Introduction
Lung cancer rates second in incidence, but first as
a cause of cancer-related mortality worldwide and in the United
States (1,2). In 2020, ~2.2 million new cases were
diagnosed worldwide and 1.8 million deaths occurred with a
mortality-to-incidence ratio of 0.82(2). The 5-year survival rate of patients
with lung cancer was 56% in 2015 for cases detected when the
disease was still localized. However, only 16% of lung cancer cases
are diagnosed at an early stage (3). This leads to only 15% of patients
with lung cancer remaining alive at 5 years, since 70% already
present with advanced stages of the diseases at the time of
diagnosis (4).
Accordingly, in order to decrease the burden of lung
cancer, solid efforts need to be made towards the early detection
and smoking cessation in addition to the optimization of
management. In fact, trends towards a decrease in lung cancer
incidence have been noted in the United States between 2009 and
2018 (by 1 and 3% annually for females and males, respectively)
(1). Recently, it was shown that
the incidence has even been steeply declining further for
advanced-stage disease, while rates for localized-stage disease
increased suddenly by 4.5% annually (1). This decline coincides and mirrors the
decrease in smoking rates in addition to the increase in lung
cancer screening. In Lebanon, lung cancer ranks second in incidence
following breast cancer, accounting for 9.2% of all cancer cases
reported between 2005 and 2015, mostly among patients >50 years
of age (89.2%) (5). This is
attributed to the high prevalence of smoking, air pollution,
exposure to asbestos and other carcinogens, and family history. A
recent study revealed that lung cancer represents 8% of all cancer
cases (11.4% in males; 5.1% in females) and ranks second after
breast cancer, with smoking again being the essential risk factor
(6). In fact, the rates of tobacco
smoking in Lebanon were reported in a study published in 2017; the
findings of that study reported a high incidence of 50.3% among
males and 34.1% among females, ranking second and first in the
Middle East and North Africa region, respectively (7). Moreover, in line with the global
trend, close to 60% of lung cancer cases in Lebanon are stage IV,
and only 12% stage IA (8).
Unfortunately, patients diagnosed with a distant metastatic disease
(stage IV) have a 1-year survival rate of around 20% compared to
81-85% for stage I (9).
In addition, Lebanon is a low-income country with
increasing rates of poverty, and recent dramatic decreases in
access to medical care and to optimal management pathways. This
translates into increasing late presentations of patients and the
late diagnosis of diseases, with limited options of treatment, as
well as the absence of a proper primary care set-up.
Hence, the expanding triad of high incidence and
mortality rates of lung cancer, high tobacco consumption and
delayed diagnosis suggests that there is an urgent need for smoking
cessation and lung cancer screening programs to be rapidly
implemented on the national level. In that perspective, the
objectives of the present consensus were to review the current
burden of lung cancer in Lebanon, to identify the gaps in achieving
early detection, and to set local recommendations for lung cancer
screening.
Drawing of recommendations and review of
evidence
A panel of experts, including members of the
Lebanese Society of Medical Oncology (LSMO) and the Lebanese
Pulmonary Society (LPS) convened and discussed all aspects and
challenges related to lung cancer screening in Lebanon.
International guidelines and important trials were presented and
discussed, as well as worldwide implementation strategies.
Accordingly, national guidelines were set, as well as
implementation recommendations, taking into consideration the
obstacles and challenges that may be faced. A voting system was
used to draw recommendations.
For the review of the evidence and the international
guidelines presentation, and to ensure that the recommendations
were evidence-based, a systematic literature review of the studies
and guidelines published between January, 2010 and 2022, was
carried out using the Medline and EMBASE databases. Global
recommendations and literature-based latest medical evidence are
also displayed in this joint statement.
Lung cancer screening in global
practice
The importance of lung cancer screening has been
demonstrated in several randomized trials and has resulted in a
significant decrease in mortality in the screened high-risk
populations and is currently uniformly acknowledged (10-12).
To date, the only recommended screening tool for that purpose is
low-dose computed tomography (CT); It has been shown to have high
sensitivity and acceptable specificity for the detection of lung
cancer and its adoption at specific intervals in high-risk
individuals leads to a decrease in lung cancer-related mortality
(10-12).
Various other modalities and combinations of tests are not
recommended, as they have not been shown to be beneficial or lack
standardization, including chest radiographies, genomics,
biomarkers and sputum cytology (13). Of note, some population-based
cohort studies have underlined the effectiveness of chest
radiographies in detecting early lung lesions and in reducing the
mortality rate of patients with lung cancer (14-16).
Additionally, in view of the evolution of artificial intelligence
in powering readings (17), there
is a need for further pilot studies for evaluation, since chest
radiographies are low in cost, readily available and are performed
in high numbers.
The two largest trials supporting the use of a
low-dose CT scan for screening are the 2011 National Lung Screening
Trial (NLST) and the 2020 NELSON studies (10,12).
These two large studies randomized 53,454 and 15,822 individuals
who were at a high-risk of developing lung cancer, respectively and
compared screening by a low-dose CT scan versus screening by chest
radiography (annually for 3 years) in the first one and to no
screening in the NELSON trial. They defined high risk according to
age and smoking history. There was a 20% reduction in mortality
from lung cancer and 6.7% fewer all-cause deaths, in the low-dose
CT scan group compared to the radiography group in the NSLT study
(10). In the NELSON study, at the
10-year data point, lung cancer was diagnosed in 5.58 cases per
1,000 person-years in the low-dose CT scan screening group versus
4.91 cases per 1,000 person-years in the control group. Lung cancer
mortality was 2.50 deaths per 1,000 person-years in the low-dose CT
scan screening group, lower than the 3.30 deaths per 1,000
person-years in the control group (12).
