic grammar,
disease ontologies and the most common key words that are presented
in the studied literature. Subsequently, all the extracted results
were displayed in WordCloud representations in order to summarize
the final output.
Results
Based on the results, >127,000 publications were
found to be related to GR and its genes of interest (Table I). A total of 274 related GR
interactome-related SNPs of utmost interest were identified
(Table SI). The annotation of the
mentioned SNPs revealed an association with 247 diseases (Tables SI and SII) and 118 genes (Tables SI and SIII). The SNPs found in the GR were
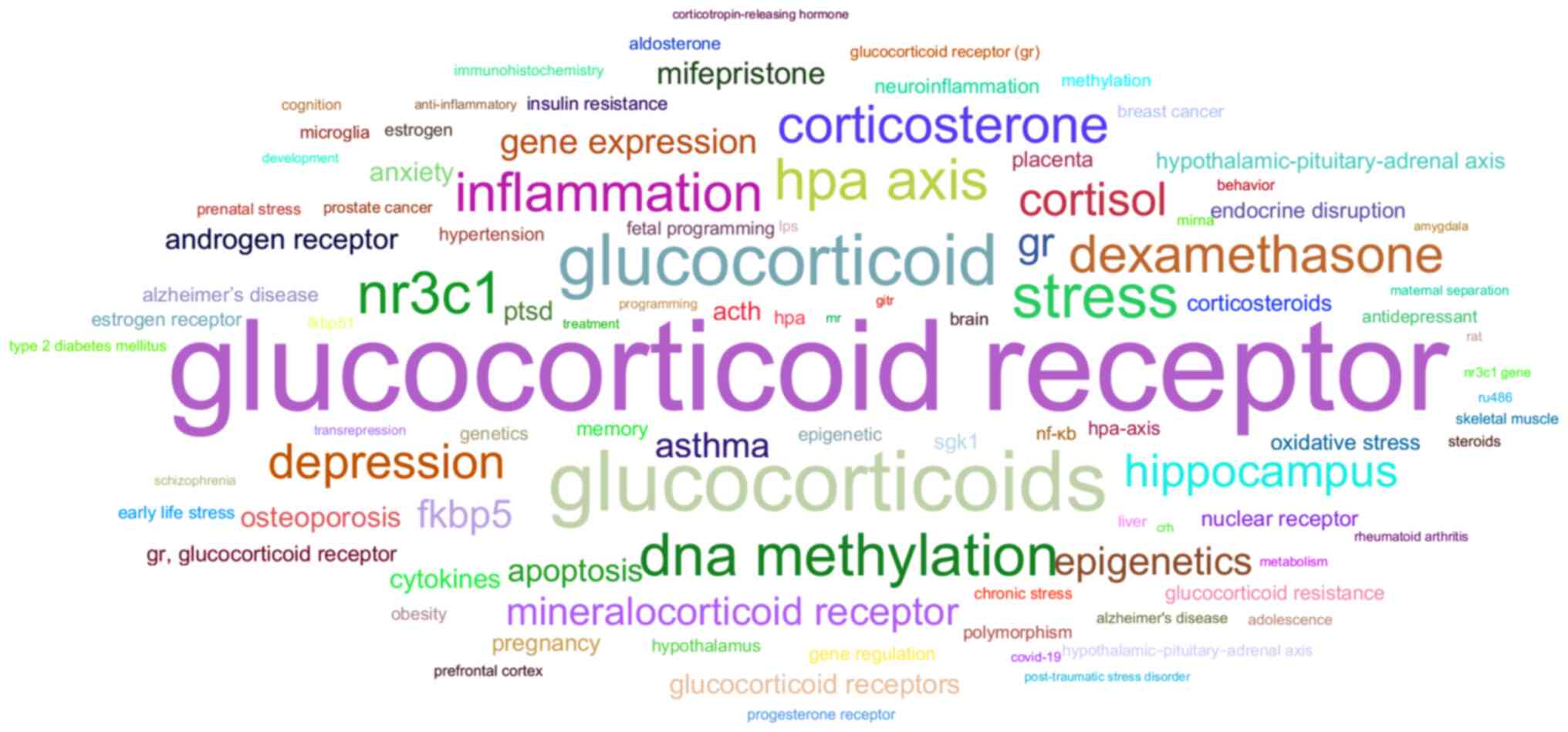
associated with specific key words in the scientific literature
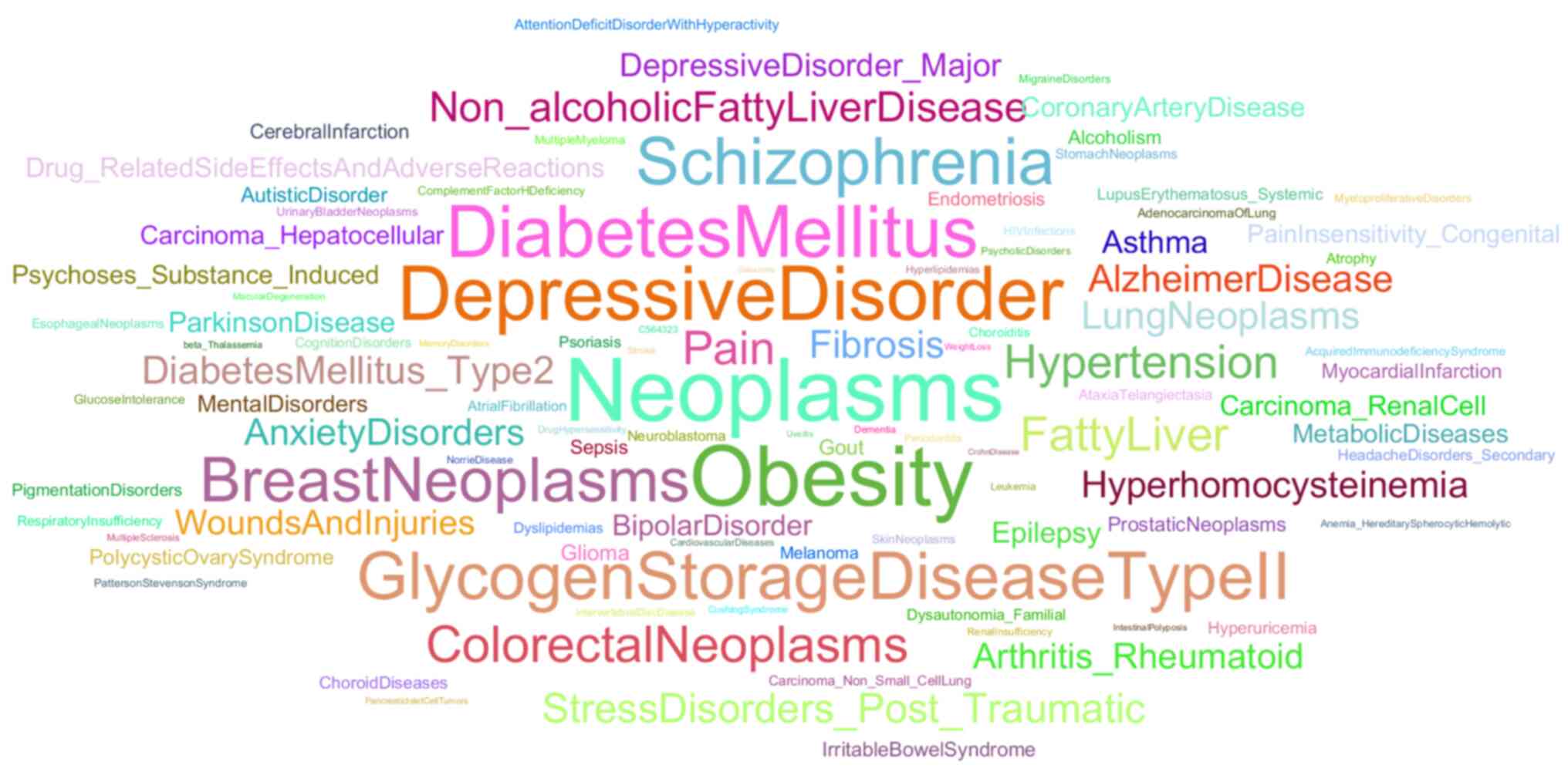
(Fig. 5). The vast majority of
these keywords can be separated into distinct groups as follows: i)
The stress system-related group, with entries such as the HPA axis,
stress and chronic stress; ii) the gene regulation-related group,
with entries such as DNA methylation and epigenetics; iii) the
immune system-related group with entries such as inflammation and
NF-κB; iv) the development-related group with entries such as fetal
programming; v) a group featuring other steroid hormone receptors,
with entries such as MR, androgen receptor and progesterone
receptor; vi) a group highlighting the role of the receptor in
metabolism with entries such as insulin resistance and obesity;
vii) a group showcasing the role of GR role in neuropsychiatric
disorders with entries such as depression, post-traumatic stress
disorder (PTSD) and schizophrenia; viii) a group highlighting the
role of the GR in brain architecture and neuronal plasticity, with
entries such as hippocampus, prefrontal cortex and microglia; ix) a
group featuring members of the GR interactome, with entries such as
FKBP5 and serum/glucocorticoid regulated kinase 1; and x) a group
featuring agonists and antagonists of the GR, such as dexamethasone
and aldosterone. Apart from the mentioned groups, several unique
key words associated with various pathological conditions emerge,
such as osteoporosis, asthma and Alzheimer's disease. Apoptosis is
also present as a unique key word, an inclusion which may be due to
the ability of the GR to promote pro-apoptotic protein expression
(39).
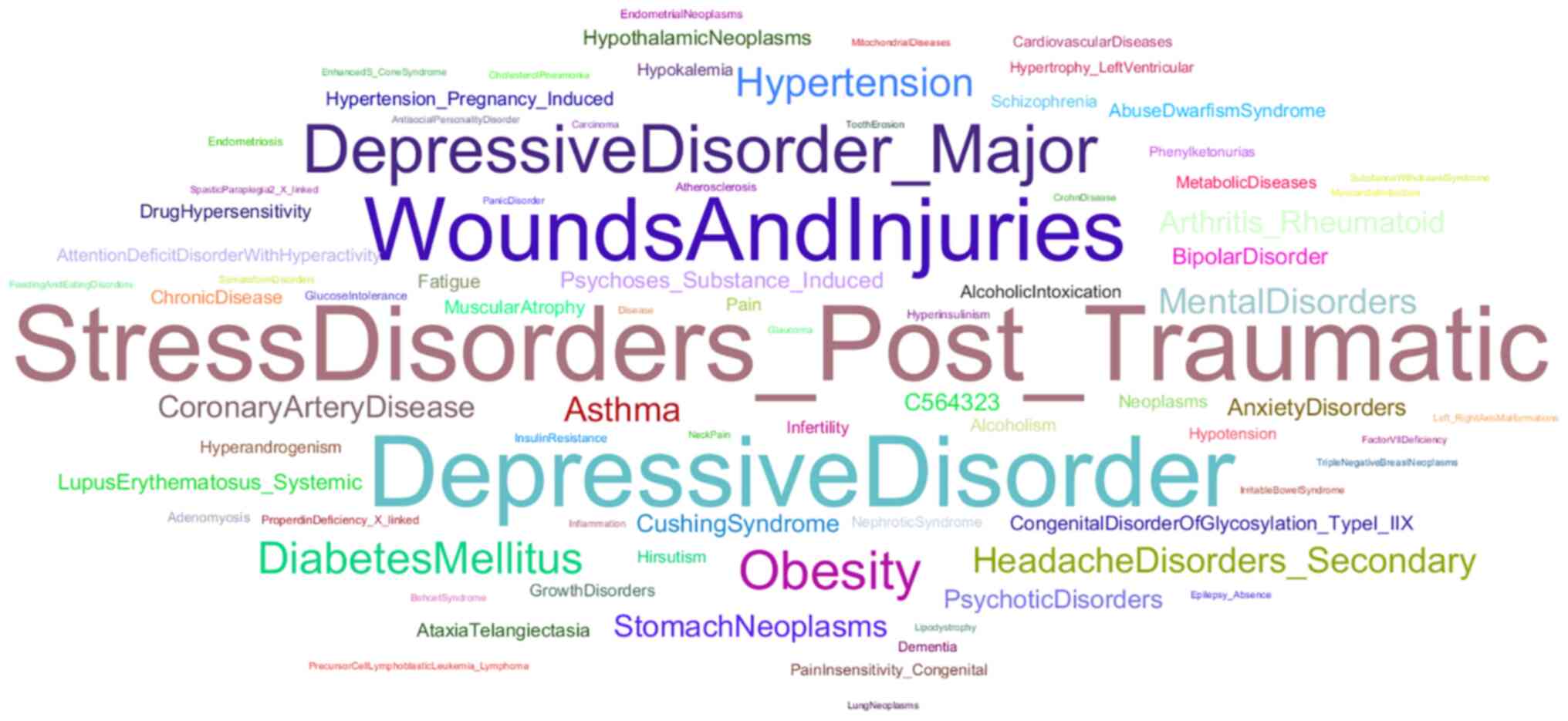
The SNPs found in the GR were studied in conjunction
with several pathological conditions (Fig. 6). The most commonly studied
pathological conditions are, as expected, neuropsychiatric
disorders, such as depression and PTSD, and metabolic disorders,
such as diabetes mellitus and obesity (40). Pathologies, such as asthma,
