Introduction
Bone disease is relatively common among patients
undergoing hemodialysis (HD) who suffer an increased frequency of
fractures (1). In routine clinical
practice, intact parathyroid hormone (iPTH) is the primary marker
that dictates the therapeutic maneuvers in chronic kidney disease
(CKD)-mineral bone disease (MBD). However, clinical studies have
demonstrated that the specificity and sensitivity of iPTH to detect
the nature of CKD-MBD are modest (2,3). In
addition, targeting iPTH levels mainly affects osteoclastic
activity, since PTH exerts its action on the bone mainly by
increasing the expression and, to a certain extent, the secretion
of receptor activator of nuclear factor-κB ligand (RANKL) by
osteoblasts. In turn, RANKL binds to RANK in osteoclasts, enhancing
their function (4).
Of note, in the general population and in patients
undergoing HD (5-8),
anthropometric indicators of nutritional status are positively
associated with increased bone loss, but are negatively associated
with fractures. In addition, decreased protein intake is associated
with reduced bone density. The latter has been attributed to the
reduced maturation and activity of osteoblasts (9-11).
Since the protein-energy wasting syndrome is prevalent among
patients undergoing HD (12), the
present study evaluated whether body mass index (BMI), a readily
available nutritional indicator, correlates with serum markers of
bone osteoblastic and osteoclastic activity in patients undergoing
HD.
In order to assess osteoblastic activity, the levels
of total procollagen type-1 aminoterminal propeptide (P1NP), a
product derived by the cleavage of procollagen produced by
osteoblasts (13), were measured.
In addition, the levels of osteocalcin (OC), another protein
produced by osteoblasts were measured. OC is the second most
abundant bone protein, next to collagen, and contributes to bone
mineralization (14). To assess
osteoclastic activity, the levels of β-isomerized C-terminal
cross-linked peptide of collagen type I (β-CTx), fragments of
collagen produced during its degradation by osteoclasts, were
measured (15). A recent study
demonstrated that the accuracy of the aforementioned markers in
detecting the nature of CKD-MBD is comparable to that of iPTH
(3).
Patients and methods
Patients
A total of 59 patients undergoing HD (mean age,
60.5±12.5 years; 40 males) participated in the present study. The
cause of end-stage renal disease was diabetes mellitus (DM) in 21
patients, primary glomerulonephritis in 9 patients, hypertension in
7 patients, interstitial nephritis in 5 patients, obstructive
nephropathy in 3 patients, autosomal dominant polycystic kidney
disease in 4 patients, and was unknown in 10 patients. All patients
were >18 years of age, and none of them suffered from any
malignancy or active infectious disease.
The patients underwent 4-h HD sessions, three times
a week, for at least 1 year prior to the study. Polysulfone
low-flux dialyzers and a bicarbonate dialysate containing 1.5
mmol/l calcium were used. The urea reduction ratio at the time of
the study was 65.4±7.4%. Serum calcium levels were 9.60±0.64 mg/dl,
serum phosphorous levels were 5.92±1.78 mg/dl and serum albumin
levels were 3.99±0.29 g/dl. All patients had anuria. None of the
patients suffered from any active infection, malignancy or
autoimmune disease, and none had a history of parathyroidectomy.
BMI was calculated using the following equation: BMI=body weight
(Kg): height (m)2.
None of the patients received corticosteroids,
cytotoxic drugs, warfarin, anticonvulsants, antidepressants,
hormone replacement therapy, bisphosphonates, or calcimimetics for
at least 6 months prior to the study. Sevelamer hydrochloride
(Renagel; Genzyme Europe) or lanthanum carbonate (Fosrenol; Shire
Pharmaceuticals Ltd.) were used as phosphate binders. A written
informed consent was obtained from each individual, and the Ethics
Committee of the University of Thessaly, Faculty of Medicine
approved the study protocol (approval no. 558/10-2-2017).
Blood sample analyses
Blood samples were obtained at the onset of the
second dialysis session of the week, and serum was stored at -80˚C.
Immunoassays for measuring P1NP, N-terminal midfragment OC, β-CTx,
iPTH and 25-hydroxy-vitamin D (25(OH)D) were performed using an
ELECSYS 2010 automatic analyzer (Roche Diagnostics GmbH).
