Introduction
Unicellular algae, including cyanobacteria, commonly
known to the general public as microalgae, have been recognized for
their potential in commercial production as functional foods. The
most widely utilized species in this category include
Chlamydomonas, Chlorella, Haematococcus
pluvialis and Arthrospira platensis. These organisms are
ubiquitous photoautotrophs, thriving in both marine and freshwater
environments. They capture solar energy and use CO2 and
mineral salts to synthesize carbohydrates for energy (1,2).
Owing to their broad applicability, microalgae are often termed
‘green biofactories’. Their increasing use in the production of
natural products, nutraceuticals, pharmaceuticals and food
ingredients is due to their palatability and rich content of
proteins, essential amino acids, vitamins and minerals (3).
Microalgae are a valuable resource, which could be
particularly beneficial in developing countries, due to their rapid
growth, energy efficiency and rich nutrient profile. They can
contain protein levels as high as 60-70% (surpassing those in meat
and milk), carbohydrates (up to 30-40%), essential minerals such as
iodine, iron and calcium, vitamins, and 10-20% omega-3, omega-6 and
omega-9 fatty acids (4). Their
versatility has attracted increasing interest from industries
aiming to develop healthier and more sustainable products,
including natural food colorants and eco-friendly biodiesel.
Microalgae are particularly appreciated for their rapid growth,
ease of harvesting, efficient drying into powder form and long
shelf life (5).
Arthrospira platensis, commonly known as
Spirulina platensis (SP), is a cyanobacterium that exists in
two phases: A green phase under optimal growth conditions and a
blue phase when exposed to stress (6). Nutritionally, SP stands out for its
high protein content and abundance of vitamins (complexes B, D, E
and K), minerals (calcium, magnesium, iron, potassium, zinc,
copper, manganese, chromium and selenium), β-carotene, and
polyunsaturated fatty acids from the omega-3 and omega-6 series
(7). Above all, Spirulina is
especially known for its phycocyanin (PC) content, a blue pigment
protein widely recognized for its significant health-promoting
properties and frequently used as a dietary supplement. This
natural pigment has been shown to exhibit strong antioxidant,
anti-inflammatory, hepatoprotective (liver-protecting) and
neuroprotective (nerve-protecting) effects in various in
vitro and in vivo studies (8). Due to its extensive therapeutic
potential, phycocyanin is increasingly incorporated into health and
wellness products aimed at supporting cellular health, reducing
oxidative stress and preventing chronic inflammation (9). Its favorable safety and
biocompatibility also support further research and application in
preventive medicine and nutraceutical development (10). These components are known for their
preventive effects on the cardiovascular system and their potent
antioxidant properties (11). SP
extracts are also used to prevent and manage conditions linked to
metabolic syndrome-related disorders (3), oxidative stress, and diseases such as
atherosclerosis, cardiac hypertrophy, heart failure and
hypertension (12).
Selenium (symbol, Se) is a key trace element present
in some microalgae, including, including SP. It plays a crucial
role in human nutrition as a structural component of antioxidant
enzymes, such as glutathione peroxidase and reductase, which help
mitigate oxidative stress (11).
Oxidative stress is a major biological event that can negatively
affect the health and function of living organisms (13). This imbalance may contribute to
conditions, such type 2 diabetes (often associated with
hyperlipidemia), ischemia, cardiovascular diseases,
neurodegenerative disorders such as Alzheimer's, and cancer; the
free radicals produced in these processes can also cause severe
organ damage (14). Over the
years, research has aimed to identify the most effective selenium
sources and, numerous investigations have indicated that selenium
nanoparticles derived from Spirulina exhibit significant anti-tumor
activity, (15) exhibiting greater
efficacy in inducing cell cycle arrest and apoptosis than larger
selenium particles (16).
The present systematic review aimed to evaluate the
antioxidant and cytotoxic efficacy of selenium-enriched SP (Se-SP)
and selenium-containing phycocyanin (Se-PC), a major bioactive
component derived from Se-SP, by synthesizing evidence from both
in vivo and in vitro studies. The primary objective
was to determine whether selenium enrichment enhances the
biological activity of these compounds compared to their
non-enriched counterparts (Spirulina or phycocyanin alone). By
comparing Se-SP and Se-PC to their respective controls, the present
systematic review sought to clarify their potential health benefits
and inform future applications in nutritional and therapeutic
interventions.
Data and methods
The present systematic review followed the Preferred
Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)
guidelines. Studies were included if they met the following
criteria: Published in the English language; investigated the
antioxidant and/or cytotoxic activities of Se-SP or Se-PC; employed
in vitro and/or in vivo experimental models; included
at least one comparative group treated with non-enriched Spirulina
or phycocyanin, in order to isolate and assess the specific
contribution of the organic selenium component to the observed
biological effects. Studies that were reviews, conference
proceedings, editorials, articles without available full texts and
those published outside the specified time frame were excluded from
the review. A comprehensive literature search was conducted using
the PubMed, Scopus and Web of Science databases, focusing the
search on title and abstract by the use of the following key words:
Selenium-containing AND phycocyanin; selenium-enriched AND
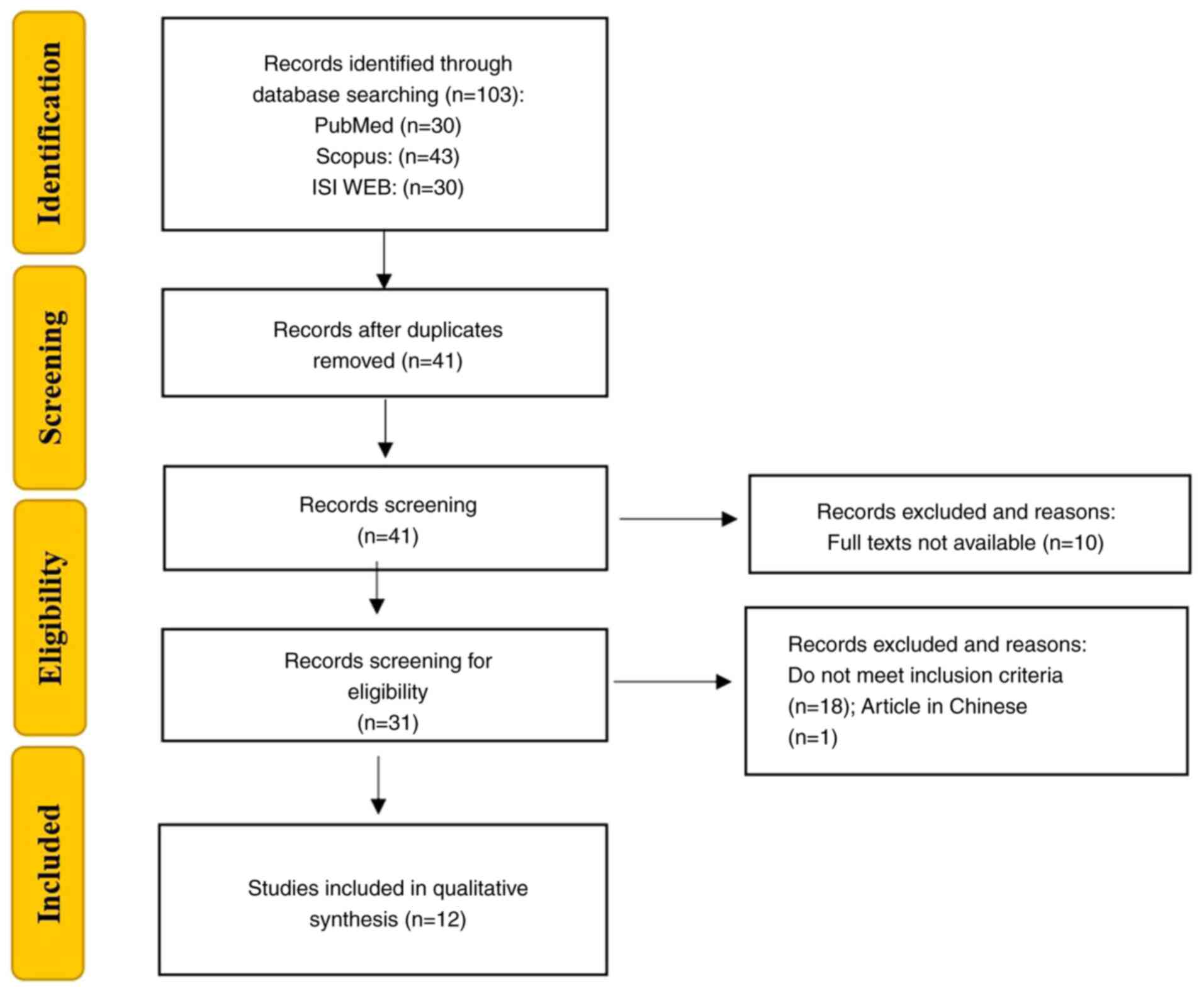
spirulina. The selection process is detailed in a PRISMA flowchart,
including both included and excluded studies with the reasons for
exclusion (Fig. 1). The selected
studies are summarized in Table I,
which outlines the study objectives, the cellular or animal models
used, the experimental conditions, and a concise summary of the
most effective treatment identified in each case.
