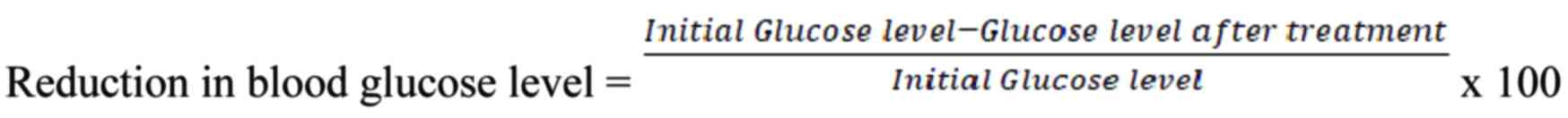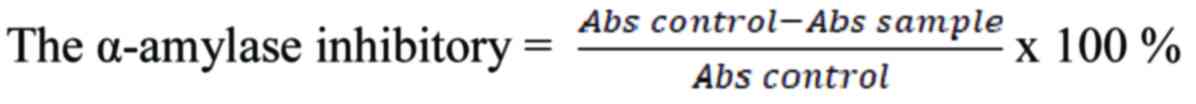Introduction
In Indonesia, diabetes mellitus (DM) constitutes a
serious health concern. With >10 million diabetic patients,
Indonesia was confirmed to have a prevalence rate of 6.2% (1). DM, particularly type 2 DM (T2DM), is
associated with the increased production of reactive oxygen species
and disruptions in the antioxidant defense system, resulting in
oxidative damage. DM is also characterized by an abnormal increase
in blood glucose levels and impaired carbohydrate, lipid and
protein metabolism due to inadequate insulin secretion and/or
action (2). Maintaining stability
and reducing blood glucose levels can be accomplished by delaying
glucose absorption in the digestive system via the inhibition of
carbohydrate hydrolyzing enzymes, such as α-glucosidase and
α-amylase. α-amylase hydrolyzes the α-bonds of α-linked
polysaccharides, such as starch and glycogen, releasing glucose and
maltose into the circulation. It is the most common type of amylase
observed in humans and other mammals. The inhibition of α-amylase
attenuates the digestion process by reducing starch breakdown in
the stomach; hence, it can be utilized as an effective strategy to
control hyperglycemic conditions (3).
Stigmasterol, a bioactive phytosterol, has been
explored for its antidiabetic activity; e.g., stigmasterol isolated
from Glycine max oil has been shown to exhibit a weak
glucose transporter type 4 (GLUT4) translocation activity in rat
skeletal myoblast cells (4).
Stigmasterol isolated from Gelidium spinosum seaweed has
also been found to exhibit antioxidant and α-amylase inhibitory
activity (5). In addition,
stigmasterol isolated from Pseuderanthemum palatiferum
leaves has been shown to exert a hypoglycemic effect in rats with
streptozotocin (STZ)-induced diabetes (6). Furthermore, stigmasterol and
β-sitosterol isolated from banana pseudostem have been found to
reduce fasting blood glucose levels in rats with alloxan-induced
diabetes (7). Another study
demonstrated that stigmasterol isolated from the aqueous ethanol
root extract of Bridelia duvigneaudii exhibited hypoglycemic
activity in albino mice with diabetes induced by oral glucose
intake (8). Previously,
β-sitosterol and stigmasterol isolated from the roots of
Indigofera heterantha were measured for their antidiabetic
activity on the basis of the glucose uptake in yeast cells
(9). Moreover, the ethanol extract
of noni fruit (Morinda citrifolia; M. citrifolia) was
previously shown to decrease blood sugar levels in mice with
STZ-induced diabetes (10).
Another study also demonstrated that the juice of M.
citrifolia fruit administered to patients with T2DM resulted in
an improvement in blood glucose levels and other pathological
parameters (11).
Generally, the identification of plant-based
anti-diabetic drugs is challenging; therefore, the present study
aimed to extract and isolate the bioactive compound in M.
citrifolia fruits and to investigate its potency as an
α-amylase inhibitor.
Materials and methods
Chemicals
The solvents used for the extraction and isolation
process were 96% ethanol (Brataco Chemica, http://bratachem.com/), n-hexane (MilliporeSigma),
ethyl acetate (MilliporeSigma), methanol (MilliporeSigma) and
chloroform (MilliporeSigma). Thin layer chromatography (TLC) was
carried out using pre-coated silica gel 60 F254 plates with a
thickness of 0.20 mm (MilliporeSigma). The human α-Amylase
Inhibitor Screening kit (cat. no. K482-100, BioVision, Inc.), which
hydrolyzes the synthetic substrate and yields a chromophore, was
used for in vitro testing. Nuclear magnetic resonance (NMR)
analyses, which included proton (1H)-NMR, carbon-13
(13C)-NMR and distortionless enhancement by polarization transfer
(DEPT) were performed using deuterated solvents (acetone-d6,
CD3OD, and/or CDCl3) on 400 mHz NMR
(JNM-ECZ500R/S1, JEOL, Ltd.) with tetramethylsilane
(MilliporeSigma) as an internal reference.
Plant materials
The fresh ripe fruits were harvested in August, 2019
(outside temperature, 25 to 30˚C) at Kendari (Google coordinates:
-4.045723429369156; 122.57746055261806), Southeast Sulawesi,
Indonesia. The fruits were taxonomically identified at the College
of Life Sciences, Bandung Institute of Technology (Bandung,
Indonesia), and were confirmed as M. citrifolia L. (sample
no. 66/I1.CO2.2). The characteristics of the plant samples matched
those described in the flowering plants' taxonomic references
(12-14).
The fruits were washed under tap water to separate
any soil, dust, and other foreign inorganic matter, and were
cleaned using standard pharmacognosy laboratory procedures, e.g.,
medicinal plant materials should be entirely free from visible
signs of contamination by molds or insects and other animal
contamination, including animal excreta. No abnormal odor,
discoloration, slime, or signs of deterioration should be detected
(15). The clean fruits were dried
at 40˚C without being exposed to sunlight. The dried samples were
mashed using a pestle in a white porcelain mortar and sealed in a
plastic container before being further processed.