Statistical analysis
IBM SPSS Statistics for Windows software, version 26
(IBM Corp.) was used for the statistical analysis. The one-sample
Kolmogorov-Smirnov test was used to evaluate whether the variables
were normally distributed. Apart from 25(OH)D, the variables did
not follow a normal distribution. Thus, non-parametric tests were
used for further statistical analyses. Spearman's Rho correlation
coefficient was calculated for detecting correlations, and the
Mann-Whitey U test was used for comparing values. Linear regression
with robust standard error estimation analysis was used to evaluate
the independent effect of BMI on the osteoblastic or osteoclastic
markers (16). The results are
expressed as the median (interquartile range), and a two-sided
P<0.05 was considered to indicate a statistically significant
difference.
Results
The median, ranges and the interquartile range
values of the evaluated factors are depicted in Table I. The correlations between BMI and
the evaluated biochemical markers of bone metabolism are presented
in Table II.
 | Table IMedian, range and interquartile range
values of the evaluated variables. |
Table I
Median, range and interquartile range
values of the evaluated variables.
| Variable | Median | Range | Interquartile
range |
|---|
| Age (years) | 65 | 49 | 18 |
| BMI | 24.57 | 28.40 | 7.40 |
| P1NP (ng/ml) | 267.60 | 857.29 | 208.20 |
| OC (ng/ml) | 221.00 | 755.58 | 210.40 |
| β-CTx (ng/ml) | 1.89 | 4.47 | 1.35 |
| iPTH (pg/ml) | 181.00 | 1083.05 | 198.47 |
| 25(OH)D (ng/ml) | 21.64 | 19.66 | 17.41 |
 | Table IICorrelations among the evaluated
variables. |
Table II
Correlations among the evaluated
variables.
| Variable | | Age, years | BMI | P1NP | OC | β-CTx | iPTH |
|---|
| Age, years | Rho | | | | | | |
| | P-value | | | | | | |
| BMI | Rho | 0.076 | | | | | |
| | P-value | 0.567 | | | | | |
| P1NP | Rho | -0.226 | -.430 | | | | |
| | P-value | 0.085 | <0.001 | | | | |
| OC | Rho | -0.523 | -0.356 | 0.769 | | | |
| | P-value | <0.001 | 0.006 | <0.001 | | | |
