Introduction
The importance and biotechnological potential of
natural products can be demonstrated by the investment in research
of approximately $480 million in 2020 by the National Institutes of
Health (NIH), and by the approval of >680 new chemical compounds
with pharmacological activities of natural origin or those derived
from natural products by the US Food and Drug Administration
between 1981 and 2010 (1,2).
Natural products are chemically and structurally
diverse and possess various biological activities, often with low
toxicity and few side-effects (3).
They can be obtained from a wide variety of sources, such as
plants, microorganisms, algae, fungi, lichens and animals (3,4).
Toxins and poisons from animals, mainly amphibians, are also used
as alternative medicines (2), and
~47 species of amphibians are recognized for their medicinal use
(5). Their poisons or skin
secretions, which are used by the animal in defense against
predators and infection, contain bioactive compounds, such as
steroids, alkaloids, biogenic amines, guanidine derivatives,
proteins and peptides (2,5), and various biological properties have
been reported, such as trypanocidal, leishmanicidal, antibacterial,
antifungal, antiproliferative, insecticide, antiviral, antitumor
and cardiotonic activities (2,5).
Toads of the family Bufonidae have a cosmopolitan
distribution, and several Bufonidae species in the genera
Rhinella and Rhaebo are found in Brazil (2). Rhaebo guttatus (R.
guttatus), a poisonous toad species, is found in tropical
forests and open areas, mainly in the Amazon Basin (2,5,6). The
chemical composition of its parotoid gland secretion and its
biological effects are poorly understood (7). Previous studies have identified
alkaloids (N-methyl-5-hidroxytryptamine, bufotenine and
dehydrobufotenin) and steroids (3β,16β-dihydroxybufa-8(14),20,22-trienolide, marinobufagin and
bufadienolides) in extracts obtained from the parotoid gland
secretion of R. guttatus (7,8).
Ferreira et al (8)
demonstrated that extracts from the secretion of the parotoid gland
of R. guttatus exhibit hemolytic and cytotoxic activity
against tumor and non-tumor cells. Toxic activity against
Plasmodium falciparum and phytopathogenic fungi has also
been reported (9,10). Oliveira et al (11) observed that a methanolic extract
from the parotoid gland secretion of R. guttatus exhibited
both mutagenic and antimutagenic activities, indicating the need
for further studies.
To enhance the current understanding of the
biological effects of the compounds in the parotoid gland secretion
of R. guttatus, the present study evaluated the activity of
its crude methanolic extract (CME) on the immune system by
analyzing its effects on cytokine production, lymphoproliferative
activity and the production of reactive species by macrophages
in vitro. In this manner, the present study aimed to
contribute to bioprospecting studies and to the development of the
bioeconomy.
Materials and methods
Poison collection and extract
preparation
Adult (males and females, from 5 to 10 animals)
R. guttatus were captured and identified by D.D.J.R. (a
permanent license for the collection of zoological material has
been obtained from IBAMA, SISBIO: 30034-1) in Nova Ubiratã, Mato
Grosso, Brazil (13˚6'16.20" S 54˚25'51.01" W). The secretion from
the parotoid gland was obtained through the manual compression of
the glands. The secretion was dried, crushed and extracted by
maceration with 99% methanol in an ultrasonic bath (Ultronique,
Indaiatuba, Brazil) for 2 h to obtain a CME of parotoid gland
secretion. The extract was filtered through filter paper (Unifil,
Curitiba, Brazil), and then macerated twice more as described
above. Finally, the extracts were pooled, and the solvent was
rotary evaporated (IKA-Werke GmbH & Co. KG) at 40˚C and kept
under vacuum in a desiccator at room temperature for 48 h. The
obtained CME was stored at 4˚C. The experimental conditions were as
previously described by Kerkhoff et al (5) and Sousa et al (2). The description of the chemical
profile of CME was presented by Sousa et al (2).
Animals and experimental design
Male Swiss mice (mean weight, 35 g, 45 days old)
obtained from the Central Bioterium of the Federal University of
Mato Grosso, Cuiabá Campus were used in the experiment. The mice
were housed in polyethylene boxes with a stainless-steel grid
during the acclimatization (15 days) and experimental periods. They
were divided into five groups of 6 mice in each and maintained
under a 12-h light/dark cycle in a temperature-controlled room
(24±1˚C, 55±2% relative humidity), with ad libitum access to
food (Nuvilab) and filtered water. During the experimental period,
the mice were administered water (control), 0.5% Tween-20
(vehicle), or various doses of the CME (8, 16 and 32 µg/ml) in a
100 µl volume per mouse per day via oral gavage for 7 or 30 days.
The doses were selected based on the study by Oliveira et al
(11). Aliquots were prepared in
microtubes, diluted in 0.5% Tween-20 and stored at 4˚C. During the
treatment period, the mice were observed daily for water and feed
consumption. The body weight of the mice was measured at the
beginning and end of the treatment period to assess body weight
gain. At the end of the treatment period (24 h later), the mice
were euthanized by cervical dislocation, and the lungs, heart,
kidneys and liver were excised to evaluate their relative and
absolute weight, and for use in histopathological analysis.
Immunological analyses were performed using cells obtained from the
spleen and peritoneal washes. The present study was registered in
the National System for the Management of Genetic Heritage and
Associated Traditional Knowledge (SISGEN) under no. A313DC9, and
approved by the UFMT Ethics Committee on the Use of Animals (CEUA),
under no. 23108.918243/2017-50.
Histopathological analysis
Fragments of the heart, liver, lungs and kidneys
were harvested, and immediately fixed with 10% formaldehyde in
sodium phosphate buffer (Synth, Diadema, Brazil), pH 7.4, at 4˚C
for 24 h. Following dehydration with alcohol and clearing with
xylol, the tissue fragments were embedded in paraffin, sectioned
(5-µm-thick) using a HYRAX M60 microtome (Zeiss GmbH),
deparaffinized and stained at room temperature (25˚C) with
hematoxylin (1 min) and eosin (2 min) (MilliporeSigma) . The
sections were examined under a AxioScope.A1 microscope (Zeiss
GmbH). For the histopathological analysis, a score was generated
(0-4) based on tissue edema, blood clots, leukocyte infiltrates and
renal tubule damage, where 0 indicates no alterations and 4
indicates a high level of alteration (12).
Analysis of total splenic cell
lymphoproliferation
The lymphoproliferation of total splenic cells was
assessed using the 3-(4,5-dimethylthiazol-2-yl)-2
5-diphenyltetrazolium bromide (MTT) colorimetric assay (Cell Groth
Determination kit, MTT based, MilliporeSigma, cat. no. CGD1)
according to the manufacturer's recommendations. Briefly, the
spleen was removed and transferred to a Petri dish containing
RPMI-1640 medium (Cultilab) and macerated with a sieve and pistil.
The cell suspension was then transferred to a falcon tube and
centrifuged at 402 x g for 10 min at room temperature (25˚C). The
cell pellet was resuspended in RPMI-1640 medium (500 µl) containing
20% fetal bovine serum (FBS; Cultilab), and the cell concentration
was adjusted to 2x104 cells/ml as determined using a
Newbauer chamber (Global New Optics) and the Trypan blue (Qhemis)
exclusion method. The cells were transferred to a 96-well
microplate, and the mitogen, concanavalin A (Con A;
MilliporeSigma), or RPMI 20% FBS (basal) was added in triplicate.
The plates were incubated for 36 h at 37˚C and 5% CO2.
MTT reagent (100 µl) was then added to each well, and the plate was
incubated at 37˚C and 5% CO2 for 4 h. Solubilizing
solution (0.1 N HCl in anhydrous isopropanol) was added, and the
absorbance was measured on an ELISA plate reader (BioClin Medical
Electronics Co.) at 630 nm.
Analysis of cytokine levels
The levels of selected cytokines in the supernatant
of total splenic cell cultures were evaluated using commercial
ELISA kits (eBioscience; IL-12p70 cat. no. 88 7121 88, IFN-g cat.
no. 88 7314 88, IL-4 cat. no. 88 7044 88, IL-10 cat. no. 88 7104
88, TNF-α cat. no. 88 7324 88, Thermo Fisher Scientific, Inc.)
according to the manufacturer's protocol. For the measurement of
interleukin (IL)-4 and IL-10 levels, the splenic cell culture was
stimulated with the mitogen Con A (3.5 µg/ml) for 24 h. For the
assessment of IL12p70 and tumor necrosis factor (TNF)-α levels, the
culture was stimulated with a formalinized aqueous suspension of
Staphylococcus aureus Cowan strain 1 (SAC, 1:5,000;
MilliporeSigma) for 48 h. The absorbance of the samples was
measured on an ELISA reader at 450 nm. The concentrations were
estimated based on a standard curve of cytokine standards in the
kit.