Extraction and isolation
Dried M. citrifolia coarse powder fruit
(3,064 g) was macerated with 20 liters of 96% ethanol three times
for 24 h to obtain 540 g extract (yield, 17.62% w/w). The extract
was then thickened using a vacuum rotary evaporator (Buchi) and
further diluted in ethyl acetate and divided into soluble (338 g or
62.59%) and insoluble (338 g or 37.40%) partitions. The insoluble
ethyl acetate partition contains polar molecules (glycosides) that
can disrupt the isolation process.
The ethyl acetate extract was then fractionated
using vacuum liquid chromatography apparatus (consisting of a glass
Buchner filter funnel and a 10-cm length glass column connected to
a vacuum pump) using 21 mobile phases or eluents (the volume of
each solvent is 150 ml) i.e., n-hexane, a combination of n-hexane
and ethyl acetate in the ratios of (9:1), (8:2), (7:3), (5:5),
(3:7), (2:8), ethyl acetate and methanol, on a 10-cm column with a
silica gel stationary phase (Silica gel 60 Merck® for
column chromatography; CAS no. 7631-86-9; MilliporeSigma) (250 g),
yielding 21 fractions observed in the chromatogram pattern using
thin layer chromatography. The fractions with the same stain
pattern were merged based on the chromatograms and were tested for
their hypoglycemic activity following the bioactivity guide
method.
Bioactivity-guided in vivo
anti-diabetic assay
A total of 30 male Swiss-Webster mice (Mus
musculus), weighing 20-30 g, were adapted to a controlled
temperature condition at 28-30˚C under a 12-h dark/light cycle
(light was turned on from 6 a.m. to 6 p.m.), with daily standard
food and water freely available, for 1 week prior to treatment. The
health and behavior of the mice were observed daily and their cages
were cleaned every 2 days to remove the feces and urine. No animals
were found dead during the acclimatization and the in vivo
experiments. Animal handling, maintenance and euthanasia procedures
were performed as approved by the Ethics Committee of Halu Oleo
University, Kendari, Indonesia. Following 1 week of
acclimatization, the mice were fasted for 18 h and their fasting
blood glucose levels were measured using a glucometer (ACCU-CHEK
Inform II, Roche Diagnostics). All the mice were then administered
STZ at a dose of 65 mg/kg body weight intraperitoneally to induce
diabetes; this was followed by a period of modification (16). STZ [chemical name,
2-deoxy-2-(3-(methyl-3-nitrosourea)-D-glucopyranose] is produced by
Streptomycetes acromogenes. Following an intraperitoneal or
intravenous administration, this antibiotic enters the pancreatic
β-cell through the Glut-2 transporter and induces the alkylation of
DNA (17).
At 48 h following the STZ administration, the blood
glucose levels of the mice were measured by obtaining blood from
the tail vein, and mice with blood glucose levels >200 mg/dl
were randomly assigned to 9 groups, with 3 mice in each group in
one cage, as follows: The negative control group (treated with a
suspension of sodium carboxymethylcellulose 0.5%); the drug control
group (treated with exogenous insulin 1 IU/kg body weight
subcutaneously); and seven treatment groups of fractions A-G (150
mg/kg body weight) in a suspension of sodium carboxymethylcellulose
0.5% by oral administration using oral gavage feeding every
afternoon at 2 p.m. for 14 consecutive days.
The blood glucose levels of the animals were
measured on days 1, 3, and 7 after treatment. The reduction in
blood glucose levels was calculated using the following
formula:
The results of the present study are expressed as
the mean ± SEM. At the end of the study period, the mice were
sacrificed using isoflurane 2% inhalation 1 liter/minute for >4
h following a procedure described elsewhere with a modification of
the dose (18). Animal death was
verified by the absence of respiration and heartbeat for a period
of >5 min.
Purification and isolation of the
compound from the active fractions with hypoglycemic activity
Active fractions with hypoglycemic activity were
further purified using a radial chromatographic method (The
Chromatotron™ model 7924T), followed by 1H-NMR, 13C-NMR,
and DEPT analyses using the JEOL JNM-ECZ500R/S1 (JEOL, Ltd.)
instrument, and were validated using liquid chromatography-mass
spectroscopy (LC-MS Waters ACQUITY UPLC I-Class in tandem with the
Xevo G2-X2 Quadrupole Time-of-Flight Mass Spectrometer; Waters
Corporation), to elucidate the chemical structure of the
isolate.
In vitro α-amylase inhibitory activity
assay
The α-amylase inhibitory activity of M.
citrifolia fruit extracts and the isolate were assayed using
the α-Amylase Inhibitor Screening Kit (BioVision Inc.). Acarbose
provided in the enzyme kit (BioVision Inc.) was employed as the
control drug and dimethyl sulfoxide (DMSO) as a blank solvent. Each
sample was dissolved in DMSO to generate a concentration of 150,
75, 37.5, 18.75, and 9.375 ppm for each sample. A total of 50 µl of
the sample (5x) was added to designated wells of a clear 96-well
microplate. The preparation of the reagents and the procedure of
this assay were performed by following the instructions provided
with the kit. The microplate was incubated at 37˚C for 25 min
before being measured at 405 nm.
The α-amylase inhibitory activity was calculated
using the following equation:
The concentration of sample required to inhibit 50%
of α-amylase activity under the above condition was defined as the
IC50 value. The α-amylase inhibitory activity of the
samples was calculated, and its IC50 values were
determined using GraphPad Prism software.
Ethical considerations
Animal handling, maintenance and euthanasia
procedures were performed as approved by the Research Ethics
Committee, Halu Oleo University, Kendari, Southeast Sulawesi,
Indonesia (document no. 1404/UN29.20/PPM/2020).