| β-CTx | Rho | -0.475 | -0.245 | 0.543 | 0.731 | | |
| | P-value | <0.001 | 0.061 | <0.001 | <0.001 | | |
| iPTH | Rho | -0.306 | -0.053 | 0.338 | 0.525 | 0.620 | |
| | P-value | 0.019 | 0.693 | 0.009 | <0.001 |
<0.001 | |
| 25(OH)D | Rho | -0.058 | 0.062 | -0.229 | -0.172 | -0.035 | 0.076 |
| | P-value | 0.665 | 0.642 | 0.08 | 0.194 | 0.793 | 0.566 |
BMI was not associated with the age of the patients.
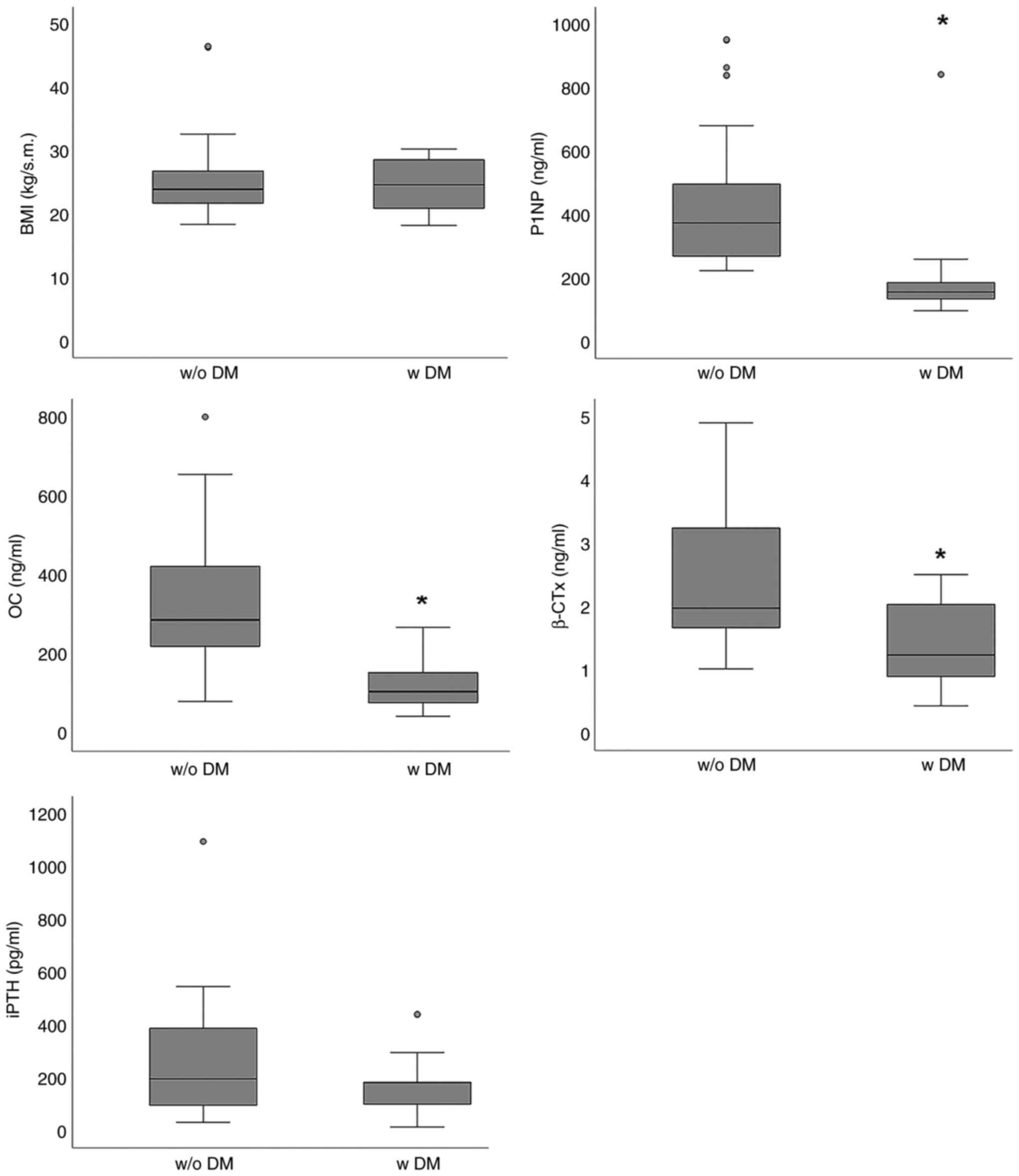
In addition, BMI did not differ markedly among the patients
undergoing HD with or without DM [24.62 (8.25) vs. 23.89 (4.74) in
patients with or without DM, respectively, P=0.868] (Fig. 1).
However, age correlated with OC (Rho -0.523,
P<0.001), β-CTx (Rho -0.475, P<0.001) and iPTH (Rho -0.306,
P=0.019) (Table II). From the
evaluated biochemical markers, diabetes mellitus affected OC, P1NP
and β-CTx. OC levels were lower in patients with DM [106.0 (77.2)
vs. 286.9(173) ng/ml; P<0.001], P1NP1 levels were lower in
patients with DM [154.6 (73.1) vs. 372.9 (250.9) ng/ml; P<0.01]
and β-CTx levels were also lower in patients with DM [1.23 (1.22)
vs. 1.97 (1.68) ng/ml; P<0.001], whereas the iPTH levels did not
differ significantly between patients with or without DM [180.1
(126.9) vs. 195.1 (237.2) pg/ml; P=0.359] (Fig. 1).
Contrary to patients' age, OC, P1NP and β-CTx levels
did not differ according to the patients' sex. OC levels were 228.4
(105.3) ng/ml in males and 219.2 (134.9) ng/ml in females
(P=0.903). P1NP levels were 257.2 (167.7) ng/ml in males and 285.2
(226.6) ng/ml in females (P=0.604). Finally, β-CTx levels were 1.85
(1.27) ng/ml in males and 1.89 (1.23) ng/ml in females (P=0.916)
(Table III). As regards 25(OH)D,
its serum concentration was 21.64 (17.41) ng/ml and it did not
correlate with OC (Rho=-0.172, P=0.194), P1NP (Rho=-0.229, P=0.08),
or β-CTx (Rho=-0.035 P=0.793) (Table
II).
 | Table IIIMedian (interquartile range) values
of OC, P1NP and β-CTx in male and female patients. |
Table III
Median (interquartile range) values
of OC, P1NP and β-CTx in male and female patients.