In the NLST trial, screening identified a
predominance of lung adenocarcinoma. In both the low-dose CT scan
and the radiography groups, a large number of adenocarcinomas and
squamous-cell carcinomas were detected at either stage I or stage
II, while small-cell lung cancer lesions were not detected at early
stages (10). In the NELSON trial,
adenocarcinomas were the most frequently detected lung cancer
subtype, 123 out of 203 screened cases. Lower proportions of
squamous cell or small-cell lung carcinomas were detected in the
screened group, compared to the non-screened group. However, the
low-dose CT scan consistently detected the different cancer
subtypes at lower stages of disease (12).
A very recent meta-analysis revealed that among
smokers screened for lung abnormalities using a low-dose CT scan,
the most frequent histologic type was adenocarcinoma, followed by
squamous cell and small cell carcinomas (18).
In addition to the NLST and NELSON trials, Table I lists the main studies adopted in
the majority of randomized controlled trials (RCTs) on the use of a
low-dose CT scan for lung cancer screening (12,19-22).
The RCTs in Table I established
low-dose CT scan as the most convenient and advantageous tool in
lung cancer screening. Therefore, governing bodies and societal
recommendations published guidelines adopting it for lung cancer
screening (Table II) (23-29).
 | Table IOverview of the major trials on
low-dose CT scans. |
Table I
Overview of the major trials on
low-dose CT scans.
| Trial | NLST (10) | DANTE (19) | DLCST (20) | NELSON (12) | MILD (21) | LUSI (22) |
|---|
| Year | 2011 | 2015 | 2016 | 2020 | 2019 | 2020 |
| Country | USA | Italy | Denmark | Belgium and The
Netherlands | Italy | Germany |
| No. of
subjects | 53,454 | 2,250 | 4,104 | 15,822 | 4,099 | 4,052 |
| Eligibility | | | | | | |
|
Age,
years | 55-74 | 60-74 | 50-70 | 50-75 | 49-75 | 50-69 |
|
Pack-years | ≥30 | ≥20 | >20 | ≥15 | ≥20 | ≥15 |
|
Quit
years | <15 | <10 | <10 | <10 | <10 | <10 |
| Comparator | Annual chest
X-ray | Annual clinical
review | - | - | - | - |
| Mortality
reduction | | | | | | |
|
Lung
cancer | 20% | 1% | 0% | 26% male | 39% | 24% |
| | | | | 61% female | | |
|
All-cause | 6.7% | 5% | 0% | - | 20% | 1% |
 | Table IIRecommendations for lung cancer
screening using a low-dose CT scan. |
Table II
Recommendations for lung cancer
screening using a low-dose CT scan.
| Organization and
year | Statements |
|---|
| 2021 | |
| National
Comprehensive Cancer Network | Recommends yearly
screening with Low-dose computed tomography to |
| | •Group 1: Adults 55
to 77 years of age who have ≥30 pack-years of smoking (current
smokers or who have quit within the past 15 years) |
| | •Group 2: Adults
≥50 years of age with ≥20 pack-years of smoking, had quit at any
time, with at least one additional risk factor beside second hand
smoke (family history of lung cancer, diagnosis of COPD,
occupational exposure to known carcinogen or personal history of
tobacco-related malignancy) (23). |
| US Preventive
Services Task Force | The USPSTF
recommends annual screening for lung cancer with low-dose CT in
adults aged 50 to 80 years who have a 20 pack-year smoking history
and currently smoke or have quit within the past 15 years (24). |
| American College of
Chest Physicians | For asymptomatic
individuals aged 50 to 80 who have smoked ≥20 pack years and either
continue to smoke or have quit within the past 15 years, annual
screening with low-dose CT is recommended (25). |
| American Society of
Clinical Oncology | ASCO recommends […]
yearly screening with a low-doseCT scan […] for people age 55 to 74
who have smoked for 30 pack years or more. It is also recommended
for those age 55 to 74 who have quit within the past 15 years
(26). |
| American Lung
Association | Screening is
recommended (for subjects who are) 50-80 years of age, have a 20
pack-year history of smoking, and are a current smoker, or have
quit within the last 15 years (27). |
| 2018 | |
| American College of
Chest Physicians | Annual screening
with low-dose CT is recommended for adults 55 to 77 years of age
with no symptoms of lung cancer who have smoked at least 30 pack
years, and who continue to smoke or have quit within the past 15
years (28). |
| 2016 | |
| The Canadian Task
Force on Preventive Health Care | Screening is
recommended for lung cancer among adults 55 to 74 years of age with
at least a 30 pack-year smoking history,who smoke or quit smoking
<15 years prior, with low-dose computed tomography (CT) every
year up to three consecutive years (29). |
Defining the high-risk population
The risk of lung cancer consistently increases with
age and smoking. The latter remains a leading risk factor for lung
cancer, contributing to >70% of worldwide lung cancer-related
deaths (30), particularly with an
elevated number of pack-years (31,32),
although lung cancer also occurs in never-smokers (33-37).
Other predisposing factors include a family history of lung cancer
(38), asbestos exposure and
second-hand smoking, as well as less obvious risk factors, such as
exposure to cooking fumes, to hormone replacement therapies and to
certain viral infections such as human papillomavirus (39).
Globally, following the NLST, several societal,
governmental and regulatory bodies and later some reimbursement
bodies, adopted the same criteria for the selection of the
high-risk population, while others shifted the definition. The
rationale behind the shift derives from studies demonstrated
social, ethnic, racial, regional and factorial disparities in the
rates of lung cancer incidence and mortality. Recently, the
National Comprehensive Cancer Network (NCCN) included a new group
in their guidelines, taking into consideration the presence of
other factors related to personal and family history (23). Therefore, there is still no
globally standardized definition of the high-risk population yet
and variable classifications are drawn and adopted by different
stakeholders (Table II).