Results
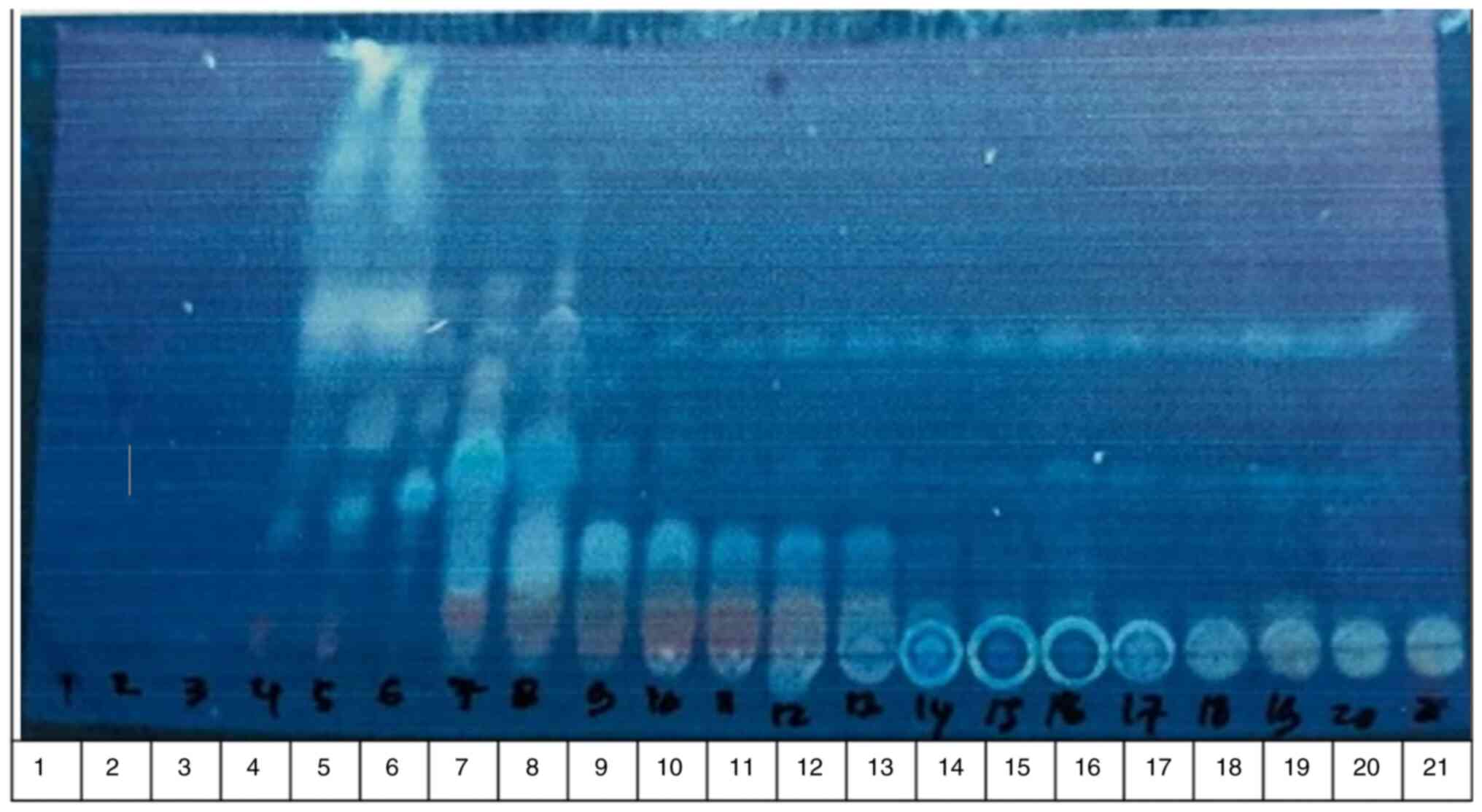
Thin-layer chromatography
technique
The 21 fractions were observed in the chromatogram
pattern using thin-layer chromatography (Fig. 1). The fractions with the same stain
pattern were merged based on the similarity of the chromatograms,
yielding 7 fractions (A-G). These fractions (A-G) were tested for
their hypoglycemic activity following the bioactivity guide
method.
Bioactivity-guided in vivo
antidiabetic assay
To determine the active fraction of M.
citrifolia fruit, fractions A-G were assayed for their
hypoglycemic activity in Swiss-Webster mice using the glucose
tolerance method. The results are presented in Table I. The findings demonstrated that
the control drug (exogenous insulin) and all fractions exhibited
notable differences (95% CI) compared with the negative control
(Na-CMC). Fraction G (48.10%), fraction C (43.08%) and fraction E
(41.27%) exhibited the most prominent suppressive effects on blood
sugar levels.
 | Table IHypoglycemic activity assay of
Morinda citrifolia fractions using the glucose tolerance
test method. |
Table I
Hypoglycemic activity assay of
Morinda citrifolia fractions using the glucose tolerance
test method.
| | Blood glucose level
following treatment (mg/dl) | |
|---|
| Groups | Fasting blood glucose
level (mg/dl) | Blood glucose level
after induction (mg/dl) | 120 min | Day 1 | Day 3 | Day 7 | Percentage reduction
of blood glucose level |
|---|
| Negative control | 76.00±15.52 | 193.30±7.63 | 196.67±20.21 | 229.30±18.82 | 262.60±40.62 | 312.60±54.04 | - |
| Control drug | 75.60±17.09 | 220.6±45.56 | N/A | 220.30±101.74 | 152.60±24.58 | 107.60±3.78 | 51.22 |
| Fraction A | 83.6±11.37 | 192.60±5.50 | 165.33±32.38 | 110.00±9.84 | 121.30±13.31 | 101.30±6.65 | 31.75 |
| Fraction B | 91.00±11.26 | 189.60±9.50 | 191.33±78.62 | 165.60±12.58 | 139.30±5.03 | 78.30±55.33 | 24.08 |
| Fraction C | 97.30±2.08 | 189.00±7.21 | 107.67±17.62 | 133.30±26.27 | 158.00±42.50 | 130.30±27.50 | 43.08 |
| Fraction D | 74.00±12.16 | 222.60±58.44 | 112.67±14.47 | 272.30±102.64 | 97.00±4.35 | 92.00±14.52 | 38.80 |
| Fraction E | 62.30±13.65 | 218.60±20.74 | 121.67±21.36 | 350.00±1.00 | 195.00±111.7 | 170.30±81.37 | 41.27 |
| Fraction F | 88.00±12.12 | 262.30±66.90 | 138.33±6.66 | 158.60±53.40 | 133.00±14.17 | 120.30±19.75 | 29.90 |
| Fraction G | 76.00±15.71 | 301.00±90.70 | 103.33±14.47 | 106.30±10.11 | 112.30±13.61 | 94.60±9.86 | 48.10 |
Purification and isolation of the
compound from the active fractions with hypoglycemic activity
By considering the TLC pattern compared to a
previous study (19) and the
hypoglycemic activity of the fraction, fraction C was selected and
proceeded to be purified using a radial chromatographic method and
yielded eight sub-fractions (C1-C8). The C1 sub-fraction exhibited
a single spot (isolate), which was further characterized using
1H-NMR, 13C-NMR, DEPT spectroscopy and LC-MS.
