Introduction
Hepatocellular carcinoma (HCC) is one of the most
aggressive malignancies. It is the third leading cause of malignant
tumor-related mortality; the fifth most common among males and the
seventh most common among females (1). At the early stages of the disease
(stages 0-A), the tumor is considered curable; however, in 60% of
HCC cases, the disease is diagnosed at advanced stages when the
average life expectancy, despite treatment, is only 8 months, while
the relevant life expectancy at the middle stages of the disease is
26 months (2,3). Furthermore, surgical treatment as an
ideal, curative treatment option is available only at the early
stages of cancer. Therefore, the early diagnosis of HCC and the
monitoring of disease recurrence are vital for the improvement of
patient survival.
Over the past decade, a number of molecular
techniques were introduced in basic and clinical oncology. Liquid
biopsy (LB) is one such diagnostic modality (4). Initially, it was used for the
detection of circulating tumor cells in blood; however, it now
includes other tumor-related components, such as DNA, RNA, microRNA
(miRNA/miR) and proteins (5,6). LB
has not yet become a routine clinical test, although its high
potential for tumor detection, molecular profiling, surgical
radicality monitoring and treatment outcome prediction is evident
(7). Compared with the tumor core
biopsy technique, LB is a less invasive procedure using body
fluids, including blood, for analyses (8). The development of the LB technique
led to the use of tumor-related nucleic acids as biomarkers for
cancer. Plasma-derived tumor-related RNA was of particular
interest.
Gankyrin, also known as PSMD10 or p28GANK, is a
small molecular protein composed of seven ankyrin domains and is a
component of the 26S proteasome (9-11).
It is encoded by the PSMD10 gene and plays a crucial role in cell
cycle progression, apoptosis and tumorigenesis. Initially, gankyrin
was identified in human HCC (12);
however, several studies have demonstrated that gankyrin expression
is upregulated in other types of cancer (for example kidney,
breast, testicular cancer and squamous cell carcinoma) as well
(13-15).
Numerous studies have demonstrated that p28GANK is
one of the main factors involved in hepatocellular carcinogenesis.
Gankyrin protein expression is increased not only in HCC tissues,
but also in cirrhotic liver, while it is absent in normal liver
samples (16-20).
In another study, the lowest level of gankyrin protein was found in
the normal liver, while its expression was notably increased in
HCC, and the level was highest in metastatic HCC (21). A previous study demonstrated that
gankyrin was associated with cell cycle and tumor progression
(22). As shown by several
studies, gankyrin can promote HCC metastasis by enhancing
epithelial-mesenchymal transition via the PI3K/Akt/HIF-1α signaling
pathway (21,23-25).
These findings indicate that gankyrin may be used as a diagnostic
and prognostic marker for HCC and for monitoring tumor
recurrence.
Despite increased evidence of the role of gankyrin
in HCC and other tumors, to the best of our knowledge, there are
currently no studies available on circulating cell-free RNA for
gankyrin in the blood plasma of patients with cancer. As a
biomarker, circulating cell-free RNA has some advantages due to its
stability, the non-invasive method used for its collection and the
accuracy of the obtained quantitative data.
The aim of the present study was to investigate the
feasibility of plasma circulating gankyrin mRNA expression as a
potential biomarker for HCC. The expression p28GANK RNA was
analyzed in the blood plasma, as well as in the tumor and non-tumor
liver tissue of patients with HCC and cirrhosis, and in healthy
individuals.
Materials and methods
Blood and formalin-fixed
paraffin-embedded (FFPE) tissue samples-study design
A total of 64 patients and healthy volunteers were
recruited in the present study, 54 individuals from whom blood was
collected and 10 archived material of FFPE (5 normal liver samples
were obtained from transplant donors and 5 from patients undergoing
cirrhotic liver biopsy) (Tables I
and II). All participants
provided written informed consent prior to enrolment. The present
study was approved by the Institutional Ethics Committee of the
Alexandre Natishvili Institute of Morphology (Ivane Javakhishvili
Tbilisi State University). All patients were admitted to the
‘Israeli-Georgian Medical Research Clinic Healthycore’.
 | Table IClinicopathological characteristics of
the patients from whom plasma was collected. |
Table I
Clinicopathological characteristics of
the patients from whom plasma was collected.
