Introduction
Vietnam is a Southeast Asian country characterized
by a tropical monsoon climate with a rich vegetation system. Out of
>12,000 plant species in Vietnam, 5,117 of these have medicinal
value (1,2), representing a key source of active
compounds for research or applications in medicine, pharmacy and
the pharmaceutical industry. The objective of the present study was
to investigate the chemical constituents and biological activities
of the essential oils (EOs) of three Melaleuca species
growing in Vietnam.
The genus Melaleuca (Myrtaceae) includes 280
species, of which four species have been found in Vietnam,
including Melaleuca alternifolia, Melaleuca cajuputi,
Melaleuca leucadendra and Melaleuca quinquenervia
(1). According to traditional
Vietnamese medicine, the species M. cajuputi, M. leucadendra
and M. quinquenervia are widely used in the treatment of
diseases, such as cold, flu, fever, malaria, indigestion, bone
pain, diarrhea, inflammatory skin diseases, allergies and eczema
(2). Previous studies on the
chemical constituents of the plant EOs have demonstrated that the
EOs of M. cajuputi are mainly composed of 1,8-cineol,
α-pinene, γ-terpinene, p-cymene, α-terpineol, caryophyllene,
α-humulene and α-gurjunene (3-6).
The EOs of M. leucadendra L. have been found to be mainly
composed of p-cymene, α-terpinene, γ-terpinene, 4-terpineol,
caryophyllene, methyleugenol and E-nerolidol (7-10).
The main components of the EOs of M. quinquenervia have been
shown to be monoterpenes (1,8-cineole, α-pinene, α-terpineol,
limonene) and sesquiterpene (viridiflorol, E-nerolidol)
(11,12). Furthermore, pharmacological studies
on the EOs from M. cajuputi, M. quinquenervia and M.
leucadendra have demonstrated their antibacterial,
antimycrobacterial, antivirus, antifungal, antiinsecticidal and
antioxidant effects (4,6,9,10,13-18);
however, other investigations have revealed that the chemical
compositions of EOs of M. cajuputi, M. quinquenervia and
M. leucadendra vary extensively in different geographic and
ecological conditions, even from different regions of the same
country, resulting in several chemotypes (7,9,10,12,18-20).
In addition, studies on the biological activities of EOs from M.
cajuputi, M. quinquenervia and M. leucadendra
species grown in Vietnam are still limited and primarily focus on
the EOs from M. cajuputi (4,6,14,18).
The present study evaluated the antimicrobial
activity and, for the first time, to the best of our knowledge the
enzyme inhibitory effects against α-amylase, α-glucosidase,
acetylcholinesterase (AChE) and xanthine oxidase (XO) of EOs
extracted from the leaves of three Melaleuca species widely
distributed in Vietnam (Fig.
1).
Materials and methods
Plant materials
The leaves of M. cajuputi and M.
quinquenervia were collected in Phong My, Phong Dien, Thua
Thien Hue, Vietnam [(16˚30'56''N, 107˚18'08''E) and (16˚30'41''N,
107˚16'56''E)] in May, 2023, while the leaves of M.
leucadendra were collected in An Minh Bac, U Minh Thuong, Kien
Giang, Vietnam (9˚37'06''N, 105˚05'50''E) in June, 2023. These
plant materials were identified by Dr Le Tuan Anh (Mientrung
Institute for Scientific Research, Vietnam National Museum of
Nature, Thua Thien Hue, Vietnam) and the voucher specimens
(MISR2023-7, MISR2023-8 and MISR2023-11) were kept in the herbarium
of the Mientrung Institute for Scientific Research, Vietnam
National Museum of Nature.
Extraction of EOs
Fresh leaves of M. cajuputi, M. quinquenervia
and M. leucadendra (200 g) were cut into small sections and
then subjected to steam distillation using a glass apparatus to
extract the EOs for 2 h at normal pressure. The EOs were then
collected, dried with 0.5 g Na2SO4 (Merck,
KGaA), stored in the dark and sealed in vials at 4˚C until further
chemical analysis and biological activity testing.
Gas chromatography-mass spectrometry
(GC-MS) analysis
The EOs extracted from the leaves of M cajuputi,
M. quinquenervia and M. leucadendra were analyzed via
GC-MS using a Shimadzu GCMS-QP2010 Plus system (Shimadzu
Corporation). This system was equipped with a flamer ionization
detector (FID) and an Equity-5 capillary column (30 m x 0.25 mm,
0.25 µm film thickness). The EOs were diluted to a concentration of
1% in n-hexane and 1.0 µl of the solutions were injected into the
instrument for analysis. The GC was operated with helium as carrier
gas at a flow rate of 1.5 ml/min. The GC oven temperature was
initiated at 60˚C for 2 min, then increased to 240˚C at a rate of
4˚C/min and maintained for 10 min before further programming to
280˚C at a rate of 5˚C/min. The injector temperature was set at
260˚C. Mass spectrometry was performed at 70 eV in a mass range of
40-500 amu with a sampling rate of 0.5 scan/sec.
Identification of the compounds
The chemical components of the EOs were identified
by comparing their relative retention indices (RI) with those of a
series of reference n-alkanes C7-C40 (Merck, KGaA). Additionally,
the identification relied on computer matching against commercial
libraries (WILEY7 Library and NIST11 Library), as well as MS and RI
data of known compounds from the literature (21,22).
The chemical structures of the compounds were drawn using ChemDraw
Ultra 8.0 (CambridgeSoft Corporation).
Determination of biological activities
of EOs: Antimicrobial activity
The antimicrobial activity of the EOs was evaluated
against a panel of five reference microorganisms, including
Staphylococcus aureus (ATCC 25923; S. aureus),
Enterococcus faecalis (ATCC 29212; E. faecalis),
Escherichia coli (ATCC 25922; E. coli),
Pseudomonas aegurinosa (ATCC 27853; P. aegurinosa)
and Candida albicans (ATCC 10231; C. albicans). The
minimum inhibitory concentrations (MICs) of the EOs against these
microorganisms were determined using the broth microdilution method
as previously reported by Dat et al (23). Briefly, the bacterial inoculum (100
µl at a concentration of 1x106 CFU/ml) was introduced
into the wells of 96-well plates containing various concentrations
of the EOs (100 µl) ranging from 1.0 to 2,560 µg/ml. The plate was
incubated at 37˚C for 24 h, followed by the measurement of
absorbance at 630 nm using an ELx800 absorbance microplate reader
(BioTek Instruments, Inc.; Agilent Technologies). The MICs of the
antibacterial EOs were defined at the lowest concentration where no
bacterial growth was observed through absorbance records at 630 nm.
