Introduction
Leishmaniasis is a neglected tropical disease caused
by an obligate intracellular, vector-borne protozoan parasite
belonging to the Trypanosomatidae family. The disease manifests in
three primary phenotypic categories: Cutaneous leishmaniasis (CL),
mucosal leishmaniasis (ML) and visceral leishmaniasis (VL). The
genus Leishmania encompasses a total of 22 species (1), each species displays specific
geographical preferences, host-related factors and characteristic
symptoms (2).
In people living with human immunodeficiency virus
(HIV; PLWH), cutaneous, mucosal and visceral manifestations can be
caused by any of the Leishmania species that infect humans
(3). Moreover, in patients with
HIV infection, the manifestations of leishmaniasis may differ from
HIV-negative individuals, with different localizations and unique
organ involvement, due to both the Leishmania
characteristics and the peculiarity of HIV (4,5).
Leishmania and HIV share the same host cell
targets and the synergistic interaction between these two diseases
can contribute to the progression of both conditions, potentially
attributed to the persistent activation of the immune system
(6-8).
In PLWH, achieving the complete eradication of
Leishmania is a rare occurrence, and the possibility of
relapse, involving different organs, exists. Consequently,
identifying predictive factors for leishmaniasis relapse has
garnered significant attention (3,9,10).
The present study describes the case of an
individual infected with HIV who experienced a rare simultaneous
occurrence of relapsing mucosal and visceral leishmaniasis,
highlighting the significance of the prompt suspicion and
recognition of the disease, as well as the challenges in the
differential diagnosis. Additionally, the implications of
HIV-coinfection on the pathogenesis, the clinical manifestations,
treatment strategies and prophylaxis measures employed for
leishmaniasis are discussed.
Case report
A 58-year-old male patient with HIV presented at the
Emergency Department of ARNAS Garibaldi Hospital (Catania, Italy),
reporting a 6-month history of dysphonia and breathing
difficulties. He also mentioned experiencing fever (up to 38˚C) in
the past month. His medical history included HIV infection since
1996 and the current use of antiretroviral therapy with
dolutegravir and doravirine. He was a smoker (~20 cigarettes daily)
and had asthmatic bronchitis for which he used inhaled steroids. He
had a previous case of visceral leishmaniasis in 2011 and had been
on secondary prophylaxis with liposomal amphotericin B at a dose of
4 mg/kg every 4 weeks.
Upon a physical examination, he displayed mobile
right-sided laterocervical lymphadenopathies measuring over a
centimeter, which were firm and non-tender. Hepatosplenomegaly was
also noted. Blood tests revealed a CD4 lymphocyte count <200
cells/mm³, an HIV viral load <20 copies/ml, a white blood cell
count of 2,100/mm³, a red blood cell count of 3,800,000/mm³, a
hemoglobin level of 8.7 mg/dl, a platelet count of 75,000/mm³ and a
B2-microglobulin level of 10 mg/dl (Table I).
 | Table ILaboratory findings (reference range)
taken at the initiation of liposomal amphotericin B therapy, at the
time of hospital discharge and at the end of therapy. |
Table I
Laboratory findings (reference range)
taken at the initiation of liposomal amphotericin B therapy, at the
time of hospital discharge and at the end of therapy.
