Introduction
The use of titanium implants has increased
substantially in dentistry to replace natural teeth due to their
high biocompatibility. Titanium or titanium admixtures are the
usual constituents of titanium implants, given their mechanical
properties and biocompatibility. There is evidence to indicate that
titanium dental implants can endure exposure to the oral
environment for more than two decades (1). The biocompatibility exhibited by
dental implants maybe attributed to the formation of a titanium
dioxide layer that prevents direct contact between the implant and
the biological environment. This, in turn, reduces the potential
for metal reactivity. The success and durability of dental implants
are contingent upon their integration with both hard and soft
tissues (2). Currently, scholars
are focusing on diverse approaches to expedite the process of
osseointegration of dental implants and to augment the surface area
of implant-bone contact. To achieve this, the osseointegration
surface areas are enlarged by creating irregularities on the
surfaces of materials used for dental implants (3). The examination of the interactions of
dental implants with biological tissues involves the use of a
titanium dioxide (TiO2) layer. When subjected to loading
conditions, the TiO2 layer is susceptible to damage
during movement between the implant and bone tissue, resulting in
implant corrosion and consequential weakening. Furthermore,
corrosion can trigger the release of minute metallic particles or
ions into the surrounding living tissues (4). A previous study revealed that the
micromotion of the abutment under cyclic loading may produce wear
particles of varying sizes between 2 and 80 nm in conical dental
implant systems. Wear debris comprises titanium particles that are
recognized to elicit a macrophage response (5). Metal nanoparticles have been widely
acknowledged for their ability to trigger an inflammatory response
through their immunomodulatory potential. This potential is
primarily exerted at the macrophage level and is characterized by
an escalation in DNA damage, protein carbonylation, lipid
per-oxidation, oxidative stress, and a reduction in superoxide
dismutase activity, total glutathione levels and total antioxidant
capacity catalase (3).
Since the seminal study by Ferguson et al
(6), the generation of metal
debris from joint replacement surgeries has been a significant
concern within the field of orthopedic surgery. The surface oxide
film layer of a metallic object implanted within the human body may
undergo disruption or degradation over time due to spontaneous
mechanical or electrochemical corrosion. This particular
interaction can yield chemically reactive metallic byproducts,
which may prompt the discharge of metal into the systemic
circulation (6,7). Bianco et al (8) investigated the dissimilarity between
the levels of titanium in the serum and urine of rabbits and Gopi
et al (9) investigated the
serum level of titanium in humans before and following implant
insertion. No noteworthy elevation was observed in either case. A
comprehensive extensive analysis of the metallic constituents in
human blood serum has yet to be performed, at least to the best of
our knowledge. It is uncertain whether the discharge of titanium or
titanium particles from dental implants may have an impact locally
or systemically. The present study thus aimed to compare the
titanium serum level before and after the placement of dental
implants, and to compare the level of serum titanium in patients
with healthy dental implants and in those with
peri-implantitis.
Patients and methods
Study design
A null hypothesis was developed for the present
prospective quasi-experimental study stating that there would be no
alteration in the serum titanium level of patients before and after
implant placement either in health or disease. The present study
was carried out at the Department of Periodontics from 2019 to 2022
and was approved by the M S Ramaiyah University, Faculty of Dental
Sciences Institutional Ethical Review Committee (EC-19/12-F-FDS).
The study design consisted of two groups of 60 patients in each,
including both males and females with an age range of 23-47 years.
Group 1 comprised healthy individuals seeking implant placements
and group 2 included patients with peri-implantitis who had
implants placed >6 months prior. A well-informed written consent
was obtained from all the patients informing them about the study
and post study publication of data and any related images.
Sample size calculation
The statistical software G*Power (version 3.1)
developed by Franz Faul at the University of Kiel (Kiel, Germany)
was employed to determine the appropriate sample size for the
present study, with a type 1α error rate of 0.05 and a power of 90%
(9). Ultimately, a sample size of
53 was selected; however, in anticipation of potential sample
attrition, the sample size was increased to 60. As per the sample
size calculation each group of the study comprises of 60 patients
contributing to total population of 120.