Structure elucidation of the
isolate
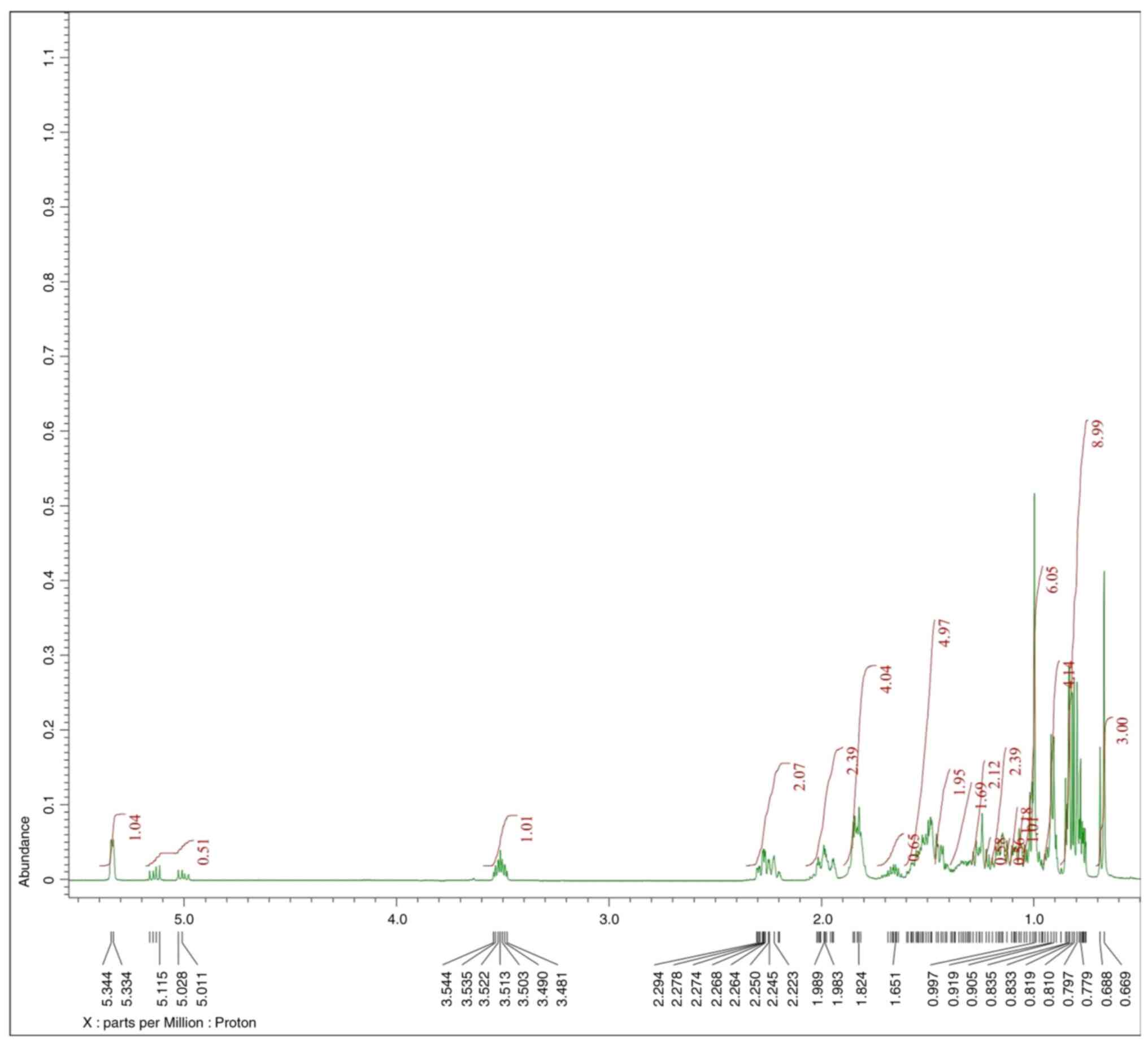
According to the 1H-NMR spectrum data,
the molecular structure of the C1 isolate consists of 48 protons,
four of which have relatively large chemical shifts of 5.34, 5.14,
5.00 and 3.51 ppm, indicating that the protons may have low
electron density. In addition, the similarity of the coupling
constant (J=15.5 Hz) values between the protons at the chemical
shifts of 5.00 and 5.14 ppm indicate that these two protons are in
trans-position. Furthermore, proton stacking with very large
integration is observed in the 1H-NMR spectrum and the
multiplicity data (m, br d and br s), that indicate
the number of neighboring protons or the position of the protons
are quite close together. This confirms the structure of the C1
isolate may belong to the steroid class.
Furthermore, the 13C-NMR spectrum of the C1 isolate
shows the 29 carbons that make up the structure. The DEPT technique
reveals that the isolate possesses three quaternary carbons, 11
methines, 9 methylene and 6 methyls. Carbons with chemical shifts
>95.0 ppm belong to the olefinic carbon (alkene) or
Csp2 carbon. One olefinic quaternary carbon indicates a
chemical shift of 140.9 ppm, while three olefinic methine carbons
indicate chemical shifts of 121.9, 129.4 and 138.5 ppm,
respectively. These four olefinic carbons enable the formation of
two double bonds.
By considering the 1H-NMR, 13C-NMR and
DEPT data, the molecular formula of the C1 isolate is
C29H48O with double bond equivalence (DBE)=6,
of which 2 come from the alkene group created by 4 Csp2
atoms and the other 4 are from cyclic carbon.