| Parameter | At the initiation
of liposomal amphotericin B therapy | At the time of
hospital discharge | At the end of
liposomal amphotericin B therapy |
|---|
| Hemoglobin, g/dl
(13.6-17.2) | 8.7 | 9.3 | 9.5 |
| RBC, 106
cells/mmc (4.3-5.7) | 3.8 | 4.1 | 3.99 |
| WBC, cells/mmc
(4,000-10,000) | 2,100 | 4,300 | 2,100 |
| Neutrophils, %
(40-75) | 50.3 | 69.2 | 45 |
| Lymphocytes, %
(25-50) | 38.5 | 22.6 | 42.3 |
| Platelets,
cells/mmc x103 (150-400) | 75 | 90 | 86 |
| AST, UI/l
(15-35) | 16 | 11 | 11 |
| ALT, UI/l
(15-35) | 6 | 9 | 6 |
| Creatinine levels
mg/dl (0.6-1.3) | 1.07 | 1.28 | 1.40 |
| C-reactive protein,
mg/dl (0.01-0.5) | 2.34 | 0.33 | 8 |
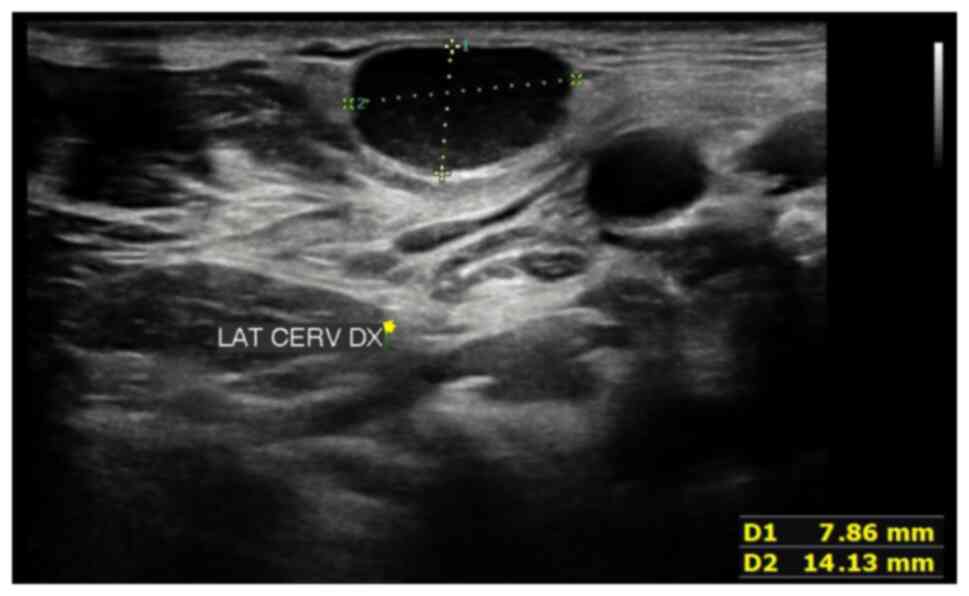
Following a neck ultrasound which revealed enlarged
lymph nodes, a subsequent computed tomography scan confirmed these
findings in the bilateral laterocervical regions, as well as in
multiple mesenteric, external iliac and para-aortic locations
(Fig. 1). PET/CT imaging indicated
significant metabolic activity with abnormal tracer accumulation in
various lymph node regions and nodules in the mesenteric area.
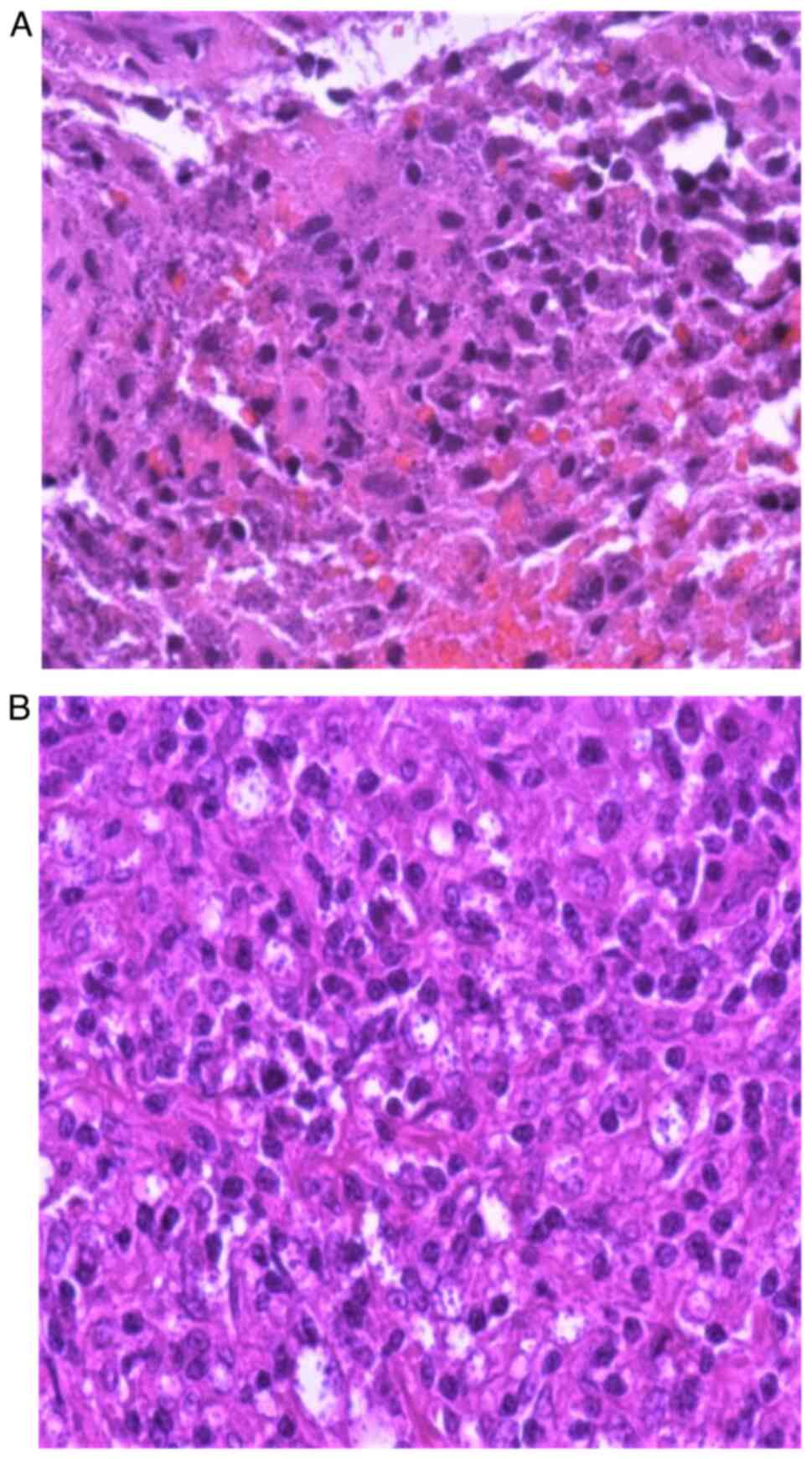
Indirect laryngoscopy identified hyperplastic
lesions on the anterior and middle thirds of the left true vocal
cord. Biopsies of the vocal cord and right supraclavicular lymph
node revealed lymphomonocytic inflammation and phagocytic activity
against Leishmania parasites, respectively (Fig. 2).
