Introduction
Lower urinary tract symptoms (LUTS) are very common
among middle-aged males (1,2). In
40% of males >50 years of age, benign prostatic hyperplasia
(BPH) is considered to be the cause of these symptoms (3). BPH is a benign hyperplasia of the
periurethral region of the prostate that causes obstructive
symptoms that significantly compromise the quality of life of
patients. Over the years, numerous therapies have been developed to
treat BPH. Although initial medications may be effective for mild
to moderate symptoms, patients with moderate to severe symptoms may
require surgical intervention. Transurethral resection of the
prostate (TURP) has been the most commonly performed procedure and
is considered the gold standard for the treatment of BPH (4). Although TURP has demonstrated
efficacy in improving urinary symptoms, acute complications and
long-term adverse events (AEs), such as erectile and ejaculatory
dysfunction, incontinence, and other complications have been
reported (5). Some studies have
indicated the efficacy and safety of a wide variety of minimally
invasive procedures for BPH, such as laser endoscopic enucleation,
green light vaporization, prostatic artery embolization and UroLift
(6-10).
All these procedures aim at avoiding or reducing complications
associated with TURP, while maintaining comparable outcomes. Water
vapor thermal therapy (WVTT) using the Rezum system, which involves
the administration of a transurethral injection of 103˚C water
steam into the prostate, is a type of minimally invasive treatment,
which has demonstrated beneficial efficacy and safety profiles for
the treatment of LUTS caused by BPH (11). A recent randomized clinical trial
reported the safety and durable efficacy of WVTT performed in an
office-based or ambulatory surgery center (12). The provision of day-case surgery
would allow for greater patient flow and improve clinical care
through increased efficiency (10).
Therefore, the present study was conducted in an aim
to assess the feasibility, safety and efficacy of day-case WVTT as
an office-based, outpatient procedure.
Patients and methods
Study design and setting
The present retrospective cohort study was conducted
at Mizuhodai Urology in Fujimi, Japan (single-unit urology clinic).
The Rezum system (Boston Scientific Corporation) was introduced at
the clinic in March, 2023. All Rezum procedures during the study
period were performed according to previously published techniques
(11,13). Spinal anesthesia was applied for
all the procedures.
Patient selection
The present study included the data of 40 patients
who underwent the Rezum procedure from March, 2023 to January,
2024, including 11 patients who were catheterized due to complete
urinary retention. The inclusion and exclusion criteria used are
presented in Table I.
 | Table IInclusion and exclusion criteria used
for the patients in the present study. |
Table I
Inclusion and exclusion criteria used
for the patients in the present study.
| Inclusion
criteria | Criteria |
|---|
| 1 | Male subjects >50
years of age who had symptomatic BPH |
| 2 | IPSS-QOL score
≥4 |
| 3 | Prostate volume
>30 cm3 to ≤90 cm3 |
| Exclusion
criteria | Criteria |
| 1 | Active or history of
UTI within the past 3 months |
| 2 | Any prior invasive
prostate intervention |
| 3 | Suspicion of prostate
cancer due to elevated PSA levels or PI-RADS ≥3 on an MRI |
Data collection and definitions
The operation time and hospitalization time on the
day of the procedure were analyzed. The patient characteristics,
International Prostate Symptom Score-Quality of Life (IPSS-QOL)
score, prostate volume (PV), post-void residual (PVR) volume
measured by transabdominal ultrasound, catheterization, medication
and AEs were monitored at baseline, and at 1, 2 and 3 months
following treatment. AEs were defined according to the
Clavien-Dindo classification (14).
Statistical analysis
Baseline and follow-up data were reviewed, with
follow-up periods of 1, 2 and 3 months. All data are reported with
appropriate descriptive statistics as follows: Normally distributed
data are reported as the mean ± standard deviation (SD) and
non-normally distributed data are reported as the median and
interquartile range (IQR). The changes observed in the patients
from baseline were analyzed using repeated measures ANOVA followed
by the Bonferroni post hoc test for each measure. The influence of
a history of urinary retention and more than six injections on
prolonged post-operative catheterization (>14 days) was assessed
using multivariate logistic regression. A value of P<0.05 was
considered to indicate a statistically significant difference.