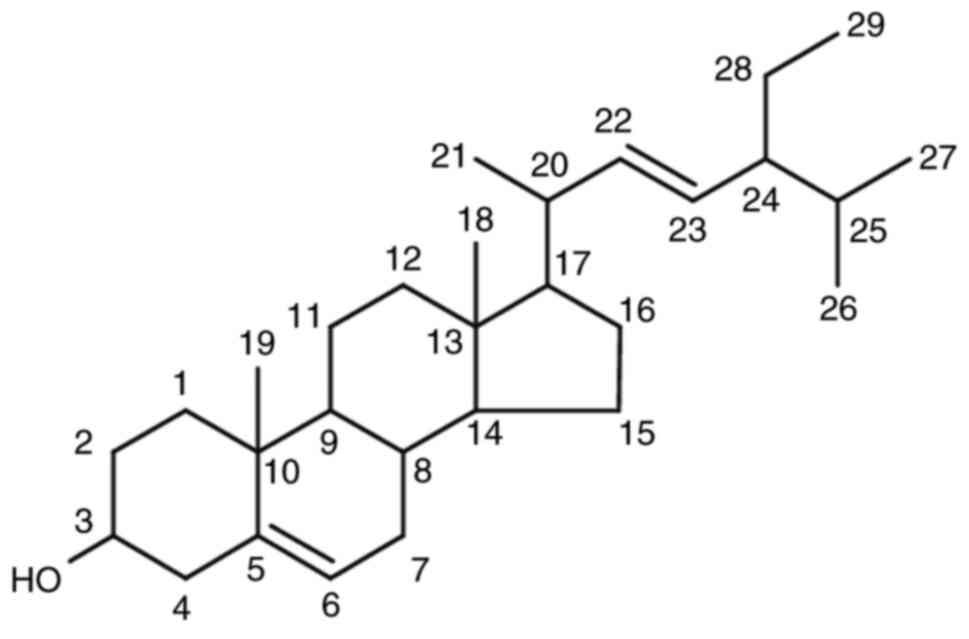
By comparing the NMR data with a Wiley library
database., the hypothesized chemical structure of the C1 isolate is
stigmasterol (Fig. 2). Further
verification was obtained by comparing the C1 isolate data with the
reference (20) (presented in
Table II).
 | Table IIComparison of the 1H-NMR
and C13-NMR data for the C1 isolate and stigmasterol. |
Table II
Comparison of the 1H-NMR
and C13-NMR data for the C1 isolate and stigmasterol.
| | C1 isolate | Stigmasterol
(16) |
|---|
| Carbon no. | δC 125
MHz (ppm) | δH (ΣH,
m, J=Hz) 500 mHz (ppm) | δC 125
MHz (ppm) | δH (ΣH,
m, J=Hz) 600 mHz (ppm) |
|---|
| 1 | 37.4 | | 37.6 | |
| 2 | 32.1 | | 32.1 | |
| 3 | 71.9 | 3.51 (1H, m) | 72.1 | 3.51 (1H, tdd,
J=4.5; 4.4; 3.8 Hz) |
| 4 | 42.5 | | 42.4 | |
| 5 | 140.9 | | 141.1 | |
| 6 | 121.9 | 5.34 (1H, m) | 121.8 | 5.31 (1H, t, J=6.1
Hz) |
| 7 | 32.1 | | 31.8 | |
| 8 | 31.8 | | 31.8 | |
| 9 | 50.2 | | 50.2 | |
| 10 | 36.7 | | 36.6 | |
| 11 | 21.2 | | 21.5 | |
| 12 | 39.9 | | 39.9 | |
| 13 | 42.4 | | 42.4 | |
| 14 | 56.9 | | 56.8 | |
| 15 | 24.3 | | 24.4 | |
| 16 | 28.4 | | 29.3 | |
| 17 | 56.1 | | 56.2 | |
| 18 | 40.7 | | 40.6 | |
| 19 | 23.2 | 0.91 (3H, d,
J=7) | 21.7 | 0.91 (3H, d, J=6.2
Hz) |
| 20 | 138.5 | 5.00 (1H, dd,
J=8.5; 15.5) | 138.7 | 4.98 (1H, m) |
| 21 | 129.4 | 5.14 (1H, dd,
J=8.5; 15.5) | 129.6 | 5.14 (1H, m) |
| 22 | 46.0 | | 46.1 | |
| 23 | 26.2 | | 25.4 | |
| 24 | 12.0 | 0.83 (3H, m) | 12.1 | 0.83 (3H, t, J=7.1
Hz) |
| 25 | 29.3 | | 29.6 | |
| 26 | 20.0 | 0.82 (3H, m) | 20.2 | 0.82 (3H, d, J=6.6
Hz) |
| 27 | 19.6 | 0.80 (3H, m) | 19.8 | 0.80 (3H, d, J=6.6
Hz) |
| 28 | 18.9 | 0.67 (3H, s) | 18.9 | 0.71 (3H, s) |
| 29 | 12.2 | 1.00 (3H, s) | 12.2 | (3H, s) |
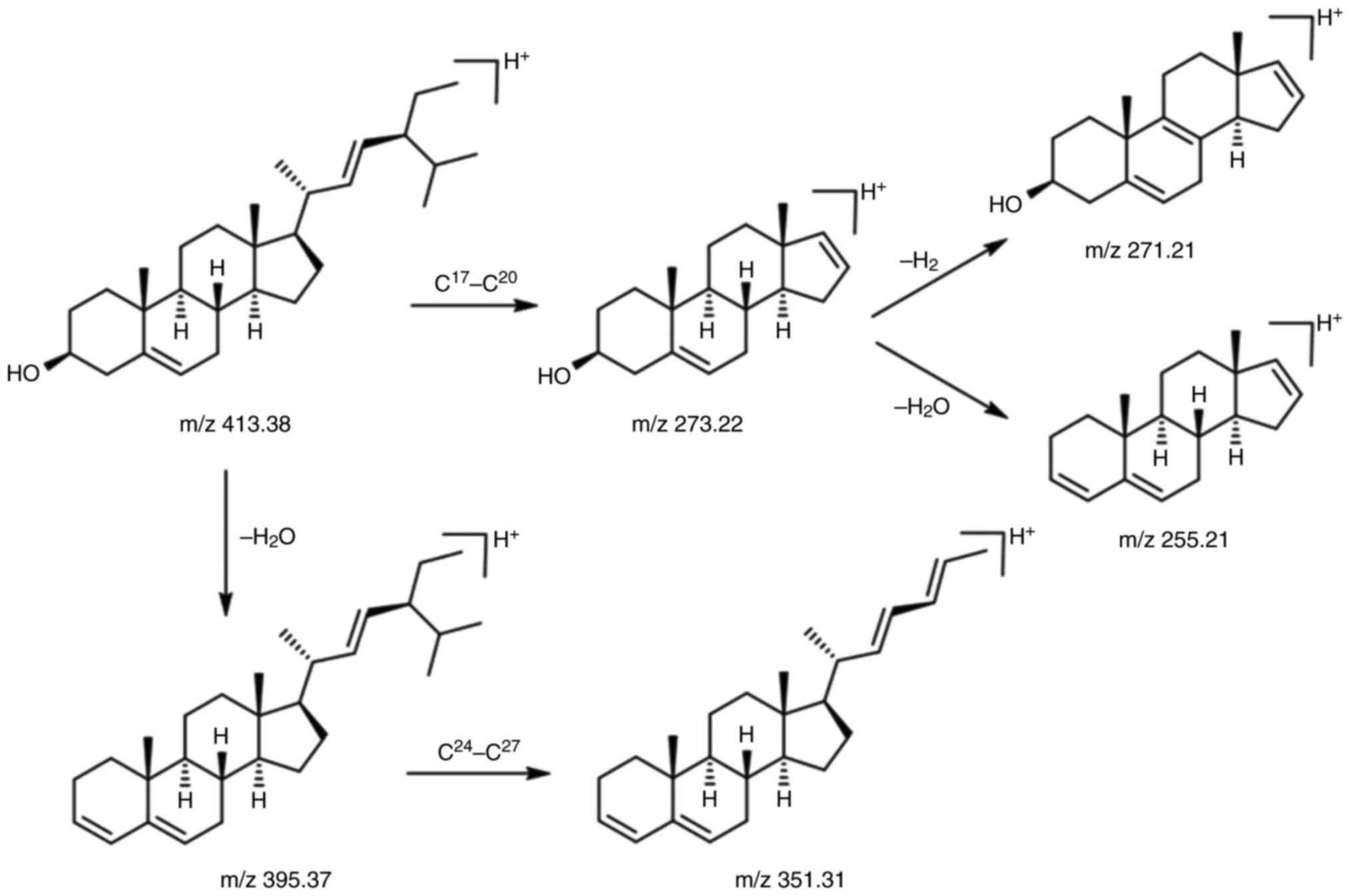
The estimated chemical structure of the C1 isolate
was validated by LC-MS/MS electrospray ionization. According to the
LC-MS/MS data, the C1 isolate exhibited [M+H]+ ion at
m/z 413.38, with molecular ion fragmentation at m/z 395.37, 351.31,
273.22, 271.21 and 255.21. The [M+H]+ ion at m/z 413.38
represents the first ionization of the C1 isolate with a positive
ion (H+). [M+H]+ ion at m/z 413.38 undergoes
fragmentation by releasing H2O molecules as confirmed by
the presence of the m/z 395.37 peaks or the m/z 273.22 to the m/z
255.21. The 1H-NMR spectrum of the C1 isolate is
presented in Fig. 3. Additionally,
bond breaking occurs in the branching area, such as C17 and C20
