Introduction
Prematurity, defined as live birth before 37 weeks
of gestation by the World Health Organization (WHO), poses a global
health challenge (1). In 2020,
there were 13.4 million cases of preterm birth (PTB), with
approximately one million resulting in mortality, as reported by
the United Nations (UN). These numbers indicate that 1 in 10 babies
worldwide are born prematurely (2). The number of PTBs has not improved
over the last decade, with 152 million PTBs occurring from 2010 to
2020. The majority of PTBs have occurred in sub-Saharan Africa and
Southern Asia, accounting for >65% of the cases globally
(2). Worldwide, there has been a
5.26% reduction in newborns born prematurely, decreasing from 16.06
million in 1990 to 15.22 million in 2019. Furthermore, deaths among
these newborns have significantly decreased by 47.71%, decreasing
from 1.27 million in 1990 to 0.66 million in 2019(3). Prematurity exposes infants to various
health risks, such as neurocognitive deficits, pulmonary issues,
heart rate irregularities, eye disorders, cerebral palsy, anemia,
neonatal sepsis and intraventricular hemorrhage (4).
PTB not only has significant medical implications,
but also poses substantial economic and social challenges for
families. In a study conducted in the USA, researchers examined the
healthcare costs associated with preterm and low-birth-weight (LBW)
infants (5). That study was a
retrospective cohort study using a national claims database of
individuals covered by Aetna, Inc. during the first 6 months of
life. The study included a total of 763,566 infants with a combined
healthcare expense of approximately $8.4 billion. Preterm infants
(n=50,511) had an average medical cost of $76,153, while LBW
infants had an average cost of $114,437. Among infants born at 24
weeks of gestation (n=418), the average cost per infant was the
highest at $603,778(5).
The pathophysiology of PTB involves various factors,
such as genetics, infection/inflammation, environment, oxidative
stress, progesterone resistance and an advanced maternal age
(6). Infection-related
inflammation is the primary cause, accounting for 40% of cases
(7). Experimental mouse models are
crucial for studying the mechanisms and potential therapies for
prematurity. Maternal inflammation in these models is induced using
bacterial lipopolysaccharide (LPS) endotoxin, which triggers the
release of cytokines, the infiltration of leukocytes and the
production of cyclooxygenase (COX)-induced prostaglandins (PGs),
endocannabinoids, reactive oxygen and nitrogen species (RONS) and
metalloproteinases (8). This
immune response leads to uterine contractions, membrane rupture and
cervical ripening (9). Mice with a
genetic predisposition to PTB (e.g., Trp53 deficiency) also
exhibit inflammation when exposed to LPS. These models provide
valuable tools for evaluating novel treatments for the challenges
associated with PTB (10,11).
The clinical management of preterm labor is
challenging as no single drug effectively addresses all its
mechanisms. Current therapeutics involve the use of tocolytics,
which delay preterm labor and enable the administration of
corticosteroids for neonatal lung maturation (12). Tocolytics include magnesium
sulfate, β-mimetics, calcium channel blockers, PG inhibitors and
oxytocin receptor antagonists (13). However, these drugs have
limitations and potential side-effects, and there is insufficient
evidence for significant neonatal benefits (14). Existing tocolytics also have
limitations and lack strong evidence for maternal or neonatal
outcomes (15,16). Consequently, efforts are underway
to discover novel tocolytic agents that are more effective and
safer. One such promising candidate is resveratrol.
Resveratrol (3,5,4'-trihydroxy-trans-stilbene) is a
natural polyphenol known for its beneficial effects on inducing
preterm labor (11,17) and other pregnancy-related
complications (18,19). It exhibits anti-inflammatory and
anti-aging properties, rendering it a potential therapeutic target
for PTB (8,17). However, there is a lack of
comprehensive studies on the specific benefits of resveratrol in
preventing prematurity. Therefore, the present systematic review
aimed to explore the potential of resveratrol in preventing
prematurity, including its pharmacological profile, content in
various plant species and therapeutic applications.
Data and methods
Protocol
The present descriptive qualitative systematic
review followed the Preferred Reporting Items for Systematic Review
and Meta-Analyses (PRISMA) statement (20).
Information sources and search
strategy
The search, conducted in July-August 2023, aimed to
identify studies on the mechanisms of resveratrol in preventing PTB
in animal models. The present systematic review followed the PICOS
format: Population (P)-Pregnancy with induced PTB; Intervention
(I)-Resveratrol; Comparison (C)-Negative control (untreated with
resveratrol); Outcome (O)-Prevention of PTB (tocolytic effect);
Study Design (S)-Preclinical studies. Specific search strategies
were used for various databases, including MEDLINE via PubMed,
ProQuest, EBSCOhost and EMBASE. The search queries and MESH
keywords combined with Boolean operators were used, such as
‘Resveratrol’ (Mesh) AND [‘Premature Birth’ (Mesh) OR ‘Obstetric
Labor, Premature’ (Mesh)].
Study selection and data collection
process
The studies were selected based on the eligibility
criteria using the PICOS strategy. The inclusion criteria were as
follows: i) English-language articles; ii) primary experimental
studies utilizing resveratrol (in vitro and in vivo);
and iii) outcomes related to PTB. The exclusion criteria
encompassed the following: i) Duplicated articles; ii) abstracts
and reviews lacking specific data; iii) clinical studies,
systematic reviews, case reports, retrospective studies, theses,
letters, editorials, opinions, surveys, guidelines, conferences,
abstracts and commentary articles. Initially, a search was
performed for human population studies; however, as none were
found, a focus was placed solely on reviewing experimental animal
studies. Articles focusing solely on the benefits of resveratrol
for preterm infants after birth were also excluded. In total, the
authors (MH, MIDR and ABP) independently screened titles and
abstracts, removing duplicates. Eligible articles had their full
texts examined for confirmation. No restrictions on publication
dates were applied to the systematic review. The search process is
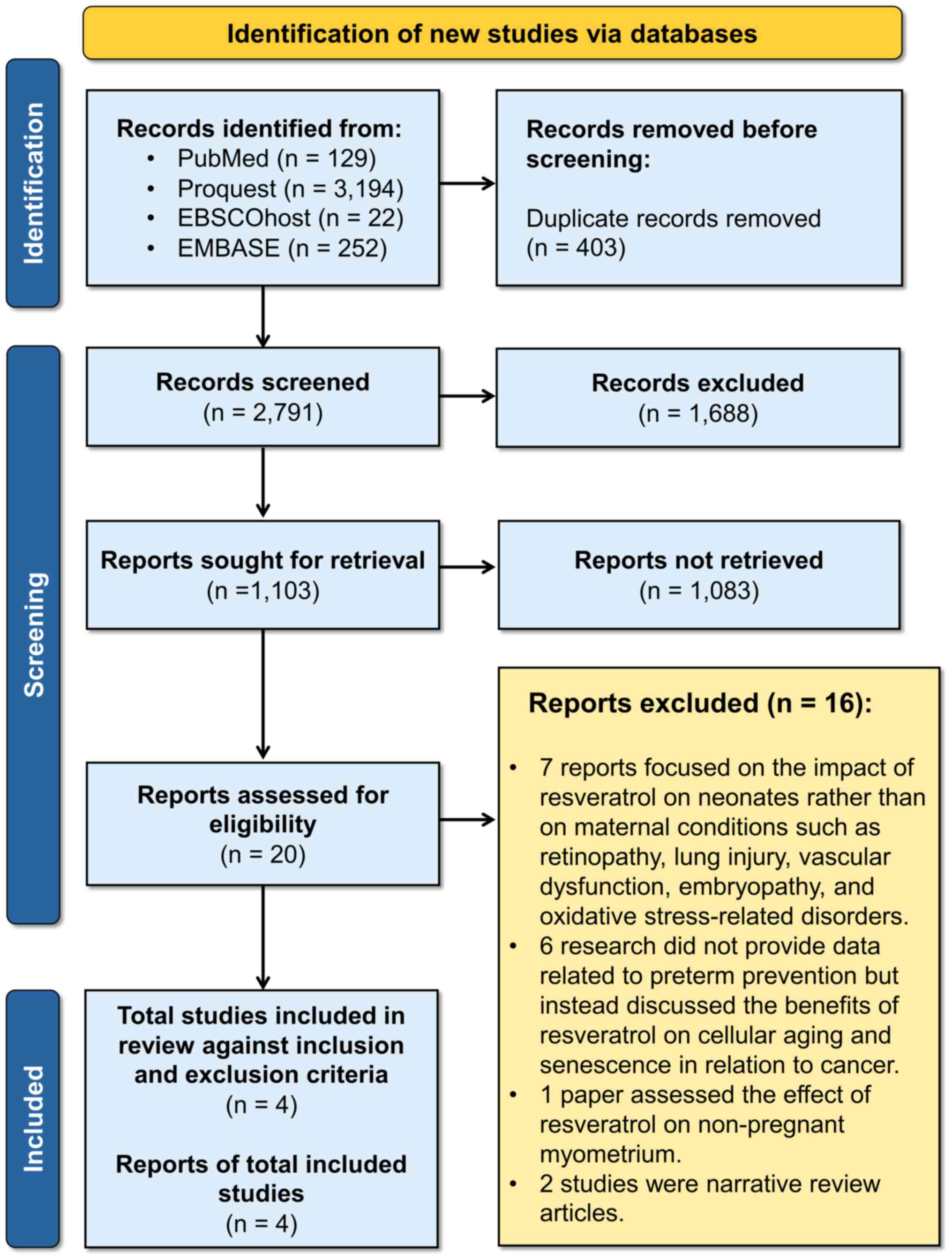
illustrated in Fig. 1.
Quality assessment and risk of
bias
To assess the quality of the studies, the ToxRTool
(Toxicological Data Reliability Assessment Tool), developed by
ECVAM (European Centre for the Validation of Alternative Methods)
(21) was used. This tool provides
criteria for evaluating the quality of pharmacology research using
in vitro and in vivo experimental study designs.
Previous systematic reviews that utilized the ToxRTool were also
considered to determine the methodological reliability and inherent
flaws in the studies (22-24).
The ToxRTool consists of a 21-point rating checklist that assesses
the methodological aspects of each study across five categories
(25). These categories cover
various aspects, including Category 1 (test substance
identification), which includes details about (1.1) the substance,
(1.2) purity, (1.3) source, and (1.4) nature and physico-chemical
properties; Category 2 (test organism characterization), which
includes (2.1) species (for in vivo studies) or test system
(for in vitro studies), (2.2) sex (for in vivo
studies) or the origin or source of the test system with the
corresponding sex characteristic (for in vitro studies),
(2.3) strain of test animals (for in vivo studies) or the
specification of cell/tissue culture (for in vitro studies),
(2.4) age or body weight of test organisms at the start of the
study (for in vivo studies) or relevant clinical
characteristics of human donors for cell/tissue cultivation (for
in vitro studies), and (2.5) housing or feeding conditions
in repeated dose studies (for in vivo studies) or
cultivation process maintenance (for in vitro studies);
Category 3 (study design description), which includes (3.1)
administration method, (3.2) administered doses or concentration in
the application media, (3.3) exposure frequency, duration, and
explanation of observation time-points, (3.4) inclusion of negative
and positive controls, (3.5) the number of animals (for in
vivo studies) or the amount of cell/tissue culture per group
(for in vitro studies), (3.6) administration scheme details
for study evaluation, and (3.7) verification of achieved
concentrations or substance stability in repeated dose studies;
Category 4 (study endpoints), which includes (4.1) clear
description and determination methods of endpoints, (4.2)
transparent and comprehensive results description for all
endpoints, and (4.3) transparent statistical methods for data
analysis; and Category 5 (study design appropriateness and
reliability), which includes (5.1) appropriate choice of study
design for substance-specific data and (5.2) reliability of
quantitative results. Studies with <13 points are deemed
unreliable, those with 13-17 points are considered reliable with
potential restrictions, and those scoring 18-21 points are deemed
reliable without restrictions.
In addition, a risk of bias (RoB) assessment tool
based on the Cochrane Handbook (26) was used with modifications that
integrated the Office of Health Assessment and Translation,
National Institute of Health (OHAT-NIH), and the National
Toxicological Program tools, as used in previous studies (27-29).
Three authors independently assessed all included studies for bias
risk across nine parameters (randomization, allocation concealment,
experimental conditions, blinding during the study, data
completeness, exposure characterization, outcome assessment,
selective reporting and data sufficiency). The responses were
categorized as low risk (yes, provided information), unclear risk,
or high risk (no, absent information) of bias following predefined
protocol criteria. Any disagreements, conflicts, or discrepancies
regarding the eligibility of studies were resolved through
discussion and consensus to determine inclusion.
