1. Introduction
The biological process of wound healing is intricate
and entails a well-coordinated series of events, such as
hemostasis, inflammation, proliferation and remodeling. The
disruption of any of these stages may attenuate or compromise the
process (1). Hence, uninterrupted
wound healing is always desirable to ensure speedy recovery without
complications. Oral wounds, particularly those linked to
periodontal disease, face distinct challenges during healing due to
the increased risk of infection attributed to the moist and warm
environment of the oral cavity combined with its continuous
exposure to bacteria. Moreover, dental plaque and calculus further
impede the healing process (1).
Periodontal disease is a chronic inflammatory
condition characterized by the breakdown of the tissues supporting
the teeth (2). It is also
recognized as one of the non-communicable diseases with established
links to systemic conditions, such as diabetes and cardiovascular
diseases. Scaling and root planing (SRP) and surgical procedures
such as flap surgery are the cornerstones to addressing the
underlying cause and for the effective treatment of periodontal
disease (3). A meticulous removal
of plaque and calculus and smoothening of the root surface can
create an environment conducive to healing post-treatment and
reduce the risk of disease recurrence. Although the healing process
following these procedures is favorable, the individual responses
may vary and, in some cases, optimal wound healing may not be
achieved (4).
Therefore, additional measures to promote wound
healing are essential for enhancing recovery and reducing patient
discomfort. Several strategies, such as maintaining proper oral
hygiene, utilizing antiseptic rinses, such as chlorhexidine and
applying regenerative materials such as enamel matrix derivatives
or collagen membranes, may be employed to promote periodontal wound
healing. Advanced techniques include platelet-rich plasma (PRP),
growth factors and low-level laser therapy (LLLT) (1). Customized treatment plans according
to the specific needs of the patient can significantly enhance
outcomes and patient comfort.
Notably, LLLT is a non-invasive technique that uses
low-intensity light to stimulate cellular processes at a molecular
level, promoting tissue repair and regeneration (5). Its ability to accelerate healing,
reduce inflammation and improve patient comfort renders it a
valuable tool in periodontal therapy. Several clinical studies have
proven the advantages of LLLT, particularly when used as an adjunct
to conventional periodontal treatment, demonstrating its
significant improvements in periodontal healing outcomes: Reduced
probing depths, improved clinical attachment levels, more rapid
epithelialization and decreased postoperative discomfort (6,7). In
addition, LLLT also has applications in other areas of dentistry,
such as in the management of temporomandibular joint disorders, the
reduction of dentinal hypersensitivity, relief from oral mucositis
and the enhancement of orthodontic tooth movement (8). The underlying mechanisms, therapeutic
parameters and clinical applications of LLLT in periodontal therapy
are discussed in the following sections.
2. History and evolution
The therapeutic use of light has roots in the
ancient medicine of Egyptians and Indians, who recognized and
utilized the healing properties of sunlight therapy to promote
health and overall well-being. However, it gained recognition and
appreciation only in the late 19th century (9). Theodore Maiman's development of light
amplification by stimulated emission of radiation (LASER) in 1960
marked a significant technological breakthrough that was grounded
in Albert Einstein's theoretical work from 1917, a significant
milestone. This innovation reignited interest in the therapeutic
applications of light energy, further advancing the field (10). Endre Mester, a Hungarian physician
and scientist, discovered that low-dose laser therapy could promote
hair growth and improve wound healing in mice [Mester et al
(11)]. He coined the term
photostimulation to describe this effect and later demonstrated its
effectiveness in treating skin ulcers in humans (12).
Although cold laser therapy and LLLT have emerged to
describe low-dose light treatments, these terms are misleading as
no actual cooling occurs, and labels such as ‘low’ and ‘level’ are
vague and imprecise. Additionally, evidence supports the
effectiveness of non-laser devices, rendering ‘laser’ an inaccurate
term. In order to address these issues, the North American
Association for Light Therapy and the World Association for Laser
Therapy agreed in 2014 to adopt the term photobiomodulation (PBM)
therapy (13).
3. PBM in periodontology
The integration of laser therapy in periodontology
dates back to the 1980s when Pick et al employed
CO2 laser for the gingivectomy of hyperplastic gingiva
(14). PBM was first introduced in
periodontal therapy in the early 2000s, yielding promising results
that marked the beginning of its widespread adoption and continuous
advancements in the field (15).
Lasers used in periodontal therapy are classified into two
categories: High-power lasers (HPLs) and low-level lasers (LLLs).
HPLs are commonly employed in periodontal treatments, including
soft tissue and bone surgeries, sulcular debridement of periodontal
pockets, root decontamination and as a part of SRP techniques.
These include Nd:YAG (1,064 nm), Er:YAG (2,940 nm), Er,Cr:YSGG
(2,780 nm) and high-power semiconductor diode laser (808-904 nm),
commonly employed for non-surgical periodontal therapy, whereas
CO2, Nd:YAG, diode laser, and Er:YAG are employed on the
root surface (16).
By contrast, LLLs are commonly used for their PBM
effects (17). PBM is a
therapeutic approach that employs non-ionizing light sources, such
as lasers and light-emitting diodes, to trigger biological
processes at the cellular level. PBM involves low-level light
therapy, typically in the red or near-infrared wavelength
(600-1,000 nm), referred to as the optical window of PBM that
triggers photochemical reactions without generating significant
heat (16). The most commonly
employed lasers are Ruby (694 nm), Argon (488 and 514 nm),
Helium-Neon (632 nm), Krypton (521, 530, 568 and 647 nm), and
low-level diode lasers in the form of Ga-Al-As (780-890 nm) or
In-Ga-AlP (630-700 nm) and Ga-As (904 nm) (18).
4. Mechanisms of action
The therapeutic effects of PBM are deeply rooted in
its ability to modulate the inflammatory response of the body
following tissue injury. In the event of acute injury, the body
initiates a complex inflammatory response to address tissue damage.
This response involves the release of mediators, such as
prostaglandins and bradykinins, leading to symptoms such as pain,
swelling and impaired function. PBM therapy provides a non-invasive
approach which can be used to alleviate inflammation and its
associated symptoms. The underlying mechanism of action is
elaborated below, explaining the ability of PBM to modulate the
inflammatory response and promote healing.