 | Table ISelected studies from the scientific
literature. |
Table I
Selected studies from the scientific
literature.
| First author, year
of publication | Highlights | In vivo
experimental groups | In vitro
experimental groups | Se-SP and Se-PC vs.
SP and PC | (Refs.) |
|---|
| Yang, 2024 | Aim: Investigate
the therapeutic potential of selenium-containing protein extracted
from Se-enriched Spirulina platensis (Se-SP) in alleviating
osteoporosis by modulating inflammation, osteoblast activity, and
osteoclasto genesis in vitro and in vivo. Animal
Model: Female C57 mice Cell line: MC3T3-E1 | Control group: Mice
that underwent a mock surgery without ovariectomy. OVX group
(Ovariectomy): Mice that underwent ovariectomy without additional
treatment. SP-treated group: OVX mice treated with 10 mg/kg SP via
intraperitoneal injections every other day for 2 months.
Se-SP-treated group: OVX mice treated with 10 mg/kg Se-SP via
intraperitoneal injections every other day for 2 months. | Control group:
Cells were cultured in a differentiation medium without any
treatment. SP-treated groups: Cells were treated with SP at
concentrations of 5 and 10 µg/ml for 14 days in the differentiation
medium. Se-SP-treated groups: Cells were treated with Se-SP at
concentrations of 5 and 10 µg/ml for 14 days in the differentiation
medium | Compared to SP
alone, Se-SP demonstrated superior efficacy in reversing bone loss
and restoring bone microarchitecture in ovariectomized mice. | (25) |
| Castel, 2024 | Aim: Evaluate the
effectiveness of Se-SP in restoring selenium levels and modulating
antioxidant enzyme activities and selenoprotein expression in rats
fed a selenium-deficient diet. Animal model: Old female Wistar
rats | Pre-treatment: 8
weeks with Se deficient diet Control: Se sufficient diet containing
0.3 mg Se/kg of food; Deficient group (D):only water for 4 weeks;
SS: SS at adose of 20 µg Se/kg b.w. day in water for 4 weeks. SP: 3
g/kg b.w. day of SP in water for 4 weeks Se-SP: 3 g/kg b.w. day of
Se-SP (providing 20 µg Se/kg b.w. day) in water for 4 weeks. | NA | Se-SP ensures
better selenium distribution and tissue bioavailability, while SS
more effectively enhances antioxidant enzyme activity and certain
selenoprotein expressions. SP alone shows only marginal antioxidant
benefit without selenium repletion. | (24) |
| Shen, 2023 | Aim: Evaluate the
efficacy of Se-PC in photodynamic therapy (PDT) against lung cancer
and compare its effects to other treatments, including PC-PDT and
PC combined with SS. Animal model: Lung carcinoma-bearing male mice
C57BL/6 Cell line: LLC cell | Control: Mice
injected with normal saline (0.2 ml), every 3 days and for 11 days.
Laser-only: Laser light (630 nm at 100 J/cm²) for 4 h after
receiving 0.2 ml of normal saline, every 3 days and for 11 days.
PC-PDT Mice injected with 0.2 ml of PC (15 mg/ml) and exposed to
laser light (630 nm at 100 J/cm²) for 4 h every 3 days and for 11
days. PC + SS-PDT: Mice injected with a combination of 0.2 ml of PC
(15 mg/ml) and SS at an equivalent Se dose to the Se-PC group,
followed by laser light (630 nm at 100 J/cm²) for 4 h, every 3 days
and for 11 days. Se-PC-PDT: Mice injected with 0.2 ml of Se-PC (15
mg/mll) and exposed to laser light (630 nm at 100 J/cm²) for 4 h,
every 3 days and for 11 days. | Control: Cells with
no treatment. PC-PDT: Cells treated with 150 µg/ml of PC, then
exposed to laser light (26 J/cm²) for 9 min and incubated for 12 h
SS + PC-PDT: Cells treated with a combination of 150 µg/ml PC and
1.14 µg/ml SS (providing an equivalent Se content to the Se-PC
group) and exposed to the same laser parameters (26 J/cm² for 9
min) and incubated for 12 h. Se-PC PDT: Cells treated with 150
µg/ml Se-PC, then exposed to laser light (26 J/cm² for 9 min) and
incubated for 12 h. | Se-PC represents
the most effective and balanced treatment, offering strong
cytotoxicity against tumor cells while preserving antioxidant
defenses in normal tissues and minimizing systemic toxicity. Its
dual action on tumor inhibition and immune activation makes it the
most promising strategy among the three approaches evaluated. | (30) |
| Castel, 2021 | Aim: Evaluate the
effects of Se-SP supplementation on sepsis outcomes in
selenium-deficient rats. Animal model: female Wistar rats | Pre-treatment: 8
weeks with Sedeficient diet Deficient group (D): Continued to
receive Sedeficient food and tap water for 4 weeks. SS: Se
supplementation in the form of SS (20 µg Se/kg b.w. day) in
drinking water for 4 weeks. SP: SP powder (3 g/kg b.w. day) in
drinking water for 4 weeks. Se-SP: Se-SP (3 g/kg b.w. day),
providing 20 µg of Se/kg b.w. day in drinking water for 4 weeks.
Post-treatment: Sepsis induced in a subgroup of each group using
the cecal ligature and puncture (CLP) method. Another subgroup
underwent a sham surgery (laparotomy only) to serve as a
control. | NA | SS remains the most
effective option in correcting selenium deficiency and mitigating
sepsis-induced oxidative and metabolic disturbances. Se-SP shows
promise in upregu-lating antioxidant genes, but fails to deliver
functional protection in the acute setting, likely due to lower
bioavailability of organic Se forms (e.g., SeMet) or matrix
interactions. SP alone, while theoretically antioxidant, offered no
protective effect and may even exacerbate early stress responses in
sepsis | (23) |
| Song, 2021 | Aim:
Neuroprotective effects of selenium-containing protein derived from
Se-SP under conditions simulating ischemic injury induced by
oxygen-glucose deprivation (OGD). Cell line: Primary hippocampal
neurons isolated from Sprague-Dawley (SD) rats | NA | Control: Cells with
no treatments. OGD: Cells subjected to oxygen-glucose deprivation
(OGD) for 6 h without any additional treatment. SP: Cells treated
with 5 or 10 µg/ml of SP during OGD exposure for 6 h. Se-SP: Cells
treated with 5 or 10 µg/ml of Se-SP during OGD exposure for 6 h.