Harvesting of peritoneal
macrophages
Following sacrifice, peritoneal macrophages were
obtained in a Class II Safety Cabinet by the addition of 10 ml
sterile, cold phosphate-buffered saline (PBS) to the peritoneal
cavity. The abdomen was massaged for 30 sec, and the collected
peritoneal fluid was transferred to 50 ml Falcon tubes. This
procedure was performed twice, to obtain a total of 20 ml fluid,
which was placed in an ice bath and then centrifuged (Novatecnica)
at 402 x g and 4˚C for 10 min. The supernatant was discarded, and
the pellet was resuspended in 1 ml RPMI-1640 medium containing 10%
FBS. The cells in the peritoneal wash were counted in a Neubauer
Chamber and adjusted to 2x106 cells/ml. The macrophages
were plated in triplicate into 96-well microplates (100 µl/well)
and incubated for 2 h at 37˚C and 5% CO2. Following
incubation, the supernatant was removed, and the wells were washed
with 100 µl RPMI. Subsequently, 200 µl RPMI containing 10% FBS were
added to each well, and the cells were incubated at 37˚C and 5%
CO2 for 36 h.
Assessment of spontaneous hydrogen
peroxide (H2O2) release by peritoneal
macrophages
The spontaneous production of
H2O2 by peritoneal macrophages was assessed
using the method developed by Pick and Mizel (13). The quantification of
H2O2 is based on the horseradish
peroxidase-dependent oxidation of phenol red by
H2O2 into a compound that is assayed for its
increased absorbance (13).
Following incubation (37˚C and 5% CO2 for 36 h), the
supernatant of the macrophage culture was collected and stored for
nitric oxide (NO.) evaluation. To measure
H2O2, 100 µl phenol red solution containing
140 mM NaCl, 10 mM K2HPO4, 5.5 mM dextrose
and 5.5 mM peroxidase were added to the cell monolayer in the
96-well microplates. The plates were incubated at room temperature
under a light for 60 min. The reaction was terminated by the
addition of 10 µl of 1 M NaOH, and the absorbance of the solution
at 630 nm was determined using an ELISA microplate reader. The
blank was phenol red and 1 M NaOH. The amount of
H2O2 produced by the macrophages was
determined based on a standard curve of known
H2O2 concentrations. The mean value of
triplicate samples was calculated.
Assessment of NO.
production by peritoneal macrophages
The NO. levels in the macrophage culture
supernatant were assessed using the colorimetric method based on
the Griess reaction, as previously described (14). Briefly, 100 µl Griess reagent [1%
N-(1-Naphthyl) ethylenediamine dihydrochloride] in distilled water
and 1% sulfanilamide diluted in 5% H3PO4 were
added to the supernatants. The reagents were mixed in equal volumes
at the time of the reaction. The absorbance of the samples at 492
nm was read on an ELISA reader. The blank was Griess reagent. The
NO. levels were calculated based on a standard curve of
known concentrations of NaNO2. The mean value of
triplicate samples was calculated.
Statistical analysis
The present study was carried out in two independent
experimental stages (A and B). The data were analyzed either
separately or together when the data obtained in experiments A and
B were homogeneous. The normal distribution of the data was
assessed using the Kolmogorov and Smirnov test. One-way ANOVA
followed by the Tukey-Kramer multiple comparisons test was used to
assess the significance of differences between experimental groups.
NO. production in experiment B at 30 days was analyzed
using an unpaired t-test. All results are expressed as the mean ±
standard deviation. A value of P<0.05 was considered to indicate
a statistically significant difference.
Results
Effects of CME from the parotoid gland
secretion of R. guttatus on body weight, food intake and organ
weights
Body weight, food intake and organ weight were
analyzed to assess the toxicity of the extract. These evaluations
are important as toxic products usually induce changes in animal
behavior, including a lack of appetite and a consequent reduction
in body weight (15). As also
previously reported by Oliveira et al (11), there were no significant
differences between the groups as regards water and feed
consumption (data not shown) or body weight gain (Table I). Although the relative weight of
the lungs was reduced in the mice treated with 8 µg/ml CME for 30
days (8 µg/ml, 0.49±0.05; control, 0.62±0.09), the absolute and
relative weights of the other organs did not differ significantly
when compared to the control groups (control and vehicle; Table I).
 | Table IBody weight gain and organ weights
(absolute and relative) of Swiss mice treated with a crude
methanolic extract of R. guttatus toad poison via daily
gavage for 7 or 30 days. |
Table I
Body weight gain and organ weights
(absolute and relative) of Swiss mice treated with a crude
methanolic extract of R. guttatus toad poison via daily
gavage for 7 or 30 days.
| 7 days |
|---|
| | Absolute weight of
organs | Relative weight of
organs |
|---|
| Group
(n=6/group) | Weight gain | Liver | Kidney | Lung | Heart | Liver | Kidney | Lung | Heart |
|---|