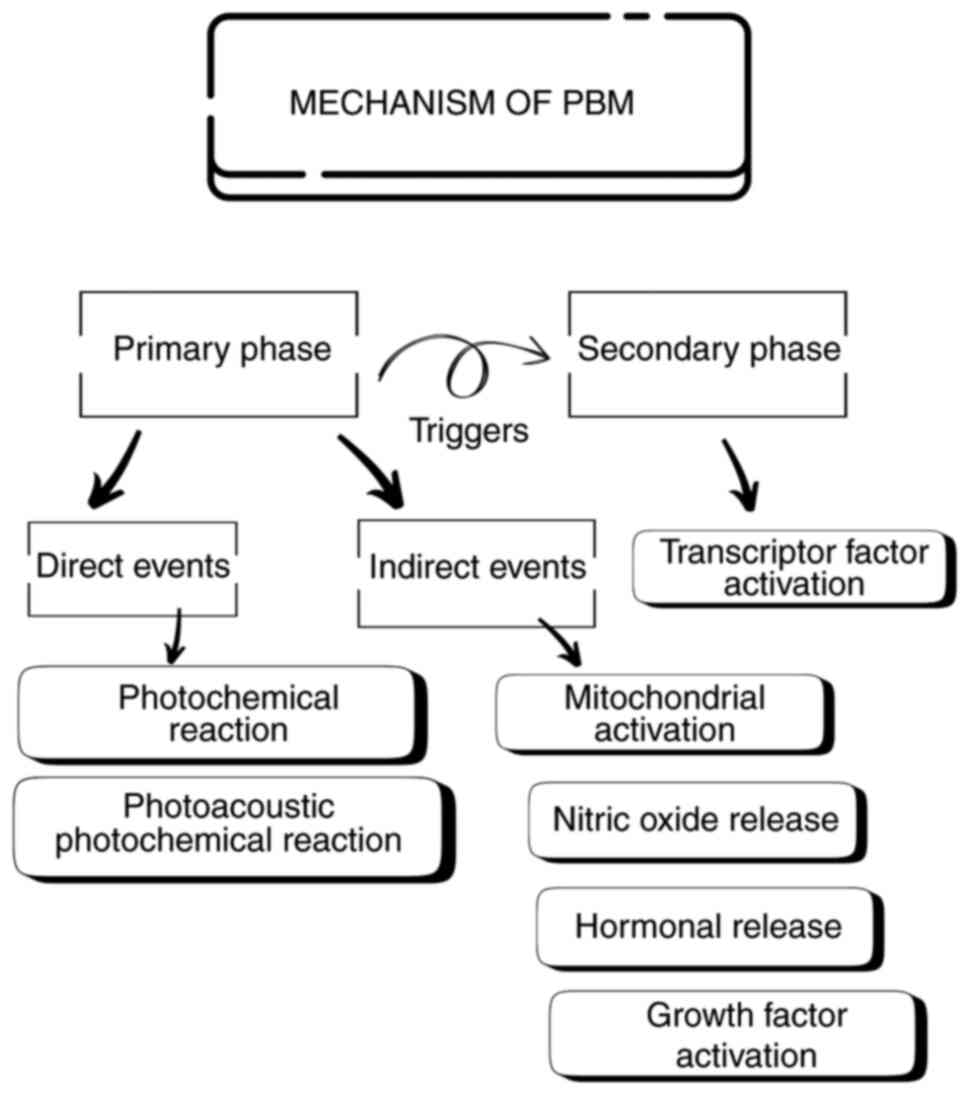
Mechanistically, the effects of PBM can be broadly
categorized as primary and secondary phases. The primary phase
comprises direct and indirect events. Direct events include
photochemical reactions and photoacoustic-photochemical effects.
Photochemical reactions occur when light is absorbed by
chromophores within cells, which in turn triggers a series of redox
reactions that lead to the generation of reactive oxygen species
(ROS). Although ROS are associated with oxidative stress, they can
function as signaling molecules at optimal levels, triggering
beneficial cellular responses. On the other hand, light absorption
in the photoacoustic-photochemical effects can induce physical
changes in tissues, such as a slight increase in temperature and
mechanical stress. These physical effects can influence cellular
processes and promote healing. Indirect events begin with
mitochondrial activation via light absorption by cytochrome
c oxidase, a vital enzyme in the electron transport chain.
This feedback loop boosts the mitochondrial function, resulting in
the increased production of ATP, which in turn stimulates the
synthesis of DNA, RNA, protein, and enzymes that support and
accelerate tissue repair and regeneration (19). Other key events include the release
of nitric oxide (NO), a potent vasodilator and signaling molecule,
which can be stimulated by PBM. NO improves blood supply, reduces
inflammation and promotes tissue healing. Another main event is
kinase activation, wherein the generated ROS activate Src kinase,
an enzyme involved in numerous cellular processes, promoting cell
survival, proliferation and migration, ultimately contributing to
tissue repair and regeneration (19,20).
Additionally, PBM can influence hormone release, which plays a
vital role in stimulating tissue growth and repair. Another
critical mechanism involves the activation of growth factors, such
as transforming growth factor-beta (TGF-β), a multifunctional
cytokine involved in various cellular processes, including wound
healing. PBM therapy generates ROS that activate latent TGF-β, in
turn triggering tissue repair by stimulating migration,
proliferation and matrix synthesis. Hence, PBM-mediated TGF-β
activation provides a promising therapeutic approach for wound
healing applications (19,21).
These primary phase events trigger a cascade of
secondary responses, including the activation of transcription
factors, such as NF-κB, AP-1 and hypoxia-inducible factor-1α, which
regulate gene expression and control cellular response. It can
induce a variety of cellular responses, including proliferation,
migration, differentiation, and matrix synthesis. It can also
accelerate healing by promoting inflammation resolution,
angiogenesis, and tissue remodeling (19) (Fig.
1).
Previous studies have stated that PBM can lead to
increased collagen production, a key component of tissue repair
(22-24).
PBM can initiate an early proliferation phase by modulating the
inflammatory response, further enhancing healing.
5. Applications in periodontal therapy
The therapeutic applications of PBM span a range of
periodontal procedures. One of its key benefits is enhancing
post-surgical healing, where it significantly reduces healing time
and reduce patient discomfort after periodontal surgery. By
reducing inflammation, stimulating cellular processes and promoting
tissue regeneration, PBM can accelerate wound healing and promote a
more rapid recovery. Various periodontal surgical procedures using
PBM have yielded promising results (25). These include gingivectomy, an
invasive procedure that often leads to delayed healing and
increased discomfort. Typically, wound healing occurs through
secondary intention, requiring ~5 weeks for complete surface
healing and 7 weeks for full tissue maturation (26). However, PBM has emerged as a
promising adjunct therapy to accelerate healing and discomfort
after gingivectomy.