With these results, a diagnosis of simultaneous ML
and VL relapse was made. Treatment commenced with liposomal
amphotericin B at 4 mg/kg/day for 5 days, followed by a dose on the
10th day and four more doses every 7 days.
Following treatment, the patient's condition
significantly improved, with resolution of dysphonia and breathing
difficulties. Hematological parameters also improved (Table I). He was discharged after the
sixth dose of liposomal amphotericin B and completed the treatment
course as an outpatient. Secondary prophylaxis continued every 4
weeks as scheduled.
Discussion
Human leishmaniasis displays a range of clinical
manifestations, which can be categorized into three main phenotypic
types: CL, ML and VL. ML, in particular, refers to the engagement
of mucous membranes in the upper respiratory tract, spanning from
the inner walls of the nostrils to the larynx, as well as the oral
cavity. This condition is caused by Leishmania spp.,
particularly New World species, such as L. braziliensis
(11).
ML can manifest concurrently with or before CL or VL
(12). In the Mediterranean area,
ML is primarily caused by species within the Leishmania
donovani complex, notably Leishmania infantum (2,13).
Visceral leishmaniasis can affect various internal organs,
particularly those belonging to the reticuloendothelial system,
including the spleen, liver, lymph nodes and bone marrow, leading
to pancytopenia with high risk of bleeding and severe infections
(14,15).
