Introduction
Tuberculosis (TB) is a contagious infection caused
by Mycobacterium tuberculosis (M.tb) that most often
affects the lungs. In 2020, 4.9 million cases of TB were reported,
down from 6.3 million the year before. This indicates that in 2020,
1.4 million individuals did not receive treatment for TB (1). Dissemination of M.tb occurs
from the lungs of the host to peripheral sites for antigen specific
T-cell mediated immunity. The potential of M.tb to
disseminate through the bloodstream and lymph has been confirmed.
As a result, TB has been reported practically in all tissues and
organs (2). Extrapulmonary TB
accounts for ~20% of all TB cases in immunocompromised patients,
and in HIV-infected individuals it is diagnosed in ~50.0% of the
cases (3). Despite this data,
research on miliary TB is limited, possibly due to the difficulty
in diagnosis and issues related to biopsy collection. During TB
infection, the host triggers an immune response that eliminates the
bacteria in a dense cellular mass called granuloma (4). The origin of new blood vessels
induced during the infection plays a salient role in the spread of
infection and restricts the penetration of anti-TB drugs from the
blood to lesions containing mycobacterial cells (5,6).
Infected macrophages often migrate from granulomas to tissues
(7). A previous study demonstrated
that granuloma structures with high vascularization and excess
endothelial cells result in impaired blood flow to the granuloma
sites and compromise drug delivery (8). This pathogenic vasculature limits the
drug concentration in the granuloma, resulting in a severe
bacterial burden. Chemokines play a predominant role in directing
immune cells to inflammatory sites that may lead to unregulated
angiogenesis and could be used to control disease severity
(9-12).
C-X-C motif chemokine receptor 4 (CXCR4) supports the migration of
leukocytes to the inflammation site and their levels correspond
with the degree of inflammation (13,14).
CXCR4 signalling induces the expression of vascular enodthelial
growth factor (VEGF), regulating morphogenesis, angiogenesis, and
immune response (15-17).
Motixafortide treatment was revealed to significantly reduce the
growth of human leukaemia and myeloma xenografts. Treatment with
motixafortide specifically was demonstrated to trigger
CXCR4-dependent apoptotic cell death in tumors. Animals treated
with motixafortide exhibited smaller tumour sizes and weights, and
larger necrotic areas (18). In
this previous study, it was demonstrated that blockade of CXCR4
induced a difference in the infection burden due to impaired
angiogenic support of granuloma formation. A chemokine antagonist
blocks selective chemokine receptors that are disease-relevant and
indispensable for disease progression. A previous study revealed
the effect of CXCR4 inhibition on VEGF expression and T-cell
infiltration (19). When combined
with chemotherapy, motixafortide may enhance the benefits of
first-line drug therapy. The aim of the present study, was to
demonstrate that inhibiting CXCR4 may suppress the induction of an
angiogenic programme in granulomas. Cellular migration and
recruitment of cells to the infected areas may not be altered in
M.tb-infected motixafortide-treated groups compared with
M.tb-infected untreated groups. The findings of the present
study indicated that blockade of CXCR4 reduces the abnormal
vasculature, increases drug absorption at the granuloma, and
reduces the spread of infection to other organs.
Materials and methods
Ethical statement
When conducting the animal research, all procedures
were carried out in accordance with the guidelines and instructions
of ARRIVE (https://arriveguidelines.org/). The experiments
involving the use of guinea pigs were approved by the Institutional
Animal Ethics Committee of the National JALMA Institute for Leprosy
and Other Mycobacterial Diseases (NJIL & OMD; Agra, India).
Lala Lajpat Rai University of Veterinary and Animal Sciences
(Hisar, India) provided healthy out-bred Hartley strain male guinea
pigs, aged 3-4 weeks (~350 g in weight). All the animal
experimental methods described in the present study were conducted
according to the relevant guidelines and regulations for handling
laboratory animals and were approved (approval no.
NJIL&OMD-3-IAEC/2019-08) by the Institutional Animal Ethics
Committee of NJIL & OMD.
M.tb infection and drug treatment
The M.tb H37Rv strain, commonly used for
animal infection studies at the laboratory of the authors (20), was cultured in Middlebrook 7H9
medium supplemented with 10% albumin dextrose catalase (ADC; both
Difco Laboratories, Inc.; BD Biosciences) at 37˚C. The culture type
of the mid-log phase was established, and aliquots were frozen at
-70˚C until needed. Before usage, cultures were diluted in sterile
normal saline and the bacterial concentration was set to
1x106 cfu/ml.
Guinea pigs were aerosol-infected with the
M.tb H37Rv strain using a Glas-Col full-body inhalation
exposure device (Glas-Col LLC) calibrated to deliver ~150
colony-forming units (CFU) to the lungs (21). The animals were housed in cages in
biological safety level 3 facilities for 10-11 weeks to allow
pathological symptoms to develop. According to the Guide for the
Care and Use of Laboratory Animals (22), ventilation was provided. Dry bulb
room temperature (20-26˚C) was maintained to avoid the stress and
strain from heat or cold conditions. Guinea pigs were maintained
under specific-pathogen-free conditions with 30-70% relative
humidiy. To ensure a normal diurnal cycle time, a controlled
lighting system was used. The animals were provided with water and
standard feed ad libitum as per the aforementioned
guidelines.
A total of 35 animals were randomly allocated to
seven groups (Table I) and
administered 10 µg/kg of motixafortide, a CXCR4 antagonist with an
IC50 value of 1 nM (MedChemExpress) by slow
intraperitonal infusion as per the recommended protocol. Guinea
pigs were injected with motixafortide subcutaneously thrice/week
for 2 weeks after the established infection with the M.tb
H37Rv strain. First line drugs, rifampicin and isoniazid
(MilliporeSigma), 25 and 10 mg/kg, respectively, were administered
orally following the early stage of M.tb infection for 5
days/week for 2 weeks. In guinea pigs, M.tb infection
usually appears during the 4th week, which is considered an early
infection (23). The animals were
sacrificed at the 4th and 6th week pre-decided time points based on
the drug treatment. The duration of the experiment was 8 weeks.
Animals were monitored daily and no adverse events requiring
euthanasia/death of the animals were reported during the
experiments. All animal welfare factors were taken into account,
including measures to reduce pain and discomfort through the use of
anaesthesia and particular housing conditions. None of the animals
succumbed during the whole experiment. For the assessment of the
bacterial burden from low-dose aerosol infection, two guinea pigs
were sacrificed following dissection and organ harvesting. Guinea
pigs were anaesthetized with 4-5% isoflurane for induction and 1-2%
for maintenance. Carbon dioxide (CO2; volume
displacement, 30-70% vol/min) method was used for euthanasia of
guinea pigs. Blood was collected (3-5 ml) from heart under
anaesthesia. During the 6th week, blood was collected 45-60 min
after treatment. The death of the guinea pigs was verified by
observing the cardiac activity.
 | Table IBasic experimental scheme. |
Table I
Basic experimental scheme.
