Introduction
Malaria represents a significant global public
health issue that affects a substantial percentage of the world's
population, particularly those residing in tropical and subtropical
regions. Despite the availability of effective antimalarial drugs
and insecticides, individuals remain at risk, particularly in
low-income countries where infection rates remain high. In Africa,
for instance, these countries continue to record the highest
incidence of infections. Several species of the parasite contribute
to human infection, with similar life cycles, but varying abilities
to persist in the human body with relapses or recrudescence
(1). Relapses are episodes caused
by Plasmodium vivax or Plasmodium ovale, whose
symptoms and asexual stages appear months to years after the
primary infection has cleared due to exoerythrocytic stages
(hypnozoites). According to the World Health Organization (WHO)
malaria terminology, a relapse is the recurrence of asexual
parasitemia in Plasmodium vivax and Plasmodium ovale
malaria that results from persisting liver stages (2). By contrast, recrudescence is the
recurrence of asexual parasitemia of the same genotype(s) that
caused the original illness following inadequate or ineffective
antimalarial treatment (3).
Recrudescence is therefore considered the result of the incomplete
clearance of asexual stages of the parasite and has only been shown
to occur with Plasmodium malariae and Plasmodium
falciparum (P. falciparum) (4). Among the plasmodia, P.
falciparum is the most dangerous due to its association with
severe clinical complications, and high morbidity and mortality
rates (1). The duration of P.
falciparum infections cannot be accurately determined and has
been controversial since the first health policy on eradication was
applied in the 1950s. Although these infections typically last no
longer than 1 year, some case reports (please see Table I) have documented suspected
recrudescence episodes within one to 13 years following primary
infection parasite clearance (5).
P. falciparum recrudescence may be caused by incomplete
anti-malarial treatment (6).
 | Table ISummary of Plasmodium
falciparum malaria cases identified in the literature which
occurred after several months or years after the last exposure. |
Table I
Summary of Plasmodium
falciparum malaria cases identified in the literature which
occurred after several months or years after the last exposure.
| First author | Time since last
exposure | Patients' risk
factors | Microscopic
evidence | (Refs.) |
|---|
| Nagley | 13-17 years | Inadequate
antimalarial treatment during previous infections | Trophozoites and
gametocytes | (24) |
| Walters | 19 months | Pregnancy | Trophozoites | (25) |
| Russell | 4 years | Unclear | Trophozoites | (26) |
| Revel | 3 years | HIV infection;
pregnancy | Trophozoites and
gametocytes | (27) |
| Kyrönseppä | 20 months | Head injury | Trophozoites | (10) |
| Krajden | 32 months | Diabetes | Trophozoites | (11) |
| Shah | 15 years | Unclear | Trophozoites | (28) |
| Giobbia | 4 years | Pregnancy | Trophozoites | (12) |
| Howden | 9 years | Previous history of
abdominal surgery | Trophozoites | (29) |
| Greenwood | 4 years | Sickle cell
disease | Trophozoites | (13) |
| D'Ortenzio | 7 months (1 case);
1 year (5 cases); 9 years (5 cases). | HIV infection (5
cases); pregnancy (5 cases); unclear (1 case). | Trophozoites | (30) |
| Theunissen | 9 years | Unclear | Trophozoites | (14) |
| Foca | 11 months | Cancer | Gametocytes | (31) |
| Szmitko | 8 years | Unclear | Trophozoites | (32) |
| Mali | 2 years (1 case); 3
(1 case); 4 years (1 case). | Unclear | Trophozoites | (33) |
| Cullen | 3 years | Unclear | Trophozoites | (34) |
| Monge-Maillo | 13 months (1 case);
14 months (1 case); 28 months (1 case) | Unclear | Trophozoites | (35) |
| Berrevoets | 2.5 years | Unclear | Trophozoites and
schizonts | (36) |
The present study describes the case of a young male
patient with potential P. falciparum recrudescence who
experienced symptoms 12 years after his last exposure to the
Plasmodium genus parasite.