rheumatoid arthritis, or systemic erythematosus lupus are also
associated with the study of the GR, which may be due to the fact
that these conditions are mainly treated through the use of
synthetic GCs (41,42). Cardiovascular diseases are also
associated with GR research, possibly due to the aforementioned
influence of the stress response system on the cardiovascular
system. Somewhat unexpected, though, is the study of wounds and
injuries, plus various neoplasms in conjunction with GR. The role
of GCs in various mechanisms underlying cancer has been largely
unexplored. Although the GR is not considered an oncogene, GCs have
been shown to arrest growth and induce apoptosis in lymphoid tissue
via GR signaling in certain patients (43). The attempt to elucidate the
mechanisms regulating the effects of GCs on cancer may be the
reason for which numerous neoplasms have been studied in
conjunction with GR. Wounds and injuries are markedly associated
with inflammation, since inflammation is a phase of the wound
healing process (44). The effects
of GCs on inflammation may affect the healing process, and thus
studies focus on the role of GR in wounds and injuries.
The SNPs found in the regulators of GR signaling
have also been studied along with specific genes in the literature
(Fig. 7). The vast majority of
these genes are the regulators themselves (FKBP5 and HSPA1L). Other
genes studied along with regulators of GR signaling include genes
coding for regulators of the HPA axis, such as CRHR1, genes coding
for main regulators of the immune system, such as complement factor
H (CFH) and nuclear factor kappa B subunit 2 (NFKB2), genes coding
for various factors influencing brain architecture, such as brain
derived neurotrophic factor antisense RNA (BDNF-AS) and
neurotrophic receptor tyrosine kinase 2 (NTRK2), genes coding for
factors influencing metabolism, such as apolipoprotein E (APOE) and
fat mass and obesity-associated protein (FTO), and the MR which
also binds GCs. Several genes which produce non-coding mRNAs are
also present, such as miR-4761. Non-coding RNAs are known to play a
crucial role in gene regulation (45), which is in accordance with the
action of GR as a transcription factor. Additional genes included
are vascular endothelial growth factor A (VEGFA) and RNA polymerase
I and III subunit c (POLR1C). VEGFA codes for the vascular
endothelial growth factor, which plays a critical role in
physiological and pathological angiogenesis (46), while POLR1C codes for the C subunit
of RNA polymerases I and III. GCs exert an angiostatic effect and
glucocorticoid treatment has been shown to influence the VEGF mRNA
levels (47). The inclusion of
POLR1C though, is of interest, and will be discussed in-depth