In the case described herein, a diagnosis of
laryngeal leishmaniasis was made in an individual living with HIV
who suffered a relapse of VL despite receiving secondary
prophylaxis for leishmaniasis. Laryngeal involvement is rare,
occurring for only 1% of cases, as indicated by Cincurá et
al (16). This discovery
renders the present case unique, being the first documented
occurrence of laryngeal involvement concurrent with a relapse of
VL. Although instances of sequential mucosal relapse in VL have
been reported, such occurrences are exceedingly uncommon (17,18).
Patients with ML frequently present with chronic
nasal symptoms, including nasal discharge, ulcerations and
epistaxis. Over time, these symptoms tend to exacerbate and can
lead to complications like dysphagia and dysphonia, as observed in
our patient, due to the gradual deterioration of soft tissues
(2,13,19,20).
The mechanism through which Leishmania reach
the mucous membranes remains a topic of ongoing debate. Three
potential pathways have been proposed. Firstly, ML can arise due to
the direct extension of contiguous facial skin lesions, as notably
observed in cases of L. major infection. Another possible
explanation for mucous membrane involvement is the direct
inoculation of parasites into the mucosa through sand fly bites,
particularly in the context of oral and nasal localizations.
Lastly, lymphatic or hematogenous dissemination is considered a
feasible route, especially in instances of L. infantum
leishmaniasis, which typically lacks preceding skin manifestations
(13).
Immunodeficiency significantly heightens
susceptibility to leishmaniasis. In individuals with a robust
immune system, the protective response against Leishmania is
characterized by a Th1 cytokine profile, which confers resistance
against infection and inhibits disease progression (21). Conversely, susceptibility to
Leishmania infection and unfavorable outcomes are linked to
a Th-2 cytokine response (22,23).
In the context of HIV infection, there is a Th1/Th2 shift provoked
by HIV, resulting in unconventional and widespread manifestations
(13). The interplay between HIV
and leishmaniasis can synergistically exacerbate the advancement of
both conditions, possibly due to the chronic activation of the
immune system (7,24,25).
Of note, PLWH who maintain higher CD4+
counts tend to experience lower rates of relapse (9). Secondary prophylaxis holds
significant importance in managing leishmaniasis. It has been
observed that the relapse rate of VL in patients with
CD4+ counts <200, who receive monthly secondary
prophylaxis, is comparable to that of patients with higher
CD4+ counts who do not receive such prophylaxis. The
occurrence of relapse not only worsens immunosuppression, but also
accelerates the progression of HIV disease. This heightened
susceptibility to opportunistic infections can lead to severe
consequences, including adverse outcomes and even mortality
(26). Consequently, implementing
secondary prophylaxis is crucial for mitigating the risk of relapse
and its detrimental effects in individuals with both HIV and VL
(27). However, although these
occur at a lower rate, relapses in patients receiving secondary
prophylaxis are frequently reported (27-29),
as demonstrated in the case in the present study.
Considerable attention is being directed towards
identifying variables that could act as predictors of leishmaniasis
relapse, aiding clinicians in identifying PLWH who are at a higher
risk. Cota et al (9)
demonstrated that factors, such as the absence of CD4+
cell count improvement during follow-up, CD4+ counts
<100 cells/ml at the onset of primary VL, the lack of secondary
prophylaxis, and a history of previous VL relapse could potentially
serve as predictive indicators for relapse. Similarly, Takele et
al (10) suggested that lower
CD4+ T cell counts, a decreased production of IFN-γ and
elevated levels of PD1 expression on CD4+ T-cells could
potentially be indicative markers of an increased risk of relapsing
disease. An elevation in lactate dehydrogenase (LDH) levels is a
useful marker for cell necrosis in such infections (30).
It has been postulated that local mucosal
immunosuppressive factors, such as tobacco smoke, systemic or
inhaled corticosteroid therapy and upper respiratory diseases can
facilitate the development of ML (13,31,32)
Fare clic o toccare qui per immettere il testo. The patient in the
present study was a smoker and received inhaled steroids to manage
asthmatic bronchitis (3).
Numerous studies have delved into the potential role
of Leishmania RNA viruses (LRVs) in the pathogenesis of ML.