Data extraction
Data extraction was performed using Microsoft Office
Excel 365. Three reviewers independently organized the data into
variables based on the review's aims and topics. These variables
included publication year, author(s), first author's country, study
design, level of evidence (LoE) according to the Oxford Centre of
Evidence-based Medicine (CEBM) criteria (30), preterm model sample, sample size,
induced diseases and definitions. The outcomes data included the
dose of resveratrol and administration details, treatment duration
and frequency, additional drugs used, and associated study
outcomes.
Synthesis of results
The relevant data of interest regarding the
aforementioned variables were collected and organized into summary
tables for study characteristics and outcomes using an electronic
spreadsheet. The studies were further grouped based on similarities
in reducing preterm rates and common underlying mechanisms.
Results
Study selection
The PRISMA flow diagram was followed for allocation
concealment, blinding, article review, and data extraction.
Initially, 3,597 results were retrieved from all databases, which
were reduced to 2,791 after removing duplicates. Screening based on
titles and abstracts resulted in 1,103 articles, and after applying
inclusion and exclusion criteria, 20 full-text articles were
assessed for eligibility. Ultimately, only four articles were
eligible for analysis and review. Additionally, searches were
conducted on Google Scholar and various Gray Literature websites
(http://www.opengrey.eu/search/,
https://v2.sherpa.ac.uk/opendoar/,
https://www.worldcat.org/, and https://ukhsalibrary.koha-ptfs.co.uk/greylit/).
However, no additional results were obtained beyond those included
in the initial selection from the central journal databases.
Study exclusion
A full-text assessment was conducted after removing
all duplicate references using Zotero version 6.0.26 as the
reference manager. The exclusion criteria included studies that
focused on the effects of resveratrol on neonates as opposed to
maternal conditions, such as retinopathy, lung injury, vascular
dysfunction, embryopathy and oxidative stress-related disorders
(n=7). Studies that discussed the benefits of resveratrol for
cellular aging and senescence in cancer were also excluded (n=6).
Additionally, a study that assessed the effects of resveratrol on
non-pregnant myometrium was excluded (n=1), as well as narrative
reviews (n=2).
Reliability and quality
assessment
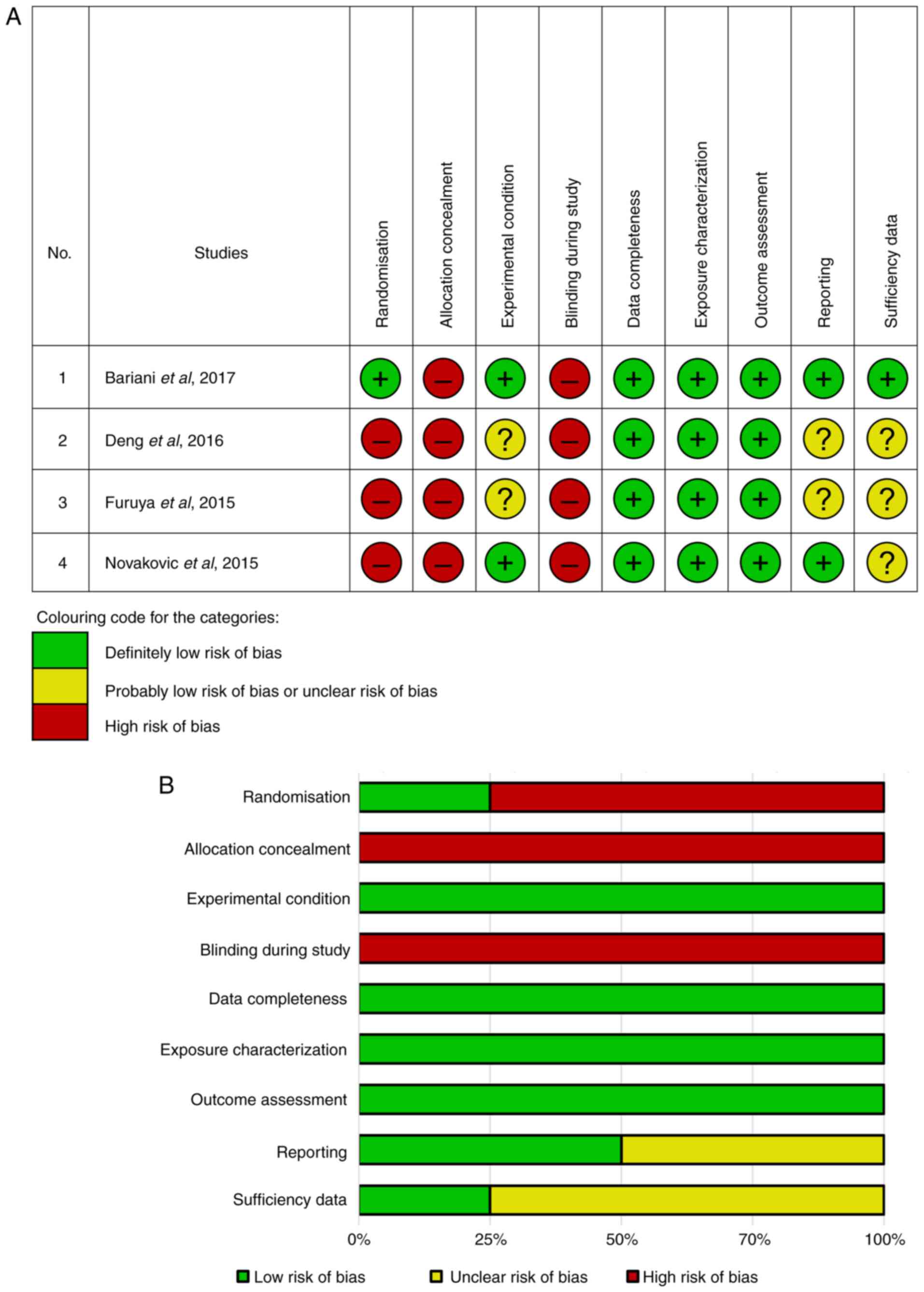
As regards the reliability assessment (Table I), two studies [Bariani et
al (8) and Novaković et
al (31)] were deemed fully
reliable and were included in the systematic review. However, two
other studies [Deng et al (11) and Furuya et al (17)] were labeled as ‘reliable with
restrictions’ due to methodological uncertainties and the absence
of critical quality measurement categories. Specifically, the study
by Deng et al (11) lacked
information on the purity and origin of resveratrol, the age and
other characteristics of the mice used, the environmental
conditions for the mice, positive controls, a precise number of
samples, and details on the stabilization and extraction/obtaining
of resveratrol. Similarly, the study by Furuya et al
(17) had limitations in sample
characteristics and conditioning during the experiment, as well as
a lack of a positive control and information on the stabilization
of resveratrol. Detailed assessments for each category are provided
in Table SI, Table SII and Table SIII.
 | Table IReliability assessment of the
selected articles according to the ToxRTool in vivo and
in vitro criteria. |
Table I
Reliability assessment of the
selected articles according to the ToxRTool in vivo and
in vitro criteria.
| | Studies included in
the systematic review [Authors/(Refs.), year of publication |
|---|
| Reliability of
study assessment | Bariani et
al (8), 2017 | Deng et al
(11), 2016 | Furuya et al
(17), 2015 | Novaković et
al (31), 2015 |
|---|
| Criteria I: Test
substance identification (maximum point 4) | 4 | 2 | 4 | 4 |
| Criteria II: Test
organism characterization (maximum point 5) | 4 | 3 | 3 | 4 |
| Criteria III: Study
design description (maximum point 7) | 5 | 4 | 5 | 5 |
| Criteria IV: Study
results documentation (maximum point 3) | 3 | 3 | 3 | 3 |
| Criteria V:
Plausibility of study design and data (maximum point 2) | 2 | 1 | 2 | 2 |
| Total score | 18 | 13 | 17 | 18 |
| Reliability
classification | Reliable without
restrictions | Reliable with
restrictions | Reliable with
restrictions | Reliable without
restrictions |
In the risk of bias summary (Fig. 2), Bariani et al (8) had a low risk of bias in seven out of
nine domains in each RoB category, with two aspects rated as high
risk. Novaković et al (31)
had a low risk of bias in five of nine domains in each RoB
category, with one aspect considered unclear risk and the remaining
three as high risk. Deng et al (11) and Furuya et al (17) each demonstrated a low risk of bias
in three domains, an unclear risk in three other domains, and three
domains with high risk. Despite all the limitations in the
reliability and quality assessment, all studies were eligible to be
assessed and reviewed in the present systematic review. According
to the CEBM levels, they all fall under the fifth level of EBM (1
to 5).
Study design and outcomes
measurement
The present systematic review included two
experimental in vivo studies [Bariani et al (8) and Furuya et al (17)] and one study combining animal and
cell experiments [Deng et al (11)]. All these studies investigated the
effect of resveratrol on preventing PTB in animal models.
Additionally, one in vitro study [Novaković et al
2015(31)] focused on the
tocolytic and myometrium relaxant effects of resveratrol using
human myometrium tissue to determine the preterm mechanisms. In
this section, several studies that examined the preventive effects
of resveratrol on PTB are presented. These studies include
information about its characteristics (Table II), as well as its beneficial
preventive effects on PTB (Table
III).
 | Table IICharacterization of the articles
included in the systematic review. |
Table II
Characterization of the articles
included in the systematic review.
| Authors, year of
publication | Region | Study Design | LoE | Sample | Sample size | Preterm induction
model | Disease induction
and operational definition | (Refs.) |
|---|
| Bariani et
al, 2017 | Argentina | In vivo | 5 | 15-days pregnant
BALB/c mice | ±35-41 mice | Inflammatory
induction: LPS i.p. (0.17 mg/kg and 0.5 mg/kg after 3 h) on day
15 | • LPS-induced
preterm labor • Preterm labor was defined as labor occurring 19
days | (8) |
| Deng et al,
2016 | USA | In vivo and
in vitro | 5 | •
Trp53fl/fl PgrCre/+ mice
(p53d/d mice) and floxed p53 mice
(p53fl/fl), which are genetically predisposed to
PTB • Human uterine fibroblast (HuF) cells, which were decidualized
in vitro and placenta obtained from term and pre- term (at
23 weeks) vaginal delivery. • p53fl/fl and
p53d/d uterine stromal cells, which were
decidualized in vitro obtained from 4-days pregnant mice •
Mouse embryonic fibroblasts (MEFs) from Prkaa1-/-
Prkaa2-/- double knockout (AMPK dKO), WT, and
p53-/ mice | • The exact number
of mice was not mentioned • Samples from 3 women were used for
human cell studies | • In vivo
model: conditional uterine deletion of the tumor suppressor gene
p53 • Superimposed inflammatory insult: LPS (10 µg)
administered 4 h following a progesterone injection (1-2 mg/mouse)
on day 16 • In vitro model: Hormone treatment to induce
decidualization in cultured uterine stromal cells | • Spontaneous PTB
in p53d/d females, both with and without dystocia
• Preterm labor was defined as labor occurring before 19 days •
Dystocia was defined as a problematic delivery lasting >12
h | (11) |
| Furuya et
al, 2015 | Japan | In vivo | 5 | 7-days pregnant
C57BL6 mice | 12 mice | Inflammatory
induction: Transvaginal administration of LPS (10 µg in 200 ml
saline) to the cervix and uterus on day 15 | • LPS-induced
preterm labor • Preterm labor was defined as labor occurring before
19 days. • PTB occurred within 48 h | (17) |
| Novaković et
al, 2015 | Serbia | In
vitro | 5 | Myometrial samples
were obtained from females undergoing an IVF program and currently
scheduled for elective C-section (due to cephalopelvic
disproportion) in the third trimester of pregnancy. Biopsies were
excised from the mid-line portion of the upper lip of the incision
in the lower uterine segment | Myometrial tissue
samples were collected from 42 non-laboring women | Contraction
inducer: Oxytocin (20 nM) | • Oxytocin-induced
phasic contractions of constant amplitude and frequency in
human-term pregnant myometrium • This study did not specifically
focus on preterm labor, but the outcomes are still relevant in the
context of tocolytic research | (31) |
 | Table IIISummary of the articles analyzed in
the systematic review focusing on the effect and potential benefit
of resveratrol in preventing preterm birth. |
Table III
Summary of the articles analyzed in
the systematic review focusing on the effect and potential benefit
of resveratrol in preventing preterm birth.