CsA + OGD: Cells pre-treated with 5 µM CsA (cyclosporine A) for 30
min, followed by OGD exposure for 6 h.CsA + SP + OGD: Cells
pre-treated with 5 µM CsA for 30 min, followed by 5 or 10 µg/ml SP
treatment during OGD exposure for 6 h. CsA + Se-SP + OGD: Cells
pre-treated with 5 µM CsA for 30 min, followed by 5 or 10 µg/ml.
Se-SP treatment during OGD exposure for 6 h. | Se-SP showed
superior antioxidant and cytoprotective effects compared to SP
alone, effectively reducing ROS, preserving mitochondrial function,
and preventing neuronal apoptosis under OGD conditions | (22) |
| Lin, 2020 | Aim: Investigate
the protective effects and underlying mechanisms of Se-SP against
high glucose-induced calcification in mouse aortic vascular smooth
muscle cells (MOVAS). Cell line: Mouse aortic vascular smooth
muscle cells (MOVAS). | NA | Control: Cells
cultured with 5 mM glucose. High glucose: Cells exposed to 10-50 mM
glucose for 48 h to investigate dose-dependent effects on
proliferation; In calcification assays, cells were exposed to 25 mM
glucose for 14 days.SP: Cells treated with 5 or 10 µg/ml SP along
with 25 mM glucose for 14 days. Se-SP: Cells treated with 5 or 10
µg/ml Se-SP along with 25 mM glucose for 14 days. GSH
pre-treatment: (specific for ROS evaluation): Cells pre-treated
with 5 mM glutathione (GSH) for 2 h before exposure to 25 mM
glucose. MAPK inhibitor: Cells treated with 10 µM SP600125 (JNK
inhibitor) along with 25 mM glucose for 14 days | Se-SP effectively
counteracts high glucose-induced oxidative stress and calcification
in vascular cells, showing superior protective and antioxidant
properties compared to SP alone. | (21) |
| Sun, 2019 | Aim: Investigate
the protective effects of Se-SP against cisplatin-induced apoptosis
Cell line: MC3T3-E1 mouse preosteoblast cells. | | Control: Cells with
no treatments. Cisplatin-only: Cells exposed to 20, 40, or 80 µg/ml
cisplatin for 24 h. Se-SP: Cells pre-treated with 5, 10, or 20
µg/ml of Se-SP for 24 h before being co-treated with 40 or 80 µg/ml
of cisplatin for another 24 h. SP: Cells were with 80 µg/ml SP or
Se-SP alone for 48 h in cytotoxicity assays. Co-treatment: Cells
treated with 10 µg/ml Se-SP and 80 µg/ml cisplatin. | Se-SP showed strong
antioxidant and cytoprotective effects by preventing mitochondrial
dysfunction and ROS-mediated apoptosis in cisplatin-injured
osteoblasts. Unlike SP, Se-SP preserved cell viability and reduced
oxidative stress through mitochondrial stabilization and DNA
protection, highlighting its potential in chemoprevention of bone
damage. | (20) |
| Fu, 2018 | Aim: Investigate
the protective effects and underlying mechanisms of Se-SP against
chronic alcohol-induced liver injury. Animal model: KM mice Cell
line: HL7702 human liver cell line. | Control: Mice
treated with physiological saline. Model: Mice treated with 30%
absolute alcohol (10 ml/kg bw) via gavage for the last 15 days of
the experiment. SP: Mice treated with 200 mg/kg bw SP via gavage
daily for 42 days, with alcohol gavage during the last 15 days.
Low-dose Se-SP: Mice treated with 100 mg/kg bw of Se-SP via gavage
daily for 42 days, with alcohol gavage during the last 15 days.
Middle-dose Se-SP: Mice treated with 200 mg/kg bw of Se-SP via
gavage daily for 42 days, with alcohol gavage during the last 15
days. High-dose Se-SP: Mice treated with 400 mg/kg bw of Se-SP via
gavage daily for 42 days, with alcohol gavage during the last 15
days. | Control: Cells
cultured in normal conditions. Alcohol-only: Cells exposed to 200
mM ethanol for 24 h. SP: Cells co-treated with 200 mM ethanol and
200 µg/ml of SP for 24 h. Se-SP: Cells co-treated with 200 mM
ethanol and varying concentrations of Se-SP (50, 100, and 200
µg/ml) for 24 h. Positive control: Cells treated with an
antioxidant or apoptosis inhibitor. | Se-SP exhibits
strong protective effects against chronic alcohol-induced liver
injury both in vitro and in vivo. It outperforms native SP
by reducing apoptosis, autophagy, and pyroptosis, while enhancing
antioxidant enzyme activity. Its optimal effectiveness is observed
at 200 mg/kg, offering a promising dietary supplement for oxidative
liver damage | (19) |
| Liu, 2018 | Aim: Evaluate the
therapeutic potential of Se-PC in enhancing the efficacy of
photodynamic therapy (PDT) for cancer treatment. Animal model:
BALB/c mice. Cell line: HepG2 and the HL7702. | Control: Mice
received no treatment. Laser-only: Mice exposed to laser
irradiation 632.8 nm, (150 J/cm²) without any photosensitizer. PC:
Mice injected with 0.2 ml PC 10 mg/ml for 10 days without laser
irradiation Se-PC: Mice injected with 0.2 ml Se-PC 10 mg/ml for 10
days without laser irradiation. PC-PDT: Mice injected with 0.2 ml
of PC 10 mg/ml for 10 days and exposed to laser irradiation (150
J/cm²). Se-PC-PDT: Mice injected with 0.2 ml of Se-PC 10 mg/ml for
10 days and exposed to laser irradiation (150 J/cm²). | Control: Cells
cultured without treatments. PC: Cells treated with 50-400 µg/ml of
PC for 4 h. Se-PC: Cells treated with 50-200 µg/ml of Se-PC for 4
h.Laser: Cells exposed to laser irradiation (632.8 nm, 45 mW/cm²,
26 J/cm²) for 9 min without photosensitizer. PC-PDT: Cells treated
with PC (50-400 µg/ml) for 4 h, followed by laser irradiation.