Flap surgeries are an integral component of
periodontal therapy that are designed to access and treat deeper
periodontal structures that cannot be adequately managed through
non-surgical means. These procedures include surgically raising a
flap that allows for the debridement of subgingival deposits, root
modification and the correction of osseous defects. Flap surgeries
aim to eliminate periodontal pockets, reduce inflammation and
restore periodontal health (27).
Flap surgeries can also be performed for recession coverage, with
techniques such as coronally advanced flap being widely used to
restore gingival tissue over the exposed root surfaces, enhancing
both function and esthetics. Some studies have consistently
demonstrated that PBM plays a crucial role in improving
post-surgical wound healing and accelerating recovery following
flap surgeries; some of these studies are listed in Table I (28-36).
 | Table IProcedural details of periodontal
therapy in recent years that incorporated PBM. |
Table I
Procedural details of periodontal
therapy in recent years that incorporated PBM.
| First author, year
of publication | Procedure | Study type | Type of laser
used | Methodology | Outcome | (Refs.) |
|---|
| Madi, 2020 | Gingivectomy | Randomized case
control | Diode | 10 out of 20
patients with inflammatory gingival enlargement (test group) were
irradiated with laser at baseline,3,5 days post-surgery, while the
control group did not receive laser treatment. Laser parameters:
Wavelength, 660 nm; duration, 3 min; power, 50 mW; technique, the
laser tip was positioned perpendicular to gingival tissue, 1 cm
away. | Significant
improvement in wound healing scores observed. | (28) |
| Uslu, 2020 | Gingivectomy | Randomized single
blind case-control study. | Diode (GaAiAs) | The study included
36 patients with inflammatory gingival enlargement, with 12
receiving laser irradiation, 12 undergoing ozone application at
baseline, 3rd and 7th days and 12 others were controls. Laser
parameters: Wavelength, 810 nm; duration, 1 min; power, 200 mW;
technique,the Laser tip was positioned perpendicular to gingival
tissue. | Oral health impact
factor was assessed, which was lower in laser group compared to
ozone group. | (29) |
| Misra, 2023 | OFD | Randomized
controlled trial. | Diode | A total of 240
sites from 40 patients with bilateral attachment loss were
included; 120 sites from the test group were irradiated with laser
and the other 120 sites were control sites. Laser parameters:
Wavelength, 890 nm; duration, 30 sec; power, 1.5 W; technique,
sweeping movements of tip in apico-coronal direction were carried
out. | Inflammatory
mediators were evaluated to assess wound healing and, significant
wound healing observed at sites irradiated with laser. | (30) |
| Shakoush, 2023 | OFD | Split mouth
randomized clinical trial. | Diode | 10 patients with
stage III periodontitis were included, where test sites were
irradiated with laser. Laser parameters: Wavelength, 808 nm;
duration, 12 sec; power, 250 mW. | Improved clinical
indices and post-operative pain observed in the PBM group. | (31) |
| Silviya, 2022 | Single-flap
periodontal surgery | Randomized
controlled clinical trial. | Diode | Of the 40 intrabony
defects included, 20 were treated with laser following surgery and
remaining 20 served a control group. Laser parameters: Wavelength,
790-810 nm; duration-4-5 min; power, not reported; technique, probe
tip was placed perpendicular in contact of defect area. | Exhibited a
decrease in pocket probing depth. However, the results were not
significant. | (32) |
| Kolamala, 2022 | OFD | Split mouth
randomized clinical trial. | Diode | 15 participants
with periodontitis were included, with 30 sites in total. 15 sites
received laser assisted surgery (test sites), and another 15 were
control sites. Laser parameters: Wavelength, 980 nm; duration, 30
sec; power, 3 W; technique, inflamed soft tissue pocket wall was
removed. | Significant
reduction in pocket depth, bleeding on probing and improved healing
was observed. | (33) |
| Guimarães,
2024 | CTG procedure | Randomized
controlled clinical trial. | Diode (GaAiAs) | Out of a total of
40 class I and II gingival recession cases, 20 test sites were
treated with the tunneling technique followed by laser irradiation;
the remaining 20 were control sites. Laser parameters: Wavelength,
660 nm; duration, 20 sec; power, 30 mW; technique, laser tip was in
contact with tissue at donor and recipient area. | Significant
difference was notedas regards post-operative discomfort in
patients treated with laser. | (34) |
| Morshedzadeh,
2022 | FGG procedure | Split mouth
randomized controlled clinical trial. | Diode (GaAiAs) | 16 patients were
treated with FGG as a part of split mouth surgery, one site was
irradiated with laser. Laser parameters: Wavelength, 940 nm;
duration, 30 sec; power, 0.21 W; technique, was employed at wound
site in non-contact mode. | Remaining wound
area was assessed to be much smaller in the region irradiated with
laser as compared to non-irradiated site. | (35) |
| Lavu, 2022 | FGG procedure | Randomized
controlled clinical trial. | Diode | A total of 38
patients with isolated gingival recession were treated using the
laterally closed tunnel technique. 19 of control group received
sham laser application, while the other 19 underwent laser
application. Laser parameters: Wavelength, 660 nm; duration, 5 sec;
power, 50 mW; technique, laser was directed perpendicularly with
slight contact to tissue at both donor and recipient area. | PBM led to the more
rapid healing of the wound site and improved patient comfort. | (36) |
Gingival tissue augmentation through free gingival
grafts and connective tissue grafts also improves healing outcomes
with PBM both at donor sites and recipient sites. These techniques
are vital for addressing gingival recession and ensuring stable
soft tissue architecture.
The key studies highlighting the application of PBM
in various periodontal surgical procedures, including grafting, are
summarized in Table I (28-36).