| Group | Treatment |
|---|
| Healthy | - |
| Infection | 150-200 CFU of
M. tuberculosis strain by aerosol inhalation on day zero
(infection),estimation of CFU at designated time points i.e., 4th
and 6th week of infection. |
| Treatment group:
Motixafortide at 10 µg/kg, INH at 25 mg kg-1 and RIF at
10 mg kg-1 respectively, by oral gavage. The 4th week
(acute stage), 8th week (chronic stage) and INH + RIF
chemotherapy. |
| Infection control
4th week (Treatment initiation: 2 weeks post-infection) | Infection:
Treatment started at 2 weeks post-infection; daily administration
of motixafortide for an additional 2 weeks. Estimation of CFU
following a wash-out period of 3 days. |
| Infection control
6th week (Treatment initiation: 4 weeks post-infection) | Infection:
Treatment started at 4 weeks post-infection; daily administration
of motixafortide for an additional 2 weeks. Estimation of CFU
following a wash-out period of 3 days. |
| Infection + anti-TB
drugs (Chemotherapy initiation: 4 weeks post-infection) | Infection:
Treatment started 4 weeks post-infection; daily administration of
INH + RIF for an additional 2 weeks. Estimation of CFU following a
wash-out period of 3 days. |
| Infection + anti-TB
drugs + motixafortide (Chemotherapy initiation: 4 weeks
post-infection) | Infection:
Treatment started 4 weeks post-infection; daily administration of
RIF + INH + motixafortide for an additional 2 week.s Estimation of
CFU following a wash-out period of 3 days. |
Determination of bacterial counts
Bacterial burdens in the lungs and spleen of
infected guinea pigs were determined at the 4th and 6th weeks of
infection, respectively. Organs were weighed and homogenized in
PBS. Tenfold serial dilutions of organ homogenates were plated on
Middlebrook 7H10 (BD Biosciences) agar plates containing 10% OADC
(BD Biosciences) and incubated at 37˚C for 21 days. Colonies on
plates were manually enumerated for bacterial burden
determination.
Segmentation of caseating granulomas
and fractal analysis
Lungs and spleen tissues were collected from
sacrificed animals, and the dissected lungs and spleen of guinea
pigs were fixed in 10% buffered formalin to avoid putrefaction and
autolysis of tissue specimens and also preserved in RNA for
subsequent analysis, for routine histopathological examination and
IHC analysis. Tissues that had been fixed in 10% buffered formalin
for 48 h at 20-22˚C were extracted and stored in cassettes before
being sent to a tissue processor. The specimens were dehydrated in
an escalating sequence of alcohols, cleaned in xylene, and then
embedded in paraffin wax in the tissue processor. The tissue
processor was programmed to maintain the specimens in each chamber
for the specified amount of time (Table II).
 | Table IIProcessing of specimens in the tissue
processor. |
Table II
Processing of specimens in the tissue
processor.
| Procedural
aspects | Reagent | Duration (h) | No. of changes (per
hour) |
|---|
| Dehydration in
ascending grades of alcohols | 70% ethanol | 1 | 1 |
| | 80% ethanol | 1 | 1 |
| | 90% ethanol | 1 | 1 |
| | Absolute
alcohol | 1 | 2 |
| Alcohol removal and
clearing of dehydrated tissue | Xylene | 1 | 2 |
| Paraffin wax
embedding (or impregnation) | Paraffin wax with
ceresin | 1.30 | 2 |
| | | 1.30 | 1 |
Sections (thickness, ~3-5 mm) were cut with a
rotator microtome after trimming of the paraffin blocks of the
tissue samples. The tissue ribbons obtained were floated onto a
water bath at 50˚C and then placed onto silane-coated glass slides.
The slides were placed on a hot plate at a temperature <50˚C for
30 min to remove residual moisture and maintain the sections firmly
adherent. The slides were either stained or stored for subsequent
staining. Prior to use, tissue sections on silane-coated slides
were maintained at 60˚C for 2 h on a hot plate to dispel any
residual moisture and to achieve maximum tissue adhesion. Sections
(5 mm) from paraffin-embedded tissues were de-waxed and stained
with haematoxylin and eosin (H&E) at room temperature, 20-25˚C
for 3-5 min, as previously described (24) and observed under an Olympus CX43
(Olympus Corporation) light microscope for histological evaluation
and to visualise bacteria in the surrounding tissues. All the
necrotic lesions of the granuloma were manually delineated and
lacunarity was observed through an Olympus BX51 (Olympus, Japan)
light microscope using MagVision software (Magnus Opto Systems
India Pvt., Ltd.). Regions of interest were drawn in images.
Segmentation of the necrotic area was performed and was loaded into
ImageJ software (version 1.53m; National Institutes of Health). The
fractal area was measured using the box counting method. Fractal
dimensions were calculated and averaged for mean values. The mean
values of all necrotic regions of different groups were calculated
and were used in further analysis.
mRNA profiling and quantification of
chemokine transcripts
mRNA extraction was performed from the serum samples
using the TRIzol-C (Sisco Research Laboratories Pvt., Ltd.) as per
the manufacturer's protocol. The resulting RNA quantification was
performed using NanoPhotometer NP-80 (Implen U.K.). RNA integrity
was evaluated by agarose gel electrophoresis.
Reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) was performed using SuperScript™ III
One-Step RT-PCR System with Platinum™ Taq DNA Polymerase
(cat. no. 12574018) and SYBR Green detection kit (Roche
Diagnostics) and signals were detected using Bio-Rad CFX-96
real-time thermal cycler. The primers were designed using
Primer3Plus software (https://www.primer3plus.com/), and the sequences are
presented in Table III. Each
reaction was performed in triplicate for each animal with 0.2
mmol/l of each primer and 50 ng of cDNA template. The cycling
conditions were as follows: Reverse transcription at 50˚C for 15
min, followed by 3 min of initial denaturation at 95˚C, 40 cycles
of denaturation at 95˚C for 10 sec, and 30 sec of
annealing/extension at 60˚C. Gene expression was normalised using
the reference gene, GAPDH. Analysis of the relative gene expression
data was performed using the 2-ΔΔCq method as previously
described (25).
 | Table IIISequences used for reverse
transcription-quantitative PCR. |
Table III
Sequences used for reverse
transcription-quantitative PCR.
| Primers | Sequences
(5'-3') | Melting temperature
(˚C) |
|---|
| VEGFA | F:
GGCCCATCGAGATGCTAGTG | 60 |
| | R:
GCCCACAGGGATTTTCTTGC | 60 |
| GAPDH | F:
ACCACAGTCCATGCCATCAC | 60 |
| | R:
TGTCGCTGTTGAAGTCA | 60 |
| CXCL-12 | F:
CCTGCCGCTTCTTTGAGA | 55 |
| | R:
CCTGGATCCACTTCAGCTTC | 55 |
| CXCR4 | F:
GTGATCTCTGCGACTGGCTT | 60 |
| | R:
CACGAGTTTGCGCGATTAGG | 59 |
Detection of VEGFA expression in lung
tissue sections using fluorescence microscopy
Dewaxing was carried out by maintaining the tissue
sections in a hot air oven for 20 min at a temperature of 60-65˚C
or until melting of the wax. Rehydration was performed in four
descending grades of alcohol (absolute alcohol, 90, 80 and 70%
alcohol) and finally in distilled water, with each change 5 min.
Antigen retrieval through sodium citrate buffer was then performed
as previously described (26).
This buffer was pre-heated until the temperature reached 90˚C and
the slides were immersed in this buffer for 1 h in the water bath.