Se-PC-PDT: Cells treated with Se-PC (50-200 µg/ml) for 4 h followed
by laser irradiation. | Se-PC combined with
PDT induces potent anticancer activity through
mitochondria-mediated apoptosis, partial inhibition of autophagy,
and enhanced antioxidant enzyme modulation. It demonstrates
stronger efficacy and higher tumour selectivity than PC alone,
offering a promising and safer strategy for liver tumour
therapy. | (29) |
| Zhang, 2011 | Aim: Evaluate Se-PC
protective effects against oxidative stress induced by AAPH
(2,2'-azobis (2-amidinopropane) dihydrochloride). Specifically, the
study explored Se-APC's ability to inhibit reactive oxygen species
(ROS) generation, prevent lipid peroxidation, and protect
antioxidant defense systems in human erythrocytes. Cell line: human
erythrocytes | NA | Control group: No
oxidative agent, no treatment (baseline erythrocyte/plasma
conditions) AAPH group (oxidative stress control): Erythrocytes or
plasma treated with 100 mM AAPH only (oxidative stress inducer)
Se-APC pretreated groups: Erythrocytes or plasma pretreated with
Se-APC at concentrations ranging from 0.3 to 1.5 µM, then exposed
to 100 mM AAPH; Also used in plasma oxidation assay at 0.06 to 0.3
µM APC pretreated groups: Erythrocytes or plasma pretreated with
APC at matching concentrations to Se-APC (e.g., 0.3 µM), followed
by AAPH exposure Positive control for antioxidant comparison:
Included Trolox as a known antioxidant for reference in the ABTS
assay | Se-APC shows
clearly superior antioxidant and cytoprotective properties compared
to native APC in vitro. It effectively scavenges radicals,
protects erythrocytes from oxidative hemolysis, and preserves
cellular antioxidant defenses, making it a promising candidate for
functional food or therapeutic applications targeting oxidative
stress-related damage. | (28) |
| Chen, 2008 | Aim: Evaluate the
antioxidant and antiproliferative activities of Se-PC. Cell line:
A375, MCF-7, erythrocytes, Hs68 | NA | A375 and MCF-7:
Control: Cells no treated. Se-PC: Cells treated with Se-PC at
varying concentrations (5, 10, 20 and 40 µM) and incubated for 72 h
to evaluate antiproliferative effects and for 24 h for apoptosis
assays.PCs: Cells treated with phycocyanin (PC) at the same
concentrations as Se-PC for comparison and incubated for 72 h to
evaluate antiproliferative effects and for 24 h for apoptosis
assays. Erythrocytes from Sprague-Dawley rats: Control: Cells
exposed to phosphate-buffered saline (PBS). AAPH: Cells treated
with 200 mM AAPH for 3 h. AAPH + Se-PC: Cells pre-treated with
Se-PC for 30 min at concentrations of 0.1 to 1.25 µM, followed by
200 mM AAPH Se-PC: Cells treated with Se-PC alone for 30 min to
confirm its non-toxic effects. | Selenium
incorporation into phycocyanin enhances both antioxidant and
antiproliferative activities in vitro. Se-PC outperformed
native PC across all assays, confirming its potential as a
selenium-based chemopreventive agent. | (27) |
| Riss, 2007 | Aim: Evaluate the
cardiovascular and oxidative stress protective effects of Se-PC
derived from Se-SP in the context of atherogenesis. Animal model:
Male Golden Syrian hamsters. | Control: Hamsters
received tap water. PC: Hamsters received native phycocyanin at a
dose of 3.63 mg/day per 100 g bw in water for 12 weeks. Se-PC:
Hamsters received Se-PC at a dose of 3.63 mg/day per 100 g bw for
12 weeks, providing 0.4 µg selenium per 100 g bw. SP: Hamsters
received crude SP at a dose of 2.66 mg/day per 100 g bw in water
for 12 weeks. Se-SP: Hamsters received Se-SP at a dose of 2.66
mg/day per 100 g bw for 12 weeks, providing 0.4 µg selenium per 100
g bw. | NA | Se-PC demonstrated
the most potent antioxidant and anti-atherogenic effects in
vivo, significantly reducing oxidative stress markers and
improving lipid profiles. Although PC and Se-SP showed beneficial
effects, Se-PC was the most effective in modulating NADPH oxidase
expression and plasma antioxidant capacity. | (26) |
Results and Discussion
Included studies
A total of 103 reports were identified, of which 62
articles were excluded due to duplications, 10 articles were
excluded as the full text was not available, 18 articles were out
of the scope and one article was published in Chinese. The
remaining 12 eligible articles were included in the systematic
review (Table I).
Se-SP
Se-SP is a biofortified form of the well-known
microalga, enhanced with selenium to boost its antioxidant and
protective properties (17). This
enriched form combines the natural benefits of Spirulina with the
potent biological activity of selenium, rendering it a promising
agent for health applications involving oxidative stress
management, immune support and cellular protection (18).
The protective effects of Se-SP against
alcohol-induced liver damage were evaluated in a study involving
HL7702 human liver cells and mice exposed to subacute alcohol
injury (19). In that study, in
vitro experiments revealed that Se-SP significantly alleviated
ethanol-related cytotoxicity in a concentration-dependent manner.
Compared to native SP, Se-SP was more effective in maintaining cell
viability, lowering the intracellular levels of reactive oxygen
species (ROS) and malondialdehyde (MDA), and boosting the activity
of key antioxidant enzymes, such as superoxide dismutase (SOD) and
glutathione peroxidase (GSH-Px or GPx). Se-SP also preserved
mitochondrial membrane potential (MMP) and reduced apoptosis, as
reflected by the lower expression of pro-apoptotic markers (Bax and
cleaved caspase-3) and higher levels of the anti-apoptotic protein,
Bcl-2. While SP also exhibited some antioxidant and cytoprotective
effects, its overall efficacy was consistently lower than that of
Se-SP (19). In vivo, mice
receiving 200 mg/kg Se-SP over a period of 42 days exhibited marked
improvement in biochemical indicators of liver injury. Serum
alanine aminotransferase and aspartate aminotransferase levels were
significantly reduced compared to the alcohol-only group and
remained closer to physiological ranges than in animals treated
with SP. Similarly, Se-SP was more effective in normalizing lipid
profiles, including total cholesterol (TC) and triglycerides,
without overshooting the normal values, an effect observed with SP
in some parameters. Se-SP also provided superior control over
oxidative stress. The activity of SOD and GSH-Px was more
significantly enhanced, and the MDA levels were more effectively
suppressed in the Se-SP-treated animals than in those treated with
SP (19). The histological
examination of liver tissues further confirmed these findings:
Se-SP more effectively preserved hepatic architecture and reduced
necrosis and inflammatory infiltration. Immunohistochemical
analyses revealed that Se-SP downregulated the levels of key
markers of apoptosis (caspase-9), autophagy (LC3) and pyroptosis
(caspase-1) more efficiently than SP. These findings underscore the
superior protective effects of Se-SP against alcohol-induced liver
cell injury, demonstrating greater efficacy than native Spirulina
in reducing apoptosis, autophagy and pyroptosis, while enhancing
antioxidant enzyme activity. Se-SP emerges as a promising dietary
supplement for the prevention and management of oxidative liver
damage (19).
The in vitro protective effects of Se-SP
against cisplatin-induced apoptosis were also previously
investigated (20). Cisplatin
exposure induced a dose-dependent increase in both early and late
apoptosis, accompanied by significant mitochondrial dysfunction,
opening of the mitochondrial permeability transition pore (MPTP)
and the excessive production of ROS. Pre-treatment with Se-SP
effectively mitigated these cytotoxic effects by preserving MMP and
restoring the balance between pro- and anti-apoptotic members of
the Bcl-2 family. This mitochondrial stabilization limited MPTP
opening and prevented the activation of the intrinsic apoptotic
cascade. Additionally, Se-SP markedly reduced ROS production and
superoxide anion levels, enhanced the activity of endogenous
antioxidant systems, such as SOD and GSH-Px, and protected cellular
DNA from oxidative damage (20).