| Variable | Males | Females | P-value |
|---|
| OC (ng/ml) | 228.4 (105.3) | 219.2 (134.9) | 0.903 |
| P1NP (ng/ml) | 257.2 (167.7) | 285.2 (226.6) | 0.604 |
| β-CTx (ng/ml) | 1.85 (1.27) | 1.89 (1.23) | 0.916 |
Furthermore, linear regression with robust standard
error estimation analysis was applied to evaluate whether the
nutritional indicator, BMI, affects the osteoblastic markers, P1NP
and OC, independently of age and DM. The usual marker of CKD-MBD
and regulator of bone metabolism, iPTH, was also included in the
model, since it correlated with P1NP and OC. Regression analysis
revealed that BMI affected the P1NP and OC levels independently of
age, DM or iPTH (Table IV).
 | Table IVUnivariate linear regression analysis
with robust standard errors estimation analysis on the effect of
BMI, age, iPTH and DM on P1NP or OC. |
Table IV
Univariate linear regression analysis
with robust standard errors estimation analysis on the effect of
BMI, age, iPTH and DM on P1NP or OC.
| A, Dependent
variable: P1NP |
|---|
| Parameter | B | Robust S.E. | t value | P-value |
|---|
| Intercept | 917.401 | 145.004 | 6.327 |
<0.001 |
| BMI | -17.332 | 3.267 | -5.305 |
<0.001 |
| Age | -2.574 | 1.678 | -1.534 | 0.131 |
| DM | -206.667 | 38.429 | -5.378 |
<0.001 |
| iPTH | 0.421 | 0.205 | 2.057 | 0.045 |
| R-squared=0.563
(adjusted R-squared=0.530) P<0.001. |
| B, Dependent
variable: OC |
| Parameter | B | Robust S.E. | t value | P-value |
| Intercept | 729.272 | 102.787 | 7.095 |
<0.001 |
| BMI | -9.739 | 1.257 | -7.745 |
<0.001 |
| Age | -4.680 | 1.161 | -4.032 |
<0.001 |
| DM | -155.544 | 18.510 | -8.403 |
<0.001 |
| iPTH | 0.491 | 0.053 | 9.186 |
<0.001 |
| R-squared=0.802
(adjusted R-squared=0.787) P<0.001. |
Discussion
Triggered by studies demonstrating an association
between nutritional status and the incidence of fractures in
patients undergoing HD (6-8),
the present study evaluated the correlation between nutritional
status and serum markers of osteoblastic or osteoclastic activity
in patients undergoing HD. The present study used the readily
available nutritional marker, BMI, widely used in the general
population and patients undergoing HD.
To evaluate osteoblastic activity, the P1NP and OC
levels were assessed (13,14), while for osteoclastic activity,
β-CTx levels were assessed (15).
These factors have a similar predictive value with iPTH, the
primary marker used in clinical practice to classify the nature of
CKD-MBD (3). To strengthen the
reliability of the results, patients undergoing HD who had anuria
were enrolled, since P1NP and β-CTx are subjected to renal
clearance (3).
BMI inversely correlated with the osteoblastic
markers, P1NP and OC, whereas no correlation was detected with the
osteoclastic marker, β-CTx. Thus, a non-optimal nutritional status
in patients undergoing HD may contribute to CKD-MBD by decreasing
osteoblastic activity. Experimental and clinical studies in other
populations detected such a correlation and incriminated the
diminishing insulin-like growth factor-1 (IGF-1) level due to a low
protein intake (9-11).
In patients undergoing HD, a low IGF-1 level is associated with
malnutrition, reduced bone mineral density and mortality (17). Protein-energy wasting syndrome is
prevalent in patients undergoing HD (12), and malnutrition is a cause of
adynamic bone disorder (18),
which is characterized by a reduced number of osteoblasts (19). Further more extensive studies are
required however, to confirm the causality between the nutritional
status and osteoblastic activity. This would lead to the
development of novel therapeutic strategies for CKD-MBD. Contrary
to current practice, which targets iPTH and osteoclastic activity,
these interventions will target osteoblastic activity.
In the present study, an intercorrelation between
iPTH and all the aforementioned osteoblastic and osteoclastic
markers was detected. These correlations are likely the result of
the coupled osteoclastic and osteoblastic activity. In the
bone-forming units, osteoblasts assemble only when iPTH-driven
osteoclasts have completed resorption. The result is a new packet
of bone that replaces the removed older bone (19).