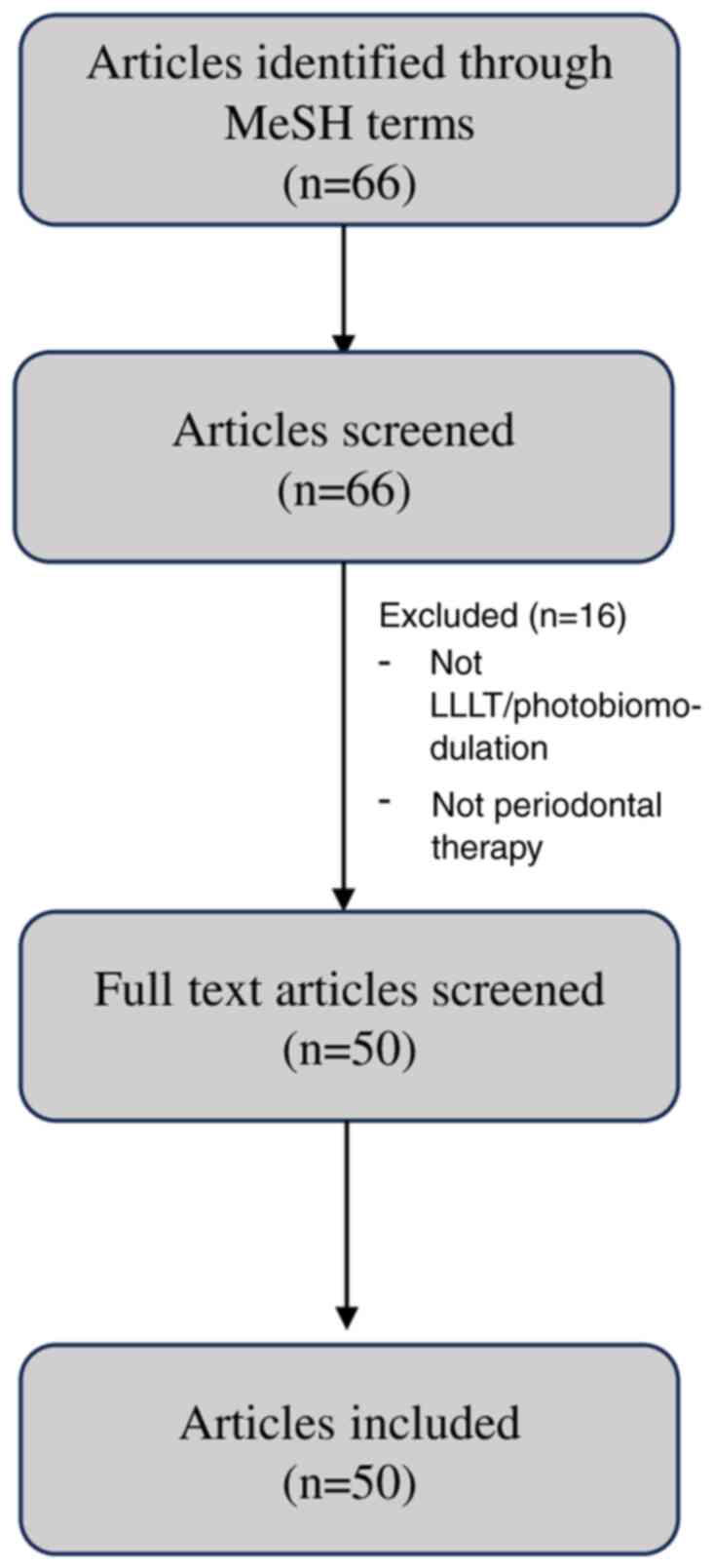
A flow diagram outlining the screening and inclusion process for
the studies in the present review is illustrated in Fig. 2. Since this article is a narrative
review and not a systematic review or meta-analysis, the PRISMA
guidelines were not applied.
Periodontal regeneration refers to the process of
rebuilding or restoring lost or damaged tissue to recover the
original form and function of the affected structures (37). PBM therapy contributes to
periodontal regeneration by influencing cellular and molecular
processes by facilitating tissue repair and bone formation. The
subsequent section describes how PBM contributes to periodontal
regeneration.
Osteoblasts are crucial for bone formation and
repair in periodontal regeneration. Diode lasers have demonstrated
promising effects on osteoblasts, stimulating cell proliferation,
viability and migration, and enhancing mineralization. These cells
also upregulate key osteogenic markers such as alkaline
phosphatase, osteocalcin and bone morphogenic proteins, while also
influencing osteoclast-related markers and signaling pathways.
Nd:YAG lasers can also enhance cell proliferation, mineralization
and the gene expression of osteogenic markers. Er:YAG lasers, under
specific conditions, can increase cell proliferation and
mineralization and modulate gene expression. CO2 lasers
have been shown to enhance bone sialoprotein expression through
specific signaling pathways (38).
Although laser irradiation, specifically with diode lasers, has
shown potential for promoting bone formation, further research is
required to optimize parameters and elucidate the underlying
mechanisms for different types of lasers (38).
Fibroblasts play a crucial role in connective
tissue, migrating to the lesion site from the late inflammatory
phase until the epithelium is formed completely. These cells
support various cellular processes involved in wound healing and
tissue regeneration. They contribute by breaking down blood clots,
secreting growth factors and cytokines, and forming new
extracellular matrix and collagen structures. Furthermore, they
play a pivotal role in promoting wound contraction. Over the years,
the biological and molecular mechanisms underlying these effects
have been actively investigated, with a particular focus on the
impact of lasers on fibroblasts. PBM stimulates fibroblast
proliferation, increasing collagen synthesis, reducing inflammation
and improving blood circulation, which further accelerates the
healing process (39). Different
laser types, such as diode, Nd:YAG, Er:YAG, Er,Cr:YSGG and
CO2 lasers can be employed for PBM. Diode lasers have
been shown to stimulate fibroblast proliferation, increase collagen
synthesis and reduce inflammation. Nd:YAG lasers can modulate
collagen synthesis and reduce inflammation, promoting tissue
repair. Er:YAG and Er,Cr:YSGG is usually used for tissue ablation
and resurfacing. However, PBM with these lasers can stimulate
fibroblast proliferation and collagen synthesis. CO2
lasers can modulate growth factor expression and reduce
inflammation. The exact mechanisms of the underlying effects of PBM
are not yet fully understood, but are considered to involve various
cellular signaling pathways (38).
The periodontal ligament (PDL) which supports and
attaches the tooth to the alveolar bone also responds positively to
PBM therapy, particularly diode and Er:YAG lasers. These have shown
promising effects on PDL cell proliferation, migration and
differentiation, and also enhance their calcification potential. By
targeting specific cellular signaling pathways, lasers can promote
tissue repair and improve periodontal health (33).