| Control | 1.32±5.14 | 2.28±0.47 | 0.63±0.10 | 0.27±0.06 | 0.22±0.04 | 5.50±0.60 | 1.47±0.32 | 0.63±0.17 | 0.50±0.07 |
| Vehicle | -0.74±8.97 | 2.18±0.45 | 0.53±0.09 | 0.28±0.10 | 0.20±0.04 | 5.13±0.84 | 1.24±0.16 | 0.68±0.27 | 0.46±0.04 |
| 8 µg/ml | 2.53±3.99 | 2.16±0.29 | 0.48±0.07 | 0.23±0.03 | 0.18±0.03 | 5.26±0.39 | 1.18±0.16 | 0.55±0.05 | 0.45±0.04 |
| 16 µg/ml | 2.19±8.50 | 2.19±0.36 | 0.54±0.14 | 0.23±0.06 | 0.19±0.04 | 5.44±0.75 | 1.36±0.33 | 0.58±0.14 | 0.48±0.09 |
| 32 µg/ml | -1.48±8.86 | 2.30±0.27 | 0.60±0.04 | 0.29±0.02 | 0.25±0.03 | 4.53±0.28 | 1.18±0.05 | 0.58±0.07 | 0.50±0.03 |
| 30 days |
| | Absolute weight of
organs | Relative weight of
organs |
| Group
(n=6/group) | Weight gain | Liver | Kidney | Lung | Heart | Liver | Kidney | Lung | Heart |
| Control | 1.67±6.65 | 2.21±0.44 | 0.52±0.06 | 0.27±0.04 | 0.20±0.03 | 4.99±0.57 | 1.18±0.15 | 0.62±0.09 | 0.45±0.07 |
| Vehicle | 2.57±5.91 | 2.34±0.22 | 0.53±0.15 | 0.27±0.04 | 0.24±0.09 | 5.10±0.38 | 1.14±0.26 | 0.59±0.05 | 0.53±0.21 |
| 8 µg/ml | 1.76±4.74 | 2.68±0.34 | 0.66±0.09 | 0.25±0.04 | 0.23±0.04 | 5.33±0.67 | 1.31±0.18 |
0.49±0.05a | 0.47±0.05 |
| 16 µg/ml | 4.83±1.90 | 2.27±0.20 | 0.63±0.08 | 0.27±0.03 | 0.24±0.03 | 4.57±0.27 | 1.26±0.14 | 0.53±0.06 | 0.48±0.05 |
| 32 µg/ml | 1.33±2.77 | 2.50±0.16 | 0.59±0.06 | 0.29±0.02 | 0.22±0.01 | 5.05±0.25 | 1.19±0.16 | 0.58±0.04 | 0.45±0.03 |
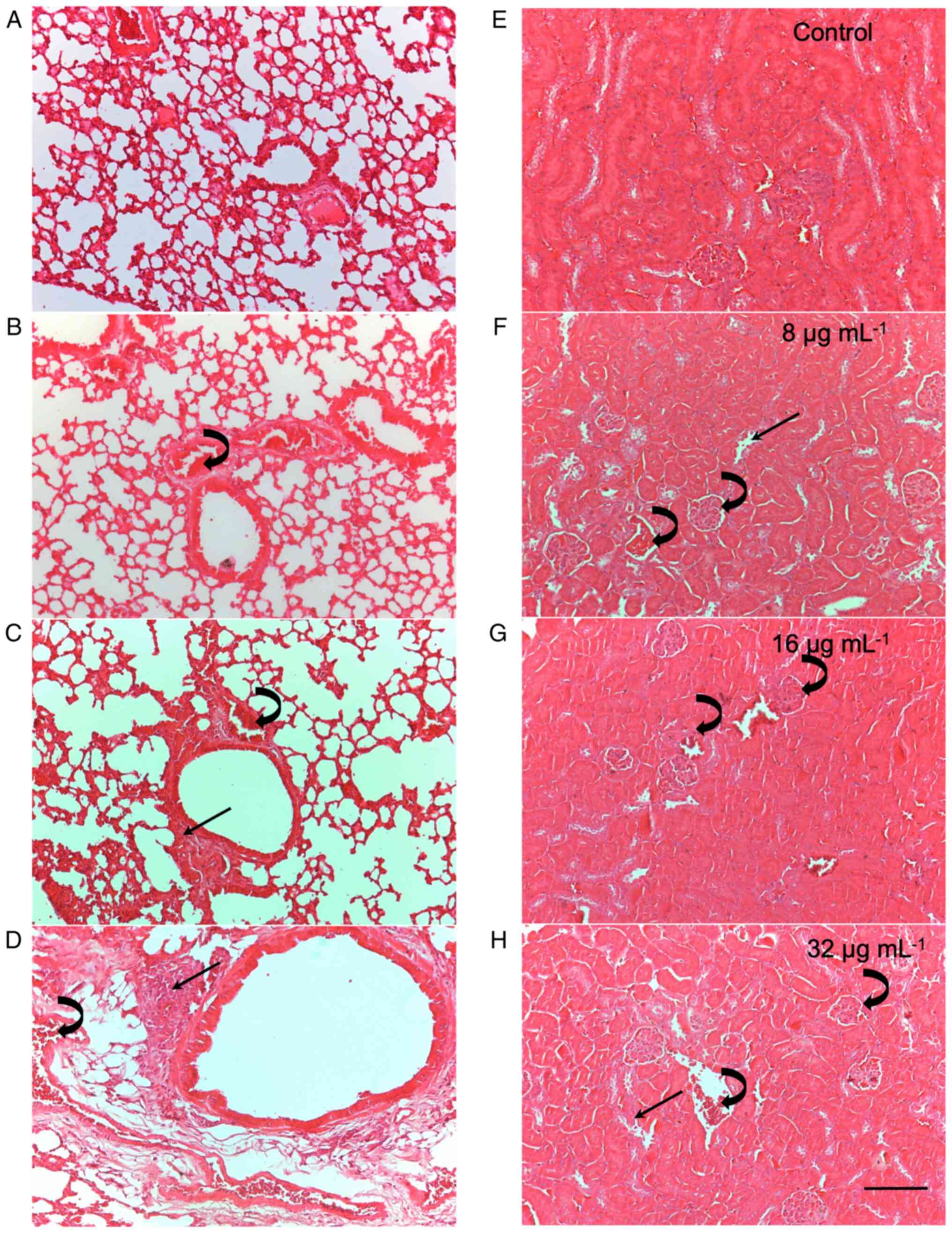
Histopathological changes induced by
CME from the parotoid gland secretion of R. guttatus
The results of the histopathological analysis are
presented in Table II and
Fig. 1 (that illustrates the
histopathological analysis of the lungs and kidneys as a
representative example of tissue damage considered in the study).
Histopathological analysis of the heart, liver, kidneys and lungs
revealed that treatment with CME from the parotoid gland secretion
of R. guttatus induced changes in these tissues, including
edema, intravascular clots, damage to the architecture of the renal
tubules and leukocyte infiltrates. After 7 days of treatment,
changes in all evaluated organs were observed, with predominant
edema and mild leukocyte infiltrates (score 1). At this time point,
the kidneys and lungs were the most affected, as they had a greater
number of alterations; the most intense alterations were in the
lungs, with scores of 2 and 3. After 30 days of treatment, the
tissue changes increased both in number and severity, with the
lungs and kidneys being the most affected organs. Tissue damage was
more noticeable after 30 days of treatment, particularly at doses
of 8 and 32 µg/ml.
 | Table IIHistopathological analysis of organs
from Swiss mice treated with a CME of R. guttatus toad
poison via daily gavage for 7 or 30 days. |
Table II
Histopathological analysis of organs
from Swiss mice treated with a CME of R. guttatus toad
poison via daily gavage for 7 or 30 days.
| 7 days |
|---|
| |
Organ
analyzed | |
|---|
| | Heart | Liver | Lungs | Kidneys | |
|---|
| Group | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Tubular damage | Total score |
|---|
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vehicle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 µg/ml | 1 (5/6) | 0 | 0 | 1 (4/6) | 0 | 0 | 1 (4/6) | 0 | 1 (4/6) | 1 (6/6) | 1 (3/6) | 0 | 5.8 |
| | | | | | | | 2 (2/6) | | 2 (2/6) | | | | |
| 16 µg/ml | 1 (5/6) | 0 | 0 | 1 (2/6) | 0 | 0 | 1 (6/6) | 0 | 1 (2/6) | 1 (6/6) | 1 (3/6) | 0 | 5.2 |
| | | | | | | | | | 2 (4/6) | | | | |
| 32 µg/ml | 1 (6/6) | 0 | 1 (2/6) | 1 (5/6) | 0 | 1 (2/6) | 1 (4/6) | 1 (2/6) | 1 (1/6) | 1 (6/6) | 1 (6/6) | 1 (1/6) | 8.5 |
| | | | | | | | 2 (2/6) | | 2 (3/6) | | | | |
| | | | | | | | | | 3 (2/6) | | | | |
| 30 days |
| |
Organ
analyzed | |
| | Heart | Liver | Lungs | Kidneys | |
| Group | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Leukocyte
infiltrates | Edema | Blood clots | Tubular damage | Total score |
| Control | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Vehicle | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 8 µg/ml | 1 (6/6) | 1 (3/6) | 1 (2/6) | 1 (5/6) | 0 | 1 (4/6) | 1 (5/6) | 1 (4/6) | 1 (1/6) | 1 (6/6) | 1 (6/6) | 1 (3/6) | 9.6 |
| | | | | | | | 2 (1/6) | | 2 (4/6) | | | | |
| | | | | | | | | | 3 (1/6) | | | | |
| 16 µg/ml | 1 (6/6) | 0 | 0 | 1 (4/6) | 0 | 0 | 1 (6/6) | 1 (3/6) | 1 (1/6) | 1 (6/6) | 1 (6/6) | 0 | 7.1 |
| | | | | | | | | | 2 (5/6) | | | | |
| 32 µg/ml | 1 (6/6) | 1 (4/6) | 1 (3/6) | 1 (5/6) | 0 | 1 (4/6) | 1 (3/6) | 1 (6/6) | 2 (3/6) | 1 (6/6) | 1 (4/6) | 1 (5/6) | 14.2 |
| | | | 2 (3/6) | 2 (1/6) | | 2 (2/6) | 2 (3/6) | | 3 (3/6) | | 2 (2/6) | 2 (1/6) | |
Immunomodulatory activity of CME from
the parotoid gland secretion of R. guttatus
The immunomodulatory activity of the CME from the
parotoid gland secretion of R. guttatus was evaluated based
on the lymphoproliferative response and cytokine production of
splenocytes from mice treated with the CME from the parotoid gland
secretion of R. guttatus for 7 and 30 days. In addition, the
capacity of peritoneal macrophages to generate NO. and
H2O2 in vitro was also evaluated.
