Introduction
A limited number of biomarkers have been used to
predict the prognosis and monitor the outcomes of patients with
breast cancer (BC), such as lactate dehydrogenase (LDH), which is
an independent prognostic marker for BC and the Ki-67 index, which
is of utmost importance to the prognosis and molecular typing of
patients with BC (1). Patients
with BC with a high Ki-67 index exhibit more pathological complete
responses (pCRs) regardless of the ER (estrogen receptor),
progesterone receptor (PR) and the human epidermal growth factor
receptor 2 (HER-2) status. Ki-67 even can be used to predict the
pCRs of patients treated with neoadjuvant therapy from Asia and
Europe, but not those from the USA (2). Even young women with BC have a higher
Ki-67 index than that of women with BC >60 years of age
(3).
In 2019, it was reported in the Guidelines of the
Chinese Society of Clinical Oncology (CSCO) that the critical value
of Ki-67 should be determined according to the practical situation
of each laboratory (4). As
recognized by the majority of Chinese experts, a Ki-67 index of
<15% indicates a low expression and one of >30% is suggestive
of a high expression. When the Ki-67 index is in the range between
15-30%, a secondary pathology consultation is suggested or clinical
decisions can be made according to the values of other indices
(4). During the practical
pathological diagnosis, it is difficult to accurately calculate the
critical value of Ki-67 with poor repeatability. The results
obtained by different laboratories are derived from technicians who
are familiar with the operation to different degrees and
trained/untrained diagnosticians. Due to these differences, the
results are not exactly the same. Sometimes, the definition of the
Ki-67 index remains controversial, particularly regarding whether
pathologists should evaluate the Ki-67 index in the ‘hot-spots’
area in the infiltrated tumor or whether they should report average
values of Ki-67(5). Notably, after
the critical value of the Ki-67 index has been reached, it is
difficult to standardize the operation or accurately calculate this
index. The accurate estimation of the Ki-67 index can be performed
using a scientific method. The potential to achieve the accurate
prognosis of patients with BC with the Ki-67 index is dependent on
whether this index is associated with the molecular subtype of BC,
the possibility of patients with different molecular types to be
applicable to the same Ki-67 index, and requires this index to
possess an interval value, facilitating clinical pathologists to
operate and increase the critical application value. These topics
are explored in the present study.
Patients and methods
Patient data and specimens
The paraffin-embedded specimens of patients with BC
hospitalized in the 989th Hospital of the PLA Joint Logistic
Support Force Hospital (Luoyang, China) during the period January,
2005 to July, 2013 were collected for the present retrospective
study. Follow-up data were obtained from 199 patients with BC with
complete information, including 1 male (0.5%) and 198 females
(99.5%) with an age range of 25-84 years (average age, 48 years).
The number of patients with an age ≤30 years was 1, that of
patients with an age range of 30-60 years was 162, and that of
patients with an age ≥60 years was 36. A total of 104 patients
exhibited no lymphatic metastasis (N0), 53 patients presented with
1-3 lymphatic metastases (N1), 31 cases had 4-9 lymphatic
metastases (N2), and 11 patients had ≥10 lymphatic metastases (N3).
As regards the American Joint Committee on Cancer (AJCC)
pathological staging (pTNM) (6),
34 patients were classified as stage IA, 72 cases as stage IIA, 45
cases as stage IIB, 35 cases as stage IIIA, 1 as stage IIIB, 11
cases as stage IIIC and 1 as stage IV. None of the patients
accepted chemotherapy or radiotherapy prior to the operation. The
specimens were fixed using 10% neutral formalin along with paraffin
embedding. The following inclusion criteria were used: i) The
patients received a definitive diagnosis via an examination
following a BC radical mastectomy; ii) no chemotherapy or
radiotherapy were provided prior to the surgery; iii) complete
follow-up visit information was provided; iv) patients with BC
metastasis or recurrence. The following exclusion criteria were
used: i) A lack of complete follow-up visit information; ii)
patients who received radiotherapy or chemotherapy prior to the
surgery; iii) the patients who were diseased not due to BC
metastasis or recurrence; iv) patients whose quality of life was
severely affected by other causes or who had succumbed to the
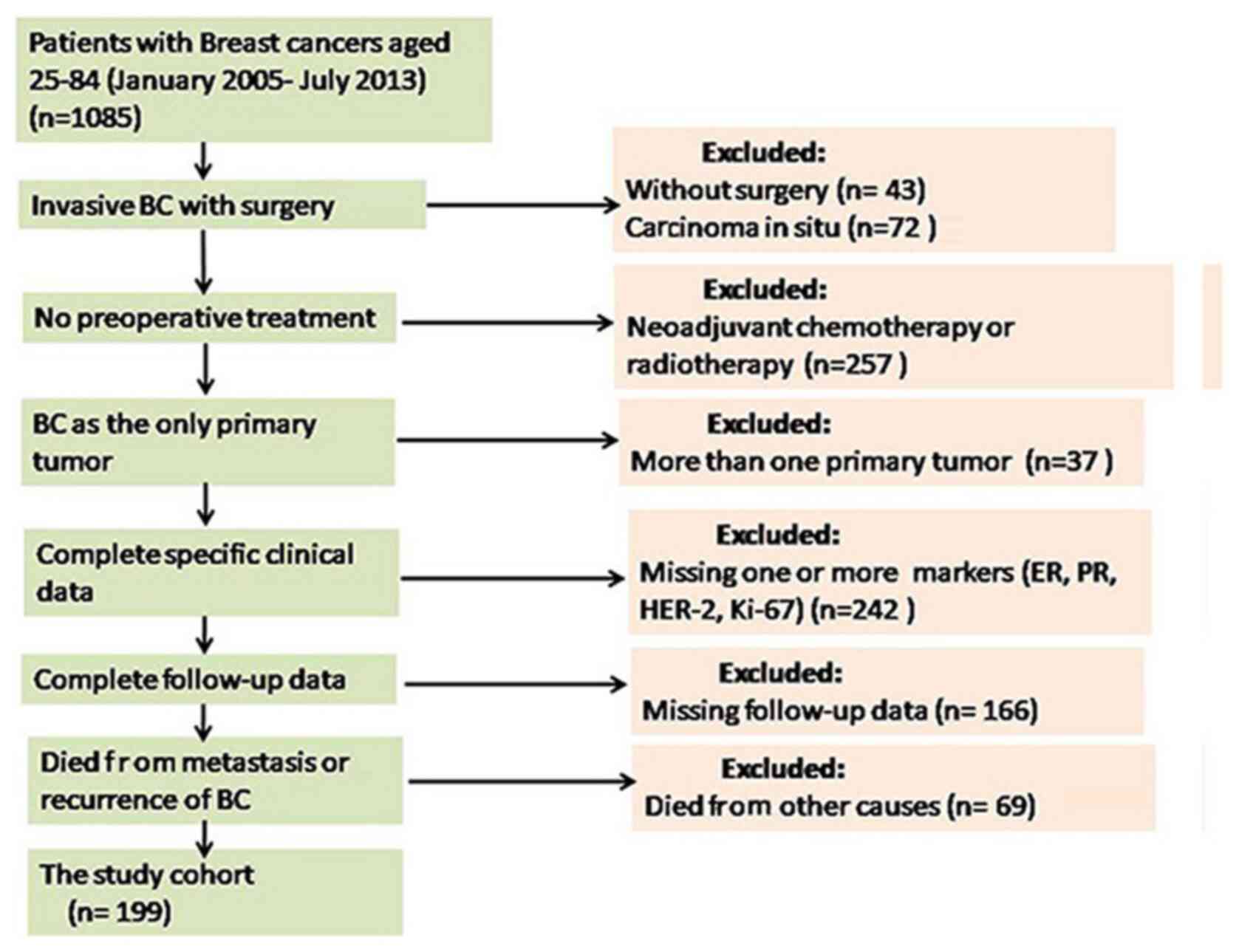
disease due to the concurrence of other tumors (Fig. 1).