| Characteristic | Patients with HCC
(n=32) | Patients with
metastatic HCC disease (n=5) | Patients with
cirrhosis (n=7) | HCV-positive patients
(n=5) | Healthy individuals
(n=5) |
|---|
| Sex, n (%) | | | | | |
|
Male | 27 (84.4) | 5(100) | 6 (85.7) | 4(80) | 4(80) |
|
Female | 5 (15.6) | 0 | 1 (14.3) | 1(20) | 1(20) |
| Age, years | | | | | |
|
Mean
(SD) | 55.7 (5.7) | 57.4 (3.6) | 57.1 (2.9) | 49.4 (1.7) | 50.0 (4.1) |
|
Median | 57.5 | 58 | 56 | 49 | 50 |
|
Range | 46-66 | 52-62 | 53-61 | 48-52 | 45-55 |
| Child-Pugh class, n
(%) | | | | | |
|
A | 18 (56.3) | NA | NA | NA | NA |
|
B | 10 (31.3) | NA | NA | NA | NA |
|
C | 4 (12.5) | NA | NA | NA | NA |
 | Table IIClinicopathological characteristics
of the patients undergoing liver tissue biopsy. |
Table II
Clinicopathological characteristics
of the patients undergoing liver tissue biopsy.
| Characteristic | Patients with
cirrhosis (n=5) | Healthy individuals
(n=5) |
|---|
| Sex, n (%) | | n (%) |
|
Male | 5(100) | 4(80) |
|
Female | 0 | 1(20) |
| Age, years | | |
|
Mean
(SD) | 50.4 (2.1) | 35.6 (3.04) |
|
Median | 50 | 35 |
|
Range | 48-53 | 32-40 |
To investigate the expression of the gankyrin/PSMD10
gene in the plasma of patients with HCC, blood samples from
patients with HCC (n=32), metastatic HCC (n=5), cirrhosis (n=7),
hepatitis C virus+ (HCV+; n=5) and healthy
individuals (n=5) were evaluated. All patients with HCC had
cirrhosis and sustained virologic response (SVR) following previous
direct-acting antiviral treatment. In the group ‘HCV+
individuals’, patients with newly-diagnosed active HCV hepatitis
without cirrhosis and HCC were included. In the group ‘patients
with cirrhosis’, patients without HCC and with SVR were included.
The ‘HCC group’ consisted of 32 patients, 5 females (15.6%) and 27
males (84.4%). The mean age of the patients was 55.7 years (±5.7;
range, 46-66 years). The group of patients with metastatic HCC
consisted of 5 male individuals with mean age 57.4 years (±3.6;
range, 52-62 years). The mean age of the 7 patients with cirrhosis
was 57.1 years (±2.9; range, 53-61 years), and among these, there
was 1 female patient (14.3%) and 6 male patients (85.7%). The group
of patients with HCV infection consisted of 5 individuals,
including 1 female (20%) and 4 males (80%) with a mean age of 49.4
years (±1.7; range 48-52 years). Among the 5 healthy volunteers,
there was 1 female (20%) and 4 males (80%) without a history of
neoplasm, liver and other organ chronic disease. The mean age of
the healthy volunteers was 50.0 years (±4.1; range, 45-55 years).
The clinical characteristics of the patients in all the study
groups are presented in Table
I.
The expression of gankyrin/PSMD10 RNA was analyzed
in tumor tissue samples from patients with HCC (tumor and adjacent;
n=32), cirrhosis (n=5) and normal tissues obtained from living
donors prior to transplantation for liver graft quality assessment
(n=5). The HCC group consisted of the same patients as in the case
of blood plasma. Tumor adjacent tissue (ADJ T) was collected from
only 27 cases, as 5 patients only had biopsy samples, as surgery
was not indicated. The group of patients with cirrhosis consisted
of 5 male individuals (100%). The mean age was 50.4 years (±2.1;
range, 48-53 years). The mean age of the healthy liver donors was
35.6 years (±3.04; range, 32-40 years), including 1 female (20%)
and 4 males (80%). The clinical characteristics of all the involved
individuals are presented in Table
II.