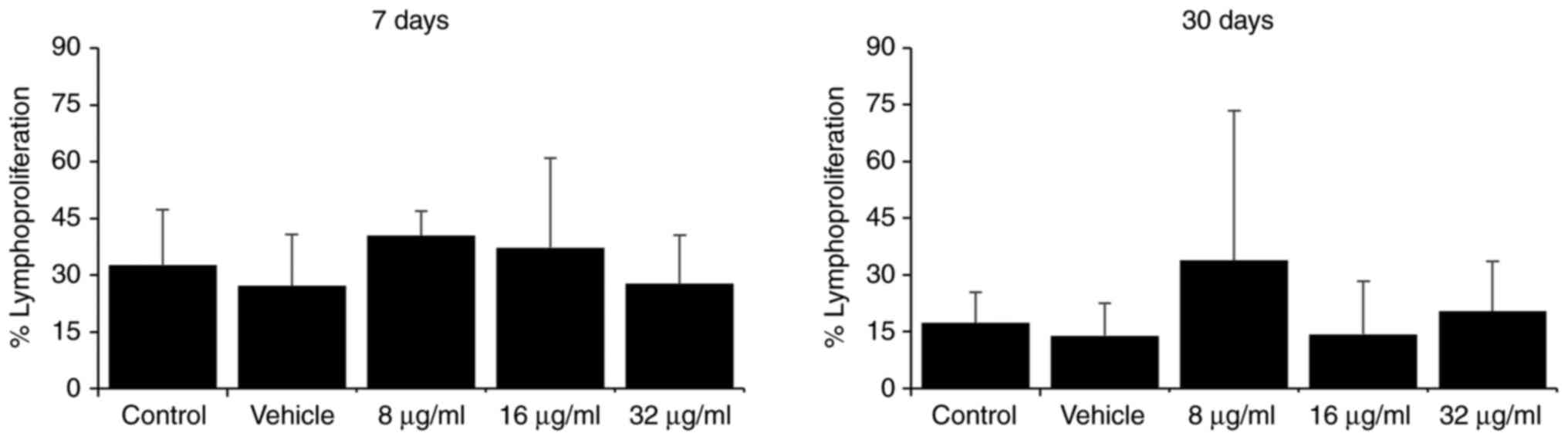
Treatment with various doses of the extract did not
markedly alter the lymphoproliferative capacity of the total
splenocytes compared to the control groups at the two evaluated
time points (7 and 30 days; Fig.
2).
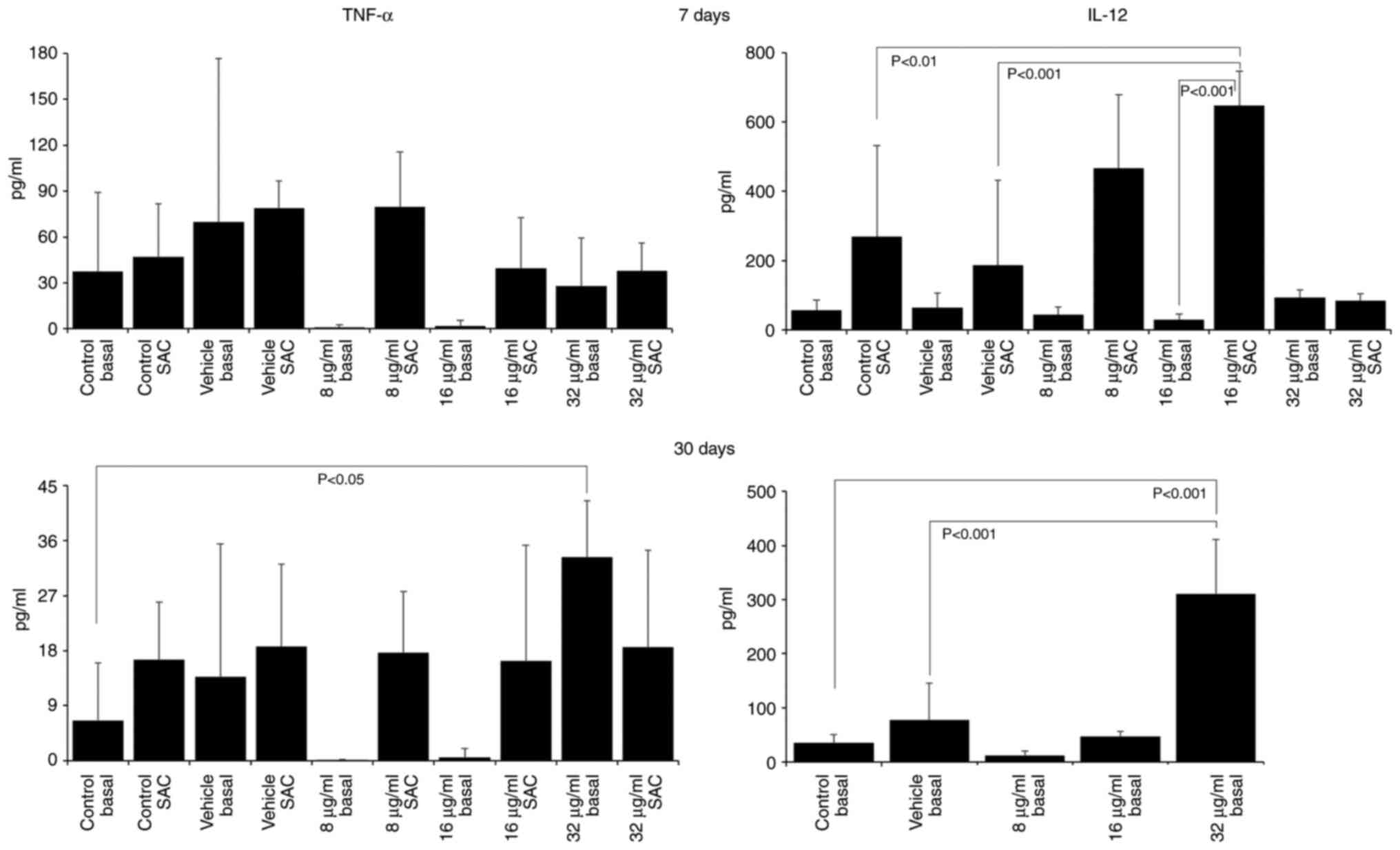
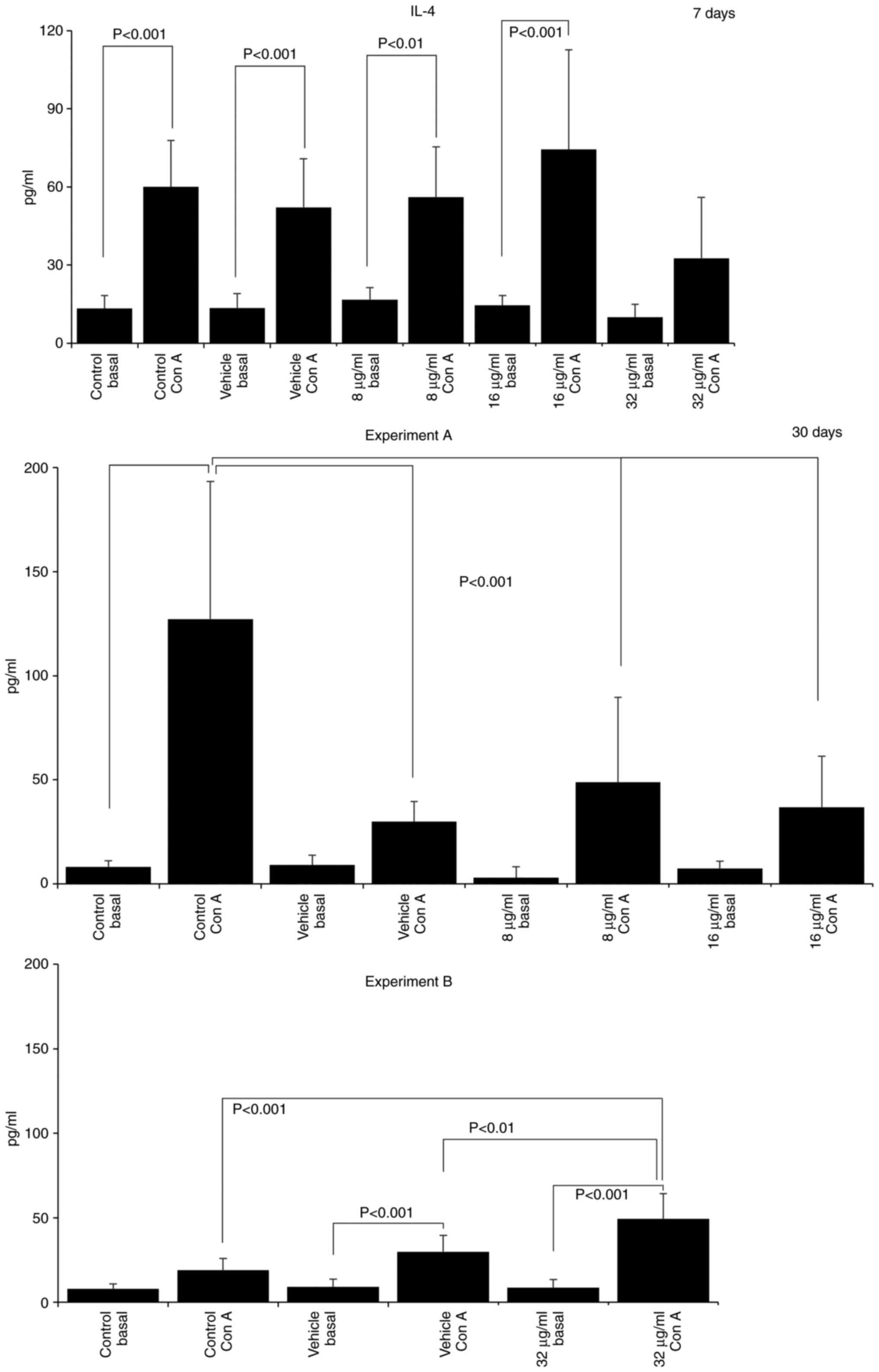
The assessment of cytokine production revealed that
treatment with the extract induced a pro-inflammatory profile, with
increases in the IL-12 and TNF-α levels, and decreases in the IL-4
and IL-10 levels. After 7 days of treatment with 16 µg/ml CME,
IL-12 production increased (SAC control, 268.10±263.47; vehicle
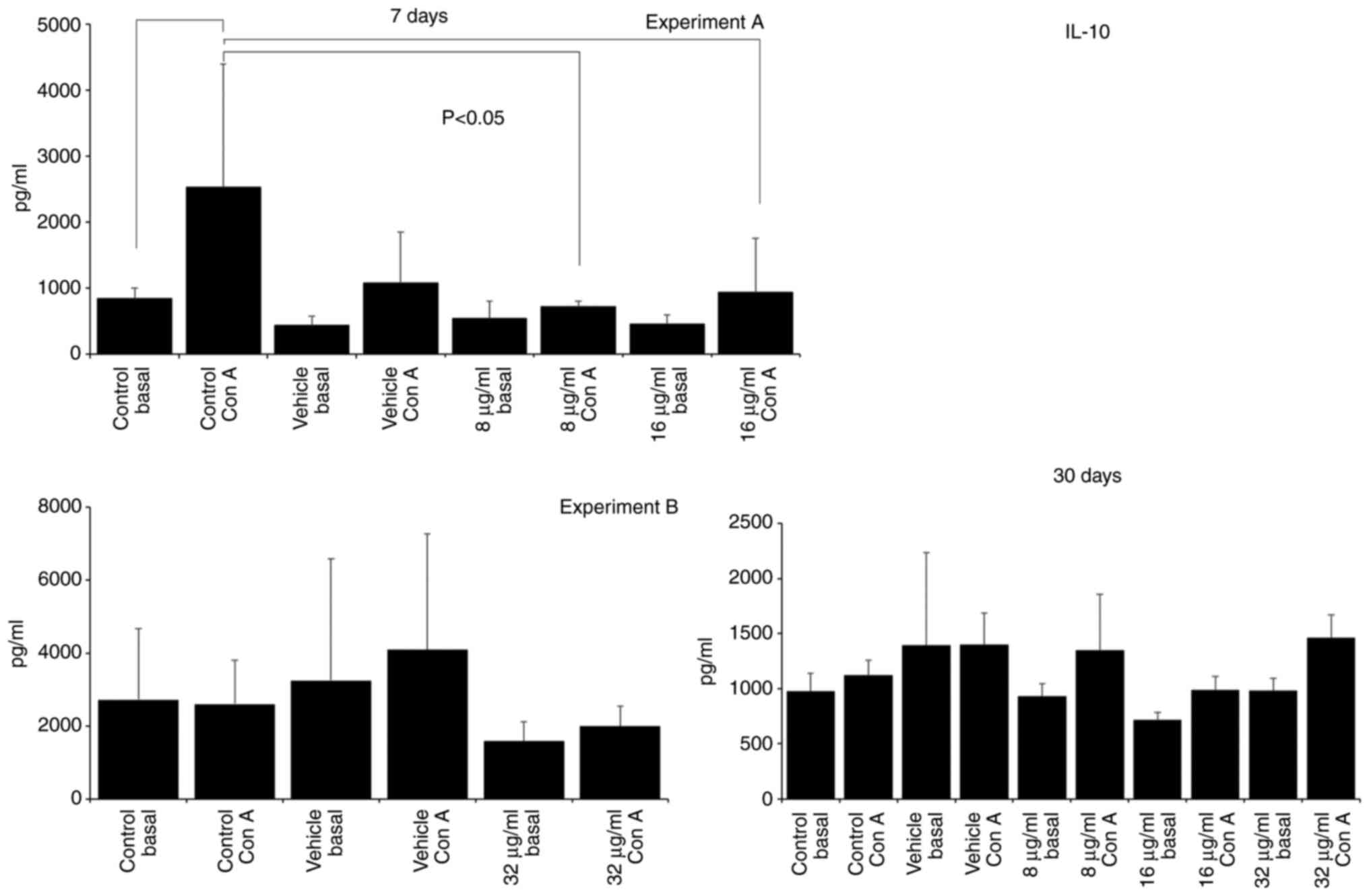
SAC, 186.27±245.10; 16 µg/ml SAC, 647.78±98.73; Fig. 3) and IL-10 production decreased
(Con A control, 2532.50±1863.00; 16 µg/ml Con A, 935.00±816.93;
Fig. 4). IL-10 production was also
decreased in the group treated with 8 µg/ml CME (8 µg/ml Con A,
720.00±79.06; Fig. 4). Treatment
of the mice with 32 µg/ml CME for 30 days increased the production
of IL-12 (basal control, 34.65±15.41; vehicle, 77.27±67.34; 32
µg/ml basal, 310.69±100.72; Fig.
3), TNF-α (basal control, 6.52±9.50; 32 µg/ml basal,
33.32±9.21; Fig. 3) and IL-4 (Con
A control, 19.01±6.82; vehicle Con A, 29.83±9.69; 32 µg/ml Con A,
49.37±14.90; Fig. 5). However, the
administration of 8 and 16 µg/ml CME reduced IL-4 production at the
same time point (Con A control, 127.22±66.02; 8 µg/ml Con A,
48.76±40.93; 16 µg/ml Con A, 36.82±24.41; Fig. 5).
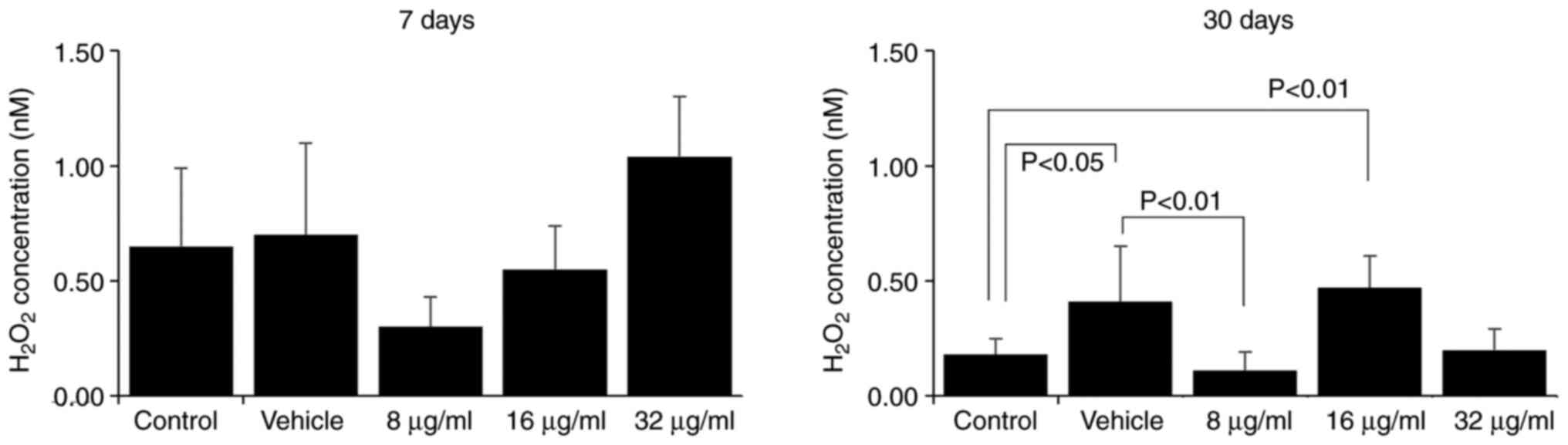
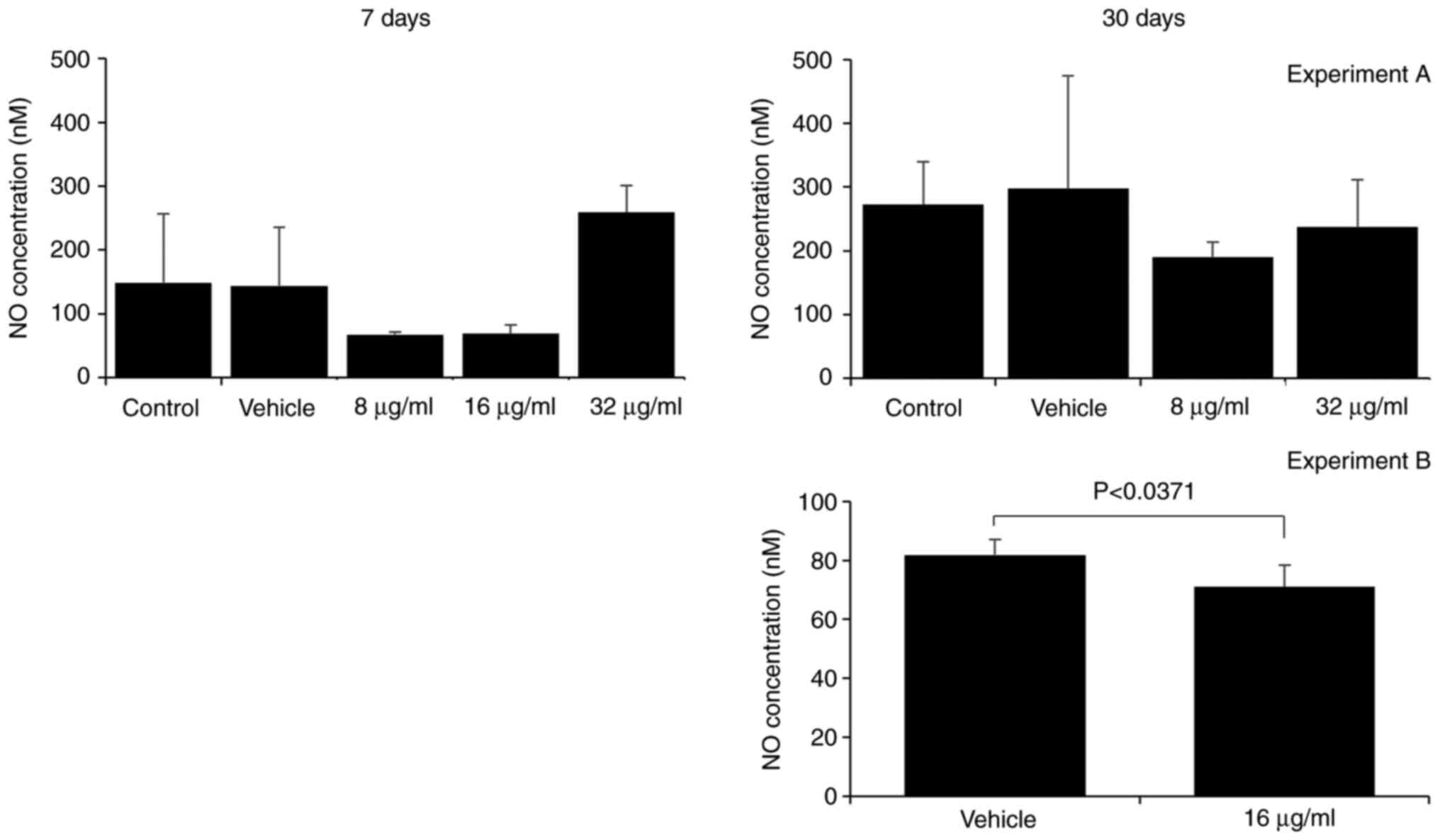
The assessment of H2O2 and
NO. production by macrophages is a method of evaluating
their effector function. Macrophages are essential components of
the innate immune response, as they respond rapidly to pathogenic
stimulation and signs of tissue damage (16,17).
The production of reactive oxygen and nitrogen species is a part of
their antimicrobial mechanism (18). Herein, the effect of the CME from
the parotoid gland secretion of R. guttatus on the
production of H2O2 and NO. by
macrophages was more evident with a longer treatment duration;
there were no marked differences between the groups at 7 days
(Figs. 6 and 7), whereas at 30 days, an increased
H2O2 production was observed in the groups
treated with 16 µg/ml extract (control, 0.18±0.07; 16 µg/ml,