| Authors, year of
publication | Resveratrol dose
and route | Duration
frequency | Additional
drug | Outcomes | (Refs.) |
|---|
| Bariani et
al, 2017 | 3 mg/kg, PO | On day 15, per 8
h | N/A | • Resveratrol
reduced the proportion of PTB compared to the control group (64%
vs. 85%) • Resveratrol reduced stillbirth compared to the negative
control group (stillbirth, 34 vs. 62%) • Resveratrol prevented
antepartum hemorrhage based on macroscopic examination. •
Resveratrol may protect against pathological PTB by reducing
uterine NOS activity, the expression of iNOS, COX-2, PGE2, and AEA
profiling (P<0.05) • Resveratrol altered the uterine
endocannabinoid profiling that was previously affected by LPS | (8) |
| Deng et al,
2016 | 30 mg/kg PO | On days 8,10,12 and
14 | • A single
injection of progesterone (1-2 mg/mouse) was administered 4 h
before LPS induction • Metformin 1 mg/kg was administered on days
8, 10 and 12 separately (not combined with resveratrol) | • The combination
of resveratrol + progesterone is an effective approach for
targeting both decidual health and compensating ovarian luteolysis
• Resveratrol or progesterone alone could not prevent the incidence
of PTB and did not increase the number of live mouse pups •
Resveratrol reduced PGE2 levels with limited effects on PGF2α
levels in both p53fl/fl and
p53d/d decidual cells, as indicated by lower
expression of Ptgs2 (encoding COX2) in mice and human
decidual cells treated with resveratrol • Resveratrol has an
inverse regulatory effect on AMPK and mTORC1 signaling in decidual
cells, controlling parturition timing to prevent PTB • Resveratrol
did not appear to have detrimental effects on pregnancy outcomes •
Progesterone injection alone showed some adverse effects on pup
viability, likely due to the normal decline in progesterone levels
approaching parturition | (11) |
| Furuya et
al, 2015 | 20 and 40 mg/kg
PO | On days 12-14
1x/day; On day 15 2x/day (at 6 to 12 h after LPS injection) | N/A | • Resveratrol
decreased the rate of PTB compared to the control group (48.6±19.4
vs. 97.8±1.9%) • Resveratrol may protect against pathological PTB
by suppressing the elevated levels of pro-inflammatory cytokines
TNF-α (16.7 vs. 53.9 pg/ml) and IL-1β (88.4 vs. 403 pg/ml), but not
IL-6 levels in peritoneal washes • Resveratrol inhibited mRNA
expression of TNF-α in uterine cervices, but not IL-1β and IL-6
levels • Resveratrol dose-dependently reduced mRNA expression of
TNF-α and IL-1β, but not IL-6 levels, in peritoneal macrophages •
Resveratrol suppressed the pro-inflammatory cytokine-mediated
elevation of COX-2 mRNA levels produced by peritoneal
macrophages | (17) |
| Novaković et
al, 2015 | A range between
1-100 µM PO | Resveratrol was
incrementally added to the bathing solution, allowing for
equilibrium response to be achieved within ~20 min | • Glibenclamide (10
µM) • Iberiotoxin (100 nM) 4-Amino- pyridine (1 mM) | • Resveratrol with
the concentration of 1-100 µM might inhibit the amplitude of
oxytocin-induced contractions in a concentration-dependent manner
(pD2 4.52+0.11, maximal responses: 82.25+1.50%) • Resveratrol
significantly reduced spontaneous rhythmic contractions and the
amplitude of phasic contractions induced by oxytocin but had no
effect on tonic contractions induced by oxytocin • Resveratrol
suppressed the contractility of human term pregnant myometrium by
modulating different myometrial K+ channels, including
the activation of KATP channels, Kv channels, and BKCa channels
through an increase in intracellular Ca2+ • Resveratrol
may induce relaxation of pregnant myometrium, at least partially,
through the activation of Kir6.2/SUR1 channels. Resveratrol at a
concentration of 10 mM showed insensitivity to all K+
channel blockers | (31) |
Discussion
Complications arising from PTB are a major
contributor to neonatal mortality and the second leading cause of
mortality among children under the age of 5 years. Surviving
children often experience lifelong disabilities. The reduction of
PTB rates is a global priority, due to its significant impact on
healthcare and the economy (5).
Addressing prematurity continues to be a significant challenge in
the field of maternal and child health, as there is no single drug
that effectively targets all the underlying pathways leading to
prematurity. Each tocolytic agent has its own limitations and
potential side-effects, with limited evidence of efficacy in
improving neonatal outcomes (32).
The present systematic review examined four articles
that explored the role of resveratrol in cases of prematurity. The
studies were conducted between 2015 and 2017, with two taking place
in developing countries (Argentina and Serbia) (8,31)
and the other two in developed countries (USA and Japan) (11,17).
The analysis included two in vivo studies conducted on mice
(8,17), one in vitro study using
human myometrial tissue (31), and
one study that combined both in vivo and in vitro
research (11). In the in
vivo studies, PTB was induced using LPS, and resveratrol was
administered orally (8,11,17).
However, the dosage and timing of resveratrol varied, ranging from
3 to 40 mg/kg on days 8 to 15. In the in vitro studies,
resveratrol was applied as a bath to cell tissue that had been
previously exposed to oxytocin (11,31).
To the best of our knowledge, there is no published study available
on the potential impact of resveratrol dosages on human tissues and
cells. However, the available studies have indicated that when
resveratrol is orally administered to mice, the compound remains
unaltered and is retained within the tissues (33).
Following oral administration, low concentrations of
resveratrol have been detected in both the plasma and tissues of
humans and experimental animals (34). Studies that involved the
incremental dosing of resveratrol from 25 to 5,000 mg consistently
demonstrated an increase in plasma concentrations without any signs
of metabolic saturation. However, even at the highest dose of 5,000
mg, the observed peak plasma levels only reached ~500 ng/ml. There
is a possibility of metabolic saturation with prolonged or repeated
dosing, which may result in elevated resveratrol levels in plasma
and tissues (35). Although it
remains a hypothesis, studies have suggested that it may be
possible to achieve biologically active concentrations of
resveratrol and/or its metabolites in human subjects through
chronic dosing (36,37).
Bariani et al (8) and Furuya et al (17) demonstrated that resveratrol
effectively reduced the incidence of PTB in mice through the
reduction of pro-inflammatory cytokines (e.g., TNF-α and IL-1β).
Novaković et al (31)
demonstrated that resveratrol significantly decreased both rhythmic
contractions and contraction amplitude. However, Deng et al
(11) argued that resveratrol
alone may not be sufficient in preventing PTB events. By analyzing
these four articles alongside supporting literature, the authors
compiled a comprehensive understanding of the risk factors and
pathophysiology of prematurity. Additionally, the present study
explored the mechanisms of action through which resveratrol targets
prematurity mechanisms, the efficacy of resveratrol as a preventive
agent for PTB, and the pharmacological characteristics of
resveratrol, from experimental settings to clinical
applications.
Pathophysiology of prematurity in
cellular and animal studies
The limited understanding of the complex etiology of
PTB is a barrier to the development of effective strategies for its
prevention. Recognized risk factors for PTB include multiple
pregnancies, chorioamnionitis, maternal diseases, genetic factors,
previous PTB and uterine abnormalities. However, not all cases of
PTB have identifiable risk factors. The pathophysiology of PTB
involves various processes, including myometrial contraction and
extracellular matrix degradation. Among these processes,
inflammation, which is initiated following implantation, plays a
crucial role in PTB (37,38). Experimental animals, primarily mice
or rats, play a critical role in studying the pathophysiology of
PTB. The commonly used inflammation-inducing agent is LPS, derived
from Escherichia coli endotoxins, administered intrauterine
or intraperitoneally (39). In
addition to LPS, various methods, including hormonal or immune
agents, can induce PTB in mice (39).
Immunity, both innate and adaptive, plays a crucial
role in the incidence of PTB. In mouse studies, macrophages induce
PTB by producing pro-inflammatory cytokines such as matrix
metallopeptidases, IL-1, IL-6 and IL-8. In the mouse model, PTB can
be prevented by inhibiting the IL-1 receptor via the NF-κβ pathway
(40). IL6-deficient mice exhibit
resistance to LPS-induced PTB (41). Complement activation is also
significant in preventing PTB, as women with spontaneous preterm
labor have increased levels of complement C3a, C4a and C5a in
infection-associated cases. Mice lacking the C5aR complement
receptor demonstrate resistance to LPS-induced PTB in vitro
(42).
In addition to the role of the innate immune system
in PTB, the adaptive immune response is significant. PTB often
occurs due to maternal intolerance to fetal antigens. Regulatory
T-cells (Tregs) and effector T-cells (Teffs) undergo changes in
frequency and phenotype. Tregs in women in preterm labor exhibit
differential activation and reduced suppressive capacity (6). Toll-like receptors (TLRs) are another
crucial component of the immune response. They activate signaling
pathways that lead to cytokine and chemokine secretion by innate
immune cells. TLR activation initiates inflammatory pathways that
result in vaginal and preterm labor, immune cell recruitment, PG
and MMP production, cervical ripening, and uterine contractions.
TLR4 and TLR2 influence the timing of delivery during pregnancy
(43,44). In addition to maternal TLR
expression, fetal TLR expression may impact birth timing, with
polymorphic alleles of fetal TLR4 and TLR2 associated with
prematurity (45).
Current research explores the role of the microbiome
role in modulating the risk of PTB. The composition of the vaginal
microbiota influences the risk of PTB, with dysbiosis linked to
increased levels of pro-inflammatory cytokines, such as CXCL10, in
early pregnancy (46). However,
administering pre-conception antibiotics to women with a history of
PTB does not reduce the rates of PTB; instead, it is associated
with a lower occurrence of LBW babies and early labor (47).
Among the various mechanisms associated with PTB,
including innate immunity, adaptive immunity and dysbiosis, there
is a notable interest in elucidating the mechanisms through which
resveratrol intervenes in these processes. Resveratrol demonstrates
antimicrobial properties against various pathogenic microorganisms,
encompassing both Gram-positive and Gram-negative bacteria, as well
as fungi. It can inhibit the growth of specific bacterial species
at concentrations below 100 µg/ml, although higher concentrations
are required to inhibit the growth of many bacterial organisms
(48). The mechanism of action of
resveratrol involves multiple pathways. It inhibits the electron
transport chain and F0F1-ATPase, resulting in reduced cellular
energy production. Resveratrol also interferes with DNA through the
formation of a Cu(II)-peroxide complex and suppresses cell division
by targeting the FtsZ gene. Moreover, it is effective in preventing
biofilm formation and acts as both an antibiofilm and an
anti-quorum sensing agent (49).
The additional proposed mechanisms of action of resveratrol have
been succinctly outlined in Fig.
3.
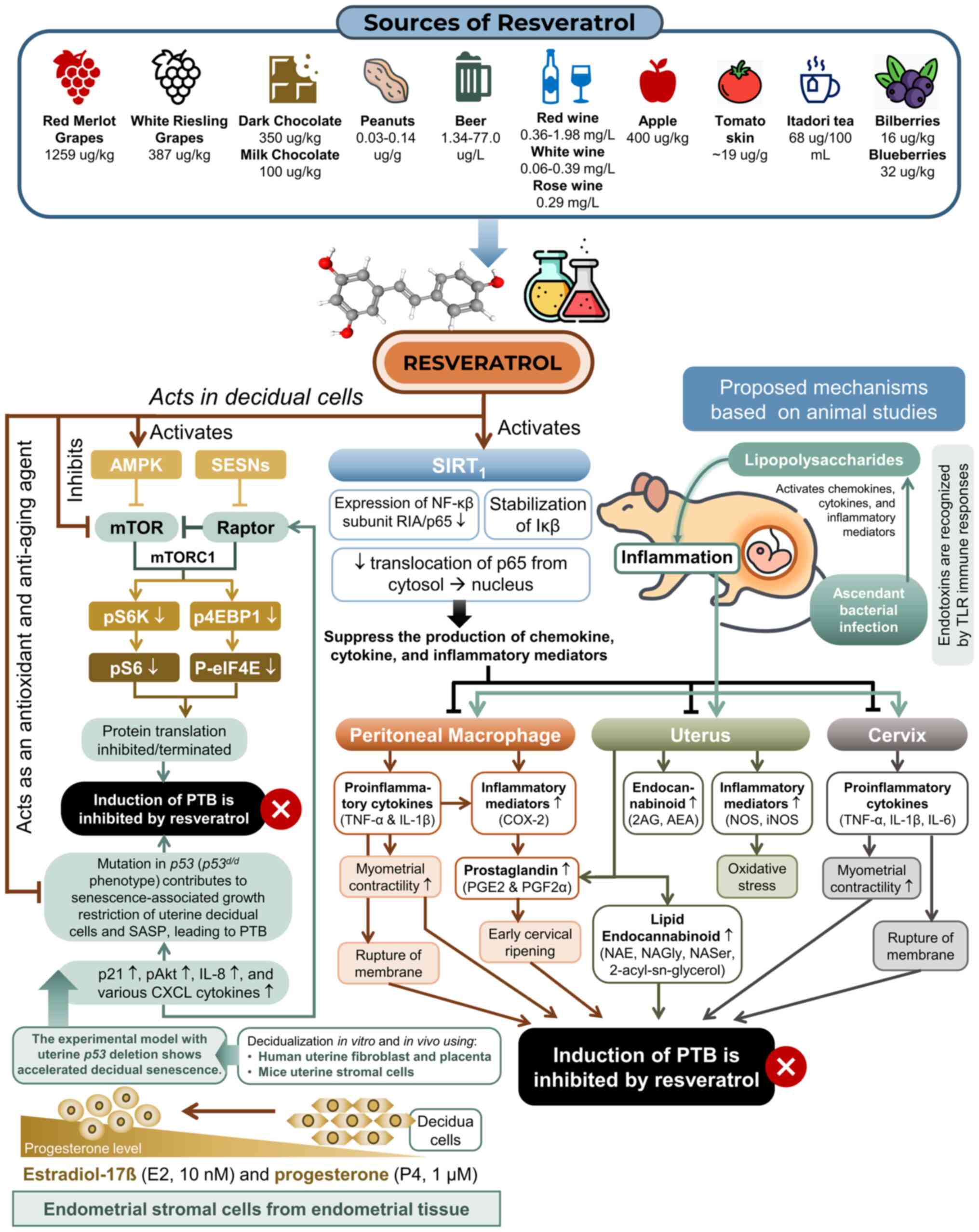 | Figure 3Proposed mechanism of action of
resveratrol in preventing PTB. The mechanism involves reducing
inflammatory mediators and cytokines in the uterus, cervix and
peritoneum, suppressing prostaglandin production, and modulating
the endocannabinoid profile. Experimental studies using a
transgenic mammalian model with uterine p53 deletion
revealed accelerated decidual senescence, characterized by elevated
levels of p21, p-Akt, IL-8 and various CXCL cytokines, collectively
contributing to the SASP. In p53d/d females, this
senescence-associated growth restriction of uterine decidual cells
occurs, and at this stage, resveratrol functions as an antioxidant
and anti-aging agent by activating AMPK and inhibiting mTORC1
signaling in decidual cells (8,11,17,31).