At the molecular level, Se-SP pre-treatment suppressed the cleavage
of PARP and reduced the activation of caspase-3, caspase-7 and
caspase-9, key mediators of apoptosis. It also inhibited the
phosphorylation of DNA damage response proteins, including ataxia
telangiectasia mutated, ataxia telangiectasia and Rad3-related, and
tumor protein p53, confirming its role in attenuating oxidative
stress-driven apoptotic signaling (20). By contrast, SP without selenium
enrichment did not provide significant protection under the same
experimental conditions. SP pre-treatment failed to improve cell
viability, did not reduce apoptosis, and had negligible effects on
oxidative markers. This direct comparison highlights the
substantial enhancement of biological activity conferred by
selenium incorporation. In summary, Se-SP displayed a markedly
superior protective profile compared to its non-enriched
counterpart, demonstrating its potential as an effective adjuvant
in preventing cisplatin-induced oxidative damage and mitochondrial
dysfunction in bone-forming cells (20).
The protective effects and underlying mechanisms of
Se-SP against high glucose-induced calcification in mouse aortic
vascular smooth muscle cells was also examined in a previous study
(21). Exposure to elevated
glucose concentrations led to significant oxidative stress,
increased ROS production and DNA damage, all contributing to
pathological calcification in vascular tissues. Pre-treatment with
Se-SP significantly mitigated these effects by reducing ROS
accumulation and limiting oxidative DNA injury (21). In addition, Se-SP modulated key
intracellular signaling cascades involved in the calcification
process. Specifically, it suppressed the overactivation of the
mitogen-activated protein kinase (MAPK) pathway and prevented the
inhibition of the phosphoinositide 3-kinase/protein kinase B
(PI3K/AKT) pathway, thereby preserving cell homeostasis and
inhibiting pro-calcific responses. When compared to native SP,
Se-SP exhibited markedly greater efficacy. SP alone exhibited only
modest antioxidant activity and did not significantly affect either
MAPK or PI3K/AKT signaling under high-glucose conditions (21). Conversely, Se-SP not only reduced
oxidative stress more effectively, but also provided broader
cytoprotective effects by interfering with multiple
calcification-related molecular pathways. Overall, Se-SP
demonstrated superior protective effects against glucose-induced
vascular damage compared to SP, emphasizing the added value of
selenium enrichment in enhancing the bioactivity of SP. These
findings support the potential application of Se-SP in the
prevention of vascular complications associated with diabetes and
metabolic disorders (21).
The neuroprotective potential of Se-SP was further
evaluated in a study examining its effects against damage induced
by oxygen and glucose deprivation (OGD) in hippocampal neurons
harvested from neonatal rats (22). Se-SP treatment significantly
improved neuronal viability under OGD conditions. Neurons treated
with 10 µg/ml Se-SP exhibited a marked increase in viability, which
increased from 57.2 to 94.5%, whereas treatment with SP alone did
not provide significant protection (22). Se-SP also substantially reduced
OGD-induced neuronal apoptosis, as evidenced by a decrease in the
number of TUNEL-positive cells from 45.6 to 6.3%. Furthermore,
Se-SP inhibited the accumulation of ROS induced by OGD, improved
MMP and modulated the expression of Bcl-2 family proteins,
effectively maintaining a balance between pro-apoptotic and
anti-apoptotic factors (22).
These findings suggest that Se-SP exerts superior antioxidant and
cytoprotective effects compared to SP alone, effectively reducing
ROS production, preserving mitochondrial function, and preventing
neuronal apoptosis under OGD conditions. Thus, Se-SP has promising
neuroprotective effects, suggesting its potential as an
intervention to support neuronal survival and prevent damage
associated with ischemic events (22).
The potential of Se-SP to enhance health outcomes
has been investigated vs. its role in managing severe conditions
such as sepsis. Notably, its efficacy was assessed in an in
vivo study involving selenium-deficient female rats (23). That study found that rats treated
with Se-SP or sodium selenite (SS) exhibited significantly longer
survival times compared to those that received standard SP.
Specifically, rats supplemented with Se-SP demonstrated increased
plasma selenium levels and longer survival post-sepsis induction,
highlighting the beneficial role of selenium-enriched
supplementation. Se-SP treatment led to a significant increase in
the levels of the anti-inflammatory cytokine, IL-10, whereas other
treatment groups exhibited lower levels. Elevated levels of the
pro-inflammatory cytokines, IL-6 and TNF-α, were observed in most
groups following sepsis induction; however, the Se-SP group
maintained a more balanced cytokine response (23). Although Se-SP improved metabolic
stability and acid-base balance, the treatment did not fully
prevent sepsis-related mortality. These results suggest that while
Se-SP exhibits potential in enhancing survival and modulating
inflammatory responses during sepsis, its effectiveness in fully
combating severe sepsis remains limited (23). Overall, SS remains the most
effective option in correcting selenium deficiency and mitigating
sepsis-induced oxidative and metabolic disturbances. Although Se-SP
exhibits promise in upregulating antioxidant genes, it fails to
deliver functional protection in the acute setting, likely due to
lower bioavailability of organic Se forms (e.g., SeMet) or matrix
interactions. Spirulina, while theoretically antioxidant, offered
no protective effect and may even exacerbate early stress responses
in sepsis (23).
The effects of SP, Se-SP and SS on restoring
selenium levels and antioxidant defenses were also investigated in
another study involving 32 female Wistar rats fed a
selenium-deficient diet over a 12-week period (24). Se-SP supplementation effectively
restored selenium concentrations in the majority of tissues, such
as the liver and kidneys, outperforming SS. While Se-SP increased
GPx activity in some tissues, SS was more effective in enhancing
SOD activity, particularly in the heart. Additionally, Se-SP
restored the expression levels of certain selenoproteins in a
tissue-dependent manner, whereas SS had a more pronounced impact on
GPx1 expression in the heart. Se-SP ensured better selenium
distribution and tissue bioavailability, while SS more effectively
enhanced antioxidant enzyme activity and certain selenoprotein
expressions. Spirulina alone exhibited only marginal antioxidant
benefits without selenium repletion (24).
The anti-osteoporotic efficacy of
selenium-containing protein extracted from Se-SP, using both in
vitro and in vivo models was also previously
investigated (25). In
vitro, MC3T3-E1 osteoblast-like cells were treated with 5 and
10 µg/ml of either Se-SP or non-enriched SP for 14 days. In
vivo, ovariectomized female mice were divided into four groups
as follows: The sham-operated, untreated ovariectomized SP-treated
(10 mg/kg) and Se-SP-treated (10 mg/kg) mice, with treatment
administered intraperitoneally every other day for 2 months
(25). That study found that Se-SP
enhanced calcium deposition, alkaline phosphatase activity and the
expression of osteoblastic markers (BMP2, RUNX2, COL-I and OCN),
while also reducing osteoclastogenesis (TRAcP and RANKL) and
promoting anti-inflammatory cytokine production (IL-4 and IL-10).
Compared to SP alone, Se-SP demonstrated superior efficacy in
reversing bone loss and restoring bone microarchitecture in
ovariectomized mice (25).