bonds or C24 and C27 bonds, since secondary ion radicals and
secondary carbocations are more stable than the primary form
(branching effect). The proposed mechanism of fragmentation is
presented in Fig. 4. Thus, it was
concluded that the molecular weight of the C1 isolate is
412.37.
In vitro α-amylase inhibitory activity
assay
M. citrifolia fruit extract exhibited potent
inhibitory activity against α-amylase with an IC50 value
of 14.16±5.72 ppm. Similarly, stigmasterol (the C1 isolate)
exhibited a prominent inhibitory activity against α-amylase with an
IC50 value of 10.29±0.76 ppm which is more potent than
that of acarbose, a known inhibitor of the enzyme,with an
IC50 value of 43.37±1.56 ppm. The results are presented
in Table III.
 | Table IIIIn vitro α-amylase inhibitory
activity of M. citrifolia extract and stigmasterol |
Table III
In vitro α-amylase inhibitory
activity of M. citrifolia extract and stigmasterol
| Sample | IC50
value (ppm) | Regression equation
and correlation coefficient (r2) |
|---|
| M.
citrifolia extract | 14.16±5.72 | y=0.01679
x + 49.92 |
| | |
r2=0.8164 |
| Stigmasterol (C1
isolate) | 10.29±0.76 | y=0.01993
x + 50.41 |
| | |
r2=0.8817 |
| Acarbose | 43.37±1.56 | y=0.11730
x + 42.77 |
| | |
r2=0.9248 |
Discussion
The present study successfully isolated a
phytosterol, stigmasterol, from the active sub-fraction C1 of M.
citrifolia fruit, by following a bioactivity-guided method.
Chemically, there are no differences in the structure of
stigmasterol extracted from various plants, the differences are
only in the amount.
The intravenous injection of the 65 mg/kg dose of
STZ in adult Wistar rats may cause the enlargement of the pancreas
followed by the deterioration of β-cells and induces experimental
DM condition (21). The
subcutaneous injection of exogenous insulin at 1 IU/kg has been
reported to significantly reduce high blood glucose levels in rats
with STZ-induced diabetes (22-24);
thus, this drug was used as the control in the present study. In
the present study, both the origin extract and the stigmasterol
isolate exhibited potent inhibitory activity against α-amylase
compared to that of acarbose.
The digestion of dietary carbohydrates is associated
with the elevation of postprandial blood glucose levels. Thus,
limiting the activity of carbohydrate digestive enzymes in the
intestinal tract is considered an important strategy. α-amylase is
the key enzyme that catalyzes the hydrolysis of carbohydrates into
smaller units, e.g., glucose (25).