Case report
A 32-year-old male patient from Chad presented at
the Umberto I Hospital (Siracusa, Southern Italy) Emergency
Department with a fever of up to 38.5˚C, chills, and general
discomfort. He had moved to Italy in 2009 and had not returned to
his home country since. Furthermore, he did not document any
travels to other malarial endemic countries. The patient reported a
not otherwise specified anti-malarial treatment for a
microscopically diagnosed episode of P. falciparum malaria
(2005) in a non-specified hospital in Chad and was monitored for
suspected mixed connective tissue disease. He denied exposure to or
contact with individuals from malaria-endemic areas, travel or stay
in airports, as well as other risk factors, such as blood
transfusions, organ transplantations, or intravenous drug use.
Moreover, the patient documented that his residential city
neighborhood was in a non-endemic area for malaria (7) and far from airports. He confirmed the
absence of any professional risk factors, noticing his profession
(warehouse worker in a shopping center). Upon his admission (May 1,
2021), he was febrile (temperature, 38˚C), his blood pressure was
140/70 mmHg, his heart rate was 110 bpm, his oxygen saturation in
room air was 96% and his respiratory rate was 20 breaths/min. The
Glasgow Coma Scale was 15 (indicating that he was fully awake,
responsive and with no issues in cognitive ability or memory). A
physical examination revealed mild abdominal pain and bilateral
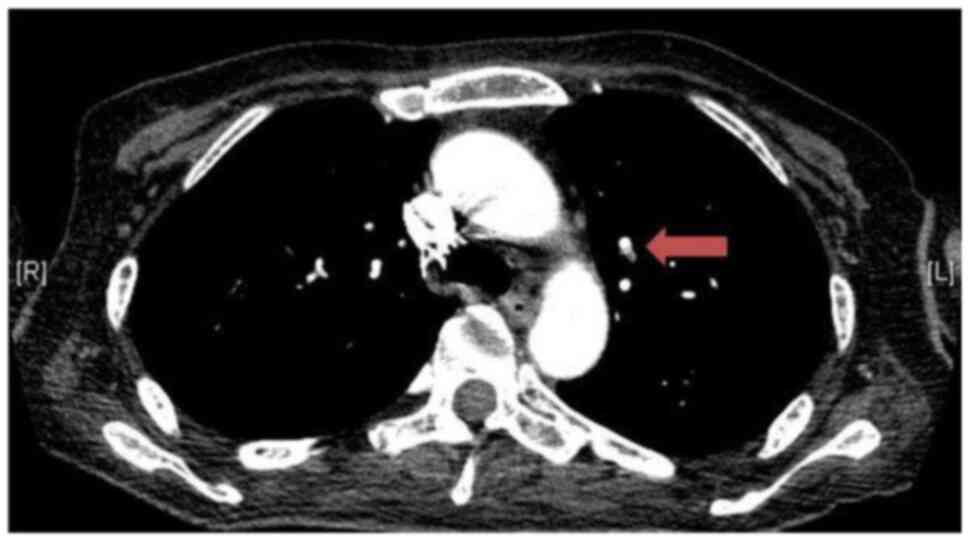
crackling breath sounds. A chest X-ray and subsequent thorax
computed tomography (CT) scan documented pulmonary microembolism
(Fig. 1), while an abdomen CT scan
revealed hepatomegaly and mesenteric lymphadenopathy (these images
are not available). Laboratory data revealed mild anemia
(hemoglobin, 10.6 g/dl; normal values, 14-16.5 g/dl) with a reduced
red blood cell count (3.55x106/mm3; normal
values, 4.52-5.90x106/mm3); his white blood
cell count was 11,100/mm3 (normal values,
4,000-11,000/mm3) with monocytosis
(1,500/mm3); his platelet count was
100,000/mm3 (normal values,
150,000-400,000/mm3); the levels of inflammatory markers
were elevated: C-reactive protein, 111 mg/l (normal values 0-5
mg/l); erythrocyte sedimentation rate, 100 mm/h (normal values,
<20 mm/h); procalcitonin level, 2 mcg/l (normal values, <0.1
mcg/l); and D-dimer level, 4,000 ng/ml (normal values, <500
ng/ml). The levels of transaminases (glutamic oxaloacetic
transaminase, 38 U/l; glutamic pyruvic transaminase, 40 U/l; normal
values, <45 U/l) and bilirubin (1 mg/dl; normal values, <1.8
mg/dl) were normal, as well as those of coagulation parameters
(international normalized ratios 0.9; normal values, <1.2). His
creatinine level was 0.9 mg/dl with an estimated glomerular
filtration rate of 131 ml/min. The patient also presented with high
ferritin levels (1,000 ng/ml). Glucose levels were normal (88
mg/dl; normal values, <100 mg/dl). The patient tested negative
for human immunodeficiency virus (HIV), hepatitis B and C virus, as
well as Epstein-Barr virus, Toxoplasma and cytomegalovirus.