0.47±0.14; Fig. 6) and reduced
production of H2O2 (vehicle, 0.41±0.24, 8
µg/ml, 0.11±0.08; Fig. 6) and
NO. (vehicle, 81.82±5.28; 16 µg/ml, 71.06±7.38; Fig. 7) in the group treated with 8 and 16
µg/ml CME, respectively.
Discussion
The present study demonstrated that a CME from the
parotoid gland secretion of R. guttatus stimulated
peritoneal macrophages to produce H2O2 and
the pro-inflammatory cytokines, IL-12 and TNF-α. The extract also
reduced the production of the anti-inflammatory cytokines, IL-10
and IL-4. To the best of our knowledge, this is the first study to
explore the immunomodulatory potential of an extract obtained from
the poison of this toad species.
The pharmacological effects of the chemical
compounds isolated from the glandular secretions of frogs have been
studied for a long time (2). For
thousands of years, Chansu, an aqueous extract obtained from the
post-auricular and skin glands of Bufo chansa gargarizans
Cantor, has been used as a Traditional Chinese Medicine for the
treatment of various conditions, such as swelling, pain, and heart
failure and, more recently, for cancers of the liver, lung, colon,
pancreas and stomach (19-21).
In Brazil, the most extensively studied amphibian-derived skin
secretions are from Rhinella marina (R. marina;
Cururu toad), which contains compounds, such as marinobufagin,
telecinobufagin, bufalin, marinobufotoxin, cholesterol,
dehydrobufotenine, and suberoyl arginine (2,22,23).
A variety of biological effects have been attributed to the
compounds in R. marina skin secretions extracts, including
cytotoxic, immunomodulatory, antifungal, antimutagenic, and
anti-plasmodial activities (9-11,22-25).
In addition to R. marina, another common frog
species in Brazil is R. guttatus, particularly in the Amazon
Basin; however the compounds in their skin secretions are poorly
characterized (2,5). Some compounds were identified, such
as N-methyl-5-hidroxytryptamine, bufotenine, dehydrobufotenine,
3β,16 β-dihydroxybufa-8(14),20,22-trienolide, marinobufagin, and
bufatrienolides (2,7,8), and
biological effects have been reported in the literature, including
cytotoxic, anti-plasmodial, fungicidal, hemolytic and antimutagenic
activities (8-11).
In the present study, it was observed that the
administration of a CME of R. guttatus to mice did not alter
food and water consumption or body weight. This was also observed