RNA extraction from peripheral blood
samples
Plasma samples were isolated from 4 ml peripheral
blood using BD Vacutainer® Blood Collection Tubes
(EDTA-K2 was used as an anticoagulant; BD Biosciences). The tubes
were centrifuged at 1,610 x g for 15 min, and 1 ml supernatant
plasma was transferred to 1.5 ml tubes and stored at -80˚C until
use. Fresh total DNA/RNA/miRNA was extracted from blood plasma
using the RecoverAll™ Total Nucleic Acid Isolation kit (Thermo
Fisher Scientific, Inc.) according to the manufacturer's
recommendations. Total nucleic acid concentration was measured
using a NanoDrop™ ND-1000 Spectrophotometer (NanoDrop Technologies;
Thermo Fisher Scientific, Inc.).
Tumor RNA extraction from FFPE tissue
samples
The starting material for RNA purification was
freshly cut sections of FFPE tissue, each with a thickness of up to
20 µm. Up to 4-20 µm sections from each FFPE tissue block were
combined and placed in a 1.5-ml microcentrifuge tube. Total DNA/RNA
was extracted from the FFPE tissues of tumor, adjacent liver and
control samples using the RecoverAll™ Total Nucleic Acid Isolation
kit for FFPE (Thermo Fisher Scientific, Inc.) according to the
manufacturer's recommendations. The RNA concentrations and purity
were determined using a Nanodrop1000 spectrophotometer (NanoDrop
Technologies; Thermo Fisher Scientific, Inc.). The A260/280 and
A260/A230 ratios were recorded along with the RNA concentration
(ng/ml). The RNA samples with a A260/A280 ratio ranging from 1.8 to
2.1 were considered of good purity.
Reverse-transcription-quantitative
polymerase chain reaction (RT-qPCR)-p28/gankyrin
RT-qPCR was performed using TaqPath™ 1-Step
Multiplex Master Mix (No ROX; cat. no. A28522; Thermo Fisher
Scientific, Inc.) according to the manufacturer's instructions.
Human 18S ribosomal RNA was used as an internal reference gene.
Each sample was run in duplicate. The RT-qPCR protocol included an
initial denaturation step at 95˚C for 20 sec and 40 cycles of
denaturation at 95˚C for 3 sec and annealing at 60˚C for 30 sec.
The relative expression levels were calculated using
2-ΔΔCq method as previously described (26). cDNA was amplified using the
following Taqman® assays: p28/Gankyrin-(Assay ID
Hs01100439_g1) and human 18S ribosomal RNA-(Assay ID Hs99999901_s1;