Similarly, for yeast, the yeast inoculum (100 µl at a concentration
of 2-5x105 CFU/ml) was added to wells containing the EOs
(100 µl) at various concentrations ranging from 1.0 to 2,560 µg/ml
in 96-well plates, which were then incubated at 28˚C for 48 h. The
MICs of the anti-yeast EOs were determined at the lowest
concentration where no yeast growth was observed through absorbance
records at 530 nm using an ELx800 absorbance microplate reader. The
antibiotics, ciprofloxacin and fluconazole (MilliporeSigma) ranging
from 0.5 to 8.0 µg/ml, served as the positive controls for bacteria
and yeast, respectively. The experiments were performed in
triplicate.
Determination of biological activities
of EOs: Enzyme inhibition activity. i) Amylase inhibitory
activity
The inhibitory effect of the EOs on α-amylase
(MilliporeSigma) was assessed according to the method previously
described by Nguyen et al (24). In brief, the starch azure solution
supplemented with 0.01 M CaCl2 in 0.05 M Tris-HCl buffer
(pH 6.9) (MilliporeSigma) was boiled for 5 min and pre-incubated at
37˚C for 5 min. The reaction containing 50 µl of the EO, 50 µl of
the substrate solution, and 25 µl of α-amylase solution (2 U/ml)
was incubated in 96-well plates at 37˚C for 10 min. The reaction
was terminated by the addition of 75 µl of 50% acetic acid,
followed by the measurement of absorbance at 650 nm using an ELx800
absorbance microplate reader (BioTek Instruments, Inc.). The
inhibitory activity was calculated as follows: Inhibition (%)=100 x
[1-(As-Abs)/(Ac-Acb)],
where: As is the absorbance of the sample,
Asb is the absorbance of the sample blank, Ac
is the absorbance of the control, and Acb is the
absorbance of the control blank. Acarbose (MilliporeSigma) was used
as a positive control with tested concentrations ranging from 10 to
200 µl/ml. The IC50 value was calculated using GraphPad
Prism v8.0 (Dotmatics). The experiments were performed in
triplicate and data are expressed as the mean ± standard
deviation.
ii) Glucosidase inhibitory activity. The
inhibitory effect of the EOs on α-glucosidase (MilliporeSigma) was
determined according to the method previously described by Nguyen
et al (24). Briefly, the
reaction containing 50 µl of the EO and 100 µl of α-glucosidase
solution (0.5 U/ml) in 0.1 M potassium phosphate buffer (pH 6.8)
(MilliporeSigma) was incubated in 96-well plates at 37˚C for 10
min. The reaction was initiated by the addition of 50 µl of 5 mM
4-Nitrophenyl β-D-glucopyranoside (MilliporeSigma), followed by
incubation at 37˚C for 30 min. The reaction was then terminated by
the addition of 75 µl of 0.2 M Na2CO3
(MilliporeSigma) and absorbance was recorded at 405 nm using an
ELx800 absorbance microplate reader. The inhibitory activity was
calculated as follows: Inhibition (%)=100 x
[1-(As-Abs)/(Ac-Acb)],
where: As is the absorbance of the sample,
Asb is the absorbance of the sample blank, Ac
is the absorbance of the control, and Acb is the
absorbance of the control blank. Acarbose (MilliporeSigma) was used
as a positive control with tested concentrations ranging from 10 to
200 µl/ml. The IC50 value was calculated using GraphPad
Prism v8.0 (Dotmatics). The experiments were performed in
triplicate and data are expressed as the mean ± standard
deviation.
iii) XO inhibitory activity. The inhibitory
effect of the EOs on XO (MilliporeSigma) was determined according
to the method described in the study by Dat et al (23). In summary, the reaction mixture
containing 50 µl of the EO, 35 µl of 70 mM phosphate buffer (pH
7.5) and 30 µl of enzyme solution (0.01 U/ml) was pre-incubated at
25˚C for 15 min, followed by the addition of 60 µl of 150 mM
xanthine (MilliporeSigma). Subsequently, the reaction was incubated
at 25˚C for 30 min, followed by the addition of 25 µl of 1.0 N HCl
(MilliporeSigma). The absorbance of the reaction was then measured
at 290 nm using an ELx800 absorbance microplate reader. The
inhibitory activity was calculated as follows: Inhibition (%)=100 x
[1-(As-Abs)/(Ac-Acb)],
where: As is the absorbance of the sample,
Asb is the absorbance of the sample blank, Ac
is the absorbance of the control and Acb is the
absorbance of the control blank. Allopurinol (MilliporeSigma) was
used as a positive control with tested concentrations ranging from
1.0 to 50 µl/ml. The IC50 was calculated using GraphPad
Prism v8.0. The experiments were performed in triplicate and the
data are expressed as the mean ± standard deviation.
iv) AChE inhibitory activity. The inhibitory
activity of the EOs on AChE (MilliporeSigma) was determined
according to the method previously described by Thai et al
(25). The reaction containing 100
µl of 3 mM 5,5-dithiobis-2-nitrobenzoate (MilliporeSigma), 20 µl of
the EO and 20 µl of AChE (0.2 U/ml; MilliporeSigma) was
pre-incubated at 25˚C for 15 min, and then initiated by the
addition of 20 µl of 15 mM acetylthiocholine iodide
(MilliporeSigma). Following incubation at 25˚C for 20 min, the
reaction was terminated by the addition of 20 µl of 4% SDS
(MilliporeSigma). Subsequently, the absorbance of the reaction was
measured at 415 nm using an ELx800 absorbance microplate reader.
The inhibitory activity was calculated as follows: Inhibition
(%)=100 x
[1-(As-Abs)/(Ac-Acb)],
where: As is the absorbance of the sample,
Asb is the absorbance of the sample blank, Ac
is the absorbance of the control and Acb is the
absorbance of the control blank. Galantamine (MilliporeSigma) was
used as a positive control with tested concentrations ranging from
1.0 to 20 µl/ml. The IC50 value was calculated using
GraphPad Prism v8.0 software (Dotmatics). The experiments were
performed in triplicate and the data are expressed as the mean ±
standard deviation.