Written informed consent was obtained from the 989th
Hospital of the PLA Joint Logistic Support Force database for the
collection of data and for the use of personal data for research
purposes, and written informed consent was obtained from the
patients or their immediate family. The present study was ethically
approved by the Ethics Committee of the 989th Hospital of the PLA
Joint Logistic Support Force review board on May 14, 2020 (Approval
no. 20200508). The patient information was obtained from the
medical records of 989th Hospital and the follow-up data were
obtained from the patients or their immediate family members.
Immunohistochemical staining
The SP immunohistochemistry (IHC) method was used to
cut the selected paraffin blocks into a thickness of 2-3 µm. Rabbit
anti-human monoclonal ER (clone no. SP1, cat. no. Kit-0012, 1:300),
rabbit anti-human monoclonal PR (clone no. SP2, cat. no. Kit-0013,
1:300), rabbit anti-human monoclonal HER-2 (clone no. MXR001, cat.
no. Kit-0043, 1:200), rabbit anti-human monoclonal antibody Ki-67
(clone no. SP6, cat. no. RMA0542, 1:500), secondary antibodies
(ready-to-use sheep anti-rabbit IgG polymer, cat. no. KIT 5010) and
color-producing reagents (DAB staining solution) were all purchased
from Fuzhou Maixin Biotech Co., Ltd. The operating steps followed
the conventional IHC method and PBS was used to replace the primary
antibodies with the negative control. Briefly, 2-3-µm-thick
sections were mounted on poly-lysine treated glass slides.
Endogenous peroxidase activity was blocked with 3.0%
H2O2 for 15 min at room temperature. The
sections were placed in a pressure cooker for 15 min in 10 mM
citrate buffer (pH 6.0) for antigen retrieval. The sections were
then incubated with primary antibodies at 4˚C overnight. The
following day, the sections were washed and incubated for 1 h at
room temperature with the secondary antibodies. Peroxidase activity
was visualized with DAB and observed under a light microscope
(Olympus Corporation) at a low magnification. The appearance of
brown particles in the membrane or the nucleus was considered as
the positive criterion.
The following result interpretation criterion was
used: ER, PR and Ki-67 were all found positive in the cell nucleus.
In the case that the presence of brown particles was observed in
>1% of BC cell nuclei, ER and PR were classified as positive
(Fig. 2A and B). The presence of yellow or brown
particles in the cell nucleus was considered to indicate positive
Ki-67 staining (Fig. 2C-E).
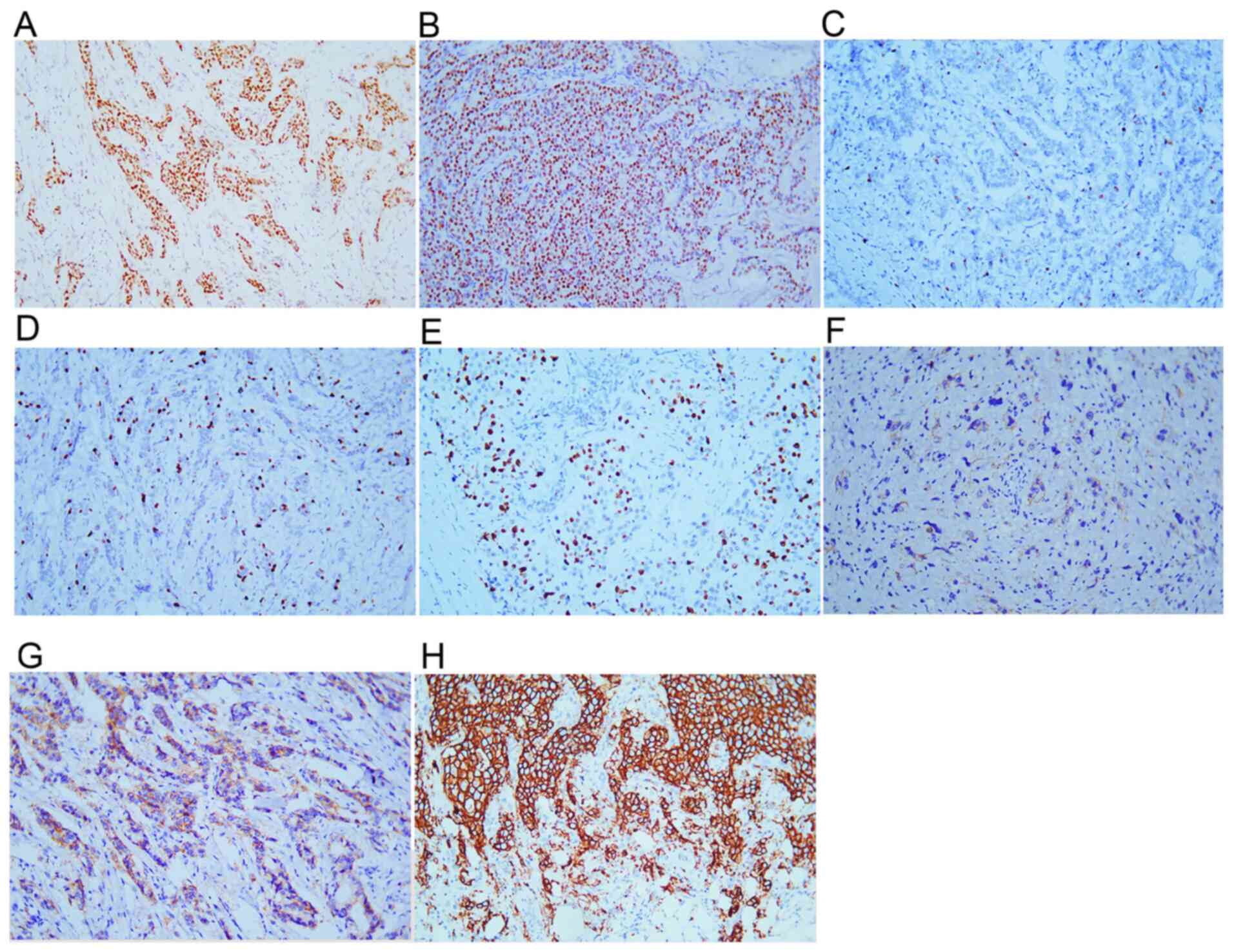 | Figure 2Immunohistochemical staining results
of ER, PR, HER-2 and Ki-67. Tumor cells positive for (A) ER, (B)
PR, (C) Ki-67 index <1%, (D) Ki-67 index 15%, (E) Ki-67 index
>30%, (F) HER-2 (1+), (G) HER-2 (2+), (H) HER-2 (3+).
Magnification, x200. PR, progestogen receptor; ER, estrogen
