Introduction
Diabetes mellitus (DM) is a disorder characterized
by high blood glucose levels and varying degrees of dysfunction in
the metabolism of proteins, lipid, and carbohydrates (1). Over the past two decades, the
understanding and management of DM, including its causes, spread,
prevention and therapy, have been firmly established (2). Damage to several biological systems,
such as the blood vessels, eyes, heart and nerves, leads to
diabetes-related complications and is associated with an elevated
risk of developing multiple illnesses (3). A metabolic disease that affects a
large number of individuals over time, is type 2 DM (T2DM)
(2). Diabetic retinopathy (DR) is
one of the microvascular complications associated with this disease
globally, that may potentially harm the eyes, particularly in
individuals between the ages of 20 and 65, resulting in visual
impairment or loss (4). When the
inner blood-retinal barrier weakens due to microvascular occlusion,
one of the most prevalent secondary microvascular complications of
diabetes is DR (4). DR may be
classified into two forms as follows: Proliferative DR (PDR) and
non-proliferative DR (NPDR) (5).
The loss of pericytes from retinal capillaries to generate
acellular capillaries, an increase in vascular permeability and the
breakdown of the inner endothelium blood retinal barrier are the
hallmarks of NPDR, an early stage of DR. Usually, there are no
symptoms. PDR is an advanced stage in which the retina forms new,
delicate and twisted blood vessels. These may result in retinal
detachment, vitreous hemorrhage and fibrovascular epiretinal
membranes, all of which are factors associated with loss of vision
(5).
Hyperglycemia causes changes in metabolic processes,
leading to the development of DR. The symptoms of diabetes result
from several interrelated processes in its intricate
pathophysiology, which include the generation of advanced glycation
end products, the stimulation of the polyol pathway and protein
kinase C, and the stimulation of the hexosamine pathway. Reactive
oxygen species (ROS) activate and disrupt these pathways, leading
to damage in the mitochondria and to an elevated death rate in
capillary cells (6).
The nuclear factor erythroid-2-related factor 2
(NFE2L2; also known as Nrf2) expertly regulates redox homeostasis.
This transcription factor belongs to the basic leucine zipper
subfamily. Under normal conditions, Nrf2 is usually bound to its
negative regulator, Keap1. However, when stress levels increase,
this bond weakens and Nrf2 moves to the nucleus. In the nucleus,
Nrf2 attaches to the antioxidant responsive element (ARE) and
activates various genes. This activation leads to a wide range of
activities, including detoxification, antioxidant activity,
cellular redox homeostasis, glutathione homeostasis and
mitochondrial biogenesis. Some of the genes activated by Nrf2
include superoxide dismutase (SOD) and heme oxygenase-1 (HO-1)
(7).
Extremely high blood pressure and blood glucose
levels can easily harm the tiny blood vessels in the retina. NFE2L2
plays a protective role in the retina (8). The deterioration of the retinal
pigment epithelium has been shown to occur in NFE2L2 knockout mice
as they age, suggesting that a lack of NFE2L2 can cause retinal
illness (9). Furthermore, during a
shared time frame with angiogenesis, NFE2L2 protects the retina
from hyperoxia-induced oxidative damage. Research using animals
deficient in NFE2L2 has indicated that the protein protects the
retina from damage caused by ischemia-reperfusion, indicating that
the pharmacological induction of NFE2L2 may be a novel approach for
the treatment of retinal illnesses, such as ischemia-reperfusion
(10). The expression of NFE2L2 is
increased in acute hyperglycemia and decreased in chronic
hyperglycemia. The downregulation of NFE2L2 expression leads to
microvascular changes that eventually lead to diabetes-related
consequences (11). The expression
of Nrf2 is regulated by interaction partners or post-translational
modifications, which subsequently influence its stability and
function (12).
The present study aimed to investigate the different
forms of gene expression of NFE2L2 and their association with blood
SOD and HO-1 levels in diabetic individuals with retinopathy.