In Lebanon, among the 1,133 cases of lung cancer
diagnosed in 2016, 53% were patients in the 55-74-year age group
(40). Pollution is an additional
risk factor contributing to the increase in lung cancer incidence.
In a country where the main governmental power grid is not
reliable, highly polluting diesel generators remain an
indispensable choice for all public and private sectors, including
households and the health, educational, economic and touristic
sectors (41). This is in addition
to overloaded traffic and unregulated waste incineration (41). Moreover, apart from the widespread
use of the combustible cigarettes, water-pipe (hookah or shisha)
smoking is very popular in Lebanon, particularly among younger
adults (42). The water-pipe has
also recently gained popularity worldwide (43,44).
While it is mistakenly perceived as a safer alternative to
combustible cigarette smoking, scientific evidence strongly refutes
this widespread belief (45). Of
note, one cigarette contains ~1 g of tobacco, while one shisha
session consumes between 8 and 12 g of tobacco (46). Plasma nicotine (cotinine) levels
following a 1-h shisha session is equivalent to ~100 cigarettes and
a single breath of shisha delivers 8-fold more smoke particles than
would a breath of cigarette (47).
In practice, comparing cigarette and Shisha smoking is difficult
since Shisha smoking occurs over prolonged durations and with each
puff, the volume inhaled is higher in addition to the presence of
other forms of known carcinogens in the constituents. It has been
shown that Shisha smoking increases the risk of lung cancer by
6-fold in comparison to non-smokers (48). Furthermore, carboxyhemoglobin forms
faster and the heart rate increases faster upon water-pipe smoking,
than with the combustible cigarette further underscoring the need
for physicians to advise their patients that water-pipe smoking
exposes them to some of the same toxicants as cigarette smoking
(49).
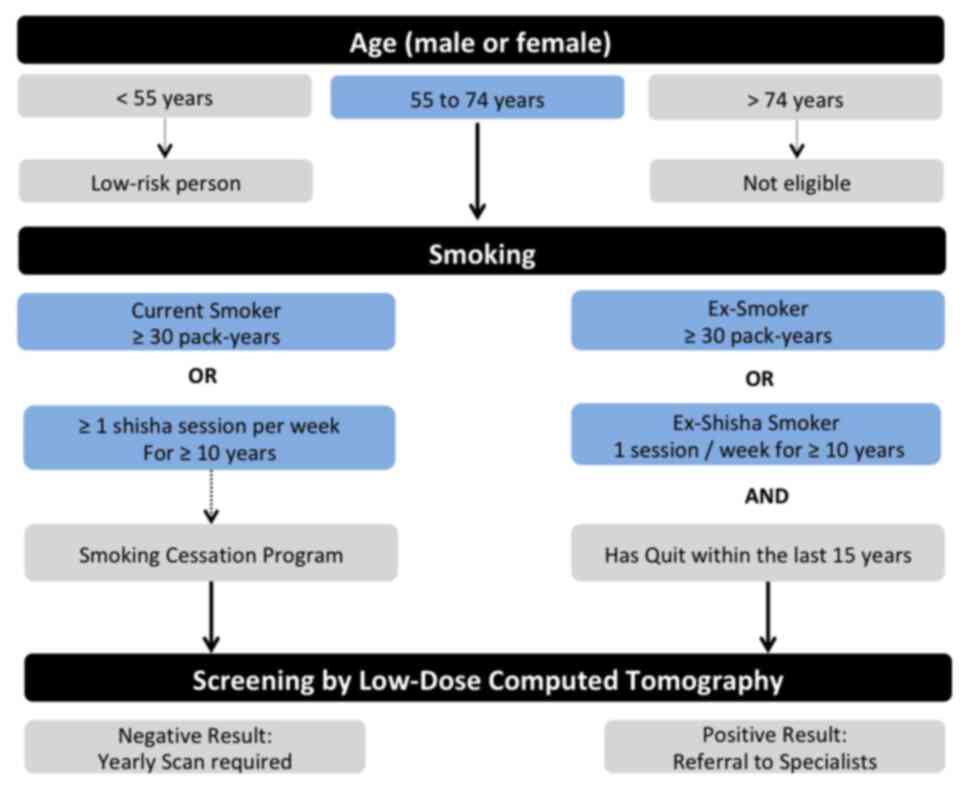
All these particularities were taken into
consideration by the panel who proposed, as displayed in Fig. 1, defining the high-risk population
eligible for screening for lung cancer using low-dose CT scan as
follows: i) Male or female subjects, between 55 and 74 years of
age, with a smoking history of 30 pack-years, whether current
smokers or having quit tobacco within 15 years; ii) or current
smokers of at least one shisha session per week for a period of
>10 years or for ex-smokers of the same rate who have quit
within 15 years; iii) these subjects should undergo yearly
screening, along with a smoking cessation counselling program, and
in the case of a diagnosis of lung cancer, they should be offered a
proper medical follow-up.
Recommendations
For screening eligibility, the following factors are
considered: i) An age between 55 to 74 years; ii) smoking history:
30 pack-years and/or at least one shisha session per week for a
period of >10 years for current smokers or for those who have
quit tobacco within 15 years.
Pillars to success
The implementation of lung screening programs
worldwide has been limited due to numerous reasons and it is
essential that a generalized awareness is key to optimize the
benefits. Practically, high-risk individuals whose low-dose CT scan
results are negative still need to be screened on a yearly basis.
The annual incidence of lung cancer detection by this approach
revolves ~1%, further underscoring the urge to set and improve such
a screening initiative (50,51).