Inclusion criteria
The inclusion criteria used in the present study
were the following: Group 1 (healthy individuals): i) Partially
edentulous patients; ii) Periodontally healthy patients; iii)
Patients with appropriate inter-occlusal distance for the placement
of the implants; iv) Patients with no history of metal allergies.
Group 2 (patients with peri-implantitis): i) The post implant
period should be >6 months, but <2 years; ii) implants having
probing depths ≥5 mm; iii) Bleeding on probing and/or suppuration;
iv) Bone loss ≥2 mm was considered in the peri-implantitis
group.
Exclusion criteria
The following exclusion criteria were used: i)
Patients using systemic or local antibiotics over the last 3
months; ii) Immuno-compromised patients, who had received
chemotherapy or radiotherapy; iii) Pregnant and lactating women;
iv) Patients with a smoking habit.
Method of assessment of
peri-implantitis
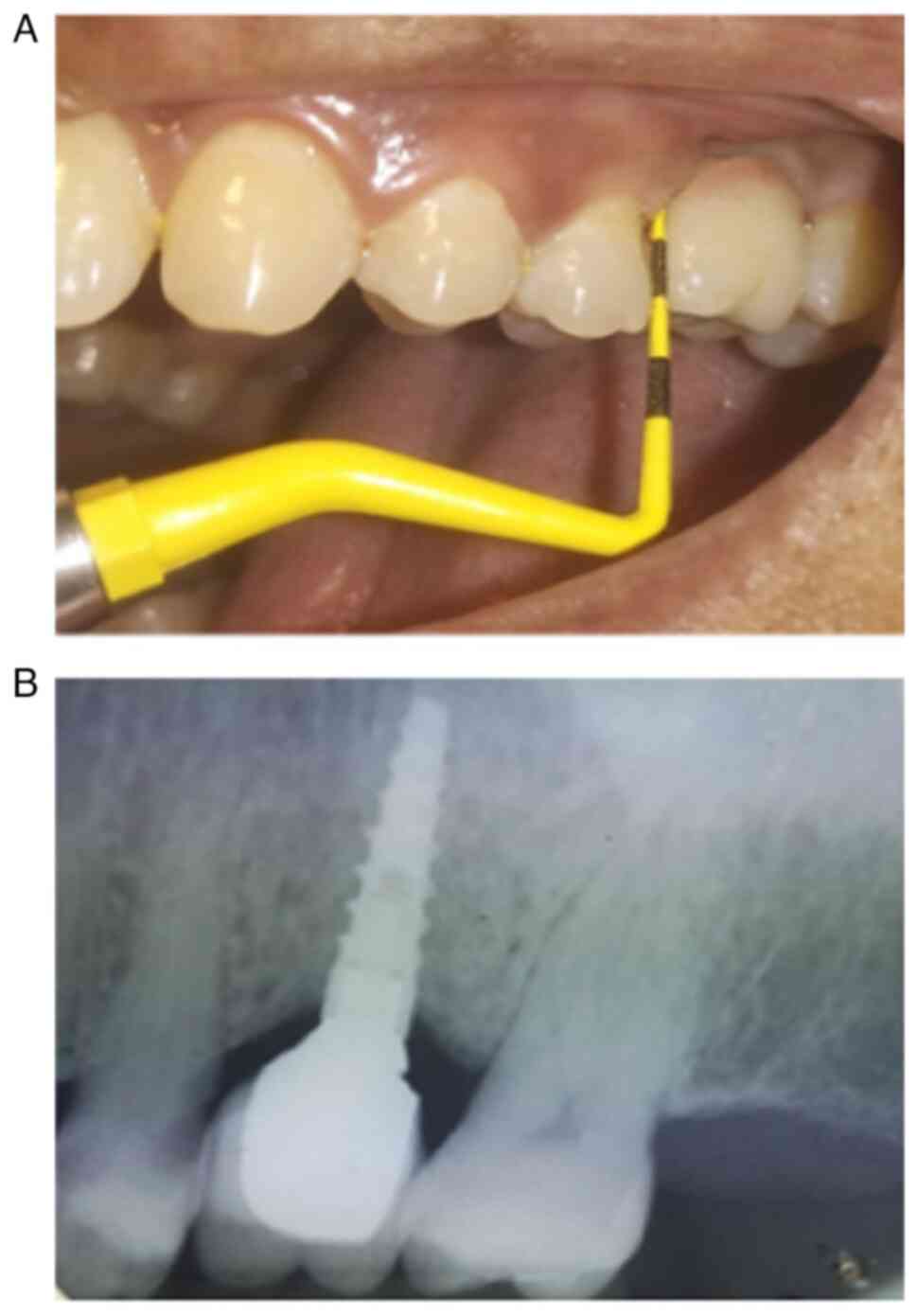
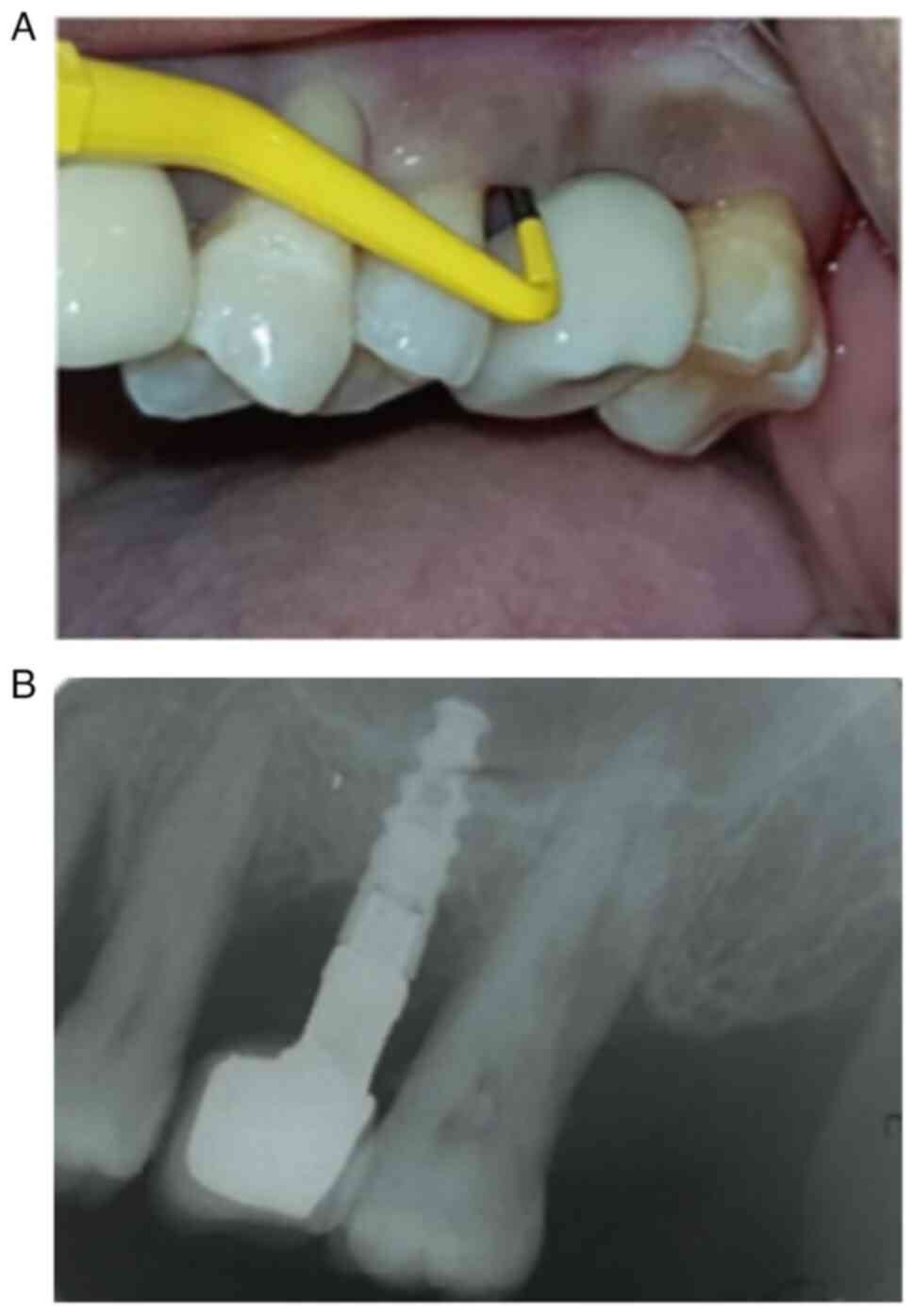
The determination of peri-implantitis diagnosis was
established through the evaluation of clinical and radiologic
criteria, in accordance with existing recommendations (10). Specifically, implants with probing
depths ≥5 mm, accompanied by bleeding upon probing and/or
suppuration, as well as a bone loss ≥2 mm, were diagnosed with
peri-implantitis (Figs. 1 and
2).