Further tests for Brucella, Salmonella and
Treponema pallidum were negative. The autoimmune panel
revealed positive results for antinuclear antibody (ANA) and
extractable nuclear antigen (ENA) 1:160. Empiric antibiotic therapy
with intravenous ceftriaxone was administered along with
anticoagulant treatment based on enoxaparin. Given the patient's
origin, it was deemed appropriate to investigate the persistence of
malaria. A rapid diagnostic test for malaria (Bioline™ Malaria Ag
P.f., Abbott Pharmaceutical Co. Ltd.) yielded positive results for
pan-malarial aldolase antigen and P. falciparum
histidine-rich protein 2. Subsequently, microscopical examinations
were performed. The microscopical assay required a 2.5% Giemsa
stain (Kaltek S.r.l.) for the thick film, while the thin film was
performed using a 10% Giemsa stain (Kaltek S.r.l.). Buffered water
(pH 7.2-7-3) was used to remove unnecessary stain drops after the
first coloration steps. Both the Giemsa stain and the buffered
water were stored at 25˚C before their application within the
diagnostic protocol. The microscopic examination of the thick film
confirmed the presence of a Plasmodium genus parasite with a
parasitemia of 4,0 asexual parasites/µl. Specifically, a total
number of 200 leukocytes were counted within 200 microscopic
fields.
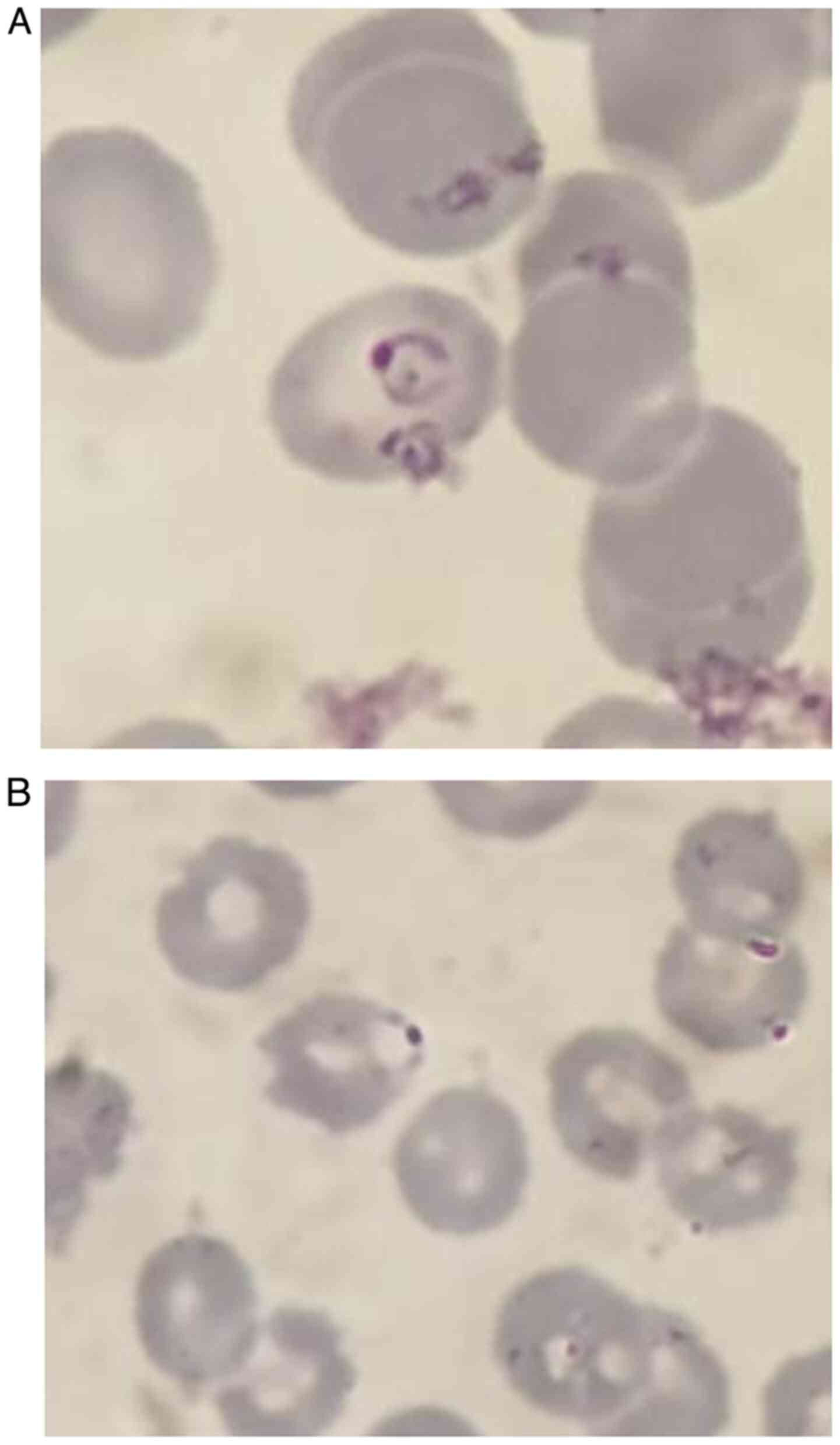
The thin smear (Fig.
2) revealed ring-shaped trophozoites, normal-sized erythrocytes
and rare banana-shaped gametocytes. P. falciparum malaria
was diagnosed. This result was confirmed by two further tests,
performed at 8 and 12 h following the first test and the beginning
of anti-malarial treatment. Specifically, anti-malarial treatment
with piperaquine/dihydroartemisinin at 300/40 mg/day for 3 days was
administered. After 24 h, malaria parasitemia was re-evaluated
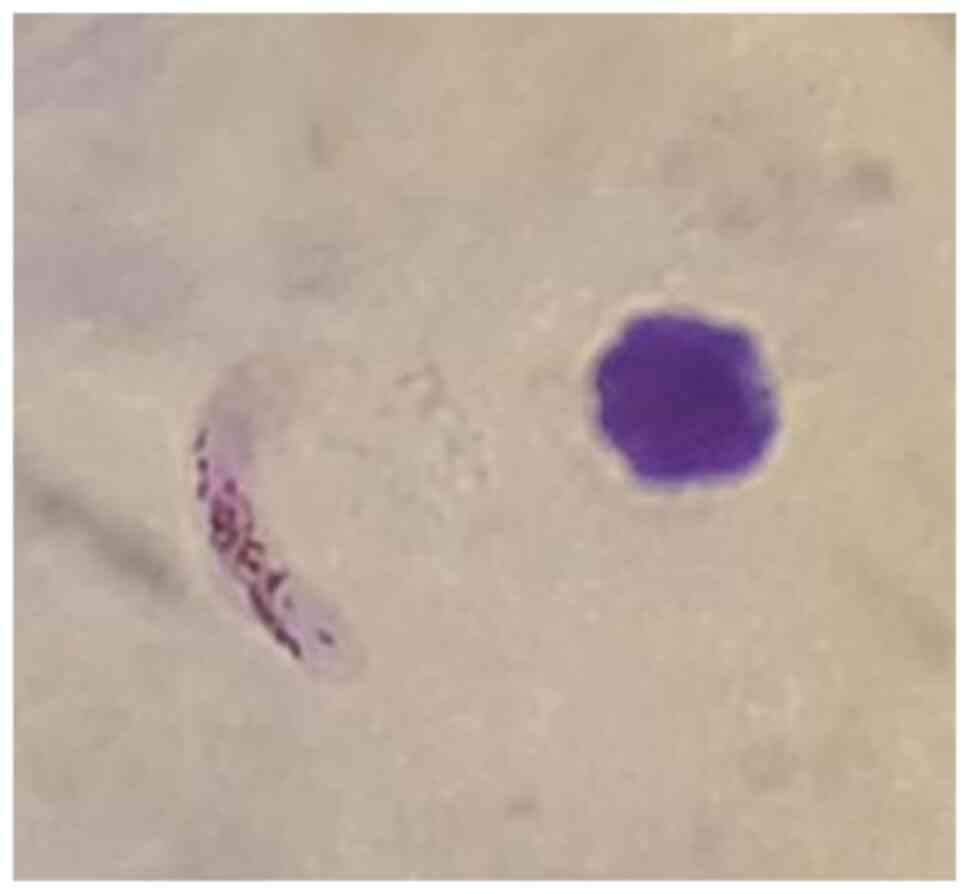
revealing only the presence of P. falciparum banana-shaped