Results and Discussion
For each of the three steam distillations of the
fresh leaves of M. cajuputi, M. quinquenervia, and M.
leucadendra (200 g), the average yield of the EOs was 1.40 g
(0.70%, wt/wt), 2.35 g (1.18%, wt/wt) and 0.62 g (0.31%, wt/wt),
respectively. The GC chromatograms of the EOs of M. cajuputi, M.
leucadendra, and M. quinquenervia are presented in the
Fig. 2, Fig. 3 and Fig. 4. The chemical constituents of the
EOs are listed in Table I and the
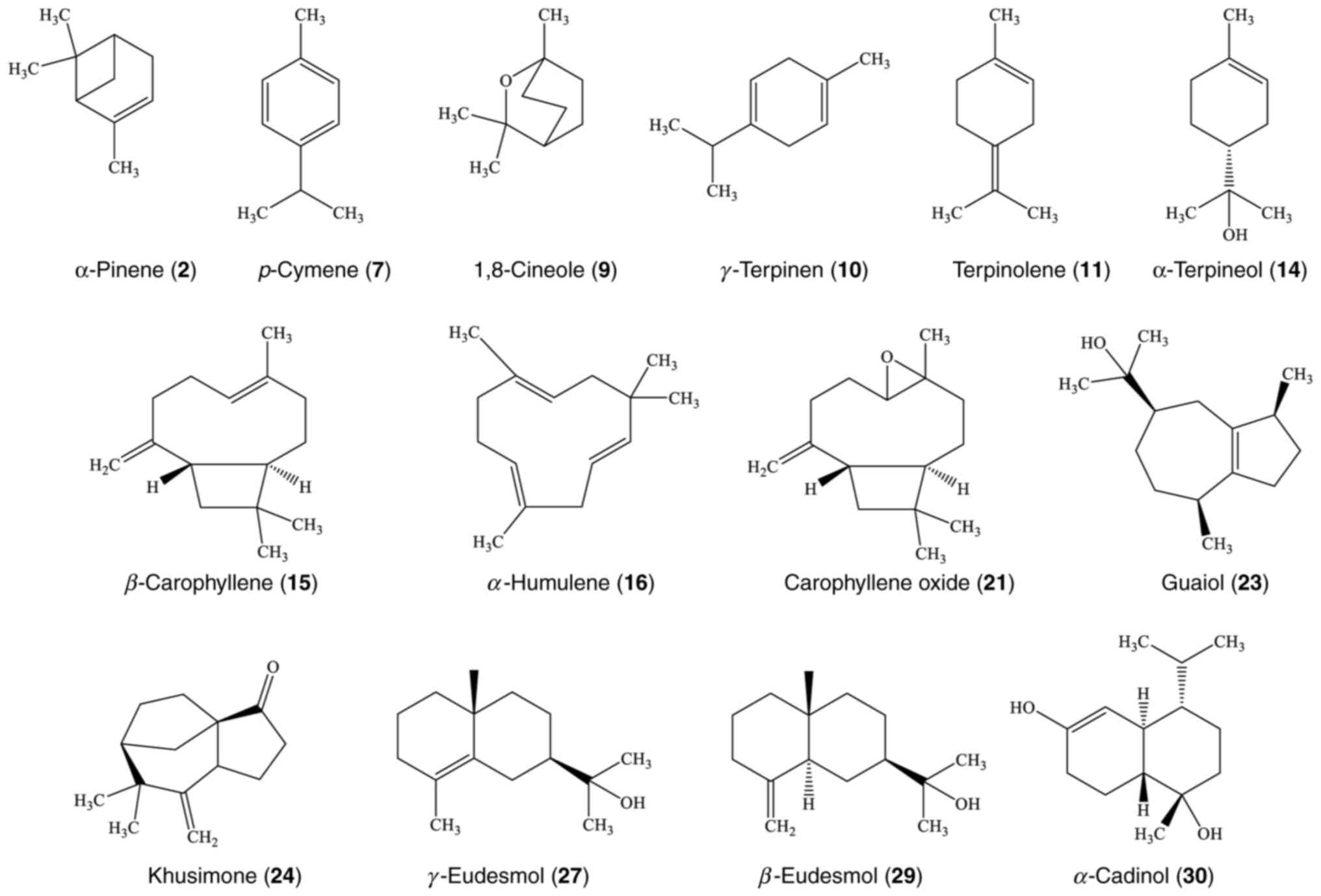
chemical structures of the main compounds are illustrated in
Fig. 5.
 | Table IChemical composition of the leaf EOs
of M. cajuputi, M. quinquenervia and M.
leucadendra. |
Table I
Chemical composition of the leaf EOs
of M. cajuputi, M. quinquenervia and M.
leucadendra.
| Compound no. | Compounds | RIa | RIb | M. cajuputi
(%) | M. quinquenervia
(%) | M. leucadendra
(%) |
|---|
| 1 | α-Thujene | 925 | 924 | - | - | 1.76 |
| 2 | α-Pipene | 932 | 932 | 0.44 | 0.84 | 7.69 |
| 3 | Benzaldehyde | 957 | 960 | 0.32 | - | - |
| 4 | β-Pinene | 975 | 974 | 0.42 | 0.79 | 0.44 |
| 5 | Myrcene | 989 | 990 | 0.58 | 0.58 | - |
| 6 | α-Phellandrene | 1,004 | 1,002 | - | - | 0.73 |
| 7 | p-Cymene | 1,023 | 1,024 | - | 0.62 | 5.38 |
| 8 | (-)-Limonene | 1,027 | 1,029 | 2.16 | 2.66 | 1.43 |
| 9 | 1,8-Cineole | 1,030 | 1,031 | 30.87 | 42.51 | - |
| 10 | γ-Terpinene | 1,057 | 1,059 | 0.35 | 0.79 | 12.94 |
| 11 | Terpinolene | 1,087 | 1,088 | - | 0.83 | 11.77 |
| 12 | Linalool | 1,099 | 1,096 | 3.86 | 3.38 | 0.55 |
| 13 | Terpinen-4-ol | 1,177 | 1,177 | 0.52 | 1.02 | 4.65 |
| 14 | α-Terpineol | 1,190 | 1,188 | 8.31 | 12.00 | 1.25 |
| 15 |
β-Caryophyllene | 1,420 | 1,419 | 1.61 | 0.95 | 14.11 |
| 16 | α-Humulene | 1,454 | 1,454 | 1.12 | 0.75 | 8.54 |
| 17 | β-Selinene | 1,487 | 1,490 | 1.17 | 0.50 | - |
| 18 | δ-Selinene | 1,492 | 1,492 | 0.52 | - | - |
| 19 | α-Selinene | 1,496 | 1,498 | 1.26 | 0.63 | 0.65 |
| 20 | Palustrol | 1,569 | 1,568 | - | - | 1.06 |
| 21 | Caryophyllene
oxide | 1,584 | 1,583 | - | - | 7.22 |
| 22 | Viridiflorol | 1,592 | 1,592 | - | - | 2.48 |
| 23 | Guaiol | 1,598 | 1,600 | 9.71 | 6.86 | - |
| 24 | Khusimone | 1,604 | 1604 | - | - | 9.87 |
| 25 |
Isolongifolanone | 1,610 | 1613 | - | - | 3.42 |
| 26 | 1-epi-cubenol | 1,627 | 1,628 | 1.59 | 1.01 | - |
| 27 | γ-Eudesmol | 1,633 | 1,632 | 6.16 | 3.90 | - |
| 28 | Alloaromadendrene
epoxide | 1,641 | 1,641 | 1.02 | - | - |
| 29 | β-Eudesmol | 1,652 | 1,650 | 9.23 | 6.53 | - |
| 30 | α-Cadinol | 1,655 | 1,654 | 11.29 | 7.81 | - |
| Total
identified | | | 92.50 | 95.95 | 95.93 |
|
-
Non-terpenoids | | | 0.32 | 0.62 | 5.38 |
|
-
Monoterpene hydrocarbons | | | 3.94 | 6.48 | 36.76 |
|
- Oxygenated
monoterpenes | | | 43.56 | 58.92 | 6.45 |
|
-
Sesquiterpene hydrocarbons | | | 5.68 | 2.82 | 23.30 |
|
- Oxygenated
sesquiterpenes | | | 39.00 | 26.11 | 24.04 |
| Total
unidentified | | | | 7.50 | 4.05 | 4.07 |
The GC-MS analysis of the leaf EO of M.