below.
Lastly, the SNPs found in the regulators of GR
signaling have been studied for their role in several diseases
(Fig. 8) (48). The diseases associated with GR
signaling regulator SNPs almost completely overlap with the
diseases studied in conjunction with GR SNPs. Several diseases
associated with metabolism or the healing process are unique to GR
signaling regulators, implying that GR may influence the mentioned
mechanisms indirectly through its interactome. It is also
intriguing that neoplasm studies are more present in GR signaling
regulators SNPs, possibly displaying that the GR may play a more
complex role in cancer than what was originally thought. As regards
disease studies which are unique to GR signaling regulators, these
include Parkinson's disease, Alzheimer's disease and epilepsy,
highlighting the role of GR signaling in proper brain function, and
polycystic ovary syndrome (PCOS). The inclusion of PCOS may be due
to the effect GCs have on the hypothalamic-pituitary-gonadal axis,
whose products play a key role in the pathophysiology of this
disease (49).
Discussion
Glucocorticoids are essential mediators of the
stress system, being the final product of the HPA axis activation
(8,18). Following their excretion from the
adrenal glands, they enter the blood circulation, find the target
cells and exert multiple actions. The majority of their actions are
carried out through gene regulation, after pairing with their
receptor; their actions also known as the genomic effects of GCs
(19). Through the transactivation
of anti-inflammatory functions and the transrepression of
pro-inflammatory genes, GCs exert their anti-inflammatory effects,
taking part in regulating inflammation and other immune system
processes (17). Among the target
genes of GR regulating attributes, one can also find pro-apoptotic
genes, mainly used in the treatment of lymphoid malignancies and
other neoplasms (50,51). During developmental phases, GCs are
involved in several fetal programming processes, resulting in
differences between treated and untreated subjects in adult life.
Embryos treated with GCs have been shown to develop earlier and
present with several physical and behavioral differences in
adulthood, compared to the untreated embryos (23). GR plays a role in several metabolic
pathways, participating in the signaling pathway of insulin in the
liver, skeletal muscles and adipose tissue (26), and are responsible for metabolic
diseases, such as diabetes and obesity (20). Other steroid receptors, biological
relatives of GR, have been studied along with GR as putative
targets for glucocorticoids, in an attempt to identify the
associated diseases (52).