receptor; HER-2, human epidermal growth factor receptor 2. |
The following HER-2 positive criteria were used: 0+
for unstained or ≤10% of invasive cancer cells presenting
incomplete and weak cell membrane staining; 1+ for >10% of
invasive cancer cells presenting incomplete and weak cell membrane
staining (Fig. 2F); 2+ for >10%
of invasive cancer cells presenting incomplete and/or moderate cell
membrane staining or ≤10% of invasive cancer cells presenting
strong and complete cell membrane staining (Fig. 2G); 3+ for >10% of invasive
cancer cells presenting strong, complete, and uniform cell membrane
staining (Fig. 2H) (7). In the presence of HER-2 2+ staining,
fluorescence in situ hybridization detection was implemented
according to the instructions of the manufacturer (Amoy
Dx®HER-2 detection kit, Amoy Diagnostics Co., Ltd.) to
further determine the status of HER-2. Briefly, 4-µm-thick sections
were dewaxed and hydration, and then placed in boiled pre-treatment
solution (pH 7.0) for 20 min. This was followed by washing with
deionized water for 1 min and washing with 2X SSC solution (pH 7.0)
for 1 min. The sections were then treated with protease K at 37˚C
for 15 min, washed with 2 X SSC solution (pH 7.0) for 1 min,
dehydrated and dried. A total of 8 µl hybridization buffer and 2 µl
of the pre-labelled HER-2 probe were added to the microcentrifuge
tube and centrifuged at 1,000 x g for 3 sec at room temperature.
Subsequently, 10 µl of the probe hybridization mixture was applied,
sealed and denatured at 78˚C for 3 min. The slides were then
incubated in a humidified chamber protect from light at 37˚C for 16
h. The slides were then washed with 2X SSC/0.3% NP-40 at 46˚C for 2
min, placed in gradient alcohol ethanol for 5 min, and finally
air-dried. A total of 10 µl DAPI was then immediately added and the
sections were observed under a fluorescence microscope (Olympus
Corporation). When the experiments were completed, at least 20
infiltrating cancer cells in ≥2 representative areas were counted
to determine the status of HER-2. The HER-2/CEP17 ratio ≥2.0 and
the mean HER-2 copy number/cell ≥4.0 indicate a positive expression
of HER-2. In the case of an HER-2/CEP17 ratio ≥2.0 and a mean HER-2
copy number/cell <4.0, it is recommended to increase the number
of cells, and once the result remains unaltered, it is judged as
negative. In the case of an HER-2/CEP17 ratio <2.0 and a mean
HER-2 copy number/cell ≥6.0, it is recommended to increase the
count of cells, and if the result remains unaltered, it is judged
positive. In the case of an HER-2/CEP17 ratio <2.0, mean HER-2
copy number/cell ≥4.0 and <6.0, and if the result of IHC HER-2
is not 3+, the signal of another 20 tumor cells was re-counted. If
the results changed, the two results were comprehensively analyzed
according to the results of IHC.