In order to determine the diagnoses, it was
necessary to obtain a consensus from three authors (MS, VS and KK)
who worked independently. Furthermore, supplementary information
regarding the age, sex, smoking habits and diabetes status of the
study subjects was also documented and presented in Table I.
 | Table IDescriptive characteristics of the
study groups. |
Table I
Descriptive characteristics of the
study groups.
| | Group 1 | Group 2 | P-value |
|---|
| Sample | 60 | 60 | |
| Male | 32 | 35 | 0.581a |
| Female | 28 | 25 | |
| Age in years (mean ±
SD) | 37±8.6 | 39±9.2 | 0.221b |
| Smoking | 0 | 0 | NA |
| Diabetes | 0 | 0 | NA |
Methodology
Blood serum samples were collected from the patients
of group 1 at three different intervals [at 1 month prior to
implant placement, at the 4th month after the surgical phase
(loading of implant) and at the 8th month after the surgical phase
(loading of implant)], and from the patients in group 2, at the
time course of peri-implantitis. A total of 2 ml blood was
withdrawn from the anterior cubital fossa of the patients. Blood
samples were stored at -20˚C (Fig.
3). Following the collection of whole blood, it was left
undisturbed in the vacutainer, allowing it to clot at room
temperature. Centrifugation was performed at 271-542 x g for 20 min
in a refrigerated (-4˚C) centrifuge. The resultant serum samples
were obtained and analysis for titanium was performed using
inductively coupled plasma-mass spectrometry (ICP-MS), Thermo
Scientific ICAP 7000 series (Thermo Fisher Scientifc, Inc.) with a
detection limit of 0.5 ng at the M S Ramaiah Advanced Drug Testing
Laboratory, Bangalore, India.
Statistical analysis
The normality of the obtained dataset was examined
using the Shapiro Wilk test. After stating the descriptive analysis
for all the groups, the Chi-squared test was applied for ordinal
data and an independent t-test was applied for the comparison of
mean age between the groups. The multiple group comparisons were
performed using one-way ANOVA followed by Tukey's post hoc test. A
P-value <0.05 was considered to indicate a statistically
significant difference. All statistical analyses were performed
using SPSS software version 22 (IBM Corp.).
Results
The present study included 120 patients, comprised
of 53 females and 67 males. The age range was 23-47 years with an
average value of 40±8 years. In the present study, no statistically
meaningful variance was observed as regards the demographic
characteristics of the participants enlisted for the research,
suggesting the absence of any bias in the selection process based
on age and sex (Table I).