cajuputi and the comparisons of relative RIs of reference
n-alkanes and the spectral databases of known compounds revealed
the presence of 21 compounds, accounting for 92.50% of the total
amount of the EO. Of these, monoterpene hydrocarbons accounted for
3.94%, oxygenated monoterpenes for 43.56%, sesquiterpene
hydrocarbons for 5.68%, oxygenated sesquiterpenes for 39.00% and
non-terpenoids for 0.32%. These results indicated that oxygenated
monoterpenes and sesquiterpenes were the main components of the EO
of M. cajuputi. Among these, 1,8-cineole and α-terpineol
were the main compounds in the group of oxygenated monoterpenes,
accounting for 30.87 and 8.31%, respectively. Moreover, guaiol
(9.71%), γ-eudesmol (6.16%), β-eudesmol (9.23%) and α-cadinol
(11.29%) were the main compounds in the oxygenated sesquiterpene
component of the EO of M. cajuputi. Previous studies have
indicated that 1,8-cineole is one of the main compounds in the leaf
EO of M. cajuputi, with widely varying concentrations
ranging of 27.78-59.90% (3-6,14).
In addition, the presence of 20 compounds was
identified in the EO of M. quinquenervia. Among these,
oxygenated monoterpenes (58.92%) accounted for the highest
proportion, whereas other compound groups, including oxygenated
sesquiterpenes, monoterpene hydrocarbons, sesquiterpene
hydrocarbons and non-terpenoids accounted for 26.11, 6.48, 2.82 and
0.62%, respectively. The compounds 1,8-cineole (42.51%),
α-terpineol (12.00%), guaiol (6.68%), β-eudesmol (6.53%) and
α-cadinol (7.81%) were the main constituents of the EO extracted
from M. quinquenervia species grown in Thua Thien Hue
province, Vietnam. However, the main compounds in the EO of M.
quinquenervia vary widely among different geographical regions.
For example, a report on the chemical composition of the EO of
M. quinquenervia in Australia and Papua New Guinea regions
indicated that EOs extracted from M. quinquenervia species
growing from Sydney, North along the East coast of Australia to
Selection Flat, New South Wales and Maryborough, Queensland,
typically contain linalool (14.0-30.0%) and E-nerolidol
(74.0-95.0%) as major components. Moreover, oils from M.
quinquenervia species in areas ranging from Sydney to Papua New
Guinea and New Caledonia often contain main constituents, such as
α-terpineol (0.5-14.0%), β-caryophyllene (0.5-28.0%), viridiflorol
(13.0-66.0%) and 1,8-cineole (10.0-75.0%) (12). The EO extracted from M.
quinquenervia species harvested in Taiwan contains main
chemical components, including α-terpineol (13.73%), viridiflorol
(14.55%), α-pinene (15.93%) and 1,8-cineole (21.60%) (11). On the other hand, the EO of M.
quinquenervia collected in Costa Rica exhibits different
compositions, including α-terpineol (6.5%), α-pinene (17.9%),
viridiflorol (21.7%) and 1,8-cineole (31.5%) (26).
The results of the chemical composition analysis of
the leaf EO of M. leucadendra identified 19 compounds,
accounting for 95.93% of the EO. Compounds, such as α-pinene
(7.69%), p-cymene (5.38%), γ-terpinene (12.94%), terpinolene
(11.77%), β-caryophyllene (14.11%), α-humulene (8.54%),
caryophyllene oxide (7.22%) and khusimone (9.87%) were the main
compounds found in the EO of M. leucadendra. Previous
investigations on the chemical composition of the EO have revealed
marked differences among M. leucadendra. In particular, the
EO extracted from M. leucadendra species harvested in
Fujian, China, has been reported to be rich in compounds, such as
α-pinene (4.96%), α-terpinene (7.82%), p-cymene (5.74%),
γ-terpinene (18.4%), α-terpineol (4.92%) and 4-terpineol (36.85%)
(8). Furthermore, compounds such
as limonene (4.8%), 1,8-cineole (61.0%), α-terpineol (15.6%) and
viridiflorol (7.9%) dominate in the composition of the EO extracted
from M. leucadendra species harvested in Havana, Cuba
(9). Moreover, the EO extracted
from M. leucadendra leaves in India has been shown to
contain (E)-nerolidol (90.85%) as the absolute predominant
compound among the 28 identified compounds present in the oil
(7). Additionally, the EO of M.
leucadendra in Senegal has been shown to contain methyleugenol
(98.4-99.5%) as the main component (10). Furthermore, a report on the
composition of the EO of M. leucadendra from Danang,
Vietnam, indicated that α-humulene (4.4%), β-selinene
(3.7%), α-selinene (3.7%), guaiol (10.9%) and α-eudesmol (17.6%)
are the major compounds in this oil (18). The findings of the present study,
as well as those of previous reports (7-10,18),
indicate that the chemical composition of the leaf EO of M.
leucadendra varies significantly due to geographical
differences.