These viruses are capable of infecting specific Leishmania
species, particularly Leishmania braziliensis and
Leishmania guyanensis. Recent research carried out in murine
models has revealed a close association between the presence of
LRVs and the severity of infection. This association leads to
modifications in the host's immune response to the parasite and is
considered a significant virulence factor contributing to the
development of ML. Indeed, when comparing the percentage of ML
samples that tested positive for LRVs with those that were
negative, the presence of the virus has been found to significantly
exacerbate the disease (33,34).
Following the Infectious Diseases Society of America
(IDSA) guidelines (3), diagnosing
leishmaniasis involves identifying Leishmania amastigotes in
tissue samples. Obtaining tissue aspirates or biopsy specimens is
highly recommended for performing smears, histopathology, parasite
culture and molecular testing. For VL, bone marrow aspiration is
the preferred diagnostic source. Nonetheless, other potential
sources, such as the liver, enlarged lymph nodes and whole blood
can also be utilized. In the case described herein, the diagnosis
of VL was established via a lymph node biopsy. For ML, it is
advised that individuals at risk undergo a comprehensive assessment
for mucosal symptoms as part of their initial evaluation. These
individuals should be promptly referred to a specialist for an
otorhinolaryngological (ORL) examination, which typically involves
fiber-optic endoscopy. This comprehensive approach ensures early
detection and appropriate management for those vulnerable to ML.
This approach, involving ORL examination, is effectively
highlighted in the case described herein as well. As shown in the
present study, a microscopic confirmation is the preferred
technique to ensure an accurate diagnosis of relapse. A positive
result from a non-quantitative PCR assay may not definitively
confirm or exclude the possibility of relapse.
As regards treatment, liposomal amphotericin B is
the preferred regimen for VL, regardless of the patient's immune
status. However, in immunocompromised individuals, the dosage
regimen requires higher daily doses, an increased administration
frequency and a greater cumulative total dose. Evaluating the
response to anti-leishmanial treatment relies mainly on clinical
criteria, eliminating the need for microscopic confirmation in
patients who display swift clinical improvement. For clinically
apparent ML, systemic anti-leishmanial therapy is advised to
prevent both morbidity (e.g., disfigurement) and mortality (e.g.,
from respiratory obstruction or aspiration pneumonia).
Additionally, the IDSA guidelines recommend considering
prophylactic corticosteroid therapy for individuals with
laryngeal/pharyngeal disease at increased risk of respiratory
obstruction. The treatment for ML primarily involves lipid
formulations of amphotericin B, particularly liposomal amphotericin
B, with cumulative total doses varying from ~20 to 60 mg/kg.
Notably, a strong association exists between clinical parameters
and microscopic responses in cases of ML. It is crucial to
emphasize that relapse in patients treated with amphotericin
formulations signifies immunological failure rather than drug
failure or resistance development. Hence, although data on this
approach are limited, managing such patients with the same drug,
possibly at higher doses or for extended periods of time, could be
considered. Finally, according to the IDSA guidelines, initiating
secondary prophylaxis with an effective anti-leishmanial drug is
recommended after completing the initial treatment course. The most
suitable agent and regimen, however, remain undetermined
definitively. Periodic parenteral liposomal amphotericin B use (3-5
mg/kg every 3-4 weeks) has shown reduced relapse rates.
Nonetheless, the risk of relapse persists, as observed in the
patient described herein who experienced relapses of both VL and ML
despite receiving secondary prophylaxis. As outlined by the IDSA
guidelines, discontinuing secondary prophylaxis could be considered
for individuals who lack evidence of active Leishmania
infection, provided their CD4 cell counts have remained between
200-350 cells/mm³ for at least 6 months. It should be noted that
even in such cases, instances of relapse have been reported
(3,35).
In conclusion, the present study underscores the
significance of maintaining a vigilant mindset towards the relapse
of VL and ML in a patient with HIV infection who previously
experienced VL, even when receiving secondary prophylaxis. In the
case of ML, the early recognition of symptoms can significantly
improve the well-being and survival rates of patients, taking into
consideration that smoking and inhaled steroids may play a critical
role in disease development. As symptoms tend to be non-specific,
it is crucial for physicians to consider the possibility of this
coinfection and include ML in the differential diagnosis,
distinguishing it from carcinoma, other malignant tumors, and
granulomatous diseases.