gametocytes (Fig. 3). The fever
disappeared together with clinical improvement and normalization of
inflammatory markers. At 48 h following the initiation of
anti-malarial therapy, a new blood sample was taken resulting in a
completely negativity for malaria infection. Within 10 days, the
patient appeared to be clinically recovered and was discharged. At
2 weeks after discharge, thoracic and abdominal CT scans (images
are not available) revealed no clinical abnormalities, indicating
the complete resolution of the previous pulmonary microembolism.
Laboratory tests documented a complete recovery. The patient was
then referred by the rheumatology specialist due to the suspicion
of mixed connective tissue disease (as suggested by ANA and
ENA).
Discussion
Malaria is currently one of the most significant
global threats, and numerous international studies have been
conducted to highlight the challenging spread and evolution of this
infectious disease (8).
Plasmodium species that infect humans follow a similar
multistage life cycle, characterized mainly by two stages: An
initial liver stage followed by proliferation in the blood
(1). The clinical impact of
malaria varies among different species, with P. falciparum
being considered the most dangerous due to its ability to cause
severe complications, such as cerebral malaria and critical
sequelae, resulting in high morbidity and mortality rates in both
adult and pediatric patients. Additionally, Plasmodium
species may differ in their persistence modes (1).
It has been documented that P. falciparum can
cause recrudescence, even a decade after the last exposure, despite
not having the ability to produce hypnozoites. Inadequate or
incomplete anti-malarial treatment is the main risk factor for
recrudescence. Incomplete anti-malarial treatment hinders the
clearance of parasites and modulates parasitemia. According to
several studies, 32 cases of P. falciparum malaria were