Although numerous studies have indicated that the EO
of Melaleuca species includes several chemotypes, the
environmental factors responsible for essential oil chemotype
distribution of these species remain unclear. Therefore, further
studies on environmental factors influencing the EO chemotype of
Melaleuca species are required. Several investigations of EO
chemotypes from other plants have revealed that the environment
factors responsible for EO chemotype distribution include soil
properties and nutrients (pH, Ca2+, K+,
organic matter, aridity and texture), bioclimatic regions
(temperature, precipitation, altitude and seasonal variation),
cultivating conditions, maturation of harvested plants, plant
storage, plant preparation and methods of extraction (27-31).
Biological activities of essential
oils. Antimicrobial activity
The antimicrobial activity of the EOs presented in
Table II demonstrated that the
EOs of the Melaleuca species examined in the present study
exhibited antimicrobial activity against all tested microorganisms
(S. aureus, E. faecalis, E. coli, P. aegurinosa, C.
albicans) with MICs in the range of 640-2560 µg/ml. The leaf EO
of M. cajuputi exhibited the highest antimicrobial activity
against E. coli and P. aegurinosa with MICs of 640
µg/ml, whereas the leaf EOs of M. quinquenervia and M.
leucadendra exhibited the highest antimicrobial activity
againts S. aureus with MIC of 640 µg/ml. The difference in
the antimicrobial activity of the EOs derived from the
Melaleuca species may be attributed to the varying chemical
composition and content of bioactive compounds present in the
EOs.
 | Table IIAntimicrobial activity of the EOs
(MIC, µg/ml). |
Table II
Antimicrobial activity of the EOs
(MIC, µg/ml).
| EOs | S.
aureus | E.
faecalis | E. coli | P.
aegurinosa | C.
albicans |
|---|
| M.
cajuputi | 1,280 | 1,280 | 640 | 640 | 2,560 |
| M.
quinquenervia | 640 | 2,560 | 1,280 | 1,280 | 2,560 |
| M.
leucadendra | 640 | 1,280 | 1,280 | 2,560 | 1,280 |
| Ciprofloxacin | 2 | 4 | 2 | 2 | - |
| Fluconazole | - | - | - | - | 4 |
Previous investigations have revealed that the EOs
of Melaleuca species exhibit antimicrobial activity against
a broad spectrum of pathogenic microbes. The EO of M.
cajuputi has been shown to inhibit the growth of a range of
Gram-positive bacteria, including Bacillus cereus, Bacillus
subtilis, Corynebacterium diphtheriae, Corynebacterium
minutissimum, Enterococcus faecium, Listeria monocytogenes,
Micrococcus luteus, S. aureus, Staphylococcus capitis,
Staphylococcus epidermidis, E. faecalis and Klebsiella
spp. at concentrations ranging of 0.4-0.6% (32,33),
Gram-negative bacteria such as Alcaligenes faecalis,
Enterobacter cloacae, E. coli and Proteus vulgaris, as
well as the fungi such as C. albicans, Gardnerella. vaginalis,
Candida glabrata, Aspergillus niger, Penicillium
notatum at concentrations ranging of 0.4-0.6% (15,34,35).
The EO of M. quinquenervia has been reported to have
effective antimicrobial activity against bacteria, including E.
coli, S. aureus, P. aeruginosa, Staphylococcus epidermidis,
Propionibacterium acnes, Streptococcus peroris, Klebsiella
pneumonia, Acinetobacter baumannii and Proteus vulgaris
with MICs of 0.5-16 mg/ml and fungi C. albicans, Candida
tropicalis, Aspergillus niger with MICs of 0.2-4 mg/ml
(17). The EO from M.
leucadendra leaf has been shown to exhibit antibacterial
activity against S. aureus and E. coli, Salmonella
thiphymurium, Proteus mirabilis, Klebsiella
pneumonie, E. coli, Enterobacter aerogenes,
Providencia rettgeri, Shigella fexnerii, P.
aeruginosa, E. faecalis, Staphylococcus
saprophyticus and S. aureus with MICs of 7.8-62.5 mg/ml
(10,36).
In vitro and in silico investigations
have demonstrated that terpenes and terpenoids are the main active
compounds in antimicrobial EOs (17,37).
Furthermore, other biomolecules, such as phenols, alcohols and
aldehydes found in the EOs, induce antimicrobial activity with
varying specificity and effectiveness. These variations are often
attributed to the functional groups within the EO and the hydrogen
bonding dynamics during their interactions (38). Terpenes are recognized for their
antimicrobial properties, mainly attributed to their ability to
disrupt cell membranes, inhibit cell growth, and interfere with
protein and DNA synthesis (39).
Specific terpenes, such as carvone, carvacrol, eugenol, thymol and
geraniol have demonstrated antibacterial effects (40), whereas compounds such as menthol,
azadirachtin, ascaridol, toosendanin, methyl eugenol and volkensin
have exhibited anti-fungal effects (41,42).
These compounds have exhibited antimicrobial effects by
compromising cellular membrane integrity (43). Monoterpenoids also exhibit
antibacterial effects by disrupting microbial multiplication and
development, as well as by interfering with their metabolic and
physiological functions (44).
Monoterpene terpineol isomers, such as terpinen-4-ol, α-terpineol
and δ-terpineol have demonstrated effective inhibition against
Gram-negative bacteria, particularly Shigella flexneri, by
inducing permeability changes in bacterial membranes, leading to
the release of nucleic acids and proteins, alongside a decrease in
membrane potential (45). Eugenol
exhibits potent bactericidal activity against Salmonella
enterica serovar Typhimurium and S. aureus (46).
Previous investigations have also demonstrated the
mechanisms of action of the main antimicrobial compounds in the EOs
of Melaleuca. For instance, limonene and 1,8-cineole enhance
the permeability of the bacterial membrane, whereas 1,8-cineole and
viridiforol inhibit enzyme peptidoglycan glycosyltransferase
(36). Limonene affects cell
membrane permeability of in Gram-positive bacteria by attacking
cell integrity and cell wall structure (47). Other compounds, such as lupene,
guaiol and 1,8-cineole exhibit antifungal effects by inhibiting the
target enzymes cellobiohydrolase, laccase and lignin peroxidase
(48).