Interpretation method for the Ki-67
index
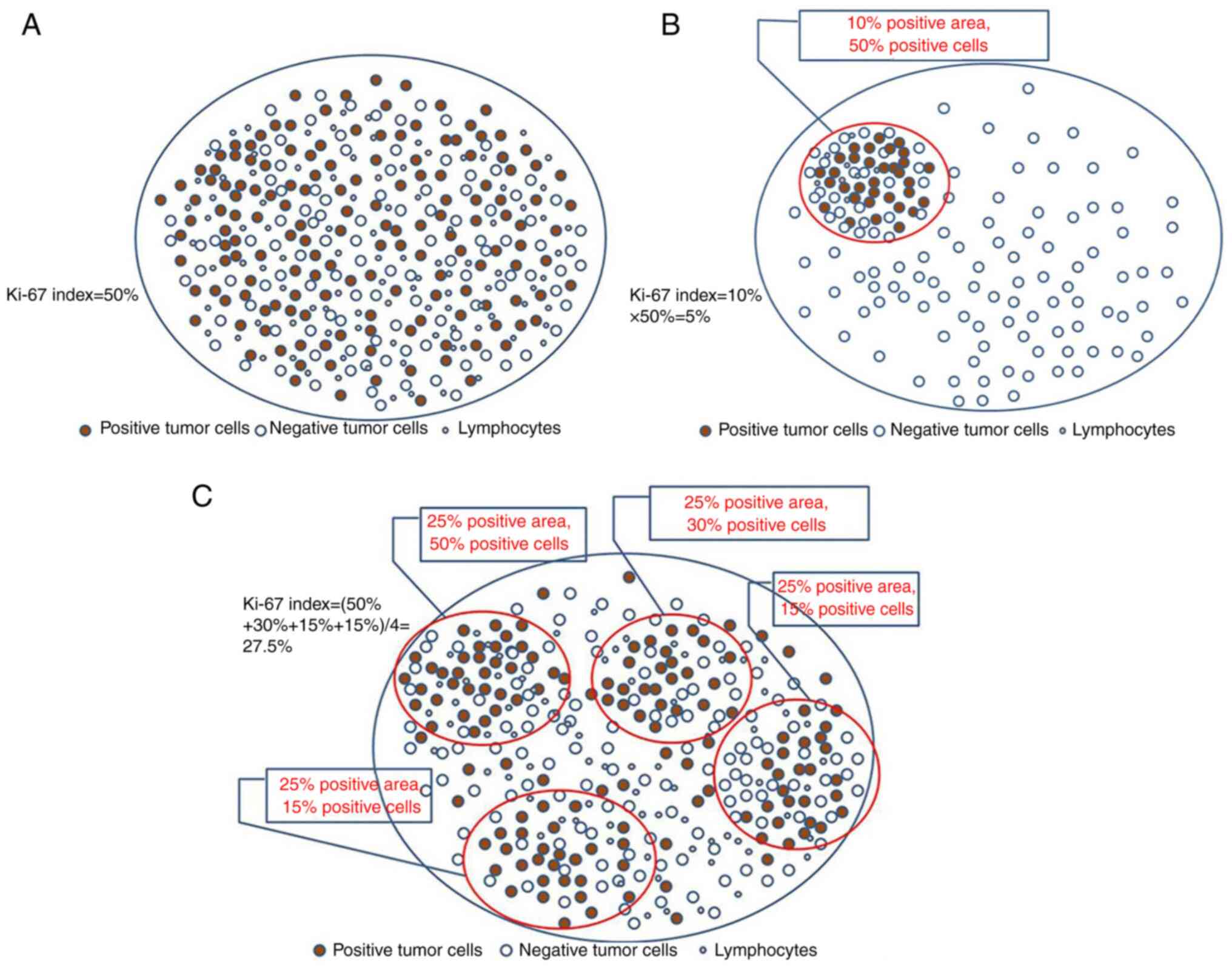
The whole section was previewed under a low power
lens of the microscope prior to the interpretation to assess
whether the Ki-67 staining was uniform. If the staining was
uniform, a total of 1,000 tumor cells were counted under a medium
and high-power lens (20-40 X objective lens), and subsequently, the
Ki-67 index was calculated (Fig.
3A).
If the staining was non-uniform, the following two
possible outcomes were considered: The positive tumor cells only
accounted for one part of the total pathological section and the
Ki-67 index was obtained by multiplying the percentage of positive
tumor cells in this region by the percentage of positive tumor
cells in the whole pathological section, namely, Ki-67
index=percentage of positive tumor cell regions in the total
pathological section x percentage of positive tumor cells in the
positive region (Fig. 3B);
secondly, the presence of focal distribution of the positive tumor
cells on the total pathological section was considered, which was
used to divide this section into several regions in order to
estimate the Ki-67 index in each region. In the end, the sum of
Ki-67 indices in all regions was divided by the number of regions
to obtain the Ki-67 index. The following formula was used: Ki-67
index=⅀ (percentage of positive tumor cells 1+ percentage of
positive tumor cells 2+ percentage of positive tumor cells
3…+percentage of positive tumor cells n)/⅀(1+2+3+……n) x100
(Fig. 3C). The staining intensity
could not be interpreted and tumor cells with an unclear contour
were not counted.
Molecular typing
The molecular typing was implemented according to
the suggestions provided in the 2019 CSCO Guidelines (low
expression if the critical value of Ki-67index <15%, and high
expression if it was >30%) (4).
When the ER+/HER-2- status and the Ki-67
index were within 15-30%, the molecular typing of BC was conducted
according to the percentage of cells with a positive PR expression.
Subsequently, the PR index >20% was considered as the cut-off
value in accordance with the 2019 CSCO Guidelines. If the PR index
was >20%, BC was classified as luminal type A, whereas if the PR
index was <20%, the BC was classified as luminal type B.
Statistical analysis
The survival data of patients with BC were
statistically analyzed using SPSS 18.0 software (SPSS, Inc.). The
Chi-square (χ2) test was used to assess the Ki-67 index,
the OS and the DFS of patients with BC, as well as the association
between the different molecular types. The Kaplan-Meier survival
analysis method (with the log-rank test) was adopted. A value of
P<0.05 was considered to indicate a statistically significant
difference.