Patients and methods
Study participants
A cohort of Iraqi individuals with T2DM diagnosed
with the disease at least 5 years prior were included in the
present observational case-control study. The participants included
in the present study were patients from the Specialized Centre for
Endocrinology and Diabetes and Ibn Al-Haitham Hospital of
Ophthalmology in Baghdad, Iraq. The recruiting commenced in
February, 2023 and was concluded in July, 2023. The research
protocol was approved by the College of Pharmacy Scientific and
Ethics Committee, University of Baghdad (REAFUBCP3112023A), Ibn Al
Haitham Teaching Eye Hospital (EAC 6332 in February 6, 2023) and
the Specialized Centre for Endocrinology and Diabetes (Registration
no. 53664 on February 1, 2023). Moreover, a written informed
consent was obtained from each participant. All participants were
interviewed by the researchers and demographic data were obtained
from them and recorded on a data collection sheet, including age,
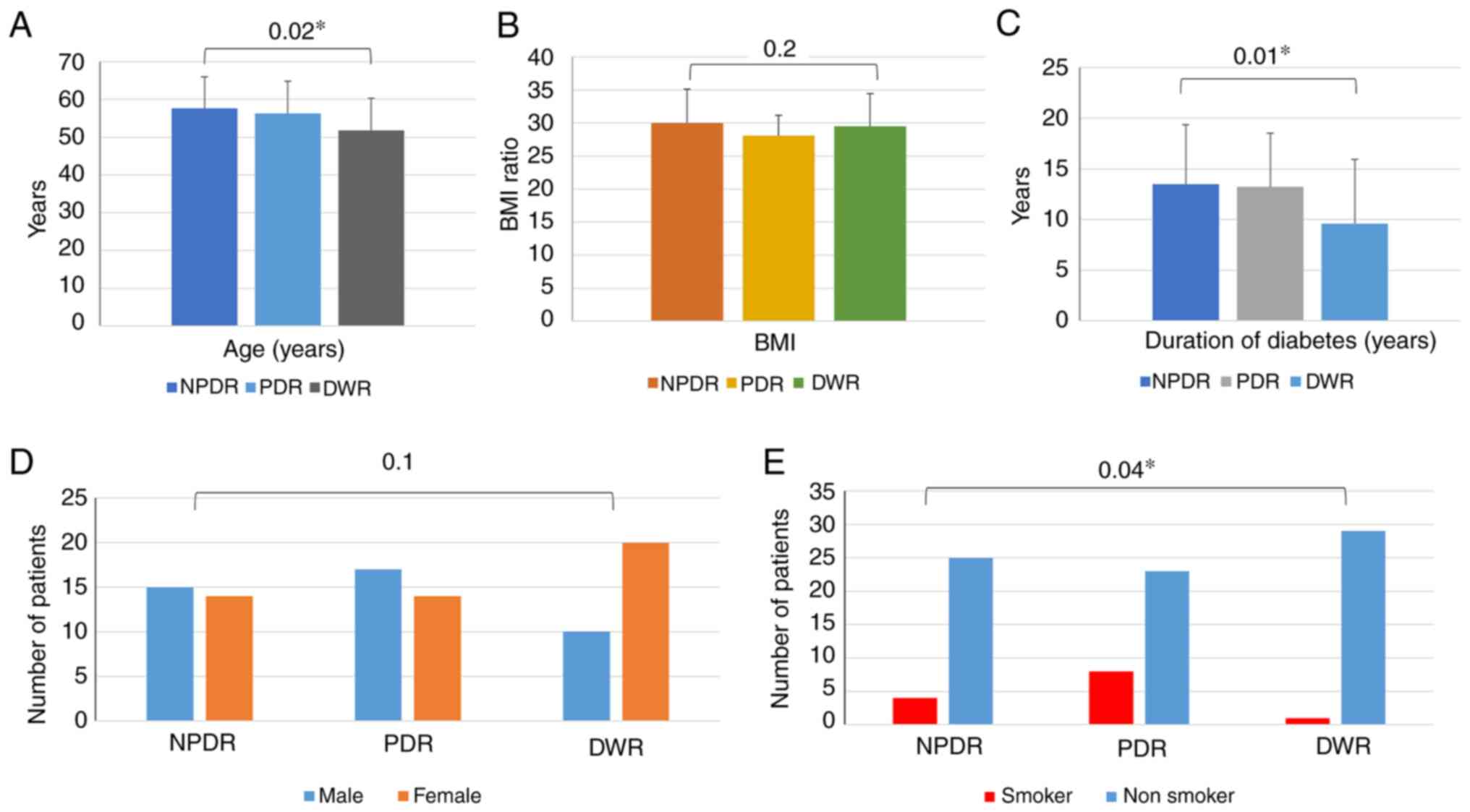
sex, the duration of disease, body weight and height (Table I).
 | Table IDemographic data for patients in the
PDR, NPDR and DWR groups. |
Table I
Demographic data for patients in the
PDR, NPDR and DWR groups.
| Characteristic | NPDR (n=29) | PDR (n=31) | DWR (n=30) | P-value |
|---|
| Age (years), mean ±
SD |
57.59±8.382a |
56.23±8.601a |
51.77±8.529b | 0.02 |
| BMI, mean ± SD | 29.9847±5.15348 | 28.0697±3.06455 | 29.4913±4.94645 | 0.2 |
| Duration of diabetes
(years), mean ± SD |
13.48±5.865a |
13.23±5.290a | 9.60
±6.333b | 0.01 |
| Sex, n (%) | | | | 0.1 |
|
Male | 15 (51.7%) | 17 (54.8%) | 10 (33.3%) | |
|
Female | 14 (48.3%) | 14 (45.2%) | 20 (66.7%) | |
| Smoking status, n
(%) | | | | 0.04 |
|
Yes | 4 (13.8%) | 8 (25.8%) | 1 (3.3%) | |
|
No | 25 (86.2%) | 23 (74.2%) | 29 (96.7%) | |
A total of 102 participants initially participated
in the study. Nevertheless, the blood samples from 12 patients were
omitted from the study due to hemolysis. The remaining 90 patients
were divided into the following groups: Group A consisted of 60
individuals who were diagnosed with T2DM and retinopathy. Their
ages ranged from 40 to 80 years. Within this group, 29 patients had
NPDR and 31 patients had PDR. Optical coherence tomography was used
by an ophthalmologist to verify the presence and location of
intra-retinal and sub-retinal fluid, retinal hemorrhages and
microaneurysms; the early treatment diabetic retinopathy study
(ETDRS) criteria were used for the diagnosis of retinopathy
(13). Group B consisted of 30
patients with T2DM without retinopathy (DWR), serving as the
control group.