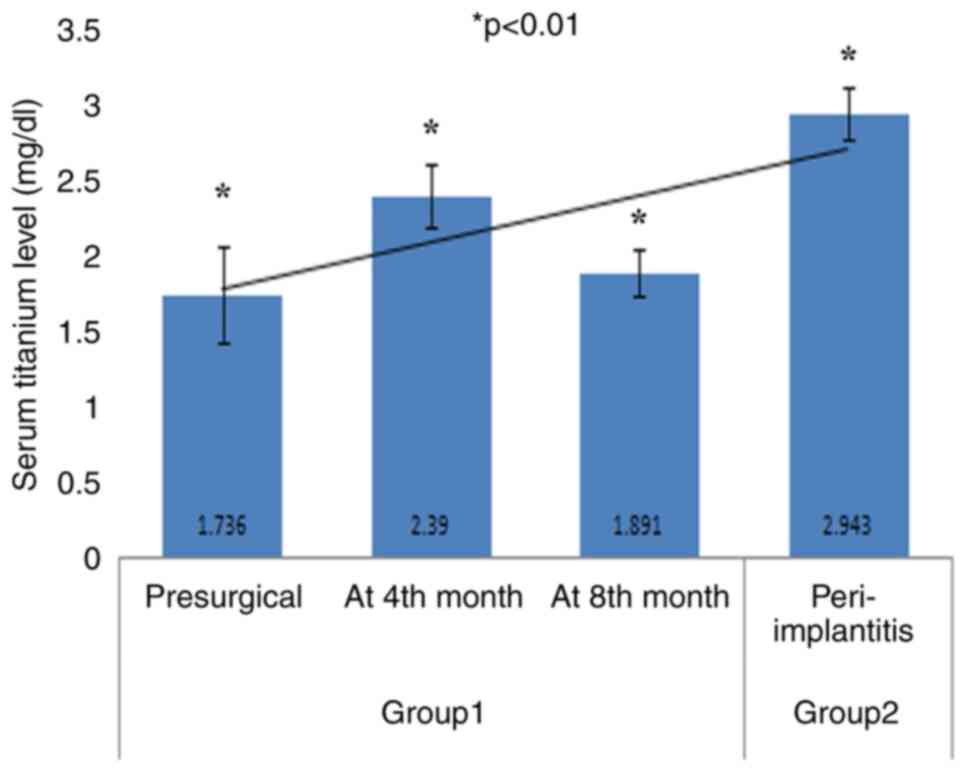
The analysis of the aggregated data revealed an
increasing trend line in serum titanium levels in the patients in
groups 1 and 2 (Fig. 3). Patients
with peri-implantitis (group 2) exhibited higher mean serum values
(2.94±0.17) compared to patients in group 1. In the patients in
group 1, the highest mean serum titanium level (2.39±0.20) was
observed at 4th month after the loading procedure (Fig. 3 and Table II). The comparative analysis of
the groups using one-way ANOVA revealed a significant difference
(P=0.001) and intra-group comparisons with Tukey's post hoc test
revealed significant differences between all parameters examined,
apart from difference in the serum titanium level between the
pre-surgical time point and the 8th month, and between the4th month
and 8th month (Tables III and
IV).
 | Table IIDescriptive analysis of estimated
serum titanium values in the study groups. |
Table II
Descriptive analysis of estimated
serum titanium values in the study groups.
| | Group 1 (n=60) | Group 2 (n=60) |
|---|
| Parameter | Pre-surgery | At the 4th month | At the 8th month | Peri-implantitis |
|---|
| Mean (mg/dl) | 1.73 | 2.39 | 1.89 | 2.94 |
| Standard
deviation | 0.31 | 0.20 | 0.14 | 0.17 |
| Shapiro-Wilk
test | 0.94 | 0.91 | 0.92 | 0.94 |
| P-value of
Shapiro-Wilk test | 0.005a | 0.001a | 0.001a | 0.009a |
 | Table IIIIntergroup comparison of mean scores
obtained using one-way ANOVA. |
Table III
Intergroup comparison of mean scores
obtained using one-way ANOVA.
| Cases | Sum of squares | df | Mean square | F value | P-value |
|---|
| Group 1 vs. group
2 | 43.808 | 3 | 14.603 | 377.705 |
<0.001a |
| Residuals | 6.843 | 177 | 0.039 | | |
 | Table IVIntra-group comparisons determined
using Tukey's post hoc test. |
Table IV
Intra-group comparisons determined
using Tukey's post hoc test.