Endothelial cells, which form the inner lining of
blood vessels, play a critical role in blood clotting, inflammation
and vascular permeability. They are essential for angiogenesis,
which is crucial for delivering oxygen and nutrients to the wound
site. PBM therapy has been shown to stimulate endothelial cell
proliferation, migration and reduce inflammation. However, the
effects of PBM on endothelial cells can vary depending on factors,
such as laser parameters, cell type and experimental conditions
(38). Similarly, epithelial cells
found on tissue, organs protect deeper tissues and support
homeostasis. They are crucial for wound healing. The effects of PBM
on various epithelial cells are limited. However, it has been
proposed that pulsed diode laser irradiation can significantly
increase the proliferation of gingival epithelial cells by
activating the MAPK/ERK pathway (38).
CO2 and Er:YAG laser irradiation have
been shown to decrease the expression of sclerostin (Sost),
a gene encoding sclerostin, a protein that inhibits bone formation.
By reducing Sost expression, laser irradiation may reduce
the inhibition of bone formation and thus promote it. Diode laser
irradiation can stimulate the differentiation and activation of
osteoclast precursor cells by upregulating RANK expression
(38).
PBM is emerging as an effective tool in non-surgical
periodontal therapy (NSPT), particularly in moderate to deep
periodontal pockets. It promotes periodontal healing by reducing
inflammation, enhancing fibroblast and osteoblast activity, and
improving tissue repair. NSPT has shown additional clinical
benefits in moderate to deep pockets when combined with laser
therapy or laser therapy alone compared to traditional mechanical
debridement. The studies by Crespi et al (40), and Eltas and Orbak (41) have demonstrated the superior
properties of Er:YAG and Nd:YAG lasers, respectively, over
traditional scaling and root planning. Notably, these positive
outcomes were particularly evident in deeper periodontal pockets.
However, the European Federation of Periodontology does not
currently recommend the routine use of PBM as an adjunct to NSPT
due to insufficient evidence supporting its efficacy (42). This is supported by Salvi et
al (43), who found no
significant benefit in probing depth reduction with adjunctive
laser use and highlighted heterogeneity of study designs and
outcomes. While PBM shows promise, its role in NSPT has yet to be
elucidated (43). Future research
is thus required to perform well-designed trials with standardized
protocols. Additionally, exploring PBM in combination with other
adjunctive therapies, such as ozone, probiotics and paraprobiotics
may provide synergistic benefits and enhance periodontal healing
outcomes (44-46).
PBM has also been applied in dental implantology,
wherein implant success hinges on both the health of the soft
tissue surrounding the implant and the secure integration of the
connective tissue to the implant surface (47). Khadra et al (47) conducted a study examining the
impact of laser therapy on enhancing fibroblast attachment to
implant surfaces. Their findings revealed that laser therapy
stimulated fibroblast activity and promoted better attachment to
the implant surface (47). That
study provided the foundation for utilizing PBM to enhance the soft
tissue interface around implants. Experimental research also
indicates that PBM can stimulate osteoblast proliferation and
differentiation, which can improve osseointegration (48). The early use of post-operative PBM
strengthens the connection between bone and implant, while boosting
bone matrix production. Dörtbudak et al (49) investigated the effects of PBM on
osteoblast activity in vitro using bone marrow-derived
mesenchymal stem cells. Their study concluded that laser treatment
enhanced osteoblastic activity, which could aid in improving
implant osseointegration (49).
Additionally, PBM has been shown to accelerate the healing around
the surgical site by the aforementioned mechanism that includes the
production of ROS and growth factors. Saini et al (50) conducted a systematic review to
evaluate the impact of PBM of dental implants. Their findings
suggest that PBM may enhance implant stability and increase density
by facilitating cellular activity, such as osteoblast stimulation
and collagen synthesis (50). PBM
has demonstrated potential in the management of peri-implantitis
and peri-implant mucositis. In their study, Al-Askar et al
(51) employed the use of PBM and
photodynamic therapy (PDT) as an adjunct to mechanical debridement
for the treatment of peri-implantitis. It was concluded that PBM
and PDT had a positive impact in reducing inflammation (51).
Recent studies have demonstrated that combining PBM
with biological adjuncts, such as PRP, platelet-rich fibrin (PRF)
and bone grafts enhances periodontal regeneration (52,53).
Systematic reviews and meta-analyses have found that PBM with
PRP/PRF stimulates tissue regeneration and improves clinical
attachment gains and bone fill compared to grafts alone (52,54).
A previous systematic review reported that PRP as an adjunct led to
greater improvements in clinical attachment level and bone level in
periodontal defects than conventional treatments (55). Additionally, as demonstrated in a
previous systematic review of in vitro studies, PBM promotes
the proliferation and osteogenic differentiation of periodontal
ligament stem cells, supporting its regenerative benefits when
paired with biomaterials (56).
Animal studies combining PBM with melatonin have
reported improved healing and reduced inflammation in periodontitis
models (57).
Microbiome-modulating agents, such as probiotics, when used
adjunctively with non-surgical therapy, help restore microbial
balance and reduce inflammation, with PBM potentially augmenting
these effects (45,58). Ozone therapy used as an adjunct to
PBM and SRP has demonstrated significant improvements in probing
depth and gingival health, with outcomes comparable to those
achieved with chlorhexidine and without added adverse effects
(59). The integration of PBM with
advanced biomaterials and microbiome modulation is gaining
interest. A nano-hydroxyapatite/chitosan (nHAp/CS) bioaerogel has
shown superior osteogenic potential in preclinical models,
indicating promise for periodontal bone regeneration. Bioactive
glasses also support osteogenesis in periodontal defects, though
PBM-specific combinations require further study (60). While these findings are promising,
the majority of available evidence stems from preclinical or small
clinical trials, highlighting the need for larger, well-designed
studies to establish definitive clinical protocols.
The present narrative review is limited by the
absence of a systematic methodology, which may introduce selection
bias. The variability in PBM protocols across studies further
limits generalizability. Additionally, the lack of quantitative
synthesis restricts the strength of conclusions drawn.
6. Conclusion and future perspectives
PBM has emerged as a promising adjunct in
periodontal therapy, demonstrating significant potential in
enhancing wound healing following periodontal procedures. By
modulating inflammatory responses, stimulating cellular processes
and promoting tissue regeneration, PBM accelerates healing while
reducing patient discomfort. The ability of PBM to enhance
fibroblast proliferation, osteoblast activity and periodontal
ligament regeneration highlights its role in improving periodontal
outcomes.