Thermo Fisher Scientific, Inc.).
Statistical analysis
The Mann-Whitney U test was used to determine the
statistical significance for comparisons data of 2-ΔΔCq
and fold change. For multiple comparisons, the non-parametric
Kruskal-Wallis test followed by the Dunn's post hoc test was.
Statistical analyses were carried out using SPSS (version 23.0; IBM
Corp.) and GraphPad Prism (version 8.0; Dotmatics).
Results
The plasma gankyrin/PSMD10 RNA level was examined
using RT-qPCR in the patients with HCC (n=32), metastatic HCC
(n=5), cirrhosis (n=7), HCV+ (n=5) and healthy
individuals (n=5). In patients with cirrhosis, HCV+ and
healthy individuals, gankyrin/PSMD10 RNA was not detected by the
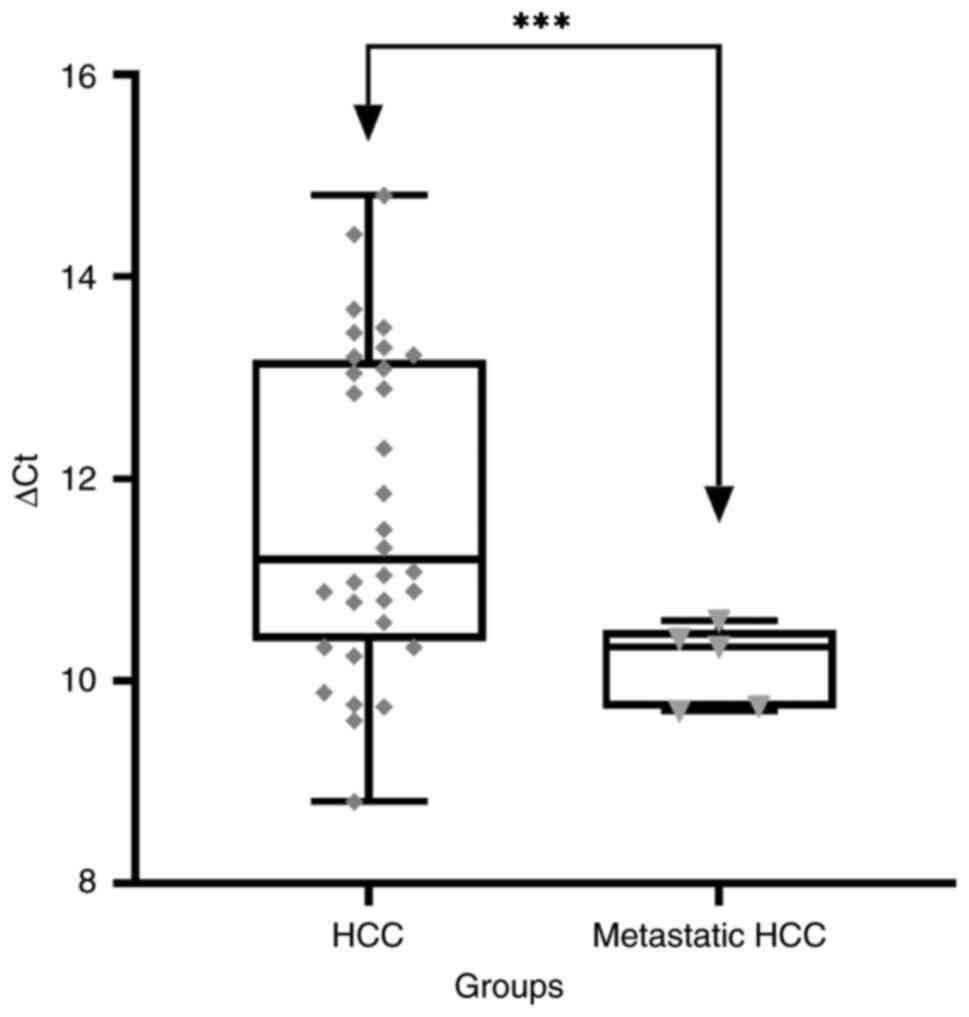
method described in the present study. ∆Cq (target gene Cq -
housekeeping gene Cq) was calculated for every sample. The mean ∆Cq
in HCC group was 11.69±1.6, and in patients with metastatic HCC it
was 10.16±0.4. A statistically significant difference in the ∆Cq
was found between the groups (mean difference, 1.532; 95% CI of
difference, 0.7444-2.3200; P<0.0002; Fig. 1).
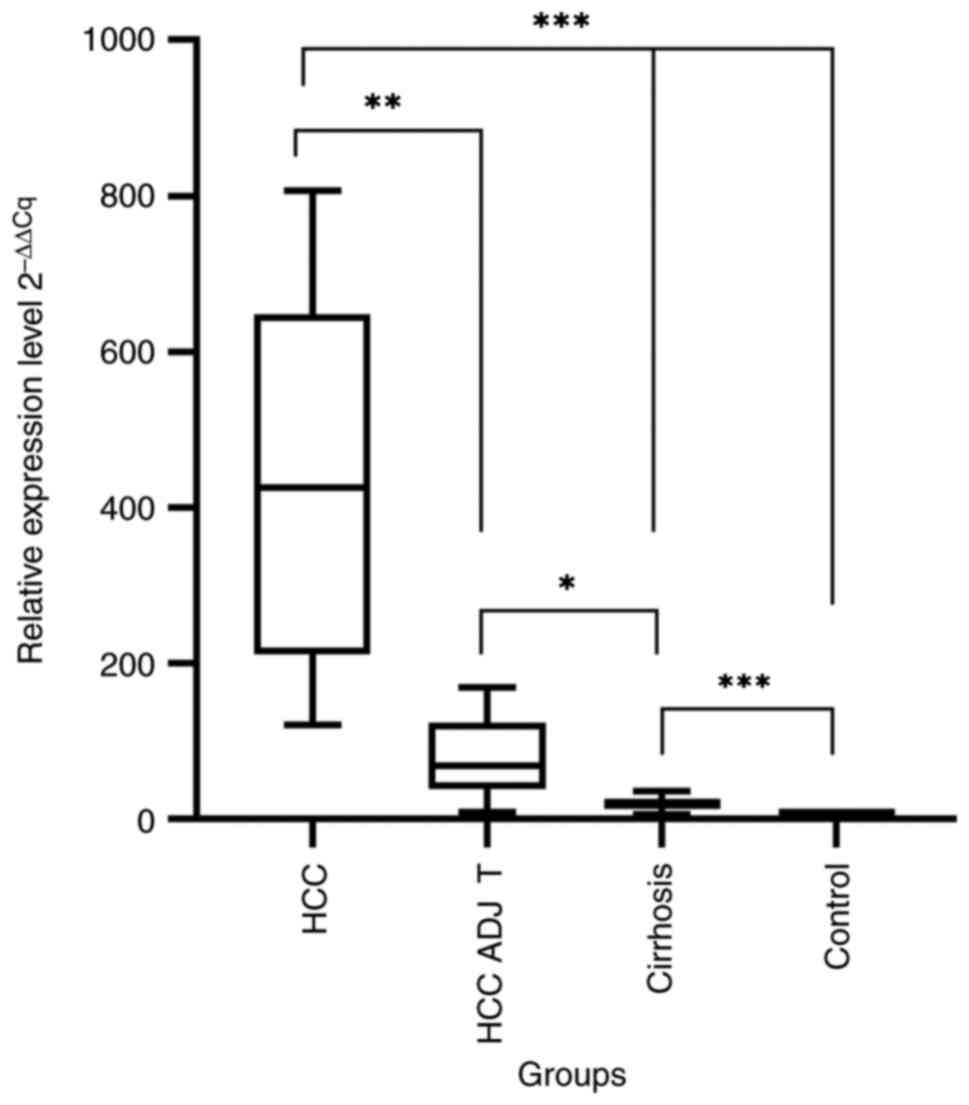
A significant difference in the gankyrin expression
level was found in the tissue samples. The 2-ΔΔCq mean
for gankyrin/PSMD10 in HCC, HCC ADJ T, cirrhosis and control group
was 418.5±220.6, 78.68±49.40, 20.62±11.32 and 1.02±0.23,
respectively. The differences between groups were statistically
significant (P<0.001; Fig.
2).
Discussion
Several studies have used LB in the clinical
management of HCC, including the research for early detection,
monitoring of disease and prediction of response (27-34).
In these studies, different molecular panels were used; however,
satisfactory levels of specificity and sensitivity were not
achieved. Thus, the identification of novel potential targets is
critical in order to obtain desirable results for clinical
implementation.
In the present study, to the best of our knowledge,
the differential expression of gankyrin RNA in the serum and liver
tissues samples from patients with HCC and from healthy individuals
was reported for the first time, identifying novel potential
biomarkers with a high diagnostic capacity.
Several findings can be highlighted in the results
of the present study. First, the gankyrin RNA level in HCC tumor