In addition, for this initiative to be sustained and successful,
there should be an in-depth analysis of the unmet needs in the
Lebanese society. In fact, the success of a lung screening is
dependent on three main pillars: i) A nationally established
standardized program with evidence-based guidelines and
high-quality services and facilities. Optimal smoking cessation
programs, a proper follow-up (and expert referral for positive
findings), and further management potentials are of significance as
well. ii) High adoption levels by individuals, physicians and
concerned bodies and societies. Complete adherence to the program
and sustainability are also primordial. iii) Successful continuous
awareness and educational, promotional and incentive campaigns.
The overall setting of a screening campaign is
summarized in the schematic diagram in Fig. 2; it displays the selection criteria
for the population at risk eligible for screening and presents the
pillars in the success of a screening initiative, tackling
different aspects from the selection of centers to the promotional
and awareness campaigns as well as the involvement of physicians
and third-party payers. Each aspect will be detailed individually
in the following sections of this manuscript.
Quality and logistics
For the expert panelists, the quality of screens is
very important and affects outcomes as well as the
cost-effectiveness of a lung screening program. In fact,
realistically, there is variability between centers in Lebanon in
relation to their qualification to perform lung cancer screening
and the program must thus include criteria to organize the
practice.
Centers
According to expert panelists, not all centers in
Lebanon are well-equipped with high-resolution CT scans, while this
is an essential component of a beneficial program. Furthermore,
even in the centers that possess such machines, set-ups and
changing the parameters might constitute a hectic task. In
addition, there is a lack in trained personnel. However, with some
facility-related restructuring and organization, the process can be
established through specific schedules, acquiring separate machines
and collaboration between centers. The panel suggests selecting no
more than 25 well-equipped centers, geographically distributed
across Lebanon and specialized in lung cancer screening. The
centers shall host trained and dedicated nurses, radiologists and
technicians, and should elaborate and implement nationwide
standards.
Radiologists
It has been recognized globally that only expert
radiologists, trained and qualified for interpretation of the scans
must be designated. In addition, for an optimal screening process,
there should be a unified and standardized classification and
reporting system. For these reasons, the panel recommends the
limitation of radiologists who are eligible to perform the
screening through the limitation of centers as mentioned above.
Each center shall include an expert radiologist according to set
criteria. These criteria along with standardized radiologic
criteria can be set by the Lebanese Ministry of Public Health
(MoPH) in alignment with the Lebanese society of radiology and it
can also be done through a consensus. Another interesting option
the panel proposed is the designation of few expert radiologists by
the MoPH to take care of the readings. Thus, with more and more
readings, more expertise can be obtained as well as more
standardization of readings. This option appears reasonable in a
small country, such as Lebanon, while the practice can be made
possible by sending the scans performed in different centers to a
central station. This is an easy possibility nowadays with all the
development that was witnessed in data transmission in the last
decade.
Recommendations
For quality and logistics, the following are
recommended: i) Well-equipped centers geographically distributed
across Lebanon implementing nationwide standards; ii) limit
recognized centers to no more than 25 in the country; iii) centers
shall include high resolution machines and trained personnel; iv)
centers shall have expert radiologists with specific criteria (set
on the national level), or central readings by a limited number of
radiologists appointed by the ministry of public health. The
possibility of expansion of the number of centers with time and
according to the need and qualification is also proposed.
Awareness to all
Referring physicians
All licensed physicians registered at the Lebanese
order of physicians are eligible and invited to participate in the
program whether a primary care physician or a specialist. However,
a recent study revealed a lack of awareness related to lung cancer
screening even among pulmonary physicians (52). Thus, the panel recommends awareness
campaigns for physicians to be organized by different stakeholders,
the launch of accredited programs and invites physicians to
self-educate on the matter, since the basics are relatively simple.
The present consensus study may also be helpful in that
perspective. Physicians shall include individuals in the program
following a medical visit and it is recommended that a shared
written informed consent should be signed after counseling by the
physician on the importance of lung cancer screening. Physicians
are also responsible of explaining the benefits of adherence and
sustainability to the program, the importance of smoking cessation
and the risks of false positives and negatives, as well as the
possible complications of further procedures if needed. In
parallel, a discussion with the persons on the possibility of
finding other pathologies through the scans, and on the decreased
risks of repeated radiation exposure with low-dose CT scans, since
this may be an anxious point they may be fearful of. Individuals
may also share their fear of diagnoses and thus physicians need to
discuss and alleviate this concern. Referrals to smoking cessation
programs shall also be proposed during this visit. Of note: i)
Every interested and prepared licensed referring physician can
participate; and ii) referrals are recommended to occur following
medical visits and after a signed informed consent.
Awareness in the population
The importance of early diagnosis is still
under-recognized in the Lebanese population, with an ambivalence
faced regarding the effectiveness of adopting health-related
behavioral changes, in addition to the fear felt by the general
population towards radiation exposure. The panel recommends
large-scale awareness campaigns targeting the general population on
the importance of lung screening and timely detection of any
lesions and on the essential role of low-dose CT scan in such
screening, and its safety profile given its very low radiation
exposure. Awareness campaigns should also promote health beliefs
and perceived benefits of risk factor mitigation, mostly smoking
cessation. Learning from previous successful campaigns can help
draw a successful strategy targeting a beneficial and successful
implementation and funding for such campaigns can be mirrored to
previous experiences. Campaigns shall focus on the importance of
early diagnosis, the decrease in burden and optimal outcomes of
treating the disease at early stages in addition to a benefit in
the reduction of mortality. Moreover, sustainability is key for the
person to completely adhere to the program and this message is to
be transmitted. In addition, smoking cessation campaigns are to be
associated to motivate cessation, to increase referrals to smoking
cessation programs and institutions, as well as to try to hinder
smoking initiation especially by the youth. Of note: i) Awareness
campaigns to the people are key; ii) focus should be placed on the
benefits of screening and of complete adherence, as well as the
harms of smoking.