in the study by Oliveira et al (11), as well as in a study using extracts
from other species, such as R. marina (23). Although the absolute and relative
organ weights were similar among the test groups, the relative
weight of the lungs was reduced in mice treated with 8 µg/ml
extract for 30 days. Histopathological analysis indicated that the
lungs and kidneys were the organs most affected by treatment with
the CME, with edema, leukocyte infiltrates and blood clots. These
effects were more prominent at 30 days and in the groups treated
with 8 and 32 µg/ml CME. These results indicated that this CME of
R. guttatus had low toxicity in the mice, since the tissue
alteration scores were low (1 and 2) and were not accompanied by
macroscopic or behavioral alterations. However, treatments >30
days may aggravate tissue damage and may impair lung and kidney
function.
The authors have previously found that the
administration of a R. guttatus CME increased the levels of
thiobarbituric acid reactive substances in the livers of mice,
which was accompanied by the increased activity of
glutathione-S-transferase (GST) and decreased levels of reduced
glutathione (GSH) (unpublished data). These data indicate that
substances present in the parotoid gland secretion activate lipid
peroxidation and induce an imbalance in the protective antioxidants
GSH and GST, corroborating the histopathological changes observed
in the present study. The livers of animals treated with various
doses of R. guttatus CME also exhibited histological
alterations indicative of local inflammation, such as the presence
of edema and leukocyte infiltrates. The metabolites of hepatotoxic
drugs can trigger the production of pro-inflammatory cytokines,
such as TNF-α, IL-1β and IL-6, resulting in liver injury (26,28).
Thus, the histopathological findings are in accordance with the
effects of CME on the production of pro-inflammatory cytokines and
the spontaneous release of H2O2. Treatment of
mice with 16 µg/ml of the extract increased IL-12 production by
splenocytes stimulated with SAC (at 7 days), stimulated the
spontaneous release of H2O2 by peritoneal
macrophages (at 30 days), and inhibited the production of IL-10 and
IL-4 by splenocytes stimulated with Con A (at 7 and 30 days,
respectively), as well as NO. release from macrophages.