tissue was significantly higher, not only compared with that in
healthy liver tissue, but also compared with that in adjacent and
cirrhotic livers. Thus, it can be added as an ancillary study for
the differential diagnosis of liver malignant and benign or
reactive nodules on biopsy. Furthermore, in the liver tissue
adjacent to the tumor, which is naturally cirrhotic liver, the
gankyrin RNA level was also higher than that in the cirrhotic liver
tissues of patients without HCC. It is not clear if this is a
consequence of adjacent ‘malignant’ process, or whether the
upregulation is a first step of ‘malignant’ transformation of
hepatocytes. If the latter was confirmed, a good prediction marker
for patients with cirrhosis would have been identified to determine
the risk of HCC development.
Secondly, the data from the expression of gankyrin
RNA in blood plasma revealed promising results. In healthy
individuals, individuals with an HCV infection and in patients with
cirrhosis, gankyrin RNA was not detectable at all in blood plasma,
while it was detectable in all patients with either local HCC or
metastatic disease. These findings indicate the potential of
gankyrin RNA alone or in combination with other genetic markers to
be used as a clinical biomarker for early detection, the monitoring
of disease and the prediction of the response in patients with
HCC.
Although additional research on gankyrin RNA is
required, the findings of the present study, together with other
clinical and instrumental data, may be informative for the
management of HCC, particularly in cases where other studies did
not provide a clear result. In addition, this marker would enable
the detection of HCC at a stage when it is impossible to make a
full diagnosis with computed tomography, ultrasound and MRT
data.
For example, in the case of the Liver Imaging
Reporting and Data System-3 (LiRads-3) lesion during cirrhosis, the
patient is monitored for a long period of time, since the
aforementioned type of lesion does not indicate a malignant tumor,
but due to the presence of pathogenic risks, it is considered a
potential danger. With the result of the LB, it may be possible to
differentiate a malignant tumor in LiRads-3 lesions from a
hyperplastic nodule, which occurs as an ordinary case in liver
cirrhosis.