The slides were allowed to cool at room temperature for 20 min. The
slides were then washed in PBS for 5 min. Quenching was performed
by treating the tissue sections with a solution of 2 ml of 30%
H2O2 in 100 ml methanol for 20-30 min (0.6%
H2O2 in methanol). Each section was then
washed for 5 min with PBS. Following digestion using 500 µg
pepsin/0.2 N HCl, for 30 min at 37˚C in a waterbath, post-fixation
was performed using glycine followed by 4% paraformaldehyde for
20-30 min at room temperature. Subsequently, 5% bovine serum
albumin (MilliporeSigma™) was used for blocking at 37˚C
for 1 h. For fluorescence immunostaining with DAPI counterstain,
AssayLite™ Mouse anti-VEGF antibody was used in
conjunction with IgG-FITC-conjugated clone (AP70032) (cat. no.
70032-05141; Assaypro LLC) for 1 h at 37˚C. The tissue sections
were incubated with the anti-VEGF antibody at a dilution of 1:100.
Excess antibody was removed by washing with phosphate-buffered
saline (PBS) at room temperature, and sections were then incubated
with the secondary detection antibody (Invitrogen™ cat.
no. G21040, lot no. 2359138; Thermo Fisher Scientific, Inc.), goat
anti-mouse conjugated to HRP at a dilution of 1:1,000 at 37˚C for 1
h. End product visualisation was achieved via a fluorescence
microscope using DAPI (Sisco Research Laboratories Pvt., Ltd.) for
15-20 min at 37˚C as the counter fluorescent stain.
Quantification of drug concentration
and analytical conditions
Plasma was separated from the blood samples
collected from the animal experiments. Plasma, up to 1 ml, was
separated and stored at -80˚C. Concentrations of rifampicin and
isoniazid were detected by following the recommended protocol
(27,28). Analysis was performed on the High
Performance Liquid Chromatography, Alliance 2996 system (Shimadzu
Corporation) and separation was carried out using a C18 column (150
mm). The sample volume was 150 µl and the flow rate was 4 ml/min.
The mobile phase consisted of water: methanol [60:40 (v/v)]
containing perchloric acid and tetrabutyl n-ammonium hydroxide. The
temperature was maintained at 25-28˚C throughout the analysis.
Based on the pharmacokinetic variables such as peak concentration
(Cmax) and area under the concentration-time curve
(AUC0-12), the plasma concentration of the drugs was
calculated using STATA 15.0 (StataCorp LLC).
Calculation of pharmacokinetic
variables and statistical analysis
Each experiment was carried out in triplicate and
outcomes were further assessed. Graphpad Prism 9 (GraphPad
Software, Inc.) and Origin 2020 (Originlab Corporation) were used
for statistical analysis. ImageJ/Fiji software version 1.53 m
(National Institutes of Health) was used to measure VEGFA
expression. The differences in CFU counts, VEGFA expression and
RT-qPCR results across the groups were analyzed using GraphPad
Prism 9 and statistically assessed using Bonferroni post hoc test
with ANOVA to compare the mean of multiple groups and an unpaired
t-test for comparisons between two groups. P≤0.05 was considered to
indicate a statistically significant difference. The normal
distribution (Gaussian) was interpreted through Shapiro-wilk test,
Kolomogorov-Smirnov test, D'Agostino-Pearson test, Anderson-Darling
test using GraphPad Prism 8. The HPLC data was assessed using
Shapiro-Wilk test and it was determined that the data followed a
normal distribution (alpha=0.05). The CFU data also assessed with
the Shapiro-Wilk test exhibited a normal distribution (apha=0.05).
Similar findings were noted for the remaining results (alpha=0.05;
Shapiro-Wilk test), however N was too small to show statistical
significance.
Results
CXCR4-induced angiogenesis permits
M.tb to disseminate
It was observed that treatment with motixafortide in
guinea pigs for 2 weeks before the start of treatment did affect
mycobacterial survival in the lungs and spleen compared to infected
non-treated animals. After 4 weeks of infection, treatment with
anti-TB drugs was initiated while motixafortide injections were
continued along with the anti-TB drugs. The combination of 10 mg/kg
rifampicin and 10 mg/kg isoniazid administered five times weekly
for two weeks revealed potent bactericidal activity and lung CFU
decreased from 6.8 log10 to 2.9 log10 CFU. However, the treatment
with motixafortide reduced mycobacterial migration to distant
organs, i.e., the spleen. Combination treatment with anti-TB drugs
further reduced the mycobacterial migration and survival in the