The inhibitory activity towards α-amylase is
categorized as very active with an IC50 value ≤25 µg/ml;
active with an IC50 value >25 and ≤50 µg/ml; less
active with an IC50 value >50 µg/ml and ≤100 g/ml;
and inactive with an IC50 value IC50 >100
g/ml (26).
The findings of the present study confirmed that
stigmasterol exhibited a potent inhibitory activity against
α-amylase; the extract was categorized as very active
(IC50 ≤25 g/ml) and acarbose was in the active category
(IC50 value >25 g/ml and ≤50 g/ml).
α-Amylase inhibitors are also known as starch
blockers due to their ability to prevent or attenuate the
absorption of starch by inhibiting the hydrolysis of 1,4-glycosidic
linkages of starch and other oligosaccharides into maltose,
maltotriose and other simple sugars (27). These findings are relevant to an
initial study by the authors that confirmed the molecular
interaction of stigmasterol towards α-amylase with a stable complex
and higher affinity compared to that of acarbose (28).
In addition to being an inhibitor of α-amylase,
stigmasterol has been reported to have anti-diabetic properties
through a variety of mechanisms. In vivo investigations
using animals have revealed that stigmasterol has the ability to
lower glucose, urea and creatinine levels. The administration of
stigmasterol isolated from plant extracts induces the release of
insulin from pancreatic α-cells, resulting in an anti-hyperglycemic
effect (5,6). In another study, stigmasterol was
demonstrated to exert an anti-diabetic effect in mice with
alloxan-induced diabetes (7).
Stigmasterol has been reported to have two
mechanisms for lowering glucose levels in vivo, the first of
which is to reduce intestinal glucose absorption or to increase in
glycolytic and glycogenic systems with a subsequent decrease in
glycogenolysis and gluconeogenesis pathways. The second mechanism
includes the activation or repair of α-cells, followed by insulin
release or insulin receptor stimulation (2,4,29).
Stigmasterol has also been found to have the ability to operate on
the GLUT4 receptor of the glucose transporter, including enhanced
translocation and expression of GLUT4(4). Moreover, a treatment of stigmasterol
was reported could prevent early apoptosis, elevated total insulin,
and improved insulin secretion in cells exposed to
glucolipotoxicity (30).
Stigmasterol isolated from the bark of Butea monosperma has
been shown to reduce blood glucose levels accompanied by elevated
plasma insulin levels in diabetic mice (31).
Although both M. citrifolia fruit extract and
stigmasterol isolated from the ethyl acetate fraction of the fruit
may have the potential to be developed as an α-amylase inhibitor,
further studies on the underlying mechanisms are warranted. The
present study did not evaluate the effects of the extract and/or
stigmasterol on the signaling pathway of insulin, such as the
ERK/MAPK pathway and/or IRS/PI3K/AKT pathway, that play a crucial
role in the glucose, protein and lipid metabolism; thus, this was a
limitation of the present study.
The present study performed the extraction,
bioactivity-guided fractionation, isolation and characterization of
stigmasterol in M. citrifolia fruits and examined its
potency as an inhibitor of α-amylase. To the best of our knowledge,
the present study is the first report the successful isolation of
stigmasterol from M. citrifolia fruits. In conclusion, both
the fruit extract and the stigmasterol isolate demonstrated a
potent inhibitory activity against α-amylase compared to acarbose.
However, further studies are required to evaluate the effects of
the extract and/or stigmasterol on the signaling pathway of
insulin, such as the ERK/MAPK pathway and/or IRS/PI3K/AKT pathways
in order to guarantee its efficacy and safety in humans.
Acknowledgements
Not applicable.
Funding
Funding: The authors thank the Rector of Universitas Padjadjaran
for funding the research via the Directorate of Research and
Community Engagement in the scheme of the Academic-Leadership
Grant.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the first author on reasonable
request.
Author's contributions
SAS, IS and JL were equally responsible for the
conception and design of the study. NL was responsible for the
formal analysis and the curation of the data. SAS, IS and JL
confirm the authenticity of all the raw data. NL contributed to the
writing of the original version of the manuscript. JL was
responsible for reviewing and revising the manuscript. All authors
have read and agreed to the published version of the
manuscript.
Ethics approval and consent to
participate
Animal handling, maintenance and euthanasia
procedures were performed as approved by the Research Ethics
Committee, Halu Oleo University, Kendari, Southeast Sulawesi,
Indonesia (document no. 1404/UN29.20/PPM/2020).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ligita T, Wicking K, Francis K, Harvey N
and Nurjannah I: How people living with diabetes in Indonesia learn
about their disease: A grounded theory study. PLoS One.
14(e0212019)2019.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Wahyuni Y: Improving the quality of life
of patients with diabetes mellitus type 2 with treatment adherence.
Media Keperawatan Indonesia. 4:234–246. 2021.
|
|
3
|
Khadayat K, Marasini BP, Gautam H, Ghaju S
and Parajuli N: Evaluation of the alpha-amylase inhibitory activity
of Nepalese medicinal plants used in the treatment of diabetes
mellitus. Clin Phytosci. 6(34)2020.
|
|
4
|
Wang J, Huang M, Yang J, Ma X, Zheng S,
Deng S, Huang Y, Yang X and Zhao P: Anti-diabetic activity of
stigmasterol from soybean oil by targeting the GLUT4 glucose
transporter. Food Nutr Res. 61(1364117)2017.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Poulose N, Sajayan A, Ravindran A,
Chandran A, Priyadharshini GB, Selvin J and Kiran GS: Anti-diabetic
potential of a stigmasterol from the seaweed Gelidium
spinosum and its application in the formulation of nanoemulsion
conjugate for the development of functional biscuits. Front Nutr.
8(694362)2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Nualkaew S, Padee P and Talubmook C:
Hypoglycemic activity in diabetic rats of stigmasterol and
sitosterol-3-O-β-D-glucopyranoside isolated from Pseuderanthemum
palatiferum (Nees) Radlk. leaf extract. J Med Plants Res.
9:629–635. 2015.
|
|
7
|
Ramu R, Shirahatti PS, Nayakavadi S, R V,
Zameer F, Dhananjaya BL and Prasad Mn N: The effect of a plant
extract enriched in stigmasterol and β-sitosterol on glycaemic
status and glucose metabolism in alloxan-induced diabetic rats.