Enzyme inhibition activity. The enzyme
inhibitory activities of the EOs presented in Table III revealed that the EOs of
Melaleuca species exerted inhibitory effects on the enzymes
α-amylase, α-glucosidase, AChE and XO. The leaf EO of M.
cajuputi exhibited inhibitory activities against the enzymes
α-amylase, α-glucosidase, AChE and XO with IC50 values
of 862.6±65.74, 1212±73.49, 765.7±26.14 and 331.9±20.64 µg/ml,
respectively; the leaf EO of M. quinquenervia exhibited
enzyme inhibitory effects with IC50 of 786.3±58.42,
1453±93.79, 815.5±20.72, and 380.1±17.85 µg/ml, respectively; and
the leaf EO of M. leucadendra exhibited enzyme inhibitory
effects IC50 of 714.3±38.09, 1066±45.01, 535.5±19.84 and
433.8±18.02 µg/ml, respectively. The variations in the composition
and content of bioactive compounds presented in the EOs of
Melaleuca species may contribute to the differences in their
enzyme inhibitory properties. Therefore, further research is needed
to identify key compounds in the EOs that inhibit the enzymes.
 | Table IIIα-Amylase, α-glucosidase,
acetylcholinesterase, and xanthine oxidase inhibitory activities of
the EOs (IC50, µg/ml). |
Table III
α-Amylase, α-glucosidase,
acetylcholinesterase, and xanthine oxidase inhibitory activities of
the EOs (IC50, µg/ml).
| | Inhibitory
activity |
|---|
| EOs | α-Amylase | α-Glucosidase |
Acetylcholinesterase | Xanthine
oxidase |
|---|
| M.
cajuputi | 862.6±65.74 | 1212±73.49 | 765.7±26.14 | 331.9±20.64 |
| M.
quinquenervia | 786.3±58.42 | 1453±93.79 | 815.5±20.72 | 380.1±17.85 |
| M.
leucadendra | 714.3±38.09 | 1066±45.01 | 535.5±19.84 | 433.8±18.02 |
| Acarbose | 60.84±2.23 | 111.5±2.96 | - | - |
| Galantamine | - | - | 5.95±0.14 | - |
| Allopurinol | - | - | - | 7.44±0.37 |
Although the antimicrobial activity of the EOs of
Melaleuca species has been reported in recent
investigations, only a limited number studies on the enzyme
inhibitory activity of Melaleuca species against α-amylase,
α-glucosidase, AChE, XO have been identified to date. As regards
the AChE and BChE enzymes, the EOs of M. cajuputi leaf and
M. citrina leaf exhibited AChE inhibitory effects with
21.18±0.54 and 71.77±2.11% inhibition, respectively at a
concentration of 100 µg/ml (49).
The methanol extract from M. cajuputi leaf also exhibited
potent AChE inhibitory effects with IC50 of 282 µg/ml
(50). The EO from M.
alternifolia leaf showed strong AChE and BChE inhibitory
effects with IC50 of 153.7±1.25 and 85.6±0.7 µg/ml,
respectively (51). In the case of
the XO enzyme, the methanol and methanol-water extracts from M.
leucadendra have been reported to have a XO inhibitory activity
with IC50 values of 76.7 and 78.9 µg/ml, respectively
(52). Involving α-amylase and
α-glucosidase enzymes, the EOs from M. alternifolia leaf and
M. viridiflora leaf demonstrated α-amylase inhibitory
activity with 14 and 28% inhibition at a concentration of 0.67
mg/ml (53). To the best of our
knowledge, the findings of the present study provide additional
insight into the enzyme inhibitory properties of the leaf EO from
Melaleuca species that have not been reported in previous
research. However, a limitation of the present study is that the
potential activity of compounds in the EOs was not investigated.
Therefore, further studies are required to determine the most
active compounds in the EO, such as molecular docking, in
silico methods for drug design and discovery, from which the
most potential.
The present study determined the chemical
composition of the leaf EOs of M. cajuputi, M.
quinquenervia and M. leucadendra with the dominance of
oxygenated monoterpenes and sesquiterpenes. The present study also
highlighted the chemical composition variation of EOs of
Melaleuca species over geographical regions, in agreement
with previous observations (7-12,14,26).
The subsequent bioassays revealed that the leaf EOs of M.
cajuputi, M. quinquenervia and M. leucadendra
exhibited antimicrobial, as well as enzyme inhibitory activities
against α-amylase, α-glucosidase, AChE and XO. The findings
presented herein provide additional insight into the enzyme
inhibitory properties of the leaf EO from Melaleuca species
not reported in previous research.
Acknowledgements
Not applicable.
Funding
Funding: The present study was funded by the Vietnam Academy of
Science and Technology (grant no. CSCL.01/23-24).
Availability of data and materials
The datasets used and/or analyzed during the
current study are available from the corresponding author on
reasonable request.
Authors' contributions
THDT and CVCL were involved in the
conceptualization and methodology of the study, as well as in the
formal analysis, writing of the original draft, and in the writing,
review and editing of the manuscript. PHT and TTTV were involved in
the methodology of the study, and in data investigation and formal
analysis. TDTP, VMN and TNMN were involved in the formal analysis.
PHT and TTTV confirm the authenticity of all the raw data. All
authors have read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ho PH: An Illustrated Flora of Vietnam.
Vol. II; 2nd Edition. Tre Publishing House, Ho Chi Minh City, pp67,
2003 (In Vietnamese).
|
|
2
|
Tap N, Trai NV, Chieu N, et al: Checklist
of medicinal plants in Vietnam, National Institute of Medical
Materials. Science and Technics Publishing House. 1:1–1191.
2016.
|
|
3
|
Sutrisno S, Retnosari R and Poerwandar
Asmaningrum H: Profile of the Indonesian essential oil from
Melaleuca cajuputi. Adv Eng Res. 171:14–19. 2018.
|
|
4
|
Bua A, Molicotti P, Donadu MG, Usai D, Le
LS Tran TTT, Ngo VQT, Marchetti M, Usai M, Cappuccinelli P and
Zanetti S: ‘In vitro’ activity of Melaleuca cajuputi against
mycobacterial species. Nat Prod Res. 34:1494–1497. 2020.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Motl O, Hodačová J and Ubik K: Composition
of Vietnamese cajuput essential oil. Flavour Fragr J. 5:39–42.