As participants of the HPA axis, GCs are associated
with structural and alterations in different parts of the brain and
subsequently also associated with several neuropsychiatric
disorders. PTSD is a disorder in which GR can be used as a
potential therapeutic target, as GCs may be able to lower the
hippocampal-mediated trauma memories. Changes in GC sensitivity of
the hippocampus could determine the risk of developing PTSD in
later life. Abnormal GC circulation and mitigated circadian
cortisol fluctuations may cause sleep disturbances observed in PTSD
cases, and morphological and functional alterations in the
hippocampus (53). It is also
known that chronic stress is a crucial factor in the development of
disorders, such as depression and it was recently proven that
neuroinflammation in the hippocampus and depression-like behavior
is mediated by activation of the GR pathway in hippocampal
microglia (54). GCs exert various
effects on prefrontal cortex functions, depending on whether the
stress is chronic or acute (55).
Acute stress can actually enhance working memory via a GR-dependent
mechanism (56). Following the
ultradian rhythm, GCs can induce changes at synapses throughout the
cortex which are involved in motor learning and other functions
(57,58). Exposure to high levels of GCs,
chronic stress and stress-related disorders may also increase the
risk of developing Alzheimer's disease (59). It has been shown that when the GR
is blocked due to stress early on in life, mice exhibit lower
cognitive flexibility and higher levels of amyloid-β in the
hippocampus. Treatment with a GR antagonist in middle-aged mice has
been shown to result in lower amyloid-β levels and recovery from
the cognitive defects (60).
GCs have been used in clinical practice for a number
of years, tapped into their ability to alleviate symptoms of
certain pathologies. Such treatments mainly involve the GC
immunosuppressive attitudes and are being administered to patients
with conditions, such as systemic erythematosus lupus, asthma and
rheumatoid arthritis (61-63).
Moreover, in cases of asthma exacerbations, GCs are used to combat
inflammation (63,64), and possibly even to induce lung
tissue regeneration (65).
However, as previously demonstrated, patients suffering from asthma
with GC resistance, were not responsive to GC treatments (63). Some mechanisms that the GR uses are
the regulation of hematopoietic cell apoptosis and the suppression
of pro-inflammatory cytokine expression (66). Sadly, several other pathologies
have emerged as adverse effects of GC use in clinical practice. The
excess of GCs in bones causes osteoporosis, as GCs activate
pro-apoptotic molecules that reduce osteocyte viability and
osteoclast apoptosis (67).
Increased GR signaling affects factors that are involved in bone
formation and calcium metabolism, finally leading to an increased
risk of fractures (68,69). Due to the adverse effects
inhibiting their unhampered prevalence, there has been extensive
research about putative agonists and antagonists that attenuate or
even eliminate these effects (70).
SNPs found in GR studies are associated with several
already mentioned pathologies, including neuropsychiatric
disorders, metabolic disorders and autoimmune diseases.
Cardiovascular diseases have also been shown, defining the role of
GR in stress and the importance of stress for coronary heart
disease. Emotional stress is a usual trigger of cardiac events and
there is even a syndrome known as stress cardiomyopathy that
supports this evidence (24).
Wounds and injuries also surfaced following the analysis of GR
SNPs, connecting the anti-inflammatory properties of GCs to another
not so obvious target. GCs can suppress the migration of
endothelial progenitor cells (EPCs) and impair wound healing,
something that should be considered before using EPCs for
autologous cell transplantation (71). On the other hand, dexamethasone has
been shown to be of assistance to wound healing in animal models of
frostbite (72). Therefore,
further studies are required to define the factors implicated in
changing the GC wound healing properties.
The role of GCs in cancer pathology and pathogenesis
is still under evaluation. The effects of GCS on tumor progression
appear to heavily rely on the cells targeted. In the case of
lymphocytic malignancies, dexamethasone, a synthetic GC, is used to
promote apoptotic cell death, while in epithelial cell tumors, GCs
mostly exert the opposite effect (73). In vitro research has
demonstrates that GCs suppress cell migration and invasion via the
downregulation of Ras homolog family member A, matrix
metalloproteinase (MMP)2, MMP9 and IL-6, or via the induction of
E-cadherin (74). On the other
hand, cancer research has also shown that an excess of GCs enhances
the proliferation of tumor cells in vitro and in vivo
(75). A poor immune system
response and a poor prognosis have also been found to be associated
with GC hypersecretion (76). GCs
have been proven to deplete T cells that infiltrate tumors of
adrenocortical carcinomas, while tumor-infiltrating lymphocytes
were more effective against tumors in other cancer types (73). Cancer signaling is extremely
complex and transcription factors, such as the GR display complex
effects (77).
The GR signaling regulators appear to play a
critical role in determining the various actions of the receptor.
The SNPs of GR regulator analysis (Fig. 7) resulted in genes that revolved
around the HPA axis, immune system, metabolism and brain
architecture. Several brain-related diseases, including Parkinson's
disease, Alzheimer's disease and epilepsy appear to be connected to
GR signaling as well. Chronic inflammation places the immune system
on alert and activates the HPA axis, producing GCs. High levels of
GCs for a long period of time activate a pro-inflammatory
environment in microglia and subsequently increase dopamine neuron
degeneration, leading to clinical manifestations of Parkinson's
disease (78). Stress is also
referred to as a possible cause for epilepsy in several patients.
Research using model mice has demonstrated that corticosterone
administration to epileptic mice results in more epileptic episodes
(79). PCOS also appears to be
connected to impaired GC signaling. GC resistance was found in
almost 67% of patients with PCOS in the study by Panayiotopoulos
et al (80).
Corticosteroids have also been used in the treatment of certain
cancer types, such as hemangiomas, taking advantage of their
ability to inhibit angiogenesis. The mechanism of dexamethasone in
this case is the inhibition of the expression of VEGFA in
hemangioma cells (81). Apart from
its anticancer VEGFA-regulating effect, GR can also inhibit the
secretion of VEGFA in endochondral ossification, thus disrupting
the normal entrance of blood vessels and creating bone growth
issues in children that have been administered GCs (82).