Awareness to regulatory and reimbursement
bodies. The benefits of lung cancer screening are important on
all levels whether medical, social, cost-related and on the
management level. In particular, oncologists, pulmonary physicians
and radiologists in addition to expert physicians should raise to
the MoPH the issue of late-stage diagnosis of lung cancer and the
tremendous cost of treating advanced stage diseases; in an attempt
to promote lung cancer screening by low-dose CT scan as a
cost-effective measure. Awareness campaigns and workshops should
target and educate concerned parties. In fact, due to the
misconception that lung cancer is exclusively a self-inflicted
disease, great resistance has been observed from third-party payers
to cover lung cancer screening tests. Additionally, the MoPH has
been reluctant to ratify the establishment of a lung cancer
committee to implement screening recommendations. The panel
suggests developing a policy in light of the clinical trial
evidence and highlighting the cost-effectiveness of lung cancer
screening for early detection to both third-party payers and the
MoPH. It may be difficult to implement with the current situation
of the paying parties; however, the panel encourages at least a
cost-effectiveness analysis that will show the benefits of
screening on the health system and in lowering the burden. Short
and long-term strategies can be established on that basis.
Awareness campaigns and workshops are to be organized and proposed
to these bodies. Of note: i) Awareness may be made through
campaigns and workshops to third-party payers; ii) delineate the
moderate-term and long-term cost benefits; iii) invitation for
cost-effective analysis and the establishment of strategies.
Coverage of lung cancer screening
Panel physicians expressed their dismay at the
difficulty of ensuring financial coverage for screening purposes.
Medical coverage schemes, whether private insurance companies,
mutuality funds or the National Social Security Fund (NSSF) tend to
block the coverage of screening tests. This is in discordance with
global practice. In the UK, CT screening is a recommended clinical
practice, subject to guidelines and reimbursement (9). This indicates the urgent need for a
nationwide supported initiative to implement lung cancer screening
among the population at high risk for lung cancer.
The success of such an initiative will translate
into the prevention of mortality and morbidity from lung cancer,
the alleviation of the healthcare burden of lung cancer, and a
reduction in economic losses due to sickness and absenteeism. From
a health economics standpoint, launching a lung cancer screening
program in Lebanon, using low-dose CT scan could, at the long run,
prove beneficial and save third-party payers substantial funds.
Between 2008 and 2013, the total average annual cost of drugs for
lung cancer was estimated at 11,397,019 USD; constituting 6.5% of
the total healthcare expenditure on major cancers (53).
Third-party payers (pharmaceutical companies,
non-governmental organizations and medical societies) could be
approached for financial support in covering, at least partially,
screening costs by low-dose CT scans in established, specialized
and properly maintained centers.
A lung cancer screening task force could be
established, including members from the LPS and LSMO. This task
force, under the patronage of the MoPH, could oversee the
nationwide implementation of the local guidelines for lung cancer
screening, in terms of health facility mapping and equipment,
training of dedicated personnel and access of the high-risk
population. Moreover, the Ministry of Economy and Trade could
impose additional taxation on tobacco products to raise their
retail price in an attempt to limit smoking (54). Of note, it may be beneficial to
develop a policy to unify the screening procedure and the financial
coverage of yearly screening in persons-at-risk by third party
payers.
Smoking cessation
According to a 2022 study, smoking prevalence in
Lebanon stands at 27%, the highest between several other countries
in the Middle East, heavily contributing to the occurrence of lung
cancer (6). An earlier study
reported a prevalence of close to 35%, with twice as many male as
female smokers, and a three-fold likelihood of hospitalization
among smokers than among non-smokers (55). While taking up smoking is a
personal decision, smokers find it difficult to quit smoking and
long-term tobacco use engenders the risk of lung cancer. Smoking
cessation initiatives, whether personal or with the aid of a
counselor, often do not yield the desired outcomes (56,57).
However, the usefulness of smoking cessation counseling depends
deeply on the counselors' knowledge of clinical practice
guidelines, on perceived support, the belief that smoking cessation
will positively impact health, and the presence of formal smoking
cessation programs (58). The
Lebanese Ministry of Public Health tobacco control program
(https://www.moph.gov.lb/en/Pages/2/3173/tobacco-program)
could serve as a platform for smoking cessation interventions and
guidance. Globally, active tobacco control programs have resulted
in decreased rates of lung cancer, attesting to their usefulness
(59-62).
In addition to public health-oriented behavioral interventions, a
pharmacological approach to smoking cessation might yield long-term
abstinence from smoking (63-65).
Nicotine-replacement preparations are efficient first-line agents
(65). Veranicline is an
FDA-approved medication for smoking cessation, with proven benefits
(63,64). Sustained-release bupropion or
clonidine can also be offered to help with smoking cessation
(65). Smoking control
interventions and disease surveillance frameworks are required to
alleviate healthcare burden and to establish health-related
policies (55). On the whole, the
following may be beneficial: i) Smoking cessation guidelines,
clinics and campaigns are essential for successful lung cancer
screening programs; ii) reliance on programs already set by the
MoPH in Lebanon.