Although the present study included a relatively
small number of patients, it provided promising and solid results,
and could lay the foundation for further research conducted towards
this direction, as well as for the detection of an improved
molecular panel together with gankyrin to diagnose early-stage
HCC.
The method described in the present study is less
invasive and enables the determination of the molecular profile
using blood plasma. LB is a simple diagnostic tool for early
detection of malignancy in risk groups, such as those infected with
HCV and hepatitis B virus for a long time, cirrhosis of the liver
and non-alcoholic fatty liver disease. In addition, gankyrin RNA as
a biomarker may be suitable for monitoring HCC recurrence.
The present study has certain limitations, which
should be mentioned. First, the present study was conducted on a
relatively small group of patients and needs to be confirmed in a
larger case series involving different centers. Second, the present
study did not include follow-up data of patients, which would be
proof of the ability of gankyrin RNA in LB to be used for the early
detection of HCC recurrence. As an LB marker, gankyrin RNA must be
validated in patients with different liver tumors and also
secondary ones.
In conclusion, the data of the present study provide
strong evidence that the expression of gankyrin RNA is
significantly increased in HCC compared with that in normal and
cirrhotic tissues. In the tumor adjacent liver tissue, the gankyrin
RNA level was higher than that in the cirrhotic liver tissues of
patients without HCC. Gankyrin RNA was not detectable in the blood
plasma of healthy individuals and in that of patients without
cancer; however, its expression was significantly increased in the
plasma of patients with HCC. The detection of gankyrin RNA in LB
can be practically used for screening patients who belong to the
risk groups for the development of HCC.
Since gankyrin is not specific for HCC, it must be
used with other molecular markers for the diagnosis of HCC, but it
can be used alone for the monitoring and early detection of tumor
recurrence. To prove this hypothesis, future studies are warranted,
which must include data regarding gankyrin RNA expression levels in
the blood samples before and after the resection of HCC and
validation at the protein level.
Acknowledgements
The authors would like to thank Professor Dimitri
Kordzaia (Director of the Aleqsandre Natishvili Institute of
Morphology) for providing the necessary research facilities.
Funding
Funding: The present study was supported by the Shota Rustaveli
National Science Foundation of Georgia SRNSFG (#PhD_F_17_33).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
All authors (LG, GL, HJS, ZB, LS, NG and MJ)
contributed to the conception and design of the study. Material
preparation was performed by LG, LS, NG and MJ. Data collection and
analysis were performed by MJ, ZB and LG. Analysis was performed by
ZB, HJS and GL. ZB and NG confirm the authenticity of all the raw
data. The first draft of the manuscript was written by LG and all
authors commented on previous versions of the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethics Committee of the Alexandre Natishvili Institute of
Morphology (Ivane Javakhishvili Tbilisi State University; approval
no. N06/18) and written informed consent was provided by the
patients. The present study was conducted in accordance with the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Goossens N and Hoshida Y: Hepatitis C
virus-induced hepatocellular carcinoma. Clin Mol Hepatol.
21:105–114. 2015.PubMed/NCBI View Article : Google Scholar
|
|
2
|
European Association For The Study Of The
Liver; European Organisation For Research And Treatment Of Cancer.
EASL-EORTC clinical practice guidelines: Management of
hepatocellular carcinoma. J Hepatol. 56:908–943. 2012.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Llovet JM, Decaens T, Raoul JL, Boucher E,
Kudo M, Chang C, Kang YK, Assenat E, Lim HY, Boige V, et al:
Brivanib in patients with advanced hepatocellular carcinoma who
were intolerant to sorafenib or for whom sorafenib failed: Results
from the randomized phase III BRISK-PS study. J Clin Oncol.
31:3509–3516. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Pantel K and Alix-Panabieres C:
Circulating tumour cells in cancer patients: Challenges and
perspectives. Trends Mol Med. 16:398–406. 2010.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Crowley E, Di Nicolantonio F, Loupakis F
and Bardelli A: Liquid biopsy: Monitoring cancer-genetics in the
blood. Nat Rev Clin Oncol. 10:472–484. 2013.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhang W, Xia W, Lv Z, Ni C, Xin Y and Yang
L: Liquid biopsy for cancer: Circulating tumor cells, circulating
free DNA or exosomes? Cell Physiol Biochem. 41:755–768.