Nevertheless, further research is warranted to gain
a deeper understanding of the risk factors associated with
leishmaniasis relapse and to devise more efficient diagnostic and
preventive approaches (36). Early
treatment has proven to enhance patient outcomes, and therefore,
investing in comprehensive studies will facilitate the development
of improved strategies for diagnosis and prevention.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (VF, EC, AM, AG, AF, EP, SS, AB, VB,
BMC, GB, BC and GN) contributed to the study conception and design.
VF, EC and AM were involved in the conceptualization of the study.
EC was involved in the methodology of the clinical case. AG, AF,
EP, SS, AB, GB, VB and BMC were involved in analyzing the patient's
data., VB, SS and AM were involved in data curation. VF and EC were
involved in the writing and preparation of the original draft. AM
was involved in the writing, reviewing and editing of the
manuscript., GB, GN and BC supervised the study. All authors have
read and agreed to the published version of the manuscript. BC and
GN confirm the authenticity of all the raw data. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient described herein.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of his data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Saydam FN, Erdem H, Ankarali H, El-Arab
Ramadan ME, El-Sayed NM, Civljak R, Pshenichnaya N, Moroti RV,
Mahmuodabad FM, Maduka AV, et al: Vector-borne and zoonotic
infections and their relationships with regional and socioeconomic
statuses: An ID-IRI survey in 24 countries of Europe, Africa and
Asia. Travel Med Infect Dis. 44(102174)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Mann S, Frasca K, Scherrer S,
Henao-Martínez AF, Newman S, Ramanan P and Suarez JA: A review of
Leishmaniasis: Current knowledge and future directions. Curr Trop
Med Rep. 2021:121–132. 2021.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Aronson N, Herwaldt BL, Libman M, Pearson
R, Lopez-Velez R, Weina P, Carvalho EM, Ephros M, Jeronimo S and
Magill A: Diagnosis and treatment of Leishmaniasis: Clinical
practice guidelines by the infectious diseases society of America
(IDSA) and the American society of tropical medicine and hygiene
(ASTMH). Clin Infect Dis. 63:e202–e264. 2016.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Marino A, Caltabiano E, Zagami A, Onorante
A, Zappalà C, Locatelli ME, Pampaloni A, Scuderi D, Bruno R and
Cacopardo B: Rapid emergence of cryptococcal fungemia,
mycobacterium chelonae vertebral osteomyelitis and gastro
intestinal stromal tumor in a young HIV late presenter: A Case
Report. BMC Infect Dis. 18(693)2018.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Celesia BM, Marino A, Del Vecchio RF,
Bruno R, Palermo F, Gussio M, Nunnari G and Cacopardo B: Is it safe
and cost saving to defer the CD4+ cell count monitoring in stable
patients on art with more than 350 or 500 cells/µl? Mediterr J
Hematol Infect Dis. 11(e2019063)2019.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ezra N, Ochoa M and Craft N: Human
immunodeficiency virus and Leishmaniasis. J Glob Infect Dis.
2:248–257. 2010.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Graepp-Fontoura I, Soeiro Barbosa D, Paes
AMA, Santos FS, Santos Neto M, Fontoura VM, Costa JML and
Abreu-Silva AL: Epidemiological, clinical and laboratory aspects of
human visceral Leishmaniasis (HVL) associated with human
immunodeficiency virus (HIV) coinfection: A systematic review.
Parasitology. 145:1801–1818. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Colović N, Jurišić V, Terzić T, Jevtovic D
and Colović M: Alveolar granulocytic sarcoma of the mandible in a
patient with HIV. Onkologie. 34:55–58. 2011.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Cota GF, de Sousa MR and Rabello A:
Predictors of visceral Leishmaniasis relapse in Hiv-infected
patients: A systematic review. PLoS Negl Trop Dis.
5(e1153)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Takele Y, Mulaw T, Adem E, Shaw CJ,
Franssen SU, Womersley R, Kaforou M, Taylor GP, Levin M, Müller I,
et al: Immunological factors, but not clinical features, predict
visceral Leishmaniasis relapse in patients co-infected with HIV.