Despite the growing body of evidence supporting the
efficacy of PBM, further research is required to optimize laser
parameters, establish standardized protocols and better understand
the underlying molecular mechanisms. With advancements being made
in laser technology and acquiring a more in-depth understanding of
the biological effects of PBM, its integration into mainstream
periodontal therapy is expected to expand. Ultimately, PBM stands
as a valuable, non-invasive and patient-friendly modality,
reinforcing its role as a promising tool for improved periodontal
wound healing and regeneration.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AS and NS were involved in designing the concept of
the review followed by conducting the literature search and
drafting the initial manuscript. AT and KSC were involved in
revising and editing the manuscript. Data authentication is not
applicable. All the authors reviewed, and have read and approved
the final manuscript.
Ethics approval and consent for
publication
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Cho YD, Kim KH, Lee YM, Ku Y and Seol YJ:
Periodontal wound healing and tissue regeneration: A narrative
review. Pharmaceuticals (Basel). 14(456)2021.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Könönen E, Gursoy M and Gursoy UK:
Periodontitis: A multifaceted disease of tooth-supporting tissues.
J Clin Med. 8(1135)2019.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Slots J: Concise evaluation and
therapeutic guidelines for severe periodontitis: A public health
perspective. Periodontol 2000. 90:262–265. 2022.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Yuan H, Liu Q, Tang T, Qin H, Zhao L, Chen
W and Guo S: Assessment of early wound healing, pain intensity,
quality of life and related influencing factors during periodontal
surgery: A cross-sectional study. BMC Oral Health.
22(596)2022.PubMed/NCBI View Article : Google Scholar
|
|
5
|
Reis CHB, Buchaim DV, Ortiz AC, Fideles
SOM, Dias JA, Miglino MA, Teixeira DDB, Pereira ESBM, da Cunha MR
and Buchaim RL: Application of fibrin associated with
photobiomodulation as a promising strategy to improve regeneration
in tissue engineering: A systematic review. Polymers (Basel).
14(3150)2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Zhao H, Hu J and Zhao L: The effect of
low-level laser therapy as an adjunct to periodontal surgery in the
management of postoperative pain and wound healing: A systematic
review and meta-analysis. Lasers Med Sci. 36:175–187.
2021.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Al Asmari D and Alenezi A: Laser
technology in periodontal treatment: Benefits, risks, and future
directions-a mini review. J Clin Med. 14(1962)2025.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Singh S, Chakraborty A, Saju AR, Singh R,
Sen A, Shrinivas S and Surana P: Comprehensive review on low-level
laser therapy in dentistry. J Pharm Bioallied Sci. 16 (Suppl
4):S3047–S3049. 2024.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Grzybowski A, Sak J and Pawlikowski J: A
brief report on the history of phototherapy. Clin Dermatol.
34:532–537. 2016.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Zhu Q, Xiao S, Hua Z, Yang D, Hu M, Zhu YT
and Zhong H: Near Infrared (NIR) light therapy of eye diseases: A
review. Int J Med Sci. 18:109–119. 2021.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Mester E, Szende B and Gärtner P: The
effect of laser beams on the growth of hair in mice. Radiobiol
Radiother (Berl). 9:621–626. 1968.PubMed/NCBI(In German).
|
|
12
|
Lawrence J and Sorra K: Photobiomodulation
as medicine: Low-level laser therapy (LLLT) for acute tissue injury
or sport performance recovery. J Funct Morphol Kinesiol.
9(181)2024.PubMed/NCBI View Article : Google Scholar
|
|
13
|
WALT/NAALT: Photobiomodulation: Mainstream
Medicine and Beyond. In: Proceedings of the WALT Biennial Congress
and NAALT Annual Conference. WALT/NAALT, Arlington, VA, USA, 2014.
https://waltpbm.org/wp-content/uploads/2021/08/WALT-NAALT-2014-Program.pdf.
|
|
14
|
Pick RM, Pecaro BC and Silberman CJ: The
laser gingivectomy. The use of the CO2 laser for the
removal of phenytoin hyperplasia. J Periodontol. 56:492–496.
1985.PubMed/NCBI View Article : Google Scholar
|
|
15
|
Dalvi S, Benedicenti S and Hanna R:
Effectiveness of photobiomodulation as an adjunct to nonsurgical
periodontal therapy in the management of periodontitis-a systematic
review of in vivo human studies. Photochem Photobiol. 97:223–242.
2021.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Theodoro LH, Marcantonio RAC, Wainwright M
and Garcia VG: LASER in periodontal treatment: Is it an effective
treatment or science fiction? Braz Oral Res. 35 (Suppl
2)(e099)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Alaijah F, Morsi A, Nasher R and Gutknecht
N: Photobiomodulation therapy in the treatment of periodontal
disease: A literature review. Lasers Dent Sci. 3:147–153. 2019.
|
|
18
|
Chhabrani A, Avinash BS, Bharadwaj RS and
Gupta M: Laser light: Illuminating the path to enhanced periodontal
care. Photodiagnosis Photodyn Ther. 46(104036)2024.PubMed/NCBI View Article : Google Scholar
|
|
19
|
Khan I and Arany P: Biophysical approaches
for oral wound healing: Emphasis on photobiomodulation. Adv Wound
Care (New Rochelle). 4:724–737. 2015.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Quirk BJ and Whelan HT: What lies at the
heart of photobiomodulation: Light, cytochrome c oxidase, and
nitric oxide-review of the evidence. Photobiomodul Photomed Laser
Surg. 38:527–530. 2020.PubMed/NCBI View Article : Google Scholar : (Epub ahead of
print).
|
|
21
|
Khan I, Rahman SU, Tang E, Engel K, Hall
B, Kulkarni AB and Arany PR: Accelerated burn wound healing with
photobiomodulation therapy involves activation of endogenous latent
TGF-β1. Sci Rep. 11(13371)2021.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Prabhu V, Rao BSS, Rao ACK, Prasad K and
Mahato KK: Photobiomodulation invigorating collagen deposition,
proliferating cell nuclear antigen and Ki67 expression during
dermal wound repair in mice. Lasers Med Sci. 37:171–180.