Results
Clinicopathological features of
patients with BC
The median age of the 199 patients with invasive
ductal carcinoma was 48 years (25-84 years old), of whom those at
stage SBRI accounted for 21.2% (42/199), those at stage II for
57.8% (115/199), and those at stage III for 21.2% (42/199). The
patients with lymphatic metastasis comprised 47.7% (95/199) of the
total sample size and those without lymphatic metastasis accounted
for 52.3% (104/199). According to the AJCC staging (6), the percentages of patients at stage
I, II, III and IV were 17.1% (34/199), 58.8% (117/199), 23.6%
(47/199) and 0.5% (1/199), respectively (Table I). The median follow-up time lasted
82 months (12-157 months). The statistical analysis indicated that
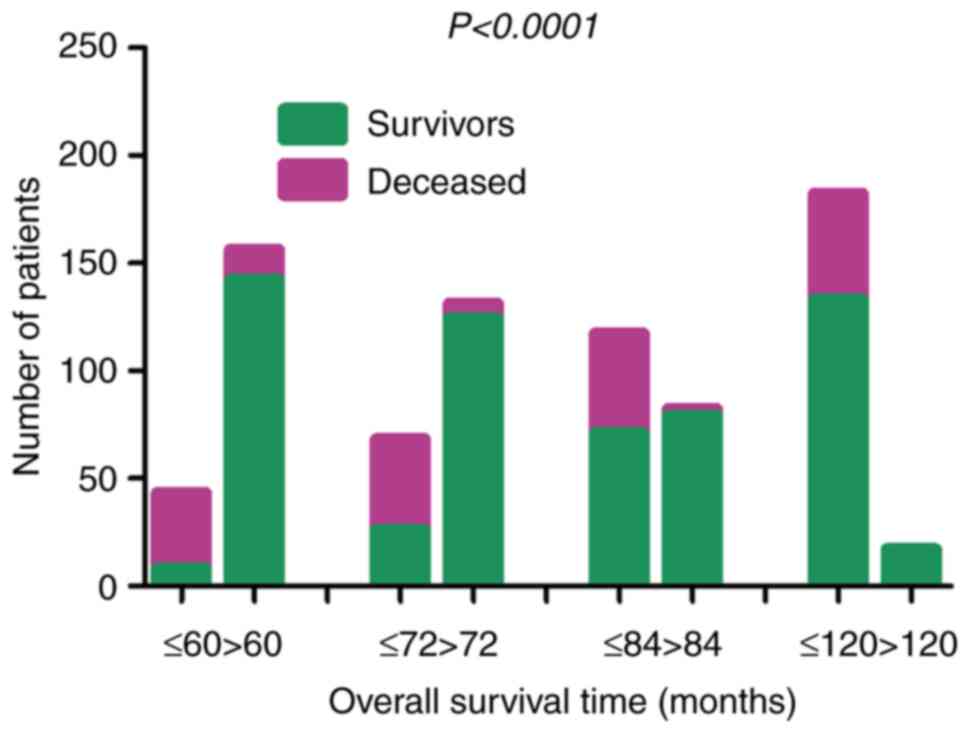
the 5-, 6-, 7- and 10-year OS rates of the patients with BC were
18.60% (8/43), 38.24% (26/68), 60.68% (71/117) and 73.08%
(133/182), respectively. The data indicated that >80% of the
patients with BC succumbed to the disease within 5 years, whereas
their survival rate was 100% following 10 years. The OS rates of
the patients with BC in the different age range groups differed
significantly (χ2=211.1, P<0.0001) (Fig. 4).
 | Table IThe clinicopathological
characteristics of 199 patients with breast cancer in the present
study. |
Table I
The clinicopathological
characteristics of 199 patients with breast cancer in the present
study.
| Characteristic | No. of patients | % |
|---|
| Sex | | |
|
Male | 1 | 0.5 |
|
Female | 198 | 99.5 |
| Age, years | | |
|
≤30 | 1 | 0.5 |
|
30-60 | 162 | 81.4 |
|
>60 | 36 | 18.1 |
| Tstage | | |
|
T1b | 13 | 6.5 |
|
T1c | 40 | 20.1 |
|
T2 | 126 | 63.3 |
|
T3 | 18 | 9.0 |
|
T4 | 2 | 1.0 |
| N stage | | |
|
0 | 104 | 52.3 |
|
N1 | 53 | 26.6 |
|
N2 | 31 | 15.6 |
|
N3 | 11 | 5.5 |
| AJCCstage | | |
|
IA | 34 | 17.1 |
|
IIA | 72 | 36.2 |
|
IIB | 45 | 22.6 |
|
IIIA | 35 | 17.6 |
|
IIIB | 1 | 0.5 |
|
IIIC | 11 | 5.5 |
|
IV | 1 | 0.5 |
| SBR grade | | |
|
I | 42 | 21.1 |
|
II | 115 | 57.8 |
|
III | 42 | 21.1 |
Associations of the Ki-67 index with
the OS and DFS of patients with BC
The number of patients with a Ki-67 index ≤1% was 16
(8.0%) and that of patients with a Ki-67 index ≥90% was 9 (4.5%).
The median percentage was 26.53% and the average value was
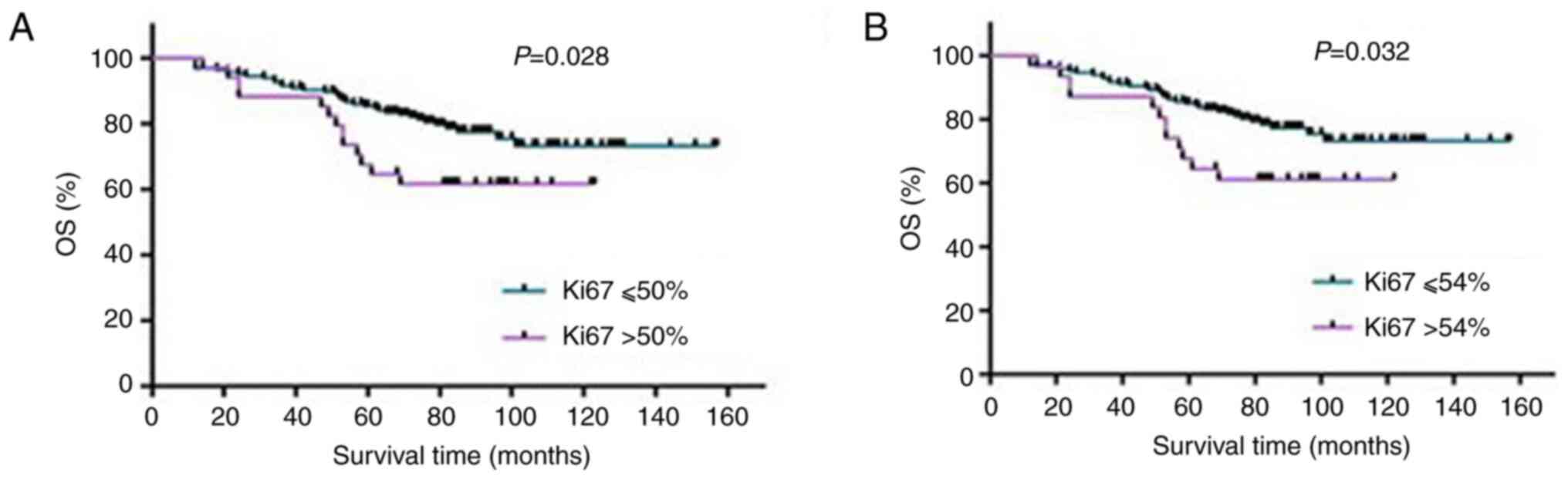
29.6±23.9%. The results of statistical analysis indicated that when
the Ki-67 index was in the range of 50-54%, the OS of the patients
with BC was questionable and the two groups exhibited significant
differences (Table II and
Fig. 5), suggesting that the Ki-67
index within the interval of 50-54% was meaningful for the
assessment of the OS of patients with BC.