Panel recommendations
The following recommendations are proposed: i) The
panel recommends a yearly low-dose CT scan for all subjects at high
risk of lung cancer, who are aged 55 to 74 years, and have a
smoking history of cigarettes and/or shisha; ii) well-equipped
centers geographically distributed across Lebanon with trained and
dedicated personnel, and implementing nationwide standards; iii)
developing a policy to unify the screening procedure and the
financial coverage of yearly screening in persons-at-risk by third
party payers; iv) awareness campaigns for the general population on
the importance of screening, the safety and efficacy of low-dose CT
scan, and the promotion of health-related behavioral changes mostly
smoking cessation; v) awareness campaigns for the physicians
involved in the screening initiative on the selection of specific
profiles for the screening.
Conclusion
The present joint statement highlights the
importance of a large-scale screening campaign for lung cancer
using low-dose CT scan. Despite the limited implementation of
low-dose CT scan for the screening of lung cancer in Lebanon, a
recent study demonstrated that a significant proportion of primary
care physicians and pulmonary specialists are still using
suboptimal screening modalities, such as chest radiography (despite
the availability and accessibility of more advanced technology),
with an inaccurate selection of the population at risk (52). This highlights the need for new
guidelines that are set by this consensus, recommending the
targeting of the general population, the physicians involved in all
phases of lung cancer care, and dedicated personnel in specialized
centers, for a successful screening campaign. In addition, the
panel seeks the support of third-party payers to make this program
marketable; including consultation, CT screening, smoking cessation
counseling and psychological/clinical follow-ups, as needed. With a
clear definition of the high-risk patient population for lung
cancer now established in Lebanon, along with recommendations on
setting up a nation-wide lung screening initiative, the present
report encourages a collective effort from medical and public
health societies with the support of third-party payers to
implement such an initiative.
Acknowledgements
The authors would like to thank the contract
research organization KBP-Biomak, Lebanon, for their assistance
with the study coordination and medical writing.
Funding
Funding: The present study was supported by an unrestricted
grant from AstraZeneca.
Availability of data and materials
Not applicable.
Authors' contributions
All authors (ZAB, NB, FEK, GJ, FN, RN, AT and SZ)
contributed to the collection of international guidelines, setting
the local guidelines, writing and reviewing of the manuscript. All
authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33.
2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
GLOBOCAN: Global cancer observatory.
Journal, 2022.
|
|
3
|
SEER Cancer Statistics Review. Cancer stat
facts: Lung and bronchus cancer. Journal, 2021.
|
|
4
|
Walters S, Maringe C, Coleman MP, Peake
MD, Butler J, Young N, Bergström S, Hanna L, Jakobsen E, Kölbeck K,
et al: Lung cancer survival and stage at diagnosis in Australia,
Canada, Denmark, Norway, Sweden and the UK: A population-based
study, 2004-2007. Thorax. 68:551–564. 2013.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Salhab HA, Fares MY, Khachfe HH and
Khachfe HM: Epidemiological Study of lung cancer incidence in
Lebanon. Medicina (Kaunas). 55(217)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Shurrab SA, Al-Badarneh AF, Nassar HI and
Almshnanah AH: Cancer in five countries of the eastern
mediterranean region: Epidemiological trends and risk implications.
Niger J Clin Pract. 25:78–84. 2022.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Rahal Z, El Nemr S, Sinjab A, Chami H,
Tfayli A and Kadara H: Smoking and lung cancer: A geo-regional
perspective. Front Oncol. 7(194)2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Salman R, Amhaz K, Hellani A, Tayara L and
Mourda B: The Epidemiology of Lung Cancer in Lebanon During 2014.
Int J Epidemiol Res. 7:63–67. 2020.
|
|
9
|
Bannister N and Broggio J: Cancer survival
by stage at diagnosis for England (experimental statistics): Adults
diagnosed. 2012, 2013 and 2014 and followed up to 2015. Journal,
2016.
|
|
10
|
National Lung Screening Trial Research
Team. Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, Fagerstrom
RM, Gareen IF, Gatsonis C, Marcus PM and Sick JD: Reduced
lung-cancer mortality with low-dose computed tomographic screening.
N Engl J Med. 365:395–409. 2011.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pinsky PF, Church TR, Izmirlian G and
Kramer BS: The national lung screening trial: Results stratified by
demographics, smoking history, and lung cancer histology. Cancer.
119:3976–3983. 2013.PubMed/NCBI View Article : Google Scholar
|
|
12
|
de Koning HJ, van der Aalst CM, de Jong
PA, Scholten ET, Nackaerts K, Heuvelmans MA, Lammers JWJ, Weenink
C, Yousaf-Khan U, Horeweg N, et al: Reduced lung-cancer mortality
with volume CT screening in a randomized trial. N Engl J Med.
382:503–513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Cainap C, Pop LA, Balacescu O and Cainap
SS: Early diagnosis and screening in lung cancer. Am J Cancer Res.
10:1993–2009. 2020.PubMed/NCBI
|
|
14
|
Dominioni L, Poli A, Mantovani W, Pisani
S, Rotolo N, Paolucci M, Sessa F, Conti V, D'Ambrosio V, Paddeu A
and Imperatori A: Assessment of lung cancer mortality reduction
after chest X-ray screening in smokers: A population-based cohort
study in Varese, Italy. Lung Cancer. 80:50–54. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kim J and Kim KH: Role of chest
radiographs in early lung cancer detection. Transl Lung Cancer Res.
9:522–531. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Nakayama T, Baba T, Suzuki T, Sagawa M and
Kaneko M: An evaluation of chest X-ray screening for lung cancer in
gunma prefecture, Japan: A population-based case-control study. Eur
J Cancer. 38:1380–1387. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Kaviani P, Digumarthy SR, Bizzo BC, Reddy
B, Tadepalli M, Putha P, Jagirdar A, Ebrahimian S, Kalra MK and
Dreyer KJ: Performance of a chest radiography AI algorithm for
detection of missed or mislabeled findings: A multicenter study.