Statistical analysis was performed using JASP (version 0.18.3, team
JASP, https://jasp-stats.org/).
Results
Patient characteristics
A total of 40 patients were included in the present
study. The median age of the patients was 71.5 years (IQR,
51.0-83.0). The mean PV was 56.9±13.8 ml. Pre-operative
catheterization, a history of urinary retention, and a median lobe
were present in 27.5, 37.5 and 45.0% of patients, respectively
(Table II).
 | Table IIPatient characteristics. |
Table II
Patient characteristics.
| Characteristic | Patients (n=40) |
|---|
| Age in years, median
(IQR) | 71.5 (51.0-83.0) |
| Performance status,
median (IQR) | 0 (0-2) |
| Preoperative
medication for BPH, n (%) | |
|
Alpha
blocker | 40(100) |
|
5-Alpha
reductase inhibitor | 30 (75.0) |
|
Phosphodiesterase-5
inhibitor | 5 (12.5) |
|
Anticoagulants/platelet aggregation
inhibitors, n (%) | 5 (12.5) |
| Preoperative IPSS-QOL
score, mean ± SD | 5.4±0.5 |
| Pre-operative PVR in
ml, mean ± SD | 291.0±419.9 |
| Pre-operative
prostate volume, mean ± SD | 56.9±13.8 |
| Preoperative
catheterization, n (%) | 11 (27.5) |
| History of urinary
retention, n (%) | 15 (37.5) |
| Median lobe, n
(%) | 18 (45.0) |
Peri-operative data
All interventions were performed within a median
period of 4.0 min (IQR, 2.0-11.0). The patients received a median
of five injections (IQR, 4-7) and were hospitalized for a mean
duration of 274.8±53.8 min. A total of 5 patients made telephone
inquiries during the first post-operative week (Table III).
 | Table IIIPeri- and post-operative efficacy
outcomes of the patients. |
Table III
Peri- and post-operative efficacy
outcomes of the patients.
| Peri- and
post-operative efficacy outcomes | Patients (n=40) |
|---|
| Duration of operation
in min, median (IQR) | 4.0 (2.0-11.0) |
| Number of injections,
median (IQR) | 5.0 (4.0-7.0) |
| Intraoperative
complications, n (%) | |
|
None | 39 (97.5) |
|
Catheter for
irrigation | 1 (2.5) |
| Duration of
hospitalization in minutes, mean ± SD | 274.8±53.8 |
| Number of phone
inquiry within 1 week after operation, no. of patients | 5 |
| Total number of
successful catheter removal, n (%) | 39 (97.5) |
| Total days until
successful catheter removal, median (IQR) | 12.0 (6.0-87.0) |
| Successful catheter
removal in 29 patients without a pre-operative catheter, n (%) | 29(100) |
| Total days until
successful catheter removal, median (IQR) | 8.0 (6.0-16.0) |
| Successful catheter
removal in 11 patients with a pre-operative catheter, n (%) | 10 (90.9) |
| Total days until
successful catheter removal, median (IQR) | 32.0 (28.0-87.0) |
| Total rate of
discontinuation of BPH medication, n (%) | 26 (65.0) |
| Total days until
discontinuation of BPH medication, median (IQR) | 58.0
(24.0-114.0) |
Catheter management and medication
use
The catheter was successfully removed following a
median of 12.0 days (IQR, 6.0-87.0) in 39 (97.5%) patients.