Se-PC
Se-PC, a compound derived from Se-SP, has attracted
marked interest for its potent antioxidant and anticancer
properties. This bioactive molecule combines the well-known
benefits of phycocyanin with the enhanced activity provided by
selenium, an essential trace element known for its antioxidant
capabilities. The studies identified in the literature collectively
highlight the diverse and significant biological activities of
Se-PC and its potential applications in various health and
therapeutic contexts. The cardiovascular and oxidative stress
protective effects of Se-PC derived from SP was first studied in
the context of atherogenesis (26). In that study conducted in
vivo on male Golden Syrian hamsters, Se-PC demonstrated
significant lipid-lowering and antioxidant effects. Se-PC reduced
plasma TC levels by 10%, outperforming native phycocyanin (PC),
which achieved a 7.5% reduction. Additionally, Se-PC significantly
decreased non-HDL cholesterol levels by 34%, a more pronounced
effect compared to PC. As regards oxidative stress markers, Se-PC
restored plasma antioxidant capacity to near-normal levels, showing
a 42% improvement over the control group, while PC, SP and Se-SP
were effective to a lesser extent (26). In cardiac tissue, Se-PC reduced
superoxide anion production by 76%, significantly exceeding the
reductions achieved by PC (54%) and spirulina variants (46-56%). In
liver tissues, GSH-Px and SOD activities were lower in all
treatment groups compared to the controls, suggesting a potential
‘sparing effect’ of the exogenous antioxidants provided by Se-PC
and PC. These results highlight the superior efficacy of Se-PC in
modulating lipid metabolism and reducing oxidative stress (26). Se-PC demonstrated the most potent
antioxidant and anti-atherogenic effects in vivo,
significantly reducing oxidative stress markers and improving lipid
profiles. Although PC and Se-SP exerted beneficial effects, Se-PC
was the most effective in modulating NADPH oxidase expression and
plasma antioxidant capacity (26).
The in vitro antioxidant and
antiproliferative activities of Se-PC were investigated in another
study with the aim of examining its role in inducing the apoptosis
of human melanoma cells (A375), human breast adenocarcinoma cells
(MCF-7) and human fibroblasts (Hs68) (27). The findings of that study revealed
that Se-PC significantly increased the percentage of depolarized
mitochondria in A375 cells from 11.6% in the control group to 39.0
and 54.7% at the 10 and 20 µM concentrations, respectively. A
similar trend was observed in MCF-7 cells, where the percentage of
depolarized mitochondria increased from 1.3% in the control to 13.2
and 20.9% at the same concentrations (27). These results suggest that Se-PC
induces apoptosis via a mitochondria-mediated pathway. Furthermore,
Se-PC demonstrated potent antioxidant properties, inhibiting
2,2'-azino-bis (3-ethylbenzothiazoline-6-sulfonate) (ABTS)
oxidation by 18.8% at 0.5 µM and 46.0% at 1.0 µM. This performance
surpassed that of regular PC, which exhibited inhibition rates of
11.7 and 31.1% at the same concentrations. Furthermore, both Se-PC
and PC led to a significantly greater inhibition of ABTS oxidation
compared to ascorbic acid and Trolox (27). Another significant finding was the
scavenging activity of Se-PC against superoxide anions, which are
biologically crucial due to their potential to decompose into more
reactive oxidative species, such as singlet oxygen and hydroxyl
radicals. That study found that Se-PC and PC inhibited superoxide
anions in a concentration-dependent manner over a range of 1-16 µM.
Additionally, Se-PC demonstrated protective effects against
H2O2-induced DNA damage. While the control
group treated with H2O2 alone exhibited DNA
damage, no significant DNA damage was observed in groups treated
with Se-PC at concentrations of 10 or 50 µM. Notably, Se-PC was
found to be a non-genotoxic compound (27). In summary, Se-PC exhibited superior
antioxidant activities compared to PC by effectively neutralizing
ABTS, superoxide anion, 2,2-diphenyl-1-picrylhydrazyl (DPPH) and
2,2'-azobis(2-methylpropionamidine) dihydrochloride (AAPH) free
radicals. It also exerted protective effects against
H2O2-induced DNA damage in blood cells. The
most noteworthy effect of Se-PC was its ability to inhibit cell
growth in A375 melanoma and MCF-7 breast adenocarcinoma cells,
primarily by inducing mitochondria-mediated apoptosis. Overall,
Se-PC outperformed native PC across all assays, confirming its
potential as a selenium-based chemopreventive agent (27).
In another study in vitro, the protective
effects of Se-PC were evaluated against oxidative stress induced by
AAPH (28). Specifically, that
study explored the ability of selenium-enriched allophycocyanin
(Se-APC) to inhibit ROS generation, prevent lipid peroxidation and
protect antioxidant defense systems in human erythrocytes. Se-APC
demonstrated superior antioxidant activity compared to regular APC,
as evidenced by its significantly higher inhibition of ABTS
radicals. It also exhibited protective effects against hemolysis,
reducing AAPH-induced hemolysis in erythrocytes in a dose-dependent
manner. Additionally, Se-APC effectively inhibited lipid
peroxidation, significantly decreasing MDA formation and restoring
levels to near control values at a concentration of 1.5 µM.
Furthermore, Se-APC protected against ROS generation, reducing ROS
levels to 195% at 0.3 µM and returning them to near control levels
at 1.5 µM (28). Finally, Se-APC
preserved the antioxidant defense system by significantly restoring
GSH levels in a concentration-dependent manner. It also prevented
the increase in GPx and GSH reductase activities, maintaining their
levels comparable to controls when used at 1.5 µM. Se-APC exhibited
clearly superior antioxidant and cytoprotective properties compared
to native APC in vitro. It effectively scavenged radicals,
protected erythrocytes from oxidative hemolysis and preserved
cellular antioxidant defenses, rendering it a promising candidate
for functional food or therapeutic applications targeting oxidative
stress-related damage (28).
The therapeutic potential of Se-PC in enhancing the
efficacy of photodynamic therapy (PDT) for cancer treatment was
evaluated both in vitro and in vivo (29,30).
In vitro experiments conducted on Lewis Lung Carcinoma (LLC)
cells demonstrated that Se-PC PDT resulted in significantly higher
levels of ROS compared to PC PDT or PC-SS PDT, indicating an
enhanced induction of oxidative stress within the cancer cells
(30). Furthermore, Se-PC PDT
treatment led to significantly reduced cell survival rates compared
to PC PDT alone, highlighting its superior efficacy in targeting
LLC cells. Se-PC represented the most effective and balanced
treatment, providing potent cytotoxicity against tumor cells, while
preserving antioxidant defenses in normal tissues and minimizing
systemic toxicity. Its dual action on tumor inhibition and immune
activation rendered it the most promising strategy among the three
approaches evaluated (30). In a
study conducted on HepG2 cells (human liver cancer cells) and
HL7702 cells (normal human liver cells), Se-PC photodynamic therapy
(Se-PC PDT) demonstrated selective cytotoxicity, significantly
reducing cell viability in HepG2 cells while sparing HL7702 cells
(29). The treatment markedly
increased intracellular ROS levels in HepG2 cells, leading to
oxidative damage and apoptosis, which was significantly more
pronounced in the Se-PC PDT group compared to the PC PDT group.
This selective induction of apoptosis highlights the potential of
Se-PC PDT as a targeted therapeutic approach against liver cancer
cells, while minimizing damage to normal liver cells (29).
In vivo, using lung carcinoma-bearing male
C57BL/6 mice, Se-PC PDT demonstrated notable efficacy with a tumor
inhibition rate of 90.1%, significantly higher than the PC PDT
group (53.1%) and the PC-SS PDT group (68.3%) (30). Se-PC PDT also inhibited metastasis,
as evidenced by reduced luminescence in major organs, such as the
liver and lungs. The treatment enhanced antioxidant defense
mechanisms, increasing the activities of SOD and GSH-Px in liver
and lung tissues, while maintaining lower levels of MDA, an
oxidative stress marker, compared to the PC-SS PDT group,
indicating reduced damage to normal tissues. Additionally, Se-PC
PDT significantly elevated serum levels of IL-6 and TNF-α,
suggesting an enhanced immune response. Mechanistically, Se-PC PDT
induced both apoptosis and pyroptosis in tumor cells, with gene
expression analysis revealing the upregulation of caspase-1,
caspase-3 and caspase-9, alongside the reduced expression of the
anti-apoptotic marker, Bcl-2. Furthermore, Se-PC PDT modulated
critical signaling pathways, including NF-κB, IL-17 and HIF-1,
involved in inflammation, tumor metabolism and immune responses.