Cell Rep Med. 3(100487)2022.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Handler MZ, Patel PA, Kapila R, Al-Qubati
Y and Schwartz RA: Cutaneous and mucocutaneous Leishmaniasis:
Differential diagnosis, diagnosis, histopathology, and management.
J Am Acad Dermatol. 73:911–926. 2015.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Bova C, de Vuono A, Ruvio M, Pignataro FS
and Fiaschi E: Visceral and mucosal Leishmaniasis mimicking wilson
disease and oral neoplasia. IDCases. 28(e01466)2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Strazzulla A, Cocuzza S, Pinzone MR,
Postorino MC, Cosentino S, Serra A, Cacopardo B and Nunnari G:
Mucosal Leishmaniasis: An underestimated presentation of a
neglected disease. Biomed Res Int. 2013(805108)2013.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Stracquadanio S, Bonomo C, Marino A,
Bongiorno D, Privitera GF, Bivona DA, Mirabile A, Bonacci PG and
Stefani S: Acinetobacter baumannii and cefiderocol, between
cidality and adaptability. Microbiol Spectr.
10(e0234722)2022.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Marino A, Stracquadanio S, Campanella E,
Munafò A, Gussio M, Ceccarelli M, Bernardini R, Nunnari G and
Cacopardo B: Intravenous fosfomycin: A potential good partner for
cefiderocol. Clinical experience and considerations. Antibiotics
(Basel). 12(49)2023.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Cincurá C, de Lima CMF, Machado PRL,
Oliveira-Filho J, Glesby MJ, Lessa MM and Carvalho EM: Mucosal
Leishmaniasis: A retrospective study of 327 cases from an endemic
area of Leishmania (Viannia) braziliensis. Am
J Trop Med Hyg. 97:761–766. 2017.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jeziorski E, Dereure J, Mac Bullen G,
Blanchet C, Ludwig C, Costes V and Rodière M: Mucosal relapse of
visceral Leishmaniasis in a child treated with anti-TNFα. Int J
Infect Dis. 33:135–136. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Darcis G, Van der Auwera G, Giot JB,
Hayette MP, Tassin F, Arrese Estrada J, Cnops L, Moutschen M, de
Leval L and Leonard P: Recurrence of visceral and muco-cutaneous
Leishmaniasis in a patient under immunosuppressive therapy. BMC
Infect Dis. 17(478)2017.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Faucher B, Pomares C, Fourcade S,
Benyamine A, Marty P, Pratlong L, Faraut F, Mary C, Piarroux R,
Dedet JP and Pratlong F: Mucosal Leishmania infantum Leishmaniasis:
Specific pattern in a multicentre survey and historical cases. J
Infect. 63:76–82. 2011.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Aliaga L, Cobo F, Mediavilla JD, Bravo J,
Osuna A, Amador JM, Martín-Sánchez J, Cordero E and Navarro JM:
Localized mucosal leishmaniasis due to Leishmania (Leishmania)
infantum: Clinical and microbiologic findings in 31 patients.
Medicine (Baltimore). 82:147–158. 2003.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Marino A, Zafarana G, Ceccarelli M,
Cosentino F, Moscatt V, Bruno G, Bruno R, Benanti F, Cacopardo B
and Celesia BM: Immunological and clinical impact of DAA-mediated
HCV eradication in a cohort of HIV/HCV coinfected patients:
Monocentric Italian experience. Diagnostics (Basel).
11(2336)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Russotto Y, Micali C, Laganà N, Marino A,
Campanella E, Celesia BM, Pellicanò GF, Venanzi Rullo E and Nunnari
G: Diagnosis, treatment, and prevention of HIV infection among
detainees: A review of the literature. Healthcare (Basel).
10(2380)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Alvar J, Aparicio P, Aseffa A, Den Boer M,
Cañavate C, Dedet JP, Gradoni L, Ter Horst R, López-Vélez R and
Moreno J: The relationship between leishmaniasis and AIDS: The
second 10 years. Clin Microbiol Rev. 21:334–359. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Cosentino F, Marino A, Anile L, Moscatt V,
Gussio M, Boscia V, Bruno R, Nunnari G, Pulvirenti A, Privitera GF,
et al: Long-term survivors in a cohort of people living with HIV
diagnosed between 1985 and 1994: Predictive factors associated with
more than 25 years of survival. Infect Dis Rep. 15:70–83.