Of note, despite having no direct connection to GR
or its interactome, a specific subunit of RNA polymerases I and III
was identified in the analysis in the present study. POLR1C is a
gene that codes for the RPAC1 subunit of ribosomal RNA polymerases
I and III. Studies from over three decades ago have shown that GCs
stimulate the production of rRNA in rat livers (83), probably making use of proteins
activating the idle form of RNA polymerase I (84), although having no particular
connection to the RPAC1 subunit. Perhaps the connection between GR
and POLR1C is indirect and involves a few mediators. Bruna et
al (85) demonstrated that the
GR inhibits the c-Jun N-terminal kinase (JNK) pathway. Upon stress,
JNK2 inactivates the TIF-IA transcription factor downstream the JNK
pathway, which makes it impossible for TIF-IA to interact with RNA
polymerase I and the initiation complex cannot be formed (86). The individual mechanisms through
which the GR can inhibit the formation of RNA polymerase I complex,
under conditions of stress are as follows: The GR LBD contains a
hormone-regulated JNK docking site. Naturally, GR in the cytoplasm
is associated with a protein complex. When GCs bind to the GR, the
ligand-receptor couple unbinds the protein complex, and the JNK
docking site is exposed. The GC-GR complex then travels to the
nucleus and binds with JNK; thus, the consequent JNK deficiency
does not allow proper signal transduction, causing the inhibition
of the pathway (85). Under stress
conditions, JNK phosphorylates TIF-IA at Thr200, inhibiting its
interaction with Pol I, resulting to inability of RNA polymerase I
to transcribe (86). An
alternative path that could connect GR to RNA polymerase I is the
mammalian target of rapamycin (mTOR) signaling pathway. The GR in
skeletal muscles targets and mainly inhibits the mTOR pathway
(87). With this pathway blocked,
mTOR is unable to activate the transcription factor TIF-IA,
altering its phosphorylation pattern, leading to no recruitment of
Pol I to the rDNA promoter (88),
and thus, to no rRNA synthesis. Additional research is required
however, to clarify the exact mechanisms underlying the complex
association of GR to RNA polymerase activity.
In conclusion, the present study established that
SNPs found in the GR interactome participate in numerous biological
processes of high importance, such as immune regulation,
metabolism, development and proper brain function. Moreover,
pathological conditions, such as autoimmune diseases,
neuropsychiatric diseases, metabolic disorders and even cancer,
appear to, in one way or another, be related with SNPs found in the
GR interactome. The study of the mentioned SNPs may provide
information on the mechanisms through which such diseases emerge
and may help to promote personalized healthcare, where therapy can
be selected based on an individual's genetic background for maximum
effectiveness. A prime example would be using the SNPs of interest
identified in the present study as diagnostic or prognostic markers
for GR-related pathologies.
Another interesting observation of the present study
was the underreported effect GR may have on rRNA synthesis through
its indirect effect on the POLR1C gene. Since rRNA synthesis
dysregulation has been associated with a broad range of diseases,
further research may expand the network of pathologies influenced
by glucocorticoids.
On the whole, the results obtained in the present
study may prove to be useful both in a clinical and a research
setting. It should be mentioned, though, that all information
presented was extracted from the currently available literature
where many articles may hold contradictory results. Moreover, as
the literature expands, specific associations may weaken or
strengthen, affecting the importance of GR in several of the
mechanisms analyzed. Nevertheless, since several associations
mentioned appear to be have been hinted at previously, the present
study appears to be in accordance with the current view of the
functions of GR.
Supplementary Material
GR interactome SNPs.
GR interactome diseases from
SNPs.
GR interactome genes from SNPs.
Acknowledgements
Not applicable.
Funding
Funding: The authors would like to acknowledge funding from the
following organizations: i) AdjustEBOVGP-Dx (RIA2018EF-2081):
Biochemical Adjustments of native EBOV Glycoprotein in Patient
Sample to Unmask target Epitopes for Rapid Diagnostic Testing. A
European and Developing Countries Clinical Trials Partnership
(EDCTP2) under the Horizon 2020 ‘Research and Innovation Actions’
DESCA; ii) ‘MilkSafe: A novel pipeline to enrich formula milk using
omics technologies’, a research co-financed by the European
Regional Development Fund of the European Union and Greek national
funds through the Operational Program Competitiveness,
Entrepreneurship and Innovation, under the call
RESEARCH-CREATE-INNOVATE (project code: T2EDK-02222); iii)
‘INSPIRED-The National Research Infrastructures on Integrated
Structural Biology, Drug Screening Efforts and Drug Target
Functional Characterization’ (grant MIS 5002550) implemented under
the Action ‘Reinforcement of the Research and Innovation
Infrastructure’, funded by the Operational Program
‘Competitiveness, Entrepreneurship and Innovation’ (NSRF 2014-2020)
and co-financed by Greece and the European Union (European Regional
Development Fund), and iv) ‘OPENSCREENGR An Open-Access Research
Infrastructure of Chemical Biology and Target-Based Screening
Technologies for Human and Animal Health, Agriculture and the
Environment’ (Grant MIS 5002691), implemented under the Action
‘Reinforcement of the Research and Innovation Infrastructure’,
funded by the Operational Program ‘Competitiveness,
Entrepreneurship and Innovation’ (NSRF 2014-2020) and co-financed
by Greece and the European Union (European Regional Development
Fund).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (MS, TM, LP, EP, ID, KP, KD, DAS, FB,
GPC, EE and DV) contributed to the conceptualization, design,
writing, drafting, revising, editing and reviewing of the
manuscript. All authors confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
DAS is the Managing Editor of the journal, but had
no personal involvement in the reviewing process, or any influence
in terms of adjudicating on the final decision, for this article.
GPC is an Editorial Advisor of the journal, but had no personal
involvement in the reviewing process, or any influence in terms of
adjudicating on the final decision, for this article. The other
authors declare that they have no competing interests.
References
|
1
|
Chrousos GP: Stress and disorders of the
stress system. Nat Rev Endocrinol. 5:374–381. 2009.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Nicolaides NC, Kyratzi E,
Lamprokostopoulou A, Chrousos GP and Charmandari E: Stress, the
stress system and the role of glucocorticoids.
Neuroimmunomodulation. 22:6–19. 2015.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Spencer RL and Deak T: A users guide to
HPA axis research. Physiol Behav. 178:43–65. 2017.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Wood CS, Valentino RJ and Wood SK:
Individual differences in the locus coeruleus-norepinephrine
system: Relevance to stress-induced cardiovascular vulnerability.