AMPK, AMP-activated protein kinase; g, gram; IκB, IκB kinase; IL,
interleukin; COX-2, cyclooxygenase 2; iNOS, inducible nitric oxide
synthase; kg, kilogram; L, liter; mg, milligram; ml, milliliter;
mTOR, mammalian target of rapamycin; mTORC1, mTOR complex 1; NAE,
N-acyl ethanolamine; NAGly, N-arachidonoyl glycine; NASer,
N-arachidonoyl serine; NF-κβ, nuclear factor kappa B; nM,
nanomolar; NOS, nitric oxide synthase; PGE2, prostaglandin E2;
PGF2α, prostaglandin F2 alpha; pAKT, phosphorylated Akt; PTB,
preterm birth; SASP, senescence-associated secretory phenotype;
SESNs, sestrin protein; TLR, Toll-like receptor; TNF-α, tumor
necrosis factor-α; µg, microgram; µM, micromolar. |
Mechanisms of action: Resveratrol
targeting the mechanism of prematurity. Reduction of inflammatory
mediators [nitric oxide (NO) synthase (NOS) and inducible NOS
(iNOS)] in the uterus
The beneficial effects of resveratrol in
inflammation-induced preterm delivery involve multiple pathways.
One of these pathways is the decrease in the activity of uterine
NOS and the protein expression of uterine iNOS. The excessive
production of NO leads to the formation of RONS, which are
associated with tissue damage, oxidative and nitrative stress
(50). To assess the activity of
NOS and the protein expression of iNOS in the uterus, measurements
were taken 5 h following the injection of LPS. The results
indicated an increase in NOS and iNOS activity in mice treated with
LPS alone. Bu contrast, mice treated with resveratrol after LPS
exposure showed no significant increase in NOS activity (51). This reduction in uterine NOS and
iNOS activity is beneficial as it prevents excessive production of
NO and its associated adverse effects, such as damage to
reproductive tissues and oxidative stress (51).
Reduction of levels of inflammatory cytokines
(TNF-α, IL-1β and IL-6) in the cervix and peritoneum.
Resveratrol is known to inhibit the activity of NF-κβ, a
transcription factor involved in the expression of pro-inflammatory
cytokines, in various immune response-related disease models
(52). This factor operates not
only in immune cells but also in the uterine myometrium, decidual
stroma and amniotic cells (17).
It promotes the production of cytokines, such as TNF-α, IL-1β, IL-6
and IL-8, which are crucial for inducing labor, particularly in
preterm cases (53,54). Resveratrol appears to inhibit this
NF-κβ/cytokine loop (17).
Furuya et al (17) focused on murine pro-inflammatory
cytokines and observed significant increases in the levels of
TNF-α, IL-1β and IL-6 in peritoneal washes and cervical tissues
following exposure to LPS. In their in vivo model,
resveratrol effectively suppressed the elevation of TNF-α and IL-1β
levels induced by LPS, with a slight reduction in IL-6 levels
(17). Although IL-6, produced by
various stromal cells, was not as markedly suppressed, it may be
due to its induction by other pro-inflammatory cytokines and direct
stimulation by LPS (55). Since
the preterm delivery model is initiated by the stimulation of TLRs
in macrophages by LPS, inhibiting the expression of TNF-α and IL-1β
in peritoneal washes and the uterine cervix directly suppresses
this signaling pathway (17).
Resveratrol effectively suppresses the production of
pro-inflammatory cytokines in the cervix, which is a key factor in
the pathogenesis of PTB. PTB often involves local inflammation
caused by microbial infection, resulting in elevated levels of
cytokines, PGs, uterine contractions, and cervical ripening
(56). In the study by Furuya
et al (17), cervical
tissues exposed to LPS in the control group exhibited significantly
higher mRNA levels of TNF-α, IL-1β and IL-6 compared to the group
treated with resveratrol(17).
Resveratrol notably reduced the peak levels of TNF-α and IL-1β
mRNA, particularly resulting in significantly lower TNF-α levels
(0.54±0.09) in the resveratrol-treated group compared to the
control group (2.45±0.93) (17).
Resveratrol also demonstrates anti-inflammatory effects on
placental tissues in pregnant women by downregulating the sirtuin-1
gene post-delivery (57). Its
mechanism involves inhibiting the transcription activity of NF-κB,
reducing the expression of the NF-κβ sub-unit RelA/p65, and
stabilizing inhibitory I-κβ (57,58).
Previous studies have demonstrated that resveratrol can suppress
inflammatory responses in endometrial stromal cells in patients
with endometriosis. The excessive production of pro-inflammatory
cytokines can further stimulate the overproduction of PG, leading
to increased uterine contractions and cervical maturation (59). Therefore, the ability of
resveratrol to suppress pro-inflammatory cytokines in the cervix
holds promising therapeutic implications for preventing PTB.
The pathogenesis of PTB involves both the cervix and
the peritoneal area. In the study by Furuya et al (17), the injection of LPS into the cervix
significantly increased the levels of pro-inflammatory cytokines
(TNF-α, IL-1β, and IL-6) in peritoneal washes, with IL-1β spiking
to levels 480 times higher (17).
This led to intrauterine inflammation and PTB in the experimental
model. Peritoneal washes assessed the local immune responses to
LPS, and the administration of resveratrol markedly decreased the
levels of TNF-α and IL-1β. TNF-α levels were reduced to one-third,
and IL-1β to about one-fourth, in mice treated with resveratrol
compared to controls. However, there was no significant difference
in IL-6 levels between the groups (17). Furuya et al (17) also demonstrated that resveratrol
prevented the increased production of TNF-α and IL-1β by peritoneal
macrophages in response to LPS. Comparing the local immune
responses within 4 h of the injection, the results of peritoneal
lavage revealed a significant decrease in TNF-α and IL-1β levels
following the administration of resveratrol (17). TNF-α levels were approximately
one-third in mice treated with resveratrol (16.7 vs. 53.9 pg/m),
while IL-1β levels were approximately one-quarter (88.4 vs. 403
pg/ml, P<0.01). However, there was no significant difference in
IL-6 levels between the resveratrol-treated mice and the controls
(17).
Reducing inflammatory mediators from peritoneal
macrophages. COX-2 enzymes are upregulated in response to
pro-inflammatory cytokines, particularly in macrophages. This leads
to elevated cytokine-mediated PGs, including PGE2 and PGF2α, which
are significant in the mechanisms of PTB (60,61).
TNF-α and IL-1β are central to the COX-2 pathway, and macrophages,
responsible for their production, are suspected to play a role in
the local inflammatory mechanism of PTB (8). In the study by Furuya et al
(17), LPS-exposed peritoneal
macrophages exhibited a significant increase in COX-2 mRNA levels
for TNF-α and IL-1β. Resveratrol treatment effectively suppressed
COX-2 production in these macrophages. This aligns with prior
observations in peritoneal washes and cervical tissues by Furuya
et al (17). Resveratrol
reduced TNF-α, IL-1β, and COX-2 mRNA levels in macrophages induced
by LPS in a concentration-dependent manner (17). Macrophages play a crucial role in
COX-2 production and subsequent PGE2 and PGF2α production. A
previous study demonstrated that omega-3 fatty acids, which also
have antioxidant and anti-inflammatory properties, can suppress
macrophage function and the elevation of PGE2 and PGF2α in
gestational tissues induced by LPS (62). These findings collectively suggest
that resveratrol primarily targets uterine-associated macrophages,
offering the potential to modulate the local inflammatory response
in PTB.
Reduction of COX-2 and PGs in the uterus.
COX-2 produces PGE2 and PGF2α, potent inducers of uterine
contractions and cervical ripening (19). PGs play a pivotal role in cervical
maturation and uterine contractions, increasing amniotic fluid
during labor (63,64), an essential process in childbirth
(65). The upregulation of COX-2
is associated with PTB pathology in both mouse models and humans.
The study by Bariani et al (8) revealed that LPS-induced uterine
inflammation increases COX-2 expression, initiating preterm labor.
This upregulation occurs in tissues such as the amnion,
choriodecidua, and myometrium, linking it to PTB pathology
(66).
The ability of resveratrol to modulate COX-2
expression, a key player in PG production, led to the assessment of
uterine PG levels, PGE2, PGF2α, 6-keto-PGF1α and PGI2(8). Resveratrol has been shown to
effectively suppress the LPS-induced elevation of COX-2 in
gestational tissues (8). Its
administration results in the downregulation of both COX-2 mRNA and
protein expression in the uterus, preventing COX-2 gene and protein
overexpression induced by LPS (8,67).
Notably, LPS alone or in combination with resveratrol does not
alter the levels of 15-hydroxyprostaglandin dehydrogenase (15-Pgdh)
mRNA and COX-1 protein levels (8),
which decrease PG production, counteracting the effects of
COX-2(68). In the study by
Bariani et al (8),
resveratrol administration prevented the increase in uterine PGE2
levels and partially affected PGF2α levels, with no significant
impact on the 6-keto-PGF1α concentration (8). The reduction in pro-inflammatory
cytokines by resveratrol may explain the lower PG expression, as
TNF-α and IL-1β induce PGE2 and PGF2α in choriodecidual tissues
(69). In summary, by decreasing
PG synthesis, resveratrol exhibits potential in helping to prevent
preterm labor.
Reduction of endocannabinoid levels. The
endocannabinoid system (ECS) consists of cannabinoid receptors CB1
and CB2, endogenous lipid ligands called endocannabinoids, and
regulating enzymes that synthesize and break down these lipids. All
endocannabinoids belong to the eicosanoid class (70). Endocannabinoids are unsaturated
fatty acid derivatives that interact with this system, eliciting
specific responses (71).
Anandamide (AEA) and 2-arachidonoylglycerol (2-AG) are key
endocannabinoids that are released enzymatically from cell membrane
phospholipid precursors in response to various stimuli, including
neurotransmitters, depolarizing agents, and hormones (71). AEA is present in the uterus, and
its levels in the blood increase during normal labor, suggesting
the involvement of the ECS in reproductive and preterm labor
processes (72-74).
The study by Bariani et al (8) demonstrated that LPS and/or
resveratrol affected uterine endocannabinoid levels. LPS did not
affect the 2-AG levels, but resveratrol alone reduced its levels.
AEA increased with LPS, but resveratrol prevented this
increase.