spleen, and the reduction was more than 2.1 log10 CFU compared to
treatment with anti-TB drugs alone (P≤0.001; Fig. 1).
 | Figure 1In guinea pigs, motixafortide
improves the anti-TB activity of the first-line regimen. (A)
Shematic diagram describing the therapeutic time period. Small
lines indicate the number of days. (B) When compared to the
control, motixafortide 10 µg/kg administered alone after 2 weeks of
aerosol infection had a significant effect on the course of acute
infection in lungs. When combined with rifampicin/isoniazid and
motixafortide the lung bacillary load was markedly reduced compared
to conventional therapy alone but not significantly. The results
are shown as the mean log10CFU/g of (B) lungs and (C)
spleen from five animals per group, at each time point, or as the
proportion of lung exhibiting inflammatory involvement. The
statistical comparison between groups was carried out using
one-wayANOVA. Notably, not a single colony was found in the spleen
of anti-motixafortide-treated guinea pigs. **P≤0.01 and
***P≤0.001 vs. 4th week INF or 6th week INF. TB,
tuberculosis; RIF, rifampicin; INH, isoniazid; ES, early stage; TS,
terminal stage; INF, infection. |
Treatment with motixafortide as an
adjunct to anti-TB drugs increases the drug levels at the granuloma
site
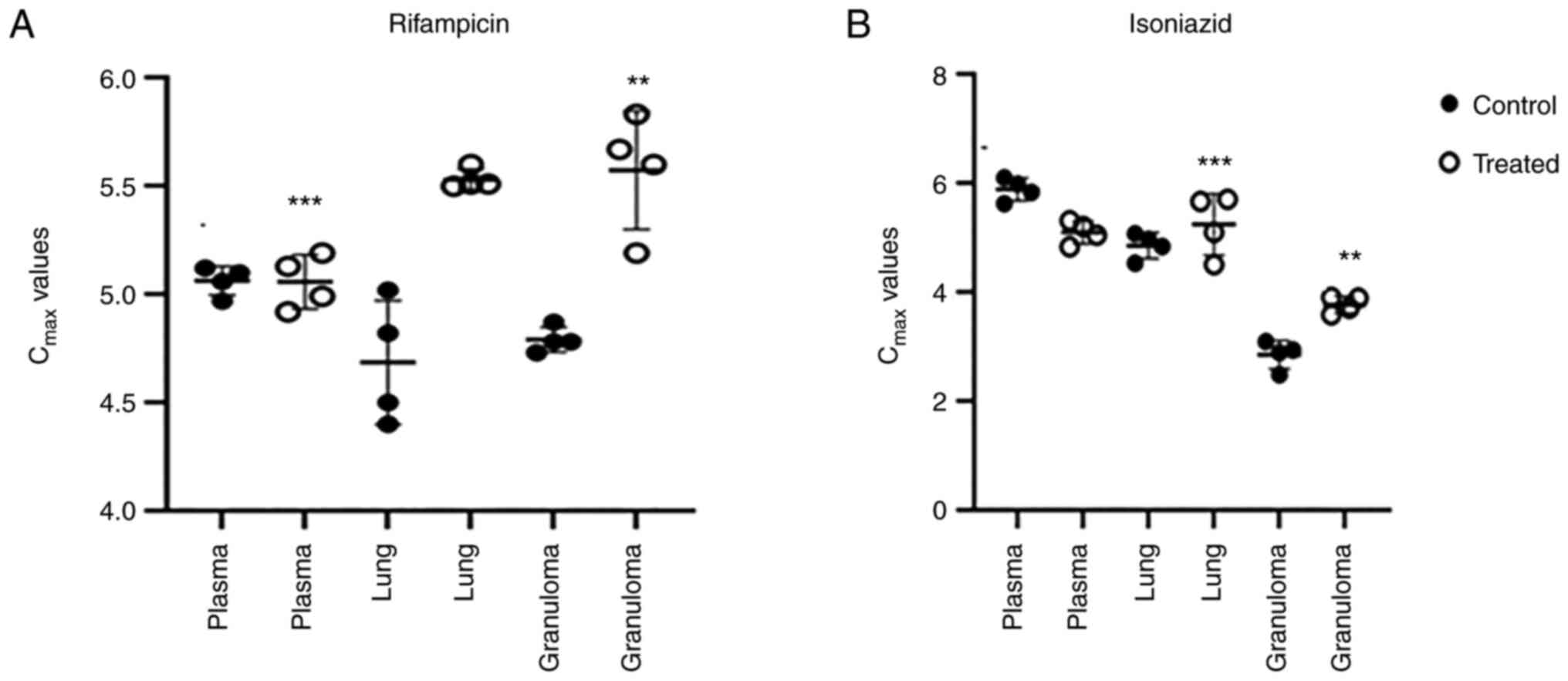
In plasma, lung and granuloma, RIF and INH were
assessed concurrently after extraction. Analysis was performed
using a C18 column at a wavelength of 267 nm. The retention times
of rifampicin and isoniazid were 3 and 5.5 min, respectively (data
not shown). Motixafortide treatment improved the drug delivery in
granulomas. M.tb lesions on the lungs were isolated from the right
apex region and caudal lung tissue was collected for drug
assessment. Normalization of vasculature and refining the vascular
perfusion increased the distribution of first line drugs in
granulomas. Anti-CXCR4 treatment normalized the granuloma
vasculature and, it was investigated whether the drug delivery can
be improved by reducing the thickness of vascularity. Rifampicin
and isoniazid delivery to TB lesions after 10 doses of anti-CXCR4
treatment compared with the controls was significantly higher
(P<0.05; Fig. 2).
Expression levels of angiogenic genes
elevated in M.tb-infected guinea pigs are reduced through
motixafortide treatment
In order to understand the effect of CXCR4
inhibition by motixafortide, the expression of all ELR+
chemokine ligands was determined. Significant changes in the
expression of the CXCL12/CXCR4 axis and VEGF-A, which were
increased following M.tb infection were also associated with
angiogenesis. CXCL12 and CXCR4 mRNA expression were significantly
increased in the lungs of guinea pigs infected with M.tb.
Treatment with motixafortide significantly reduced the expression
of the CXCL12/CXCR4 axis (P<0.05). Treatment with anti-TB drugs
attenuated the mRNA level of CXCL12/CXCR4, which was further
reduced in guinea pigs treated with anti-TB drugs and motixafortide
combination. Similar results were observed for VEGF-A, where a
combination of motixafortide and anti-TB drugs significantly
reduced VEGF-A compared to anti-TB drugs alone, bringing the levels
of angiogenic factors to a normal level (Fig. 3).
 | Figure 3Expression levels of VEGFA, CXCR4 and
CXCL-12. (A) Bar charts showing the levels of VEGFA in different
groups. A one-way ANOVA multiple comparisions was used to compare
the treated conditions to the infection control. The
motixafortide-treated group at the 6th week exhibited a significant
reduction in VEGFA levels when compared with the infection group
and anti-TB drug treatment alone. (B) Bar charts showing the CXCR4
levels in different groups, revealed enhanced expression of CXCR4
in infected guinea pigs. Following motixafortide therapy, CXCR4
levels were significantly decreased. (C) Following M. tb
infection, an increase in the CXCL-12 profile from 4th-week
infection to 6th-week infection groups was revealed but no
significant changes in CXCL-12 expression were revealed with
motixafortide treatment (P≥0.01, based on one-way ANOVA; n=5).
**P≤0.01 and ***P≤0.001 vs. 6th week INF or
6th week INF + RIF + INH. VEGFA, vascular endothelial growth
factor-A; CXCR4, C-X-C motif chemokine receptor 4; M. tb,
Mycobacterium tuberculosis; TB, tuberculosis; RIF,
rifampicin; INH, isoniazid. |
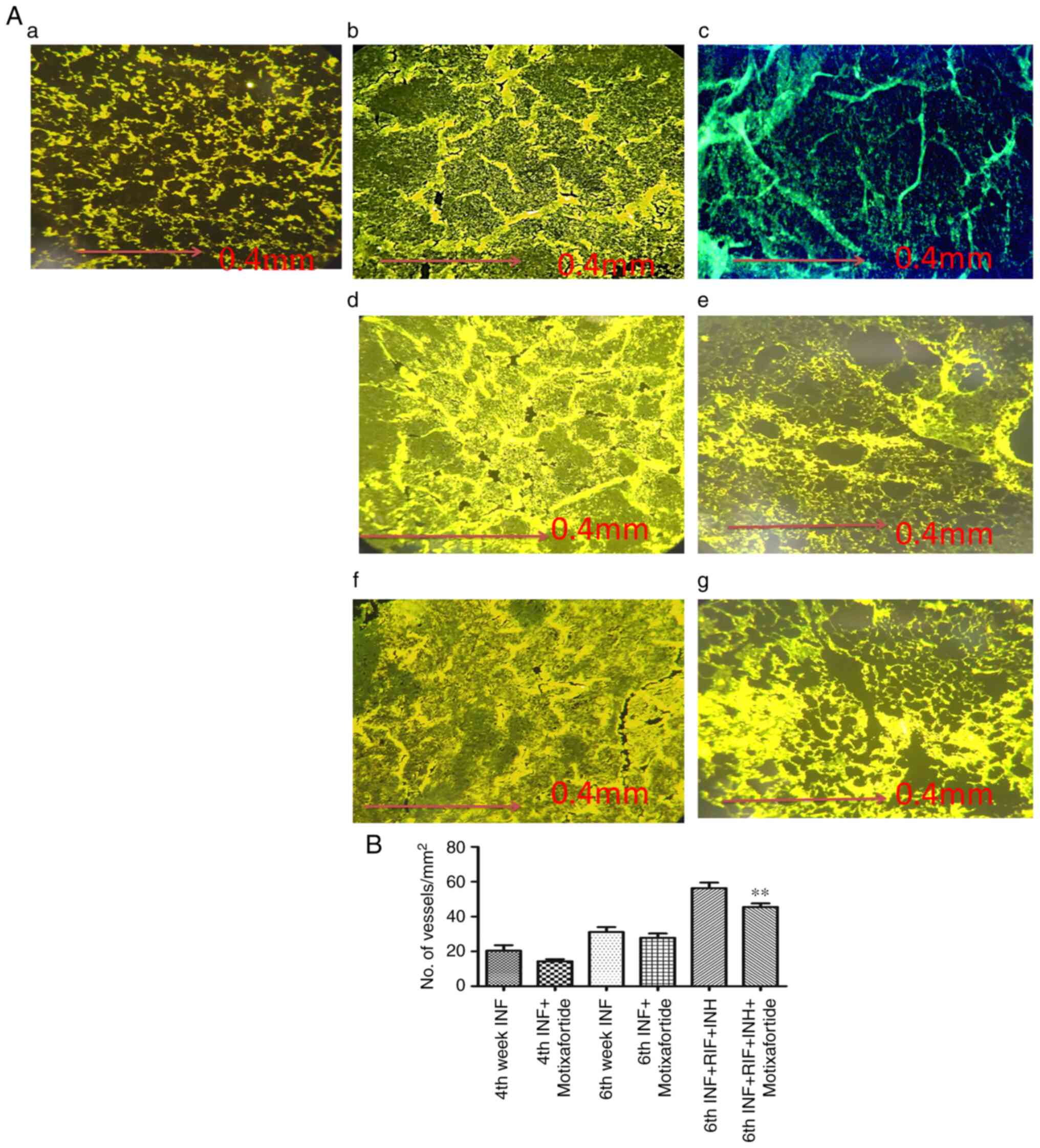
CXCR4 inhibition reduces VEGFA
expression
The vasculature was visualised in the lung tissues.