1990.
|
|
6
|
Toan TQ, Thao LAI, Chien NQ, Van NTH,
Phuong ÐL, Huong TT, Quan PM, Inh CT, Minh PTH, Bich HT, et al:
Determination of chemical composition and antimicrobial activity of
Melaleuca cajuputi essential oil from Quang Tri province,
Vietnam. Asian J Chem. 32:2203–2207. 2020.
|
|
7
|
Padalia RC, Verma RS, Chauhan A and
Chanotiya CS: The essential oil composition of Melaleuca
leucadendra L. grown in India: A novel source of (E)-nerolidol.
Ind Crops Prod. 69:224–227. 2015.
|
|
8
|
Zhang J, Wu H, Jiang D, Yang Y, Tang W and
Xu K: The antifungal activity of essential oil from Melaleuca
leucadendra (L.) L. grown in China and its synergistic effects
with conventional antibiotics against Candida. Nat Prod Res.
33:2545–2548. 2019.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Monzote L, Scherbakov AM, Scull R, Satyal
P, Cos P, Shchekotikhin AE, Gille L and Setzer WN: Essential oil
from Melaleuca leucadendra: Antimicrobial,
antikinetoplastid, antiproliferative and cytotoxic assessment.
Molecules. 25(5514)2020.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Diallo A, Tine Y, Sène M, Diagne M, Diop
A, Ngom S, Ndoye I, Boye CSB, Sy GY, Costa J, et al: The essential
oil of Melaleuca leucadendra L. (Myrtaceae) from Fatick
(Senegal) as a source of methyleugenol. Composition, antibacterial
and anti-inflammatory activities. J Essent Oil Res. 34:322–328.
2022.
|
|
11
|
Chao WW, Su CC, Peng HY and Chou ST:
Melaleuca quinquenervia essential oil inhibits
α-melanocyte-stimulating hormone-induced melanin production and
oxidative stress in B16 melanoma cells. Phytomedicine. 34:191–201.
2017.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Ireland BF, Hibbert DB, Goldsack RJ, Doran
JC and Brophy JJ: Chemical variation in the leaf essential oil of
Melaleuca quinquenervia (Cav.) S.T. Blake. Biochem Syst
Ecol. 30:457–470. 2002.
|
|
13
|
Noor AAM, Yusuf SM, Wahab WNAWA, Adam MFIC
and Sul'ain MD: Evaluation on composition, antioxidant and toxicity
of Melaleuca cajuputi leaves. Adv Tradit Med. 21:693–699.
2021.
|
|
14
|
My TTA, Loan HTP, Hai NTT, Hieu LT, Hoa
TT, Thuy BTP, Quang DT, Triet NT, Anh TTV, Dieu NTX, et al:
Evaluation of the inhibitory activities of COVID-19 of Melaleuca
cajuputi oil using docking simulation. ChemistrySelect.
5:6312–6320. 2020.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dahiya P: Evaluation of in vitro
antimicrobial potential and phytochemical analysis of spruce,
cajeput and jamrosa essential oil against clinical isolates. Int J
Green Pharm. 10:2016.
|
|
16
|
Bakar AABU, Ahmad H, Sulaiman S, Omar B
and Ali RMAT: Evaluation of in vitro bioactivity of Melaleuca
cajuputi powell essential oil against Aedes aegypti (L.)
and Aedes albopictus (Skuse). Sains Malays. 48:1919–1926.
2019.
|
|
17
|
Aumeeruddy-Elalfi Z, Gurib-Fakim A and
Mahomoodally MF: Chemical composition, antimicrobial and antibiotic
potentiating activity of essential oils from 10 tropical medicinal
plants from Mauritius. J Herb Med. 6:88–95. 2016.
|
|
18
|
An NTG, Huong LT, Satyal P, Tai TA, Dai
DN, Hung NH, Ngoc NTB and Setzer WN: Mosquito larvicidal activity,
antimicrobial activity, and chemical compositions of essential oils
from four species of Myrtaceae from central Vietnam. Plants
(Basel). 9(544)2020.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Van HT, Phan UTX and Tran GB: Chemical
diversity of the Melaleuca cajuputi leaf oils from six
locations in southern Vietnam. Agric Conspec Sci. 84:391–397.
2019.
|
|
20
|
Kim JH, Liu KH, Yoon Y, Sornnuwat Y,
Kitirattrakarn T and Anantachoke C: Essential leaf oils from
Melaleuca cajuputi. Acta Hortic. 680:65–72. 2005.
|
|
21
|
Joulain D and Koenig WA: The atlas of
spectral data of sesquiterpene hydrocarbons. E.B. Verlag, Hamburg,
1989.
|
|
22
|
Adams RP: Identification of essential oil
components by gas chromatography/mass spectrometry, 4.1th edition.
Allured Publishing, Carol Stream, IL, USA, pp804, 2017.
|
|
23
|
Dat TTH, Cuc NTK, Cuong PV, Smidt H and
Sipkema D: Diversity and antimicrobial activity of vietnamese
sponge-associated bacteria. Mar Drugs. 19(353)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Nguyen TK, Thuy Thi Tran L, Ho Viet D,
Thai PH, Ha TP, Ty PV, Duc LP, Ton That Huu D and Cuong LCV:
Xanthine oxidase, α-glucosidase and α-amylase inhibitory activities
of the essential oil from Piper lolot: In vitro and in silico
studies. Heliyon. 9(e19148)2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Thai NM, Dat TTH, Hai NTT, Bui TQ, Phu NV,
Quy PT, Triet NT, Pham DT, De Tran V and Nhung NTA: Identification
of potential inhibitors against Alzheimer-related proteins in
Cordyceps militaris ethanol extract: Experimental evidence and
computational analyses. 3 Biotech. 13(292)2023.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Chaverri C and Cicció J: Chemical
composition of essential oils of the tree Melaleuca
quinquenervia (Myrtaceae) cultivated in Costa Rica. UNED Res J.
13(3327)2021.
|
|
27
|
Barra A: Factors affecting chemical
variability of essential oils: A review of recent developments. Nat
Prod Commun. 4:1147–1154. 2009.PubMed/NCBI
|
|
28
|
Moghaddam M and Mehdizadeh L: Chapter
13-Chemistry of essential oils and factors influencing their
constituents. In: Soft Chemistry and Food Fermentation. Grumezescu
AM and Holban AM (eds). Academic Press, New York, pp379-419,
2017.
|
|
29
|
Yavari A, Nazeri V, Sefidkon F and Hassani
ME: Influence of some environmental factors on the essential oil
variability of Thymus migricus. Nat Prod Commun. 5:943–948.