Inclusion and exclusion criteria
The inclusion criteria were as follows: Patients
were selected to be previously diagnosed with T2DM according to the
American Diabetic Association (ADA) diagnostic criteria (14). The age of the diabetic patients had
to be between 40-80 years. The duration of DM in the patients need
to be >5 years.
The following exclusion criteria were used: Patients
with type 1, gestational DM, patients on insulin therapy, diabetic
patients with cardiovascular, liver and renal diseases, acute
bacterial and viral infection, autoimmune diseases and ocular
diseases, diabetic patients using multivitamin supplements, and
those with DR on anti-VEGF drugs were excluded from the study.
Specimen collection and handling
From each participant, 10 ml of venous blood were
drawn by venipuncture. A total of 5 ml of the obtained blood sample
was transferred into an EDTA blood collection tube (Ningbo Greetmed
Medical Instruments Co., Ltd.) for HbA1c assay by modified
enzymatic reagent for the in vitro determination of HbA1C in
human blood; 250 µl of the contents of the EDTA tube were
transferred to a 750-µl TRIzol Eppendorf tube (Shandong Leader
Technology Co., Ltd.) and frozen at (-20˚C) for RNA extraction and
analysis. Following 30 min of coagulation, 5 ml of the remaining
whole blood were transferred to a gel tube. The tube was then
centrifuged at 1,008 x g for 10 min at room temperature to extract
the serum. Some serum was utilized by the laboratory of the medical
center (Specialized Centre for Endocrinology and Diabetes, Baghdad,
Iraq) to determine fasting serum glucose (FSG) levels using an
enzymatic colorimetric technique on the same day of sample
collection. Aliquots of the remaining serum were stored in
Eppendorf tubes and then frozen at -20˚C) until all samples were
collected. Following this, SOD and HO-1 levels were measured using
ELISA kits (MyBioSource; SOD kit cat. no. MBS005068 and HO-1 kit
cat. no. MBS268886).
Analysis of gene expression
RNA was extracted from all samples using a pre-made
solution, namely TRIzol® LS reagent (Guangzhou Dongsheng
Biotech Co., Ltd.), following the manufacturer's protocol. The
concentration and purity of the extracted RNA were assessed using a
Nanodrop spectrophotometer (Thermo Fisher Scientific, Inc.) to
determine the quality of the samples for later analysis using
reverse transcription-quantitative PCR (RT-qPCR). By using an
EasyScript® One-Step gDNA Removal and cDNA Synthesis
SuperMix kit, total RNA were reverse transcribed to complementary
DNA (cDNA) and stored for expression analysis. The NFE2L2 gene
expression levels were determined using RT-qPCR. Alpha DNA Ltd.
created and produced the primer sequences for the NFE2L2 gene
(Table II), which were then
freeze-dried and kept at a temperature of -20˚C. The GAPDH
housekeeping gene was used as an internal control to determine the
ΔCt value (Table II). To
normalize the quantities of mRNA that are produced by the NFE2L2
gene, the levels of the internal control gene GAPDH were amplified
and analyzed. A smart cycler real-time PCR System was used. The
components of TransStart® Top Green qPCR Super Mix kits
TransGen Biotech Co., Ltd. were used to measure the threshold cycle
(Cq), which allowed for the determination of the fold change and
the levels of gene expression, as shown in Table III.
 | Table IIPrimers sequence for used for the
analysis of the gene expression of GAPDH and NFE2L2. |
Table II
Primers sequence for used for the
analysis of the gene expression of GAPDH and NFE2L2.
| Gene | Primer sequence
(5'→3' direction) | Primer size (bp) | Product size
(bp) | Temperature (˚C) |
|---|
| NFE2L2 | | | | |
|
Forward |
ACCCTTGTCACCATCTCAGG | 20 | 134 | 52 |
|
Reverse |
AGCGGCTTGAATGTTTGTCT | 20 | | |
| GAPDH | | | | |
|
Forward |
GAAATCCCATCACCATCTTCCAGG | 24 | 160 | 58 |
|
Reverse |
GAGCCCCAGCCTTCTCCATG | 20 | | |
 | Table IIIConditions used for the RT-qPCR
analysis of GAPDH and NFE2L2 genes. |
Table III
Conditions used for the RT-qPCR
analysis of GAPDH and NFE2L2 genes.
| Step | Temperature | Time | No. of cycles |
|---|
| Initial
denaturation | 94˚C | 5 Min | 1 |
| Denaturation | 94˚C | 10 Sec | |
| Annealing | 52˚C
(NFE2L2) 58˚C (GAPDH) | 15 Sec | 40 |
| Extension | 72˚C | 20 Sec | |
| Final
extension | 72˚C | 5 Min | 1 |
Calculation of gene expression
The determination of fold differences in the
quantitative expression of mature RNAs was accomplished using the
relative cycle threshold (2-∆∆Cq) methodology (15). The real-time cycler software was
used in order to establish a threshold cycle (Cq) for each sample.
The Cq values for the housekeeping gene, GAPDH, and the target
gene, NFE2L2, being tested in the patients and controls were
documented.