The effects of AEA on the uterus are conflicting,
with some studies demonstrating the relaxation of the pregnant
myometrium (75), while others
have reported that the activation of CB1 receptors leads to the
increased production of PGE2 in the amnion and choriodecidua
(76). CB1 receptor activation is
associated with the increased production of PGF2α in
inflammation-induced preterm labor (77). Prolonged endocannabinoid signaling
is linked to adverse pregnancy outcomes (78). Bariani et al (8) found that LPS altered AEA and related
endocannabinoid-like lipid levels in the uterus, consistent with
previous research (75,76). LPS-induced cytokines can affect
endocannabinoid production by modulating the expression and
activity of fatty acid amide hydrolase (FAAH) (79,80).
In the study by Bariani et al (8) treatment with resveratrol restored AEA
and other lipid levels to the control levels, although the exact
mechanisms involved remain unclear. The increased AEA production
induced by LPS was associated with a higher incidence of preterm
labor, while the tocolytic effects of resveratrol correlated with
reduced uterine AEA content (8).
Bariani et al (8) discovered that LPS increased the AEA
levels in the uterus, resulting in a greater likelihood of preterm
labor. However, resveratrol prevented this increase, indicating its
tocolytic effect in reducing uterine endocannabinoids during
inflammation (8). In addition to
endocannabinoids, endocannabinoid-like lipids from the N-acyl
ethanolamine, N-arachidonoyl glycine (NAGlys), N-arachidonoyl
serine (NASers) and 2-acyl-sn-glycerols families can undergo
changes in composition during LPS-induced pregnancy inflammation
(8). The specific molecular
mechanism through which resveratrol modifies uterine
endocannabinoids and lipid composition remains unclear. However, it
is known that NAGlys and NASers, which are part of these lipids, do
not activate CB1 or CB2 receptors (81). NASers have been observed to
regulate calcium-activated potassium channels (82) and N-type calcium channels (83). Additionally, NAGlys can be
metabolized by COX-2 and FAAH, potentially functioning as
endogenous enzyme inhibitors (84).
There was a 1.5-fold increase in the average plasma
levels of AEA from 1.20±0.57 nM in non-labor conditions to
1.82±0.87 nM during labor (P<0.0001) (72). A plasma AEA concentration exceeding
1.095 nM was found to be a better predictor of PTB before 37 weeks
of gestation compared to currently used predictors, such as
cervical length, oncofetal fibronectin and cervical insulin-like
growth factor binding protein 1. It demonstrated 87.14%
specificity, 25.93% sensitivity, a 70.24% negative predictive value
(NPV), and a 61.2% positive predictive value (PPV), surpassing
existing predictive tests (85).
The average plasma AEA level significantly increased in women with
a positive fetal fibronectin test compared to those with a negative
result, providing additional evidence of the role of AEA in labor
and its potential use as a predictor for PTB (85). AEA levels are regulated through
cellular uptake by the AEA transporter and enzymatic degradation by
FAAH on the cell membrane (86).
LPS-induced cytokines influence endocannabinoid production by
affecting FAAH protein expression and enzymatic activity (87). Anti-inflammatory cytokines (IL-4
and IL-10) enhance FAAH activity, while pro-inflammatory cytokines
(IL-12 and IFN-γ) reduce FAAH activity and protein expression in
peripheral mononuclear cells (lymphocytes) (8,88).
During pregnancy, FAAH levels in peripheral mononuclear cells show
an inverse correlation with blood AEA levels (89). Low AEA levels with high FAAH are
crucial for the successful development of pregnancy (90,91).
The reduced activity and expression of anandamide hydrolase in
peripheral lymphocytes can serve as an early indication of
spontaneous abortion before eight weeks of gestation, offering
potential diagnostic utility for pregnancy monitoring and fertility
assessment (91).
Modulating 5' AMP-activated protein kinase
(AMPK) and mammalian target of rapamycin complex (mTORC)
pathways. Inflammation and oxidative stress are widely
recognized risk factors for PTB. Studies conducted on mouse models,
specifically those with spontaneous and inflammation-induced PTB
with p53 deletion (p53d/d females), have
indicated the involvement of mTOR activation in decidual senescence
and the excessive production of PGs derived from COX2, ultimately
leading to PTB (11). In female
mice with the p53d/d phenotype, a noticeable
decrease in the growth of uterine decidual cells due to accelerated
senescence has been observed (92,93).
This condition is accompanied by elevated levels of pAkt, p21, IL-8
and various CXCL cytokines, collectively contributing to the
senescence-associated secretory phenotype, which further promotes
decidual senescence and PTB (93).
Additionally, this p53 deletion has been associated with
PTB, which can also be influenced by factors, such as increased ROS
production due to conditions such as pre-eclampsia, as well as
environmental factors such as smoking and pollution (93).
Deng et al (11) demonstrated that resveratrol, an
antioxidant and anti-aging agent, activated AMPK signaling and
inhibited mTORC1 signaling in decidual cells without causing any
adverse effects on the mother or offspring. Consequently,
resveratrol offers protection against both spontaneous and
inflammation-induced PTB in p53d/d females.
Similar beneficial effects have also been observed with the use of
AMPK activators such as metformin, which enhances decidual health
and reduces PTB rates in mouse models (11). Human fetal membranes contain both
AMPK and its phosphorylated form (p-AMPK). Membranes obtained from
spontaneous term labor and pre-labor rupture display significantly
lower levels of p-AMPK compared to intact membranes (11). Pre-treatment of human fetal
membranes with AMPK activators reduces the production of
inflammatory cytokines in response to LPS, indicating the
anti-inflammatory effects of p-AMPK on fetal membranes (94). Resveratrol decreases PGE2 levels by
suppressing Ptgs2, the COX2-encoding gene, in decidual cells. It
also inhibits hyperactive mTORC1 through both AMPK-dependent and/or
AMPK-independent pathways, underscoring its role in the regulation
of AMPK and mTORC1 signaling in an inverse manner (95).
Other potential benefits of resveratrol: Smooth
muscle relaxation. Resveratrol has the ability to relax smooth
muscle, which may have an impact on PTB by targeting potassium
(K+) channels (31,96-98).
The proper functioning of K+ channels is crucial for
maintaining a relaxed uterine state during pregnancy by aiding in
membrane repolarization (99). The
dysregulation of K+ channels can lead to abnormal
uterine activity, contributing to conditions such as preterm labor
(31). Poorly functioning or less
active K+ channels can decrease the repolarizing current
in myometrial smooth muscle cells, resulting in untimely uterine
contractions and preterm delivery. Conversely, excessive expression
of K+ channels in the later stages of pregnancy may
hinder synchronized uterine muscle activity needed for full-term
labor (98). Various types of
K+ channels, including big Ca2+-sensitive
K+ (BKCa) channels, ATP-sensitive K+ (KATP)
channels, Kv channels and SK channels, induce relaxation in both
non-pregnant and pregnant myometrium, demonstrating the complex
regulation of uterine tone (31,96-98).
BKCa channels, which hold a particular influence on uterine smooth
muscle, play a significant role in inducing smooth muscle
relaxation and repolarizing K+ current (99). Their activity varies throughout
pregnancy, and their modulation is regulated by 17b-estradiol
(31). BKCa channels have a more
pronounced relaxant effect during mid-gestation (100,101), indicating their importance in
maintaining uterine quiescence (99).
The regulation of myometrial tone during pregnancy
involves the modulation of ion channels. β-receptor agonists, used
to delay preterm labor, interact with BKCa channels via ß2- and
ß3-receptors, resulting in uterine relaxation (99). Human studies suggest increased
levels of BKCa channel in late pregnant non-laboring myometrium but
decreased expression in preterm and term laboring myometrium
(99). KATP channels, composed of
Kir6 channels and sulfonylurea receptors, play a role in myometrial
quiescence; however, their specific function in the myometrium is
not yet clear. The downregulation of KATP channels, particularly
Kir6.1 and SUR2B subunits, may increase uterine excitability and
labor contractions in term pregnancy (101). The regulation of KATP channels
varies with the stage of gestation and the incidence of labor
contractions in the human myometrium, necessitating further
research for a comprehensive understanding (101). Aberrant K channel function or
expression is associated with conditions such as PTB and
preeclampsia (96,102). Resveratrol can modulate K channel
activity, potentially offering new treatments for these conditions
by reducing intracellular calcium levels and inhibiting various
contractions (31). Resveratrol
effectively inhibits uterine smooth muscle contractions, which can
lead to infertility, endometriosis, miscarriage, or PTB (31,96,97,99).
It targets KATP, BKCa and Kv channels to achieve relaxation effects
on the uterus (97). This
relaxation potential extends to pregnancy-related disorders, such
as PTB, by relaxing the myometrial smooth muscle and fetoplacental
blood vessels, inhibiting contractions induced by oxytocin, PGs and
acetylcholine (96).
Efficacy of resveratrol in preventing
preterm birth
Resveratrol shows promise as an herbal preventive
agent for PTB. In the study by Bariani et al (8), the administration of resveratrol
resulted in a 64% reduction in PTB rates and a 28% decrease in
mortality rates. These effects may be attributed to resveratrol's
impact on uterine NOS activity, iNOS expression, COX-2, PGE2, and
AEA profiling. Additionally, this treatment led to a decrease in
antepartum hemorrhage, fetal toxicity, and offspring mortality.
Importantly, mice treated with resveratrol did not experience
vaginal bleeding, and their uterine morphology improved (8). Deng et al (11) demonstrated that resveratrol has the
potential to prevent PTB through its modulation of AMPK and mTORC1
signaling in decidual cells. The administration of resveratrol
offers several advantages, including no adverse effects on
pregnancy outcomes, effective targeting of decidual health, and
compensation for ovarian luteolysis (11). The study conducted by Furuya et
al (17) also supports the
tocolytic effects of resveratrol by suppressing peritoneal
macrophage-induced inflammation and preventing spontaneous PTB in a
genetic model of accelerated decidual senescence.
Furthermore, Furuya et al (17) discovered that resveratrol had a
significant impact on reducing the rate of PTB in mice. At a dosage
of 20 mg/kg, the PTB rate was 48.6±19.4% compared to 97.8±1.9% in
the control group (P=0.03). Morever, at a dosage of 40 mg/kg, the
PTB rate was 57.1±13.8% compared to 83.7±13.2% in the control group
(P=0.15). By orally administering resveratrol, the occurrence of
LPS-induced prematurity in mice was reduced through the suppression
of inflammatory mediators, PGs, peritoneal macrophages and
endocannabinoid compounds in the maternal reproductive organs
(17). Novaković et al
(31) demonstrated that
resveratrol effectively prevents PTB by inhibiting uterine
contractions, including those stimulated by oxytocin. Resveratrol
achieves this by regulating various myometrial K+
channels, such as KATP, Kv and BKCa channels, by increasing
intracellular Ca2+ levels. Moreover, it induces partial
relaxation of the myometrium by activating Kir6.2/SUR1 channels.
Nonetheless, there are certain limitations to consider, such as the
failure of a single resveratrol treatment to prevent PTB in the
study conducted by Deng et al (11) and its lack of effectiveness against
tonic contractions induced by oxytocin (31).
Pharmacological profile of
resveratrol: From bench to bedside
Oral resveratrol is readily absorbed but undergoes
rapid metabolism (102). Azachi
et al (103) conducted a
study in which red grape cell (RGC) resveratrol exhibited two
plasma concentration peaks: One at 1 h following administration and
another at 5 h following administration. The initial peak is likely
a result of the extensive glucuronidation and sulfation of
resveratrol in the enterocytes of the small intestine. By contrast,
the second peak occurs due to the flow of bile-containing
metabolites from the liver to the intestines, a normal occurrence
after food consumption that may prolong the pharmacological effect
of certain substances and their metabolites (103,104).
The median time to peak drug concentration
(Tmax) for total resveratrol at a dose of 150 mg RGC is
4 h (range, 0.67-6.00), and at 50 mg it is 1 h (range, 0.33-8.00).
Moreover, the median Tmax for free resveratrol at a dose
of 150 mg RGC is 1 h (range, 0.33-4.00) (103). Resveratrol exhibits a high oral
absorption but rapid metabolism, resulting in poor overall
bioavailability. Even with a high dose of 5 g, it only reaches a
peak plasma-free concentration of 538 ng/ml. This limited
bioavailability may be attributed to low solubility, similar to
other polyphenol aglycones, which struggle to form hydrogen bonds
due to hydrophobic interactions with hydroxyl or aromatic groups
(103). One approach to enhance
solubility is through glycosylation of the resveratrol parent
compound (103).