2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Wang J, Chang S, Li G and Sun Y:
Application of liquid biopsy in precision medicine: Opportunities
and challenges. Front Med. 11:522–527. 2017.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pinzani P, D'Argenio V, Del Re M,
Pellegrini C, Cucchiara F, Salvianti F and Galbiati S: Updates on
liquid biopsy: Current trends and future perspectives for clinical
application in solid tumors. Clin Chem Lab Med. 59:1181–1200.
2021.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Qin X, Wang X, Liu F, Morris LE, Wang X,
Jiang B and Zhang Y: Gankyrin activates mTORC1 signaling by
accelerating TSC2 degradation in colorectal cancer. Cancer Lett.
376:83–94. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Wang C, Li X, Ren L, Ma C, Wu M, Liang W,
Zhao J, Li S, Tan Q, Liao Y, et al: Gankyrin as potential biomarker
for colorectal cancer with occult liver metastases. Front Oncol.
11(656852)2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Xu X, Lou Y, Tang J, Teng Y, Zhang Z, Yin
Y, Zhuo H and Tan Z: The long non-coding RNA Linc-GALH promotes
hepatocellular carcinoma metastasis via epigenetically regulating
Gankyrin. Cell Death Dis. 10(86)2019.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Higashitsuji H, Itoh K, Nagao T, Dawson S,
Nonoguchi K, Kido T, Mayer RJ, Arii S and Fujita J: Reduced
stability of retinoblastoma protein by gankyrin, an oncogenic
ankyrin-repeat protein overexpressed in hepatomas. Nat Med.
6:96–99. 2000.PubMed/NCBI View
Article : Google Scholar
|
|
13
|
Jahangiri R, Mosaffa F, EmamiRazavi A,
Gharib M and Jamialahmadi K: Increased expression of gankyrin and
stemness factor oct-4 are associated with unfavorable clinical
outcomes and poor benefit of tamoxifen in breast carcinoma
patients. Pathol Oncol Res. 26:1921–1934. 2020.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Camacho-Moll ME, Macdonald J, Looijenga
LHJ, Rimmer MP, Donat R, Marwick JA, Shukla CJ, Carragher N,
Jørgensen A and Mitchell RT: The oncogene Gankyrin is expressed in
testicular cancer and contributes to cisplatin sensitivity in
embryonal carcinoma cells. BMC Cancer. 19(1124)2019.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Li H, Zhang J, Zhen C, Yang B and Feng L:
Gankyrin as a potential target for tumor therapy: evidence and
perspectives. Am J Transl Res. 10:1949–1960. 2018.PubMed/NCBI
|
|
16
|
Fu XY, Wang HY, Tan L, Liu SQ, Cao HF and
Wu MC: Overexpression of p28/gankyrin in human hepatocellular
carcinoma and its clinical significance. World J Gastroenterol.
8:638–643. 2002.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Zhao X, Fu J, Xu A, Yu L, Zhu J, Dai R, Su
B, Luo T, Li N, Qin W, et al: Gankyrin drives malignant
transformation of chronic liver damage-mediated fibrosis via the
Rac1/JNK pathway. Cell Death Dis. 6(e1751)2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Su B, Luo T, Zhu J, Fu J, Zhao X, Chen L,
Zhang H, Ren Y, Yu L, Yang X, et al:
Interleukin-1beta/Iinterleukin-1 receptor-associated kinase 1
inflammatory signaling contributes to persistent Gankyrin
activation during hepatocarcinogenesis. Hepatology. 61:585–597.
2015.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Jing H, Zhang G, Meng L, Meng Q, Mo H and
Tai Y: Gradually elevated expression of Gankyrin during human
hepatocarcinogenesis and its clinicopathological significance. Sci
Rep. 4(5503)2014.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Llovet JM, Chen Y, Wurmbach E, Roayaie S,
Fiel MI, Schwartz M, Thung SN, Khitrov G, Zhang W, Villanueva A, et
al: A molecular signature to discriminate dysplastic nodules from
early hepatocellular carcinoma in HCV cirrhosis. Gastroenterol.