Catheter removal was successful in 29 (100%) patients without a
pre-operative catheter following a median of 8 (IQR, 6.0-16.0)
days. In 11 patients with a pre-operative catheter, catheters were
successfully removed in 10 (90.9%) patients following a median of
32 (IQR, 28.0-87.0) days (Table
III). BPH medications were discontinued by 26 of 40 (65.0%)
patients. The median time to discontinuation was 58.0 days (IQR,
24.0-114.0) (Table III). A
history of urinary retention and more than six injections during
the procedure increased the risk of prolonged postoperative
catheterization (>14 days) (Table
IV).
 | Table IVPotential risk factors for prolonged
postoperative catheterization (>14 days). |
Table IV
Potential risk factors for prolonged
postoperative catheterization (>14 days).
| Parameters/potential
risk factors | OR (CI), P-value |
|---|
| History of urinary
retention | 32.450
(3.247-324.276), 0.003 |
| No. of injections
≥6 | 15.578
(1.677-144.689), 0.016 |
Functional outcomes
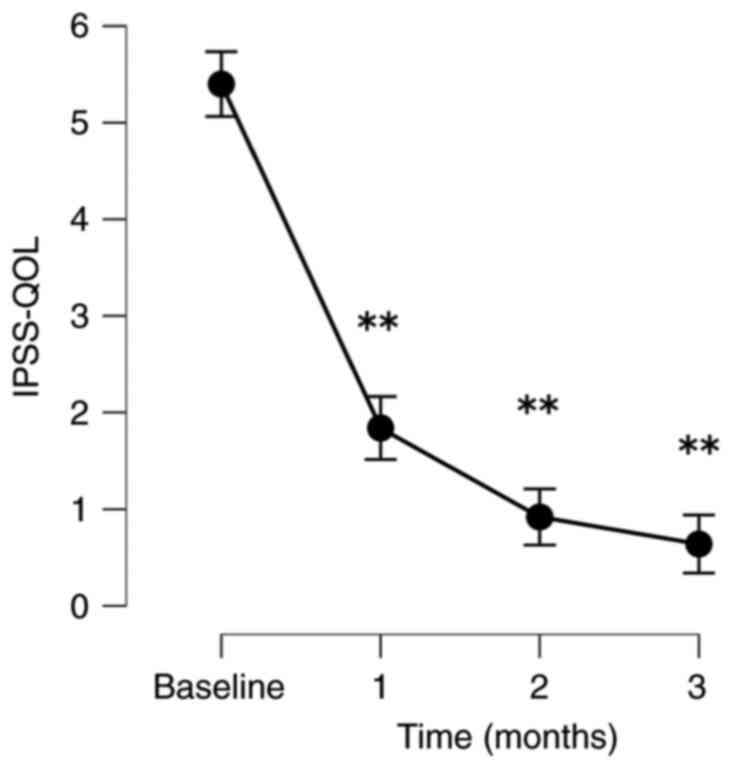
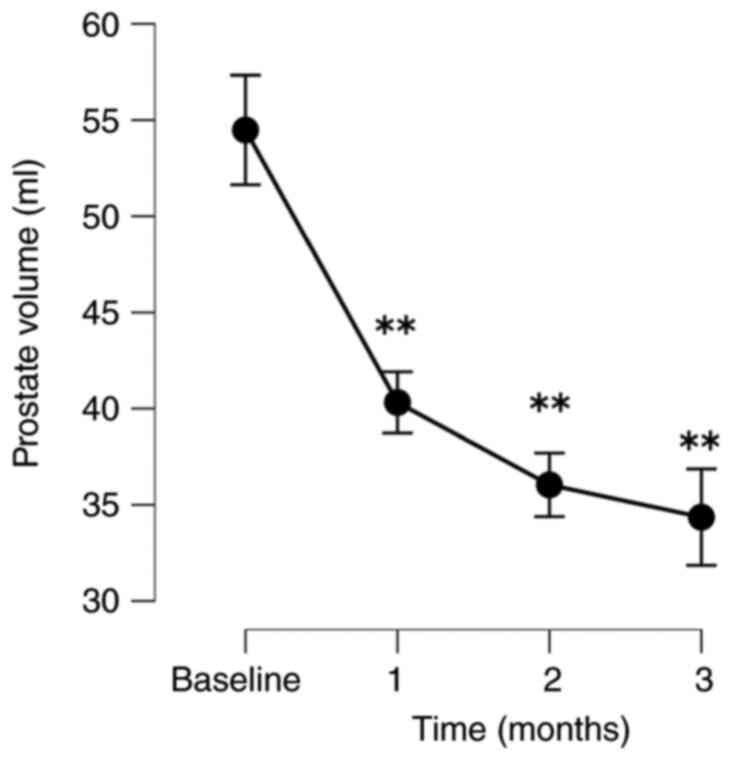
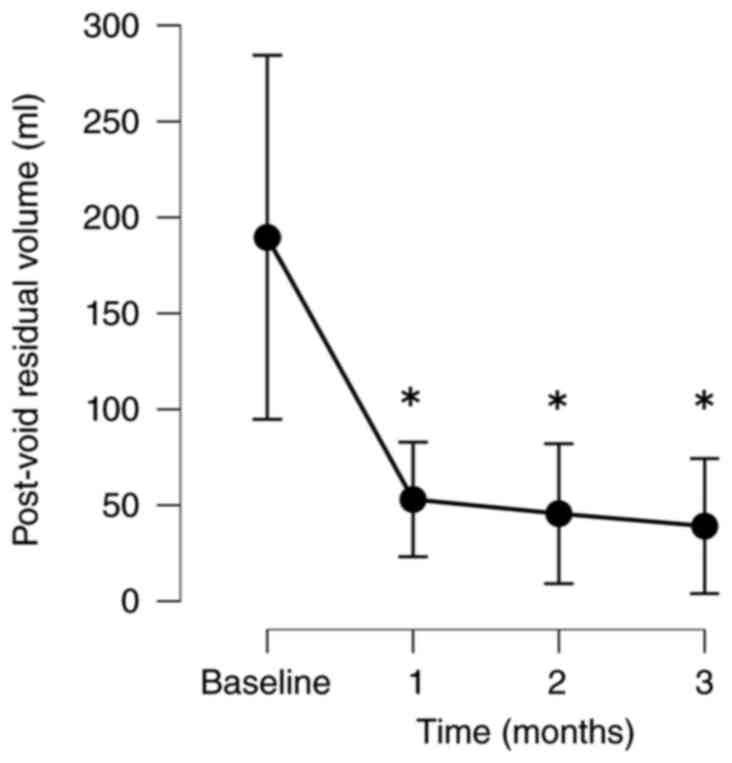
The patients exhibited significant improvements in
their QOL based on their IPSS-QOL scores. The PV and PVR volumes
decreased significantly. When comparing baseline to follow-up, the
mean post-operative IPSS-QOL scores at 1, 2 and 3 months following
treatment decreased significantly by 66.6, 81.7 and 88.0%,
respectively. PV significantly decreased by 25.3, 34.0 and 37.2% at
1, 2 and 3 months following treatment, respectively. The PVR volume
also decreased significantly from baseline to 1, 2 and 3 months
following treatment by 24.0, 23.2 and 40.8%, respectively (Fig. 1, Fig.
2 and Fig. 3). All the related
results are presented in Table
V.
 | Table VChanges in the outcomes of patients
from baseline to 3 months. |
Table V
Changes in the outcomes of patients
from baseline to 3 months.