Statistical analysis
Data were analyzed using SPSS version 25 software
(IBM Corp.). The Shapiro-Wilk test was used to test the normality
of the results. Continuous variables are expressed as the mean ±
SD, while numbers and frequencies were used for presenting
categorical data. For normally distributed data, one-way analysis
of variance (ANOVA) was utilized for more than two groups for
continuous variables. When the latter test results were
significant, a post-hoc analysis was performed with Duncan's
multiple range test. Fisher's exact or Chi-squared tests were
utilized to measure the group differences between categorical
variables. Pearson's correlation analysis was performed and the
correlation coefficient (R) was used to calculate the correlation
between parameters. Receiver operation characteristic curve (ROC
curve) analysis was also used. A P-value <0.05 was considered to
indicate a statistically significant difference.
Results
Demographical data of the two groups
of patients with T2DM with retinopathy and in those without
retinopathy
The differences between the PDR, NPDR and DWR groups
were analyzed. Statistically significant differences were found for
age, the duration of diabetes and smoking status between these
groups, whereas the mean values of BMI and sex did not exhibit any
significant differences between the groups (Fig. 1).
Biochemical characteristics of
patients with DM with and without retinopathy
Although the PDR group exhibited elevated levels of
FSG compared with the NPDR and DWR groups, there were no
significant differences among the three groups. The results of the
analysis of HbA1c revealed significantly higher levels in the PDR
group than in the NPDR and DWR groups. Conversely, the HbA1c levels
exhibited no significant difference between the NPDR and DWR groups
(Table IV).
 | Table IVBiochemical characteristics of
diabetic patients with two stages of retinopathy and without
retinopathy. |
Table IV
Biochemical characteristics of
diabetic patients with two stages of retinopathy and without
retinopathy.
| Marker | PDR (n=31), mean ±
SD | NPDR (n=29), mean ±
SD | DWR (n=30), mean ±
SD | P-value |
|---|
| FSG mg/dl | 221.61±71.307 | 193.07±72.896 | 204.90±72.844 | 0.3 |
| HbA1C |
9.69±1.724a |
8.23±1.733b |
8.361.736b | 0.002 |
| HO-1 (ng/ml) |
4.295±0.609b |
4.259±0.656b |
7.697±0.921a | 0.0001 |
| SOD (U/ml) |
70.799±20.313b |
68.271±22.740b |
246.013±50.619a | 0.0001 |
In addition, significantly lower serum levels of SOD
and HO-1 were observed in PDR and NPDR groups, compared with the
DWR group. However, there were no significant differences between
the PDR and NPDR groups as regards the SOD and HO-1 levels
(Table IV).
Comparison of gene expression of
NFE2L2 among the different groups
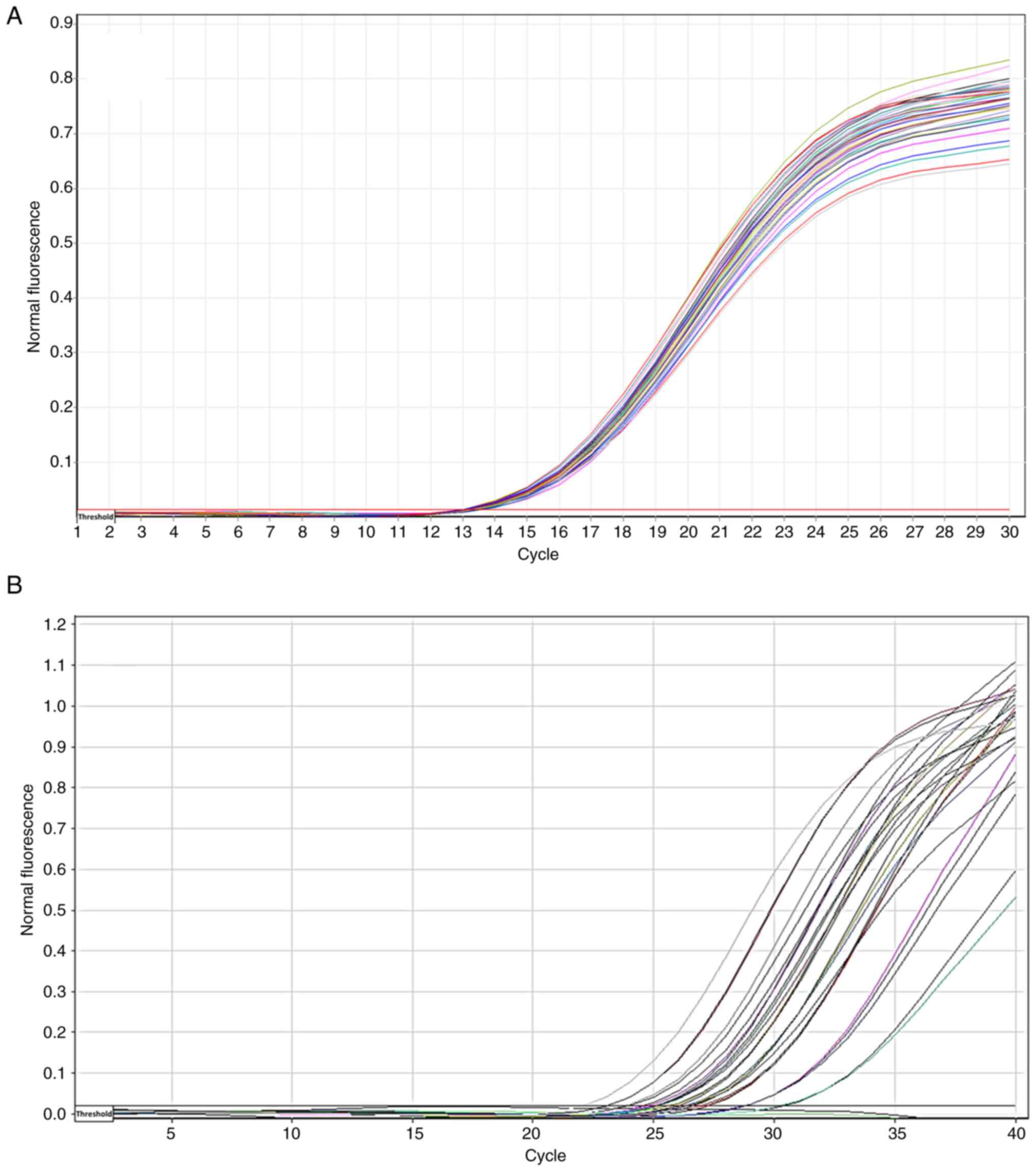
The amplification plots of GAPDH and NFE2L2 mRNA
expression were determined as a Cq value (Fig. 2). A lower Cq value indicated the
presence of larger copies of the target, whereas a higher Cq value
indicated the presence of smaller copies. As regards gene
expression, high Cq values indicate low expression, and low Cq
values indicates a high expression (Fig. 2).