At 30 days, the basal production of TNF-α and IL-12 increased in
the mice treated with 32 µg/ml of the extract. Treatment with 8
µg/ml of the extract inhibited the production of
H2O2 (at 30 days), IL-10 (at 7 days) and IL-4
(at 30 days). Taken together, these results demonstrate that the
CME of R. guttatus induced a pro-inflammatory state in
treated mice, by promoting the production of IL-12 and TNF-α and
inhibiting the production of IL-4 and IL-10.
TNF-α is a pro-inflammatory cytokine produced mainly
by macrophages that regulates several cellular functions, such as
leukocyte activation, cytokine and chemokine production, and the
release of reactive oxygen and nitrogen species (29). It is one of the main
inflammation-inducing cytokines and promotes vascular changes, such
as the activation of endothelial cells, increased vascular
permeability and vasodilation, and leukocyte influx, in addition to
being a potent neutrophil activator (18,29,30).
IL-12 is also produced by macrophages and is considered to function
as a bridge between the innate and specific immune responses by
promoting the cell-mediated response, including differentiation of
T-helper (Th) 1 and cytotoxic T-lymphocytes, and the activation of
microbicidal functions and the oxidative burst of macrophages via
interferon-γ (IFN-γ)-induced feedback (18,30,31).
The oxidative burst results from the formation of active NADPH
oxidase within the macrophage phagolysosome, resulting in increased
oxygen consumption (18). This
generates superoxide anion radicals in the lumen of the
phagolysosome, which are converted to H2O2 by
superoxide dismutase (18). In the
present study, the mitogenic stimulus (SAC) was commonly used to
stimulate macrophages in vitro and measure the subsequent
IL-12 and TNF-α production as it is rich in immunostimulant
components, such as peptidoglycans and lipoteichoic acid (32). Thus, the results revealed that the
compounds present in the CME of R. guttatus are able to
modulate the function of macrophages to a pro-inflammatory profile,
characteristic of M1-type cells.
Treatment with the extract also led to decreases in
IL-10, IL-4 and NO. production. IL-10 is an
anti-inflammatory cytokine produced by diverse cells, including
macrophages and T-lymphocytes (30). IL-10 inhibits the production of
cytokines, such as IL-12, TNF-α and IFN-γ, by activated phagocytes
and T-lymphocytes, and reduces lymphocyte activation by inhibiting
antigen presentation by dendritic cells and macrophages (30,33).
IL-4 is produced by T-lymphocytes, mast cells, basophils and
eosinophils, and, in contrast to the effects of IL-12, IL-4 favors
the differentiation of Th 2 lymphocytes, characteristic of the
humoral response of allergic inflammation and parasitic infection
(30,34). The Th 2 response-promoting effect
of IL-4 results in inhibition of the Th 1 response and,
consequently, in the decreased production of IL-12, TNF-α and IFN-γ
(33). In addition, IL-4
suppresses macrophage activity, inhibiting the microbicidal
activity of the cell, including the production of NO.
(18,33). Thus, these effects are in
accordance with the results obtained in the present study and
reinforce the pro-inflammatory effect of the compounds present in
the extract.
However, the administration of 32 µg/ml extract
promoted IL-4 production by splenocytes stimulated with Con A after
30 days of treatment. Despite the inhibitory effect of IL-4 on the
Th 1 cellular response and its anti-inflammatory character, this
cytokine is a key cofactor in the production of IL-12 and IFN-γ,
which are characteristic of the Th 1 profile (35,36).
Some studies have shown that a lack of IL-4 results in the
development of a deficient Th 1 response, with impairs responses to
tumors and infections caused by Candida albicans and
Leishmania major (35,36).
Thus, the increased production of IL-4, observed in the present
study, may also have contributed to the development of the
pro-inflammatory profile.
The results of the present study are reinforced by
similar findings in previous studies on bufadienolides obtained
from other amphibian species, including the promotion of the Th 1
response (37), the
differentiation of M1 macrophages (38), and the stimulation of IL-12 and
TNF-α (38) and reactive oxygen
species (39,40).
Finally, it should be noted that the dose of 16
µg/ml CME induced the most prominent immunomodulatory response with
the lowest histopathological damage score, corroborating the
observation that intermediate doses are optimal as they induce
immunological responses with maximum effector action (41).
In conclusion, the results of the present study
demonstrated that a CME of R. guttatus has low toxicity in
Swiss mice and an immunomodulatory effect; it promotes the
development of a pro-inflammatory response. Further studies are
required however, to identify the main compounds and intracellular
pathways involved in this process. Thus, R. guttatus is a
promising species for bioprospecting studies, particularly for
conditions in which stimulation of the immune response is
advantageous, such as neoplastic diseases.
Nature is a rich source of diverse bioactive
compounds. As an example, the marine algal polysaccharides with
several pharmaceutical activities have already been described,
including antioxidant, anti-inflammatory, immunomodulatory and
antidiabetic effects, being beneficial to human health and
nutrition (42). Thus, studies
with natural products become essential for the development of the
bioeconomy.
Acknowledgements
The authors would like to thank Dr Amilcar Sabino
Damazo, Department of Basic Science in Health, Faculty of Medical
Sciences, Federal University of Mato Grosso, Cuiabá, Brazil, for
his assistance in the histopathological analysis.
Funding
Funding: The authors wish to acknowledged the CAPES Foundation
for the availability of a scholarship.
Availability of data and materials
The datasets generated and analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
VDGS, DDJR, LC and APS conceived the study and
designed the experiments. VDGS and LC participated in the design
and interpretation of the data. SRDNP, EBRDS and LC performed the
experiments and the data analysis. LC and VDGS wrote the manuscript
and participated in the manuscript revisions. LC, SRNP and EBRS
confirm the authenticity of all the raw data. All authors have read
and approved the final manuscript.
Ethics approval and consent to
participate
The present study was registered in the National
System for the Management of Genetic Heritage and Associated
Traditional Knowledge (SISGEN) under no. A313DC9, and approved by
the UFMT Ethics Committee on the Use of Animals (CEUA), under no.
23108.918243/2017-50.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Still P, Chen W, Weber W and Hopp DC:
NCCIH priorities for natural products research. Planta Med.
88:698–701. 2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Sousa LQ, Machado KC, Oliveira SFC, Araújo
LS, Monção-Filho ES, Melo-Cavalcante AM, Vieira-Júnior GM and
Ferreira PMP: Bufadienolides from amphibians: A promising source of
anticâncer prototypes for radical innovation, apoptosis triggering
and Na+/K+-ATPase inhibition. Toxicon.
127:63–76. 2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Deng LJ, Qi M, Li N, Lei YH, Zhang DM and
Chen JX: Natural products and their derivatives: Promising
modulators of tumor immunotherapy. J Leukoc Biol. 108:493–508.
2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Ekiert HM and Szopa A: Biological
activities of natural products II. Molecules.
27(1519)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Kerkhoff J, Noronha JC, Bonfilio R,
Sinhorin AP, Rodrigues DJ, Chaves MH and Junior GMV: Quantification
of bufadienolides in the poisons of Rhinella marina and Rhaebo
guttatus by HPLC-UV. Toxicon. 119:311–318. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Lötters S, De la Riva I, Reichle S and
Soto G: First records of Bufo guttatus from Bolivia with comments
on Bufo glaberrimus (Amphibia: Bufonidae). Bonner Zoologische
Beiträege. 49:75–78. 2000.
|
|
7
|
Souza EBR, Júnior PT, Vasconcelos LG,
Rodrigues DJ, Sinhorin VDG, Kerkhoff J, Pelissari SRN and Sinhorin
AP: Comparative study of the chemical profile of the parotoid gland
secretions from Rhaebo guttatus from different regions of the
Brazilian Amazon. Toxicon. 179:101–106. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Ferreira PMP, Lima DJB, Debiasi BW, Soares
BM, Machado KC, Noronha JC, Rodrigues DJ, Sinhorin AP, Pessoa C and
Vieira GM Jr: Antiproliferative activity of Rhinella marina and
Rhaebo guttatus venom extracts from Southern Amazon. Toxicon.
72:43–51. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Banfi FF, Guedes KES, Andrighetti CR,
Aguiar AC, Debiasi BW, Noronha JAC, Rodrigues DJ, Júnior GMV and
Sanchez BAM: Antiplasmodial and cytotoxic activities of toad
poisons from southern amazon, Brazil. Korean J Parasitol.