Resveratrol interacts with cytochrome P450 enzymes,
inhibiting CYP3A4, CYP2D6 and CYP2C9, while inducing CYP1A2,
potentially affecting drugs metabolized by these enzymes (105). Urinary excretion studies
demonstrate high absorption rates (at least 70%) after oral
resveratrol intake, with 53.4 to 84.9% of total radioactivity
recovered in urine. However, rapid biotransformation leads to
minimal unmodified resveratrol in circulation and relatively high
levels (maximum, 2 µM) of resveratrol metabolites after a 25 mg
oral dose, resulting in near-zero resveratrol bio-availability
(104). The intravenous injection
of a 0.2 mg dose shows no second peak and a rapid decline in plasma
total radioactivity over the first hour, indicating extensive
distribution. Plasma half-life durations are similar after both
routes (9.2 h orally and 11.4 h intravenously), with dose-dependent
resveratrol plasma levels reaching up to 530 ng/ml with higher
doses (up to 5 g) (104). The
administration of resveratrol during pregnancy appears to be
relatively safe for both the mother and fetus, with no reported
toxicity or adverse pregnancy outcomes (11,102).
Resveratrol is primarily sourced from grapes, grape
skins, peanuts, red wine, cranberries and Japanese knotweed
(Polygonum cuspidatum) (102,105). The quantity of resveratrol varies
among these sources. For example, RGCs contain between 726 to 916
mg/kg (103), while agricultural
red grapes range from 0 to 42.5 mg/kg (103). Other examples include jamun fruit
with 11.19 to 34.87 mg/g of dry weight, mulberries with 35.8 to
50.61 mg/g, jackfruit with 0.07 to 3.56 mg/g (106), apples with 47 mg/g, broccoli with
18 mg/g, onions with 18 mg/g and black tea with 11.3 mg/g (107). Typical concentrations of
resveratrol in various food products to meet the recommended daily
allowance (RDA) of 1 g are as follows: Red grapes (92-1,604 mg/kg
fresh weight), white grapes (59-1,759 mg/kg fresh weight), peanuts
without seed coats (0.03-0.14 mg/g), red wines (0.361-1.972 mg/l),
white wines (0-1.089 mg/l), rosé wines (0.29 mg/l), beers
(1.34-77.0 mg/l), tomato skins (19 mg/g dry weight), dark chocolate
(350 mg/kg), milk chocolate (100 mg/kg), Itadori tea (68 mg/100 ml)
and apples (400 mg/kg fresh weight) (108).
Due to its limited solubility in water, resveratrol
is primarily absorbed through passive diffusion in the colon and
undergoes significant pre-systemic metabolism in the liver. This
liver metabolism markedly reduces the levels of free resveratrol
before it enters systemic circulation. Resveratrol is conjugated to
more soluble glucuronides (e.g., resveratrol-3-O-glucuronide,
resveratrol-4-O-glucuronide) and sulfates (e.g.,
resveratrol-trisulfate) in phase II metabolism or binds to albumin
and lipoproteins. As a result, the therapeutic effectiveness of
oral resveratrol is relatively low. Efforts to improve the
bioavailability of resveratrol or delay its phase II metabolism
through the use of resveratrol prodrugs are crucial for its
biomedical applications (108,109).
The oral supplementation of 80 mg resveratrol in
overweight pregnant women has demonstrated a reduction in the
incidence of gestational diabetes mellitus, improvement in lipid
profiles, and a decrease in glucose levels within a 60-day period.
Additionally, resveratrol administration at a dose of 50 mg for up
to 5 doses has been found to lower blood pressure in patients with
preeclampsia (102). However, it
should be noted that there is limited clinical research available
on the use of resveratrol during pregnancy, with more extensive
studies available for patients with diabetes, cardiovascular issues
and metabolic disorders (105).
Challenges in the clinical application of resveratrol include the
wide range of dosages used (ranging from 5 mg to 5 g daily), the
diverse dose-effect associations observed in the SIRT1 pathway,
concerns regarding renal toxicity associated with micronized oral
formulations such as SRT501 (commercially marketed as a supplement,
rather than a definitive treatment drug) and gastrointestinal
side-effects at high doses. Furthermore, it has been observed that
resveratrol can activate estrogen-regulated genes that are
associated with estrogen-dependent neoplasms (105).
Strength and limitations
This presented systematic review highlights a
unique academic perspective on the prevention of PTB using
resveratrol. It offers original contributions to the field of
preclinical investigations of natural compound medicine for PTB.
Notably, to the best of our knowledge, this systematic review is
the first to specifically examine the impact of resveratrol in
preventing PTB and highlights its promising benefits. The present
study goes beyond a mere summary of the literature by providing a
comprehensive framework that explains the preventive mechanisms of
resveratrol. It also includes a visualized image that consolidates
the scattered literature. By adopting this approach, the present
study contributes significantly to the development of strategies to
combat PTB. Furthermore, the present study stands out for its
meticulous quality assessment, employing stringent predetermined
criteria to critically evaluate the included studies.
In the present systematic review, the authors were
not able to conduct a meta-analysis due to two main reasons. First,
there was a limited number of articles included, which makes it
impractical. Second, the data and results presented in these
studies were presented in a heterogeneous manner, rendering it
impossible to pool the data. The main limitation of this systematic
review was the diverse nature of how data and results are presented
across the included studies, which prevents a meta-analysis from
being practically feasible. Additionally, while the significant
prevalence of favorable outcomes in the present systematic review
is encouraging, it is important to consider the possibility of
publication bias, where studies with unfavorable results may remain
unpublished. Furthermore, the strict inclusion criteria resulted in
the exclusion of several studies that did not directly involve
resveratrol in PTB. Nonetheless, the insights from these excluded
studies are still integrated into the discussion section, adding to
the broader conversation on the subject.
Despite the potential of resveratrol as a tocolytic
agent for preventing PTB, particularly in cases involving
intrauterine inflammation, there is a scarcity of human studies
evaluating its effectiveness in this regard, and no current human
clinical trials have been conducted, at least to the best of our
knowledge. While mouse models provide valuable information, their
limitations stem from significant anatomical and physiological
differences compared to humans, and findings from in vitro
and in vivo studies may not directly translate to human
outcomes due to inherent species disparities. However, this
juncture presents an opportune moment for researchers to validate
the potential benefits of resveratrol through dedicated human
studies. The current insights from various models offer valuable
information about labor and delivery mechanisms, underscoring the
need for well-designed prospective studies to assess the efficacy
of resveratrol in human populations.
In conclusion, the findings presented herein
indicate that resveratrol may have the ability to prevent preterm
labor induced by LPS by inhibiting pro-inflammatory mediators,
reducing PG levels, improving endocannabinoid profiles, relaxing
smooth muscle and preventing excessive uterine contractions through
ion channels. Considering its ability to cross the placenta, future
studies are required to investigate its potential protective
effects on embryos. Moreover, a better understanding of the role of
resveratrol in human or similar uterine physiology necessitates
further investigation. Urgent clinical trials are necessary to
determine the optimal dosage of resveratrol for PTB therapy using
standardized formulations. Given the low oral bioavailability of
resveratrol, improving its pharmacological properties is pivotal.
Structural optimization and the use of resveratrol-encapsulated
nanoparticles can enhance efficacy, reduce dosages, minimize
side-effects and target specific organs. Additionally, exploring
the potential of locally derived resveratrol from plants and
conducting oligomer research shows promise. Long-term studies are
required to substantiate the scientific potential of
resveratrol.
Supplementary Material
Details of reliability assessment
according to ToxRtool (21,25).
Summary of scoring reliability
assessment (21,25).
Interpretation of reliability
assessment (21,25).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
MH served as the principal investigator for the
present study, conceiving the research and making the decision to
publish. In the role of guarantor, MH took full responsibility for
the work. MH, MIDR, and ABP jointly designed the methodology. MH,
MIDR and ABP conducted the investigation, had complete access to
the literature data, contributed to data analysis and curation,
drafted the manuscript and secured funding. MH and ABP utilized
software to create visualizations of the study findings and managed
the project. Additionally, MH provided resources, validated all
evidence analyses and supervised the study process meticulously.
MH, MIDR and ABP confirm the authenticity of all the raw data. All
authors have read and approved the final version for
publication.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
World Health Organization (WHO): Factsheet
of Preterm Birth. WHO, Geneva, 2018. https://www.who.int/news-room/fact-sheets/detail/preterm-birth.
|
|
2
|
Pan American Health Organization (PAHO):
152 million babies born preterm in the last decade. PAHO,
Washington, DC, 2013. https://www.paho.org/en/news/15-6-2023-152-million-babies-born-preterm-last-decade#:~:text=An%20estimated%2013.4%20million%20babies,37%20weeks%20of%20pregnancy)%20worldwide.
Accessed Sep 28, 2023.
|
|
3
|
Cao G, Liu J and Liu M: Global, regional,
and national incidence and mortality of neonatal preterm birth,
1990-2019. JAMA Pediatr. 176(787)2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Bolisetty S, Dhawan A, Abdel-Latif M,
Bajuk B, Stack J and Oei JL: New South Wales and Australian Capital
Territory Neonatal Intensive Care Units' Data Collection.
Intraventricular hemorrhage and neurodevelopmental outcomes in
extreme preterm infants. Pediatrics. 133:55–62. 2014.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Beam AL, Fried I, Palmer N, Agniel D, Brat
G, Fox K, Kohane I, Sinaiko A, Zupancic JAF and Armstrong J:
Estimates of healthcare spending for preterm and low-birthweight
infants in a commercially insured population: 2008-2016. J
Perinatol. 40:1091–1099. 2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Romero R, Dey SK and Fisher SJ: Preterm
labor: One syndrome, many causes. Science. 345:760–765.
2014.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Bastek JA, Gómez LM and Elovitz MA: The
role of inflammation and infection in preterm birth. Clin
Perinatol. 38:385–406. 2011.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Bariani MV, Correa F, Leishman E,
Domínguez Rubio AP, Arias A, Stern A, Bradshaw HB and Franchi AM:
Resveratrol protects from lipopolysaccharide-induced inflammation
in the uterus and prevents experimental preterm birth. Mol Hum
Reprod. 23:571–581. 2017.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Tency I: Inflammatory response in maternal
serum during preterm labour. Facts Views Vis Obgyn. 6:19–30.
2014.PubMed/NCBI
|
|
10
|
Elovitz MA, Wang Z, Chien EK, Rychlik DF
and Phillippe M: A new model for Inflammation-Induced preterm
birth. Am J Pathol. 163:2103–2111. 2003.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Deng W, Cha J, Yuan J, Haraguchi H, Bartos
A, Leishman E, Viollet B, Bradshaw HB, Hirota Y and Dey SK: p53
coordinates decidual sestrin 2/AMPK/mTORC1 signaling to govern
parturition timing. J Clin Invest. 126:2941–2954. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Simhan HN and Caritis SN: Prevention of
preterm delivery. N Engl J Med. 357:477–487. 2007.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Abramovici A, Cantu J and Jenkins SM:
Tocolytic therapy for acute preterm labor. Obstet Gynecol Clin
North Am. 39:77–87. 2012.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Alfirevic Z: Tocolytics: Do they actually
work? BMJ. 345:e6531. 2012.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Croen LA, Connors SL, Matevia M, Qian Y,
Newschaffer C and Zimmerman AW: Prenatal exposure to β2-adrenergic
receptor agonists and risk of autism spectrum disorders. J Neurodev
Disord. 3:307–315. 2011.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Gidaya NB, Lee BK, Burstyn I, Michael Y,
Newschaffer CJ and Mortensen EL: In utero exposure to
β-2-Adrenergic receptor agonist drugs and risk for autism spectrum
disorders. Pediatrics. 137(e20151316)2016.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Furuya H, Taguchi A, Kawana K, Yamashita
A, Inoue E, Yoshida M, Nakamura H, Fujimoto A, Inoue T, Sato M, et
al: Resveratrol protects against pathological preterm birth by
suppression of Macrophage-Mediated Inflammation. Reprod Cie.
22:1561–1568. 2015.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bourque SL, Dolinsky VW, Dyck JRB and
Davidge ST: Maternal resveratrol treatment during pregnancy
improves adverse fetal outcomes in a rat model of severe hypoxia.
Placenta. 33:449–452. 2012.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Poudel R, Stanley JL, Rueda-Clausen CF,
Andersson IJ, Sibley CP, Davidge ST and Baker PN: Effects of
resveratrol in pregnancy using murine models with reduced blood
supply to the uterus. PLoS One. 8(e64401)2013.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372(n71)2021.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Schneider K, Schwarz M, Burkholder I,
Kopp-Schneider A, Edler L, Kinsner-Ovaskainen A, Hartung T and
Hoffmann S: ‘ToxRTool’, a new tool to assess the reliability of
toxicological data. Toxicol Lett. 189:138–144. 2009.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Valenti C, Billi M, Pancrazi GL, Calabria
E, Armogida NG, Tortora G, Pagano S, Barnaba P and Marinucci L:
Biological effects of cannabidiol on human cancer cells: Systematic
review of the literature. Pharmacol Res. 181(106267)2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Moser VC, Morris-Schaffer K, Richardson JR
and Li AA: Glyphosate and neurological outcomes: A systematic
literature review of animal studies. J Toxicol Environ Health B
Crit Rev. 25:162–209. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Gupta M, Singh D, Rastogi S, Siddique HR,
Al-Dayan N, Ahmad A, Sikander M and Sarwat M: Anti-cancer activity
of guggulsterone by modulating apoptotic markers: A systematic
review and meta-analysis. Front Pharmacol.