The mean Cq value of GAPDH, the housekeeping gene
used in the present study, and the mean Cq value of the NFE2L2 gene
are presented in Table V. The gene
expression of NEF2L2 was 3-fold higher in the DR groups than in the
DWR group (Table V). In addition,
a a significant increase in the folds of gene expression was
observed in the DR groups compared with the DWR group (Fig. 3).
 | Table VFold of NFE2L2 expression as per the
2-ΔΔCq method. |
Table V
Fold of NFE2L2 expression as per the
2-ΔΔCq method.
| Groups | Mean Cq of
NFE2L2 | Mean Cq of
GAPDH | ΔΔCq (mean Cq of
NFE2L2) |
2-ΔΔCq |
Experimentalgroup/control group | Fold of gene
expression |
|---|
| PDR (n=31) | 25.97 | 13.98 | 11.99 | 0.000246 |
0.000246/0.000076 | 3.2 |
| NDPR (n=29) | 26.24 | 14.20 | 12.03 | 0.000239 |
0.000239/0.000076 | 3.1 |
| DWR (n=30) | 27 | 13.30 | 13.67 | 0.000076 |
0.000076/0.000076 | 1.00 |
Analysis of NFE2L2 gene expression
using a ROC curve
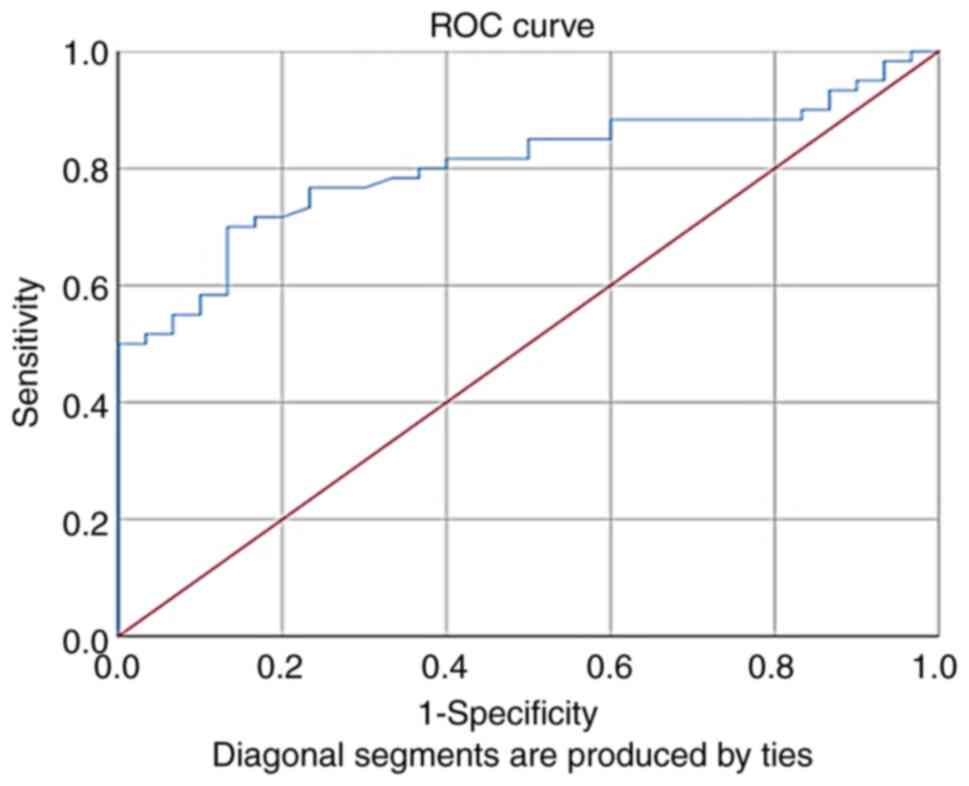
The validity of the NFE2L2 fold of expression as a
marker for the diagnosis of retinopathy was assessed using ROC
curves. The results revealed that the fold of gene expression was a
good indicator for retinopathy in patients with T2DM, with an area
under the ROC curve of 0.8, a sensitivity of 70% and a specificity
of 87% (Fig. 4 and Table VI).