 | Table IIAssociation between the Ki-67 index
and the overall survival of patients with breast cancer. |
Table II
Association between the Ki-67 index
and the overall survival of patients with breast cancer.
| Ki-67 index | Patients who
succumbed (n, %) | Patients who
survived (n, %) | Survival rate
(%) | χ2
value | P-value |
|---|
| ≤50% | 36 (21.82) | 129 (78.18) | 78.18 | 4.83 | 0.028 |
| >50% | 13 (38.24) | 21 (61.76) | 61.76 | | |
| ≤54% | 37 (22.02) | 131 (77.98) | 77.98 | 4.60 | 0.032 |
| >54% | 12 (38.71) | 19 (61.29) | 61.29 | | |
Following the termination of the follow-up time
period, 24 cases among the 199 patients with BC experienced
single-organ and/or multi-organ metastasis (lung, brain and bone)
or chest wall in situ recurrence; a total of 175 patients
succumbed to the disease or were under a progression-free survival
status after the follow-up time.
Prognosis of patients with BC with
different molecular types via the Ki-67 index
The molecular subtyping of the 199 patients with BC
was implemented in accordance with the 2019 CSCO standard (4). The results indicated that 54 patients
were classified as luminal type A; 67 cases were classified as
luminal type B, including 28 patients with luminal B HER-2 (-) and
39 with luminal B HER-2 (+) type; 39 patients were classified as
the HER-2 overexpression type and 39 had triple-negative BC (TNBC)
type (Table III). Of note, the
patients with luminal type A BC and a Ki-67 index ≤4% survived and
their follow-up time period lasted 69-156 months, suggesting that
the survival time of patients with luminal type A BC and a low
Ki-67 expression was long (data not shown).
 | Table IIIMolecular subtype of the 199 patients
with breast cancer according to the 2019 CSCO guidelines. |
Table III
Molecular subtype of the 199 patients
with breast cancer according to the 2019 CSCO guidelines.
| Molecular
subtype | Patients who
succumbed (n, %) | Patients who
survived (n, %) | Total (n) |
|---|
| Luminal A | 9 (16.7) | 45 (83.3) | 54 |
| Luminal B
HER-2(-) | 6 (21.4) | 22 (78.6) | 28 |
| Luminal B
HER-2(+) | 9 (23.1) | 30 (76.9) | 39 |
| Overexpression of
HER-2 | 13 (33.3) | 26 (66.7) | 39 |
|
Triple-negative | 12 (30.8) | 27 (69.2) | 39 |
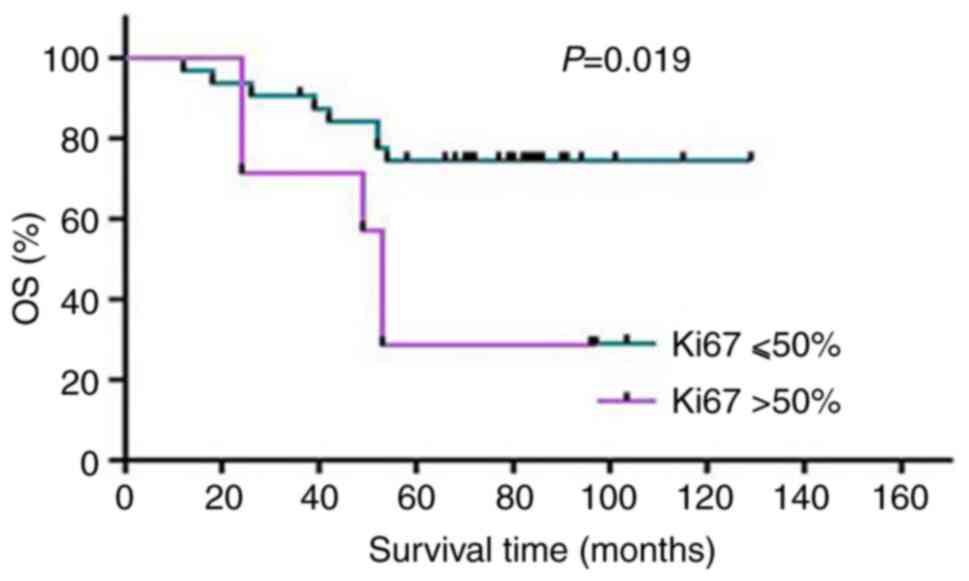
Among the patients with HER-2-overexpressing BC, 50%
was considered as the cut-off value of Ki-67, and their mortality
rate was 71.43% (5/7) when the Ki-67 index was >50%, which was
significantly different from the group with a Ki-67 index ≤50%
(χ2=5.54, P=0.019). The data indicated that this cut-off
value for the Ki-67 index was significant for the prognosis of
patients with HER-2-overexpressing BC; specifically, the patients
with HER-2-overexpressing BC and a Ki-67 index >50% had a poor
prognosis (Fig. 6).
Discussion
Ki-67, which has been widely applied in clinical
pathology, is a nucleoprotein discovered and determined in
Hodgkin's lymphoma in the 1980s (8). Its application to BC is based on the
following two aspects: i) It is used for molecular typing and to
treatment guidance; its use is particularly crucial for
distinguishing luminal type A from luminal type B, since patients
with luminal type A only require endocrinotherapy, while patients
with luminal type B obtain an optimal clinical effect by using the
systematic chemotherapy combined with endocrinotherapy (9); ii) it is applied to the prognosis of
patients with BC and several studies have shown that patients with
BC and a high Ki-67 index exhibit a poor prognosis and are more
susceptible to recurrence (10,11).
However, the results regarding the application of the Ki-67 index
in BC at home and abroad are inconsistent and even mutually
contradictory. It has been demonstrated that patients with BC and a
high Ki-67 index will acquire improved pCRs and will not require
the assessment of their hormone receptor levels (ER or PR) and
HER-2 status (2). However, it has
also been shown that the Ki-67 index is unrelated to the prognosis
of patients with BC; therefore, the lower the index, the poorer the
prognosis (12). According to the
study by Kadivar and Aram (13),
other clinicopathological features, such as histological grade, the
presence or absence of lymphatic metastasis, the tumor volume, and
vascular invasion should be combined when the Ki-67 index is used
to assess the influence of clinical treatment on patients with BC.