14(1155163)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
EU Science Hub: ToxRTool-Toxicological
data reliability assessment tool. In: European Commission for
Scientific Tools and Databases. European Commission, 2023.
https://joint-research-centre.ec.europa.eu/scientific-tools-and-databases/toxrtool-toxicological-data-reliability-assessment-tool_en.
Accessed Sep 21, 2023.
|
|
26
|
University of Pittsburgh: Health Research
Reporting Guidelines, Study Execution Manuals, Critical Appraisal,
Risk of Bias, and Non-reporting Biases: From Cochrane Handbook for
Systematic Reviews of Interventions [Internet]. University of
Pittsburgh: Health Sciences Library System Guides, 2023. https://hsls.libguides.com/reporting-study-tools/risk-of-bias.
Accessed Sep 21, 2023.
|
|
27
|
Osmanovic A, Halilovic S, Kurtovic-Kozaric
A and Hadziabdic N: Evaluation of periodontal ligament cell
viability in different storage media based on human PDL cell
culture experiments-A systematic review. Dental Traumatol.
34:384–393. 2018.PubMed/NCBI View Article : Google Scholar
|
|
28
|
National Toxicology Program: Handbook for
conducting a literature-based health assessment using OHAT approach
for systemic review and evidence integration. National Institute of
Environmental Health Science, pp1-102, 2019.
|
|
29
|
Puidokas T, Kubilius M, Stumbras A and
Juodzbalys G: Effect of leukocytes included in platelet
concentrates on cell behaviour. Platelets. 30:937–945.
2019.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Center for Evidence-Based Medicine (CEBM):
OCEMB Levels of Evidence. CEBM, Oxford, 2011. https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence.
Accessed Sep 21, 2023.
|
|
31
|
Novaković R, Radunović N,
Marković-Lipkovski J, Ćirović S, Beleslin-Čokić B, Ilić B, Ivković
B, Heinle H, Živanović V and Gojković-Bukarica LJ: Effects of the
polyphenol resveratrol on contractility of human term pregnant
myometrium. Mol Hum Reprod. 21:545–551. 2015.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Wilson A, Hodgetts-Morton VA, Marson EJ,
Markland AD, Larkai E, Papadopoulou A, Coomarasamy A, Tobias A,
Chou D, Oladapo OT, et al: Tocolytics for delaying preterm birth: A
network meta-analysis. Cochrane Database Syst Rev.
8(CD014978)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Abd El-Mohsen M, Bayele H, Kuhnle G,
Gibson G, Debnam E, Kaila Srai S, Rice-Evans C and Spencer JP:
Distribution of [3H]trans-resveratrol in rat tissues following oral
administration. Br J Nutr. 96:62–70. 2006.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Walle T, Hsieh F, DeLegge MH, Oatis JE and
Walle UK: High absorption but very low bioavailability of oral
resveratrol in humans. Drug Metab Dispos. 32:1377–1382.
2004.PubMed/NCBI View Article : Google Scholar
|
|
35
|
Walle T: Bioavailability of resveratrol.
Ann N Y Acad Sci. 1215:9–15. 2011.PubMed/NCBI View Article : Google Scholar
|
|
36
|
la Porte C, Voduc N, Zhang G, Seguin I,
Tardiff D, Singhal N and Cameron DW: Steady-State pharmacokinetics
and tolerability of Trans-Resveratrol 2000 mg twice daily with
food, quercetin and alcohol (Ethanol) in healthy human subjects.
Clin Pharmacokinet. 49:449–454. 2010.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Brown VA, Patel KR, Viskaduraki M, Crowell
JA, Perloff M, Booth TD, Vasilinin G, Sen A, Schinas AM, Piccirilli
G, et al: Repeat dose study of the cancer chemopreventive agent
resveratrol in healthy volunteers: Safety, pharmacokinetics, and
effect on the Insulin-like growth factor axis. Cancer Res.
70:9003–9011. 2010.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Gracie S, Pennell C, Ekman-Ordeberg G, Lye
S, McManaman J, Williams S, Palmer L, Kelley M, Menon R and Gravett
M: PREBIC ‘-Omics’ Research Group. An integrated systems biology
approach to the study of preterm birth using ‘-omic’ technology-a
guideline for research. BMC Pregnancy Childbirth.
11(71)2011.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Green ES and Arck PC: Pathogenesis of
preterm birth: Bidirectional inflammation in mother and fetus.
Semin Immunopathol. 42:413–429. 2020.PubMed/NCBI View Article : Google Scholar
|
|
40
|
McCarthy R, Martin-Fairey C, Sojka DK,
Herzog ED, Jungheim ES, Stout MJ, Fay JC, Mahendroo M, Reese J,
Herington JL, et al: Mouse models of preterm birth: Suggested
assessment and reporting guidelines. Biol Reprod. 99:922–937.
2018.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Romero R and Tartakovsky B: The natural
interleukin-1 receptor antagonist prevents interleukin-l-induced
preterm delivery in mice. Am J Obstet Gynecol. 167:1041–1045.
1992.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Robertson SA, Christiaens I, Dorian CL,
Zaragoza DB, Care AS, Banks AM and Olson DM: Interleukin-6 is an
essential determinant of on-time parturition in the mouse.
Endocrinology. 151:3996–4006. 2010.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Montalbano AP, Hawgood S and Mendelson CR:
Mice deficient in surfactant protein A (SP-A) and SP-D or in TLR2
manifest delayed parturition and decreased expression of
inflammatory and contractile genes. Endocrinology. 154:483–498.
2013.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Gonzalez JM, Franzke CW, Yang F, Romero R
and Girardi G: Complement activation triggers metalloproteinases
release inducing cervical remodeling and preterm birth in mice. Am
J Pathol. 179:838–849. 2011.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Wahid HH, Dorian CL, Chin PY, Hutchinson
MR, Rice KC, Olson DM, Moldenhauer LM and Robertson SA: Toll-Like
Receptor 4 Is an essential upstream regulator of On-Time
parturition and perinatal viability in mice. Endocrinology.
156:3828–3841. 2015.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Krediet TG, Wiertsema SP, Vossers MJ,
Hoeks SBEA, Fleer A, Ruven HJ and Rijkers GT: Toll-like receptor 2
polymorphism is associated with preterm birth. Pediatr Res.
62:474–476. 2007.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Bayar E, Bennett PR, Chan D, Sykes L and
MacIntyre DA: The pregnancy microbiome and preterm birth. Semin
Immunopathol. 42:487–499. 2020.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Andrews WW, Goldenberg RL, Hauth JC,
Cliver SP, Copper R and Conner M: Interconceptional antibiotics to
prevent spontaneous preterm birth: A randomized clinical trial. Am
J Obstet Gynecol. 194:617–623. 2006.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Abedini E, Khodadadi E, Zeinalzadeh E,
Moaddab SR, Asgharzadeh M, Mehramouz B, Dao S and Samadi Kafil H: A
comprehensive study on the antimicrobial properties of resveratrol
as an alternative therapy. Evid Based Complement Alternat Med.
2021(8866311)2021.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Ma DSL, Tan LTH, Chan KG, Yap WH,
Pusparajah P, Chuah LH, Ming LC, Khan TM, Lee LH and Goh BH:
Resveratrol-Potential antibacterial agent against foodborne
pathogens. Front Pharmacol. 9(102)2018.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Roberts RA, Laskin DL, Smith CV, Robertson
FM, Allen EMG, Doorn JA and Slikker W: Nitrative and oxidative
stress in toxicology and disease. Toxicol Sci. 112:4–16.
2009.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Meng T, Xiao D, Muhammed A, Deng J, Chen L
and He J: Anti-Inflammatory action and mechanisms of resveratrol.
Molecules. 26(229)2021.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Gómez-Chávez F, Correa D,
Navarrete-Meneses P, Cancino-Diaz JC, Cancino-Diaz ME and
Rodríguez-Martínez S: NF-κB and its regulators during pregnancy.
Front Immunol. 12(679106)2021.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Malaguarnera. Influence of resveratrol on
the immune response. Nutrients. 11(946)2019.PubMed/NCBI View Article : Google Scholar
|
|
55
|
Liu T, Zhang L, Joo D and Sun SC: NF-κB
signaling in inflammation. Signal Transduct Target Ther.
2(17023)2017.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Klawitter M, Hakozaki M, Kobayashi H,
Krupkova O, Quero L, Ospelt C, Gay S, Hausmann O, Liebscher T,
Meier U, et al: Expression and regulation of toll-like receptors
(TLRs) in human intervertebral disc cells. Eur Spine J.
23:1878–1891. 2014.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Lappas M, Mitton A, Lim R, Barker G, Riley
C and Permezel M: SIRT1 Is a novel regulator of key pathways of
human labor1. Biol Reprod. 84:167–178. 2011.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Manna SK, Mukhopadhyay A and Aggarwal BB:
Resveratrol suppresses TNF-induced activation of nuclear
transcription factors NF-kappa B, activator protein-1, and
apoptosis: Potential role of reactive oxygen intermediates and
lipid peroxidation. J Immunol. 164:6509–6519. 2000.PubMed/NCBI View Article : Google Scholar
|
|
59
|
Zhu X, Liu Q, Wang M, Liang M, Yang X, Xu
X, Zou H and Qiu J: Activation of sirt1 by resveratrol inhibits
TNF-α induced inflammation in fibroblasts. PLoS One.
6(e27081)2011.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Taguchi A, Yamashita A, Kawana K,
Nagamatsu T, Furuya H, Inoue E, Osuga Y and Fujii T: Recent
progress in therapeutics for inflammation-associated preterm birth:
A review. Reprod Sci. 24:7–18. 2017.PubMed/NCBI View Article : Google Scholar
|
|
61
|
Taguchi A, Wada-Hiraike O, Kawana K, Koga
K, Yamashita A, Shirane A, Urata Y, Kozuma S, Osuga Y and Fujii T:
Resveratrol suppresses inflammatory responses in endometrial
stromal cells derived from endometriosis: A possible role of the
sirtuin 1 pathway. J Obstet Gynaecol Res. 40:770–778.
2014.PubMed/NCBI View Article : Google Scholar
|
|
62
|
Vidal MS, Lintao RCV, Severino MEL,
Tantengco OAG and Menon R: Spontaneous preterm birth: Involvement
of multiple feto-maternal tissues and organ systems, differing
mechanisms, and pathways. Front Endocrinol (Lausanne).
13(1015622)2022.PubMed/NCBI View Article : Google Scholar
|
|
63
|
Wood EM, Hornaday KK and Slater DM:
Prostaglandins in biofluids in pregnancy and labour: A systematic
review. PLoS One. 16(e0260115)2021.PubMed/NCBI View Article : Google Scholar
|
|
64
|
Yamashita A, Kawana K, Tomio K, Taguchi A,
Isobe Y, Iwamoto R, Masuda K, Furuya H, Nagamatsu T, Nagasaka K, et
al: Increased tissue levels of omega-3 polyunsaturated fatty acids
prevents pathological preterm birth. Sci Rep.
3(3113)2013.PubMed/NCBI View Article : Google Scholar
|
|
65
|
Li WJ, Lu JW, Zhang CY, Wang WS, Ying H,
Myatt L and Sun K: PGE2 vs PGF2α in human parturition. Placenta.
104:208–219. 2021.PubMed/NCBI View Article : Google Scholar
|
|
66
|
Alfirevic Z, Keeney E, Dowswell T, Welton
NJ, Dias S, Jones LV, Navaratnam K and Caldwell DM: Labour
induction with prostaglandins: A systematic re view and network
meta-analysis. BMJ. 350(h217)2015.PubMed/NCBI View Article : Google Scholar
|
|
67
|
Sykes L, MacIntyre DA, Teoh TG and Bennett
PR: Anti-inflammatory prostaglandins for the prevention of preterm
labour. Reproduction. 148:R29–R40. 2014.PubMed/NCBI View Article : Google Scholar
|
|
68
|
Challis JR, Lockwood CJ, Myatt L, Norman
JE, Strauss JF and Petraglia F: Inflammation and Pregnancy. Reprod
Sci. 16:206–215. 2009.PubMed/NCBI View Article : Google Scholar
|
|
69
|
Zhang Y, Desai A, Yang SY, Bae KB, Antczak
MI, Fink SP, Tiwari S, Willis JE, Williams NS, Dawson DM, et al:
Inhibition of the prostaglandin-degrading enzyme 15-PGDH
potentiates tissue regeneration. Science.