2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Dzopalić T, Božić-Nedeljković B and
Jurišić V: Function of innate lymphoid cells in the immune-related
disorders. Hum Cell. 32:231–239. 2019.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Celesia BM, Marino A, Borracino S,
Arcadipane AF, Pantò G, Gussio M, Coniglio S, Pennisi A, Cacopardo
B and Panarello G: Successful extracorporeal membrane oxygenation
treatment in an acquired immune deficiency syndrome (AIDS) patient
with acute respiratory distress syndrome (ARDS) complicating
pneumocystis jirovecii pneumonia: A challenging case. Am J Case
Rep. 21(e919570)2020.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Diro E, Edwards T, Ritmeijer K, Fikre H,
Abongomera C, Kibret A, Bardonneau C, Soipei P, Mutinda B, Omollo
R, et al: Long term outcomes and prognostics of visceral
leishmaniasis in HIV infected patients with use of pentamidine as
secondary prophylaxis based on CD4 level: A prospective cohort
study in Ethiopia. PLoS Negl Trop Dis. 13(e0007132)2019.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Araújo CF, Oliveira IBN, Silva MVT,
Pereira LIA, Pinto SA, Silveira MB, Dorta ML, Fonseca SG, Gomes RS
and Ribeiro-Dias F: New world Leishmania spp. infection in people
living with HIV: Concerns about relapses and secondary prophylaxis.
Acta Trop. 224(106146)2021.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Mohammed R, Fikre H, Schuster A, Mekonnen
T, van Griensven J and Diro E: Multiple relapses of visceral
leishmaniasis in HIV co-infected patients: A case series from
Ethiopia. Curr Ther Res Clin Exp. 92(100583)2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Jurisic V, Radenkovic S and Konjevic G:
The actual role of LDH as tumor marker, biochemical and clinical
aspects. Adv Exp Med Biol. 867:115–224. 2015.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Cocuzza S, Strazzulla A, Pinzone MR,
Cosentino S, Serra A, Caltabiano R, Lanzafame S, Cacopardo B and
Nunnari G: Isolated laryngeal leishmaniasis in immunocompetent
patients: an underdiagnosed disease. Case Rep Infect Dis. 2013:1–7.
2013.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Patel TA, Scadding GK, Phillips DE and
Lockwood DN: Case report: Old world mucosal leishmaniasis: Report
of five imported cases to the hospital for tropical diseases,
London, United Kingdom. Am J Trop Med Hyg. 97:1116–1119.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
de Carvalho RVH, Lima-Junior DS, da Silva
MVG, Dilucca M, Rodrigues TS, Horta CV, Silva ALN, da Silva PF,
Frantz FG, Lorenzon LB, et al: Leishmania RNA virus exacerbates
Leishmaniasis by subverting innate immunity via TLR3-mediated NLRP3
inflammasome inhibition. Nat Commun. 10:1–17. 2019.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Cantanhêde LM, da Silva Júnior CF, Ito MM,
Felipin KP, Nicolete R, Salcedo JM, Porrozzi R, Cupolillo E and
Ferreira Rde G: Further evidence of an association between the
presence of Leishmania RNA virus 1 and the mucosal manifestations
in tegumentary Leishmaniasis patients. PLoS Negl Trop Dis.
9(e0004079)2015.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Di Luca M, Iannella G, Montevecchi F,
Magliulo G, De Vito A, Cocuzza S, Maniaci A, Meccariello G,
Cammaroto G, Sgarzani R, et al: Use of the transoral robotic
surgery to treat patients with recurrent lingual tonsillitis. Int J
Med Robot. 16(e2106)2020.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Marletta S, L'Imperio V, Eccher A,
Antonini P, Santonicco N, Girolami I, Dei Tos AP, Sbaraglia M,
Pagni F, Brunelli M, et al: Artificial intelligence-based tools
applied to pathological diagnosis of microbiological diseases.
Pathol Res Pract. 243(154362)2023.PubMed/NCBI View Article : Google Scholar
|