Physiol Behav. 172:40–48. 2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Bari BA, Chokshi V and Schmidt K: Locus
coeruleus-norepinephrine: Basic functions and insights into
Parkinson's disease. Neural Regen Res. 15:1006–1013.
2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mariotti A: The effects of chronic stress
on health: New insights into the molecular mechanisms of brain-body
communication. Future Sci OA. 1(FSO23)2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Yaribeygi H, Panahi Y, Sahraei H, Johnston
TP and Sahebkar A: The impact of stress on body function: A review.
EXCLI J. 16:1057–1072. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Smith SM and Vale WW: The role of the
hypothalamic-pituitary-adrenal axis in neuroendocrine responses to
stress. Dialogues Clin Neurosci. 8:383–395. 2006.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Vyas S, Rodrigues AJ, Silva JM, Tronche F,
Almeida OF, Sousa N and Sotiropoulos I: Chronic stress and
glucocorticoids: From neuronal plasticity to neurodegeneration.
Neural Plast. 2016(6391686)2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Porter BA, Ortiz MA, Bratslavsky G and
Kotula L: Structure and Function of the nuclear receptor
superfamily and current targeted therapies of prostate cancer.
Cancers (Basel). 11(1852)2019.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Weikum ER, Liu X and Ortlund EA: The
nuclear receptor superfamily: A structural perspective. Protein
Sci. 27:1876–1892. 2018.PubMed/NCBI View
Article : Google Scholar
|
|
12
|
Kaziales A, Barkovits K, Marcus K and
Richter K: Glucocorticoid receptor complexes form cooperatively
with the Hsp90 co-chaperones Pp5 and FKBPs. Sci Rep.
10(10733)2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Baker JD, Ozsan I, Rodriguez Ospina S,
Gulick D and Blair LJ: Hsp90 Heterocomplexes Regulate steroid
hormone receptors: From stress response to psychiatric disease. Int
J Mol Sci. 20(79)2018.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Timmermans S, Souffriau J and Libert C: A
General introduction to glucocorticoid biology. Front Immunol.
10(1545)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Louw A: GR Dimerization and the impact of
GR dimerization on GR protein stability and half-life. Front
Immunol. 10(1693)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Robertson S, Hapgood JP and Louw A:
Glucocorticoid receptor concentration and the ability to dimerize
influence nuclear translocation and distribution. Steroids.
78:182–194. 2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Frego L and Davidson W: Conformational
changes of the glucocorticoid receptor ligand binding domain
induced by ligand and cofactor binding, and the location of
cofactor binding sites determined by hydrogen/deuterium exchange
mass spectrometry. Protein Sci. 15:722–730. 2006.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Vandevyver S, Dejager L and Libert C: On
the trail of the glucocorticoid receptor: Into the nucleus and
back. Traffic. 13:364–374. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Hudson WH, Youn C and Ortlund EA: The
structural basis of direct glucocorticoid-mediated transrepression.
Nat Struct Mol Biol. 20:53–58. 2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Vegiopoulos A and Herzig S:
Glucocorticoids, metabolism and metabolic diseases. Mol Cell
Endocrinol. 275:43–61. 2007.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Groeneweg FL, van Royen ME, Fenz S, Keizer
VI, Geverts B, Prins J, de Kloet ER, Houtsmuller AB, Schmidt TS and
Schaaf MJ: Quantitation of glucocorticoid receptor DNA-binding
dynamics by single-molecule microscopy and FRAP. PLoS One.
9(e90532)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Whirledge S and DeFranco DB:
Glucocorticoid signaling in health and disease: Insights from
tissue-specific GR knockout mice. Endocrinology. 159:46–64.
2018.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wilson KS, Tucker CS, Al-Dujaili EA,
Holmes MC, Hadoke PW, Kenyon CJ and Denvir MA: Early-life
glucocorticoids programme behaviour and metabolism in adulthood in
zebrafish. J Endocrinol. 230:125–142. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Steptoe A and Kivimäki M: Stress and
cardiovascular disease. Nat Rev Cardiol. 9:360–370. 2012.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Liu B, Zhang TN, Knight JK and Goodwin JE:
The glucocorticoid receptor in cardiovascular health and disease.
Cells. 8(1227)2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kuo T, McQueen A, Chen TC and Wang JC:
Regulation of glucose homeostasis by glucocorticoids. Adv Exp Med
Biol. 872:99–126. 2015.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Akalestou E, Genser L and Rutter GA:
Glucocorticoid metabolism in obesity and following weight loss.
Front Endocrinol (Lausanne). 11(59)2020.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Quatrini L and Ugolini S: New insights
into the cell- and tissue-specificity of glucocorticoid actions.
Cell Mol Immunol. 18:269–278. 2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Petta I, Dejager L, Ballegeer M, Lievens
S, Tavernier J, De Bosscher K and Libert C: The interactome of the
glucocorticoid receptor and its influence on the actions of
glucocorticoids in combatting inflammatory and infectious diseases.
Microbiol Mol Biol Rev. 80:495–522. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Rao NA, McCalman MT, Moulos P, Francoijs
KJ, Chatziioannou A, Kolisis FN, Alexis MN, Mitsiou DJ and
Stunnenberg HG: Coactivation of GR and NFKB alters the repertoire
of their binding sites and target genes. Genome Res. 21:1404–1416.
2011.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shimba A and Ikuta K: Control of immunity
by glucocorticoids in health and disease. Semin Immunopathol.
42:669–680. 2020.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Myers B, McKlveen JM and Herman JP:
Glucocorticoid actions on synapses, circuits, and behavior:
Implications for the energetics of stress. Front Neuroendocrinol.
35:180–196. 2014.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Fietta P and Fietta P: Glucocorticoids and
brain functions. Riv Biol. 100:403–418. 2007.PubMed/NCBI
|
|
34
|
MacArthur J, Bowler E, Cerezo M, Gil L,
Hall P, Hastings E, Junkins H, McMahon A, Milano A, Morales J, et
al: The new NHGRI-EBI Catalog of published genome-wide association
studies (GWAS Catalog). Nucleic Acids Res. 45:D896–D901.