In the present study, the serum 25(OH)D level was
21.84±5.24 ng/ml. Thus, 25(OH)D was below the recommended lower
limit of 30 ng/ml in the majority of patients. A high incidence of
25(OH)D insufficiency is common among patients undergoing HD
(20). Notably, in the present
study 25(OH)D was not correlated with OC, P1NP or β-CTx. Previous
studies have also demonstrated a lack of an association between
serum 25(OH)D levels and markers of osteoblastic or osteoclastic
activity (21,22).
In the present study, BMI was not associated with
the patients' age. In addition, the BMI values did not differ among
the patients undergoing HD with or without DM. In addition, BMI was
not correlated with iPTH. However, age and DM affect bone
metabolism in patients undergoing HD, and adynamic bone disease is
more common among elderly and diabetic patients undergoing HD
(23). Indeed, in the present
study, age negatively correlated with OC, β-CTx and iPTH in the
patients undergoing HD. Furthermore, the patients with DM
undergoing HD had lower median OC, P1NP and β-CTX values. Of note,
in the present cohort of patients undergoing HD, and contrary to
patients' age, the OC, P1NP and β-CTx levels did not differ
according to the patients' sex. The latter may result from heavily
disrupted bone metabolism that characterizes patients undergoing
HD, which may prevail to hormonal sex differences.
Regression analysis revealed that BMI determines
P1NP and OC levels significantly and independently of age, DM and
the levels of iPTH. As it is already known, CKD-MBD significantly
contributes to mortality in patients undergoing HD (24). Hence, to a certain extent, the
effect of nutritional status on bone metabolism detected in the
present study may explain the reverse epidemiology of the higher
the BMI, the lower the mortality observed in this population
(25).
Certainly, the findings obtained herein require
validation from further studies using larger cohorts of patients
undergoing HD. Additionally, the simultaneous assessments of bone
structure alongside measurements of bone mineral density or bone
biopsy with histological examinations, which were not conducted in
the present study, would provide greater clarity on this matter. In
conclusion, BMI inversely correlates with markers of osteoblastic
activity in patients undergoing HD. Further studies are warranted
to confirm causality in this correlation. This could lead to the
development of strategies with which to enhance osteoblastic
activity by improving nutritional status in patients with
CKD-MBD.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
TE designed the study. TE, GA, GP, EN and IS
contributed to the acquisition, analysis and interpretation of the
data. TE wrote the manuscript. TE and GA confirm the authenticity
of all the row data. All authors drafted the manuscript, critically
revised the manuscript, agree to be fully accountable for ensuring
the integrity and accuracy of the work, and have read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study was conducted according to the
Declaration of Helsinki, and was approved by the Ethics Committee
of the University of Thessaly, Faculty of Medicine (approval no.
558/10-2-2017). A written informed consent was obtained from all
subjects involved in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tentori F, McCullough K, Kilpatrick RD,
Bradbury BD, Robinson BM, Kerr PG and Pisoni RL: High rates of
death and hospitalization follow bone fracture among hemodialysis
patients. Kidney Int. 85:166–173. 2014.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Qi Q, Monier-Faugere MC, Geng Z and
Malluche HH: Predictive value of serum parathyroid hormone levels
for bone turnover in patients on chronic maintenance dialysis. Am J
Kidney Dis. 26:622–631. 1995.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Salam S, Gallagher O, Gossiel F, Paggiosi
M, Khwaja A and Eastell R: Diagnostic accuracy of biomarkers and
imaging for bone turnover in renal osteodystrophy. J Am Soc
Nephrol. 29:1557–1565. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Boyce BF and Xing L: Functions of
RANKL/RANK/OPG in bone modeling and remodeling. Arch Biochem
Biophys. 473:139–146. 2008.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Rizzoli R, Biver E and Brennan-Speranza
TC: Nutritional intake and bone health. Lancet Diabetes Endocrinol.
9:606–621. 2021.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Chen SC, Chung WS, Wu PY, Huang JC, Chiu
YW, Chang JM and Chen HC: Associations among Geriatric Nutrition
Risk Index, bone mineral density, body composition and handgrip
strength in patients receiving hemodialysis. Nutrition. 65:6–12.
2019.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Lee H, Kim K, Ahn J, Lee DR, Lee JH and
Hwang SD: Association of nutritional status with osteoporosis,
sarcopenia, and cognitive impairment in patients on hemodialysis.