diagnosed in patients who had left an endemic area 7 months to 15
years prior (9-13).
Of these cases, three were solely related to P. falciparum
gametocytaemia observed on blood films.
According to the literature (9-14),
these reports suggested that some patients were able to easily
clear the infection themselves. Out of the 32 cases reported, 5
patients had taken anti-malarial medication in the previous months,
while 13 cases were associated with comorbidities, pregnancy, or
trauma. A summary of the cases of P. falciparum malaria
mentioned above, occurring months or years after the last exposure
is presented in Table I.
Based on the patient's medical history, it appears
that a previous malarial episode was not properly treated due to
incomplete intake or ineffectiveness of the prescribed
anti-malarial treatment. It is reasonable to assume that the
patient's current infection was caused by P. falciparum,
which was the predominant species in Chad between 2005 and 2010
(2-4,15).
Other potential risk factors such as diabetes, HIV infection,
anemia, and head injury have been ruled out. Therefore, physical
stress and incomplete antimalarial treatment are the only factors
that could be related to the patient's current condition.
The scientific literature on P. falciparum
has provided evidence of subclinical infections that can keep
patients at risk of developing recrudescence and becoming a
reservoir for malaria mosquitoes. When there is suspicion of a
recrudescence episode, specific attention is required in the
malaria diagnostic process (16).
Moreover, data confirm the importance of monitoring countries that
have achieved malaria elimination carefully. Patients with
asymptomatic infections can represent a silent reservoir for the
survival of the parasite in some countries. Therefore, it is
recommended to perform supervision for 3 years after declaring
malaria elimination in a previous malaria-endemic country (9).