Varied vascular morphologies were observed in the different groups
of guinea pigs (Fig. 4Aa-g). The
vessel thickness was measured and plotted (Fig. 4B). Motixafortide treatment had no
effect on the microvessel density of granulomas in guinea pig
tissue sections. However, there was a reduction in the thickness of
the vessels. VEGF expression was considerably high in the affected
regions of the lung tissue. The number of vessels was not affected
by anti-CXCR4 treatment. It was observed that vessels associated
with the internal region of the granulomas at the caseating region
were collapsed, which was normalised through anti-CXCR4 treatment.
Treatment with anti-TB drugs attenuated the level of VEGFA which
was further reduced in guinea pigs treated with anti-TB drugs and
motixafortide combination respectively (Fig. 4).
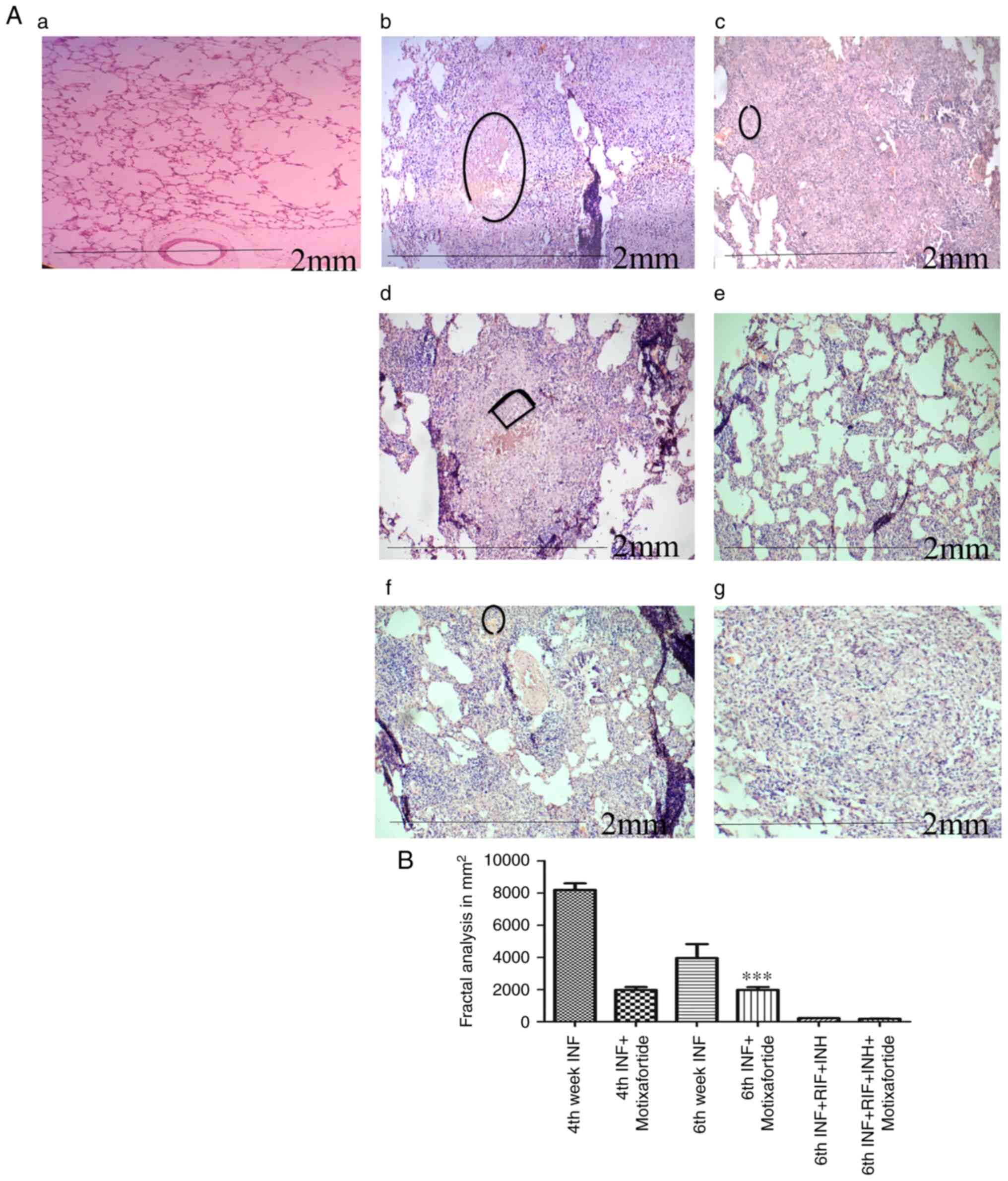
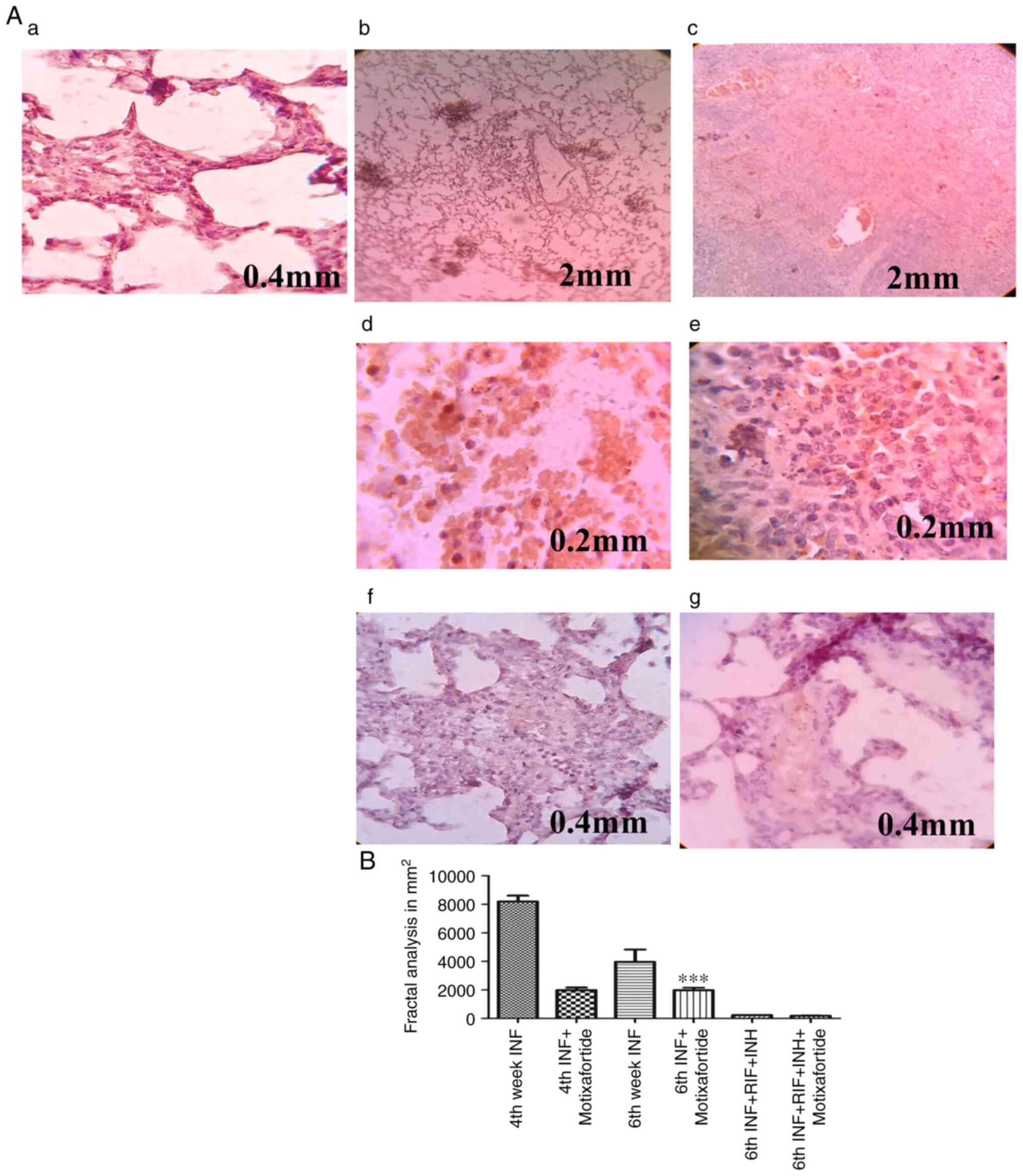
CXCR4 impairment reduces granulomatous
necrotic regions
The necrotic core was markedly evident by the 4th
week, which appears to indicate the development of a necrotic
region before the emergence of an acquired immune response. During
the late stage of infection, multiple scattered areas were
observed, resulting in the deconstructed granuloma following the
spread of the infection via the deposition of debris in the airways
(Fig. 5).
Anti-CXCR4 treatment reduces
CD4+ T cell exhaustion
Because adaptation of anti-mycobacterial defence in
the lungs is caused by immunological T-cell activation of
macrophages (29), it is critical
to investigate the role of anti-CXCR4 in the adaptive immune
response. Significant differences in the migration of
CD4+ T cells to the granuloma site in
M.tb-infected anti-CXCR4 treated guinea pigs and
M.tb-infected guinea pigs during the early stages of TB
infection were observed, indicating that excessive migration of T
cells induces inflammation but has no effect on bacterial severity.
During the 6th week, anti-CXCR4 used in conjunction with anti-TB
medications decreased CD4+ T cells when compared to
anti-TB drug therapy alone, indicating early bacterial load
reduction (Fig. 6).
Discussion
CXCR4 signalling is necessary for the start of the
granuloma-associated proangiogenic programme. Poor vascularization
of the developing granulomas in cxcr4b-deficient zebrafish embryos
also inhibited bacterial growth. Since cxcr4b mutants shared
similar microbicidal abilities against initial mycobacterial
infection and shared a cellular composition with wild-type siblings
in granulomatous lesions, the suppressed infection expansion in
cxcr4b mutants could not be explained by a general lack of
leukocyte recruitment or by a different intramacrophage bacterial
growth rate (30). The expression
of vegfaa was upregulated to a similar degree in cxcr4b mutants and
wild-types, indicating that vascular endothelial growth factor is
not required for the granuloma vascularization phenotype of cxcr4b
mutants (30). Using anti-CXCR4
treatment in a guinea pig animal model, the function of the