| Group | Comparison made,
vs. | Mean difference | SE | t value | P-value |
|---|
| Group 1
pre-surgical | Group 1 at the 4th
month | -0.654 | 0.036 | -18.218 |
<0.001a |
| | Group 1 at the 8th
month | -0.162 | 0.036 | -1.313 | 0.108 |
| | Group 2
peri-implantitis | -1.207 | 0.036 | -33.623 |
<0.001a |
| Group 1 at the 4th
month | Group 1 at the 8th
month | 0.032 | 0.036 | 0.905 | 0.367 |
| | Group 2
peri-implantitis | -0.553 | 0.036 | -15.405 |
<0.001a |
| Group 1 at the 8th
month | Group 2
peri-implantitis | -0.585 | 0.036 | -16.31 |
<0.001a |
Discussion
Since ~1981, titanium has been utilized for the
construction of dental implants. The primary alloys employed are
commercially pure titanium (cpTi) and Ti-6Al-4V, both of which
exhibit clinical success rates of up to 99% after a decade. These
alloys possess biocompatibility when in contact with bone and
gingival tissues, and have the ability to undergo osseointegration
(9).
The corrosion behavior of metal implants is a
crucial determinant of their biocompatibility. This is due to the
potential detrimental effects resulting from the release of metal
ions during the corrosion process. The tissue in the immediate
vicinity of the implant, as well as the systemic environment, can
be affected by these factors, potentially leading to allergic
reactions. The presence of an oxide layer on the surface of the
implant significantly influences the outcome of osseointegration.
The utilization of dental implants may result in elevated levels of
titanium in both the bloodstream and serum (11,12).
Gopi et al (9) conducted an assessment on the
liberation of titanium, aluminium and vanadium from dental implants
through a comparison of the serum concentrations of these ions
prior to and following surgical procedures. Notably, a marginal
variation was observed in the post-operative levels of titanium
(2.31 mg/dl) in relation to the preoperative levels (2.28 mg/dl),
without any statistically significant difference (P>0.5)
(9). The present study assessed
significant differences in the concentrations of titanium in the
bloodstream before (1.79 mg/dl) and after (2.39 mg/dl) the 4thmonth
of the loading of the implant, and the findings obtained were not
in accordance with those of the study conducted by Gopi et
al (9). Another study
demonstrated a comparable lack of significance in the association
between the average serum concentration of titanium at the
beginning, after 8 weeks and after 6 months, with values of 2.39,
2.35 and 2.38 mg/dl, respectively (13). The present study also found no
significant difference in the titanium level between the
pre-surgical phase and at the 8th month of post-loading of the
implant. This indicates that serum titanium levels significantly
increase immediately after the loading of the implant; however,
with time, the concentration decreases.
The release of titanium particles from the surface
of the implant has a detrimental effect on both the nearby and
far-reaching tissue, as it infiltrates the surrounding tissues and
enters the bloodstream (14). In
the localized region of the dental implant known as the
peridontium, the presence of titanium particles can trigger an
inflammatory condition known as peri-implantitis, characterized by
an escalation in inflammatory mediators, such as macrophages,
cytokines, TNF-α and IL-6. Previous studies have assessed the
concentration of titanium and inflammatory mediators in serum;
however, no substantial findings have been attained (15,16).
The present study, on the other hand, revealed a statistically
significant difference in the serum concentration of titanium
between individuals with uncompromised dental implants and those
experiencing peri-implantitis.
Previous research has demonstrated the existence of
titanium particles in the tissues surrounding dental implants.
Nevertheless, no conclusive statistical evidence has been presented
to establish a connection between dissolved titanium and
peri-implantitis (17). The
present study assessed the levels of titanium in the serum of
patients with both healthy implants and implants affected by
peri-implantitis. The findings obtained demonstrated significant
differences, which is in accordance with the findings in the study
by Olmedo et al (14).
Although the occurrence of inflammation is observed
as a healing response promptly following the loading of an implant
and is accompanied by heightened levels of titanium in serum
(10), a similar response can also
manifest in cases of peri-implantitis. During peri-implantitis,
macrophages that are recruited engulf wearable titanium particles,
resulting in an elevation of titanium levels in serum (14,16).
The potential of titanium particles that have undergone corrosion
to induce an immune response may result in inflammation of the
periodontium and the subsequent degradation of bone tissue. During
the process of immune activation, a variety of inflammatory
cytokines is discharged, which encompass granulocyte-macrophage
colony-stimulating factor, prostaglandin, TNF-α, IL-1β and IL-6.