The P. falciparum parasites constantly affect
the human immune system through various mechanisms to gain benefits
for their survival (17).
Scientific data have also suggested an association between the
persistence of P. falciparum and the presence of antigenic
variants of the parasite (18).
P. falciparum attempts an immune escape strategy by
producing antigenic variants different from the original infectious
strain. These variants have differential expression levels of
var genes that encode PfEMP1 proteins. Although some
variants may evade the immune response, they are less able to bind
receptors and form erythrocytic adhesions. It may be hypothesized
that less virulent antigenic variants can persist post-clearance
with minimal or no clinical manifestations. It is reasonable to
assume that some variants reduce their fitness to allow long-term
persistence in the human host (18). This could provide a basis for
further studies, as ideally, less virulent P. falciparum
variants should be investigated in every doubtful case of malaria
(19).
Some cases of suspected P. falciparum
recrudescence can be categorized as cryptic malaria. This term is
defined by the Centers for Disease Control and Prevention (CDC) as
‘a case of malaria where epidemiologic investigations fail to
identify an apparent mode of acquisition (this term applies mainly
to cases found in non-endemic countries)’ (20). Cryptic malaria can occur when there
is an unreliable patient travel history, diagnostic delays,
suspected importation of an infected mosquito, or transmission
through contact with infected blood or tissue (3,21).
However, in the case described in the present study,
risky conditions such as transfusions, transplantations,
intravenous drug intake, airport proximity, travel and contact with
individuals from endemic areas were ruled out by the patient. The
objective was to investigate a doubtful case where the origin is
uncertain and to determine whether it is a case of cryptic malaria
or a recrudescence of P. falciparum.
To achieve this goal, the patient's epidemiological
and clinical history was examined. He had left Chad, an endemic
region for malaria, 12 years prior, and was currently presenting
with high procalcitonin and parasitemia with P. falciparum.
It is well known that procalcitonin levels can be elevated during
malaria, particularly when there is a long interval between the
first symptoms and the diagnosis (22). Thus, the high procalcitonin values
of the patient described herein, without any signs of sepsis
supported the suspicion of malaria infection. Furthermore, it was
suspected that the patient may have experienced transient
immunodepression due to a stressful life situation (a business
trip) and a suspected mixed connective tissue disease.
A positive blood culture several days following a
diagnosis of malaria never developed into overt sepsis, and no
further positive tests followed. Therefore, it was concluded that
the high procalcitonin level may be related to the malaria episode
rather than bacteremia. The patient firmly denied all possible
predisposing factors for cryptic malaria. As a result, the authors
are confident that this may be one of the rare cases of P.
falciparum recrudescence with low parasitemia, 12 years after
the patient's last exposure. In summary, the predisposition to
believe in a P. falciparum recrudescence could be supported
by previous literature data about insufficient antimalarial
treatment and parasite recrudescence after years.
In conclusion, the case presented herein underscores
the importance of screening for malaria when faced with fever of
unknown origin (FUO). Moreover, it highlights the need to consider
malaria persistence as a potential diagnosis in certain patients.
Fortunately, blood sampling and malaria diagnostics are simple and
cost-effective procedures that can yield invaluable information
without breaking the bank. Notably, the current guidelines from the
Infectious Diseases Society of America (IDSA) recommend a physical
examination, imaging tests and laboratory analyses, such as
surveillance and blood cultures for the management of FUO (23). It is suggested that this algorithm
be augmented with routine blood sampling for malaria diagnosis.
Given the diverse mechanisms through which malaria can be
transmitted, not all of which are easily recognizable, it is
crucial to include this test in the workup of all FUO cases. This
protocol expansion is necessary to ensure prompt and accurate
diagnosis, and ultimately, improved patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Data sharing is not applicable to this article as no
datasets were generated or analyzed during the current study.
Authors' contributions
All authors (AM, MC, GM, EdC, AF, ElC, LT and IP)
contributed to the study conception and design.