 | Table VIReceiver operating characteristic
curve analysis of NFE2L2 fold of gene expression. |
Table VI
Receiver operating characteristic
curve analysis of NFE2L2 fold of gene expression.
| Parameter | AUC | Explanation | P-value | Best cut off | Sensitivity
(%) | Specificity
(%) |
|---|
| Fold | 0.80 | Very good | 0.001 | 2.5928 | 70 | 87 |
Correlation of fold for gene
expression with the studied biomarkers
Correlation analysis revealed that NFE2L2 gene
expression was significantly high, with a decrease in the of SOD
and HO-1 serum levels, thus indicating a negative correlation
between NFE2L2 gene expression, and SOD and HO-1 serum levels
(Table VII).
 | Table VIIPearson's correlation analysis
between serum biomarker levels included in the present study and
fold change. |
Table VII
Pearson's correlation analysis
between serum biomarker levels included in the present study and
fold change.
| Parameter | HO-1 | SOD |
|---|
| Fold change | | |
|
R value | -0.357 | -0.364 |
|
P-value | 0.001 | 0.0001 |
Discussion
Previous research has demonstrated that the
transcription factor NFE2L2 plays a crucial protective role against
DR by regulating ARE-antioxidant genes (16,17).
This suggests that NFE2L2 may be a promising therapeutic target for
DR. The present study observed the increased expression of the
NFE2L2 gene among patients with DR. In addition, its expression
negatively correlated with the downstream antioxidant enzymes, SOD
and HO-1. A number of studies analyzing the expression of the
NFE2L2 gene have demonstrated similar results. The study conducted
by Sun et al (18) revealed
the increased of expression Nrf2 in the retina of rats with DM,
suggesting the beginning of an endogenous oxidative stress system
in these animals. It was discovered that rat retinal tissues under
DM conditions had an abnormally high oxidative stress marker, with
considerably lower levels of SOD and such a diabetic status can
further induce the upregulation of Nrf2 expression (18). In the study by Cao et al
(19), retinal ganglion cells
exhibited an increased apoptosis and ROS content under high glucose
induction, suppressed cellular anti-oxidation indices such as SOD,
an increased expression of NFE2L2, and a decreased expression of
the negative regulatory protein for Nrf2, Keap1. In another study
by Xu et al (10), retinal
tissues from mice with DM exhibited a significantly increased Nrf2
expression and nuclear translocation, together with potentiated
peroxidation products. Based on these findings, it appears that the
antioxidation potency of the body is still lacking, with abnormally
high ROS concentrations, even if the oxidative stress mechanism has
been started.
HO-1, a crucial activator of NFE2L2/ARE-dependent
signaling, has the ability to protect retinal neurons and vascular
endothelial cells from damage caused by DR. HO-1 has marked
anti-inflammatory, antioxidant and antiproliferative properties.
Retinal pigment epithelial cells, microglia and neurons all contain
HO-1(20).
Compared with the diabetic group without
retinopathy, the HO-1 level in the DR group was markedly lower,
according to previous studies (21,22).
These results corroborate the findings of the present study,
demonstrating that HO-1 influences the development of DR,
particularly the malfunction and death of retinal endothelial cells
(21,22).
As a catalyst for the conversion of superoxide to
hydrogen peroxide, SOD is responsible for its elimination. Hydrogen
peroxide retains some active, but is less so than superoxide; it
plays a crucial role in typical cellular signaling. It is possible
to further break down hydrogen peroxide to water by using catalase
or peroxidases. The retina of diabetic mice exhibits a reduced SOD
activity (23). One way to prevent
the development of acellular retinal capillaries in diabetes is to
overexpress SOD in transgenic mice. As a result, it keeps electron
transport chain complex III active, inhibits the increase in
retinal superoxide caused by diabetes, and prevents the
mitochondria from becoming more permeable. This suggests that the
restoration of SOD activity exerts a protective effect (23).
Consistent with previous studies (24,25),
the present study demonstrated that high HbA1c levels increased the
risk of retinopathy (in the PDR and NPDR groups) and that stringent
blood glucose control lowers both the risk and severity of
retinopathy.
In the case of diabetic microvascular issues,
glycemic control is crucial. There is a 37% decrease in the risk of
developing microvascular complications for every 1% decrease in the
revised mean of HbA1c (26).