chemokine receptor CXCR4 in granuloma formation and the spread of
mycobacterial infection was revealed. It was determined that
inhibition of CXCR4 induced an attenuated infection, which could be
related to pathogenic angiogenesis. The lungs sections were stained
for CD4+ T-cell expression to compare the difference in
the number of lung T cells between anti-CXCR4-treated and
M.tb-infected guinea pigs. The findings of the present study
confirm the research that revealed that early TB spread of
M.tb infection was associated with the onset of
antimycobacterial immunity (31).
The defect in the granuloma may be recapitulated by pharmacological
inhibitors of CXCR4, such as motixafortide (BKT140 4-fluorobenzoyl)
(32). Chemotaxis depends heavily
on CXCR4. Additionally, the association between this receptor and
angiogenesis is becoming more and more clear. Angiogenesis was
recently discovered to be crucial for the growth of cellular
clusters called granulomas, which contain the mycobacteria and are
the hallmark of the TB disease, in the well-known
zebrafish-Mycobacterium marinum (M. marinum) TB
model. Supporting the study that CXCR4 induces angiogenesis, a
positive association between the survival of mycobacteria and
angiogenesis that is normally observed in M.tb-infected
guinea pigs was restricted in anti-CXCR4-treated guinea pigs. A
pharmacological antagonist of VEGFR has been proposed as a
host-targeted therapy to suppress the formation of granulomas in TB
patients (33). Based on the data
of the present study, CXCR4 antagonists may be a novel adjunct
combined with anti-TB drugs acting as anti-angiogenic TB therapy.
The results indicate a role of CXCR4 in eliciting angiogenesis and
inducing VEGF expression. Adaptation of anti-mycobacterial defence
in the lungs is due to the activation of macrophages by immune T
cells. Thus, it is important to analyse the involvement of
anti-CXCR4 in the adaptive immune response, which was not addressed
in the present study and is a limitation. Direct target or complete
inhibition of such host factors may reveal an adverse effect in
spite of decreasing disease progression. Inflammatory chemokines
CCL2 and CXCL5 were suppressed and classical complement pathway
factors were activated following dexamethasone treatment, while the
effects were absent with anti-VEGF treatment (34). The CXCR4/CXCL12 axis has been
revealed to induce tumour progression and angiogenesis by
activating the PI3K/Akt pathway, resulting in increased expression
of VEGF (35). VEGFR inhibitors
act as extensive angiogenesis blockers, involving the generation of
small vessels from inter-segmental vessels. CXCR4 exhaustion
uniquely inhibits granuloma-induced angiogenesis (36). The significance of inflammation in
triggering granuloma-induced angiogenesis and the exact mechanisms
through which CXCR4 regulates new blood vessel formation requires
further elucidation. Notably, the results also suggest that
pathogenic angiogenesis may contain the dispersion of inflammation.