Food Funct. 7:3999–4011. 2016.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Credo D, Masimba PJ, Machumi F and
Heydenreich M: Isolation of stigmasterol from 80% aqueous ethanol
root extract of Bridelia duvigneaudii J.Leon and its hypoglycaemic
activity on oral glucose loaded white albino mice. Int J Res Pharm
Chem. 8:492–501. 2018.
|
|
9
|
Zeb MA, Khan SU, Rahman TU, Sajid M and
Seloni S: Isolation and biological activity of β-sitosterol and
stigmasterol from the roots of Indigofera heterantha. Pharm
Pharmacol Int J. 5:204–207. 2017.
|
|
10
|
Lolok N, Sahidin I, Sumiwi A and Muhtadi
A: Antidiabetes effect of noni fruit (Morinda citrifolia l.)
on mice with oral glucose tolerance method and streptozotocin
induction method. Res J Pharm Technol. 14:5067–5071. 2021.
|
|
11
|
Algenstaedt P, Stumpenhagen A and
Westendorf J: The effect of Morinda citrifolia L. fruit
juice on the blood sugar level and other serum parameters in
patients with diabetes type 2. Evid Based Complement Alternat Med.
2018(3565427)2018.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Backer CA and Bakhuizen van den Brink Jr
RC: 1965. Flora of Java. Vol II. NVP Noordhoff, Groningen, The
Netherlands. pp351, 1965.
|
|
13
|
Cronquist A: An Integrated System
Classification of Flower Plants. Columbia University Press. New
York, NY, USA. ppxiii-xviii, 1981.
|
|
14
|
Kesonbua W and Chantaranothai P: The genus
Morinda (Rubiaceae) in Thailand. Science Asia. 39:331–339.
2013.
|
|
15
|
World Health Organization: Quality control
methods for medicinal plant materials, ISBN 92 4 154510 0, Geneva,
1998.
|
|
16
|
Damasceno DC, Netto AO, Iessi IL, Gallego
FQ, Corvino SB, Dallaqua B, Sinzato YK, Bueno A, Calderon IM and
Rudge MV: Streptozotocin-induced diabetes models:
Pathophysiological mechanisms and fetal outcomes. Biomed Res Int.
2014(819065)2014.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Szkudelski T: The mechanism of alloxan and
streptozotocin action in B cells of the rat pancreas. Physiol Res.
50:537–546. 2001.PubMed/NCBI
|
|
18
|
Xiao XL, Wu JT, Zhang HZ, Wang YD, Zhang
JQ, Liu LF, Yu-Chen Min-Li, Yang PB, Wu XL and Liu JX: The
neurotoxic effect of isoflurane on age-defined neurons generated
from tertiary dentate matrix in mice. Brain Behav.
11(e01949)2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Charisma SL, Susilawati Y, Muhtadi A and
Sadino A: Separation of ethyl acetate fraction of mengkudu fruit
(Morinda citrifolia L.) and its hypoglycemic activity by
glucose tolerance method. Res J Chem Environ. 23:4–9. 2019.
|
|
20
|
Moko EM and Rahardiyan D: Structure of
Stigmasterols in Bran of Red Rice from Minahasa Regency, North
Sulawesi, Indonesia. Fullerene J Chem. 5(16)2020.
|
|
21
|
Singh MP and Pathak K: Animal models for
biological screening of anti-diabetic drugs: An overview. Euro J
Exp Bio. 5:37–48. 2015.
|
|
22
|
Qinna NA and Badwan AA: Impact of
streptozotocin on altering normal glucose homeostasis during
insulin testing in diabetic rats compared to normoglycemic rats.
Drug Des Devel Ther. 9:2515–2525. 2015.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Luippold G, Bedenik J, Voigt A and
Grempler R: Short- and longterm glycemic control of
streptozotocin-induced diabetic rats using different insulin
preparations. PLoS One. 11(e0156346)2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Raj A, Shuklan P, Madan P, Chauhan K,
Phogat J and Rani S: Comparative attenuating impact of camel milk
and insulin in streptozotocin-induced diabetic albino rats. ACS
Omega. 8:29270–29280. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Sales PM, Souza PM, Simeoni LA and
Silveira D: α-Amylase inhibitors: A review of raw material and
isolated compounds from plant source. J Pharm Pharm Sci.
15:141–183. 2012.PubMed/NCBI View
Article : Google Scholar
|
|
26
|
Kazeem MI, Adamson JO and Ogunwande IA:
Modes of inhibition of α-amylase and α-glucosidase by aqueous
extract of Morinda lucida Benth leaf. Biomed Res Int.
2013(527570)2013.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Wickramaratne MN, Punchihewa JC and
Wickramaratne DB: In-vitro alpha amylase inhibitory activity of the
leaf extracts of Adenanthera pavonina. BMC Complement Altern Med.
16(466)2016.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Lolok N, Ramadhan DSF, Sumiwi SA, Sahidin
I and Levita J: Molecular docking of β-Sitosterol and stigmasterol
isolated from Morinda citrifolia with α-amylase,
α-glucosidase, dipeptidylpeptidase-IV, and peroxisome
proliferator-activated receptor-γ. Rasayan J Chem. 15:20–30.
2022.
|
|
29
|
Indradevi S, Ilavenil S, Kaleeswaran B,
Srigopalram S and Ravikumar S: Ethanolic extract of Crinum
asiaticum attenuates hyperglycemia-mediated oxidative stress and
protects hepatocytes in alloxan induced experimental diabetic rats.
J King Saud Univer Sci. 24:171–177. 2012.
|
|
30
|
Ward MG, Li G, Barbosa-Lorenzi VC and Hao
M: Stigmasterol prevents glucolipotoxicity induced defects in
glucose-stimulated insulin secretion. Sci Rep.
7(9536)2017.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Panda S, Jafri M, Kar A and Meheta BK:
Thyroid inhibitory, antiperoxidative and hypoglycemic effects of
stigmasterol isolated from Butea monosperma. Fitoterapia.
80:123–126. 2009.PubMed/NCBI View Article : Google Scholar
|