Consistent with the findings of the present study,
Hou et al (27)
demonstrated that hyperglycemia induces the presence of ROS, such
as superoxide and hydrogen peroxide, in the body and is caused by
the auto-oxidation of glucose, lipid peroxidation and protein
glycation. This leads to a decrease in SOD activity and an increase
in malondialdehyde levels in diabetic individuals with retinopathy
compared to those without retinopathy (27). Diabetic individuals with
retinopathy exhibit a significant decrease in SOD activity,
suggesting a deficiency in antioxidants. This deficiency directly
leads to the production of ROS (28). The function and activity of NFE2L2
may be hindered by a number of factors, which lead to an increased
expression of the NRF2L2 gene without an increased ARE-target gene
expression (SOD and HO-1). First, the transcriptional regulation in
which the NFE2L2 promoter contains a binding site for NF-κB, allows
it to be induced by inflammatory stimuli. A high basal activity of
NFE2L2 has been attributed to the constitutive NF-κB-mediated
upregulation of the NFE2L2 gene. Second, post-transcriptional
regulation also plays a role: MicroRNAs are endogenous
single-stranded, non-coding RNAs with an average of 22 nucleotides
in length that repress gene expression by sequence-specific binding
with mRNA molecules and subsequent inhibition of protein
translation and destabilization of mRNA. Third, as regards the
regulation of the Nrf2 transcriptional activation of its target
genes, gene transcription profiles have revealed that not all genes
in the vicinity of NFE2LE are transcriptionally regulated by NFE2L2
binding. The regulation of NFE2L2 activity is not limited to the
control of its abundance, but can also be modulated by the
availability of its binding partners. Fourth, the as regards the
regulation of Nrf2 protein stability, NFE2L2 possesses seven
conserved NRF2-ECH homology (Neh) domains with different functions
to control NFE2L2 transcriptional activity. Neh6 domain contains
two redox-independent degrons that bind to E3 ubiquitin ligase
β-transducin repeat-containing protein, which mediates NFE2L2
degradation in oxidatively stressed cells. The Neh7 domain mediates
interaction with retinoic X receptor alpha, which represses NFE2L2
activity. These domains modulate NFE2L2 stability and
transcriptional activation of its target genes at multiple levels,
including transcriptional and post-transcriptional and
post-translational regulation in response to various insults. Thus,
the aforementioned possible factor may alter the activity of NFE2L2
at the levels of transcription, translation, post-translational
modifications, nuclear translocation, and binding to the promoters
of regulated genes (29).
The present study had certain limitations which
should be mentioned. One was that it only included two diabetic
endocrine centers in the city of Baghdad. Furthermore, the
measurement of NFE2L2 in vitreous fluid was not possible in the
present study. In addition, the present study was not able to
accommodate a sufficient amount of time for follow-up between pre-
and post-treatment groups of diabetic retinopathy patients owing to
capacity constraints. However, one of the primary limitations of
the present study was the small sample size. The limited number of
participants may reduce the generalizability of the results, making
it difficult to apply the findings to a broader population. The
limited financing has restricted the authors' ability to obtain
more kits to assess the aforementioned markers. Future studies with
larger sample sizes are thus required to validate these findings
and enhance their applicability.
In conclusion, the present study demonstrated that
the serum SOD and HO-1 levels were significantly lower in the DR
groups than in the DWR group. The expression of the NFE2L2 gene was
increased by 3-fold in diabetic patients with retinopathy. The
correlation analysis revealed a negative correlation between the
fold change and serum SOD and HO-1 levels in the DR groups. The
decline in SOD and HO-1 levels in the DR groups indicated the
consumption of antioxidant capacity in detoxifying ROS due to
uncontrolled hyperglycemia, thus increasing the expression of
NFE2L2 to counteract the oxidative stress conditions in DR.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHM was involved in the conception and design of the
study, in the literature search, clinical and data analysis,
statistical analysis, and in the preparation and manuscript
reviewing of the manuscript. SHA was involved in the conception and
design of the study, in data analysis, and in the preparation and
manuscript reviewing of the manuscript. SHM and SHA confirm the
authenticity of all the raw data. Both authors have read and
approved the final manuscript. SHM and SHA confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
Prior to sample collection, a statement of patient
by written informed consent to participate in the study as
specified in the Declaration of Helsinki was sought from each
patient. Administrative ethical approval was granted by the
University of Baghdad/College of Pharmacy Ethics Committee
(registered under REAFUBCP3112023A), Ibn Al Haitham Teaching Eye
Hospital (EAC 6332 in February 6, 2023) and the Specialized Centre
for Endocrinology and Diabetes (Registration no. 53664 on February
1, 2023).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Faris Raheem M, Ali SH, Al-Nuaimi AMA and
Shareef GL: Impact of serum vitamin D level on selected
bone-related markers in obese-type 2 diabetes patients. [version 1;
peer review: awaiting peer review]. F1000Research. 12(56)2023.
|
|
2
|
Ali IA and Ali SH: Impact of osteocalcin
level on vascular calcification in type 2 diabetics in relation to
fibroblast growth factor-23 (FGF-23). Iraqi J Pharm Sci. 27:42–54.
2018.
|
|
3
|
Shaheed HS and Ali SH: Association of
carnosinase-1 gene polymorphism with serum carnosine and
carnosinease-1 isoform levels in type 2 diabetics with
cardiovascular diseases in Iraq. Al-Rafidain J Med Sci. 4:109–117.
2023.
|
|
4
|
Kadhim SA, Saleh ES and Jaafer AD:
Assessment of serum levels of advanced oxidation protein products
in type 2 diabetic patients with and without retinopathy taking
different antidiabetic treatments. Iraqi J Pharm Sci. 32:74–82.
2023.
|
|
5
|
Gui F, You Z, Fu S, Wu H and Zhang Y:
Endothelial dysfunction in diabetic retinopathy. Front Endocrinol
(Lausanne). 11(591)2020.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Mhaibes SH and Ali SH: Biomarkers of
oxidative stress in diabetic microvascular complications. Iraqi J
Pharm Sci. 33(3)2024.(In press).
|
|
7
|
He F, Ru X and Wen T: NRF2, a
transcription factor for stress response and beyond. Int J Mol Sci.