Asia Pac J Clin Nutr. 29:712–723. 2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Yamada S, Arase H, Yoshida H, Kitamura H,
Tokumoto M, Taniguchi M, Hirakata H, Tsuruya K, Nakano T and
Kitazono T: Malnutrition-Inflammation complex syndrome (MICS) and
bone fractures and cardiovascular events in patients undergoing
hemodialysis: The Q-Cohort study. Kidney Med.
4(100408)2022.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tahimic CG, Wang Y and Bikle DD: Anabolic
effects of IGF-1 signaling on the skeleton. Front Endocrinol
(Lausanne). 4(6)2013.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Bonjour JP: The dietary protein, IGF-I,
skeletal health axis. Horm Mol Biol Clin Investig. 28:39–53.
2016.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Zanker CL and Cooke CB: Energy balance,
bone turnover, and skeletal health in physically active
individuals. Med Sci Sports Exerc. 36:1372–1381. 2004.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Kovesdy CP and Kalantar-Zadeh K: Why is
protein-energy wasting associated with mortality in chronic kidney
disease? Semin Nephrol. 29:3–14. 2009.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Ebeling PR, Peterson JM and Riggs BL:
Utility of type I procollagen propeptide assays for assessing
abnormalities in metabolic bone diseases. J Bone Miner Res.
7:1243–1250. 1992.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Takahashi M, Kushida K, Nagano A and Inoue
T: Comparison of the analytical and clinical performance
characteristics of an N-MID versus an intact osteocalcin
immunoradiometric assay. Clin Chim Acta. 294:67–76. 2000.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Okabe R, Nakatsuka K, Inaba M, Miki T,
Naka H, Masaki H, Moriguchi A and Nishizawa Y: Clinical evaluation
of the Elecsys beta-CrossLaps serum assay, a new assay for
degradation products of type I collagen C-tlopeptides. Clin Chem.
47:1410–1414. 2001.PubMed/NCBI
|
|
16
|
Hayes AF and Cai L: Using
heteroskedasticity-consistent standard error estimators in OLS
regression: An introduction and software implementation. Behav Res
Methods. 39:709–722. 2007.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Jia T, Gama Axelsson T, Heimbürger O,
Bárány P, Lindholm B, Stenvinkel P and Qureshi AR: IGF-1 and
Survival in ESRD. Clin J Am Soc Nephrol. 9:120–127. 2014.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Haarhaus M and Evenepoel P: European Renal
Osteodystrophy (EUROD) workgroup; Chronic Kidney Disease Mineral
and Bone Disorder (CKD-MBD) working group of the European Renal
Association–European Dialysis and Transplant Association
(ERA-EDTA). Differentiating the causes of adynamic bone in advanced
chronic kidney disease informs osteoporosis treatment. Kidney Int.
100:546–558. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Manolagas SC: Birth and death of bone
cells: Basic regulatory mechanisms and implications for the
pathogenesis and treatment of osteoporosis. Endocr Rev. 21:115–137.
2000.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Gonzalez EA, Sachdeva A, Oliver DA and
Martin KJ: Vitamin D insufficiency and deficiency in chronic kidney
disease. A single center observational study. Am J Nephrol.
24:503–510. 2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Jørgensen HS, Winther S, Bøttcher M, Hauge
EM, Rejnmark L, Svensson M and Ivarsen P: Bone turnover markers are
associated with bone density, but not with fracture in end stage
kidney disease: A cross-sectional study. BMC Nephrol.
18(284)2017.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Lv J, Xie W, Wang S, Zhu Y, Wang Y, Zhang
P and Chen J: Associated factors of osteoporosis and vascular
calcification in patients awaiting kidney transplantation. Int Urol
Nephrol: Apr 24, 2023 (Epub ahead of print).
|
|
23
|
Brandenburg VM and Floege J: Adynamic bone
disease-bone and beyond. NDT Plus. 1:135–147. 2008.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Li D, Zhang L, Zuo L, Jin CG, Li WG and
Chen JB: Association of CKD-MBD markers with all-cause mortality in
prevalent hemodialysis patients: A cohort study in beijing. PLoS
One. 12(e0168537)2017.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Leavey SF, McCullough K, Hecking E,
Goodkin D, Port FK and Young EW: Body mass index and mortality in
‘healthier’ as compared with ‘sicker’ haemodialysis patients:
Results from the dialysis outcomes and practice patterns study
(DOPPS). Nephrol Dial Transplant. 16:2386–2394. 2001.PubMed/NCBI View Article : Google Scholar
|