The catalyst responsible for this activation is the presence of
titanium particles, which subsequently initiates the activation of
the NLRP3 inflammasome, ultimately leading to the release of a
mature IL-1β (18). The primary
focus during the management of peri-implantitis was previously
centered on mitigating inflammation. However, the emphasis should
be on reducing the corrosion of titanium implants.
While the serum titanium level does not reach toxic
levels in instances of dental implants, it is important to note
that these titanium particles have the potential to be transported
through the bloodstream to various regions of the body, thereby
inducing toxic consequences (19).
The exposure of titanium in dental implants to fluoride ions can
occur through mouth rinses, toothpastes, drinking water or food.
Consequently, the utilization of fluoride as a potential
confounding factor should be taken into consideration in
forthcoming confirmatory investigations that aim to evaluate the
connection between titanium corrosion and peri-implantitis
(20,21). In order to mitigate the leaching of
titanium particles, a previous study was conducted using an aqueous
solution of lactic acid and phosphate-buffered saline. However, it
was discovered that there was no discernible connection between the
augmentation of surface roughness and the release of ions, both in
experimental and biological circumstances (20).
The present study has certain limitations, which
should be mentioned. The present study utilized the ICP-MS
technique to evaluate the serum titanium level which present in
minute amounts. Although a significant association between healthy
implant and implants with peri-implantitis was obtained, the status
of inflammation in soft tissues around the implant was not
assessed. Further studies are thus warranted to evaluate the
titanium level and inflammatory components in gingival tissues and
blood serum.
In conclusion, understanding the complex association
between titanium corrosion and peri-implantitis is crucial for
improving the long-term success and safety of dental implants.
Further research is required to explore these connections and
potential mitigation strategies to ensure the continued well-being
of patients with dental implants.
Acknowledgements
Not applicable.
Funding
Funding: The present study was supported by the Indian Council
of Medical Research (grant no. 5/4/2-15/2019-NCD-II).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MS made a substantial contribution to the conception
and design of the study, as well as in the critical reviewing of
the manuscript. VS was involved in patient selection and in the
design of the study, in the analysis and interpretation of the
data, as well as in the critical reviewing of the manuscript. KK
was involved in the analysis and interpretation of the data, as
well as in the critical reviewing of the manuscript. SS was
involved in the laboratory investigation and data segregation, as
well as in the critical reviewing of the manuscript. GC was
involved in data analysis and in the critical reviewing of the
manuscript. BA was involved in implant surgery and patient
selection along with the critical reviewing of the manuscript for
important intellectual content. All authors have read and approved
the final manuscript. MS and VS confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
The present study was approved by the Institutional
Ethical Review Committee of the Faculty of Dental Sciences
(EC-19/12-F-FDS) at Ramaiah University of Applied Sciences
Bangalore. Written informed consent was obtained from the patients
for their participation in the present study.
Patient consent for publication
Written informed consent was obtained from the
patients for the publication of the present study and any related
images.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Dhaliwal JS, Abd Rahman NA, Ming LC,
Dhaliwal SKS, Knights J and Albuquerque Junior RF: Microbial
biofilm decontamination on dental implant surfaces: A mini review.
Front Cell Infect Microbiol. 11(736186)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Baseri M, Radmand F, Hamedi R, Yousefi M
and Kafil HS: Immunological aspects of dental implant rejection.
Biomed Res Int. 2020(7279509)2020.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Bressan E, Ferroni L, Gardin C, Bellin G,
Sbricoli L, Sivolella S, Brunello G, Schwartz-Arad D, Mijiritsky E,
Penarrocha M, et al: Metal nanoparticles released from dental
implant surfaces: Potential contribution to chronic inflammation
and peri-implant bone loss. Materials (Basel).
12(2036)2019.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Vinayak R, Rosh RM, Praveen J and Anirban
Chatterjee: Evaluation of titanium ions levels in blood in patients
with endosseous titanium dental implants using inductively coupled
plasma mass spectrometry-a retrospective study. Int J Sci Res.