54:415–421. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Raasch-Fernandes LD, Bonaldo SM, Rodrigues
DJ, Ferrarini SR, Verçosa AGA and Oliveira DL: In vitro
antimicrobial activity of methanolic extracts from cutaneous
secretions of Amazonian amphibians against phytopathogens of
agricultural interest. Acta Amazonica. 51:145–155. 2021.
|
|
11
|
Oliveira AF, Castoldi L, Vieira Jr GM,
Filho ES, Chaves MH, Rodriques DJ and Sugui MM: Evaluation of
antimutagenic and cytotoxic activity of skin secretion extract of
Rhinella marina and Rhaebo guttatus (Anura, Bufonidae). Acta
Amazonica. 49:145–151. 2019.
|
|
12
|
Duan W, Chan JH, Wong CH, Leung BP and
Wong WS: Anti-inflammatory effects of mitogen-activated protein
kinase Amazonia inhibitor U0126 in an asthma mouse model. J
Immunol. 172:7053–7059. 2004.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Pick E and Mizel D: Rapid microassays for
the measurement of superoxide and hydrogen peroxide production by
macrophages in culture using an automatic enzyme immunoassay
reader. J Immunol Methods. 46:211–226. 1981.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Green LC, De Luzuriaga KR, Wagner DA, Rand
W, Istfan N, Young VR and Tannenbaum SR: Nitrate biosynthesis in
man. Proc Natl Acad Sci USA. 78:7764–7768. 1981.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Pires OC, Taquemasa AVC, Akisue G,
Oliveira FO and Araújo CEP: Preliminary comparative analysis of the
acute toxicity and median lethal dose (DL50) of the
fruit of the Brazilian Black Pepper (Schinus terebinthifolius
Raddi) and Black pepper (Piper nigrum L.). Acta Farm Bonaerense.
23:176–182. 2004.
|
|
16
|
Kolliniati O, Ieronymaki E, Vergadi E and
Tsatsanis C: Metabolic regulation of macrophage activation. J
Innate Immun. 14:51–67. 2022.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Locati M, Curtale G and Mantovani A:
Diversity, mechanisms and significance of macrophage plasticity.
Annu Rev Pathol. 15:123–147. 2020.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Lendeckel U, Venz S and Wolke C:
Macrophages: Shapes and functions. ChemTexts. 8(12)2022.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Qi F, Li A, Inagaki Y, Xu H, Wang D, Cui
X, Zhang L, Kokudo N, Du G and Tang W: Induction of apoptosis by
cinobufacini preparation through mitochondria- and Fas-mediated
caspase-dependent pathways in human hepatocellular carcinoma cells.
Food Chem Toxicol. 50:295–302. 2021.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wie X, Si N, Zhang Y, Zhao H, Yang J, Wang
H, Wang L, Han L and Bian B: Evaluation of bufadienolides as the
main antitumor components in cinobufacin injection for liver and
gastric cancer therapy. PLoS One. 12(e0169141)2017.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Yin JH, Zhu XY, Shi WD and Liu LM:
Huachansu injection inhibits metastasis of pancreatic cancer in
mice model of human tumor xenograft. BMC Complement Altern Med.
14(483)2014.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Pelissari SRN, Sinhorin VDG, Castoldi L,
Vasconcelos LG, Rodrigues DJ, Ribeiro EBS, Kerkhoff J and Sinhorin
AP: Methanolic extract of Rhinella marina poison: Chemical
composition, antioxidant and immunomodulatory activities. J Braz
Chem Soc. 32:1584–1597. 2021.
|
|
23
|
Filho ESM, Chaves MH, Ferreira PMP, Pessoa
C, Lima DJB, Maranhão SSA, Rodrigues DJ and Júnior GMV: Cytotocity
potencial of chemical constituents isolated and derivatised from
Rhinella marina venon. Toxicon. 194:37–43. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Machado KC, Sousa LQ, Lima DJB, Soares BM,
Cavalcanti BC, Maranhão SSA, Noronha JC, Rodrigues DJ, Militão GCG,
Chaves MH, et al: Marinobufagin, a molecule from poisonous frogs,
causes biochemical, morphological and cell cycle changes in human
neoplasms and vegetal cells. Toxicol Lett. 285:121–131.
2018.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Schmeda-Hirschmann G, Quispe C, Arana GV,
Theoduloz C, Urra FA and Cárdenas C: Antiproliferative activity and
chemical composition of the venom from the Amazonian toad Rhinella
marina (Anura: Bufonidae). Toxicon. 121:119–129. 2016.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Schemitt EG, Hartmann RM, Colares JR,
Licks F, Salvi JO, Marroni CA and Marroni NP: Protective action of
glutamine in rats with severe acute liver failure. World J Hepatol.
11:273–286. 2019.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Zakaria Z, Othman ZA, Suleiman JB, Jalil
NAC, Ghazali WSW, Nna VU and Mohamed M: Hepatoprotective effect of
bee bread in metabolic dysfunction-associated fatty liver disease
(MAFLD) rats: Impact on oxidative stress and inflammation.
Antioxidants (Basel). 10(2031)2021.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Ijaz MU, Shahad MS, Samad A, Ashraf A,
Al-Ghanim K, Mruthinti SS and Mahboob S: Tangeretin ameliorates
bisphenol induced hepatocyte injury by inhibiting inflammation and
oxidative stress. Saudi J Biol Sci. 29:1375–1379. 2022.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Lona JMF, Martínez MS, Alarcón GV, Rodas
AB and Bello JR: Tumor necrosis factor alfa (TNF-α) in
cardiovascular diseases: Molecular biology and genetics. Gac Méd
Mex. 149:521–530. 2013.PubMed/NCBI(In Spanish).
|
|
30
|
Borish LC and Steinke JW: 2. Cytokines and
chemokines. J Allergy Clin Immunol. 111:S460–S475. 2003.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Trinchieri G: Interleukin-12 and the
regulation of innate resistance and adaptive immunity. Nat Rev
Immunol. 3:133–146. 2003.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Zhu FG and Pisetsky DS: Role of the heat
shock protein 90 in immune response stimulation by bacterial DNA
and synthetic oligonucleotides. Infect Immun. 69:5546–5552.
2011.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Opal SM and DePalo VA: Anti-inflammatory
cytokines. Chest. 117:1162–1172. 2000.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Iwaszko M, Bialy S and Bogunia-Kubik K:
Significance of interleukin (IL)-4 and IL-13 in inflammatory
arthritis. Cells. 10(3000)2021.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Morris SC, Orekhova T, Meadows MJ, Heidorn
SM, Yang J and Finkelman FD: IL-4 induces in vivo production of
IFN-gamma by NK and NKT cells. J Immunol. 176:5299–5305.
2006.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Kalinski P, Smits HH, Schuitemaker JHN,
Vieira PL, Eijk MV, Jong EC, Wierenga EA and Kapsenberg ML: IL-4 is
a madiator of IL-12p70 induction by human Th2 cells: Reversal of
polarized Th2 phenotype by dendrict cells. J Immunol.
165:1877–1881. 2000.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Wu SC, Fu BD, Shen HQ, Yi PF, Zhang LY, Lv
S, Guo X, Xia F, Wu XL and Wei XB: Telocinobufagin enhances the Th1
immune response and protects against Salmonella typhimurium
infection. Int Immunopharmacol. 25:353–362. 2015.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Yu Z, Li Y, Li Y, Zhang J, Li M, Ji L,
Tang Y, Zheng Y, Sheng J, Han Q, et al: Bufalin stimulates
antitumor immune response by driving tumor-infiltrating macrophage
toward M1 phenotype in hepatocellular carcinoma. J Immunother
Cancer. 10(e004297)2022.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Khalaf FK, Dube P, Kleinhenz AL, Malhotra
D, Gohara A, Drummond CA, Tian J, Haller ST, Xie Z and Kennedy DJ:
Proinflammatory effects of cardiotonic steroids mediated by NKA α-1
(Na+/K+-ATPase α-1)/Src complex in renal
epithelial cells and immune cells. Hypertension. 74:73–82.
2019.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Khalaf FK, Tassavvor I, Mohamed A, Chen Y,
Malhotra D, Xie Z, Tian J, Haller ST, Westfall K, Tang WHW and
Kennedy DJ: Epithelial and endothelial adhesion of immune cells is
enhanced by cardiotonic steroid signiling through
Na+/K+-ATPase-α-1. J Am Heart Assoc.
9(e013933)2020.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Abbas AK, Lichtman AH and Pillai S:
Imunologia celular e molecular (ed.) Elsevier Brasil, p552,
2012.
|
|
42
|
Cheong KL, Yu B, Chen J and Zhong S: A
comprehensive review of the cardioprotective effect of marine algae
polysaccharide on the gut microbiota. Foods.
11(3550)2022.PubMed/NCBI View Article : Google Scholar
|