The treatment also downregulated genes associated with angiogenesis
and tumor progression, such as Vegfa, Mmp13, and Serpine1,
highlighting its multifaceted anti-tumor effects (30). In a study conducted on BALB/c mice,
the Se-PC PDT group exhibited the most significant reduction in
tumor volume and weight compared to all other groups (29). This treatment also effectively
decreased oxidative stress markers, such as MDA, while enhancing
the activity of antioxidant enzymes, including SOD and GSH-Px, in
tumor tissues. Additionally, the Se-PC PDT group exhibited a marked
increase in apoptosis within tumor cells, highlighting its potent
antitumor and antioxidative effects (30). These findings collectively
emphasize Se-PC combined with PDT induces potent anticancer
activity through mitochondria-mediated apoptosis, partial
inhibition of autophagy, and enhanced antioxidant enzyme
modulation. It demonstrates stronger efficacy and higher tumor
selectivity than PC alone, offering a promising and safer strategy
for liver tumor therapy (30).
In conclusion, the present systematic review
provides robust evidence that selenium enrichment significantly
enhances the biological efficacy of both SP and its key
pigment-protein complex, phycocyanin extracted from Se-SP, across a
wide range of experimental settings. Compared to their native
counterparts, both Se-SP and Se-PC consistently show superior
antioxidant, cytoprotective, anti-inflammatory, and in some cases,
anticancer activities.
Specifically, Se-SP exhibits markedly more potent
protective effects than SP alone, as demonstrated in models of
alcohol-induced liver damage, cisplatin-induced cytotoxicity, high
glucose-induced vascular calcification, and oxygen-glucose
deprivation in neurons. Se-SP improves mitochondrial function,
reduces oxidative markers (e.g., ROS and MDA), enhances antioxidant
enzyme activity (e.g., SOD and GPx), and modulates
apoptosis-related pathways more effectively than non-enriched
Spirulina. Notably, the superiority of Se-SP emerges most clearly
in models of hepatic injury, neuroprotection and bone loss, where
its selenium-mediated modulation of signaling pathways plays a
decisive role. However, Se-SP appears less bioavailable and less
effective than inorganic selenium (e.g., SS) in acute systemic
conditions such as sepsis, limiting its applicability in urgent or
high-burden clinical contexts.
Likewise, Se-PC significantly outperforms native PC
in antioxidant capacity, radical scavenging activity, mitochondrial
protection and selective cytotoxicity in cancer models. In
particular, Se-PC demonstrates enhanced efficacy in PDT for cancer,
exerting dual effects of tumor apoptosis and immune activation
while maintaining low systemic toxicity. Compared to PC, Se-PC more
potently modulates oxidative stress, preserves redox homeostasis,
and activates apoptosis-related gene pathways, making it a
promising candidate for targeted therapeutic strategies,
particularly in oncology.
Despite the compelling results, the available
literature presents several limitations, including the scarcity of
direct comparative studies between Se-SP and Se-PC, and between
these selenium-enriched compounds and conventional selenium forms
or combinatorial strategies. Variability in enrichment protocols,
dosage regimens, and biological models further complicates
comparative interpretation.
Future research is required to prioritize direct
head-to-head comparisons of Se-SP vs. Se-PC under standardized
conditions, both to elucidate their mechanistic divergences and to
optimize their use in specific pathological contexts. Long-term
in vivo studies and clinical trials will be crucial for
validating safety profiles, bioavailability, and dose-response
relationships. Exploring combinatorial therapies and synergistic
interactions with other bioactive may further expand their
application in preventive and functional nutrition, as well as in
integrative medicine.
Acknowledgements
The present study was supported by the Ministry of
University and Research (MUR) as part of the FSE REACT-EU-PON
2014-2020 ‘Research and Innovation’ resources-Green/Innovation
Action-DM MUR 1062/2021-Title of the Research ‘GrEEnoncoprev’. The
present study was also supported by the Department of Medical,
Surgical and Advanced Technologies ‘G.F. Ingrassia’, University of
Catania, Italy.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CS conceptualized the study. CS, CF and CC were
involved in the study methodology. CS, CF, CC, PR carried out the
search and screening of the articles for inclusion in the
systematic review. For the records whose inclusion was in doubt, a
focus group was carried out with MF, GOC and MC. The focus group
approved the final eligibility of records. PR validated the
methodological approach. CC, GOC, and MC were involved in assessing
the risk of bias for the articles screening. CF, PR, MC, MF, and
GOC were involved in data curation, writing and preparation of the
original draft of the manuscript. CC, GOC, MF and MC reviewed and
edited the final draft of manuscript. MF and GOC, supervised, and
edited the final draft. GOC and MF confirm the authenticity of all
the raw data. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Leong YK and Chang JS: Integrated role of
algae in the closed-loop circular economy of anaerobic digestion.
Bioresour Technol. 360(127618)2022.PubMed/NCBI View Article : Google Scholar
|
|
2
|
Copat C, Favara C, Tomasello MF, Sica C,
Grasso A, Dominguez HG, Conti GO and Ferrante M: Astaxanthin in
cancer therapy and prevention (review). Biomed Rep.
22(66)2025.PubMed/NCBI View Article : Google Scholar
|
|
3
|
Gutiérrez-Salmeán G, Fabila-Castillo L and
Chamorro-Cevallos G: Nutritional and toxicological aspects of
spirulina (Arthrospira). Nutr Hosp. 32:34–40. 2015.PubMed/NCBI View Article : Google Scholar
|
|
4
|
Zakaria SM and Kamal SMM: Subcritical
water extraction of bioactive compounds from plants and algae:
Applications in pharmaceutical and food ingredients. Food Eng Rev.
8:23–34. 2016.
|
|
5
|
Abdel-Moneim AME, El-Saadony MT, Shehata
AM, Saad AM, Aldhumri SA, Ouda SM and Mesalam NM: Antioxidant and
antimicrobial activities of Spirulina platensis extracts and
biogenic selenium nanoparticles against selected pathogenic
bacteria and fungi. Saudi J Biol Sci. 29:1197–1209. 2022.PubMed/NCBI View Article : Google Scholar
|
|
6
|
Maddiboyina B, Vanamamalai HK, Roy H,
Ramaiah null, Gandhi S, Kavisri M and Moovendhan M: Food and drug
industry applications of microalgae Spirulina platensis: A
review. J Basic Microbiol. 63:573–583. 2023.PubMed/NCBI View Article : Google Scholar
|
|
7
|
Khan Z, Bhadouria P and Bisen PS:
Nutritional and therapeutic potential of Spirulina. Curr Pharm
Biotechnol. 6:373–379. 2005.PubMed/NCBI View Article : Google Scholar
|
|
8
|
Pak W, Takayama F, Mine M, Nakamoto K,
Kodo Y, Mankura M, Egashira T, Kawasaki H and Mori A:
Anti-oxidative and anti-inflammatory effects of spirulina on rat
model of non-alcoholic steatohepatitis. J Clin Biochem Nutr.
51:227–234. 2012.PubMed/NCBI View Article : Google Scholar
|
|
9
|
Zhang Y, Li L, Qin S, Yuan J, Xie X, Wang
F, Hu S, Yi Y and Chen M: C-phycocyanin alleviated
cisplatin-induced oxidative stress and inflammation via gut
microbiota-metabolites axis in mice. Front Nutr.