The emphases of the present study is based on the ability to
effectively solve these issues, to contribute to improve the
operability and accuracy of the Ki-67 index when used in BC, and
accurately measure the Ki-67 index and apply it to the prognosis of
patients with BC. In addition, the unspecific grouping by the ‘high
Ki-67 index’ and ‘low Ki-67 index’ should be avoided as much as
possible, as the distribution interval of ‘high and low Ki-67
index’ can be too large to operate in practice; for example, the
low Ki-67 index can range from 0 to 28.6% (14,15).
Another difficulty faced by pathologists is the
accurate estimation of the Ki-67 index. In order to optimize the
counting method and reduce the differences among observers to the
greatest extent, the international Ki-67 in the Breast Cancer
Working Group has provided certain suggestions; namely, the cells
should be counted at least under three high power fields (40 X
objective lens) or at least 500-1,000 cancer cells should be
counted, including the invasion edge and hotspot area of the tumor
(16). Although this method is
both time- and labor-consuming, it is still the most economical and
practical method, earning considerable acceptance from pathologists
(17). The Ki-67 counting by
computer-aided means has also been reported (18). In the present study, the Ki-67
index was calculated using the artificial partitioning calculation
method, which enabled more accurate calculations. Specifically, the
Ki-67 index was calculated by two senior pathologists in each case,
and the average number was finally calculated as the Ki-67 index of
each patient. With simple and convenient operation, this method can
not only reduce the errors among different observers, but can also
consider the non-uniform staining of tumor cells.
Following the counting of the percentage of
Ki-67-positive cells in the 199 BC samples and statistical
analysis, an interval of the Ki-67 index was noted for the
prognosis of patients with BC; when the Ki-67 index was within the
range of 50-54%, it was valuable for assessing the OS of patients
with BC. Previous research has indicated that when 10% is
considered as the cut-off value for Ki-67, the OS and
recurrence-free survival of patients with BC in the low PR
expression group (<20%) are both higher than those in the high
PR expression group (>20%), irrespective of the group
classification [high-expression (≥10%) group or low-expression
(<10%) group]; notably, the patients presenting with a high
Ki-67 expression and a low PR expression exhibit the poorest
prognosis (19). When 14% is
considered as the cut-off value for Ki-67, the pCR rate of patients
with luminal type BC, a high Ki-67 expression (≥14%), and low PR
expression (<50%) following neoadjuvant chemotherapy is
relatively high (20). These
conclusions have indicated that the Ki-67 index is meaningful for
the prognosis of patients with BC; however, the cut-off value is
inconsistent, which is closely related to the grouping conditions
of patients, the interpretation method of the Ki-67 index, and
notably, the standardization of the Ki-67 interpretation.
In the present study, the results indicated that the
patients with luminal type A BC and a low Ki-67 index (≤4%)
exhibited a favorable prognosis, and all patients survived through
the 13-year follow-up time period. The mean OS of the patients with
HER-2-overexpressing BC was significantly different when 50% was
considered as the cut-off value of Ki-67. However, Jain et
al (21) suggested the use of
35% as the cut-off value to predict the pathological response of
patients with BC to neoadjuvant chemotherapy. Moreover, it has been
demonstrated that when the Ki-67 index is within 10-20%, high
intergroup associations manifest among different respondents
despite the presence of group differences (22). It is suggested that the clinical
use of ‘low Ki-67 expression’ and ‘high Ki-67 expression’ may be
more meaningful. When the hotspot areas are counted, the number of
observers reported with a high Ki-67 expression is apparently
higher than that of the average level. When the Ki-67 staining is
weak, the observers are also prone to a high Ki-67 expression
(23). Accordingly, in the study
by Zhu et al (24), the
cut-off value of Ki-67 was 30% and was used as an independent
prognostic factor affecting the OS and DFS of patients with TNBC.
The latter patients with Ki-67 >30% had a poor prognosis
(24). Therefore, the prognosis of
patients with BC could be predicted more accurately by using the
different Ki-67 indices for the different molecular subtypes of BC.
Concomitantly, as demonstrated in a previous study, the activity of
LDH and catalase (CAT) in BC tissues aided the identification of
the characteristics of cancer aggressiveness (25). In practice, every biomarker which
was used to predict the prognosis of patients with BC has
limitations, such as Ki-67, LDH and CAT. Thus, a few markers need
to be used jointly to determine the prognosis of patients with BC
objectively. For the Ki-67 index, the different cut-off values
should be used to predict the survival of patients with different
molecular subtypes of BC. At the same time, this conclusion may
have a limitation as the data obtained herein were drawn from
patients in China; thus, further multicenter studies are warranted
to draw more objective conclusions.
In conclusion, the present study provided a
systematic exploration of the interpretation of the Ki-67 index
based on the partitioning calculation method and assessed its
clinical value for the prognosis of patients with BC. The results
indicated that the Ki-67 index obtained through the partitioning
calculation method was very meaningful for assessing the OS of
patients with BC. Different cut-off values of Ki-67 should be
adopted for providing information for patients with BC with
different molecular subtypes. The cut-off value of Ki-67 should
fall into a certain interval, but should not be used at a fixed
point.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Medical Science
and Technique Program of Henan Province (grant no.
LHGJ20210823).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW was involved in the conception and design of the
study. XL and NF were involved in the development of the study
methodology. NM and FL were involved in the acquisition of data. YW
and YC were involved in data analysis. CW and TY were involved in
data interpretation. CW, TY, YC and NF were involved in the writing
and reviewing/revision of the manuscript. NF and CW supervised the
study. XL, NF and YC confirm the authenticity of all the raw data.
All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the 989th
Hospital of the PLA Joint Logistic Support Force database for the
collection of data and for the use of personal data for research
purposes, and written informed consent was obtained from the
patients or their immediate family. The present study was ethically
approved by the Ethics Committee of the 989th Hospital of the PLA
Joint Logistic Support Force review board on May 14, 2020 (Approval
no. 20200508).
Patient consent for publication
Written informed consent was obtained from all
patients or their immediate family members for the publication of
their data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–124. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Chen XY, He C, Han DD, Zhou M, Wang Q,
Tian J, Li L, Xu F, Zhou E and Yang K: The predictive value of
Ki-67 before neoadjuvant chemotherapy for breast cancer: A
systematic review and meta-analysis. Future Oncol. 13:843–857.