GM was involved in the study methodology. AF and EC
were involved in examining the patient and the patient's data. LT
was involved in data curation. AM and MC were involved in the
writing and preparation of the original draft. IP was involved in
the writing, review and editing of the manuscript and supervised
the study. All authors have read and approved, and have agreed to
the published version of the final manuscript. LT and IP confirm
the authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was obtained from the
patient for the inclusion of his data in the present study.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication his data and any related images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sato S: Plasmodium-a brief introduction to
the parasites causing human malaria and their basic biology. J
Physiol Anthropol. 40(1)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
World Health Organization: WHO Malaria
terminology, 2021 update. https://www.who.int/publications/i/item/9789240038400.
Accessed September 10, 2023.
|
|
3
|
Malvy D, Torrentino-Madamet M, L'Ollivier
C, Receveur MC, Jeddi F, Delhaes L, Piarroux R, Millet P and
Pradines B: Plasmodium falciparum Recrudescence two years
after treatment of an uncomplicated infection without return to an
area where malaria is endemic. Antimicrob Agents Chemother.
62:e01892–17. 2018.PubMed/NCBI View Article : Google Scholar
|
|
4
|
World Health Organization: Guidelines for
the Treatment of malaria, Third edition, 2015. https://www.afro.who.int/publications/guidelines-treatment-malaria-third-edition.
Accessed September 10, 2023.
|
|
5
|
Buck E and Finnigan NA: Malaria. 2023. In:
StatPearls (Internet). Treasure Island (FL), StatPearls Publishing,
2023.
|
|
6
|
Zekar L and Sharman T: Plasmodium
falciparum malaria. In: StatPearls (Internet). Treasure Island
(FL), StatPearls Pub-Lishing, 2021.
|
|
7
|
World Health Organization: World malaria
report 2022. https://www.who.int/teams/global-malaria-programme/reports/world-malaria-report-2022.
Accessed September 10, 2023.
|
|
8
|
Saydam FN, Erdem H, Ankarali H, El-Arab
Ramadan ME, El-Sayed NM, Civljak R, Pshenichnaya N, Moroti RV,
Mahmuodabad FM, Maduka AV, et al: Vector-borne and zoonotic
infections and their relationships with regional and socioeconomic
statuses: An ID-IRI survey in 24 countries of Europe, Africa and
Asia. Travel Med Infect Dis. 44(102174)2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Ashley EA and White NJ: The duration of
Plasmodium falciparum infections. Malar J.
13(500)2014.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Kyrönseppä H, Tiula E, Repo H and
Lähdevirta J: Diagnosis of falciparum malaria delayed by long
incubation period and mis-leading presenting symptoms: Life-saving
role of manual leucocyte differential count. Scand J Infect Dis.
21:117–118. 1989.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Krajden SP, Panisko DM, Tobe B, Yang J and
Keystone JS: Prolonged infection with Plasmodium falciparum
in a semi-immune patient. Trans R Soc Trop Med Hyg. 85:731–732.
1991.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Giobbia M, Tonon E, Zanatta A, Cesaris L,
Vaglia A and Bisoffi Z: Late recrudescence of Plasmodium
falciparum malaria in a pregnant woman: A case report. Int J
Infect Dis. 9:234–235. 2005.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Greenwood T, Vikerfors T, Sjoberg M,
Skeppner G and Farnert A: Febrile Plasmodium falciparum
malaria 4 years after expo-sure in a man with sickle cell disease.
Clin Infect Dis. 47:e39–e41. 2008.PubMed/NCBI View
Article : Google Scholar
|
|
14
|
Theunissen C, Janssens P, Demulder A,
Nouboussie D, Van-Esbroeck M, Van-Gompel A and Van-Denende J:
Falciparum malaria in patient 9 years after leaving malaria-endemic
area. Emerg Infect Dis. 15:115–116. 2009.PubMed/NCBI View Article : Google Scholar
|
|
15
|
World Malaria Report-World Health
Organization, 2010. https://www.who.int/publications/i/item/9789241564106.
Accessed September 10, 2023.
|
|
16
|
Calle CL, Mordmüller B and Singh A:
Immunosuppression in malaria: Do Plasmodium falciparum
parasites hijack the host? Pathogens. 10(1277)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Marletta S, L'Imperio V, Eccher A,
Antonini P, Santonicco N, Girolami I, Dei Tos AP, Sbaraglia M,
Pagni F, Brunelli M, et al: Artificial intelligence-based tools
applied to pathological diagnosis of microbiological diseases.