| Parameter | Baseline | 1 month | 2 months | 3 months |
|---|
| IPSS-QOL | | | | |
|
No. of
patients analyzed | 40 | 33 | 32 | 25 |
|
Absolute,
mean (SD) | 5.4 (0.5) | 1.8 (0.9) | 1.0 (1.1) | 0.6 (1.0) |
|
Change, mean
(SD) | | -3.6 (1.1) | -4.4 (1.1) | -4.8 (1.1) |
|
% Change,
mean | | -66.6 (17.3) | -81.7 (19.3) | -88.0 (18.0) |
|
P-value | | <0.001 | <0.001 | <0.001 |
| PV | | | | |
|
No. of
patients analyzed | 40 | 34 | 32 | 25 |
|
Absolute,
mean (SD) | 56.9 (13.8) | 43.1 (15.4) | 37.0 (13.8) | 34.4 (13.0) |
|
Change, mean
(SD) | | -14.0 (7.8) | -18.6 (8.6) | -20.1 (10.1) |
|
% Change,
mean | | -25.3 (14.5) | -34.0 (15.7) | -37.2 (17.6) |
|
P-value | | <0.001 | <0.001 | <0.001 |
| PVR | | | | |
|
No. of
patients analyzed | 40 | 34 | 32 | 24 |
|
Absolute,
mean (SD) | 291.0 (419.9) | 50.2 (58.2) | 48.2 (46.9) | 39.1 (43.3) |
|
Change, mean
(SD) | | -214.4 (355.6) | -234.6 (382.3) | -150.4 (265.5) |
|
% Change,
mean | | -24.0 (78.8) | -23.2 (80.5) | -40.8 (39.1) |
|
P-value | | 0.004 | 0.002 | 0.001 |
Safety outcomes
Over the course of the follow-up period, AEs were
observed in 11 patients, including Clavien-Dindo grade II gross
hematuria in 2 (5%) patients and grade II urinary tract infection
(UTI) in 1 (2.5%) patient. In total, 4 patients (10%) had an
episode of UTI, and 2 patients (5%) had urinary frequency and
dysuria. In addition, 1 patient (2.5%) had an episode of urinary
tract pain. No grade ≥III Clavien-Dindo events occurred in any of
the patients (Table VI).
 | Table VISafety outcomes. |
Table VI
Safety outcomes.
| | Patients
(n=40) |
|---|
| Safety
outcomes | Clavien-Dindo
classification (14) |
|---|
| Adverse events, n
(%) | Grade I | Grade II |
|
Gross
hematuria | 0 | 2 (5.0) |
|
Urinary
frequency | 2 (5.0) | 0 |
|
Urinary
tract infection | 3 (7.5) | 1 (2.5) |
|
Urinary
tract pain | 1 (2.5) | 0 |
|
Dysuria | 2 (5.0) | 0 |
Discussion
The aim of the present study was to evaluate the
feasibility, efficacy and safety profile of day-case WVTT as an
office-based outpatient procedure in a real-world cohort. All
interventions were performed within a median period of 4.0 min
without intraoperative complications. No case required unscheduled
post-operative visits or hospitalization. These results suggest
that day-case, office-based WVTT is feasible, similar to other
minimally invasive therapies for BPH (10,15,16).
In the present study, spinal anesthesia was applied on all
procedures, and all patients could leave the office in ~4 h on the
day of the procedure. Although a recent meta-analysis revealed that
intravenous anesthesia was mainly applied during WVTT (17), the results of the present study
suggest that spinal anesthesia may also be considered as an
option.
In previous studies, the time to post-operative
catheter removal was between 0 and 7 days (11,12,18-20).
In the series of patients in the present study, the catheterization
time was 8 days in patients without pre-operative
catheter-dependent urinary retention (Table III). Furthermore, the present
study demonstrated that the catheter-free rate in the subgroup of
patients with a pre-operative catheter was 90.9% following a median
of 32 days. These results are in accordance with those of a
previous study (20). These data
also suggest that the time to the first trial of post-operative
catheter removal should be prolonged in pre-operatively
catheterized patients. Furthermore, the data presented herein
indicate that a history of urinary retention significantly
increases the risk of prolonged post-operative catheterization. The
first trial of post-operative catheter removal should also be
prolonged in patients with a history of catheterization. According
to the logistic regression analysis performed herein, more than six
injections during the procedure significantly increased the risk of
prolonged post-operative catheterization. As reported in a previous
study, the use of more injections may result in a greater degree of
inflammation and tissue edema, which may result in a longer
catheterization period (21).
The data of the present study indicate a significant
improvement of QOL with a reduction in IPSS-QOL by 66.6% at 1 month
following the procedure. This confirms data from prior studies
(12,13,22-24).
The PV and PVR volume decreased by ~40% at 3 months following
treatment. These results are consistent with those of previous
studies (12,13,22).
In terms of safety outcomes, AEs were observed in 11
patients, including grade II UTI and gross hematuria; however, no
patients had a grade ≥III event. As these results are comparable to
those of previous studies (11,12,20),
it can be assumed that WVTT can be safely performed as an
office-based, outpatient procedure.