2022.PubMed/NCBI View Article : Google Scholar
|
|
23
|
de Farias Marques AC, Albertini R, Serra
AJ, da Silva EAP, de Oliveira VLC, Silva LM, Leal-Junior ECP and de
Carvalho PDTC: Photobiomodulation therapy on collagen type I and
III, vascular endothelial growth factor, and metalloproteinase in
experimentally induced tendinopathy in aged rats. Lasers Med Sci.
31:1915–1923. 2016.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Zhang P, Zhang X and Zhu H:
Photobiomodulation at 660 nm promotes collagen synthesis via
downregulation of HIF-1α expression without photodamage in human
scleral fibroblasts in vitro in a hypoxic environment. Graefes Arch
Clin Exp Ophthalmol. 261:2535–2545. 2023.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Ebrahimi P, Hadilou M, Naserneysari F,
Dolatabadi A, Tarzemany R, Vahed N, Nikniaz L, Fekrazad R and
Gholami L: Effect of photobiomodulation in secondary intention
gingival wound healing-a systematic review and meta-analysis. BMC
Oral Health. 21(258)2021.PubMed/NCBI View Article : Google Scholar
|
|
26
|
Pavlov SB, Babenko NM, Kumetchko MV,
Litvinova OB and Mikhaylusov RN: Experimental study of the effect
of photobiomodulation therapy on the regulation of the healing
process of chronic wounds. Int J Photoenergy.
2021(3947895)2021.
|
|
27
|
Moreno Rodríguez JA and Ortiz Ruiz AJ:
Periodontal granulation tissue preservation in surgical periodontal
disease treatment: A pilot prospective cohort study. J Periodontal
Implant Sci. 52:298–311. 2022.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Madi M and Mahmoud MM: The evaluation of
healing effect of low-level laser treatment following gingivectomy.
Beni-Suef Univ J Basic Appl Sci. 9(25)2020.
|
|
29
|
Uslu MÖ and Akgül S: Evaluation of the
effects of photobiomodulation therapy and ozone applications after
gingivectomy and gingivoplasty on postoperative pain and patients'
oral health-related quality of life. Lasers Med Sci. 35:1637–1647.
2020.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Misra P, Kalsi R, Anand Arora S, Singh KS,
Athar S and Saini A: Effect of low-level laser therapy on early
wound healing and levels of inflammatory mediators in gingival
crevicular fluid following open flap debridement. Cureus.
15(e34755)2023.PubMed/NCBI View Article : Google Scholar
|
|
31
|
Shakoush G, Albonni H and Almahdi W:
Low-level laser therapy has an additional effect with open flap
debridement on the treatment of stage III periodontitis: A
split-mouth randomized clinical trial. Quintessence Int.
54:274–286. 2023.PubMed/NCBI View Article : Google Scholar
|
|
32
|
Silviya S, C M A, Prakash PSG, Bahammam
SA, Bahammam MA, Almarghlani A, Assaggaf M, Kamil MA, Subramanian
S, Balaji TM and Patil S: The efficacy of low-level laser therapy
combined with single flap periodontal surgery in the management of
intrabony periodontal defects: A randomized controlled trial.
Healthcare (Basel). 10(1301)2022.PubMed/NCBI View Article : Google Scholar
|
|
33
|
Kolamala N, Nagarakanti S and Chava VK:
Effect of diode laser as an adjunct to open flap debridement in
treatment of periodontitis-a randomized clinical trial. J Indian
Soc Periodontol. 26:451–457. 2022.PubMed/NCBI View Article : Google Scholar
|
|
34
|
Guimarães CC, Ferreira AJ and Sperandio M:
Effect of photobiomodulation on root covering associated with the
connective tissue grafting technique-randomized controlled clinical
study. Res Sq. 3:rs–5300074. 2024.
|
|
35
|
Morshedzadeh G, Aslroosta H and Vafaei M:
Effect of GaAlAs 940 nm Photobiomodulation on palatal wound healing
after free gingival graft surgery: A split mouth randomized
controlled clinical trial. BMC Oral Health. 22(202)2022.PubMed/NCBI View Article : Google Scholar
|
|
36
|
Lavu V, Gutknecht N, Vasudevan A, S K B,
Hilgers RD and Franzen R: Laterally closed tunnel technique with
and without adjunctive photobiomodulation therapy for the
management of isolated gingival recession-a randomized controlled
assessor-blinded clinical trial. Lasers Med Sci. 37:1625–1634.
2022.PubMed/NCBI View Article : Google Scholar
|
|
37
|
Liang Y, Luan X and Liu X: Recent advances
in periodontal regeneration: A biomaterial perspective. Bioact
Mater. 5:297–308. 2020.PubMed/NCBI View Article : Google Scholar
|
|
38
|
Ohsugi Y, Niimi H, Shimohira T, Hatasa M,
Katagiri S, Aoki A and Iwata T: In vitro cytological responses
against laser photobiomodulation for periodontal regeneration. Int
J Mol Sci. 21(9002)2020.PubMed/NCBI View Article : Google Scholar
|
|
39
|
Sakurai Y, Yamaguchi M and Abiko Y:
Inhibitory effect of low-level laser irradiation on LPS-stimulated
prostaglandin E2 production and cyclooxygenase-2 in human gingival
fibroblasts. Eur J Oral Sci. 108:29–34. 2000.PubMed/NCBI View Article : Google Scholar
|
|
40
|
Crespi R, Capparè P, Toscanelli I,
Gherlone E and Romanos GE: Effects of Er:YAG laser compared to
ultrasonic scaler in periodontal treatment: A 2-year follow-up
split-mouth clinical study. J Periodontol. 78:1195–1200.
2007.PubMed/NCBI View Article : Google Scholar
|
|
41
|
Eltas A and Orbak R: Effect of 1,064-nm
Nd:YAG laser therapy on GCF IL-1β and MMP-8 levels in patients with
chronic periodontitis. Lasers Med Sci. 27:543–550. 2012.PubMed/NCBI View Article : Google Scholar
|
|
42
|
Sanz M, Herrera D, Kebschull M, Chapple I,
Jepsen S, Beglundh T, Sculean A and Tonetti MS: EFP Workshop
Participants and Methodological Consultants. Treatment of stage
I-III periodontitis-The EFP S3 level clinical practice guideline. J
Clin Periodontol. 47 (Suppl 22):S4–S60. 2020.PubMed/NCBI View Article : Google Scholar
|
|
43
|
Salvi GE, Stähli A, Schmidt JC, Ramseier
CA, Sculean A and Walter C: Adjunctive laser or antimicrobial
photodynamic therapy to non-surgical mechanical instrumentation in
patients with untreated periodontitis: A systematic review and
meta-analysis. J Clin Periodontol. 47 (Suppl 22):S176–S198.