8:115–123. 2019.
|
|
5
|
Fretwurst T, Buzanich G, Nahles S, Woelber
JP, Riesemeier H and Nelson K: Metal elements in tissue with dental
per-iimplantitis: A pilot study. Clin Oral Implants Res.
27:1178–1186. 2016.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Ferguson AB Jr, Laing PG and Hodge ES: The
ionization of metal implants in living tissues. J Bone Joint Surg
Am. 42-A:77–90. 1960.PubMed/NCBI
|
|
7
|
Mercuri LG, Miloro M, Skipor AK, Bijukumar
D, Sukotjo C and Mathew MT: Serum Metal levels in maxillofacial
reconstructive surgery patients: A pilot study. J Oral Maxillofac
Surg. 76:2074–2080. 2018.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bianco PD, Ducheyne P and Cuckler JM:
Titanium serum and urine levels in rabbits with a titanium implant
in the absence of wear. Biomaterials. 17:1937–1942. 1996.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Gopi G, Shanmugasundaram S, Krishnakumar
Raja VB and Afradh KM: Evaluation of serum metal ion levels in
dental implant patients: A prospective study. Ann Maxillofac Surg.
11:261–265. 2021.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Renvert S, Persson GR, Pirih FQ and
Camargo PM: Peri-implant health, peri-implant mucositis, and
peri-implantitis: Case definitions and diagnostic considerations. J
Periodontol. 89 (Suppl 1):S304–S312. 2018.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Nuevo-Ordóñez Y, Montes-Bayón M,
Blanco-González E, Paz-Aparicio J, Raimundez JD, Tejerina JM, Peña
MA and Sanz-Medel A: Titanium release in serum of patients with
different bone fixation implants and its interaction with serum
biomolecules at physiological levels. Anal Bioanal Chem.
401:2747–2754. 2011.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Temiz M, Dayi E and Saruhan N: Evaluation
of blood titanium levels and total bone contact area of dental
implants. Biomed Res Int. 2018(4121639)2018.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Saini RS and Kaur K: Analysis of serum
metal ion levels in dental implant patients. Int J Health Sci. 6
(S5):5645–5649. 2022.
|
|
14
|
Olmedo DG, Nalli G, Verdú S, Paparella ML
and Cabrini RL: Exfoliative cytology and titanium dental implants:
A pilot study. J Periodontol. 84:78–83. 2013.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Kilic S, Kazancioğlu H, Küçüksezer U,
Deniz G and Gülsüm AK: Evaluation of inflammatory cytokine and
plasma titanium levels in dental implant treated patients. Curr Res
Dent Sci. 24:199–205. 2015.
|
|
16
|
Mabilleau G, Bourdon S, Joly-Guillou ML,
Filmon R, Baslé MF and Chappard D: Influence of fluoride, hydrogen
peroxide and lactic acid on the corrosion resistance of
commercially pure titanium. Acta Biomater. 2:121–129.
2006.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Safioti LM, Kotsakis GA, Pozhitkov AE,
Chung WO and Daubert DM: Increased levels of dissolved titanium are
associated with peri-implantitis-a cross-sectional study. J
Periodontol. 88:436–442. 2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Kheder W, Al Kawas S, Khalaf K and
Samsudin AR: Impact of tribocorrosion and titanium particles
release on dental implant complications-A narrative review. Jpn
Dent Sci Rev. 57:182–189. 2021.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Kasai Y, Iida R and Uchida A: Metal
concentrations in the serum and hair of patients with titanium
alloy spinal implants. Spine (Phila Pa 1976). 28:1320–1326.
2003.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Wennerberg A, Ide-Ektessabi A, Hatkamata
S, Sawase T, Johansson C, Albrektsson T, Martinelli A, Södervall U
and Odelius H: Titanium release from implants prepared with
different surface roughness. Clin Oral Implants Res. 15:505–512.
2004.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Siirila HS and Kononen M: The effect of
oral topical fluorides on the surface of commercially pure
titanium. Int J Oral Maxillofac Implants. 6:50–54. 1991.PubMed/NCBI
|