The present study has certain limitations, which
should be mentioned. The present study was a single-office,
retrospective study with a select number of patients. Additionally,
a fundamental limitation of the present study was that follow-up
time points were not tightly controlled. Despite these limitations,
significant improvements in QOL and urinary function were observed
at all follow-up time points.
In conclusion, the present study demonstrates that
day-case WVTT is feasible, effective and safe as an office-based,
outpatient procedure. Further investigations are required however,
to determine patient groups for whom WVTT may be indicated and to
identify the advantages of WVTT for other minimally invasive
treatments for BPH.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed in the current
study are available from the corresponding author on reasonable
request.
Author's contributions
TO conceived and designed the study, obtained the
patients' data, performed data analysis, and wrote and edited the
manuscript. The author has read and approved the final manuscript.
TO confirms the authenticity of all the raw data.
Ethics approval and consent to
participate
Written informed consent was obtained from all study
subjects for their participation in the present study. Ethical
approval was obtained from the Ethics Committee of Mizuhodai
Urology (Fujimi, Japan; reference no. 1001). Written informed
consent was obtained from the patients for publication of the
present study and any related images.
Patient consent for publication
Not applicable.
Competing interests
The author declares that he has no competing
interests.
References
|
1
|
Vuichoud C and Loughlin KR: Benign
prostatic hyperplasia: Epidemiology, economics and evaluation. Can
J Urol. 22 (Suppl 1):S1–S6. 2015.PubMed/NCBI
|
|
2
|
Wei JT, Calhoun E and Jacobsen SJ:
Urologic diseases in America project: Benign prostatic hyperplasia.
J Urol. 173:1256–1261. 2005.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Zhang SJ, Qian HN, Zhao Y, Sun K, Wang HQ,
Liang GQ, Li FH and Li Z: Relationship between age and prostate
size. Asian J Androl. 15:116–120. 2013.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Foster HE, Dahm P, Kohler TS, Lerner LB,
Parsons JK, Wilt TJ and McVary KT: Surgical Management of lower
urinary tract symptoms attributed to benign prostatic hyperplasia:
AUA guideline amendment 2019. J Urol. 202:592–598. 2019.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Friedl A, Schneeweiss J, Stangl K,
Mühlstädt S, Zachoval R, Hruby S, Gründler T, Kivaranovic D,
Fornara P, Lusuardi L and Brössner C: The adjustable transobturator
male system in stress urinary incontinence after transurethral
resection of the prostate. Urology. 109:184–189. 2017.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Brassetti A, DE Nunzio C, Delongchamps NB,
Fiori C, Porpiglia F and Tubaro A: Green light vaporization of the
prostate: Is it an adult technique? Minerva Urol Nefrol.
69:109–118. 2017.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Checcucci E, Veccia A, De Cillis S,
Piramide F, Volpi G, Amparore D, Pecoraro A, Piana A, Granato S,
Verri P, et al: New ultra-minimally invasive surgical treatment for
benign prostatic hyperplasia: A systematic review and analysis of
comparative outcomes. Eur Urol Open Sci. 33:28–41. 2021.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Jones P, Rai BP, Nair R and Somani BK:
Current status of prostate artery embolization for lower urinary
tract symptoms: Review of world literature. Urology. 86:676–681.
2015.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Jones P, Rajkumar GN, Rai BP, Aboumarzouk
OM, Cleaveland P, Srirangam SJ and Somani BK: Medium-term Outcomes
of Urolift (Minimum 12 Months Follow-up): Evidence from a
systematic review. Urology. 97:20–24. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Yilmaz M, Karaaslan M, Polat ME, Tonyali
S, Aybal HÇ, Şirin ME, Toprak T, Tunç L, Gratzke C and Miernik A:
Is day-case surgery feasible for laser endoscopic enucleation of
the prostate? A systematic review. World J Urol. 41:2949–2958.