9(996614)2022.PubMed/NCBI View Article : Google Scholar
|
|
10
|
Li X, Ma L, Zheng W and Chen T: Inhibition
of islet amyloid polypeptide fibril formation by
selenium-containing phycocyanin and prevention of beta cell
apoptosis. Biomaterials. 35:8596–8604. 2014.PubMed/NCBI View Article : Google Scholar
|
|
11
|
Surai PF, Fisinin VI and Karadas F:
Antioxidant systems in chick embryo development. Part 1. Vitamin E,
carotenoids and selenium. Anim Nutr. 2:1–11. 2016.PubMed/NCBI View Article : Google Scholar
|
|
12
|
Wu Q, Liu L, Miron A, Klímová B, Wan D and
Kuča K: The antioxidant, immunomodulatory, and anti-inflammatory
activities of Spirulina: an overview. Arch Toxicol. 90:1817–1840.
2016.PubMed/NCBI View Article : Google Scholar
|
|
13
|
Tajvidi E, Nahavandizadeh N, Pournaderi M,
Pourrashid AZ, Bossaghzadeh F and Khoshnood Z: Study the
antioxidant effects of blue-green algae Spirulina extract on ROS
and MDA production in human lung cancer cells. Biochem Biophys Rep.
28(101139)2021.PubMed/NCBI View Article : Google Scholar
|
|
14
|
Han P, Li J, Zhong H, Xie J, Zhang P, Lu
Q, Li J, Xu P, Chen P, Leng L and Zhou Z: Anti-oxidation properties
and therapeutic potentials of spirulina. Algal Res.
55(102240)2021.
|
|
15
|
Yang F, Tang Q, Zhong X, Bai Y, Chen T,
Zhang Y, Li Y and Zheng W: Surface decoration by Spirulina
polysaccharide enhances the cellular uptake and anticancer efficacy
of selenium nanoparticles. Int J Nanomedicine. 7:835–844.
2012.PubMed/NCBI View Article : Google Scholar
|
|
16
|
Alipour S, Kalari S, Morowvat MH, Sabahi Z
and Dehshahri A: Green synthesis of selenium nanoparticles by
cyanobacterium Spirulina platensis (abdf2224): Cultivation
condition quality controls. BioMed Res Int.
2021(6635297)2021.PubMed/NCBI View Article : Google Scholar
|
|
17
|
Menon S, Ks SD, R S, S R and S VK:
Selenium nanoparticles: A potent chemotherapeutic agent and an
elucidation of its mechanism. Colloids Surf B Biointerfaces.
170:280–292. 2018.PubMed/NCBI View Article : Google Scholar
|
|
18
|
Pires R, Costa M, Pereira H, Cardoso H,
Ferreira L, Lapa N, Silva J and Ventura M: Microalgae as a selenium
vehicle for nutrition: A review. Discov Food. 4(84)2024.
|
|
19
|
Fu X, Zhong Z, Hu F, Zhang Y, Li C, Yan P,
Feng L, Shen J and Huang B: The protective effects of
selenium-enriched Spirulina platensis on chronic
alcohol-induced liver injury in mice. Food Funct. 9:3155–3165.
2018.PubMed/NCBI View Article : Google Scholar
|
|
20
|
Sun JY, Hou YJ, Fu XY, Fu XT, Ma JK, Yang
MF, Sun BL, Fan CD and Oh J: Selenium-containing protein from
selenium-enriched Spirulina platensis attenuates
cisplatin-induced apoptosis in MC3T3-E1 mouse preosteoblast by
inhibiting mitochondrial dysfunction and ros-mediated oxidative
damage. Front Physiol. 9(1907)2019.PubMed/NCBI View Article : Google Scholar
|
|
21
|
Lin C, Zhang LJ, Li B, Zhang F, Shen QR,
Kong GQ, Wang XF, Cui SH, Dai R, Cao WQ and Zhang P:
Selenium-containing protein from selenium-enriched Spirulina
platensis attenuates high glucose-induced calcification of
MOVAS cells by inhibiting ROS-mediated DNA damage and regulating
MAPK and PI3K/AKT pathways. Front Physiol. 11(791)2020.PubMed/NCBI View Article : Google Scholar
|
|
22
|
Song X, Zhang L, Hui X, Sun X, Yang J,
Wang J, Wu H, Wang X, Zheng Z, Che F and Wang G:
Selenium-containing protein from selenium-enriched Spirulina
platensis antagonizes oxygen glucose deprivation-induced
neurotoxicity by inhibiting ROS-mediated oxidative damage through
regulating MPTP opening. Pharm Biol. 59:629–638. 2021.PubMed/NCBI View Article : Google Scholar
|
|
23
|
Castel T, Theron M, Pichavant-Rafini K,
Guernec A, Joublin-Delavat A, Gueguen B and Leon K: Can
selenium-enriched spirulina supplementation ameliorate sepsis
outcomes in selenium-deficient animals? Physiol Rep.
9(e14933)2021.PubMed/NCBI View Article : Google Scholar
|
|
24
|
Castel T, Léon K, Gandubert C, Gueguen B,
Amérand A, Guernec A, Théron M and Pichavant-Rafini K: Comparison
of sodium selenite and selenium-enriched spirulina supplementation
effects after selenium deficiency on growth, tissue selenium
concentrations, antioxidant activities, and selenoprotein
expression in rats. Biol Trace Elem Res. 202:685–700.
2024.PubMed/NCBI View Article : Google Scholar
|
|
25
|
Yang Y, Yang H, Feng X, Song Q, Cui J, Hou
Y, Fu X and Pei Y: Selenium-containing protein from
selenium-enriched Spirulina platensis relieves osteoporosis
by inhibiting inflammatory response, osteoblast inactivation, and
osteoclastogenesis. J Food Biochem. 2024(3873909)2024.
|
|
26
|
Riss J, Décordé K, Sutra T, Delage M,
Baccou JC, Jouy N, Brune JP, Oréal H, Cristol JP and Rouanet JM:
Phycobiliprotein C-phycocyanin from Spirulina platensis is
powerfully responsible for reducing oxidative stress and NADPH
oxidase expression induced by an atherogenic diet in hamsters. J
Agric Food Chem. 55:7962–7967. 2007.PubMed/NCBI View Article : Google Scholar
|
|
27
|
Chen T and Wong YS: In vitro antioxidant
and antiproliferative activities of selenium-containing phycocyanin
from selenium-enriched Spirulina platensis. J Agric Food
Chem. 56:4352–4358. 2008.PubMed/NCBI View Article : Google Scholar
|
|
28
|
Zhang H, Chen T, Jiang J, Wong YS, Yang F
and Zheng W: Selenium-containing allophycocyanin purified from
selenium-enriched Spirulina platensis attenuates
AAPH-induced oxidative stress in human erythrocytes through
inhibition of ROS generation. J Agric Food Chem. 59:8683–8690.
2011.PubMed/NCBI View Article : Google Scholar
|
|
29
|
Liu Z, Fu X, Huang W, Li C, Wang X and
Huang B: Photodynamic effect and mechanism study of
selenium-enriched phycocyanin from Spirulina platensis
against liver tumours. J Photochem Photobiol B. 180:89–97.
2018.PubMed/NCBI View Article : Google Scholar
|
|
30
|
Shen J, Xia H, Zhou X, Zhang L, Gao Q, He
K, Liu D and Huang B: Selenium enhances photodynamic therapy of
C-phycocyanin against lung cancer via dual regulation of
cytotoxicity and antioxidant activity. Acta Biochim Biophys Sin
(Shanghai). 55:1925–1937. 2023.PubMed/NCBI View Article : Google Scholar
|