2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Ossom Williamson P and Minter CIJ:
Exploring PubMed as a reliable resource for scholarly
communications services. J Med Libr Assoc. 107:16–29.
2019.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Sherry ST, Ward MH, Kholodov M, Baker J,
Phan L, Smigielski EM and Sirotkin K: dbSNP: The NCBI database of
genetic variation. Nucleic Acids Res. 29:308–311. 2001.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Landrum MJ, Lee JM, Riley GR, Jang W,
Rubinstein WS, Church DM and Maglott DR: ClinVar: Public archive of
relationships among sequence variation and human phenotype. Nucleic
Acids Res. 42 (Database Issue):D980–D985. 2014.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Allot A, Peng Y, Wei CH, Lee K, Phan L and
Lu Z: LitVar: A semantic search engine for linking genomic variant
data in PubMed and PMC. Nucleic Acids Res. 46:W530–W536.
2018.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Gruver-Yates AL and Cidlowski JA:
Tissue-specific actions of glucocorticoids on apoptosis: A
double-edged sword. Cells. 2:202–223. 2013.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Raftopoulou S, Nicolaides NC, Papageorgiou
L, Amfilochiou A, Zakinthinos SG, George P, Eliopoulos E, Chrousos
GP and Vlachakis D: Structural Study of the DNA: Clock/Bmal1
complex provides insights for the role of cortisol, hGR, and HPA
axis in stress management and sleep disorders. Adv Exp Med Biol.
1195:59–71. 2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Strehl C, Ehlers L, Gaber T and Buttgereit
F: Glucocorticoids-All-rounders tackling the versatile players of
the immune system. Front Immunol. 10(1744)2019.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Nicolaides NC, Skyrla E, Vlachakis D,
Psarra AM, Moutsatsou P, Sertedaki A, Kossida S and Charmandari E:
Functional characterization of the hGRalphaT556I causing Chrousos
syndrome. Eur J Clin Invest. 46:42–49. 2016.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Pufall MA: Glucocorticoids and cancer. Adv
Exp Med Biol. 872:315–333. 2015.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Guo S and Dipietro LA: Factors affecting
wound healing. J Dent Res. 89:219–229. 2010.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Patil VS, Zhou R and Rana TM: Gene
regulation by non-coding RNAs. Crit Rev Biochem Mol Biol. 49:16–32.
2014.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shibuya M: Vascular Endothelial Growth
Factor (VEGF) and Its Receptor (VEGFR) signaling in angiogenesis: A
crucial target for anti- and pro-angiogenic therapies. Genes
Cancer. 2:1097–105. 2011.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Liu B and Goodwin JE: The Effect of
glucocorticoids on angiogenesis in the treatment of solid tumors. J
Cell Signal. 1:42–49. 2020.PubMed/NCBI
|
|
48
|
Nicolaides NC, Geer EB, Vlachakis D,
Roberts ML, Psarra AM, Moutsatsou P, Sertedaki A, Kossida S and
Charmandari E: A novel mutation of the hGR gene causing Chrousos
syndrome. Eur J Clin Invest. 45:782–791. 2015.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Valkenburg O, Uitterlinden AG, Themmen AP,
de Jong FH, Hofman A, Fauser BC and Laven JS: Genetic polymorphisms
of the glucocorticoid receptor may affect the phenotype of women
with anovulatory polycystic ovary syndrome. Hum Reprod.
26:2902–2911. 2011.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Schmidt S, Rainer J, Ploner C, Presul E,
Riml S and Kofler R: Glucocorticoid-induced apoptosis and
glucocorticoid resistance: Molecular mechanisms and clinical
relevance. Cell Death Differ. 11 (Suppl 1):S45–S55. 2004.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Greenstein AE and Hunt HJ: Glucocorticoid
receptor antagonism promotes apoptosis in solid tumor cells.
Oncotarget. 12:1243–1255. 2021.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Kino T and Chrousos GP: Glucocorticoid and
mineralocorticoid receptors and associated diseases. Essays
Biochem. 40:137–155. 2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Szeszko PR, Lehrner A and Yehuda R:
Glucocorticoids and hippocampal structure and function in PTSD.
Harv Rev Psychiatry. 26:142–157. 2018.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Feng X, Zhao Y, Yang T, Song M, Wang C,
Yao Y and Fan H: Glucocorticoid-Driven NLRP3 inflammasome
activation in hippocampal microglia mediates chronic stress-induced
depressive-like behaviors. Front Mol Neurosci.
12(210)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
McEwen BS, Nasca C and Gray JD: Stress
effects on neuronal structure: Hippocampus, amygdala, and
prefrontal cortex. Neuropsychopharmacology. 41:3–23.
2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Yuen EY, Liu W, Karatsoreos IN, Feng J,
McEwen BS and Yan Z: Acute stress enhances glutamatergic
transmission in prefrontal cortex and facilitates working memory.
Proc Natl Acad Sci USA. 106:14075–14079. 2009.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Liston C, Cichon JM, Jeanneteau F, Jia Z,
Chao MV and Gan WB: Circadian glucocorticoid oscillations promote
learning-dependent synapse formation and maintenance. Nat Neurosci.
16:698–705. 2013.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Liston C and Gan WB: Glucocorticoids are
critical regulators of dendritic spine development and plasticity
in vivo. Proc Natl Acad Sci USA. 108:16074–16079. 2011.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Canet G, Pineau F, Zussy C, Hernandez C,
Hunt H, Chevallier N, Perrier V, Torrent J, Belanoff JK, Meijer OC,
et al: Glucocorticoid receptors signaling impairment potentiates
amyloid-β oligomers-induced pathology in an acute model of
Alzheimer's disease. FASEB J. 34:1150–1168. 2020.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Lesuis SL, Weggen S, Baches S, Lucassen PJ
and Krugers HJ: Targeting glucocorticoid receptors prevents the
effects of early life stress on amyloid pathology and cognitive
performance in APP/PS1 mice. Transl Psychiatry.