2017.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Erić I, Petek Erić A, Kristek J, Koprivčić
I and Babić M: Breast cancer in young women: Pathologic and
immunohistochemical features. Acta Clin Croat. 57:497–502.
2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Li JB and Jiang ZF: Update and
interpretation of 2019 guideline of Chinese Society of clinical
oncology (CSCO): Breast cancer. Chin J Surg Oncol. 11:155–160.
2019.
|
|
5
|
Shet T: Ki-67 in breast cancer: Simulacra
and simulation. Indian J Cancer. 57:231–233. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Teichgraeber DC, Guirguis MS and Whitman
GJ: Breast cancer staging: Updates in the AJCC cancer staging
manual, 8th edition, and current challenges for radiologists, from
the AJR special series on cancer staging. AJR Am J Roentgenol.
217:278–290. 2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Guideline Recommendations for HER2
Detection in Breast Cancer Group. Guidelines for HER2 detection in
breast cancer, the 2014 version. Zhonghua Bing Li Xue Za Zhi.
43:262–267. 2014.PubMed/NCBI(In Chinese).
|
|
8
|
Gerdes J, Schwarting R and Stein H: High
proliferative activity of Reed Sternberg associated antigen Ki-1
positive cells in normal lymphoid tissue. J Clin Pathol.
39:993–997. 1986.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Goldhirsch A, Winer EP, Coates AS, Gelber
RD, Piccart-Gebhart M, Thürlimann B and Senn HJ: Panel members.
Personalizing the treatment of women with early breast cancer:
Highlights of the St Gallen international expert consensus on the
primary therapy of early breast cancer. Ann Oncol. 24:2206–2223.
2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Nishimura R, Osako T, Nishiyama Y, Tashima
R, Nakano M, Fujisue M, Toyozumi Y and Arima N: Prognostic
significance of Ki-67 index value at the primary breast tumor in
recurrent breast cancer. Mol Clin Oncol. 2:1062–1068.
2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Pathmanathan N, Balleine RL, Jayasinghe
UW, Bilinski KL, Provan PJ, Byth K, Bilous AM, Salisbury EL and
Boyages J: The prognostic value of Ki67 in systemically untreated
patients with node-negative breast cancer. J Clin Pathol.
67:222–228. 2014.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kurozumi S, Matsumoto H, Hayashi Y, Tozuka
K, Inoue K, Horiguchi J, Takeyoshi I, Oyama T and Kurosumi M: Power
of PgR expression as a prognostic factor for
ER-positive/HER2-negative breast cancer patients at intermediate
risk classified by the Ki67 labeling index. BMC Cancer.
17(354)2017.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Kadivar M and Aram F: Assessment of Ki67
in breast cancer: A comparison between the Eye-10 method, stepwise
counting strategy, and international system of Ki67 evaluation.
Iran J Pathol. 15:13–18. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Urruticoechea A, Smith IE and Dowsett M:
Proliferation marker Ki-67 in early breast cancer. J Clin Oncol.
23:7212–7220. 2005.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Stuart-Harris R, Caldas C, Pinder SE and
Pharoah P: Proliferation markers and survival in early breast
cancer: A systematic review and meta-analysis of 85 studies in
32,825 patients. Breast. 17:323–334. 2008.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Fulawka L and Halon A: Ki-67 evaluation in
breast cancer: The daily diagnostic practice. Indian J Pathol
Microbiol. 60:177–184. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Dowsett M, Nielsen TO, A'Hern R, Bartlett
J, Coombes RC, Cuzick J, Ellis M, Henry NL, Hugh JC, Lively T, et
al: Assessment of Ki67 in breast cancer: recommendations from the
international Ki67 in breast cancer working group. J Natl Cancer
Inst. 103:1656–1664. 2011.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Ašoklis R, Kadziauskienė A, Paulavičienė
R, Petroška D and Laurinavičius A: Quantitative histopathological
assessment of ocular surface squamous neoplasia using digital image
analysis. Oncol Lett. 8:1482–1486. 2014.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kang YJ, Lee HB, Kim YG, Han J, Kim Y, Yoo
TK, Lee ES, Moon HG, Noh DY and Han W: Ki-67 expression is a
significant prognostic factor only when progesterone receptor
expression is low in estrogen receptor-positive and HER2-negative
early breast cancer. J Oncol. 2019(7386734)2019.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Silva LRD, Vargas RF, Shinzato JY,
Derchain SFM, Ramalho S and Zeferino LC: Association of menopausal
status, expression of progesterone receptor and Ki67 to the
clinical response to neoadjuvant chemotherapy in Luminal breast
cancer. Rev Bras Ginecol Obstet. 41:710–717. 2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jain P, Doval DC, Batra U, Goyal P, Bothra
SJ, Agarwal C, Choudhary DK, Yadav A, Koyalla VPB, Sharma M, et al:
Ki-67 labeling index as a predictor of response to neoadjuvant
chemotherapy in breast cancer. Jpn J Clin Oncol. 49:329–338.
2019.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Li XR, Liu M, Zhang YJ, Wang JD, Zheng YQ,
Li J, Ma B and Song X: Evaluation of ER, PgR, HER-2, Ki-67, cyclin
D1, and nm23-H1 as predictors of pathological complete response to
neoadjuvant chemotherapy for locally advanced breast cancer. Med
Oncol. 28 (Suppl 1):S31–S38. 2011.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Røge R, Nielsen S, Riber-Hansen R and
Vyberg M: Ki-67 proliferation index in breast cancer as a function
of assessment method: A NordiQC experience. Appl Immunohistochem
Mol Morphol. 29:99–104. 2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhu X, Chen L, Huang B, Wang Y, Ji L, Wu
J, Di G, Liu G, Yu K, Shao Z and Wang Z: The prognostic and
predictive potential of Ki-67 in triple-negative breast cancer. Sci
Rep. 10(225)2020.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Radenkovic S, Milosevic Z, Konjevic G,
Karadzic K, Rovcanin B, Buta M, Gopcevic K and Jurisic V: Lactate
dehydrogenase, catalase, and superoxide dismutase in tumor tissue
of breast cancer patients in respect to mammographic findings. Cell
Biochem Biophys. 66:287–295. 2013.PubMed/NCBI View Article : Google Scholar
|