348(aaa2340)2015.PubMed/NCBI View Article : Google Scholar
|
|
70
|
Mitchell MD, Rice GE, Vaswani K, Kvaskoff
D and Peiris HN: Differential regulation of eicosanoid and
endocannabinoid production by inflammatory mediators in human
choriodecidua. PLoS One. 11(e0148306)2016.PubMed/NCBI View Article : Google Scholar
|
|
71
|
Maia J, Fonseca B, Teixeira N and
Correia-da-Silva G: The fundamental role of the endocannabinoid
system in endometrium and placenta: Implications in
pathophysiological aspects of uterine and pregnancy disorders. Hum
Reprod Update. 26:586–602. 2020.PubMed/NCBI View Article : Google Scholar
|
|
72
|
Nallendran V, Lam PM, Marczylo TH, Bankart
MJ, Taylor AH, Taylor DJ and Konje JC: The plasma levels of the
endocannabinoid, anandamide, increase with the induction of labour.
BJOG. 117:863–869. 2010.PubMed/NCBI View Article : Google Scholar
|
|
73
|
Bariani MV, Domínguez Rubio AP, Cella M,
Burdet J, Franchi AM and Aisemberg J: Role of the endocannabinoid
system in the mechanisms involved in the LPS-induced preterm labor.
Reproduction. 150:463–472. 2015.PubMed/NCBI View Article : Google Scholar
|
|
74
|
Komarnytsky S, Rathinasabapathy T, Wagner
C, Metzger B, Carlisle C, Panda C, Le Brun-Blashka S, Troup JP and
Varadharaj S: Endocannabinoid system and its regulation by
polyunsaturated fatty acids and full spectrum hemp oils. Int J Mol
Sci. 22(5479)2021.PubMed/NCBI View Article : Google Scholar
|
|
75
|
Rava A and Trezza V: Emerging roles of
endocannabinoids as key lipid mediators for a successful pregnancy.
Int J Mol Sci. 24(5220)2023.PubMed/NCBI View Article : Google Scholar
|
|
76
|
Dennedy MC, Friel AM, Houlihan DD,
Broderick VM, Smith T and Morrison JJ: Cannabinoids and the human
uterus during pregnancy. Am J Obstet Gynecol. 190:2–9.
2004.PubMed/NCBI View Article : Google Scholar
|
|
77
|
Mitchell MD, Sato TA, Wang A, Keelan JA,
Ponnampalam AP and Glass M: Cannabinoids stimulate prostaglandin
production by human gestational tissues through a tissue- and
CB1-receptor-specific mechanism. Am J Physiol Endocrinol Metab.
294:E352–E356. 2008.PubMed/NCBI View Article : Google Scholar
|
|
78
|
Kozakiewicz ML, Grotegut CA and Howlett
AC: Endocannabinoid system in pregnancy maintenance and labor: A
mini-review. Front Endocrinol (Lausanne). 12(699951)2021.PubMed/NCBI View Article : Google Scholar
|
|
79
|
Booker L, Kinsey SG, Abdullah RA, Blankman
JL, Long JZ, Ezzili C, Boger DL, Cravatt BF and Lichtman AH: The
fatty acid amide hydrolase (FAAH) inhibitor PF-3845 acts in the
nervous system to reverse LPS-induced tactile allodynia in mice. Br
J Pharmacol. 165:2485–2496. 2012.PubMed/NCBI View Article : Google Scholar
|
|
80
|
Sun X, Deng W, Li Y, Tang S, Leishman E,
Bradshaw HB and Dey SK: Sustained endocannabinoid signaling
compromises decidual function and promotes inflammation-induced
preterm birth. J Biol Chem. 291:8231–8240. 2016.PubMed/NCBI View Article : Google Scholar
|
|
81
|
Donvito G, Nass SR, Wilkerson JL, Curry
ZA, Schurman LD, Kinsey SG and Lichtman AH: The endogenous
cannabinoid system: A budding source of targets for treating
inflammatory and neuropathic pain. Neuropsychopharmacology.
43:52–79. 2018.PubMed/NCBI View Article : Google Scholar
|
|
82
|
Connor M, Vaughan CW and Vandenberg RJ:
N-Acyl amino acids and N-acyl neurotransmitter conjugates:
Neuromodulators and probes for new drug targets. Br J Pharmacol.
160:1857–1871. 2010.PubMed/NCBI View Article : Google Scholar
|
|
83
|
Godlewski G, Offertáler L, Osei-Hyiaman D,
Mo FM, Harvey-White J, Liu J, Davis MI, Zhang L, Razdan RK, Milman
G, et al: The endogenous brain constituent N-Arachidonoyl l-Serine
Is an activator of large conductance Ca 2+-Activated K+ channels. J
Pharmacol Exp Ther. 328:351–361. 2009.PubMed/NCBI View Article : Google Scholar
|
|
84
|
Guo J, Williams DJ and Ikeda SR:
N-Arachidonoyl L-Serine, a putative endocannabinoid, alters the
activation of N-Type Ca2+ Channels in sympathetic neurons. J
Neurophysiol. 100:1147–151. 2008.PubMed/NCBI View Article : Google Scholar
|
|
85
|
Grazia Cascio M, Minassi A, Ligresti A,
Appendino G, Burstein S and Di Marzo V: A structure-activity
relationship study on N-arachidonoyl-amino acids as possible
endogenous inhibitors of fatty acid amide hydrolase. Biochem
Biophys Res Commun. 314:192–196. 2004.PubMed/NCBI View Article : Google Scholar
|
|
86
|
Bachkangi P, Taylor AH, Bari M, Maccarrone
M and Konje JC: Prediction of preterm labour from a single blood
test: The role of the endocannabinoid system in predicting preterm
birth in high-risk women. Eur J Obstet Gynecol Reprod Biol.
243:1–6. 2019.PubMed/NCBI View Article : Google Scholar
|
|
87
|
Deutsch DG: A personal retrospective:
Elevating anandamide (AEA) by targeting fatty acid amide hydrolase
(FAAH) and the fatty acid binding proteins (FABPs). Front
Pharmacol. 7(370)2016.PubMed/NCBI View Article : Google Scholar
|
|
88
|
Liu J, Bátkai S, Pacher P, Harvey-White J,
Wagner JA, Cravatt BF, Gao B and Kunos G: Lipopolysaccharide
induces anandamide synthesis in macrophages via
CD14/MAPK/phosphoinositide 3-kinase/NF-kappaB independently of
platelet-activating factor. J Biol Chem. 278:45034–45039.
2003.PubMed/NCBI View Article : Google Scholar
|
|
89
|
Nagarkatti P, Pandey R, Rieder SA, Hegde
VL and Nagarkatti M: Cannabinoids as novel anti-inflammatory drugs.
Future Med Chem. 1:1333–1349. 2009.PubMed/NCBI View Article : Google Scholar
|
|
90
|
Maccarrone M, Valensise H, Bari M,
Lazzarin N, Romanini C and Finazzi-Agrò A: Relation between
decreased anandamide hydrolase concentrations in human lymphocytes
and miscarriage. Lancet. 355:1326–1329. 2000.PubMed/NCBI View Article : Google Scholar
|
|
91
|
Dong C, Chen J, Harrington A, Vinod KY,
Hegde ML and Hegde VL: Cannabinoid exposure during pregnancy and
its impact on immune function. Cell Mol Life Sci. 76:729–743.
2019.PubMed/NCBI View Article : Google Scholar
|
|
92
|
Hirota Y, Cha J, Yoshie M, Daikoku T and
Dey SK: Heightened uterine mammalian target of rapamycin complex 1
(mTORC1) signaling provokes preterm birth in mice. Proc Natl Acad
Sci USA. 108:18073–18078. 2011.PubMed/NCBI View Article : Google Scholar
|
|
93
|
Wolfson ML, Aisemberg J, Correa F and
Franchi AM: Peripheral blood mononuclear cells infiltration
downregulates decidual FAAH activity in an LPS-Induced embryo
resorption model. J Cell Physiol. 232:1441–1447. 2017.PubMed/NCBI View Article : Google Scholar
|
|
94
|
Cox LS and Redman C: The role of cellular
senescence in ageing of the placenta. Placenta. 52:139–145.
2017.PubMed/NCBI View Article : Google Scholar
|
|
95
|
Singh M, Parent S, Leblanc V and Asselin
E: Resveratrol modulates the expression of PTGS2 and cellular
proliferation in the normal rat endometrium in an AKT-Dependent
Manner1. Biol Reprod. 84:1045–1052. 2011.PubMed/NCBI View Article : Google Scholar
|
|
96
|
Rajkovic J, Djokic V, Gostimirovic M,
Gojkovic-Bukarica L, Martorell M, Sharifi-Rad J and Novakovic R:
Potassium channels on smooth muscle as a molecular target for
plant-derived Resveratrol. Cell Mol Biol (Noisy-le-Grand).
66:133–144. 2020.PubMed/NCBI
|
|
97
|
Carreiro JN, Magnani M, Jobling P, van
Helden DF, Nalivaiko E and Braga VA: Resveratrol restores uterine
contractions during hypoxia by blockade of ATP-sensitive potassium
channels. J Funct Foods. 33:307–313. 2017.
|
|
98
|
Lim R, Barker G and Lappas M: Activation
of AMPK in human fetal membranes alleviates infection-induced
expression of pro-inflammatory and pro-labour mediators. Placenta.
36:454–462. 2015.PubMed/NCBI View Article : Google Scholar
|
|
99
|
Novakovic R, Ilic B, Beleslin-Cokic B,
Radunovic N, Heinle H, Scepanovic R and Gojkovic-Bukarica L: The
effect of resveratrol on contractility of non-pregnant rat uterus:
The contribution of K(+) channels. J Physiol Pharmacol. 64:795–805.
2013.PubMed/NCBI
|
|
100
|
Okawa T, Longo M, Vedernikov YP, Chwalisz
K, Saade GR and Garfield RE: Role of nucleotide cyclases in the
inhibition of pregnant rat uterine contractions by the openers of
potassium channels. Am J Obstet Gynecol. 182:913–918.
2000.PubMed/NCBI View Article : Google Scholar
|
|
101
|
Brainard AM, Korovkina VP and England SK:
Potassium channels and uterine function. Semin Cell Dev Biol.
18:332–339. 2007.PubMed/NCBI View Article : Google Scholar
|
|
102
|
Zheng S, Feng Q, Cheng J and Zheng J:
Maternal resveratrol consumption and its programming effects on
metabolic health in offspring mechanisms and potential
implications. Biosci Rep. 38(BSR20171741)2018.PubMed/NCBI View Article : Google Scholar
|
|
103
|
Azachi M, Yatuv R, Katz A, Hagay Y and
Danon A: A novel red grape cells complex: Health effects and
bioavailability of natural resveratrol. Int J Food Sci Nutr.
65:848–855. 2014.PubMed/NCBI View Article : Google Scholar
|
|
104
|
Wang P and Sang S: Metabolism and
pharmacokinetics of resveratrol and pterostilbene. BioFactors.
44:16–25. 2018.PubMed/NCBI View Article : Google Scholar
|
|
105
|
Pollack RM and Crandall JP: Resveratrol:
Therapeutic potential for improving cardiometabolic health. Am J
Hypertens. 26:1260–1268. 2013.PubMed/NCBI View Article : Google Scholar
|
|
106
|
Shrikanta A, Kumar A and Govindaswamy V:
Resveratrol content and antioxidant properties of underutilized
fruits. J Food Sci Technol. 52:383–390. 2015.PubMed/NCBI View Article : Google Scholar
|
|
107
|
Cione E, La Torre C, Cannataro R, Caroleo
MC, Plastina P and Gallelli L: Quercetin, Epigallocatechin gallate,
curcumin, and resveratrol: From dietary sources to human MicroRNA
modulation. Molecules. 25(63)2019.PubMed/NCBI View Article : Google Scholar
|
|
108
|
Weiskirchen S and Weiskirchen R:
Resveratrol: How much wine do you have to drink to stay healthy?
Adv Nutr. 7:706–718. 2016.PubMed/NCBI View Article : Google Scholar
|
|
109
|
Kuršvietienė L, Stanevičienė I,
Mongirdienė A and Bernatonienė J: Multiplicity of effects and
health benefits of resveratrol. Medicina (Kaunas). 52:148–155.
2016.PubMed/NCBI View Article : Google Scholar
|