Diagnostics (Basel). 12(2086)2022.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Hunger T, Wanka-Pail E, Brix G and Griebel
J: Lung cancer screening with low-dose CT in smokers: A systematic
review and meta-analysis. Diagnostics (Basel).
11(1040)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Infante M, Cavuto S, Lutman FR, Passera E,
Chiarenza M, Chiesa G, Brambilla G, Angeli E, Aranzulla G, Chiti A,
et al: Long-term follow-up results of the DANTE trial, a randomized
study of lung cancer screening with spiral computed tomography. Am
J Respir Crit Care Med. 191:1166–1175. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wille MMW, Dirksen A, Ashraf H, Saghir Z,
Bach KS, Brodersen J, Clementsen PF, Hansen H, Larsen KR, Mortensen
J, et al: Results of the randomized danish lung cancer screening
trial with focus on high-risk profiling. Am J Respir Crit Care Med.
193:542–551. 2016.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Pastorino U, Silva M, Sestini S, Sabia F,
Boeri M, Cantarutti A, Sverzellati N, Sozzi G, Corrao G and
Marchianò A: Prolonged lung cancer screening reduced 10-year
mortality in the MILD trial: New confirmation of lung cancer
screening efficacy. Ann Oncol. 30:1162–1169. 2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Becker N, Motsch E, Trotter A, Heussel CP,
Dienemann H, Schnabel PA, Kauczor HU, Maldonado SG, Miller AB,
Kaaks R and Delorme S: Lung cancer mortality reduction by LDCT
screening-results from the randomized German LUSI trial. Int J
Cancer. 146:1503–1513. 2020.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wood DE, Kazerooni EA, Baum SL, Eapen GA,
Ettinger DS, Hou L, Jackman DM, Klippenstein D, Kumar R, Lackner
RP, et al: Lung cancer screening, version 3.2018, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
16:412–441. 2018.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Dyer O: US task force recommends extending
lung cancer screenings to over 50s. BMJ. 372(n698)2021.PubMed/NCBI View
Article : Google Scholar
|
|
25
|
Mazzone PJ, Silvestri GA, Souter LH,
Caverly TJ, Kanne JP, Katki HA, Wiener RS and Detterbeck FC:
Screening for lung cancer: CHEST guideline and expert panel report.
Chest. 160:e427–e494. 2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
American Society of Clinical Oncology.
Lung cancer-non-small cell: Screening. 2021 27 January 2022].
Available from: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/screening.
|
|
27
|
American Lung Association. Is lung cancer
screening right for me? 2021 27 January 2022]. Available from:
https://www.lung.org/lung-health-diseases/lung-disease-lookup/lung-cancer/saved-by-the-scan/resources/is-lung-cancer-screening-right.
|
|
28
|
Armstrong C: Lung cancer screening
recommendations from the ACCP. Am Fam Physician. 98:688–689.
2018.PubMed/NCBI
|
|
29
|
Canadian Task Force on Preventive Health
Care. Recommendations on screening for lung cancer. CMAJ.
188:425–432. 2016.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Ordóñez-Mena JM, Schöttker B, Mons U,
Jenab M, Freisling H, Bueno-de-Mesquita B, O'Doherty MG, Scott A,
Kee F, Stricker BH, et al: Quantification of the smoking-associated
cancer risk with rate advancement periods: Meta-analysis of
individual participant data from cohorts of the CHANCES consortium.
BMC Med. 14(62)2016.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Bach PB, Kattan MW, Thornquist MD, Kris
MG, Tate RC, Barnett MJ, Hsieh LJ and Begg CB: Variations in lung
cancer risk among smokers. J Natl Cancer Inst. 95:470–478.
2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Katki HA, Kovalchik SA, Berg CD, Cheung LC
and Chaturvedi AK: Development and validation of risk models to
select ever-smokers for CT lung cancer screening. JAMA.
315:2300–2311. 2016.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Ball D: The IASLC multidisciplinary
approach to thoracic oncology. Aurora, CO: International
Association for the Study of Lung Cancer, 2014.
|
|
34
|
McCarthy WJ, Meza R, Jeon J and Moolgavkar
SH: Chapter 6: Lung cancer in never smokers: Epidemiology and risk
prediction models. Risk Anal. 32 (Suppl 1):S69–S84. 2012.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Alberg AJ, Wallace K, Silvestri GA and
Brock MV: Invited commentary: The etiology of lung cancer in men
compared with women. Am J Epidemiol. 177:613–616. 2013.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Planchard D and Besse B: Lung cancer in
never-smokers. Eur Respir J. 45:1214–1217. 2015.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Subramanian J and Govindan R: Lung cancer
in ‘never-smokers’: A unique entity. Oncology (Williston Park).
24:29–35. 2010.PubMed/NCBI
|
|
38
|
Lorenzo Bermejo J and Hemminki K: Familial
lung cancer and aggregation of smoking habits: A simulation of the
effect of shared environmental factors on the familial risk of
cancer. Cancer Epidemiol Biomarkers Prev. 14:1738–1740.
2005.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Bae JM: Modifiable risk factors of lung
cancer in ‘never-smoker’ women. Epidemiol Health.
37(e2015047)2015.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Ministry of Public Health: NCR
tables-counts of cases. Journal, 2016.
|
|
41
|
Jaafar W, Zaherddine V, Hussein F, Saliba
NA and Hayeck N: Poor regulation implications in a low and middle
income country based on PAH source apportionment and cancer risk
assessment. Environ Sci Process Impacts. 23:1986–1996.