21(4777)2020.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Xu X, Luo P, Wang Y, Cui Y and Miao L:
Nuclear factor (erythroid-derived 2)-like 2 (NFE2L2) is a novel
therapeutic target for diabetic complications. J Int Med Res.
41:13–19. 2013.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhao Z, Chen Y, Wang J, Sternberg P,
Freeman ML, Grossniklaus HE and Cai J: Age-related retinopathy in
NRF2-deficient mice. PLoS One. 6(e19456)2011.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Xu Z, Wei Y, Gong J, Cho H, Park JK, Sung
ER, Huang H, Wu L, Eberhart C, Handa JT, et al: NRF2 plays a
protective role in diabetic retinopathy in mice. Diabetologia.
57:204–213. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Kumar A and Mittal R: Nrf2: A potential
therapeutic target for diabetic neuropathy. Inflammopharmacology.
25:393–402. 2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Dodson M, Shakya A, Anandhan A, Chen J,
Garcia JGN and Zhang DD: NRF2 and diabetes: The good, the bad, and
the complex. Diabetes. 71:2463–2476. 2022.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Solomon SD and Goldberg MF: ETDRS grading
of diabetic retinopathy: Still the gold standard? Ophthalmic Res.
62:190–195. 2019.PubMed/NCBI View Article : Google Scholar
|
|
14
|
American Diabetes Association Professional
Practice Committee. 2. Diagnosis and classification of diabetes:
Standards of care in diabetes-2024. Diabetes Care. 47 (Suppl
1):S20–S42. 2024.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408.
2001.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Deliyanti D, Alrashdi SF, Tan SM, Meyer C,
Ward KW, de Haan JB and Wilkinson-Berka JL: Nrf2 activation is a
potential therapeutic approach to attenuate diabetic retinopathy.
Invest Ophthalmol Vis Sci. 59:815–825. 2018.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Li C, Miao X, Li F, Wang S, Liu Q, Wang Y
and Sun J: Oxidative stress-related mechanisms and antioxidant
therapy in diabetic retinopathy. Oxid Med Cell Longev.
2017(9702820)2017.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Sun Y, Xiu C, Liu W, Tao Y, Wang J and Qu
YI: Grape seed proanthocyanidin extract protects the retina against
early diabetic injury by activating the Nrf2 pathway. Exp Ther Med.
11:1253–1258. 2016.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Cao Y, Li X, Wang CJ, Li P, Yang B, Wang
CB and Wang LX: Role of NF-E2-related factor 2 in neuroprotective
effect of l-carnitine against high glucose-induced oxidative stress
in the retinal ganglion cells. Biomed Pharmacother. 69:345–348.
2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
B Domènech E and Marfany G: The relevance
of oxidative stress in the pathogenesis and therapy of retinal
dystrophies. Antioxidants (Basel). 9(347)2020.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Fan J, Xu G, Jiang T and Qin Y:
Pharmacologic induction of heme oxygenase-1 plays a protective role
in diabetic retinopathy in rats. Invest Ophthalmol Vis Sci.
53:6541–6556. 2012.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Bucolo C, Drago F, Maisto R, Romano GL,
D'Agata V, Maugeri G and Giunta S: Curcumin prevents high glucose
damage in retinal pigment epithelial cells through ERK1/2-mediated
activation of the Nrf2/HO-1 pathway. J Cell Physiol.
234:17295–17304. 2019.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Kanwar M, Chan PS, Kern TS and Kowluru RA:
Oxidative damage in the retinal mitochondria of diabetic mice:
Possible protection by superoxide dismutase. Invest Ophthalmol Vis
Sci. 48:3805–3811. 2007.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Setareh J, Hoseinzade G, Khoundabi B,
Kamali M, Ebrahimi A, Fazlollahpour-Naghibi A, Zareei M,
Mohamaditabar M and Makaremi A: Can the level of HbA1C predict
diabetic retinopathy among type II diabetic patients? BMC
Ophthalmol. 22(415)2022.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Kant D, Kumari J and Singh RK: Correlation
of blood sugar and HbA1C levels in different stage of diabetes
retinopathy: A hospital based prospective study. Int J Health Sci.
6:1867–1876. 2022.
|
|
26
|
Salih BH, Ali SH and Allehibi KI: Serum
aldosterone levels in patients with diabetic nephropathy in
relation to vascular calcification. Iraqi J Pharm Sci. 28:53–63.
2019.
|
|
27
|
Hou Y, Lin M, Qiu X, He M, Zhang Y and Guo
F: Effect of type-2 diabetes mellitus in retinopathy patients on
MDA, SOD activity and its correlation with hba1c. Braz Arch Biol
Technol. 64(e21200075)2022.
|
|
28
|
Filla LA and Edwards JL: Metabolomics in
diabetic complications. Mol Biosyst. 12:1090–1105. 2016.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Hyttinen JMT, Kannan R, Felszeghy S,
Niittykoski M, Salminen A and Kaarniranta K: The Regulation of
NFE2L2 (NRF2) signalling and epithelial-to-mesenchymal transition
in age-related macular degeneration pathology. Int J Mol Sci.
20(5800)2019.PubMed/NCBI View Article : Google Scholar
|