2023.PubMed/NCBI View Article : Google Scholar
|
|
11
|
McVary KT, Gange SN, Gittelman MC,
Goldberg KA, Patel K, Shore ND, Levin RM, Rousseau M, Beahrs JR,
Kaminetsky J, et al: Minimally invasive prostate convective water
vapor energy ablation: A multicenter, randomized, controlled study
for the treatment of lower urinary tract symptoms secondary to
benign prostatic hyperplasia. J Urol. 195:1529–1538.
2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
McVary KT, Gittelman MC, Goldberg KA,
Patel K, Shore ND, Levin RM, Pliskin M, Beahrs JR, Prall D,
Kaminetsky J, et al: Final 5-year outcomes of the multicenter
randomized sham-controlled trial of a water vapor thermal therapy
for treatment of moderate to severe lower urinary tract symptoms
secondary to benign prostatic hyperplasia. J Urol. 206:715–724.
2021.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Dixon CM, Cedano ER, Pacik D, Vit V, Varga
G, Wagrell L, Larson TR and Mynderse LA: Two-year results after
convective radiofrequency water vapor thermal therapy of
symptomatic benign prostatic hyperplasia. Res Rep Urol. 8:207–216.
2016.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Dindo D, Demartines N and Clavien PA:
Classification of surgical complications: A new proposal with
evaluation in a cohort of 6336 patients and results of a survey.
Ann Surg. 240:205–213. 2004.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Miller LE, Chughtai B, Dornbier RA and
McVary KT: Surgical reintervention rate after prostatic urethral
lift: Systematic review and meta-analysis involving over 2,000
patients. J Urol. 204:1019–1026. 2020.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Sciacqua LV, Vanzulli A, Di Meo R,
Pellegrino G, Lavorato R, Vitale G and Carrafiello G: Minimally
invasive treatment in benign prostatic hyperplasia (BPH). Technol
Cancer Res Treat. 22(15330338231155000)2023.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Yang J, Wu W, Amier Y, Li X, Wan W, Liu C,
Zhang Y and Yu X: Efficacy and safety of Water Vapor Thermal
Therapy in the treatment of benign prostate hyperplasia: A
systematic review and single-arm Meta-analysis. BMC Urol.
23(72)2023.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Bole R, Gopalakrishna A, Kuang R, Alamiri
J, Yang DY, Helo S, Ziegelmann MJ and Köhler TS: Comparative
postoperative outcomes of rezūm prostate ablation in patients with
large versus small glands. J Endourol. 34:778–781. 2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Johnston MJ, Noureldin M, Abdelmotagly Y,
Paramore L, Gehring T, Nedas TG, Rajkumar G, Emara A and Hindley
RG: Rezum water vapour therapy: Promising early outcomes from the
first UK series. BJU Int. 126:557–558. 2020.PubMed/NCBI View Article : Google Scholar
|
|
20
|
McVary KT, Holland B and Beahrs JR: Water
vapor thermal therapy to alleviate catheter-dependent urinary
retention secondary to benign prostatic hyperplasia. Prostate
Cancer Prostatic Dis. 23:303–308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Babar M, Loloi J, Azhar U, Tang K, Ines M,
Singh S, Iqbal N and Ciatto M: Rezum outcomes in relationship to
number of injections: Is less more? J Endourol. 37:157–164.
2023.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bausch K, Zahiti L, Schrutt M, Wetterauer
C, Halbeisen FS, Ebbing J and Seifert HH: Water vapor thermal
therapy of lower urinary tract symptoms due to benign prostatic
obstruction: Efficacy and safety analysis of a real-world cohort of
211 patients. World J Urol. 41:1605–1612. 2023.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Elterman D, Bhojani N, Vannabouathong C,
Chughtai B and Zorn KC: Rezūm therapy for ≥80-mL benign prostatic
enlargement: A large, multicentre cohort study. BJU Int.
130:522–527. 2022.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Whiting D, Noureldin M, Abdelmotagly Y,
Johnston MJ, Brittain J, Rajkumar G, Emara A and Hindley R:
Real-world early outcomes and retreatment rates following water
vapour ablative therapy for symptomatic benign prostatic
hyperplasia. Eur Urol Open Sci. 39:72–78. 2022.PubMed/NCBI View Article : Google Scholar
|