Pathol Res Pract. 243(154362)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Jensen AR, Adams Y and Hviid L: Cerebral
Plasmodium falciparum malaria: The role of PfEMP1 in its
pathogenesis and Im-munity, and PfEMP1-based vaccines to prevent
it. Immunol Rev. 293:230–252. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Marino A, Bivona DA and Bonacci P: Updates
in central nervous system malaria: Literature review and
considerations. Curr Opin Infect Dis. 35:255–261. 2022.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Centers for Disease Control and
Prevention: Malaria Glossary. https://www.cdc.gov/malaria/glossary.html. Accessed
September 10, 2023.
|
|
21
|
Cryptic malaria guidance-Travel and
Migrant Health Section (TMHS), Health Protection Services,
2011.
|
|
22
|
Uzzan B, Izri A, Durand R, Deniau M,
Bouchard O and Perret GY: Serum procalcitonin in uncomplicated
falciparum malaria: A preliminary study. Travel Med Infect Dis.
4:77–80. 2006.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Wright WF and Auwaerter PG: Fever and
fever of unknown origin: Review, recent advances, and lingering
dogma. Open Forum Infect Dis. 7(ofaa132)2020.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nagley L: Probable relapse of malignant
tertian malaria after thirteen years. Lancet. 2(773)1945.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Walters J: Quiescent malarial parasites.
Br Med J. 1:1206–1207. 1960.
|
|
26
|
Russell PF, West LS, Manwell RD and
Macdonald G: Practical malariology. 2nd edition, Oxford University
Press, London, pp750, 1963.
|
|
27
|
Revel MP, Datry A, Saint Raimond A, Lenoir
G, Danis M and Gentilini M: Plasmodium falciparum malaria
after three years in a non-endemic area. Trans R Soc Trop Med Hyg.
82(832)1988.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Shah S, Filler S, Causer LM, Rowe AK,
Bloland PB, Barber AM, Roberts JM, Desai MR, Parise ME and Steketee
RW: Malaria Surveillance-United States, 2002. MMWR Surveill Summ.
53:21–34. 2004.PubMed/NCBI
|
|
29
|
Howden BP, Vaddadi G, Manitta J and
Grayson ML: Chronic falciparum malaria causing massive splenomegaly
9 years after leaving an endemic area. Med J Aust. 182:186–188.
2005.PubMed/NCBI View Article : Google Scholar
|
|
30
|
D'Ortenzio E, Godineau N, Fontanet A,
Houze S, Bouchaud O, Matheron S and Le Bras J: Prolonged
Plasmodium falciparum infection in immigrants, Paris. Emerg
Infect Dis. 14:323–326. 2008.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Foca E, Zulli R, Buelli F, De Vecchi M,
Regazzoli A and Castelli F: P. falciparum malaria
recrudescence in a cancer patient. Infez Med. 17:33–34.
2009.PubMed/NCBI
|
|
32
|
Szmitko PE, Kohn ML and Simor AE:
Plasmodium falciparum malaria occurring 8 years after
leaving an endemic area. Diagn Microbiol Infect Dis. 63:105–107.
2009.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Mali S, Kachur SP and Arguin PM: Malaria
Surveillance-United States, 2010. MMWR Surveil Summ. 61:1–17.
2012.PubMed/NCBI
|
|
34
|
Cullen KA and Arguin PM: Division of
parasitic diseases and malaria, center for global health, centers
for disease control and prevention (CDC). Malaria
Surveillance-United States, 2011. MMWR Surveill Summ. 62:1–17.
2013.PubMed/NCBI
|
|
35
|
Monge-Maillo B, Norman F, Pérez-Molina JA,
Díaz-Menéndez M, Rubio JM and López-Vélez R: Plasmodium
falciparum in asymptomatic immigrants from sub-Saharan Africa,
Spain. Emerg Infect Dis. 18:356–357. 2012.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Berrevoets MA, Sprong T, Meis JF and
Dofferhoff AS: Plasmodium falciparum malaria recrudescence
occurring 2.5 years after leaving an endemic country. Neth J Med.
71:426–428. 2013.PubMed/NCBI
|