2020.PubMed/NCBI View Article : Google Scholar
|
|
44
|
Colombo M, Gallo S, Garofoli A, Poggio C,
Arciola CR and Scribante A: Ozone gel in chronic periodontal
disease: A randomized clinical trial on the anti-inflammatory
effects of ozone application. Biology (Basel).
10(625)2021.PubMed/NCBI View Article : Google Scholar
|
|
45
|
Meng N, Liu Q, Dong Q, Gu J and Yang Y:
Effects of probiotics on preventing caries in preschool children: A
systematic review and meta-analysis. J Clin Pediatr Dent.
47:85–100. 2023.PubMed/NCBI View Article : Google Scholar
|
|
46
|
Butera A, Pascadopoli M, Nardi MG, Ogliari
C, Chiesa A, Preda C, Perego G and Scribante A: Clinical use of
paraprobiotics for pregnant women with periodontitis: Randomized
clinical trial. Dent J (Basel). 12(116)2024.PubMed/NCBI View Article : Google Scholar
|
|
47
|
Khadra M, Kasem N, Lyngstadaas SP, Haanaes
HR and Mustafa K: Laser therapy accelerates initial attachment and
subsequent behaviour of human oral fibroblasts cultured on titanium
implant material. A scanning electron microscope and
histomorphometric analysis. Clin Oral Implants Res. 16:168–175.
2005.PubMed/NCBI View Article : Google Scholar
|
|
48
|
Teo JW, Tan KB, Nicholls JI, Wong KM and
Uy J: Three-dimensional accuracy of plastic transfer impression
copings for three implant systems. Int J Oral Maxillofac Implants.
29:577–584. 2014.PubMed/NCBI View Article : Google Scholar
|
|
49
|
Dörtbudak O, Haas R and Mallath-Pokorny G:
Biostimulation of bone marrow cells with a diode soft laser. Clin
Oral Implants Res. 11:540–545. 2000.PubMed/NCBI View Article : Google Scholar
|
|
50
|
Saini RS, Kanji MA, Okshah A, Alshadidi
AAF, Binduhayyim RIH, Vyas R, Aldosari LIN, Vardanyan A, Mosaddad
SA and Heboyan A: Comparative efficacy of photobiomodulation on
osseointegration in dental implants: A systematic review and
meta-analysis. Photodiagnosis Photodyn Ther.
48(104256)2024.PubMed/NCBI View Article : Google Scholar
|
|
51
|
Al-Askar MH, Abdullatif FA, Alshihri AA,
Ahmed A, Divakar DD, Almoharib H and Alzoman H: Comparison of
photobiomodulation and photodynamic therapy as adjuncts to
mechanical debridement for the treatment of peri-implantitis.
Technol Health Care. 30:389–398. 2022.PubMed/NCBI View Article : Google Scholar
|
|
52
|
Valamvanos K, Valamvanos TF, Toumazou S
and Gartzouni E: The combined use of photobiomodulation therapy and
platelet-rich fibrin for the management of two MRONJ stage II
cases: An alternative approach. Front Dent Med. 3(973738)2022.
|
|
53
|
Vigliar MFR, Marega LF, Duarte MAH,
Alcalde MP, Rosso MPO, Ferreira Junior RS, Barraviera B, Reis CHB,
Buchaim DV and Buchaim RL: Photobiomodulation therapy improves
repair of bone defects filled by inorganic bone matrix and fibrin
heterologous biopolymer. Bioengineering (Basel).
11(78)2024.PubMed/NCBI View Article : Google Scholar
|
|
54
|
Dipalma G, Inchingolo AM, Patano A,
Palumbo I, Guglielmo M, Trilli I, Netti A, Ferrara I, Viapiano F,
Inchingolo AD, et al: Photobiomodulation and growth factors in
dentistry: A systematic review. Photonics. 10(1095)2023.
|
|
55
|
Roselló-Camps À, Monje A, Lin GH, Khoshkam
V, Chávez-Gatty M, Wang HL, Gargallo-Albiol J and Hernandez-Alfaro
F: Platelet-rich plasma for periodontal regeneration in the
treatment of intrabony defects: A meta-analysis on prospective
clinical trials. Oral Surg Oral Med Oral Pathol Oral Radiol.
120:562–574. 2015.PubMed/NCBI View Article : Google Scholar
|
|
56
|
Mylona V, Anagnostaki E, Chiniforush N,
Barikani H, Lynch E and Grootveld M: Photobiomodulation effects on
periodontal ligament stem cells: A systematic review of in vitro
studies. Curr Stem Cell Res Ther. 19:544–558. 2024.PubMed/NCBI View Article : Google Scholar
|
|
57
|
Mohamed Abdelgawad L, Gamal Mahmoud
Ibrahim Salem Y and El Tayeb EAA: Impact of photobiomodulation and
melatonin on periodontal healing of periodontitis in
immunosuppressed rats. J Lasers Med Sci. 15(e39)2024.PubMed/NCBI View Article : Google Scholar
|
|
58
|
Yu S, Zhang Y, Zhu C, Zhou H, Liu J, Sun
J, Li A and Pei D: Adjunctive diode laser therapy and probiotic
Lactobacillus therapy in the treatment of periodontitis and
peri-implant disease. J Vis Exp, 2022.
|
|
59
|
Cetiner DO, Isler SC, Ilikci-Sagkan R,
Sengul J, Kaymaz O and Corekci AU: The adjunctive use of
antimicrobial photodynamic therapy, light-emitting-diode
photobiomodulation and ozone therapy in regenerative treatment of
stage III/IV grade C periodontitis: A randomized controlled
clinical trial. Clin Oral Investig. 28(426)2024.PubMed/NCBI View Article : Google Scholar
|
|
60
|
Souto-Lopes M, Grenho L, Manrique Y, Dias
MM, Lopes JCB, Fernandes MH, Monteiro FJ and Salgado CL: Bone
regeneration driven by a nano-hydroxyapatite/chitosan composite
bioaerogel for periodontal regeneration. Front Bioeng Biotechnol.
12(1355950)2024.PubMed/NCBI View Article : Google Scholar
|