2021.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Waked M, Salameh P and Aoun Z: Water-pipe
(narguile) smokers in Lebanon: A pilot study. East Mediterr Health
J. 15:432–442. 2009.PubMed/NCBI
|
|
43
|
Jawad M, Charide R, Waziry R, Darzi A,
Ballout RA and Akl EA: The prevalence and trends of waterpipe
tobacco smoking: A systematic review. PLoS One.
13(e0192191)2018.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Warren CW, Lea V, Lee J, Jones NR, Asma S
and McKenna M: Change in tobacco use among 13-15 year olds between
1999 and 2008: Findings from the global youth tobacco survey. Glob
Health Promot. 16 (2 Suppl):S38–S90. 2009.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Badran M and Laher I: Waterpipe (shisha,
hookah) smoking, oxidative stress and hidden disease potential.
Redox Biol. 34(101455)2020.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Shihadeh A: Investigation of mainstream
smoke aerosol of the argileh water pipe. Food Chem Toxicol.
41:143–152. 2003.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Monn C, Kindler P, Meile A and Brändli O:
Ultrafine particle emissions from waterpipes. Tob Control.
16:390–393. 2007.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Awan KH, Siddiqi K, Patil Sh and Hussain
QA: Assessing the effect of waterpipe smoking on cancer outcome-a
systematic review of current evidence. Asian Pac J Cancer Prev.
18:495–502. 2017.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Eissenberg T and Shihadeh A: Waterpipe
tobacco and cigarette smoking: Direct comparison of toxicant
exposure. Am J Prev Med. 37:518–523. 2009.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Henschke CI, Naidich DP, Yankelevitz DF,
McGuinness G, McCauley DI, Smith JP, Libby D, Pasmantier M, Vazquez
M, Koizumi J, et al: Early lung cancer action project: Initial
findings on repeat screenings. Cancer. 92:153–159. 2001.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Swensen SJ, Jett JR, Sloan JA, Midthun DE,
Hartman TE, Sykes AM, Aughenbaugh GL, Zink FE, Hillman SL, Noetzel
GR, et al: Screening for lung cancer with low-dose spiral computed
tomography. Am J Respir Crit Care Med. 165:508–513. 2002.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Bou Akl I, K Zgheib N, Matar M, Mukherji
D, Bardus M and Nasr R: Primary care and pulmonary physicians'
knowledge and practice concerning screening for lung cancer in
Lebanon, a middle-income country. Cancer Med. 10:2877–2884.
2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Elias F, Khuri FR, Adib SM, Karam R, Harb
H, Awar M, Zalloua P and Ammar W: Financial burden of cancer drug
treatment in Lebanon. Asian Pac J Cancer Prev. 17:3173–3177.
2016.PubMed/NCBI
|
|
54
|
Bader P, Boisclair D and Ferrence R:
Effects of tobacco taxation and pricing on smoking behavior in high
risk populations: A knowledge synthesis. Int J Environ Res Public
Health. 8:4118–4139. 2011.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Sibai AM, Iskandarani M, Darzi A, Nakkash
R, Saleh S, Fares S and Hwalla N: Cigarette smoking in a Middle
Eastern country and its association with hospitalisation use: A
nationwide cross-sectional study. BMJ Open.
6(e009881)2016.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Babb S, Malarcher A, Schauer G, Asman K
and Jamal A: Quitting smoking among adults-United States,
2000-2015. MMWR Morb Mortal Wkly Rep. 65:1457–1464. 2017.PubMed/NCBI View Article : Google Scholar
|
|
57
|
United States Public Health Service Office
of the Surgeon General; National Center for Chronic Disease
Prevention and Health Promotion (US) Office on Smoking and Health:
Smoking cessation: A report of the surgeon general. Chapter 6,
Interventions for smoking cessation and treatments for nicotine
dependence. Washington (DC): US Department of Health and Human
Services, 2020.
|
|
58
|
Knudsen HK, Studts CR and Studts JL: The
implementation of smoking cessation counseling in substance abuse
treatment. J Behav Health Serv Res. 39:28–41. 2012.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000-14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Cheng TYD, Cramb SM, Baade PD, Youlden DR,
Nwogu C and Reid ME: The international epidemiology of lung cancer:
Latest trends, disparities, and tumor characteristics. J Thorac
Oncol. 11:1653–1671. 2016.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Ferlay J, Colombet M, Soerjomataram I,
Parkin DM, Piñeros M, Znaor A and Bray F: Cancer statistics for the
year 2020: An overview. Int J Cancer: Apr 5, 2021 (Epub ahead of
print).
|
|
62
|
Johnson DH, Fehrenbacher L, Novotny WF,
Herbst RS, Nemunaitis JJ, Jablons DM, Langer CJ, DeVore RF III,
Gaudreault J, Damico LA, et al: Randomized phase II trial comparing
bevacizumab plus carboplatin and paclitaxel with carboplatin and
paclitaxel alone in previously untreated locally advanced or
metastatic non-small-cell lung cancer. J Clin Oncol. 22:2184–2191.
2004.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Russo C, Walicka M, Caponnetto P, Cibella
F, Maglia M, Alamo A, Campagna D, Frittitta L, Di Mauro M, Caci G,
et al: Efficacy and safety of varenicline for smoking cessation in
patients with type 2 diabetes: A randomized clinical trial. JAMA
Netw Open. 5(e2217709)2022.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Courtney RJ, McRobbie H, Tutka P, Weaver
NA, Petrie D, Mendelsohn CP, Shakeshaft A, Talukder S, Macdonald C,
Thomas D, et al: Effect of cytisine vs varenicline on smoking
cessation: A randomized clinical trial. JAMA. 326:56–64.
2021.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Corelli RL and Hudmon KS: Medications for
smoking cessation. West J Med. 176:131–135. 2002.PubMed/NCBI
|