In addition, CXCR4 inhibition or VEGF signalling attenuation has
been revealed to lead to the formation of constructed granuloma and
to reduce granuloma expansion (37). Infected miR-206-knockdown zebrafish
embryos exhibited upregulation of cxcl12a and cxcr4b, and enhanced
neutrophil recruitment to infection sites. Increased neutrophil
response and decreased bacterial burden brought on by miR-206
knockdown depended on the Cxcl12/Cxcr4 signalling axis using
CRISPR/Cas9-mediated knockdown of cxcl12a and cxcr4b expression and
AMD3100 inhibition of Cxcr4(38).
Necrosis is induced by bacterial lysis and forms at the centre of
the granuloma, resulting in the spreading of the infection and
destruction of the lung tissue. Extracellular bacilli could be
found in the necrotic region and released to the luminal side of
the cavity, resulting in the spread of the infection (39). The inefficiency and long-term
treatment strategy of anti-TB drugs are considered to be sources of
pharmacokinetic factors (40).
Aside from the complex array of diseased sites, the vasculature
within granuloma impedes drug delivery (41). According to a previous study, CXCR4
has been linked to tumour angiogenesis via transcriptional
regulation of VEGF signalling (42). It has been shown that M.
marinum infection stimulates angiogenesis in zebrafish and
supports the spread of bacterial infection (6). To verify the role of CXCR4 in
dissemination of tuberculosis infection in guinea pigs, infected
guinea pigs were injected with motixafortide subcutaneously thrice
per week for 2 weeks after the established infection with the
M.tb H37Rv strain, and VEGF expression was observed.
Chemokines serve a critical function in directing immune cells to
the inflammatory site, which may lead to unregulated angiogenesis
and could be decisive in controlling the disease severity (43). In this regard, it is important to
study the regulatory mechanisms involved in the production of
chemokines linked with increased angiogenesis. The members of the
CXC chemokine family that contain the ELR motif, as compared with
those that lack these three amino acids, are potent inducers of
angiogenic activity. When the concentration of anti-TB drugs was
delineated in relation to the vascular density at the granuloma
site, a steep gradient of reduced drug levels was found with
increased vasculature. The restricted permeability of anti-TB drugs
leads to drug tolerance that is associated with latent bacterial
populations. Extensive drug distribution profiling should be
considered during drug discovery to optimise drug penetration to
infection sites such as granuloma and lesions. To reach the bacilli
inside the macrophages, anti-TB drugs should be distributed into
various parts by overcoming barriers such as lesions in the lungs
with higher cellular composition and vascularization. From the
blood, the drug enters into the interstitial space of lesions and
then penetrates. In the present study it was demonstrated that the
distribution of drug concentration decreased in the plasma, lungs,
and granuloma, respectively. A limited number of animals was also a
limitation of the present study. In the present study, inhibition
of CXCR4 affected the expression of VEGFA and granuloma
vascularisation. It was revealed that CXCR4 induced new blood
vessel formation during TB infection. Obstruction of pathological
angiogenesis with CXCR4 inhibitors is an alternative therapeutic
strategy to normalise the vasculature at granuloma sites to
increase the penetration of drugs, and reduce the bacterial count
in the lungs. The pivotal step in preventing drug resistance in TB
and curing this disease may be achieved through efficient
combination therapy, which distributes the drug into the lesion and
lesion compartments targeting the vulnerable bacterial
population.
Acknowledgements
We would like to thank Mr Nilesh Pal (Animal
Experimentation Laboratory; ICMR-NJIL & OMD, Agra, India), and
Mr Amit Kumar Rajput (Technician-C, Animal Experimentation
Laboratory; ICMR-NJIL & OMD) for technical assistance.
Funding
Funding: The present study was supported by the Department of
Science and Technology (DST), Government of India (New Delhi,
India), DST-INSPIRE Fellowship (no. DST/INSPIRE
Fellowship/2018/IF180175) awarded to KSD.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
KSD and AKS conceived the study, analysed the data
and wrote the manuscript. KSD and DSC designed the study. VK, AVS
and SVS participated in data analysis. KSD and DSC confirm the
authenticity of all the raw data. All the authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
All the animal experimental methods described in the
present study were conducted according to the relevant guidelines
and regulations for handling laboratory animals and were approved
(approval no. NJIL&OMD-3-IAEC/2019-08) by the Institutional
Animal Ethics Committee of NJIL & OMD (Agra, India).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WHO report 2019, https://apps.who.int/iris/bitstream/handle/10665/329368/9789241565714-eng.pdf.
|
|
2
|
Polena H, Boudou F, Tilleul S,
Dubois-Colas N, Lecointe C, Rakotosamimanana N, Pelizzola M,
Andriamandimby SF, Raharimanga V, Charles P, et al: Mycobacterium
tuberculosis exploits the formation of new blood vessels for its
dissemination. Sci Rep. 6(33162)2016.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Golden MP and Vikram HR: Extrapulmonary
tuberculosis: An overview. Am Fam Physician. 72:1761–1768.
2005.PubMed/NCBI
|
|
4
|
Russell DG, Barry CE III and Flynn JL:
Tuberculosis: What we don't know can, and does, hurt us. Science.
328:852–856. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Dartois V: The path of anti-tuberculosis
drugs: From blood to lesions to mycobacterial cells. Nat Rev
Microbiol. 12:159–167. 2014.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Oehlers SH, Cronan MR, Scott NR, Thomas
MI, Okuda KS, Walton EM, Beerman RW, Crosier PS and Tobin DM:
Interception of host angiogenic signalling limits mycobacterial
growth. Nature. 517:612–615. 2015.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Dvorak HF, Brown LF, Detmar M and Dvorak
AM: Vascular permeability factor/vascular endothelial growth
factor, microvascular hyperpermeability, and angiogenesis. Am J
Pathol. 146:1029–1039. 1995.PubMed/NCBI
|
|
8
|
Jain RK: Normalizing tumor vasculature
with anti-angiogenic therapy: A new paradigm for combination
therapy. Nat Med. 7:987–989. 2001.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Chackerian AA, Alt JM, Perera TV, Dascher
CC and Behar SM: Dissemination of Mycobacterium tuberculosis is
influenced by host factors and precedes the initiation of T-cell
immunity. Infect Immun. 70:4501–4509. 2002.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Davis JM and Ramakrishnan L: The role of
the granuloma in expansion and dissemination of early tuberculous
infection. Cell. 136:37–49. 2009.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Monin L and Khader SA: Chemokines in
tuberculosis: The good, the bad and the ugly. Semin Immunol.
26:552–558. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Keeley EC, Mehrad B and Strieter RM:
Chemokines as mediators of neovascularization. Arterioscler Thromb
Vasc Biol. 28:1928–1936. 2008.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Perez AL, Bachrach E, Illigens BM, Jun SJ,
Bagden E, Steffen L, Flint A, McGowan FX, Del Nido P,
Montecino-Rodriguez E, et al: CXCR4 enhances engraftment of muscle
progenitor cells. Muscle Nerve. 40:562–572. 2009.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Hoshino Y, Tse DB, Rochford G, Prabhakar
S, Hoshino S, Chitkara N, Kuwabara K, Ching E, Raju B, Gold JA, et
al: Mycobacterium tuberculosis-induced CXCR4 and chemokine
expression leads to preferential X4 HIV-1 replication in human
macrophages. J Immunol. 172:6251–6258. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
García-Cuesta EM, Santiago CA,
Vallejo-Díaz J, Juarranz Y, Rodríguez-Frade JM and Mellado M: The
role of the CXCL12/CXCR4/ACKR3 axis in autoimmune diseases. Front
Endocrinol (Lausanne). 10(585)2019.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gil M, Seshadri M, Komorowski MP, Abrams
SI and Kozbor D: Targeting CXCL12/CXCR4 signaling with oncolytic
virotherapy disrupts tumor vasculature and inhibits breast cancer
metastases. Proc Natl Acad Sci USA. 110:E1291–E1300.