8(53)2018.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Porta S, Danza A, Arias Saavedra M,
Carlomagno A, Goizueta MC, Vivero F and Ruiz-Irastorza G:
Glucocorticoids in systemic lupus erythematosus. Ten Questions and
Some Issues. J Clin Med. 9(2709)2020.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Hua C, Buttgereit F and Combe B:
Glucocorticoids in rheumatoid arthritis: Current status and future
studies. RMD Open. 6(e000536)2020.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Henderson I, Caiazzo E, McSharry C, Guzik
TJ and Maffia P: Why do some asthma patients respond poorly to
glucocorticoid therapy? Pharmacol Res. 160(105189)2020.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Alangari AA (ed): The Use of
Glucocorticoids in the Treatment of Acute Asthma Exacerbations,
2012 doi: 10.5772/53221.
|
|
65
|
Freishtat RJ, Nagaraju K, Jusko W and
Hoffman EP: Glucocorticoid efficacy in asthma: Is improved tissue
remodeling upstream of anti-inflammation. J Investig Med. 58:19–22.
2010.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Flammer JR and Rogatsky I: Minireview:
Glucocorticoids in autoimmunity: Unexpected targets and mechanisms.
Mol Endocrinol. 25:1075–1086. 2011.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Compston J: Glucocorticoid-induced
osteoporosis: An update. Endocrine. 61:7–16. 2018.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Chotiyarnwong P and McCloskey EV:
Pathogenesis of glucocorticoid-induced osteoporosis and options for
treatment. Nat Rev Endocrinol. 16:437–447. 2020.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Briot K and Roux C: Glucocorticoid-induced
osteoporosis. RMD Open. 1(e000014)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
McMaster A and Ray DW: Drug Insight:
Selective agonists and antagonists of the glucocorticoid receptor.
Nat Clin Pract Endocrinol Metab. 4:91–101. 2008.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Carolina E, Kato T, Khanh VC, Moriguchi K,
Yamashita T, Takeuchi K, Hamada H and Ohneda O: Glucocorticoid
impaired the wound healing ability of endothelial progenitor cells
by reducing the expression of CXCR4 in the PGE2 pathway. Front Med
(Lausanne). 5(276)2018.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Tu H, Zhang D, Barksdale AN, Wadman MC,
Muelleman RL and Li YL: Dexamethasone improves wound healing by
decreased inflammation and increased vasculogenesis in mouse skin
frostbite model. Wilderness Environ Med. 31:407–417.
2020.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Volden PA and Conzen SD: The influence of
glucocorticoid signaling on tumor progression. Brain Behav Immun.
30 (Suppl):S26–S31. 2013.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Lin KT and Wang LH: New dimension of
glucocorticoids in cancer treatment. Steroids. 111:84–88.
2016.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Gündisch S, Boeckeler E, Behrends U,
Amtmann E, Ehrhardt H and Jeremias I: Glucocorticoids augment
survival and proliferation of tumor cells. Anticancer Res.
32:4251–4261. 2012.PubMed/NCBI
|
|
76
|
Landwehr LS, Altieri B, Schreiner J,
Sbiera I, Weigand I, Kroiss M, Fassnacht M and Sbiera S: Interplay
between glucocorticoids and tumor-infiltrating lymphocytes on the
prognosis of adrenocortical carcinoma. J Immunother Cancer.
8(e000469)2020.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Yao W, Qiu HM, Cheong KL and Zhong S:
Advances in anti-cancer effects and underlying mechanisms of marine
algae polysaccharides. Int J Biol Macromol. 221:472–485.
2022.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Herrero MT, Estrada C, Maatouk L and Vyas
S: Inflammation in Parkinson's disease: Role of glucocorticoids.
Front Neuroanat. 9(32)2015.PubMed/NCBI View Article : Google Scholar
|
|
79
|
van Campen JS, Hessel EVS, Bohmbach K,
Rizzi G, Lucassen PJ, Lakshmi Turimella S, Umeoka EHL, Meerhoff GF,
Braun KPJ, de Graan PNE and Joëls M: Stress and corticosteroids
aggravate morphological changes in the dentate gyrus after
early-life experimental febrile seizures in mice. Front Endocrinol
(Lausanne). 9(3)2018.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Panayiotopoulos A, Bhangoo A, Khurana D,
Ten S, Michl J and Ghanny S: Glucocorticoid resistance in premature
adrenarche and PCOS: From childhood to adulthood. J Endocr Soc.
4(bvaa111)2020.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Greenberger S, Boscolo E, Adini I,
Mulliken JB and Bischoff J: Corticosteroid suppression of VEGF-A in
infantile hemangioma-derived stem cells. N Engl J Med.
362:1005–1013. 2010.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Koedam JA, Smink JJ and van Buul-Offers
SC: Glucocorticoids inhibit vascular endothelial growth factor
expression in growth plate chondrocytes. Mol Cell Endocrinol.
197:35–44. 2002.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Frey A and Seifart KH: Glucocorticoids
directly affect the synthesis of ribosomal RNA in rat-liver cells.
Mol Cell Endocrinol. 28:161–172. 1982.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Matsui H, Yazawa H, Suzuki N and Hosoya T:
Effects of glucocorticoid and cycloheximide on the activity and
amount of RNA polymerase I in nuclei of rat liver. Biochem J.
235:699–705. 1986.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Bruna A, Nicolàs M, Muñoz A, Kyriakis JM
and Caelles C: Glucocorticoid receptor-JNK interaction mediates
inhibition of the JNK pathway by glucocorticoids. EMBO J.
22:6035–6044. 2003.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Mayer C, Bierhoff H and Grummt I: The
nucleolus as a stress sensor: JNK2 inactivates the transcription
factor TIF-IA and down-regulates rRNA synthesis. Genes Dev.
19:933–941. 2005.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Shimizu N, Yoshikawa N, Ito N, Maruyama T,
Suzuki Y, Takeda S, Nakae J, Tagata Y, Nishitani S and Takehana K:
Crosstalk between glucocorticoid receptor and nutritional sensor
mTOR in skeletal muscle. Cell Metab. 13:170–182. 2011.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Mayer C, Zhao J, Yuan X and Grummt I:
mTOR-dependent activation of the transcription factor TIF-IA links
rRNA synthesis to nutrient availability. Genes Dev. 18:423–434.
2004.PubMed/NCBI View Article : Google Scholar
|