131:1758–1767. 2006.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fu J, Chen Y, Cao J, Luo T, Qian YW, Yang
W, Ren YB, Su B, Cao GW, Yang Y, et al: p28GANK overexpression
accelerates hepatocellular carcinoma invasiveness and metastasis
via phosphoinositol 3-kinase/AKT/hypoxia-inducible factor-1alpha
pathways. Hepatology. 53:181–192. 2011.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Iwai A, Marusawa H, Kiuchi T, Higashitsuji
H, Tanaka K, Fujita J and Chiba T: Role of a novel oncogenic
protein, gankyrin, in hepatocyte proliferation. J Gastroenterol.
38:751–758. 2003.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Chao J, Zhao S and Sun H:
Dedifferentiation of hepatocellular carcinoma: Molecular mechanisms
and therapeutic implications. Am J Transl Res. 12:2099–2109.
2020.PubMed/NCBI
|
|
24
|
Han J, Wang F, Lan Y, Wang J, Nie C, Liang
Y, Song R, Zheng T, Pan S, Pei T, et al: KIFC1 regulated by
miR-532-3p promotes epithelial-to-mesenchymal transition and
metastasis of hepatocellular carcinoma via gankyrin/AKT signaling.
Oncogene. 38:406–420. 2019.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Wang WP, Sun Y, Lu Q, Zhao JB, Wang XJ,
Chen Z, Ni YF, Wang JZ, Han Y, Zhang ZP, et al: Gankyrin promotes
epithelial-mesenchymal transition and metastasis in NSCLC through
forming a closed circle with IL-6/ STAT3 and TGF-β/SMAD3 signaling
pathway. Oncotarget. 8:5909–5923. 2017.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Oussalah A, Rischer S, Bensenane M, Conroy
G, Filhine-Tresarrieu P, Debard R, Forest-Tramoy D, Josse T,
Reinicke D, Garcia M, et al: Plasma mSEPT9: A novel circulating
cell-free DNA-based epigenetic biomarker to diagnose hepatocellular
carcinoma. EBioMedicine. 30:138–147. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Qu C, Wang Y, Wang P, Chen K, Wang M, Zeng
H, Lu J, Song Q, Diplas BH, Tan D, et al: Detection of early-stage
hepatocellular carcinoma in asymptomatic HBsAg-seropositive
individuals by liquid biopsy. Proc Natl Acad Sci USA.
116:6308–6312. 2019.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Xu RH, Wei W, Krawczyk M, Wang W, Luo H,
Flagg K, Yi S, Shi W, Quan Q, Li K, et al: Circulating tumour DNA
methylation markers for diagnosis and prognosis of hepatocellular
carcinoma. Nat Mater. 16:1155–1161. 2017.PubMed/NCBI View
Article : Google Scholar
|
|
30
|
Kisiel JB, Dukek BA, Kanipakam RVS, Ghoz
HM, Yab TC, Berger CK, Taylor WR, Foote PH, Giama NH, Onyirioha K,
et al: Hepatocellular carcinoma detection by plasma methylated DNA:
Discovery, phase I pilot, and phase II clinical validation.
Hepatology. 69:1180–1192. 2019.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Sohn W, Kim J, Kang SH, Yang SR, Cho JY,
Cho HC, Shim SG and Paik YH: Serum exosomal microRNAs as novel
biomarkers for hepatocellular carcinoma. Exp Mol Med.
47(e184)2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
von Felden J, Schulze K, Krech T, wald F,
Nashan B, Pantel K, Lohse AW, Riethdorf S and Wege H: Circulating
tumor cells as liquid biomarker for high HCC recurrence risk after
curative liver resection. Oncotarget. 8:89978–89987.
2017.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Sun YF, Xu Y, Yang XR, Guo W, Zhang X, Qiu
SJ, Shi RY, Hu B, Zhou J and Fan J: Circulating stem cell-like
epithelial cell adhesion molecule-positive tumor cells indicate
poor prognosis of hepatocellular carcinoma after curative
resection. Hepatology. 57:1458–1468. 2013.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Ogle LF, Orr JG, Willoughby CE, Hutton C,
McPherson S, Plummer R, Boddy AV, Curtin NJ, Jamieson D and Reeves
HL: Imagestream detection and characterisation of circulating
tumour cells-A liquid biopsy for hepatocellular carcinoma? J
Hepatol. 65:305–313. 2016.PubMed/NCBI View Article : Google Scholar
|