2013.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Peled A, Abraham M, Avivi I, Rowe JM,
Beider K, Wald H, Tiomkin L, Ribakovsky L, Riback Y, Ramati Y, et
al: The high-affinity CXCR4 antagonist BKT140 is safe and induces a
robust mobilization of human CD34+ cells in patients with multiple
myeloma. Clin Cancer Res. 20:469–479. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Beider K, Begin M, Abraham M, Wald H,
Weiss ID, Wald O, Pikarsky E, Zeira E, Eizenberg O, Galun E, et al:
CXCR4 antagonist 4F-benzoyl-TN14003 inhibits leukemia and multiple
myeloma tumor growth. Exp Hematol. 39:282–292. 2011.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Salcedo R, Wasserman K, Young HA, Grimm
MC, Howard OM, Anver MR, Kleinman HK, Murphy WJ and Oppenheim JJ:
Vascular endothelial growth factor and basic fibroblast growth
factor induce expression of CXCR4 on human endothelial cells: In
vivo neovascularization induced by stromal-derived factor-1alpha.
Am J Pathol. 154:1125–1135. 1999.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Davuluri KS, Singh AK, Kumar V, Singh SV,
Singh AV, Kumar S, Yadav R, Kushwaha S and Chauhan DS: Stimulated
expression of ELR+ chemokines, VEGFA and TNF-AIP3 promote
mycobacterial dissemination in extrapulmonary tuberculosis patients
and Cavia porcellus model of tuberculosis. Tuberculosis (Edinb).
135(102224)2022.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Klinkenberg LG, Lee JH, Bishai WR and
Karakousis PC: The stringent response is required for full
virulence of Mycobacterium tuberculosis in guinea pigs. J Infect
Dis. 202:1397–1404. 2010.PubMed/NCBI View
Article : Google Scholar
|
|
22
|
National Research Council (US) Committee
for the Update of the Guide for the Care and Use of Laboratory
Animals. Guide for the care and use of laboratory animals. 8th
edition. Washington (DC): National Academies Press (US), 2011.
|
|
23
|
Kashino SS, Napolitano DR, Skobe Z and
Campos-Neto A: Guinea pig model of Mycobacterium tuberculosis
latent/dormant infection. Microbes Infect. 10:1469–1476.
2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Fischer AH, Jacobson KA, Rose J and Zeller
R: Hematoxylin and eosin staining of tissue and cell sections. CSH
Protoc. 2008(pdb.prot4986)2008.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Kim SW, Roh J and Park CS:
Immunohistochemistry for pathologists: Protocols, pitfalls, and
tips. J Pathol Transl Med. 50:411–418. 2016.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Goutal S, Auvity S, Legrand T, Hauquier F,
Cisternino S, Chapy H, Saba W and Tournier N: Validation of a
simple HPLC-UV method for rifampicin determination in plasma:
Application to the study of rifampicin arteriovenous concentration
gradient. J Pharm Biomed Anal. 123:173–178. 2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Aït Moussa L, El Bouazzi O, Serragui S,
Soussi Tanani D, Soulaymani A and Soulaymani R: Rifampicin and
isoniazid plasma concentrations in relation to adverse reactions in
tuberculosis patients: A retrospective analysis. Ther Adv Drug Saf.
7:239–247. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Sia JK and Rengarajan J: Immunology of
Mycobacterium tuberculosis Infections. Microbiol Spectr 7:
10.1128/microbiolspec.GPP3-0022-2018, 2019.
|
|
30
|
Torraca V, Tulotta C, Snaar-Jagalska BE
and Meijer AH: The chemokine receptor CXCR4 promotes granuloma
formation by sustaining a mycobacteria-induced angiogenesis
programme. Sci Rep. 7(45061)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Ramonell KM, Zhang W, Hadley A, Chen CW,
Fay KT, Lyons JD, Klingensmith NJ, McConnell KW, Coopersmith CM and
Ford ML: CXCR4 blockade decreases CD4+ T cell exhaustion and
improves survival in a murine model of polymicrobial sepsis. PLoS
One. 12(e0188882)2017.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Reyes AWB, Arayan LT, Huy TXN, Vu SH, Kang
CK, Min W, Lee HJ, Lee JH and Kim S: Chemokine receptor 4 (CXCR4)
blockade enhances resistance to bacterial internalization in
RAW264.7 cells and AMD3100, a CXCR4 antagonist, attenuates
susceptibility to Brucella abortus 544 infection in a murine model.
Vet Microbiol. 237(108402)2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Pozzobon T, Goldoni G, Viola A and Molon
B: CXCR4 signaling in health and disease. Immunol Lett. 177:6–15.
2016.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Mirabelli P, Mukwaya A, Lennikov A,
Xeroudaki M, Peebo B, Schaupper M and Lagali N: Genome-wide
expression differences in anti-Vegf and dexamethasone treatment of
inflammatory angiogenesis in the rat cornea. Sci Rep.
7(7616)2017.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Mousavi A: CXCL12/CXCR4 signal
transduction in diseases and its molecular approaches in
targeted-therapy. Immunol Lett. 217:91–115. 2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Liang Z, Brooks J, Willard M, Liang K,
Yoon Y, Kang S and Shim H: CXCR4/CXCL12 axis promotes VEGF-mediated
tumor angiogenesis through Akt signaling pathway. Biochem Biophys
Res Commun. 359:716–722. 2007.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Kawaguchi N, Zhang TT and Nakanishi T:
Involvement of CXCR4 in normal and abnormal development. Cells.
8(185)2019.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Wright K, de Silva K, Plain KM, Purdie AC,
Blair TA, Duggin IG, Britton WJ and Oehlers SH: Mycobacterial
infection-induced miR-206 inhibits protective neutrophil
recruitment via the CXCL12/CXCR4 signalling axis. PLoS Pathog.
17(e1009186)2021.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Philips JA and Ernst JD: Tuberculosis
pathogenesis and immunity. Annu Rev Pathol. 7:353–384.
2012.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Xu Y, Wu J, Liao S and Sun Z: Treating
tuberculosis with high doses of anti-TB drugs: Mechanisms and
outcomes. Ann Clin Microbiol Antimicrob. 16(67)2017.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Viaggi B, Cangialosi A, Langer M, Olivieri
C, Gori A, Corona A, Finazzi S and Di Paolo A: Tissue penetration
of antimicrobials in intensive care unit patients: A systematic
review-part II. Antibiotics (Basel). 11(1193)2022.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Tong RT, Boucher Y, Kozin SV, Winkler F,
Hicklin DJ and Jain RK: Vascular normalization by vascular
endothelial growth factor receptor 2 blockade induces a pressure
gradient across the vasculature and improves drug penetration in
tumors. Cancer Res. 64:3731–3736. 2004.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Mehrad B, Keane MP and Strieter RM:
Chemokines as mediators of angiogenesis. Thromb Haemost.
97:755–762. 2007.PubMed/NCBI
|