2010.PubMed/NCBI
|
|
30
|
Rajčević N, Dodoš T, Novaković J,
Kuzmanović N, Janaćković P and Marin P: Are environmental factors
responsible for essential oil chemotype distribution of Balkan
Juniperus communis var. saxatilis populations? Plant Biosyst.
157:102–111. 2023.
|
|
31
|
Boira H and Blanquer A: Environmental
factors affecting chemical variability of essential oils in
Thymus piperella L. Biochem Syst Ecol. 26:811–822. 1998.
|
|
32
|
Ashrafi B, Rashidipour M, Marzban A,
Soroush S, Azadpour M, Delfani S and Ramak P: Mentha
piperita essential oils loaded in a chitosan nanogel with
inhibitory effect on biofilm formation against S. mutans on
the dental surface. Carbohydr Polym. 212:142–149. 2019.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Hamoud R, Sporer F, Reichling J and Wink
M: Antimicrobial activity of a traditionally used complex essential
oil distillate (Olbas(®) Tropfen) in comparison to its
individual essential oil ingredients. Phytomedicine. 19:969–976.
2012.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Chao SC, Young DG and Oberg CJ: Screening
for inhibitory activity of essential oils on selected bacteria,
fungi and viruses. J Essent Oil Res. 12:639–649. 2000.
|
|
35
|
Christoph F, Stahl-Biskup E and Kaulfers
PM: Death kinetics of Staphylococcus aureus exposed to
commercial tea tree oils s.L. J Essent Oil Res. 13:98–102.
2001.
|
|
36
|
Bautista-Silva JP, Seibert JB, Amparo TR,
Rodrigues IV, Teixeira LFM, Souza GHB and Dos Santos ODH:
Melaleuca leucadendra essential oil promotes loss of cell
membrane and wall integrity and inhibits bacterial growth: An in
silico and in vitro approach. Curr Microbiol. 77:2181–2191.
2020.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Masyita A, Mustika Sari R, Dwi Astuti A,
Yasir B, Rahma Rumata N, Emran TB, Nainu F and Simal-Gandara J:
Terpenes and terpenoids as main bioactive compounds of essential
oils, their roles in human health and potential application as
natural food preservatives. Food Chem X. 13(100217)2022.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Skaltsa HD, Demetzos C, Lazari D and
Sokovic M: Essential oil analysis and antimicrobial activity of
eight Stachys species from Greece. Phytochemistry.
64:743–752. 2003.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Álvarez-Martínez FJ, Barrajón-Catalán E,
Herranz-López M and Micol V: Antibacterial plant compounds,
extracts and essential oils: An updated review on their effects and
putative mechanisms of action. Phytomedicine.
90(153626)2021.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Gallucci MN, Oliva M, Casero C, Dambolena
J, Luna A, Zygadlo J and Demo M: Antimicrobial combined action of
terpenes against the food-borne microorganisms Escherichia coli,
Staphylococcus aureus and Bacillus cereus. Flavour Fragr
J. 24:348–354. 2009.
|
|
41
|
Pandey AK, Sonker N and Singh P: Efficacy
of some essential oils against Aspergillus flavus with
special reference to lippia alba oil an inhibitor of fungal
proliferation and aflatoxin B1 production in green gram seeds
during storage. J Food Sci. 81:M928–M934. 2016.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Pandey AK, Kumar P, Singh P, Tripathi NN
and Bajpai VK: Essential oils: Sources of antimicrobials and food
preservatives. Front Microbiol. 7(2161)2017.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Guimarães AC, Meireles LM, Lemos MF,
Guimarães MCC, Endringer DC, Fronza M and Scherer R: Antibacterial
activity of terpenes and terpenoids present in essential oils.
Molecules. 24(2471)2019.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Burt S: Essential oils: Their
antibacterial properties and potential applications in foods-a
review. Int J Food Microbiol. 94:223–253. 2004.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Huang J, Yang L, Zou Y, Luo S, Wang X,
Liang Y, Du Y, Feng R and Wei Q: Antibacterial activity and
mechanism of three isomeric terpineols of Cinnamomum
longepaniculatum leaf oil. Folia Microbiol (Praha). 66:59–67.
2021.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Devi KP, Nisha SA, Sakthivel R and Pandian
SK: Eugenol (an essential oil of clove) acts as an antibacterial
agent against Salmonella typhi by disrupting the cellular
membrane. J Ethnopharmacol. 130:107–115. 2010.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Han Y, Sun Z and Chen W: Antimicrobial
susceptibility and antibacterial mechanism of limonene against
Listeria monocytogenes. Molecules. 25(33)2019.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Patramurti C, Amin R, Nastiti CMRR and
Hariono M: A review on the potency of Melaleuca leucadendron
leaves solid waste in wood preservation and its in silico
prediction upon biological activities. Int J For Res.
2020(8885259)2020.
|
|
49
|
Petrachaianan T, Chaiyasirisuwan S,
Athikomkulchai S and Sareedenchai V: Screening of
acetylcholinesterase inhibitory activity in essential oil from
Myrtaceae. Thai J Pharm Sci. 43:63–68. 2019.
|
|
50
|
Isah M, Zengin G, Wan Abdul Wahab WNA,
Abdullah H, Sul'ain MD, Uba AI, Ishak WRW and Jamil S: Antioxidant,
enzyme inhibition, toxicity, and molecular docking analysis of
Melaleuca cajuputi leaf extract and fractions. Nat Resour
Hum Health. 4:89–97. 2024.
|
|
51
|
Zafar F, Shahid M, Fatima H, Riaz M, Anjum
F, Mushtaq Z, Zia S, Jahangir MM and Aslam MA: Antibiofilm and
quorum sensing inhibition (QSI) potential of Lagerstroemia
speciosa leaves extract. Dose Response.
20(15593258221132080)2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Nguyen MT, Awale S, Tezuka Y, Tran QL,
Watanabe H and Kadota S: Xanthine oxidase inhibitory activity of
Vietnamese medicinal plants. Biol Pharm Bull. 27:1414–1421.
2004.PubMed/NCBI View Article : Google Scholar
|
|
53
|
Capetti F, Cagliero C, Marengo A, Bicchi
C, Rubiolo P and Sgorbini B: Bio-guided fractionation driven by in
vitro α-amylase inhibition assays of essential oils bearing
specialized metabolites with potential hypoglycemic activity.
Plants (Basel). 9(1242)2020.PubMed/NCBI View Article : Google Scholar
|